Abstract
Diabetic retinopathy is characterized by blood-retinal barrier (BRB) breakdown and neurotoxicity. These pathologies have been associated with oxidative stress and proinflammatory cytokines, which may operate by activating their downstream target p38 MAP kinase. In the present study, the protective effects of a nonpsychotropic cannabinoid, cannabidiol (CBD), were examined in streptozotocin-induced diabetic rats after 1, 2, or 4 weeks. Retinal cell death was determined by terminal dUTP nick-end labeling assay; BRB function by quantifying extravasation of bovine serum albumin-fluorescein; and oxidative stress by assays for lipid peroxidation, dichlorofluorescein fluorescence, and tyrosine nitration. Experimental diabetes induced significant increases in oxidative stress, retinal neuronal cell death, and vascular permeability. These effects were associated with increased levels of tumor necrosis factor-α, vascular endothelial growth factor, and intercellular adhesion molecule-1 and activation of p38 MAP kinase, as assessed by enzyme-linked immunosorbent assay, immunohistochemistry, and/or Western blot. CBD treatment significantly reduced oxidative stress; decreased the levels of tumor necrosis factor-α, vascular endothelial growth factor, and intercellular adhesion molecule-1; and prevented retinal cell death and vascular hyperpermeability in the diabetic retina. Consistent with these effects, CBD treatment also significantly inhibited p38 MAP kinase in the diabetic retina. These results demonstrate that CBD treatment reduces neurotoxicity, inflammation, and BRB breakdown in diabetic animals through activities that may involve inhibition of p38 MAP kinase.
Diabetic retinopathy is the leading cause of blindness in working age adults, affecting nearly 16 million people in the United States alone.1 Diabetic retinopathy is characterized by early breakdown of the blood-retinal barrier (BRB) that causes loss of vision through macular edema and/or vitreoretinal neovascularization.2–4 Recent studies have suggested that neurodegeneration of the retina is a critical component of diabetic retinopathy.5–7 Neurotoxicity causes permanent impairment of visual function due to cell death of the inner retinal and ganglion cells. We have reported that BRB breakdown is associated with increases in oxidative stress and tyrosine nitration in early experimental diabetes.8 However, the causal role of oxidative stress in diabetes-induced neurodegeneration has not been elucidated.
The initial insult that leads to vascular dysfunction and neurotoxicity in the diabetic retina probably occurs very early. Early increases in vascular endothelial growth factor (VEGF) and tumor necrosis factor-α (TNF-α) have been correlated with vascular hyperpermeability in diabetic retinas, implying a proinflammatory component of the pathology.3,8,9 During early stages of experimental diabetes, leukocyte adhesion (leukostasis) occurs due to hyperglycemia-induced oxidative stress, the actions of proinflammatory cytokines and the consequent activation of endothelial intercellular adhesion molecule-1 (ICAM-1).10,11 Leukostasis also leads to vascular occlusion and tissue ischemia, which have been associated with changes in neuronal function, edema, and neuronal cell loss.12
The p38 MAP kinase, a stress-activated serine/threonine protein kinase, is a downstream target of proinflammatory cytokines and oxidative stress. Activation of p38 MAP kinase has been reported in sensory neurons and endothelial cells maintained under high glucose conditions13,14 and in diabetic retinas.15,16 Activation of p38 MAP kinase is implicated in apoptotic death of retinal ganglion cells17 and in N-methyl-d-aspartic acid (NMDA)-induced neural retinal death.18 Activation of p38 MAP kinase has also been implicated in vascular hyperper-meability in diabetic retinas19 and in VEGF-induced permeability.20,21
Cannabinoids are known to possess therapeutic properties including inhibition of oxidation,22,23 NMDA receptor-activation,24 and inflammation.25,26 The best-known cannabinoids from marijuana are (−)-Δ9-tetrahydrocannabinol (THC) and (−)-cannabidiol (CBD). The nonpsychotropic CBD has been shown to decrease interleukin-1, TNF-α, and interferon-γ in murine collagen-induced arthritis26 and to prevent central nervous system neuronal damage in gerbils caused by cerebral ischemia.27 We have recently demonstrated the neuroprotective effect of both THC and CBD via antioxidant action in NMDA-induced retinal neurotoxicity in rats.28 We have also demonstrated the BRB-preserving effects of blocking oxidative stress in diabetic rats.8 The effects of CBD on neuro-inflammation and BRB breakdown in hyperglycemia, however, have not been studied. The present study evaluates the ability of CBD to reduce oxidative stress, preserve BRB function, and prevent neural cell death in experimental diabetes.
Materials and Methods
Animal Preparation
All procedures with animals were performed in accordance with the Public Health Service Guide for the Care and Use of Laboratory Animals (Department of Health, Education, and Welfare publication, NIH 80-23), The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and Medical College of Georgia guidelines. Diabetes was induced in male Sprague-Dawley rats after fasting overnight by intravenous injection of streptozotocin (60 mg/kg) dissolved in 0.01 mol/L sodium citrate buffer, pH 4.5. Detection of glucose in the urine of injected animals and blood glucose levels >250 mg/dl were the markers to indicate diabetic status. Three sets of animals were prepared for a total of 127 rats to study the effects of 1, 2, or 4 weeks of induced diabetes. The CDB-treated control or diabetic group received intraperitoneal injections of CBD (10 mg/kg) every other day for the duration of the study. CBD dose was selected based on previous studies showing maximum protection of CBD at the level of 5 mg/kg/day.26,27 In the current study, CBD was injected 10 mg/kg every 2 days to decrease the frequency of injection. CBD was obtained from National Institute of Drug Abuse (Research Triangle Park, NC), and a fresh solution in 0.25 ml of 1:1:18 of alcohol:cremorphol:Ringer solution was prepared. Control groups received vehicle injections at the same time points.
Measurement of BRB Function
Integrity of the BRB was measured as previously described.8 After 2 weeks of diabetes induction, rats received tail vein injections of 10 mg/kg of bovine serum albumin-fluorescein conjugate (Molecular Probes, Eugene, OR) 30 minutes before sacrifice. Plasma was assayed for fluorescein concentration using a CytoFluor 4000 spectrofluorometer (CytoFluor, Foster City, CA). The eyes were enucleated, embedded in OCT medium, and frozen on dry ice. Frozen eye sections (10 μm) collected from each group (n = 6 to 7) were analyzed with a fluorescence microscope fitted with a spot camera. Images were collected from 10 retinal nonvascular areas (200 μm2) along the vertical meridian within 4 mm of the optic disk. The average retinal fluorescence intensity was calculated and normalized to plasma fluorescence intensity for each animal. Through serial sectioning of each eye and detection of extravasation of bovine serum albumin-fluorescein, this technique enabled quantification of increased vascular permeability in the retina.
Evaluation of Neural Cell Death in Rat Retina
Terminal dUTP nick end-labeling (TUNEL) assay was performed after 4 weeks of induced diabetes to detect retinal cell death by using horseradish peroxidase (HRP) detection (TUNEL-HRP; Intergen, Purchase, NY) in whole-mount retina.5 Formalin-fixed retinas were flat-mounted with photoreceptor side facing the slide, dehydrated in ethanol, defatted by xylenes, and rehydrated. After permeabilization and endogenous peroxidase quenching, TUNEL-HRP staining with AEC was performed following the manufacturer’s instructions. The total number of TUNEL-HRP-positive cells was counted in each retina. TUNEL was also performed in 10-μm OCT-frozen eye sections using the ApopTAG in situ cell death detection kit (TUNEL-FITC) as described previously.28
Measurement of Retinal Lipid Peroxidation
Lipid peroxide concentration in the 2-week diabetic rat retinas was determined by a method that measures the amount of thiobarbituric acid reactivity by the amount of malondialdehyde (MDA) formed during acid hydrolysis of the lipid peroxide compound. Retinas were homogenized in a Mini-Bead beater with treated Ottawa sand in 250 μl of modified RIPA buffer containing 50 mmol/L Tris, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40, 0.25% deoxycholate, supplemented with 40 mmol/L NaF, 2 mmol/L Na3VO4, and 1:100 (v/v) of proteinase inhibitor cocktail (Sigma, St. Louis, MO). Insoluble material was removed by centrifugation at 12,000 × g at 4°C for 30 minutes. The reaction mixture contained 0.03 ml of retina homogenate, 0.02 ml of 8.1% sodium dodecyl sulfate, 0.15 ml of 20% acetic acid solution (buffered to pH 3.5), and 0.15 ml of 0.8% thiobarbituric acid. The mixture was then incubated at 95°C for 1 hour. After cooling, 0.1 ml of distilled water followed by 0.5 ml of the mixture of n-butanol and pyridine (15:1, v/v) was added, and the final mixture was shaken vigorously. After centrifugation (1500 × g, 10 minutes), absorbance of the solvent layer was measured at 532 nm. The reagent 1,1,3,3-tetraethoxypropane was used as external standard. Lipid peroxide level was expressed in terms of nm of MDA per mg of protein.
Dichlorofluorescein (DCF) Assay
DCF is the oxidation product of the reagent 2′,7′-dichlorofluorescein diacetate (H2DCFDA; Molecular Probes), a marker of cellular oxidation by hydrogen peroxides and peroxynitrite.29 H2DCFDA was directly applied to frozen eye sections of 2-week diabetic and control retinas and oxidized to the fluorescent compound 2′,7′-dichlorofluorescein (DCF). The fluorescence of DCF was measured and analyzed at 10 adjacent locations (200 μm2) along the vertical meridian within 4 mm of the optic disk. The average retinal fluorescence intensity (10 fields/retina, n = 6 in each group) was analyzed using fluorescence microscopy and Ultra-View morphometric software.
Measurement of Retinal Nitrotyrosine
Nitrotyrosine immunoreactivity was measured as an indicator for ONOO− formation. The distribution of nitrotyrosine in frozen eye sections was analyzed using immunolocalization techniques as described previously.8 After 2 weeks of induced diabetes, frozen eye sections were fixed with 4% paraformaldehyde then reacted with a polyclonal rabbit anti-nitrotyrosine antibody (Upstate Biotechnology, Lake Placid, NY) followed by Oregon Green-conjugated goat anti-rabbit antibody (Molecular Probes). Data (10 fields/retina, n = 6 in each group) were analyzed using fluorescence microscopy and Ultra-View morphometric software to quantify intensity of immunostaining.
Immunohistochemical Localization and Quantification of VEGF and ICAM-1
The distribution and quantification of VEGF and ICAM-1 expression in retinal sections was analyzed after 2 or 4 weeks of induced diabetes. OCT-frozen sections (10 μm) of eyes were fixed using 4% paraformaldehyde solution in phosphate-buffered saline (PBS) for 10 minutes. Sections were washed several times with PBS and were reacted with a monoclonal mouse anti-ICAM-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by Texas Red-conjugated goat anti-mouse antibody (Molecular Probes). For VEGF, retinal sections were reacted with a polyclonal rabbit anti-VEGF antibody (Abcam Inc., Cambridge, MA) followed by Oregon Green-conjugated goat anti-rabbit antibody (Molecular Probes). Data (10 fields/retina, n = 6 in each group) were analyzed using fluorescence microscopy and Ultra-View morphometric software to quantify the density of immunostaining.
Enzyme-Linked Immunosorbent Assay (ELISA) for TNF-α
The TNF-α protein levels were estimated with the ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions after 1, 2, or 4 weeks of induced diabetes. Each retina was homogenized in 125 μl of solution, as described previously.9 The reaction was stopped and the absorption was measured in a plate reader at 450 nm. Nonspecific absorption was measured at 562 nm. We performed all measurements in quadruplicate. The tissue sample concentration of TNF-α was calculated from a standard curve and corrected for protein concentration.
Retinal Protein Extraction and Western Blot Analysis
After 2 or 4 weeks of induced diabetes, rats were anesthetized with an intraperitoneal injection of 400 mg/kg of chloral hydrate and sacrificed by decapitation. Individual retinas were dissected and homogenized in a Mini-Bead beater with treated Ottawa sand in 250 μl of modified RIPA buffer, containing 50 mmol/L Tris, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40, 0.25% deoxycholate, supplemented with 40 mmol/L NaF, 2 mmol/L Na3VO4, and 1:100 (v/v) of proteinase inhibitor cocktail (Sigma). Insoluble material was removed by centrifugation at 12,000 × g at 4°C for 30 minutes. Antibodies for phospho-p38 MAP kinase and p38 MAP kinase were purchased from Cell Signaling Technology (Beverly, MA). Retinal protein extract of 100 μg, was boiled in 6× Laemmli sample buffer, separated on a gradient (4 to 20%) sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad, Hercules, CA) by electrophoresis, transferred to nitrocellulose membrane, and incubated with specific antibodies. The primary antibody was detected using a horseradish peroxidase-conjugated sheep anti-rabbit antibody (Amersham BioSciences, Buckinghamshire, UK) and enhanced chemiluminescence. Intensity of immunoreactivity was measured by densitometry (n = 6 in each group).
Data Analysis
The results are expressed as mean ± SEM. Differences among experimental groups were evaluated by analysis of variance, and the significance of differences between groups was assessed by the posthoc test (Fisher’s PLSD) when indicated. Significance was defined as P < 0.05.
Results
CBD Treatment Does Not Alter Body Weight or Blood Glucose Levels
Table 1 shows the changes of body weight and blood glucose levels for diabetic, CBD-treated diabetic, and age-matched nondiabetic control groups at duration of 1, 2, or 4 weeks after streptozotocin-induced diabetes. These parameters were similar in CBD-treated and untreated rats. Significant increases of blood glucose level (>350 mg/dl) were observed in the diabetic rats compared to control rats (∼100 mg/dl). Treatment with CBD had no effect on the body weight or the blood glucose levels in diabetic rats (>350 mg/dl) or in treated controls.
Table 1-6715.
Effects of STZ-Induced Diabetes on Body Weight and Blood Glucose Levels in Control and Diabetic, Diabetic Treated with CBD Animals, or Control Treated with CBD
| Animal group | n | Start weight | End weight | Blood glucose (mg/dL) | |
|---|---|---|---|---|---|
| 1 week | Control | 6 | 163 ± 1.9 | 225 ± 2.8 | 100 ± 5.9 |
| Diabetic | 7 | 160 ± 1.5 | 197 ± 2.9 | 354 ± 15.1 | |
| Diabetic + CBD | 6 | 157 ± 1.8 | 182 ± 6.6 | 378 ± 25.1 | |
| Control + CBD | 6 | 159 ± 1.7 | 221 ± 3.6 | 103 ± 5.1 | |
| 2 weeks | Control | 12 | 192.8 ± 3.6 | 327 ± 4 | 100 ± 3.1 |
| Diabetic | 14 | 180.8 ± 4.8 | 256 ± 7.4 | 375 ± 9.5 | |
| Diabetic + CBD | 12 | 175.6 ± 2.1 | 269 ± 12 | 375 ± 12.6 | |
| Control + CBD | 12 | 189 ± 2.6 | 321 ± 3.9 | 104 ± 3.1 | |
| 4 weeks | Control | 12 | 132 ± 8.8 | 330 ± 26.3 | 100 ± 5.1 |
| Diabetic | 14 | 126.6 ± 10.4 | 218 ± 21.2 | 473 ± 9.5 | |
| Diabetic + CBD | 14 | 129 ± 9.5 | 241 ± 13.6 | 433 ± 12.6 | |
| Control + CBD | 12 | 130 ± 4.7 | 341 ± 3.7 | 101 ± 4.3 |
Data are mean ± SE. Animals were made diabetic by a single STZ injection (60 mg/ kg) in freshly prepared 10 mmol/L sodium citrate buffer, pH 4.5.
CBD Prevents BRB Breakdown in Experimental Diabetes
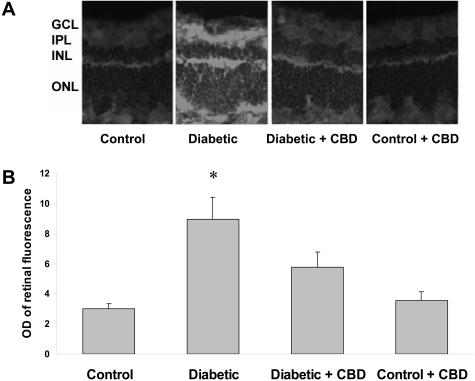
Breakdown of the BRB is a well-established feature of both clinical and experimental diabetes.30 Assessment of BRB function via detection of extravasation of bovine serum albumin-fluorescein has been previously shown to be sensitive and quantifiable.31 Previous studies using this assay have shown increased permeability as early as 2 weeks after induction of diabetes.8 Microscopic images showed prominent fluorescence that was diffusely distributed through the retinal parenchyma in the diabetic retina (Figure 1A). The average retinal fluorescence intensity was calculated and normalized to plasma fluorescence intensity for each animal. Quantitative analysis showed an approximately fourfold increase (P < 0.001) in the fluorescence intensity in the diabetic retinas compared to untreated control (Figure 1B). Treatment with CBD significantly inhibited diabetes-induced hyperpermeability. The control rat retinas were not affected by CBD treatment.
Figure 1-6715.
BRB-protection by CBD in experimental diabetes. Vascular permeability was determined by intravenous injection of bovine serum albumin-fluorescein (100 mg/kg) in streptozotocin-induced diabetic and control rat retina. Animals were sacrificed after 30 minutes and eyes were enucleated and snap-frozen. A: Representative images show the albumin extravasation in different retinal layers; the ganglion cell layer (GCL), inner plexiform layer (IPL), the inner nuclear layer (INL), and the outer nuclear layer (ONL) B: Morphometric analysis of fluorescence intensity in serial sections of rat eyes shows that diabetic rats had a fourfold increase in fluorescence compared with controls. Treatment of diabetic rats with CBD (10 mg/kg/2days) blocked the permeability increase. Treatment of control rats with CBD (10 mg/kg every 2 days) did not alter BRB breakdown in control animals. Data shown is the mean ± SEM of six or seven animals in each group. *P < 0.001. Original magnifications, ×200.
CBD Prevents Neural Cell Death in the Retina of Diabetic Rats
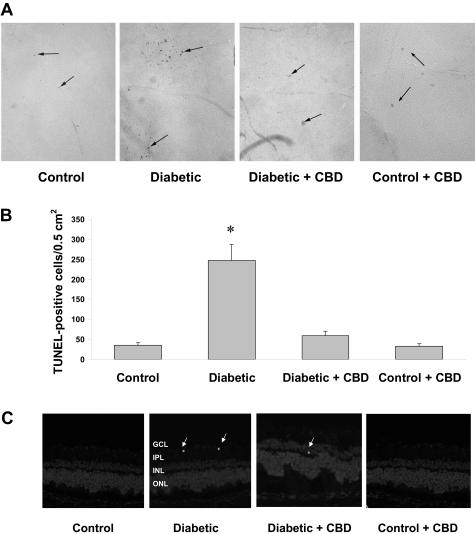
Accelerated death of retinal ganglion cells and neurons has been reported to be significant in experimental diabetes as early as 4 weeks.5 We have demonstrated the neuroprotective effects of CBD in NMDA-induced retinal toxicity.28 Therefore, we tested the hypothesis that CBD prevents death of inner neurons in streptozotocin-induced diabetic rat retinas after 4 weeks. Quantitative analysis of TUNEL-HRP-labeled cells in the whole-mounted retinas showed a sevenfold increase in the frequency of retinal cell death in the retinas after 4 weeks of induced diabetes (Figure 2, A and B). CBD (10 mg/kg every 2 days) significantly reduced the number of TUNEL-positive cells (P < 0.001). TUNEL+ nuclei were not associated with the capillary wall (Figure 2A) and did not co-localize with the endothelial marker B4-isolectin (data not shown), suggesting that most of the cells undergoing death are not vascular and are more likely to be either neurons or glial cells. We further confirmed the neural cell death rather than nonvascular cells by staining frozen sections using TUNEL-FITC. These studies showed that scattered TUNEL+ cells were present in the ganglion cell and inner nuclear layers, with occasional distribution in the outer nuclear layer of diabetic rat retinas (Figure 2C). Treatment of control rats with CBD (10 mg/kg every 2 days) did not alter the number of TUNEL+ cells in flat mount or retinal sections. These results confirm the previous reports that neurodegeneration is an early and important component of diabetic retinopathy. These results also suggest a potential therapeutic role of CBD in diabetes.
Figure 2-6715.
Retinal neuroprotective effect of CBD in experimental diabetes. A: Numerous TUNEL HRP-labeled cells (arrows) were detected in whole-mounted retinas from 4-week diabetic rats as compared with untreated controls and the CBD-treated group. B: Total number of TUNEL HRP-positive cells counted in each retina, expressed per 0.5 cm2. The diabetic rats had significantly more TUNEL HRP-positive cells than controls and the CBD-treated group (*P < 0.001; n = 5 to 6). Treatment with CBD (10 mg/kg every 2 days) blocked cell death in the diabetic retinas but did not alter number of TUNEL+ cells in control rats. C: A representative image shows the TUNEL labeling of frozen eye sections from the diabetic rats (4 weeks) in different retinal layers; the ganglion cell layer (GCL), inner plexiform layer (IPL), the inner nuclear layer (INL), and the outer nuclear layer (ONL). TUNEL+ cells (arrows) were distributed mainly in the inner retinal layers. Original magnifications, ×100 (A).
CBD Reduces Diabetes-Induced Oxidative and Nitrative Stress
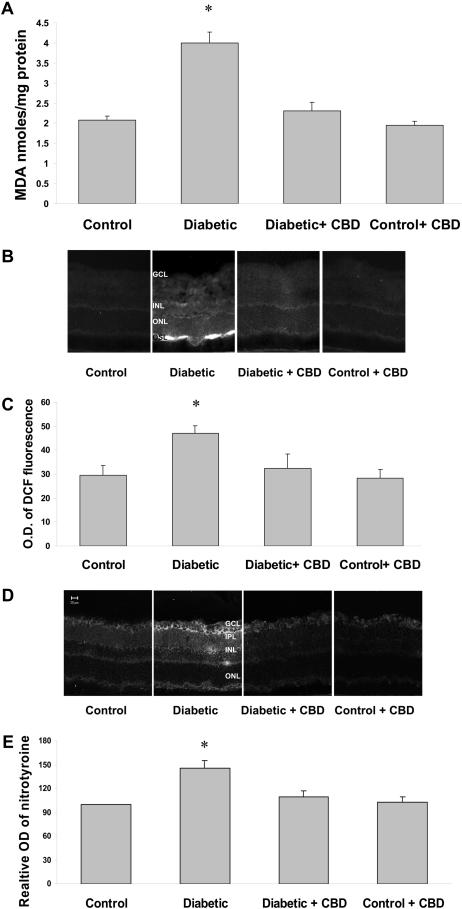
The neural retina has a high content of polyunsaturated fatty acids and hence, is extremely susceptible to oxidative insult by reactive oxygen species (ROS).32 We have previously shown that inhibition of oxidative stress prevents BRB breakdown in experimental diabetes and prevents retinal neural cell death in the NMDA-induced neurotoxicity rat model.8,28 To elucidate the mechanism of CBD’s neuroprotective activity and BRB-preserving actions in the diabetic retina, we determined the effects of CBD on reducing oxidative and nitrative stress in the rat retina after 2 weeks of experimental diabetes using several techniques. Levels of lipid peroxides were measured by the thiobarbituric acid test and expressed as nm concentration of MDA. MDA levels were increased approximately twofold in the diabetic retinas in comparison with the controls (Figure 3A). This effect was significantly reduced by CBD treatment (10 mg/kg every 2 days). MDA formation was not altered by CBD alone.
Figure 3-6715.
CBD reduces oxidative and nitrative stress in diabetic retinas. A: CBD reduces lipid peroxidation in the retinas of diabetic rats. Lipid peroxidation was determined by the amount of MDA formed in rat retina. Treatment of diabetic rats with CBD (10 mg/kg every 2 days) inhibited MDA formation while treatment of controls did not affect MDA formation. Data shown is the mean ± SEM of six or seven animals in each group. (*P < 0.05). B: CBD reduces peroxides in the retinas of diabetic rats as represented by DCF fluorescence in rat retina. A representative image shows the fluorescence distribution in different retinal layers; the ganglion cell layer (GCL), the inner nuclear layer (INL), and the outer nuclear layer (ONL), outer segment layer (OSL). C: Morphometric analysis of fluorescence intensity in serial sections of rat eyes shows that diabetic rats had a significant increase in fluorescence compared with controls. Treatment with CBD (10 mg/kg every 2 days) inhibited ROS formation in diabetic rats but not normal controls. Data shown is the mean ± SEM of six or seven animals in each group (*P < 0.05). D: CBD reduces nitrotyrosine in the retina of diabetic rats. A representative image shows the fluorescence distribution in different retinal layers; the ganglion cell layer (GCL), inner plexiform layer (IPL), the inner nuclear layer (INL), the outer nuclear layer (ONL), and the retinal pigment epithelium (RPE). E: Morphometric analysis of fluorescence intensity in serial sections of rat eyes shows that diabetic rats had a significant increase in fluorescence compared with controls. Treatment with CBD (10 mg/kg every 2 days) inhibited nitrotyrosine formation in the diabetic rats but not in the normal controls. Data shown is the mean ± SEM of six or seven animals in each group (*P < 0.05). Original magnifications, ×200.
Fluorescence measurement of 2′,7′-dichlorofluorescein (DCF) has been used extensively as a marker for both oxidative and nitrative stress and is suggested to reflect the overall status of the redox state of the cell.29 As shown in Figure 3B, images from diabetic retinas showed increased DCF fluorescence within the inner and outer plexiform, the inner and outer nuclear layers and a prominent accumulation of the fluorescence in the outer segment layer. Quantitative analysis showed a significant increase (P < 0.05) in the fluorescence intensity in the diabetic retinas compared to normal controls (Figure 3C). Treatment with CBD blocked diabetes-induced oxidative stress as indicated by significant inhibition of fluorescence accumulation in the diabetic rats. The normal control rat retinas were not affected by CBD treatment. We further confirmed the antioxidant effects of CBD by measuring tyrosine nitration. As shown in Figure 3D, diabetes-induced neurotoxicity involved significant tyrosine nitration within retinal layers with strongest immunoreactivity within the ganglion cell layer. Quantitative analysis showed that levels of tyrosine nitration increased ∼1.6-fold in the diabetic retinas compared with the controls (Figure 3E). This tyrosine nitration was almost completely eliminated by CBD (10 mg/kg every 2 days). Tyrosine nitration formation in untreated rats was not altered by CBD treatment.
CBD Reduces VEGF Expression in Experimental Diabetes
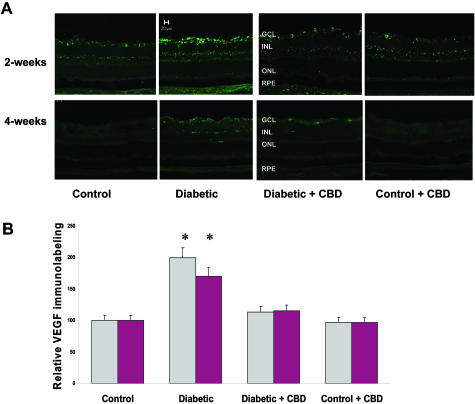
VEGF is a known mediator of the pathogenesis of both background and proliferative diabetic retinopathy. Increased VEGF levels in diabetic rats and humans have been correlated with the breakdown of BRB. The distribution and relative amount of VEGF after 2 or 4 weeks of experimental diabetes was analyzed using immunolocalization techniques. As shown in Figure 4A, diabetic retinas showed high levels of VEGF expression, which is localized in the ganglion cell layer, nerve fiber, and inner retinal layers. Densitometry and statistical analysis showed 2-fold and 1.7-fold) increases in diabetic retinas after 2 and 4 weeks of diabetes, respectively, compared to untreated controls (Figure 4B). Treatment with CBD (10 mg/kg every 2 days) significantly blocked the increases in VEGF expression in diabetic retinas after 2 or 4 weeks of experimental diabetes. VEGF expression in normal controls was not altered by CBD treatment.
Figure 4-6715.
CBD reduces VEGF expression in experimental diabetes. The distribution and quantification of VEGF in retinal sections were analyzed by immunolabeling using VEGF antibody followed by Oregon Green-conjugated secondary antibody. A: Representative images showing the fluorescence distribution in different retinal layers; the ganglion cell layer (GCL), the inner nuclear layer (INL), the outer nuclear layer (ONL), and the retinal pigment epithelium (RPE) after 2 or 4 weeks of induced diabetes. B: Morphometric analysis shows that diabetic retinas have significant increases of VEGF expression (twofold, 1.7-fold) after 2 and 4 weeks of induced diabetes, respectively, compared to untreated controls. Treatment with CBD (10 mg/kg every 2 days) significantly blocked the increase in VEGF expression. CBD treatment did not alter amount or distribution of VEGF in the treated controls. Data shown is the mean ± SEM of six animals in each group (*P < 0.05). Original magnifications, ×200.
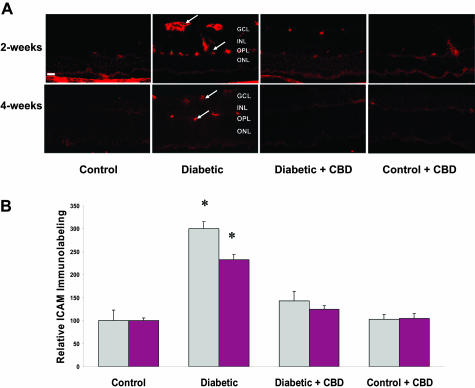
CBD Reduces ICAM-1 Expression in Experimental Diabetes
Increases in expression of ICAM-1 have been correlated with increased retinal vascular permeability in diabetes. Up-regulated ICAM-1 expression has been correlated also with retinal neural death in retinal ischemia.12 To elucidate whether the protective effects of CBD on both vascular and neural retina under diabetic conditions involve alteration of ICAM-1, we determined ICAM-1 protein expression in the frozen eye sections after 2 or 4 weeks of experimental diabetes. As shown in Figure 5A, diabetic retinas showed high levels of ICAM-1 expression, which was localized in the vascular layers of retina including the nerve fiber and outer-plexiform layers. Densitometry and statistical analysis showed 3-fold and 2.5-fold increases in ICAM expression in diabetic retinas compared to normal controls after 2 or 4 weeks, respectively (Figure 5B). Treatment with CBD (10 mg/kg every 2 days) significantly blocked the increase in ICAM-1 expression in diabetic retinas at 2 or 4 weeks. ICAM expression in normal controls was not altered by CBD treatment.
Figure 5-6715.
CBD reduces ICAM-1 expression in experimental diabetes. The distribution and quantification of ICAM-1 in retinal sections were analyzed by immunofluorescence using anti-ICAM-1 antibody followed by Texas Red-conjugated secondary antibody. A: Representative images showing the fluorescence distribution of ICAM-1 in the vascular layers of the retina after 2 or 4 weeks of induced diabetes (arrows point to the blood vessels in the nerve layer and outer plexiform layer (OPL). Other retinal layers include the ganglion cell layer (GCL), the inner nuclear layer (INL), and the outer nuclear layer (ONL). B: Morphometric analysis shows that diabetes is associated with high levels of ICAM-1 expression (threefold, 2.5-fold increases in 2 and 4 weeks of induced diabetes, respectively). Treatment with CBD (10 mg/kg every 2 days) significantly blocked the increase in ICAM-1 expression in the diabetic retinas. CBD treatment did not alter amount or distribution of ICAM-1 in the treated controls. Data shown is the mean ± SEM of six animals in each group (*P < 0.05). Original magnifications, ×200.
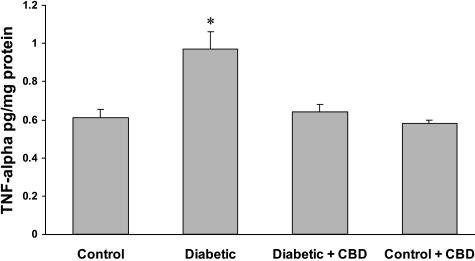
CBD Reduces TNF-α Levels in Experimental Diabetes
TNF-α is a proinflammatory cytokine that has been implicated in the pathogenesis of diabetic retinopathy. To investigate anti-inflammatory effect of CBD, we measured retinal TNF-α levels with a sandwich ELISA after 1, 2, or 4 weeks of experimental diabetes. Compared with age-matched nondiabetic animals, the retinas of diabetic animals demonstrated a significant increase in normalized TNF-α levels only after 1 week (Figure 6). Treatment with CBD (10 mg/kg every 2 days) reduced the retinal TNF-α levels in diabetic animals to levels comparable with those found in nondiabetic animals. After 2 or 4 weeks of induced diabetes, diabetic retinas showed a modest, but not significant, increase in TNF-α expression, suggesting that diabetes-induced increases in TNF-α were early and transient.
Figure 6-6715.
CBD reduces TNF-α levels in experimental diabetes. After 1 week of experimental diabetes with or without CBD treatment, retinal TNF-α levels were measured with a sandwich ELISA. Diabetic animals demonstrated a significant increase in normalized TNF-α levels compared with the control. Treatment with CBD (10 mg/kg every 2 days) reduced the retinal TNF-α levels in diabetic animals but not the normal controls. Data shown is the mean ± SEM of seven animals in each group (*P < 0.05).
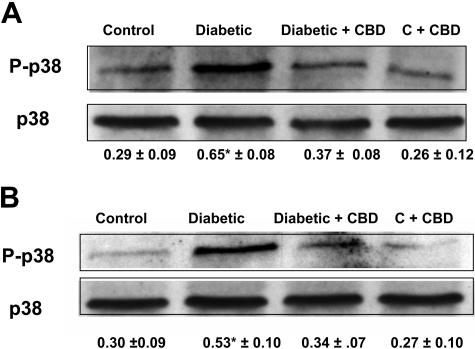
CBD Blocks Activation of p38 MAP Kinase in the Retinas of Experimental Diabetes
The above data suggest that hyperglycemia-induced oxidative stress and inflammation induce breakdown of BRB and accelerate retinal neuron cell death. P38 MAP kinase, a downstream target of proinflammatory cytokines and oxidative stress, has been shown to be a key regulator of vascular permeability and cell death. We therefore examined the role of p38 MAP kinase in diabetic retinas and whether CBD treatment blocks this pathway. Diabetic rats or age-matched nondiabetic control rats were treated with CBD (10 mg/kg every 2 days) for 2 or 4 weeks. Western blot analysis of the phosphorylation of p38 MAP kinase in the diabetic retinas showed significant increases (2-fold and 1.6-fold) in phosphorylation of p38 MAP kinase after 2 and 4 weeks, respectively, of induced diabetes (Figure 7, A and B). Treatment with CBD significantly blocked the increases in phosphorylation of p38 MAP kinase at 2 or 4 weeks without altering the level of expression or phosphorylation in treated control.
Figure 7-6715.
CBD inhibits the activation of p38 MAP kinase in the retinas of experimental diabetes. Western analysis of the phosphorylation of p38 MAP kinase showed that experimental diabetes significantly increased phosphorylation of p38 MAP kinase (P-p38) (approximately twofold) in the 2-week diabetic retinas (A) and (∼1.6-fold) in the 4-week diabetic retinas (B). Treatment with CBD significantly blocked the increase in phosphorylation of p38 MAP kinase at both time points. Data shown is the mean ± SEM of six animals in each group (*P < 0.05). Treatment with CBD did not alter levels of p38 MAP kinase phosphorylation in the treated controls.
Discussion
There are several major findings of this study. 1) During early stages of diabetes in rats, the induction of inner retinal neuronal cell death and breakdown of the BRB correlated with increases in the formation of ROS and proinflammatory cytokines and with activation of p38 MAP kinase. 2) Treatment with CBD reduced ROS formation; blocked the increased expression of TNF-α, VEGF, and ICAM-1; and prevented the activation of p38 MAP kinase. 3) Treatment with CBD prevented two functional components of diabetic retinopathy—vascular permeability and neural cell death. To the best of our knowledge, our study is the first to demonstrate the neuroprotective and BRB-preserving effects of CBD in early experimental diabetes via antioxidant and anti-inflammatory actions of CBD.
The breakdown of the BRB is a clinical hallmark of early diabetic retinopathy that continues with the progression of the disease.30 Our data confirmed our previous finding of a breakdown of the BRB at 2 weeks after the onset of diabetes. Our results also showed a significant increase in neuronal cell death, particularly in the ganglion cell layer in diabetic retinas compared to controls. In good agreement with our results, several groups have reported increased neuronal cell death5–7,33 and BRB breakdown in diabetic retinas.3,4,8,31 The BRB breakdown and retinal neural cell death were associated with oxidative and nitrative stress as indicated by significant increases in lipid peroxidation, tyrosine nitration, and DCF fluorescence. Treatment of diabetic animals with CBD maintained the integrity of the BRB, significantly reduced the number of apoptotic cells, and blocked the increases in oxidative and nitrative stress. In line with these results, the role of oxidative stress and tyrosine nitration in causing the BRB breakdown has been demonstrated in previous studies.8,34 Neurons are highly susceptible to oxidative stress that can induce both neural necrosis and apoptosis; therefore, it is likely that diabetes-induced oxidative stress leads to neuronal injury.35 Several reports have described the neuroprotective effects of CBD via blocking lipid peroxidation or nitrotyrosine formation in glutamate-induced cell death in vitro in neuron cultures and in vivo in NMDA-induced neurotoxicity.22,28,36 These findings suggest that diabetes-induced oxidative stress is likely to drive both neural cell death and BRB breakdown and that the naturally occurring nonpsychotropic cannabinoid CBD is a potentially useful therapeutic agent for the treatment of diabetic retinopathy.
Diabetes-induced oxidative stress has been well documented in patients and animals.8,32,37–39 One of the major sources of increased ROS in the diabetic retina is nitric oxide synthase (NOS). The significance of nitric oxide contribution to neuronal and vascular injury is indicated by the actions of NOS inhibitors in blocking neural damage in the ischemic-stroke model40 and the NMDA neurotoxicity model,28 and in preventing BRB breakdown in diabetes.4,8 Diabetes-induced glutamate accumulation may also increase the release of ROS leading to neuron cell death.41,42 Whether diabetes affects vascular or neural retina cells, both the microglial and macroglial cells are activated, and the function of macroglia in metabolizing glutamate is impaired in the diabetic retina.41,43 Formation of advanced glycation end products (AGE) has been shown to be a source of oxidative stress in retinal endothelial cells via the interaction with AGE receptor.44 In addition, activation of the polyol pathway under the diabetic condition leads to depletion of NADPH, which compromises antioxidant defense mechanisms of both neuronal and endothelial cells.6,35,45
Under diabetic conditions, BRB breakdown is thought to occur because of diabetes-induced oxidative stress that results in increased activity of proinflammatory cytokines including VEGF.2–4,8,9 VEGF plays a central role in the vascular hyperpermeability in the retina and participates in the pathogenesis of both background and proliferative diabetic retinopathy.2,3,31 Here we demonstrate that in the diabetic retina there are significant increases in the levels of ICAM-1 and VEGF after 2 weeks of experimental diabetes while at the same time there is increased permeability. Our results showed a significant increase in VEGF expression in diabetic retinas after 2 weeks that was sustained for 4 weeks. We also show significant increases in ICAM-1 expression after 2 weeks of diabetes that were maintained elevated after 4 weeks. These results confirm the inflammatory nature of diabetes and support the causal role of these mediators in triggering BRB breakdown. Treatment of diabetic animals with CBD blocked the increases in VEGF and ICAM-1 expression and preserved BRB. In support of these findings, a recent study showed that a nonpsychoactive cannabinoid (JWH-133) blocked the increases in VEGF expression and prevented vascular permeability in a mouse model of tumor angiogenesis.46 Previous work has established the role of ICAM-1 in the pathogenesis of early diabetes-induced leukostasis and BRB breakdown.10,11,47 Leukostasis has also been reported to play a causal role in vascular occlusion and tissue ischemia, which in turn leads to changes in neuronal function, edema, and neuronal cell loss.12 In agreement, our data show that CBD’s action in blocking diabetes-induced VEGF and ICAM-1 expression in the retina and normalizing the BRB is also associated with a positive neuroprotective effect.
We next screened the anti-inflammatory action of CBD on retinal TNF-α levels after 2 or 4 weeks of induced diabetes. Diabetic retinas showed modest but not significant increases in TNF-α levels compared to normal controls. After 1 week of experimental diabetes, retinal TNF-α levels were increased significantly in diabetic retinas compared to normal controls. This effect was prevented by CBD treatment. Our results suggest that the increases in TNF-α were transient and did not coincide with the course of vascular dysfunction or neural cell death.
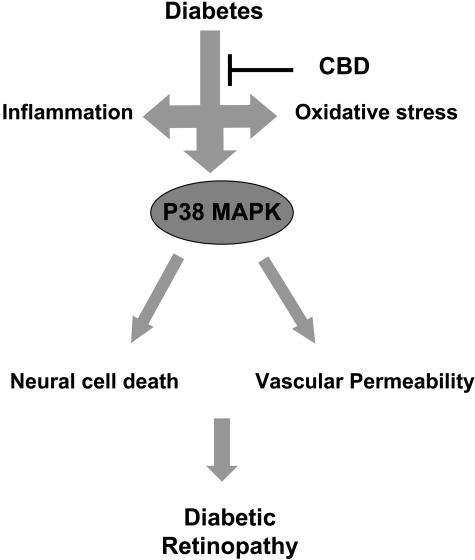
The p38 MAP kinase, a stress-activated protein kinase, is a downstream target of proinflammatory cytokines and oxidative stress. Our data showed significant increases in phosphorylation of p38 MAP kinase in diabetic retinal homogenate after 2 weeks of experimental diabetes, a time point that coincides with increases in BRB breakdown. The increase in phosphorylation of p38 MAP kinase in diabetic retina was sustained after 4 weeks of experimental diabetes, a time point that coincides with accelerated neural cell death. These effects were blocked by treatment of diabetic animals with CBD for 2 or 4 weeks. Consistent with our data, activation of p38 MAP kinase has been reported in neurons cultured in high glucose,16 in endothelial cells maintained in high glucose,13,14 and in diabetic retinas15 and has been implicated in apoptotic death of retinal ganglion cells17 and in NMDA-induced neural retinal death.18 Activation of p38 MAP kinase has also been implicated in vascular hyperpermeability in diabetic retinas,19 in TNF-α-induced permeability,48,49 and in VEGF-induced permeability.20,21 The mechanisms by which p38 MAP kinase mediates vascular permeability have not been elucidated. However, p38 MAP kinase activation has been described to induce transcription-independent effects such as induction of actin reorganization and cellular motility.50 Our data suggest that diabetes-induced oxidative stress and proinflammatory signaling pathways converge at the level of a secondary messenger, p38 MAP kinase, to mediate two different aspects of diabetic retinopathy—neuronal cell death and BRB breakdown (Figure 8).
Figure 8-6715.
Schematic figure that summarizes the findings of the current study showing that experimental diabetes causes increases in both oxidative stress and inflammatory mediators that lead to activation of P38 MAP kinase, increases in vascular permeability, and neural cell death.
Cannabinoids produce their biological effects by acting through at least three receptors. The cannabinoid receptor CB1 is responsible for psychoactivity and is expressed predominately in the brain and retinal neurons.51,52 Receptor CB2 is expressed predominately in immune cells, cerebral microglial cells, and in the retina.53,54 The third receptor, abnormal-cannabidiol (abn-CBD)-sensitive receptor, is not cloned but is pharmacologically defined in the cerebral microglial cells and endothelial cells in mice lacking receptors CB1 and CB2.53,55 CBD has very low affinity to either CB1 or CB2 but is a partial agonist of abn-CBD-sensitive receptor.55 The anti-inflammatory effects of CBD may be attributable to its activity on the abn-CBD-sensitive receptor.25,26,53 The neuroprotective effect of CBD may depend on its antioxidant ability directly to scavenge ROS22,23 or indirectly to inhibit reuptake/degradation of endogenous cannabinoids, including arachidonoyl-ethanolamide and 2-arachidonoyl glycerol. These endogenous cannabinoids act presynaptically to activate CB1, which inhibits N-type calcium channels and so further reduces calcium influx and glutamate release.56 CBD has been shown to inhibit the reuptake and degradation of arachidonoyl-ethanolamide in vitro and is a candidate for an anti-ischemia drug.56 Because the release of arachidonoyl-ethanolamide or 2-arachidonoyl glycerol occurs only in damaged regions and does not produce psychotropic effects, compounds that inhibit their transport/degradation may be of therapeutic utility without producing psychotropic effects. Current efforts are in progress to study the role of CB1 receptor by following the effects of CBD in CB1 receptor knockout mice. The nonpsychotropic CBD is a promising candidate for anti-inflammatory and neuroprotective therapeutics.
Acknowledgments
We thank Ms. Sonya Liu for outstanding technical help with confocal microscopy and Ms. Penny Roon for tissue sectioning.
Footnotes
Address reprint requests to Gregory I. Liou, Ph.D., Department of Ophthalmology, Medical College of Georgia, 1120 15th St., Augusta, GA 30912. E-mail: giliou@mcg.edu.
Supported by the Knights Templar Educational Foundation of Georgia, the Fight for Sight (to G.I.L.), the American Heart Association (Scientist Development Grant to A.B.E.), and the National Institutes of Health (grants EY04618 and EY11766 to R.B.C.).
References
- Neely KA, Gardner TW. Ocular neovascularization: clarifying complex interactions. Am J Pathol. 1998;153:665–670. doi: 10.1016/S0002-9440(10)65607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- Takeda M, Mori F, Yoshida A, Takamiya A, Nakagomi S, Sato E, Kiyama H. Constitutive nitric oxide synthase is associated with retinal vascular permeability in early diabetic rats. Diabetologia. 2001;44:1043–1050. doi: 10.1007/s001250100588. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D’Amore PA, Shima DT, Adamis AP. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Nishijima K, Kiryu J, Honda Y, Yue BY, Sawamura T. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol. 2002;120:1707–1713. doi: 10.1001/archopht.120.12.1707. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Yamamoto K, Yoshimura SI, Taniyama Y, Aoki M, Matsubara H, Kim S, Kaneda Y, Ogihara T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes. 2001;50:1472–1481. doi: 10.2337/diabetes.50.6.1472. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–252. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Tenneti L, Lipton SA. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J Neurosci. 2000;20:5037–5044. doi: 10.1523/JNEUROSCI.20-13-05037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe S, Lipton SA. Divergent NMDA signals leading to proapoptotic and antiapoptotic pathways in the rat retina. Invest Ophthalmol Vis Sci. 2003;44:385–392. doi: 10.1167/iovs.02-0187. [DOI] [PubMed] [Google Scholar]
- Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M, Sunderkotter C, Sveinbjornsson B, Hippenstiel S, Willuweit A, Marino M, Haas E, Seljelid R, Scheurich P, Suttorp N, Grell M, Risau W. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood. 2001;97:1321–1329. doi: 10.1182/blood.v97.5.1321. [DOI] [PubMed] [Google Scholar]
- Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M. p38 MAP kinase—a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Pegorini S, Arcidiacono MV, Consalez GG, Croci L, Sala M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci Lett. 2003;346:61–64. doi: 10.1016/s0304-3940(03)00569-x. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI. Neuroprotective effect of (−)delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Engler C, Krogsaa B, Lund-Andersen H. Blood-retina barrier permeability and its relation to the progression of diabetic retinopathy in type 1 diabetics. An 8-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 1991;229:442–446. doi: 10.1007/BF00166307. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Armstrong D, al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med. 1991;11:433–436. doi: 10.1016/0891-5849(91)90161-u. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG. Nitric oxide donor-induced increase in permeability of the blood-brain barrier. Brain Res. 2000;866:101–108. doi: 10.1016/s0006-8993(00)02254-x. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Mercuri F, Quagliaro L, Assaloni R, Motz E, Tonutti L, Taboga C. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- Fathallah L, Obrosova IG. Increased retinal lipid peroxidation in early diabetes is not associated with ascorbate depletion or changes in ascorbate redox state. Exp Eye Res. 2001;72:719–723. doi: 10.1006/exer.2001.0994. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38:385–390. doi: 10.1016/s0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75:95–108. doi: 10.1016/s0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Casanova ML, Planas A, Del Pulgar TG, Villanueva C, Fernandez-Acenero MJ, Aragones J, Huffman JW, Jorcano JL, Guzman M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–531. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwariaku FE, Chang J, Zhu X, Liu Z, Duffy SL, Halaihel NH, Terada L, Turnage RH. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock. 2002;18:82–85. doi: 10.1097/00024382-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol. 2003;95:1889–1895. doi: 10.1152/japplphysiol.00225.2003. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, McIntosh HH, Deutsch DG. Immunocytochemical localization of cannabinoid CB1 receptor and fatty acid amide hydrolase in rat retina. J Comp Neurol. 1999;415:80–90. doi: 10.1002/(sici)1096-9861(19991206)415:1<80::aid-cne6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Straiker A, Maguire G. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis Neurosci. 2000;17:91–95. doi: 10.1017/s0952523800171093. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]