Abstract
Podocytes are crucial for the permeability of the glomerular filtration barrier. In glomerular disease, however, reactive oxygen species (ROS) may be involved in podocyte injury and subsequent proteinuria. Here, we describe ROS-dependent gene induction in differentiated podocytes stimulated with H2O2 or xanthine/xanthine-oxidase. Superoxide anions and H2O2 increased mRNA and protein expression of GAS5 (growth arrest-specific protein 5) and CHOP (C/EBP homology protein). Cultured podocytes overexpressing CHOP showed increased generation of superoxide anions compared to controls. In addition, the expression of α3/β1 integrins, crucial for cell-matrix interaction of podocytes, was down-regulated, leading to increased cell-matrix adhesion and cell displacement. The altered cell-matrix adhesion was antagonized by the ROS scavenger 1,3-dimethyl-2-thiourea, and the increase in cell displacement could be mimicked by stimulating untransfected podocytes with puromycin, an inductor of ROS. We next performed immunohistochemical staining of human kidney tissue (normal, membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy) as well as sections from rats with puromycin nephrosis, a model of minimal change nephropathy. CHOP was weakly expressed in podocytes of control kidneys but up-regulated in most proteinuric human kidneys and in rat puromycin nephrosis. Our data suggest that CHOP—via increased ROS generation—regulates cell-matrix adhesion of podocytes in glomerular disease.
The podocyte plays a crucial role in the maintenance of the permeability properties of the glomerular filtration barrier.1 Damage of the podocyte leads to proteinuria, which is a hallmark of almost all glomerular diseases.2 The mechanisms by which podocytes are damaged are so far incompletely understood but reactive oxygen species (ROS) have been found to induce podocyte injury and proteinuria under a variety of circumstances.3 In addition, direct intra-arterial infusion of H2O2 causes proteinuria without a change in glomerular filtration rate, renal plasma flow, or structural changes of the glomerular filtration barrier.4 Overproduction of ROS has been detected in several glomerular diseases including puromycin nephrosis, a model of minimal change disease;5 Heyman nephritis, a model of membranous nephropathy;6,7 and the Mpv17−/− mouse, a model for steroid-resistant focal segmental sclerosis.8 In these glomerulopathies, pretreatment of animals with ROS scavengers prevented foot process effacement and proteinuria.5,7,9 In addition, in biopsies of patients with membranous nephropathy, oxidatively modified proteins are found in podocytes, mesangial cells, and basal membranes.10
Because ROS seem to play a direct role in the damage of podocytes, it would be helpful to understand the cellular mechanisms by which ROS induce podocyte damage and proteinuria. Many cellular processes are accompanied by changes in gene expression. Recently, we introduced an in vitro model in which genes that are differentially regulated by ROS could be identified by polymerase chain reaction (PCR)-based suppressive subtractive hybridization (PCR-SSH) in podocytes.11 In these studies members of the growth arrest gene family were identified. The family member CHOP (C/EBP homology protein) heterodimerizes with members of the C/EBP1 family of transcription factors.12 The expression of CHOP is markedly induced by a variety of cellular stresses. The dimerization of CHOP with C/EBP inhibits the function of C/EBP by preventing its DNA binding to the promoters of a subset of genes. In addition, the CHOP/C/EBP heterodimer binds to a specific DNA sequence and stimulates the transcription of specific genes.13 CHOP also heterodimerizes with other non-C/EBP subfamilies of basic region leucine zipper proteins, eg, activating transcription factor 3 and Jun/Fos. Several functions have been proposed for CHOP. Heterotopic overexpression of CHOP induces growth arrest in fibroblasts,14 inhibits adipocyte differentiation,15 and induces apoptosis in vitro.16 Here we show that CHOP is differentially induced by ROS in podocytes and that CHOP regulates important properties of podocyte function.
Materials and Methods
Cell Culture
Conditionally immortalized mouse podocytes were cultured as reported elsewhere.17 In brief, podocytes were maintained in RPMI 1640 medium (Life Technologies, Eggenstein, Germany) supplemented with 5% fetal calf serum (Biochrom, Berlin, Germany), 100 kU/L penicillin, and 100 mg/L streptomycin (Life Technologies). To propagate podocytes, cells were cultivated at 33°C on type I collagen (permissive conditions) in culture medium supplemented with 10 U/ml recombinant interferon-γ. To induce differentiation, podocytes were maintained on type I collagen (Biochrom) at 37°C without supplementation with interferon-γ (nonpermissive conditions). Only differentiated podocytes between passages 10 and 16 were used in our experiments. Cells were switched to media that contained 1% fetal calf serum 24 hours before the experiments and then exposed to various treatments.
PCR-Based Suppressive Subtractive Hybridization (PCR-SSH)
PCR-SSH was performed according to the method recently described.11 In brief, RNA was isolated from control cells and cells stimulated with superoxide anions (O2−) generated from the xanthine/xanthine-oxidase reaction (X/XO; 50 μmol/L/5 or 50 mU/ml) for 4 hours. cDNA synthesis using 1 μg of total RNA from each cell population was performed with the SMART PCR cDNA synthesis kit (Clone Tech, Palo Alto, CA) and subsequent long-distance PCR according to the manufacturer’s instructions. Virtual Northern analysis showed that cDNA from one clone hybridized to a 0.5-kb transcript that was strongly up-regulated in cells that had been treated with X/XO. The insert was then sequenced and identified as part of the mouse GAS5 cDNA sequence (accession no. X59728).
Expression of GAS5 and CHOP mRNA in Podocytes
RNA preparation, reverse transcription, and polymerase chain reaction (PCR) amplification were performed according to the method described recently.18 In brief, total RNA from cultured mouse podocytes was isolated with the acid guanidinium-thiocyanate-phenol-chloroform extraction method, and the amount of RNA was measured by spectrophotometry. For first strand synthesis, 0.2 μg of total RNA were mixed in 5× reverse transcription buffer containing 0.5 mmol/L dNTP, 10 μmol/L random primers, 10 mmol/L dithiothreitol, 4 U ribonuclease inhibitor, and 20 U M-MLV reverse transcriptase (Promega, Mannheim, Germany) (reverse transcriptase was also omitted to control for the amplification of contaminating DNA). Reverse transcription was performed at 42°C for 1 hour followed by 95°C for 5 minutes. PCR was performed in duplicates in a total volume of 20 μl, each containing 4 μl of reverse transcription reaction and 16 μl of PCR master mixture with 10 pmol each of sense and anti-sense primer and 1.0 U Taq DNA polymerase. The cycle profile included denaturation for 60 seconds at 94°C, annealing for 60 seconds at 63°C (CHOP), or 64°C (GAS5) and extension for 60 seconds at 72°C. In the experiments for the analysis of the mRNA expression of CHOP, 30 cycles of PCR were performed, for the analysis of GAS5, 33 cycles of PCR were performed. The amplification products of 10 μl from each PCR reaction were separated on a 1.5% agarose gel, stained with ethidium bromide and visualized by UV irradiation. PCR amplification of reverse transcriptase reactions without reverse transcriptase revealed no PCR product, thereby excluding amplification of genomic DNA. All PCR products were sequenced to ensure identity of the fragments with published sequences.
Semiquantitative Analysis
The amplification products of 10 μl of PCR product were separated on a 1.5% agarose gel, stained with ethidium bromide, visualized with UV irradiation and digitally photographed. Band densities were analyzed by imaging software (Image Quant; Molecular Dynamics, Krefeld, Germany). The data were normalized to GAPDH/β-actin mRNA expression. The following primers were used: β-actin: sense 5′-TGTTACCAACTGGGACGACA-3′; anti-sense 5′-TCTCAGCTGTGGTGGTGAAG-3′; GAS5: sense 5′-TGTGGCAAAGGAGGATGAAG-3′; anti-sense 5′-CCAGGCACCTCAGAAACAAA-3′; CHOP: sense 5′-CACATCCCAAAGCCCTCG-3′; anti-sense 5′-CTCAGTCCCCTCCTCAGC-3′.
Plasmids and Viral Vector Construction
The CHOP cDNA-containing plasmids used to generate the retroviral transfer vectors have been described elsewhere.19 The plasmid pLXSN was kindly provided by D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA). The plasmids pMD-G, and pMD-gp were a kind gift of R. Mulligan (Harvard Medical School, Boston, MA). The retroviral transfer vectors pLXSN-CHOP-IRES-gfp were generated using standard cloning techniques.
Cell Culture, Virus Production, and Transduction
The retroviral vector was produced by co-transfection of HEK 293T cells with three plasmids (2.5 μg of pMD-G, 7.5 μg of pMD-gp, and 10 μg of the retroviral transfer vector) using the calcium phosphate method. The supernatant was harvested, centrifuged to remove cellular debris, and filtered. Cells were infected in the presence of 8 μg/ml polybrene for 4 hours, and were selected 3 to 4 days after viral transduction in neomycin (500 μg/ml) to achieve 100% positive cells. Virus transduction and transgene expression were monitored by fluorescent microscopy of the co-expressed GFP protein and Western blot analysis.
Immunohistochemical Analysis
Fixation and preparation of tissue for immunohistochemical analyses were performed as recently described.20 In brief, human kidney biopsies or unaltered human kidney tissue from patients after carcinomectomy were incubated in cold (4°C) phosphate-buffered saline (PBS) followed by 5 ml of 4% paraformaldehyde. Thereafter, kidney samples were incubated for 24 hours at 4°C in 4% paraformaldehyde solution, embedded in paraffin, and cut into 2- to 4-μm-thick slices. Slices were deparaffinized in xylol for 1 hour, gradually hydrated through graded alcohols (100 to 70%), and washed in deionized water. After incubation in 1% H2O2 for 30 minutes, slices were rehydrated with PBS, and antigen unmasking was performed by incubation of the slices in 10 μm citrate buffer (pH 6.0) for 10 minutes. Blocking was performed using a 1% bovine serum albumin solution for 10 minutes. Thereafter, sections were incubated for 24 hours, in a humidified chamber at 4°C, with antibodies against CHOP (rabbit anti-GADD153; Santa Cruz Biotechnology, Santa Cruz, CA). The slices were washed extensively with PBS and incubated for 45 minutes with a secondary antibody using a commercially available ABC kit (Vectastain rabbit peroxidase; Vector Laboratories, Burlingame, CA). Slices were washed with PBS, incubated with avidin-biotin for 45 minutes, and stained with 3-amino-9 ethylcarbazole or by using the Neufuchsin chromogen. Sections were examined in a blinded manner by two independent scientists with a conventional light microscope (Zeiss LSM 510). Negative controls were performed by elimination or heat denaturation of the primary antibody.
Western Blotting
Cultured mouse podocytes were washed once with PBS, scraped, centrifuged (2000 × g, 4°C, 5 minutes), and homogenized in ice-cold Tris-buffered saline containing 2 mmol/L EDTA, 100 mmol/L NaCl, 20 mmol/L Tris, 2 mmol/L EGTA, 2 mmol/L phenylmethyl sulfonyl fluoride, a proteinase inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and 1% Nonidet P-40. Samples were resuspended in Laemmli sample buffer, boiled (5 minutes), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer electrophoresis. The transblots were probed with the primary antibodies anti-CHOP, anti-α3 integrin, anti-β1 integrin, anti-α-actin, anti-β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), or anti-α-dystroglycan (Biomol, Hamburg, Germany), followed by peroxidase-labeled secondary antibodies (sheep anti-mouse, donkey anti-rabbit; Amersham Pharmacia Biotech, Piscataway, NJ). Signal was detected with chemiluminescence detection reagents (Amersham Pharmacia Biotech). All transblots were stained with Ponceau solution or reprobed with anti-α-actin antibodies or anti-β-tubulin antibodies to prove equal amounts of protein were loaded on the membrane.
Measurement of NADPH Oxidase Activity
Podocytes were rinsed twice with ice-cold PBS and scraped with KREBS solution (pH 7.35) containing 99 mmol/L NaCl, 4.7 mmol/L KCl, 1.8 mmol/L CaCl2, 1.2 mmol/L MgCl2, 25 mmol/L NaHCO3, 1.03 mmol/L K2HPO4, 20 mmol/L Na-HEPES, and 11.1 mmol/L glucose and centrifuged (200 × g, 4°C, 5 minutes). The supernatant was discarded and the pellet resuspended in fresh KREBS buffer. The cell suspension (100 μl) was added to KREBS solution containing 5 μmol/L lucigenin and stimulated with 100 μmol/L NADPH. Bioluminescence was measured with Lumat LB9501 (Berthold GmbH, Wildbad, Germany). Cells were subsequently lysed and protein content measured by the Lowry method. To calculate the amount of superoxide produced, total counts were analyzed by integrating the area under the signal curve. These values were compared with a standard curve that was generated by using xanthine/xanthine oxidase as described.21 Superoxide generation was expressed as nmol O2− generated per mg cellular protein per minute.
Cell Adhesion Assay
Differentiated CHOP-overexpressing and control cells were switched to media that contained 1% fetal calf serum 24 hours before the experiments. Cells were trypsinized, centrifuged, and resuspended in RPMI medium without fetal calf serum. Cells were counted in a Neubauer chamber, plated on 96-well plates coated with collagen IV (10,000 cells/well), and incubated for 1 to 5 hours at 37°C. Thereafter, cells were washed with PBS, fixed, and stained with 0.2% crystal violet dissolved in 20% methanol for 10 minutes at room temperature. Cells were washed twice with PBS, solubilized with a 1% aqueous sodium dodecyl sulfate solution, and the extinction measured at 550 nm in an enzyme-linked immunosorbent assay reader (Thermolab System; Multiskan EX, Dreieich, Germany). In some experiments, cells were preincubated with the potent ROS scavenger 1,3-dimethyl-2-thiourea (DMTU) (10 mmol/L) and cell adhesion measured in the presence of DMTU.
Cell Proliferation Assay
Proliferation assay was performed with untransfected differentiated podocytes stimulated with either H2O2 or X/XO using the CellTrace CFSE proliferation kit (Molecular Probes, Leiden, The Netherlands) according to the manufacturer’s instructions. CFSE (carboxyfluorescein diacetate, succinimidyl ester) is an amine-reactive dye that is incorporated into cells and divided equally between daughter cells during proliferation. The number of cell divisions can be determined using flow cytometry to measure the mean fluorescence intensity.22 In brief, cells were incubated with 10 μmol/L CFSE for 15 minutes at 37°C and washed with PBS before culture. Thereafter either H2O2 (250 μmol/L) or X/XO [50 μmol/L/(50 mU/ml)] was added to the culture medium for the indicated times. Proliferation was assessed by flow cytometry on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Cell Displacement Experiments
Migration of individual podocytes was captured by means of time lapse video microscopy. Differentiated podocytes (CHOP-overexpressing, CHOP mock transfected, untransfected podocytes stimulated with 50 μg/ml puromycin for 24 hours, and untransfected podocytes stimulated with vehicle for 24 hours) were seeded on a collagen IV matrix, kept in culture medium with 1% fetal calf serum for 24 hours, and placed in a heating chamber (37°C) on the stage of a phase contrast microscope (Axiovert 25, ×32; Zeiss, Oberkochen, Germany). Migration was monitored for 245 minutes. Data acqui-sition was done with a video camera (Hamamatsu, Hersching, Germany) controlled by HiPic software (Hamamatsu). Images were taken in 25-minute intervals and stored as stacks of TIFF files. The outlines of individual cells were marked throughout the entire image stack with Amira software (TGS, France; http://www.amiravis.com/). These segmentation data were used for further processing. Quantitative data analysis and calculation of parameters were performed with JAVA programs developed by us. Migration was determined as the movement of the cell center with time as described recently.23 We calculated the average speed of migrating cells (estimated from 25-minute time intervals applying a three point difference quotient) and their mean displacement, ie, the distance covered within 245 minutes.
Induction of Puromycin Aminonucleoside Nephrosis in Rats
Wistar rats (n = 6, ∼150 g) fed with standard rat chow and free access to water were randomly divided into an experimental group (n = 3) and a control group (n = 3). The rats in the experimental group received a single intraperitoneal injection (15 mg/100 g) of puromycin aminonucleoside (PAN) (Sigma Chemical Co., St. Louis, MO), and the rats in the control group were given 2 ml of normal saline as described.24 Urine collections were performed daily to measure protein excretion. Rats were sacrificed at day 5 and kidneys harvested for immunohistochemistry.
Statistical Analyses
Data expressed as mean ± SEM were analyzed by analysis of variance for repeated measures when comparing within groups and one-way analysis of variance when comparing among groups; Student’s t-test was used for a two-group comparison. P < 0.05 was considered significant.
Results
To identify genes induced by ROS, we used a PCR-based cDNA subtractive hybridization strategy to generate a cDNA library of genes that were differentially expressed by ROS in podocytes. One hundred clones from the differentially expressed cDNA library were further analyzed by differential screening, and 27 clones generated an increased hybridization signal when hybridized to RNA from X/XO-treated podocytes compared with RNA from control cells.11 Of these 27 clones, we detected a 0.5-kb transcript that was highly up-regulated in podocytes that had been stimulated with ROS. This cDNA clone was sequenced and identified by BLAST analysis as a 228-bp fragment of the mouse GAS5 cDNA. GAS5 belongs to the group of growth-sensitive genes represented by growth arrest-specific (GAS) and growth arrest and DNA damage (GADD) genes. Preliminary analysis of other members of this family (GAS2, GAS3, GAS5, GAS6, and CHOP) revealed that in addition to GAS5, only CHOP was differentially regulated by ROS (data for GAS2, GAS3, and GAS5 not shown). We therefore further studied the function of GAS5 and CHOP in podocytes.
O2− and H2O2 Increase GAS5 and CHOP mRNA Expression in Podocytes
Reverse transcriptase (RT)-PCR studies showed that podocytes expressed mRNA for the growth arrest genes GAS5 and CHOP (Figure 1). For GAS5, a double band representing two splice variants was found. To confirm the results found with the PCR-based cDNA subtractive hybridization strategy, podocytes were stimulated with X/XO and the time-dependent induction of GAS5 and CHOP mRNA expression was characterized. In control cells only little mRNA expression for GAS5 and CHOP could be detected. Stimulation of differentiated podocytes with X/XO for 1, 4, 12, 24, and 48 hours induced a time-dependent significant increase in GAS5 mRNA expression at 4 and 12 hours and in CHOP mRNA expression at 1, 4, and 12 hours (Figure 1). Like X/XO, H2O2 (250 μmol/L) induced a time-dependent increase in CHOP mRNA expression (Figure 1). The response to H2O2 was concentration-dependent with a threshold concentration of 50 μmol/L H2O2 (Figure 2). To assure that quantification was obtained in the linear portion of the PCR amplification curve, cycle dependency of RT-PCR experiments was shown (Figure 2). Pretreatment of podocytes with the ROS scavenger N-acetylcysteine (10 mmol/L) for 30 minutes completely inhibited the H2O2-induced increase in CHOP mRNA expression (Figure 2).
Figure 1.
GAS5 and CHOP mRNA is time dependently expressed in X/XO and H2O2-treated differentiated mouse podocytes. Time course for the induction of GAS5 and CHOP mRNA by X/XO and H2O2. Gas5 and CHOP mRNA induction was studied in control podocytes or in podocytes that have been incubated with X/XO (50 μmol/L/50 mU/ml) (A) or H2O2 (250 μmol/L) (B) for the indicated times. Experiments were performed by using RT or no RT in each set-up (RT− not shown). The densitometric analysis of mRNA expression profiles for CHOP was normalized with GAPDH mRNA expression. Studies were performed with primers derived from published mouse cDNA sequences resulting in a 379-bp fragment for CHOP and two 344- and 301-bp fragments for GAS5. Sequence analysis of the resulting amplification products revealed sequence identity for the amplified fragments with published sequences (n = 3 to 15, values are means ± SEM, *P < 0.05 versus control, t-test).
Figure 2.
Top left: Cycle dependency of RT-PCR experiments. Experiments were performed to assure that quantification was obtained in the linear portion of the PCR amplification curve. Densitometric analysis of the bands revealed a linear correlation between band densities and amount of cycles (data not shown). In all experiments 21 cycles were used for β-actin and 30 cycles for CHOP amplification. Top right: Concentration response curve of the effect of H2O2 on CHOP mRNA expression in podocytes (n = 3, values are means ± SEM, *P < 0.05 versus 0 μmol/L H2O2, analysis of variance, Scheffé’s test). Bottom left: Effect of N-acetylcysteine on H2O2-mediated increase in CHOP mRNA expression. Podocytes were incubated with vehicle (Co), H2O2 (250 μmol/L), H2O2 (250 μmol/L) + n-ACC (10 mmol/L), or n-ACC (10 mmol/L) for 1 hour. N-acetylcysteine was added 60 minutes before stimulation with H2O2. The densitometric analysis of mRNA expression profiles for CHOP by RT-PCR was normalized with β-actin mRNA expression. Bottom right: Statistical analysis (n = 3, values are means ± SEM, *P < 0.05 versus control, t-test).
Recent data show that despite four alternative splicing patterns for GAS5, no cellular protein is expressed.25,26 We therefore concentrated our efforts on the analysis of CHOP protein expression. To test whether the increased CHOP mRNA expression levels in response to ROS resulted in a subsequent increase in protein expression, CHOP protein was measured in H2O2-treated, xanthine/xanthine oxidase-treated, and control podocytes by Western blotting. An ∼10-fold increase in H2O2-mediated protein expression and an approximately threefold increase in superoxide-mediated CHOP protein expression were detected after 24-hour stimulation with H2O2 or xanthine/xanthine oxidase, respectively (Figure 3).
Figure 3.
Effect of H2O2 and X/XO on CHOP protein expression. Podocytes were stimulated with H2O2 (250 μmol/L) or X/XO (50 μmol/L/50 mU/ml) or vehicle (Co) for the indicated times and protein expression for CHOP (∼32 kd) was analyzed by Western blot. Ponceau staining was used to ensure equal protein loading. There is significant up-regulation of CHOP expression after 24 hours and 48 hours of stimulation with H2O2 and after 24 hours of stimulation with X/XO. Statistical analysis (n = 3 to 4, values are means ± SEM, *P < 0.05 versus control, t-test).
CHOP Overexpression Increases NADPH-Dependent O2− Generation in Podocytes
To further investigate a possible role of CHOP on podocyte function, a mouse podocyte cell line overexpressing CHOP was developed. Figure 4 shows a Western blot experiment demonstrating expression levels for CHOP in podocytes overexpressing CHOP and control cells transfected with vector only. It has been suggested that the generation of ROS in the glomerulus leads to podocyte foot effacement and proteinuria,2 but the source of ROS generation in glomerulopathies is little characterized. Activation of the NADPH oxidoreductase enzyme complex leading to the generation of ROS has been shown in podocytes in vivo and in vitro.6,18 We therefore tested the hypothesis that CHOP might modify ROS generation in podocytes. Stimulation of the NAD(P)H-oxidase enzyme complex with NADPH (0.1 mmol/L) led to a small increase in O2− production in podocytes transfected with vector only. In contrast, addition of NADPH (0.1 mmol/L) to podocytes overexpressing CHOP increased O2− production by ∼2.5-fold. Figure 4 shows chemiluminescence experiments of NADPH-dependent O2− generation in CHOP-overexpressing podocytes and controls with a summary of the total superoxide anion production throughout 15 minutes (areas under the curves). To test the hypothesis that GAS5 and CHOP are both regulated by ROS and interact in podocytes GAS5 mRNA expression was determined by semiquantitative RT-PCR in CHOP-overexpressing cells. Interestingly, GAS5 expression was down-regulated in CHOP-overexpressing cells (Figure 4).
Figure 4.
NADPH-mediated superoxide anion production in control cells and in CHOP-overexpressing podocytes. Top left: Expression levels for CHOP in podocytes overexpressing CHOP and control cells transfected with vector only. Top right: Statistical analysis (n = 5, values are means ± SEM, *P < 0.05 versus control, t-test). Middle left: Recording of the time course and magnitude of NADPH-oxidase activation in control cells and CHOP-overexpressing podocytes. Middle right: Summary of the experiments. O2− generation by NADPH-oxidase activity was calculated by integrating the total counts during the first 15 minutes of stimulation. Values are expressed as nmol O2− generated per mg cellular protein per minute (n = 6, values are means ± SEM, *P < 0.05 versus control, t-test). Bottom: CHOP down-regulates GAS5 mRNA expression in CHOP-overexpressing cells (CHOP) compared to vector-only transfected cells (Co). GAPDH expression was not different in both cell lines.
The Expression of α3 and β1 Integrin Is Reduced in CHOP-Overexpressing Podocytes
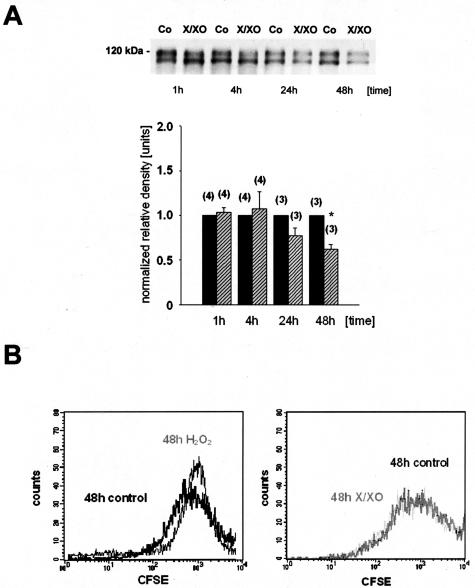
The α3/β1 integrin complex is a major extracellular matrix receptor complex expressed by podocytes, which plays a critical role in adhering foot processes to the glomerular basal membrane (GBM).27 α3 integrin-deficient mice are not able to produce intact foot processes.28 In addition, anti-β1 integrin antibodies lead to an increase of albumin permeability in isolated glomeruli, suggesting that β1 integrin is involved in maintaining the permeability barrier.2,9 To study whether CHOP might be involved in the regulation of α3/β1 integrin expression in podocytes, the expression of these integrins was investigated in control cells and CHOP-overexpressing podocytes. Figure 5 shows that the expression of β1 integrin and α3 integrin is reduced in CHOP-overexpressing cells. In contrast no alterations of the protein expression of the slit membrane proteins CD-2AP and NEPH-1 could be detected in CHOP-overexpressing podocytes (data not shown). To investigate whether the expression of β1 integrin was influenced by ROS we performed experiments with untransfected mouse podocytes that were stimulated with superoxide anions generated by the xanthine/xanthine-oxidase reaction or with vehicle. Figure 6A shows, that stimulation of mouse podocytes with xanthine/xanthine-oxidase time dependently reduces the β1 integrin expression after 48 hours of stimulation compared to vehicle stimulated cells.
Figure 5.
Expression of α3 and β1 integrins is reduced in CHOP-overexpressing podocytes. Western blot experiments showing the significant reduction in β1 integrin and α3 integrin protein expression in CHOP-overexpressing podocytes compared to control cells. All transblots were reprobed for either α-actin or β-tubulin to prove equal amounts of protein were loaded on the membrane (n = 5 to 6, values are means ± SEM, *P < 0.05 versus control, t-test).
Figure 6.
A: Expression of β1 integrin is down-regulated after stimulation with superoxide anions. Time course of β1 integrin expression after stimulation with X/XO or vehicle. There is a significant down-regulation of β1 integrin after 48 hours of stimulation with X/XO compared to vehicle-treated cells. Values were normalized with corresponding controls. All transblots were stained with Ponceau solution to prove equal amounts of protein were loaded on the membrane. In addition blots were reprobed with α-actin (data not shown) (n = 5 to 6, values are means ± SEM, *P < 0.05 versus control, t-test). B: Proliferation profiles of differentiated CFSE-loaded podocytes after stimulation with either H2O2 (250 μmol/L) or X/XO (50 μmol/L/50 mU/ml) for 48 hours. There was nearly no cell division detectable after 48 hours of stimulation in either group, indicating that H2O2 or X/XO does not induce cell proliferation and dedifferentiation in these cells.
Stimulation with H2O2 and X/XO Does Not Induce Proliferation in Mouse Podocytes
To test the hypothesis that ROS induce cell proliferation and dedifferentiation in podocytes, we stimulated differentiated podocytes with either H2O2 or X/XO and measured cell proliferation after incubation of the cells with CFSE, an amine-reactive dye that is incorporated into cells and distributed equally between daughter cells during division proliferation. There was nearly no cell divisiondetectable after 48 hours of stimulation in either group, indicating that there was no change in proliferation or differentiation during stimulation with H2O2 or X/XO (Figure 6B).
Cell-Matrix Adhesion Is Increased in CHOP-Overexpressing Cells
Recent data suggest that the α3/β1 integrin complex plays an important role for the adhesion of podocyte foot processes to the GBM.28,29 To test whether CHOP overexpression reduces cell adhesion to collagen IV, an important extracellular matrix protein of the GBM, we performed adhesion assays in CHOP-overexpressing cells and control cells. Cell adhesion to collagen IV increased in cells-overexpressing CHOP compared to control cells, a mechanism that was antagonized by the ROS scavenger DMTU (10 mmol/L) (Figure 7A).
Figure 7.
A: Cell adhesion is increased in CHOP-overexpressing cells. Increased cell-matrix adhesion (collagen IV-coated plates) in CHOP-overexpressing compared to control cells. This effect could be antagonized in the presence of DMTU (10 mmol/L), a potent ROS scavenger (n = 3 to 5, between 15 and 48 data points were used for each experiment, values are means ± SEM, *P < 0.05 versus control, t-test). B, top: Cell migration velocity (v) and cell displacement (dis) are increased in CHOP-overexpressing cells and puromycin-stimulated cells compared to controls (CHOP: V = 0.23 ± 0.06 μm/minute, dis = 19.7 ± 2.2 μm/250 minutes, n = 28; CHOP-control: V = 0.04 ± 0.003 μm/minute, dis = 6.8 ± 0.9 μm/250 minutes, n = 33; pur: V = 0.2 ± 0.03 μm/minute, dis = 23.4 ± 2.2 μm/250 minutes, n = 51; pur-control: V = 0.06 ± 0.007 μm/minute, dis = 11.5 ± 1.7 μm/250 minutes, n = 43 (values are means ± SEM, *P < 0.05 versus control, t-test). B, bottom: Motion trajectories of CHOP-overexpressing and control cells. Mean displacement distance is indicated by the circle.
Cell Mobility and Displacement Velocity Is Increased in CHOP-Overexpressing Cells and Untransfected Podocytes Stimulated with Puromycin
Recent data show an increase in cell mobility in podocytes stimulated with puromycin.30 To test the possibility of an increase in cell mobility in CHOP-overexpressing cells, we analyzed cell displacement (dis) and cell velocity (V) in CHOP-overexpressing podocytes, puromycin-stimulated untransfected podocytes (pur), and their controls (CHOP-control, pur-control). Cell velocity and cell displacement were significantly increased in CHOPoverexpressing podocytes and podocytes stimulated with puromycin (50 μg/ml) compared to their controls (Figure 7B).
Podocyte CHOP Protein Expression Is Increased in Human Membranous Nephropathy, Focal and Segmental Glomerulosclerosis, and Minimal Change Nephropathy
Overproduction of ROS has been detected in many glomerulopathies such as Heyman nephritis, a model of membranous nephropathy,6,7 and PAN nephrosis.31 To test the hypothesis that CHOP might be involved in the pathogenetic process of glomerular disease, we stained human biopsy samples of patients with membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy, and control samples from patients undergoing nephrectomy because of renal cancer. Very little CHOP was expressed in normal human podocytes. In contrast, expression of CHOP was increased in podocytes of most tissue samples from patients with membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy as compared to control samples (Figure 8). Interestingly, an increase in CHOP expression was not found in all biopsies. Analysis of staining intensity showed that in some biopsies CHOP expression was not different from control samples (Table 1).
Figure 8.
CHOP protein expression is increased in podocytes of patients with membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy. Immunohistochemical staining of kidney tissues from patients with membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy, or from patients undergoing nephrectomy because of renal cancer. There was a strong increase in CHOP protein expression in podocytes from most patients with proteinuric disease. At least seven different tissue samples were tested in each group (Table 1).
Table 1.
Staining Intensity of CHOP in Biopsies of Patients with Minimal Change Nephropathy, Focal Segmental Glomerulosclerosis and Membranous Nephropathy
| Staining intensity | 0 | 1+ | 2+ |
|---|---|---|---|
| Minimal change nephropathy | n = 4 | n = 2 | n = 2 |
| Focal segmental glomerulosclerosis | n = 2 | n = 4 | n = 2 |
| Membranous nephropathy | n = 1 | n = 1 | n = 5 |
Podocyte CHOP Protein Expression Is Increased in Rat Puromycin Nephrosis
To test if CHOP might be involved in the pathogenetic process of PAN nephrosis in rats, a model of ROS-mediated glomerular injury, we induced PAN in Wistar rats. ROS supposedly play an important role in the pathogenesis of PAN.32 Little CHOP was expressed in sections of control rats whereas an up-regulation of CHOP was found in rats with PAN (Figure 9). In contrast, α-dystroglycan, a member of the dystroglycan/dystrophin complex of adhesion molecules, was down-regulated in kidneys from rats with PAN compared to vehicle-treated rats.
Figure 9.
CHOP protein expression is increased in podocytes of rats with PAN compared to vehicle-treated rats. Immunohistochemical staining of CHOP in podocytes from vehicle-treated rats (top left) and from rats with PAN (top right). Kidneys were harvested at day 5 after induction of PAN. Bottom left: Time course for proteinuria in vehicle-treated rats and rats with PAN. The maximum of proteinuria was reached at day 4 after induction of PAN. Significant α-dystroglycan (160 kd) down-regulation in kidneys from vehicle-treated rats and from rats with PAN (n = 3 for each group, values are means ± SEM, *P < 0.05 versus control, t-test). To prove that equal amounts of protein were loaded on the membrane all transblots were reprobed with antibodies against α-actin (data not shown).
Discussion
Overproduction of ROS has been detected in several glomerular diseases and ROS scavengers have been shown to reduce foot process effacement and proteinuria.2–8 However, despite the evidence of the role of ROS in podocyte injury, the clinical benefit derived from these insights has yet to come, and most studies show that only pretreatment with ROS scavenger prevents glomerular injury.3 One reason for the lacking efficiency of ROS scavengers in reversing established glomerular injury may be that ROS itself changes several signaling cascades in podocytes, which then maintain podocyte injury by mechanisms distinct from ROS. Alternatively, ROS might regulate protective and deleterious effects on cell functions at the same time with subsequent ineffectiveness of ROS scavengers. This study used previous data from a PCR-based cDNA subtractive hybridization strategy11 to characterize expression differences for CHOP in podocytes that have been treated with ROS. ROS, produced from both the xanthine/xanthine-oxidase reaction as well as H2O2, induced a time-dependent expression of CHOP mRNA.
CHOP belongs to the group of growth arrest and DNA damage (GADD) genes.33 During development these genes are expressed at different times before and after implantation and seem to play a role in the growth of different organs such as the cardiovascular system, lung, and kidney. The mRNAs for the GADD gene family are increased in growth-arrested cells. Interestingly, stable overexpression of CHOP in our cell line did not increase cell death or growth arrest compared to control cells. Similar results have been found in an osteoblastic cell line,34 but conflicting data exist. A rat1 cell line constitutively expressing GADD153 could not be established, presumably because of the growth-arresting properties of GADD153.35 CHOP is a nuclear protein that serves as a dominant-negative inhibitor of C/EBP transcription factors.12 Cellular stress, particularly in response to toxic and metabolic insults that perturb function of the endoplasmic reticulum, strongly induces the expression of CHOP.36 Several reports indicate that ROS can up-regulate the transcription of CHOP.37–42 However, contradictory data exist, reporting down-regulation of CHOP by H2O2 in neuronal cells.43 In addition, up-regulation of CHOP by superoxide dismutase was found in MCF7 cells.44 CHOP serves as a growth arrest gene, blocking cells from progressing from G1 to S phase.14 Besides its function as a growth arrest gene CHOP has been implicated in several other important cellular functions such as intracellular acidification,45 differentiation,15 apoptosis, and regeneration.46
Induction of GAS5 and CHOP mRNA in podocytes was immediate and relatively long-lasting, given that elevated CHOP mRNA was still detectable 12 hours after podocytes had been exposed to exogenous ROS. Because recent data have shown that no cellular protein is expressed despite four alternative splicing patterns for GAS5, no confirmation of GAS5 expression on protein level was performed.25,26 Western blot analysis of CHOP expression showed that the induction of CHOP protein by H2O2 and xanthine/xanthine oxidase was not immediate but occurred after 24 and 48 hours of stimulation, indicating that CHOP may play a prolonged role in the damage of podocytes induced by ROS. Recent data indicate that CHOP and GAS5 expression is increased after nutrient deprivation.47 In addition, data suggest that GAS5 might regulate CHOP expression through snoRNA.26 To test the hypothesis that GAS5 and CHOP can interact in podocytes, GAS5 mRNA expression was determined in CHOP-overexpressing cells. GAS5 expression was down-regulated in CHOP-overexpressing cells suggesting a negative feedback mechanism of CHOP on GAS5 expression.
CHOP protein could also be detected in podocytes in the glomerulus, suggesting that it plays a role in podocyte function in vivo. As a consequence of the high degree of differentiation of podocytes, it has been shown that they are unable to proliferate under normal conditions, and that in contrast to the proliferative capacity of the neighboring mesangial cells, the cell-cycle quiescence in podocytes is tightly controlled.48 An escape of the podocyte from cell-cycle arrest leads to injury of the glomerular structure followed by a rapid deterioration of kidney function, as shown by the deleterious course of collapsing nephropathy.49 Our data show that proliferation in CHOP-overexpressing podocytes is not altered, but cell displacement is markedly increased. In addition, stimulation of untransfected podocytes with puromycin had a similar effect. This effect has been shown before30 and might reflect the known migration tendency of podocytes in nephritic syndrome. Our data also indicate that CHOP protein expression is increased in membranous nephropathy, focal segmental glomerulosclerosis, and minimal change disease suggesting a functional role of CHOP in this disease. Interestingly, expression of CHOP was not found in all biopsies studied. This might be caused by differences in the severity of disease, time course of the disease, or subforms of disease. Further studies will be necessary to clarify this phenomenon.
To investigate possible functions of CHOP in podocytes, CHOP was constitutively overexpressed in a mouse cell line. In CHOP-overexpressing podocytes an increased generation of O2− could be detected. Podocytes are known to be not only the target but also the source of ROS. In Heyman nephritis sublytic C5b-9 attack on podocytes causes up-regulation of the NADPH oxidoreductase enzyme complex by podocytes, which is translocated to the cell surface.2 Subsequently, ROS are generated, reach the GBM matrix, and initiate lipid peroxidation and degradation of GBM collagen IV, leading to proteinuria.2 Therefore, induction of CHOP might—via activation of NADPH-dependent generation of superoxide anions—increase podocyte injury and proteinuria. Recently, it has been demonstrated that elevated CHOP expression results in an increased ROS production, reduction of cellular glutathione levels, and down-regulation of BclII in fibroblasts.35
Attachment of podocytes to the glomerular basement membrane is thought to be mediated in part by the α3/β1 integrin complex and by other cytoskeletal proteins including dystroglycan, actin, talin, vinculin, and α-actinin.49 The α3/β1 integrin complex plays an important role for kidney development and function.27,28 α3 integrin knockout mice show a disorganized GBM and podocytes with immature foot processes.27 Cross-linking of β1 integrin by antibody binding has been shown to inhibit adhesion of podocytes in vitro and to increase glomerular permeability for albumin.50,51 A reduced expression of α3/β1 integrin on podocytes has been demonstrated in podocytes of both humans and rats with diabetes52 and in PAN, an experimental model for human minimal change disease.31 In the latter study treatment of animals with phosphatidyl choline-bound superoxide dismutase reduced proteinuria and preserved the expression of α3 integrin expression in podocytes.31 Interestingly, in our experiments with rats treated with puromycin to induce PAN, up-regulation of CHOP was found exclusively in podocytes. In addition, Western blot analysis of α3/β1 integrins in podocytes showed that the expression of both integrins is reduced in CHOP-overexpressing podocytes. In contrast the expression of CD2AP or NEPH-1, two important proteins of the podocyte slit diaphragm53 was not altered (data not shown). The data indicate that CHOP may be a regulator of α3/β1 integrin expression in podocytes and could play a role in the maintenance of podocyte adherence to the GBM. Down-regulation of α3/β1 integrins in podocytes might depend on extracellular ROS because stimulation of untransfected podocytes with xanthine/xanthine oxidase reduced β1 integrin expression similar to the reduction seen in CHOP-overexpressing cells. Surprisingly, cell adhesion of CHOP-overexpressing cells was increased. This effect could be antagonized with the ROS scavenger DMTU. An increased cell-matrix adhesion of podocytes in the presence of reduced α3 integrin expression has been shown very recently in PAN, but the mechanism involved is poorly understood.31 In the latter study, down-regulation of α3 integrin protected against PAN-induced podocyte detachment. It therefore might be possible, that CHOP—via down-regulation of integrins—inhibits the detachment of podocyte foot processes in glomerular disease. Further studies will be necessary to differentiate between deleterious and beneficial effects of ROS on various cell functions in the glomerulus.
In summary the data indicate that podocytes regulate CHOP expression in response to ROS. The expression of CHOP in podocytes is increased in some forms of proteinuric nephropathies and PAN. CHOP regulates important functions in podocytes: it increases NADPH-dependent generation of O2−, it reduces the expression of α3/β1 integrins, and alters cell attachment and cell displacement. Thus, CHOP might play a critical role in the glomerular injury.
Acknowledgments
In remembrance of Stefan Greiber, a brilliant nephrologist and good friend, who died during the experimentation of this publication.
We thank C. Hupfer, P. Daemisch, and P. Kulick for their excellent technical assistance; and Peter Mundel, Albert Einstein College of Medicine, New York, NY, for the generous gift of podocyte cells.
Footnotes
Address reprint requests to Prof. Hermann Pavenstaedt, M.D., Chief of the Department of Medicine, Division of Nephrology, University Clinics Muenster, Albert-Schweitzer-Str. 33, 48149 Muenster, Germany. E-mail: hermann.pavenstaedt@ukmuenster.de.
Supported by the Deutsche Forschungsgemeinschaft (grant PA 483/5-1) and by Interdisziplinäres Zentrum für Klinische Forschung Muenster (grant Pa2/108/04).
S. Greiber died in December 2003.
References
- Pavenstadt H. Roles of the podocyte in glomerular function. Am J Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Neale TJ. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol. 1996;7:2518–2526. doi: 10.1681/ASN.V7122518. [DOI] [PubMed] [Google Scholar]
- Gwinner W, Grone HJ. Role of reactive oxygen species in glomerulonephritis. Nephrol Dial Transplant. 2000;15:1127–1132. doi: 10.1093/ndt/15.8.1127. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Ichikawa I, Fogo A. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J Am Soc Nephrol. 1991;2:902–912. doi: 10.1681/ASN.V24902. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, Bertram JF, Ryan GB. Antioxidants protect podocyte foot processes in puromycin aminonucleoside-treated rats. J Am Soc Nephrol. 1994;4:1974–1986. doi: 10.1681/ASN.V4121974. [DOI] [PubMed] [Google Scholar]
- Neale TJ, Ullrich R, Ojha P, Poczewski H, Verhoeven AJ, Kerjaschki D. Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc Natl Acad Sci USA. 1993;90:3645–3649. doi: 10.1073/pnas.90.8.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SV. Evidence suggesting a role for hydroxyl radical in passive Heymann nephritis in rats. Am J Physiol. 1988;254:F337–F344. doi: 10.1152/ajprenal.1988.254.3.F337. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol. 1999;154:1067–1075. doi: 10.1016/S0002-9440(10)65359-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone HJ, Walli AK, Grone EF. The role of oxidatively modified lipoproteins in lipid nephropathy. Contrib Nephrol. 1997;120:160–175. doi: 10.1159/000059835. [DOI] [PubMed] [Google Scholar]
- Grone HJ, Grone EF, Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab Invest. 2002;82:5–14. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- Greiber S, Müller B, Daemisch P, Pavenstädt H. Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony-stimulating factor. J Am Soc Nephrol. 1996;13:86–95. doi: 10.1681/ASN.V13186. [DOI] [PubMed] [Google Scholar]
- Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of CHOP. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994;8:453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- Batchvarova N, Wang XZ, Ron D. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J. 1995;14:4654–4661. doi: 10.1002/j.1460-2075.1995.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–147. doi: 10.1016/0014-5793(96)01016-2. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia BA, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Greiber S, Munzel T, Kastner S, Muller B, Schollmeyer P, Pavenstadt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int. 1998;53:654–663. doi: 10.1046/j.1523-1755.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- Nickel C, Benzing T, Sellin L, Gerke P, Karihaloo A, Liu ZX, Cantley LG, Walz G. The polycystin-1 C-terminal fragment triggers branching morphogenesis and migration of tubular kidney epithelial cells. J Clin Invest. 2002;109:481–489. doi: 10.1172/JCI12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek MJ, Wahle S, Muller B, Benzing T, Huber TB, Kretzler M, Cohen C, Busse-Grawitz A, Pavenstadt H. Stra13, a prostaglandin E2-induced gene, regulates the cellular redox state of podocytes. FASEB J. 2003;17:682–684. doi: 10.1096/fj.02-0250fje. [DOI] [PubMed] [Google Scholar]
- Bek MJ, Reinhardt HC, Fischer KG, Hirsch JR, Hupfer C, Dayal E, Pavenstadt H. Up-regulation of early growth response gene-1 via the CXCR3 receptor induces reactive oxygen species and inhibits Na+/K+-ATPase activity in an immortalized human proximal tubule cell line. J Immunol. 2003;170:931–940. doi: 10.4049/jimmunol.170.2.931. [DOI] [PubMed] [Google Scholar]
- Holcombe H, Mellman I, Janeway CA, Jr, Bottomly K, Dittel BN. The immunosuppressive agent 15-deoxyspergualin functions by inhibiting cell cycle progression and cytokine production following naive T cell activation. J Immunol. 2002;169:4982–4989. doi: 10.4049/jimmunol.169.9.4982. [DOI] [PubMed] [Google Scholar]
- Schwab A, Wulf A, Schulz C, Kessler W, Nechyporuk-Zloy V, Römer M, Reinhardt J, Weinhold D, Dieterich P, Stock C, Hebert SC. Subcellular distribution of Ca2+ sensitive K+ channels in migrating cells. J Cell Physiol. 2006;206:86–94. doi: 10.1002/jcp.20434. [DOI] [PubMed] [Google Scholar]
- Guan N, Ding J, Deng J, Zhang J, Yang J. Key molecular events in puromycin aminonucleoside nephrosis rats. Pathol Int. 2004;54:703–711. doi: 10.1111/j.1440-1827.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- Raho G, Barone V, Rossi D, Philipson L, Sorrentino V. The gas 5 gene shows four alternative splicing patterns without coding for a protein. Gene. 2000;256:13–17. doi: 10.1016/s0378-1119(00)00363-2. [DOI] [PubMed] [Google Scholar]
- Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol. 1998;18:6897–6909. doi: 10.1128/mcb.18.12.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Symons JM. Integrins in kidney development, function, and disease. Am J Physiol. 2000;279:F233–F242. doi: 10.1152/ajprenal.2000.279.2.F233. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Adler S. Integrin receptors in the glomerulus: potential role in glomerular injury. Am J Physiol. 1992;262:F697–F704. doi: 10.1152/ajprenal.1992.262.5.F697. [DOI] [PubMed] [Google Scholar]
- Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and a3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- Kojima K, Matsui K, Nagase M. Protection of alpha(3) integrin-mediated podocyte shape by superoxide dismutase in the puromycin aminonucleoside nephrosis rat. Am J Kidney Dis. 2000;35:1175–1185. doi: 10.1016/s0272-6386(00)70056-4. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Lovett D, Lehrer RI, Couser WG, Klebanoff SJ. Role of oxidants and proteases in glomerular injury. Kidney Int. 1994;45:352–359. doi: 10.1038/ki.1994.45. [DOI] [PubMed] [Google Scholar]
- Fleming JV, Fontanier N, Harries DN, Rees WD. The growth arrest genes gas5, gas6, and CHOP-10 (gadd153) are expressed in the mouse preimplantation embryo. Mol Reprod Dev. 1997;48:310–316. doi: 10.1002/(SICI)1098-2795(199711)48:3<310::AID-MRD2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Delany AM, Canalis E. CCAAT/enhancer binding protein homologous protein (DDIT3) induces osteoblastic cell differentiation. Endocrinology. 2004;145:1952–1960. doi: 10.1210/en.2003-0868. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- Pomerance M, Carapau D, Chantoux F, Mockey M, Correze C, Francon J, Blondeau JP. CCAAT/enhancer-binding protein-homologous protein expression and transcriptional activity are regulated by 3′,5′-cyclic adenosine monophosphate in thyroid cells. Mol Endocrinol. 2003;17:2283–2294. doi: 10.1210/me.2002-0400. [DOI] [PubMed] [Google Scholar]
- Lovat PE, Oliverio S, Corazzari M, Ranalli M, Pearson AD, Melino G, Piacentini M, Redfern CP. Induction of GADD153 and Bak: novel molecular targets of fenretinide-induced apoptosis of neuroblastoma. Cancer Lett. 2003;197:157–163. doi: 10.1016/s0304-3835(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Ikeyama S, Wang XT, Li J, Podlutsky A, Martindale JL, Kokkonen G, van Huizen R, Gorospe M, Holbrook NJ. Expression of the pro-apoptotic gene gadd153/chop is elevated in liver with aging and sensitizes cells to oxidant injury. J Biol Chem. 2003;278:16726–16731. doi: 10.1074/jbc.M300677200. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Stevens JL, Lau SS, Monks TJ. Quinone thioether-mediated DNA damage, growth arrest, and gadd153 expression in renal proximal tubular epithelial cells. Mol Pharmacol. 1996;50:592–598. [PubMed] [Google Scholar]
- Patton GW, Paciga JE, Shelley SA. NR8383 alveolar macrophage toxic growth arrest by hydrogen peroxide is associated with induction of growth-arrest and DNA damage-inducible genes GADD45 and GADD153. Toxicol Appl Pharmacol. 1997;147:126–134. doi: 10.1006/taap.1997.8227. [DOI] [PubMed] [Google Scholar]
- Luethy JD, Fargnoli J, Park JS, Fornace AJ, Jr, Holbrook NJ. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem. 1990;265:16521–16526. [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T, Althausen S, Hotop S. Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem. 2001;76:1916–1924. doi: 10.1046/j.1471-4159.2001.00206.x. [DOI] [PubMed] [Google Scholar]
- Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D. CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol. 1999;19:495–504. doi: 10.1128/mcb.19.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JV, Hay SM, Harries DN, Rees WD. Effects of nutrient deprivation and differentiation on the expression of growth-arrest genes (gas and gadd) in F9 embryonal carcinoma cells. Biochem J. 1998;330:573–579. doi: 10.1042/bj3300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W. Progressive renal failure—inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996;11:1738–1742. [PubMed] [Google Scholar]
- Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S, Chen X. Anti-Fx1A antibody recognizes a beta 1-integrin on glomerular epithelial cells and inhibits adhesion and growth. Am J Physiol. 1992;262:F770–F776. doi: 10.1152/ajprenal.1992.262.5.F770. [DOI] [PubMed] [Google Scholar]
- Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH. Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci. 2000;67:2345–2353. doi: 10.1016/s0024-3205(00)00815-8. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Wartiovaara J. Molecular basis of glomerular perm-selectivity. Curr Opin Nephrol Hypertens. 2001;10:543–549. doi: 10.1097/00041552-200107000-00009. [DOI] [PubMed] [Google Scholar]