Abstract
Fas/FasL signaling is best known for induction of apoptosis. However, there is an alternate pathway of Fas signaling that induces inflammatory cytokines, particularly tumor necrosis factor (TNF)-α and interleukin (IL)-8. This pathway is prominent in cells that express high levels of anti-apoptotic molecules such as Bcl-xL. Because TNF-α is central to the pathogenesis of psoriasis and psoriatic epidermis has a low apoptotic index with high expression of Bcl-xL, we hypothesized that inflammatory Fas signaling mediates induction of psoriasis by activated lymphocytes. Noninvolved skin from psoriasis patients was grafted to beige-severe combined immunodeficiency mice, and psoriasis was induced by injection of FasL-positive autologous natural killer cells that were activated by IL-2. Induction of psoriasis was inhibited by injection of a blocking anti-Fas (ZB4) or anti-FasL (4A5) antibody on days 3 and 10 after natural killer cell injection. Anti-Fas monoclonal antibody significantly reduced cell proliferation (Ki-67) and epidermal thickness, with inhibition of epidermal expression of TNF-α, IL-15, HLA-DR, and ICAM-1. Fas/FasL signaling is an essential early event in the induction of psoriasis by activated lymphocytes and is necessary for induction of key inflammatory cytokines including TNF-α and IL-15.
Boehncke and colleagues1 have demonstrated that lymphocytes are capable of “pulling the trigger on psoriasis.” Psoriasis requires immune activation, and treatments specifically targeting T lymphocytes can clear psoriasis.2,3 Psoriasis can be induced in noninvolved skin from psoriasis donors when it is grafted onto severe combined immunodeficiency (SCID) mice combined with injection of autologous lymphocytes activated by bacterial superantigens.4–6 Natural killer (NK) cells can also induce psoriasis in noninvolved skin grafts from psoriasis donors.7,8 It is likely that there is a primary skin defect responsible for psoriasis, but the triggering of this defect, and the resulting pathology, requires immune activation.9 The major outstanding question is by what mechanism do activated T cells or NK cells induce psoriasis?
Blocking of tumor necrosis factor (TNF)-α can clear psoriasis in clinical studies, indicating it has an essential role in psoriasis pathogenesis.10,11 TNF-α also has a critical role in a human skin graft/immunodeficient mouse model of psoriasis.12 Interleukin (IL)-15 inhibition by monoclonal antibody (mAb) injection inhibits psoriasis induction in human skin grafts.13 Any model of psoriasis pathology must thus explain this central role for IL-15 and TNF-α.
Fas (CD95) activation generally induces apoptosis. However, there is evidence from multiple cell types that Fas has an alternative signaling pathway that induces inflammatory cytokines including TNF-α and IL-8. This inflammatory pathway may become predominant in the absence of apoptosis.14 Psoriatic epidermis expresses increased levels of Fas along with the anti-apoptotic molecule Bcl-xL.15–17 Keratinocytes isolated from psoriasis donors are relatively resistant to apoptosis.18 Elevated expression of anti-apoptotic factors in psoriatic epidermis should inhibit Fas-mediated apoptosis and may promote the production of TNF-α in response to FasL/Fas signaling. We hypothesized that psoriasis is induced by FasL/Fas signaling by activated lymphocytes, resulting in inflammatory cytokine (eg, TNF-α and IL-8) production by keratinocytes.
Materials and Methods
Animals
C.B-17/IcrHsd-scid-bg (beige-SCID) mice (Harlan Laboratories Ltd., Jerusalem, Israel), 2 to 3 months of age, were used in this study. The mice were raised in the pathogen-free animal facility of the B. Rappaport Faculty of Medicine, Technion-Israel Institute of Technology. Animal care and research protocols were in accordance with institutional guidelines and were approved by the institutional committee on animal use.
Patients
After receiving approval of the institutional ethics committee, nine psoriatic patients were included in this study. All patients had classic plaque psoriasis. None of the patients were treated. Nonlesional psoriatic skin was obtained from the thighs of each patient by electrical dermatome (Brown 666 Dermatome; Zimmer, Warsaw, IN).
Culture of Cells with NK Activity and Receptors
Peripheral blood mononuclear cells were isolated from autologous psoriatic donors by centrifugation on Ficoll/Hypaque (Amersham Biosciences, Piscataway, NJ). The peripheral blood mononuclear cells were then cultured with 100 U/ml IL-2 (Pepro Tech Inc., Rocky Hill, NJ) in medium composed of RPMI 1640, 10% human AB serum (Sigma, St. Louis, MO), 1% glutamine, and 1% antibiotics (media components; Biological Industries, Kibbutz Beit Haemeck, Israel). Medium was changed as needed. After 21 days the cells were injected into human skin explants on beige-SCID mice. Such cell lines express heterogeneous NK cell markers and exhibit NK cytotoxicity.9 NK cells generated by IL-2 stimulation (phenotyped from five donors) were heterogeneous with respect to phenotype, with 49 to 80% of cells positive for FasL and a mean of 38% of cells positive for both CD56 and FasL.
Skin Transplantation and Injection of Lymphocytes
Skin transplantation was performed as described previously.9 Skin from a single donor was divided into three or four segments and grafted onto one mouse for each group, with three to four groups per donor. Skin from each of nine psoriatic donors was divided and grafted onto one mouse for each group, with three to four groups per donor. Each mouse had only one graft, so that each donor was represented by one mouse per group. NK cells were injected 4 weeks after skin engraftment. NK cells were suspended in complete medium (RPMI 1640, 10% human AB serum, 1% glutamine, 1% antibiotics, 100 U/ml IL-2), at 107 cells/ml, and injected intradermally in 0.7 ml (7 × 106 cells). Four weeks after lymphocyte injection (8 weeks after engraftment), the grafts were harvested. Grafts were analyzed by histology and immunohistochemistry.
Anti-Fas (CD95) and Anti-FasL Antibody Injections
Blocking anti-human Fas (CD95) monoclonal antibody ZB4 and anti-human FasL monoclonal antibody (4A5) were obtained from MBL (Nagoya, Japan). ZB4 does not induce apoptosis and blocks Fas-mediated apoptosis.19 Neither antibody contained azide. Antibodies were injected intradermally into the grafts 3 days and 10 days after NK cell injections. Mice received 25 μg of antibody in a volume of 0.3 ml on days 3 and 10, for a total of 50 μg. Control mice received isotype control mouse IgG.
Determination of Epidermal Thickness
Histological assessment of the grafts was performed by light microscopy both before and after transplantation. Two blinded observers performed evaluation, one who was not aware of the design of the study. Epidermal thickness was determined with an ocular micrometer at a minimum of 50 points along the epidermis selected to represent points of maximal and minimal thickness. Thickness of the suprapapillary plate was similarly measured at 50 points for each sample.
Immunohistochemical Staining of Frozen Sections
Monoclonal antibodies to human antigens were as follows for immunohistochemistry of frozen sections: anti-HLA-DR (Becton Dickinson, San Jose, CA), anti-CD54 (ICAM-1) (Biodesign), and anti-Fas (9CD95) (DAKO, Carpinteria, CA). Purified murine IgG was used as a control for the above antibodies. Immunohistochemistry was performed on OCT-embedded specimens with a biotin-avidin system (Vectastain; Vector Laboratories, Burlingame, CA).
Immunohistochemical Staining of Paraffin Sections
Goat anti-human TNF-α (R&D Systems, Minneapolis, MN) was used on deparaffinized and peroxidase-blocked slides. Sections were treated with citrate buffer, pH 6, in the microwave oven for 20 minutes, cooled for 30 minutes at room temperature, and blocked for nonspecific binding as well as avidin-biotin. All washes were performed with phosphate-buffered saline-saponin. Anti-TNF-α was applied overnight at 4°C. Slides were next incubated with biotinylated rabbit anti-goat IgG (DAKO), followed by streptavidin-horseradish-peroxidase (Jackson Immunoresearch, West Grove, PA). The color was developed with 3-amino-9-ethylcarbazole. FasL was detected on deparaffinized paraffin sections as described above using mouse anti-human FasL (BD Transduction Laboratories, Lexington, KY), followed by biotinylated horse anti-mouse IgG (Vector Laboratories), and streptavidin horseradish peroxidase (Jackson Immunoresearch). IL-15 was detected using the above method with mouse anti-human IL-15 (R&D Systems). Ki-67 was detected with mouse anti-human Ki-67 (Zymed, South San Francisco, CA) using the above procedure, except that antigen retrieval was achieved with ethylenediaminetetraacetic acid, pH 8, buffer.
RNA Preparation and cDNA Synthesis
Total RNA was extracted from 4-mm-diameter skin-punch biopsies using RNA STAT 60 (Tel-Test Inc., TX,) according to the manufacturer’s instructions, with the addition of proteinase K and DNase digestion steps. RNA concentration and purity were determined spectrophotometrically by measuring fluorescence at 260 nm and 280 nm. Total RNA (1 μg) was reverse-transcribed into cDNA in a total volume of 20 μl (100 μg/μL) using transcription reagents (Promega, Madison, WI) according to the manufacturer’s instructions.
TaqMan Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
Approximately 10 ng (1 μl) of input RNA was used in subsequent TaqMan analysis. Sequence-specific primers and probes for TaqMan quantitative PCR analysis of mRNA expression of GAPDH and TNF-α genes were purchased from Applied Biosystems (Foster City, CA) (assay on demand). PCR 1× (50°C for 2 minutes, 95°C for 10 minutes), 45× (95°C for 15 seconds, 60°C for 1 minute) was performed in the presence of 10 μl of TaqMan Universal PCR Master Mix (PE Applied Biosystems), 1 μl of forward and reverse primers, and a sequence-specific fluorescent probe. Real-time PCR was performed on an ABI Prism 7700 sequence detector (TaqMan), in which fluorescent output, measured as cycle threshold (Ct), was directly proportional to input cDNA concentration.
Statistical Analysis
All multiple statistical comparisons were performed using analysis of variance, Tukey-Kramer multiple comparison tests. Single comparisons were performed with χ2 as indicated. P < 0.05 was considered statistically significant.
Results
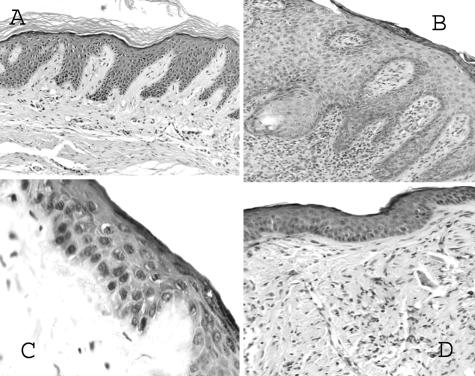
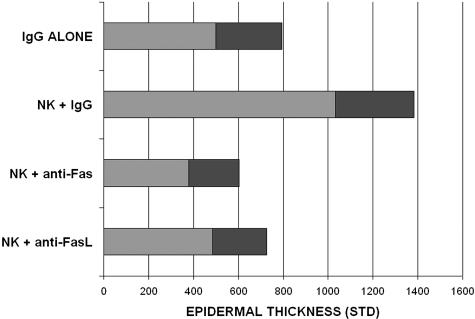
Skin from noninvolved sites of psoriasis donors was grafted onto C.B-17/IcrHsd-scid-bg (beige-SCID) mice. All of the features of psoriasis were replicated in all (nine of nine) grafts with injection of NK cells, including parakeratosis, hyperkeratosis, absence of granular layer, regular acanthosis (increased epidermal thickness), elongation of rete ridges, and increased dermal vasculature. Injection of blocking anti-Fas monoclonal antibody (ZB4) resulted in absence of all of the above features in grafts (zero of seven displayed characteristics), as did injection of anti-FasL in grafts (zero of eight displayed characteristics) (Figure 1). Munro’s microabscesses with neutrophils were observed in five of nine positive control grafts injected with NK cells, but not in the antibody-injected groups. Blocking with either anti-Fas or anti-FasL monoclonal antibody (mAb) resulted in significant (P < 0.01) reduction in epidermal thickness (Figure 2). Anti-Fas mAb treatment also resulted in significant (P < 0.01) reduction in proliferation index. Anti-FasL mAb was less effective and did not result in a significant difference in proliferation index.
Figure 1.
Histology of human grafts on beige-SCID mice injected with control mouse IgG (A), NK cells plus control IgG (B), NK cells plus blocking anti-human Fas (ZB4) mAb (C), and NK cells plus anti-human FasL mAb (D).
Figure 2.
Epidermal thickness of human grafts injected with control mouse IgG, NK cells plus control IgG, NK cells plus blocking anti-human Fas (ZB4), and NK cells plus anti-human FasL. Epidermal thickness is shown in nm with shaded area (1 STD).
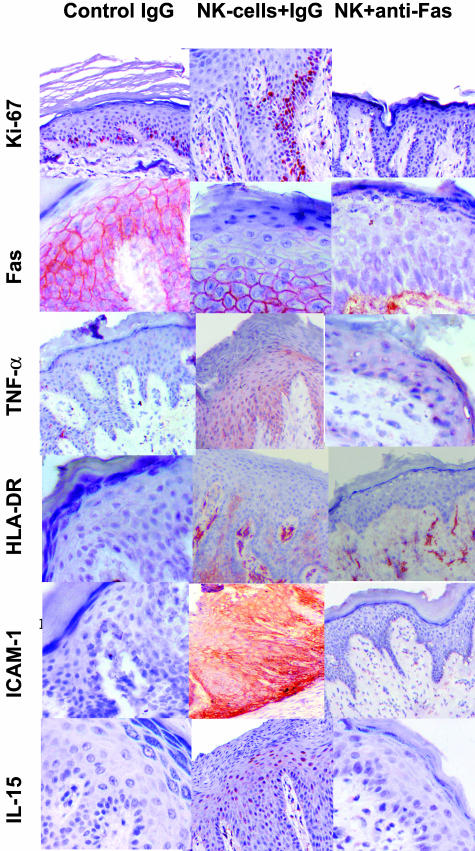
NK cells expressed FasL (49 to 80% positive) and epidermis expressed Fas and this is consistent with our hypothesis. The NK cell lines were heterogeneous with a mean of 38% of cells positive for both CD56 and FasL. Fas was expressed in epidermis of all (six of six) grafts injected with NK cells, as well as (five of six) grafts not injected (Figure 3). Grafts injected with both anti-Fas mAb and NK cells did not express Fas (zero of four versus six of six; P < 0.05 by χ2). Injection of anti-FasL did not block epidermal expression of Fas. It is possible that the lack of expression of Fas by anti-Fas-treated grafts reflects masking of epitopes by the blocking antibody.
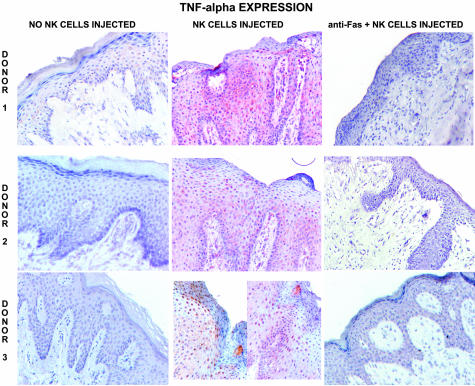
Figure 3.
Expression of TNF-α, IL-15, Ki-67, HLA-DR, ICAM-1, and Fas by grafts injected with control mouse IgG, NK cells plus control IgG, and NK cells plus blocking anti-human Fas (ZB4) mAb.
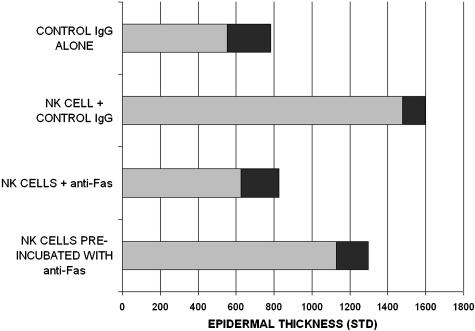
It was necessary to determine whether the anti-Fas mAb (ZB4) was acting directly on the epidermis or the injected NK cells. ZB4 has been previously shown to block Fas-mediated apoptosis and not to induce apoptosis.19 Skin from each of six donors was grafted onto four beige-SCID mice to form four treatment groups. Autologous NK cells and antibodies were injected as above. Group 1 received injections of control IgG alone. Group 2 received injections of autologous NK cells plus control IgG. Group 3 received autologous NK cell injections followed by anti-Fas (ZB4). NK cells for group 4 mice were preincubated with anti-Fas (ZB4) for 30 minutes at 4°C and washed, before injection into the grafts. Injection of autologous NK cells (group 2) induced epidermal hyperplasia as in the previous experiments (P < 0.001 versus no NK cells; Figure 4). Anti-Fas treatment of grafts on days 3 and 10 after NK cell injection (group 3) inhibited this increased epidermal thickness (P < 0.001 compared to NK cells alone). However, NK cells preincubated with anti-Fas before injection (group 4) induced epidermal hyperplasia comparable to that of NK cells alone (P < 0.01 versus both groups 1 and 3). All comparisons were performed by analysis of variance corrected for multiple comparisons. This data indicates that the anti-Fas (ZB4) mAb prevented psoriasis induction by acting on the epidermis rather than the injected NK cells.
Figure 4.
Anti-Fas (ZB4) mAb preincubation with NK cells; epidermal thickness of grafts injected with control IgG alone, NK cells plus control IgG, NK cells plus anti-Fas (ZB4), and NK cells preincubated with anti-Fas (ZB4) for 30 minutes and then washed before injection. Epidermal thickness is shown in nm with shaded area (1 STD).
Epidermis of all grafts injected with NK cells expressed TNF-α (eight of eight grafts; Figures 3 and 5). Injection of anti-Fas mAb blocked TNF-α expression, with expression seen in only one of six grafts (difference in graft numbers indicates stain not performed). Suppression of TNF-α by blocking anti-Fas mAb was statistically significant (P < 0.05 by χ2). Anti-FasL injection was less effective in blocking TNF-α expression, with strong expression in one graft and weak expression in two grafts (total three of seven grafts). IL-15 was expressed by epidermis in all (nine of nine) grafts after injection of NK cells versus only one of nine control noninjected grafts. Injection of anti-Fas mAb resulted in focal IL-15 expression in two of seven grafts.
Figure 5.
TNF-α expression by grafts from three donors injected with control IgG, NK cells plus control IgG, and NK cells plus blocking anti-Fas (ZB4).
TNF-α mRNA was also measured by real-time reverse transcriptase (RT)-PCR using material from the second set of experiments (five donors). For four of five donors the level of TNF-α mRNA decreased with the injection of anti-Fas mAb (Table 1). The remaining donor had elevated levels of TNF-α mRNA. However, this one donor with elevated mRNA levels also did not exhibit suppression of epidermal thickness, which was similar to the level observed with injection of NK cells plus control IgG. Fas blocking antibody was able to suppress TNF-α mRNA in concert with epidermal thickness.
Table 1.
TNF-α mRNA by Real-Time RT-PCR
| P132 | P129 | P136 | P134 | P158 | |
|---|---|---|---|---|---|
| Injected NK cells and anti-Fas mAb | 0.058 | 0.00 | 0.134 | 1.70 | 0.00 |
| Injected NK cells and control IgG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
TNF-α mRNA levels determined by real-time RT-PCR expressed relative to NK cells plus control IgG arbitrarily set at 1. Each column represents one donor. Donor P134 epidermal thickness with anti-Fas was comparable to NK cells plus control IgG.
Skin grafts injected with NK cells expressed immunological markers of psoriasis in addition to histological markers. Epidermis was positive for ICAM-1 (six of six) and HLA-DR (five of six) in grafts injected with NK cells (Figure 3). Injection of anti-Fas in addition to NK cells blocked both HLA-DR and ICAM-1, with no expression (zero of four) in treated grafts (P < 0.05 by χ2). However, anti-FasL did not block HLA-DR, with expression in all (five of five) grafts and ICAM-1 expression in two of five grafts.
Discussion
Blocking anti-Fas and anti-FasL monoclonal antibodies were able to prevent the induction of psoriasis by injection of NK cells. As previously reported by us and others,7–9 injection of NK cells induced classic psoriasis histology and immunohistochemistry in noninvolved skin grafts from psoriasis donors. Blocking anti-Fas as well as anti-FasL mAb were able to inhibit these psoriatic changes. Preincubation of anti-Fas mAb with NK cells did not inhibit psoriasis generation, demonstrating that this ZB4 mAb did not act directly on the NK cells. Fas was expressed by epidermis of grafts that received only control IgG and was up-regulated with the injection of NK cells. FasL was expressed by a large proportion of the heterogeneous NK cells. Both TNF-α and IL-15 were expressed by psoriatic epidermis after NK cell injection, and expression of both cytokines, as well as TNF-α mRNA, was blocked by injection of blocking anti-Fas mAb. TNF-α and IL-15 are both critical to psoriasis pathogenesis.10–13 These data indicate that Fas signaling is upstream to both mediators in the induction of psoriasis.
Anti-Fas antibody (ZB4) and anti-FasL were both effective in significantly blocking histological changes of psoriasis, including increased epidermal thickness. Anti-Fas was also able to significantly block increased epidermal proliferation and expression of TNF-α, HLA-DR, and ICAM-1. However, anti-FasL was less effective in blocking TNF-α and did not significantly block epidermal proliferation (Ki-67) or expression of HLA-DR and ICAM-1. It must be emphasized that ZB4 anti-Fas mAb blocks Fas-mediated apoptosis and does not induce apoptosis.19 Anti-Fas mAb can directly block Fas signaling at the keratinocyte, whereas anti-FasL mAb is likely to act indirectly on the FasL-positive NK cells. Evidently, with this experimental protocol, direct blocking by anti-Fas mAb was more effective. However, the anti-FasL mAb did result in significant blocking of histological changes and epidermal thickness, providing evidence that induction of psoriasis by activated lymphocytes was mediated by blocking of Fas signaling. The ability of both anti-Fas and anti-FasL to inhibit psoriasis induction argues that the effects of the mAb are not simply directed against lymphocytes. The mAbs were injected 3 and 10 days after the NK cells to minimize interference of the mAb with the NK cells. Further evidence that anti-Fas mAb did not directly inactivate NK cells was provided by lack of effect of this antibody when preincubated directly with NK cells before injection.
These experiments were not designed to determine the relative role of NK cells versus T cells or other activated lymphocytes in the pathogenesis of psoriasis. Neither were they designed to yield information on the nature of autoantigen(s) in psoriasis. The information on Fas triggering of psoriasis may be relevant to any of the above potential mechanisms of psoriasis regardless of the nature of the autoantigen and whether induced by NK cells, NK-T cells, or T cells.
Fas-induced apoptosis has a role in the pathogenesis of eczematous conditions,20,21 and Fas-induced cleavage of E-cadherin is a feature of spongiosis.22 Keratinocyte expression of Fas is up-regulated by interferon-γ treatment in vitro, and activating anti-Fas antibody (nonblocking) is able to induce apoptosis of interferon-γ-treated keratinocytes.23 Despite the expression of Fas, apoptosis is not a feature of psoriasis, and both spongiosis and loss of E-cadherin are absent.24,25
Induction of inflammatory cytokines by Fas activation has been demonstrated with multiple cell types. Bronchiolar epithelial cells produce IL-8 in response to anti-Fas treatment.26 In contrast to Fas-induced apoptosis, IL-8 production does require protein synthesis. Similar Fas induction of IL-8 has been reported for rheumatoid arthritis synoviocytes27 and colonic epithelial cells.28 Fas activation can induce phosphorylation of Iκ-Bα, which activates the nuclear factor-κB pathway, thereby inducing production of IL-8 and TNF-α.29 nuclear factor-κB is also a survival factor for human keratinocytes, reducing sensitivity to apoptosis and inducing anti-apoptotic factors.30 Fas signaling can induce angiogenesis in mice.31 Because angiogenesis is a prominent feature of psoriasis, this may be an additional factor in induction of psoriasis by lymphocyte FasL/Fas signaling. Psoriasis differs from eczematous dermatitis in that there is a low level of apoptosis and minimal spongiosis24,25 despite expression of Fas by keratinocytes and FasL by infiltrating lymphocytes.
What then explains the discrepancy between up-regulation of Fas and lack of apoptosis in psoriasis? As noted above, psoriasis epidermis expresses Bcl-xL, which has anti-apoptotic properties. In addition, IL-15 inhibits keratinocyte apoptosis induced by Fas, and both IL-15 and IL-15 receptor are expressed at high levels in lesional psoriatic plaques.32 Protection of keratinocytes from Fas-mediated apoptosis seems to be a factor in induction of the Fas inflammatory cytokine (eg, TNF-α) pathway. Keratinocyte expression of IL-15 and IL-15 receptor may protect keratinocytes from Fas-mediated apoptosis. Alternately, the primary defect in psoriasis may be a constitutive increase in keratinocyte anti-apoptotic factors (eg, Bcl-xL), allowing Fas induction of inflammatory cytokines (eg, IL-8 and TNF-α).
Here we show that Fas/FasL signaling is essential for the induction of psoriasis by activated NK cells. Blocking of Fas signaling prevents downstream events, including induction of TNF-α production by keratinocytes and psoriasis histological changes. Fas signaling by the nonapoptotic pathway appears to be an essential early event in psoriasis induction.
Footnotes
Address reprint requests to Dr. Amos Gilhar, Laboratory for Skin Research, Rappaport Building, Technion Faculty of Medicine, P.O. Box 9649, Bat-Galim, Haifa, 31096, Israel. E-mail: fligilhar@matat.health.gov.il.
References
- Boehncke WH, Dressel D, Zollner TM, Kaufmann R. Pulling the trigger on psoriasis. Nature. 1996;379:777. doi: 10.1038/379777a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb SL, Gilleaudeau P, Jonson R, Estes L, Woodworth TG, Gottlieb AB, Krueger JG. Response of psoriasis to a lymphocyte-selective toxin (DAB389 IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442–447. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- Gilhar A, David M, Ullmann Y, Berkutski T, Kalish RS. T-Lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J Invest Dermatol. 1997;109:283–288. doi: 10.1111/1523-1747.ep12335758. [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Zollner TM, Dressel D, Kaufmann R. Induction of psoriasiform inflammation by a bacterial superantigen in the SCID-hu xenogeneic transplantation model. J Cutan Pathol. 1997;24:1–7. doi: 10.1111/j.1600-0560.1997.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999;155:145–158. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch Dermatol. 1999;135:546–552. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Bonish B, Huang BB, Porcelli SA. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212–225. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Ullmann Y, Kerner H, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Psoriasis is mediated by a cutaneous defect triggered by activated immunocytes: induction of psoriasis by cells with NK receptors. J Invest Dermatol. 2002;119:384–391. doi: 10.1046/j.1523-1747.2002.01812.x. [DOI] [PubMed] [Google Scholar]
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomized trial. Lancet. 2001;357:1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB, Etanercept Psoriasis Study Group Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen LS, Schuurman J, Beurskens F, Dam TN, Dagnaes-Hasen F, Skov L, Rygaard J, Voorhorst-Ogink MM, Gerritsen AF, van Dijk MA, Parren PWHI, Baadsgaard O, van de Winkel JGJ. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112:1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J, Lee TH. Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem. 2001;276:47100–47106. doi: 10.1074/jbc.M107385200. [DOI] [PubMed] [Google Scholar]
- Wrone-Smith T, Johnson T, Nelson B, Boise LH, Thompson CB, Nunez G, Nickoloff BJ. Discordant expression of Bcl-x and Bcl-2 by keratinocytes in vitro and psoriatic keratinocytes in vivo. Am J Pathol. 1995;146:1079–1088. [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Manabe A, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Aberrant expression of apoptosis-related molecules in psoriatic epidermis. J Dermatol Sci. 2002;28:187–197. doi: 10.1016/s0923-1811(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nishioka K. Alteration of the expression of Bcl-2, Bcl-x, Bax, Fas, and Fas ligand in the involved skin of Psoriasis vulgaris following topical anthralin therapy. Skin Pharmacol Appl Skin Physiol. 2003;16:50–58. doi: 10.1159/000068289. [DOI] [PubMed] [Google Scholar]
- Wrone-Smith T, Mitra RS, Thompson CB, Jasty R, Castle VP, Nickoloff BJ. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–1329. [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Thorpe CJ, Yonehara S, Chiodl F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1996;9:201–209. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, Girolomoni G, Cavani A. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol. 2000;165:3058–3064. doi: 10.4049/jimmunol.165.6.3058. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Altznauer F, Akdis M, Simon HU, Disch R, Brocker EB, Blaser K, Adkis CA. The differential fate of cadherins during T-cell induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Dermatol. 2001;117:927–934. doi: 10.1046/j.0022-202x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- Sayama K, Yonehara S, Watanabe Y, Miki Y. Expression of Fas antigen on keratinocytes in vivo and induction of apoptosis in cultured keratinocytes. J Invest Dermatol. 1994;103:330–334. doi: 10.1111/1523-1747.ep12394858. [DOI] [PubMed] [Google Scholar]
- Laporte M, Galand P, Fokan D, de Graef C, Heenen M. Apoptosis in established and healing psoriasis. Dermatology. 2000;200:314–316. doi: 10.1159/000018394. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Doi H, Ito Y, Shibata MA, Yoshinaka R, Otsuki Y. Evaluation of cell death and proliferation in psoriatic epidermis. J Dermatol Sci. 2004;35:207–214. doi: 10.1016/j.jdermsci.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Kawasaki M, Yoshimi M, Kaneko Y, Kunitake R, Maeyama T, Tanaka T, Hara N. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol. 1999;21:436–445. doi: 10.1165/ajrcmb.21.3.3397. [DOI] [PubMed] [Google Scholar]
- Sekine C, Yagita H, Kobata T, Hasunuma T, Nishioka K, Okumura K. Fas-mediated stimulation induces IL-8 secretion by rheumatoid arthritis synoviocytes independently of CPP32-mediated apoptosis. Biochem Biophys Res Commun. 1996;228:14–20. doi: 10.1006/bbrc.1996.1610. [DOI] [PubMed] [Google Scholar]
- Abreu-Martin MT, Vidrich A, Lynch DH, Targan SR. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol. 1995;155:4147–4154. [PubMed] [Google Scholar]
- Russo MP, Bennett BL, Manning AM, Brenner DA, Jobin C. Differential requirement for NF-kappaB-inducing kinase in the induction of NF-kappaB by IL-1beta, TNF-alpha, and Fas. Am J Physiol. 2002;283:C347–C357. doi: 10.1152/ajpcell.00166.2001. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Bacon P, Chaturvedi V, Nickoloff BJ. Role of NF-kappaB activity in apoptotic response of keratinocytes mediated by inter-feron-gamma, tumor necrosis factor-alpha, and tumor-necrosis-factor-related apoptosis-inducing ligand. J Invest Dermatol. 2001;117:898–907. doi: 10.1046/j.0022-202x.2001.01477.x. [DOI] [PubMed] [Google Scholar]
- Biancone L, De Martino A, Orlandi V, Conaldi PG, Toniola A, Carmussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckert R, Asadullah K, Seifert M, Budagian VM, Arnold R, Trombotto C, Paus R, Bulfone-Paus S. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol. 2000;165:2240–2250. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]