Abstract
Polyamines are essential for normal cellular growth and function. Activation of polyamine catabolism in transgenic rats overexpressing spermidine/spermine N1-acetyltransferase, the key enzyme in polyamine catabolism, results in severe acute pancreatitis. Here, we investigated the role of polyamine catabolism in pancreatitis and studied the effect of polyamine analogues on the outcome of the disease. Polyamine depletion was associated with arginine- and cerulein-induced pancreatitis as well as with human acute necrotizing and chronic secondary pancreatitis. Substitution of depleted polyamine pools with methylspermidine partially prevented arginine-induced necrotizing pancreatitis whereas cerulein-induced edematous pancreatitis remained unaffected. Transgenic rats receiving methylated polyamine analogues after the induction of pancreatitis showed less pancreatic damage than the untreated rats. Most importantly, polyamine analogues dramatically rescued the animals from pancreatitis-associated mortality. Induction of spermidine/spermine N1-acetyltransferase in acinar cells isolated from transgenic rats resulted in increased trypsinogen activation. Pretreatment of acini with bismethylspermine prevented trypsinogen activation, indicating that premature proteolytic activation is one of the effects triggered by polyamine depletion. Our data suggest that activation of polyamine catabolism is a general pathway in the pathogenesis of acute pancreatitis and that experimental disease can be ameliorated with stable polyamine analogues.
Multiple mediators play a role in the development of acute pancreatitis. Initial phases in the pathophysiology of severe acute pancreatitis include activation and release of pancreatic enzymes from the acinar cells, as well as activation of proinflammatory cytokines.1 In severe disease, autodigestion of the pancreas, and eventually multiple organ failure due to systemic factors, leads to the complications associated with high mortality. Intracellular activation of pancreatic trypsinogen is considered to be the critical early event in the onset of pancreatitis.
Genetically modified mice, mainly gene-disrupted mice, have been used to study the mediators of acute pancreatitis.2 The studies have proven the existence of numerous mediators in the immune system but failed to demonstrate that inactivation of any of these alone would prevent the local pancreatic or remote organ damage. It thus seems that an efficient therapy to treat acute pancreatitis would consist of a combination of drugs affecting different mediator pathways or metabolic routes.
The polyamines spermidine and spermine and their diamine precursor putrescine are necessary for normal cellular growth and differentiation, but their exact physiological functions remain to be proven.3 The first indication of the association of polyamines in pancreatic integrity was obtained recently with transgenic rats that overexpressed heavy metal-inducible metallothionein promoter (MT)-driven gene encoding spermidine/spermine N1-acetyltransferase (SSAT), the key enzyme in polyamine catabolism. We showed that activation of polyamine catabolism in these rats caused extensive depletion of spermidine and spermine and resulted in the development of severe acute pancreatitis within 24 hours.4 Using this rat model, we were also able to show that a polyamine analogue, α-methylspermidine (MeSpd), which is supposed to fulfill the putative cellular functions of spermidine but is resistant to SSAT-dependent catabolism, prevented acute pancreatitis when administered before the induction of the transgene.5 Polyamine catabolism-induced retardation of liver regeneration could likewise be restored by a prior administration of the polyamine analogue.5,6 We recently showed that another polyamine analogue, namely bis-α-methylspermidine (Me2Spm), was effective in supporting growth both in vivo and in vitro.6 The aim of the present study was to investigate whether the changes in polyamine pools occur in other rat models of experimental pancreatitis and in human pancreatitis, to investigate the possible effects of pharmacological intervention with α-methylated polyamine analogues on experimental pancreatitis including our transgenic rat model, and to elucidate the mechanism of pancreatitis caused by activation of polyamine catabolism using acinar cell culture.
Materials and Methods
Chemicals
MeSpd7 and Me2Spm8 were synthesized as described earlier. The polyamine analogues were dissolved in saline, neutralized, and administered intraperitoneally in a volume of 0.1 ml/kg body weight. Zinc sulfate heptahydrate was purchased form Merck (Darmstadt, Germany) and administered intraperitoneally in distilled water. l-Arginine was a product of Sigma (St. Louis, MO) and was dissolved in saline immediately before use. Cerulein (Takus) was obtained from Pharmacia & Upjohn, Erlangen, Germany.
Patients
Human pancreatic specimens were obtained at the Tampere University Hospital from patients undergoing pancreaticoduodenal resection for a mass in the head of the pancreas (14 patients) or necrosectomy for acute necrotizing pancreatitis (two patients). In the patients with surgical resection, a 2 × 2 mm2 specimen of pancreas was cut from the resection line. In the patients with necrosectomy, a 2 × 2 mm2 specimen was cut immediately from the best preserved area of the removed part of pancreas. The sample was immediately immersed in liquid nitrogen and stored in −70°C before assays.
The human specimens were grouped as follows: group 1 (n = 4): normal pancreas, from patients with bile duct or duodenal adenocarcinoma; group 2 (n = 2): acute pancreatitis, from patients with acute necrotizing pancreatitis and extensive necrosis (alcoholic etiology); group 3 (n = 4): chronic pancreatitis (alcoholic etiology); group 4 (n = 6): chronic secondary pancreatitis, from patients with pancreatic carcinoma or ampullary adenocarcinoma. The study was approved by the Institutional Review Board of Tampere University Hospital, Finland.
Animals
The production of transgenic Wistar rats harboring metallothionein (MT) I promoter-driven SSAT gene construct has been described.4 Severe necrotizing pancreatitis was induced by activating the transgene expression via the MT I promoter with the administration of a nontoxic dose (10 mg zinc/kg i.p.) of zinc in a volume of 0.2 ml/kg body weight.4 The therapeutic effects of MeSpd and Me2Spm were studied by injecting the analogues at 4 and 8 hours after induction of pancreatitis, ie, at times when first signs of pancreatitis were evident. Nontransgenic Wistar rats were used in the studies of l-arginine- and cerulein-induced pancreatitis. l-Arginine was administered intraperitoneally as a single dose of 2.5 g/kg, which produced necrotizing pancreatitis within 24 hours as described by Tani and colleagues.9 Cerulein was given as seven hourly intraperitoneal injections of 50 μg/kg.10 The preventive effect of MeSpd was studied by injecting the analogue at 20 and 4 hours before induction of pancreatitis to allow accumulation of the MeSpd. Both male and female rats were used in the experiments. Blood for hematocrit measurement was collected from femoral vein into heparinized glass microcapillaries (Miles Inc., Elkhart, IN). At the end of the experiments, the rats were sacrificed by decapitation and the blood was drained onto heparinized dishes. Alternatively, the rats were anesthetized with CO2, thoracotomized, and blood was collected by puncturing the heart before decapitation. The pancreas was removed, the tail of the organ was fixed for histology, and the rest frozen in liquid nitrogen and stored at −70°C until analyzed. Where indicated, other tissues were taken after the removal of the pancreas and handled similarly. The Institutional Animal Care and Use Committee of the University of Kuopio and the Provincial Government approved the animal experiments.
Isolation and Analyses of Pancreatic Acini
Dispersed acini (intact secretory units of 20 to 50 acinar cells) were freshly prepared from the pancreas of male syngeneic and transgenic rats by collagenase (Serva, Heidelberg, Germany) digestion.11 Acinar biovolume was determined by a CASY system (Schärfe, Reutlingen, Germany) and adjusted to a concentration of 2 mm3/ml. Acini were incubated in a culture medium containing 24.5 mmol/L HEPES, 96 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L MgCl2, 2.5 mmol/L Na2HPO4, 0.5 mmol/L CaCl2, 11.5 mmol/L glucose, 5 mmol/L sodium pyruvate, 5 mmol/L sodium glutamate, and 5 mmol/L sodium fumarate, with minimum essential medium (1% v/v) and bovine serum albumin, fraction V (1% w/v), at pH 7.4.12 At a temperature of 37°C, acini were gently mixed (50 rpm) and periodically gassed with oxygen.
In the first experiment, we examined the effect of Zn2+ on intracellular trypsinogen activation. Pancreatic acini of untreated syngeneic and transgenic rats were isolated as described above. Freshly prepared acini were incubated for 2 hours by addition of ZnSO4 to the culture medium at different concentrations (0 to 250 μmol/L). At the end of incubation acini were washed and intracellular trypsinogen activation was analyzed.
In the second experiment, we examined the influence of the polyamine analogue Me2Spm on zinc-induced trypsinogen activation. Therefore, syngeneic and transgenic rats were treated by administration of Me2Spm (25 mg/kg i.p.) as two doses 24 and 4 hours before sacrifice. Moreover, Me2Spm (1 mmol/L) was added to all buffers of acinar isolation and incubation in vitro. As described in the first experiment, isolated acini were incubated with ZnSO4 (50 μmol/L or 100 μmol/L) for 2 hours after analysis of trypsinogen activation. In parallel to all in vitro experiments, separate aliquots of acini were incubated without ZnSO4 but stimulated with the cholecystokinin analogue cerulein (10 nmol/L, 60 minutes). Under these conditions intracellular trypsinogen activation reached a maximum.
To measure intracellular trypsinogen activation, acini were washed, resuspended in fresh medium (without zinc or cerulein), transferred to 96-well microtiter plates, and the cell-permeable and trypsin-specific substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 (Molecular Probes, Eugene, OR) was added (10 μmol/L). Intracellular tryptic substrate cleavage and the released fluorochrome rhodamine-110 were quantified by cytofluorometry continuously for 60 minutes (excitation, 485 nm; emission, 530 nm; CytoFluor 2350; Millipore, Bedford, MA).13 Trypsinogen activation corresponds to the linear increase of rhodamine fluorescence (ΔF) per time interval (Δt) and was expressed as slope of linear regression (ΔF/Δt).14 Zinc-induced trypsinogen activation was calculated as a percentage of the maximum activatable trypsinogen detected by the corresponding cerulein experiments.
Analytical Methods
SSAT activity was assayed according to Bernacki and colleagues.15 Polyamines, their acetylated derivatives and the methylated polyamine analogues were determined by high-performance liquid chromatography as described by Hyvönen and colleagues.16 Plasma α-amylase and alanine aminotransferase (ALAT) activities were determined using the analyzer system Microlab 200 from Merck (Darmstadt, Germany). Hematocrit values were measured in blood samples taken from femoral vein using an Ames microspin centrifuge (Bayer Diagnostic GmbH, Speer, Germany) according to the manufacturer’s instructions.
Histological Analyses
Formalin-fixed tissue specimens were embedded in paraffin, cut into 5-μm-thick slices and stained with hematoxylin and eosin. The sections were evaluated according to Niederau and colleagues.17 For necrosis and vacuolization, the scores refer to an approximate percentage of cells involved. Definitions for histological evaluation in Table 1 in terms of necrosis and/or vacuolization are normal (0 to 5% of necrosis), mild (5 to 30% of necrosis), moderate (30 to 50% of necrosis), and severe (>50% of necrosis).
Table 1-6704.
Pancreatic SSAT Activities, Polyamine Pools, α-Amylase Activities, and Histology in Nontransgenic Rats after Treatment with l-Arginine or Cerulein with or without Pretreatment with MeSpd
| Group (number of animals) | SSAT (pmol/mg/10 minutes) | Putrescine (pmol/mg tissue weight) | Spermidine (pmol/mg tissue weight) | Spermine (pmol/mg tissue weight) | MeSpd (pmol/mg tissue weight) | α -Amylase (U/L) | Histology‡ |
|---|---|---|---|---|---|---|---|
| Control (9) | 3.6 ± 0.8 | 7 ± 9 | 4405 ± 1708 | 862 ± 364 | 1913 ± 462 | Normal | |
| l-arginine (8) | 10.5 ± 6.4* | 293 ± 340 | 2138 ± 1297† | 406 ± 170† | 20,785 ± 8681† | Moderate to severe | |
| l-arginine + | 13.7 ± 7.0† | 492 ± 353* | 3276 ± 1420† | 584 ± 183* | 1669 ± 705 | 5221 ± 3838 | Normal to mild |
| MeSpd (11) | |||||||
| Control (5) | 4.4 ± 1.6 | 32 ± 3 | 4100 ± 320 | 750 ± 260 | 2650 ± 210 | Normal | |
| Cerulein (6) | 31.9 ± 4.7† | 185 ± 47† | 1750 ± 230† | 340 ± 63† | 69,850 ± 8040† | Mild to moderate | |
| Cerulein + | 34.9 ± 5.2† | 197 ± 56† | 1770 ± 224† | 320 ± 25† | ND | 80,310 ± 15,230† | Mild to moderate |
| MeSpd (5) |
The rats received l-arginine (2.5g/kg i.p.) 24 hours before sacrifice or cerulein (50 μg/kg i.p. in seven hourly injections) before being sacrificed 1 hour after the last cerulein injection. MeSpd (50 mg/kg i.p.) was administered as two doses 20 hours and 4 hours before l-arginine or cerulein. The data are expressed as means ± SD. ND, not detectable. Numbers of animals per group are given in parentheses after each group definition.
P < 0.05 and
P < 0.01 as compared with untreated control animals.
For definition of evaluation, see Materials and Methods.
Statistical Analyses
The data are expressed as means ± SD. One-way analysis of variance with Dunnett’s post hoc test for multiple comparisons was used for statistical analyses with the aid of the software package GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). Survival data were analyzed using Fisher’s exact test and the Kaplan-Meier method with log-rank test.
Results
Polyamine Catabolism in l-Arginine-Induced Pancreatitis
To study whether activation of polyamine catabolism could be observed in other pancreatitis models besides our transgenic model, which is specifically directed to induction of SSAT, we induced necrotizing pancreatitis in normal rats with an excessive dose of l-arginine. As seen in Table 1, the treatment caused threefold increase in SSAT activity, and consequently, ∼50% depletion of spermidine and spermine with a 40-fold accumulation of putrescine. These changes are distinct characteristics of activated polyamine catabolism. Pancreatitis was assessed on the basis of elevated plasma amylase activity and histological scoring representing mild to severe pancreatitis manifested as edema, vacuolization, presence of inflammatory cells, and appearance of extensive necrosis (in average 35 to 60% cells involved). Despite the replenishment of total polyamine pools, pretreatment of the rats with MeSpd before induction of pancreatitis did not prevent the activation of polyamine catabolism, as evidenced by increased SSAT activity and depleted pools of the natural polyamines, although to a lesser extent than with l-arginine alone (Table 1). However, plasma amylase activity was nearly normalized in rats treated with the polyamine analogue and the histological status of pancreas was clearly improved. The range of pancreatic necrosis in these animals was 0 to 30%. Essentially similar results were obtained with another polyamine analogue, Me2Spm at 25 mg/kg in an identical experimental setting (results not shown).
Polyamine Catabolism in Cerulein-Induced Pancreatitis
Mild edematous pancreatitis with variable level of edema and vacuolization but virtually without necrosis was caused by cerulein. Activation of polyamine catabolism was also evident in this model of pancreatitis (Table 1). In addition to depleted spermidine and spermine pools, accumulation of putrescine was seen. Traces of N1-acetylspermidine, which is normally undetectable in nontransgenic animals, were also detected in cerulein-treated animals (not shown). Plasma amylase activity was highly elevated in cerulein-treated rats. The histological hallmarks observed were edema, inflammation, and abundant intracellular vacuolization with minimal necrosis. Pretreatment of the rats with MeSpd did not change the polyamine pattern, the histological status or amylase activity from those seen in rats treated with cerulein alone (Table 1). MeSpd was not detectable at the time of sacrifice (Table 1).
Treatment of Polyamine Catabolism-Induced Pancreatitis in Transgenic Rats
Our earlier studies showed that pretreatment of transgenic rats with MeSpd before induction of pancreatitis totally prevented the disease. We now investigated the possibility to interfere with the pathogenesis by treating the rats with a polyamine analogue as late as several hours after induction of pancreatitis. The choice of the polyamine analogue used here was based on preliminary experiments in which spermine analogue bismethylspermine proved to be as efficient as MeSpd in supporting cellular function.6 Table 2 summarizes the results of 24- and 48-hour treatment of rats with zinc alone or followed by administration of Me2Spm given at the time when the induction of polyamine catabolism has clearly commenced (50% depletion of spermidine at 4 hours) and the first signs of developing pancreatitis (edema, inflammation, and occasional necrosis) are evident. Immense induction of SSAT was followed by near total depletion of spermidine and spermine by 24 hours. Indicative of greatly enhanced acetylation of spermidine by SSAT, there was an increase in the N1-acetylspermidine pool, which is normally undetectable (Table 2). Treatment with Me2Spm had little effect on the depleted concentrations of the natural polyamines but led to compensatory accumulation of Me2Spm and its oxidation product MeSpd. Analysis of the rats at 48 hours after zinc gave similar results (Table 2).
Table 2-6704.
Pancreatic SSAT Activities and Polyamine Concentrations in Transgenic Rats after Treatment with Zinc and Me2Spm
| Group | SSAT (pmol/mg/10 minutes) | Putrescine (pmol/mg tissue) | N1-acetyl-spermidine (pmol/mg tissue) | Spermidine (pmol/mg tissue) | Spermine (pmol/mg tissue) | MeSpd (pmol/mg tissue) | Me2Spm (pmol/mg tissue) |
|---|---|---|---|---|---|---|---|
| Control | 29 ± 7 | 3130 ± 1020 | 49 ± 6 | 4140 ± 977 | 437 ± 45 | ND | ND |
| Zn 24 hours | 8600 ± 7020 | 3478 ± 502* | 119 ± 55 | 150 ± 36* | 53 ± 14* | ND | ND |
| Zn 24 hours + Me2Spm | 8820 ± 6660 | 1850 ± 357 | 67 ± 6 | 92 ± 8* | 30 ± 7* | 129 ± 18 | 493 ± 45 |
| Zn 48 hours | 9560 ± 8910 | 1580 ± 256 | 108 ± 53 | 380 ± 76* | 81 ± 25* | ND | ND |
| Zn 48 hours + Me2Spm | 7010 ± 8410 | 2430 ± 850 | 70 ± 10 | 311 ± 84* | 52 ± 19* | 389 ± 112 | 665 ± 180 |
The rats received zinc (10 mg/kg i.p.) without or with Me2Spm (25 mg/kg i.p.) administered 4 and 8 hours after zinc. The animals were sacrificed 24 or 48 hours after zinc. The data are given as means ± SD. There were three to seven rats per group. ND, not detected.
P < 0.01 as compared with untreated transgenic control animals.
The histological evaluation of pancreatic tissue at 24 hours revealed edema, vacuolization, and necrosis involving ∼50% of cells in zinc-treated animals when compared to untreated animals (Figure 1, a and b). Necrosis appeared to be less extensive (10 to 50% of the cells involved) after Me2Spm (Figure 1c). In correlation with the therapeutic effect, there was less ascites fluid on necropsy in the intraperitoneal cavity of animals that responded to the analogue treatment. After a 48-hour treatment with zinc, the pancreas exhibited massive necrosis and inflammation (Figure 1d) that was alleviated by the supplementation of Me2Spm (Figure 1e). A representative pancreatic section of an animal treated similarly with zinc and the spermidine analogue, MeSpd, likewise showed only partial necrosis (Figure 1f). Whether or not treated with the polyamine analogues, there were no overt macroscopical or histological abnormalities in other tissues examined (liver, heart, kidney, and lung) apart from occasional foci of inflammatory cells seen in liver and lung (not shown). It should also be noted that tissue polyamine levels in heart, kidney, and lung remained virtually normal in zinc-treated animals (not shown), excluding the possibility that local polyamine depletion caused tissue failure in these organs.
Figure 1-6704.
Histology of transgenic rat pancreas after treatment with zinc and methylated polyamine analogues. Control pancreas (a), pancreas 24 hours after zinc (b), pancreas 24 hours after with zinc and Me2Spm given 4 and 8 hours after zinc (c), pancreas 48 hours after zinc (d), pancreas 48 hours after and Me2Spm given 4 and 8 hours after zinc (e), pancreas 48 hours after zinc and MeSpd given 4 and 8 hours after zinc (f), pancreas 2 weeks after zinc and Me2Spm (g), and pancreas 2 weeks after zinc and MeSpd (h). Scale bar, 100 μm. Original magnifications, ×200.
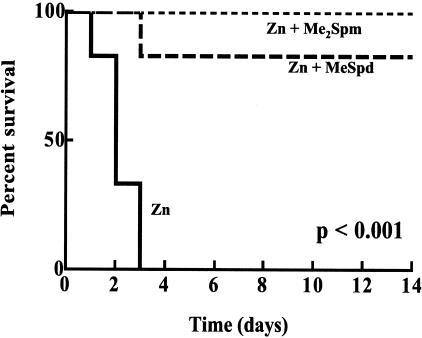
ALAT activities, as a measure of liver damage, and hematocrit values determined at 24 hours after zinc are shown in Table 3. Both values were significantly elevated in zinc-treated rats with pancreatitis. ALAT activity decreased to some extent whereas the hematocrit value was totally normalized by Me2Spm treatment (Table 3). Without any intervention the overall mortality of zinc-treated rats was 100% within 72 hours (Figure 2). Treatment of rats with either MeSpd or Me2Spm after zinc dramatically protected them from early death as well as from later mortality for up to 2 weeks of follow-up (Figure 2).
Table 3-6704.
Plasma ALAT Activities and Blood Hematocrit Values in Transgenic Rats after Treatment with Zinc and Me2Spm
| Group | P-ALAT (U/L) | Hematocrit (%) |
|---|---|---|
| Control | 69 ± 15 (6) | 52.5 ± 3.0 (11) |
| Zn 24 hours | 208 ± 82 (7)† | 64.6 ± 8.3† (8) |
| Zn 24 hours + Me2Spm | 169 ± 23 (4)* | 52.2 ± 3.9 (5) |
The rats received zinc (10 mg/kg i.p.) without or with Me2Spm (25 mg/kg) administered 4 and 8 hours after zinc. The animals were sacrificed 24 hours after zinc. The data are given as means ± SD. Numbers of animals per group are given in parentheses after the value.
P < 0.05 and
P < 0.01 as compared with untreated transgenic control animals.
Figure 2-6704.
Survival of rats treated with zinc and MeSpd or Me2Spm. The rats received zinc (10 mg/kg i.p.) and MeSpd (50 mg/kg i.p.) or Me2Spm (25 mg/kg i.p.) 4 and 8 hours after zinc. Solid line, rats treated with zinc alone; dashed line, rats treated with zinc and MeSpd; dotted line, rats treated with zinc and Me2Spm. There were six animals in each group.
Histological examination of long-term survivors showed that Me2Spm was able to prevent pancreatic necrosis and subsequent fibrosis almost totally (Figure 1g). The protective effect of MeSpd was essentially similar to that caused by Me2Spm (Figure 1h). Due to expression of MT in liver, hepatic polyamine catabolism is activated in MT-SSAT transgenic animals by zinc, which may contribute to any potential pancreatitis-related hepatic damage. However, the histological changes after zinc treatment were only mild and transient, and liver totally recovered after 2 weeks in rats receiving polyamine analogues. Kidney, heart, and lung were practically normal in the surviving animals, even in those exhibiting sectional fibrosis of pancreas.
Polyamine Analysis of Human Pancreatic Specimens
A small number of human pancreatic biopsies were analyzed for polyamine contents. Normal specimens (group 1) revealed polyamine levels comparable to those reported earlier18 for healthy human pancreas, ie, 4 ± 1, 140 ± 63, and 136 ± 50 nmol/mg DNA for putrescine, spermidine, and spermine, respectively. Concentrations of spermidine and spermine constituted only 2 and 24% of normal levels, respectively, in group 2 (acute pancreatitis) whereas they were normal in group 3 (chronic alcoholic pancreatitis). Interestingly, in group 4 (chronic secondary pancreatitis), an accumulation of putrescine (3.5-fold compared with normal) together with decreased levels of spermidine (39% of normal) and spermine (78% of normal) were found. Acetylated spermidine was not detectable in any of the samples.
Protease Activation in Isolated Acini
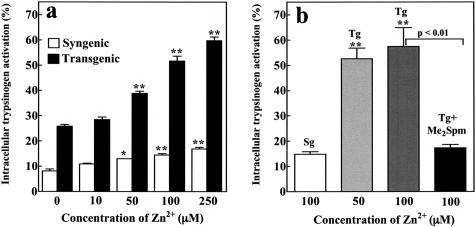
Intracellular activation of digestive zymogens is the initiating factor in the course of acute pancreatitis. To investigate the hypothesis that a zinc-induced activation of polyamine catabolism and decrease of spermidine and spermine pools leads to an intracellular activation of trypsinogen in isolated acinar cells, we used the cell-permeable and trypsin-specific substrate (CBZ-IlePro-Arg)2-rhodamine-110 as described previously.13 Trypsinogen activation by a supramaximal concentration (10 nmol/L) of cerulein revealed an identical activation pattern in acini from syngenic and transgenic animals with or without pretreatment with Me2Spm (not shown). Treatment of the acini with different concentrations of ZnSO4 for 120 minutes to induce transgene expression resulted in a dose-dependent and significantly higher trypsinogen activation in the acini from transgenic animals in comparison with syngenic acini (Figure 3a). Furthermore, pretreatment of the animals with Me2Spm completely blocked the intracellular trypsinogen activation, indicating that the depletion of intracellular polyamine pools is responsible for trypsinogen activation (Figure 3b).
Figure 3-6704.
Zinc-induced trypsinogen activation in isolated pancreatic acini. a: Zinc (ZnSO4) was added in vitro at different concentrations (0, 10, 50, 100, 250 μmol/L) for 2 hours. Zinc-induced trypsinogen activation is shown as a percentage of the maximal activated trypsin activity in response to cerulein (10 nmol/L). Values denote means +/− SEM of triplicates, representative of experiments with five animals. b: Influence of Me2Spm on zinc-induced (2 hours) trypsinogen activation in isolated pancreatic acini. Activation is shown as a percentage of the maximal activated trypsin activity in response to cerulein (10 nmol/L). Means +/− SEM of triplicates of three to nine animals in each group.
Discussion
The involvement of enhanced polyamine catabolism in the development of acute pancreatitis was first found in transgenic rats overexpressing SSAT, the key enzyme in polyamine catabolism. We now studied different established rat models of the disease to reveal the universality of our initial finding. l-Arginine causes severe acute necrotizing pancreatitis that is manifested in hyperamylasemia and histology very similar to the human disease19 and to our transgenic model of pancreatitis. Our present results show that polyamine catabolism is involved in the development of arginine-induced pancreatitis and possibly contributes to the pathogenesis caused by other arginine-mediated mechanisms, such as generation of oxygen-derived free radicals, proinflammatory cytokines, and nitric oxide.20,21 Results from experiments with the cerulein model of pancreatitis further support the view that activated polyamine catabolism is a general phenomenon in the pathogenesis of pancreatitis. The fact that MeSpd had no effect on the edematous pancreatitis caused by cerulein is explained by the lack of compensatory accumulation of MeSpd in pancreas (Table 1). Also, other mediators besides polyamine depletion may have a critical role in the mechanism of edematous pancreatitis caused by this secretagogue. This conclusion is supported by our finding that Me2Spm prevented zinc-induced but not cerulein-induced trypsinogen activation in isolated acini (Figure 3). In both pancreatitis models in which substantial necrosis develops (transgenic SSAT induction model and l-arginine model), polyamine supplementation reduced necrosis, suggesting that polyamine changes are associated with the development of necrosis. Spermine and spermidine levels appeared to be decreased in human necrotizing pancreatitis, although the small number of clinical samples available does not allow us to draw conclusions of the involvement of polyamine catabolism in human pancreatitis.
α-Methylated analogues of spermidine and spermine have been considered metabolically more stable than the natural polyamines. They are not substrates for SSAT and MeSpd is a poor substrate for spermine synthase.22 In contrast with the stability of MeSpd, Me2Spm is, however, converted to MeSpd to some extent in vivo.6 These analogues appear to fulfill cellular functions of polyamines in promoting the conversion of B-DNA to Z-DNA and restoring growth of polyamine-depleted cells.22,23 Our recent work with MeSpd was the first report of a methylated polyamine analogue being used in vivo.5 Only recently, with a sufficient supply of Me2Spm, have we been able to study the potential of this analogue in supporting pancreatic integrity. The present results distinctly show that Me2Spm is at least as effective as MeSpd in its protective action. Most importantly, the analogues given 4 to 8 hours after the induction of acute pancreatitis alleviated pancreatitis, especially acinar cell necrosis, prevented hemoconcentration and dramatically improved the survival of the animals. The finding that Me2Spm was partially metabolized to MeSpd also in pancreas, by the action of polyamine oxidase and/or spermine oxidase, offers the advantage that depletion of both spermidine and spermine can be overcome by the administration of Me2Spm. It should be noted that similar therapy with the natural polyamines is not feasible, as they would undergo rapid degradation due to activated acetylation.
According to our accumulated experience with the SSAT-overexpressing transgenic rat model, development of severe pancreatitis is inevitable when the cellular concentration of spermidine falls clearly below the value of 1000 pmol/mg tissue weight. Polyamine catabolism-induced severe pancreatitis was associated with ultimate death of the transgenic animals unless depleted pools of polyamines were substituted by administration of polyamine analogues. The specific cause of death of zinc-treated rats with pancreatitis is not clear at the moment, as other tissues studied up to 48 hours after administration of zinc showed little histopathological changes. The minor histological changes and metabolic effects seen in liver (Table 3) in this transgenic animal model were most likely not related to severe pancreatitis but were associated with hepatic SSAT induction because the metallothionein promoter-driven SSAT transgene is also expressed in liver. However, our earlier studies showed that MeSpd and Me2Spm restored early liver regeneration5,6 suggesting that polyamine analogues may also protect liver from potential damage during the course of pancreatitis. This was now verified in the long-term survivors whose livers showed normal appearance. It is possible that the methylated polyamine analogues exert a protective effect in tissues other than pancreas and liver. Hemoconcentration resulting from enhanced vascular permeability and manifested as abnormally high hematocrit values 24 hours after induction of pancreatitis (Table 3) is likely to cause hypovolemic shock, hypercoagulability, and microthrombi, leading to deterioration of tissue microcirculation and functional activity. The finding that hematocrit values were normalized by the treatment with Me2Spm strongly suggests that the surviving animals are protected from systemic complications because of less severe pancreatitis. In fact, although methylated polyamine analogues alleviate pancreatic damage their life-supporting effect may primarily be targeted to systemic phenomena. This hypothesis is supported by recent reports showing that acylhomospermines and lysine-spermine conjugates may be useful in the treatment of endotoxic shock24,25 and that oral spermine administration may have therapeutic effect in systemic inflammation and intestinal damage in a mouse endotoxin model.26
Severe distortion of polyamine homeostasis is likely to exert a deleterious effect in tissue integrity and/or function. Exocrine pancreatic tissue has the highest spermidine concentration in the mammalian body27 and the molar ratio of spermidine and spermine is also exceptionally high in pancreas,4 which is typical of a tissue with high rate of protein synthesis or active proliferation. Profound depletion of polyamines destabilizes DNA, interferes with transcription, and results in cell death via activation of caspase-3 and disruption of the mitochondrial membrane potential.28 Polyamines participate in the maintenance of tissue structure via nonspecific ionic or more specific molecular interactions with cellular components and play a role in immune response and contribute to acute phase inflammation.29
Acute pancreatitis is caused by premature activation of the inactive zymogen enzymes with its subsequent consequences of radical formation and cytokine release. The initial factors triggering this activation have not been clarified.30 It is possible that the higher polyamines directly inhibit protease activity.31 Their depletion would therefore result in a direct activation of the digestive enzymes. Our present results show that induction of polyamine catabolism in acinar cells is clearly associated with intracellular trypsinogen activation.
Acknowledgments
We thank Tuula Reponen, Sisko Juutinen, Aune Heikkinen, Uta Naumann, and Simone Rackow for skillful technical assistance; and Dr. Anne Uimari for helpful suggestions in the revision of the manuscript.
Footnotes
Address reprint requests to Dr. Leena Alhonen, A.I. Virtanen Institute for Molecular Sciences, University of Kuopio, P.O. Box 1627, FI-70211 Kuopio, Finland. E-mail: leena.alhonen@uku.fi.
Supported by the Academy of Finland (project number 50317).
References
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Pastor AM, Frossard J-L. Are genetically modified mice useful for the understanding of acute pancreatitis? FASEB J. 2001;15:893–897. doi: 10.1096/fj.00-0672rev. [DOI] [PubMed] [Google Scholar]
- Jänne J, Alhonen L, Pietilä M, Keinänen TA. Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem. 2004;271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- Alhonen L, Parkkinen JJ, Keinänen T, Sinervirta R, Herzig KH, Jänne J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc Natl Acad Sci USA. 2000;97:8290–8295. doi: 10.1073/pnas.140122097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen TL, Alhonen L, Sinervirta R, Keinänen T, Herzig KH, Suppola S, Khomutov AR, Vepsäläinen J, Jänne J. A polyamine analogue prevents acute pancreatitis and restores early liver regeneration in transgenic rats with activated polyamine catabolism. J Biol Chem. 2002;277:39867–39872. doi: 10.1074/jbc.M205967200. [DOI] [PubMed] [Google Scholar]
- Järvinen A, Grigorenko N, Khomutov AR, Hyvönen MT, Uimari A, Vepsäläinen J, Sinervirta R, Keinänen TA, Vujcic S, Alhonen L, Porter CW, Jänne J. Metabolic stability of alpha-methylated polyamine derivatives and their use as substitutes for the natural polyamines. J Biol Chem. 2005;280:6595–6601. doi: 10.1074/jbc.M412788200. [DOI] [PubMed] [Google Scholar]
- Grigorenko NA, Vepsäläinen J, Järvinen A, Keinänen TA, Alhonen L, Jänne J, Kritzyn AM, Khomutov AR. A new synthesis of α-methylspermidine. Bioorgan Khim (Moscow) 2004;30:441–445. doi: 10.1023/b:rubi.0000037268.98349.ce. [DOI] [PubMed] [Google Scholar]
- Grigorenko NA, Vepsäläinen J, Järvinen A, Keinänen TA, Alhonen L, Jänne J, Khomutov AR. Novel syntheses of α-methyl- and α,α′-dimethylspermine. Bioorgan Khim (Moscow) 2005;31:183–188. doi: 10.1007/s11171-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990;35:367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- Niederau C, Niederau M, Luthen R, Strohmeyer G, Ferrell LD, Grendell JH. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990;99:1120–1127. doi: 10.1016/0016-5085(90)90633-c. [DOI] [PubMed] [Google Scholar]
- Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger B, Lerch MM, Tessenow W. Direct detection of premature protease activation in living pancreatic acinar cells. Lab Invest. 1998;78:763–764. [PubMed] [Google Scholar]
- Guilbault GG. Fluorescence in enzymology. Guilbault GG, editor. New York: M. Dekker,; Practical Fluorescence. 1990:pp 683–773. [Google Scholar]
- Bernacki RJ, Oberman EJ, Seweryniak KE, Atwood A, Bergeron RJ, Porter CW. Preclinical antitumor efficacy of the polyamine analogue N1,N11-diethylnorspermine administered by multiple injections or continuous infusion. Clin Cancer Res. 1995;1:847–857. [PubMed] [Google Scholar]
- Hyvönen T, Keinänen TA, Khomutov AR, Khomutov RM, Eloranta TO. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J Chromatogr. 1992;574:17–21. doi: 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser C, Fölsch UR, Paprotny C, Creutzfeld W. Polyamine concentrations in pancreatic tissue, serum, and urine of patients with pancreatic cancer. Pancreas. 1990;5:119–127. doi: 10.1097/00006676-199003000-00001. [DOI] [PubMed] [Google Scholar]
- Hegyi P, Rakonczay Z, Jr, Sari R, Gog C, Lonovics J, Takacs T, Czako L. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004;10:2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czako L, Takacs T, Varga IS, Hai DQ, Tiszlavicz L, Hegyi P, Mandi Y, Matkovics B, Lonovics J. The pathogenesis of L-arginine-induced acute necrotizing pancreatitis: inflammatory mediators and endogenous cholecystokinin. J Physiol Paris. 2000;94:43–50. doi: 10.1016/s0928-4257(99)00104-7. [DOI] [PubMed] [Google Scholar]
- Takacs T, Czako L, Morschl E, Laszlo F, Tiszlavicz L, Rakonczay Z, Jr, Lonovics J. The role of nitric oxide in edema formation in L-arginine-induced acute pancreatitis. Pancreas. 2002;25:277–282. doi: 10.1097/00006676-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Lakanen JR, Coward JK, Pegg AE. Alpha-methyl polyamines: metabolically stable spermidine and spermine mimics capable of supporting growth in cells depleted of polyamines. J Med Chem. 1992;35:724–734. doi: 10.1021/jm00082a013. [DOI] [PubMed] [Google Scholar]
- Byers TL, Lakanen JR, Coward JK, Pegg AE. The role of hypusine depletion in cytostasis induced by S-adenosyl-L-methionine decarboxylase inhibition: new evidence provided by 1-methylspermidine and 1,12-dimethylspermine. Biochem J. 1994;303:363–368. doi: 10.1042/bj3030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Suresh Kumar EV, Wood SJ, Cromer JR, Datta A, David SA. Lipopolysaccharide sequestrants: structural correlates of activity and toxicity in novel acylhomospermines. J Med Chem. 2005;48:2589–2599. doi: 10.1021/jm049449j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MR, Wood SJ, Miller KA, Nguyen T, Cromer JR, David SA. Lysine-spermine conjugates: hydrophobic polyamine amides as potent lipopolysaccharide sequestrants. Bioorg Med Chem. 2005;13:2523–2536. doi: 10.1016/j.bmc.2005.01.038. [DOI] [PubMed] [Google Scholar]
- ter Steege JC, Forget PP, Buurman WA. Oral spermine administration inhibits nitric oxide-mediated intestinal damage and levels of systemic inflammatory mediators in a mouse endotoxin model. Shock. 1999;11:115–119. doi: 10.1097/00024382-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Löser C, Fölsch UR, Cleffmann U, Nustede R, Creuzfeldt W. Role of ornithine decarboxylase and polyamines in camostate (Foy-305)-induced pancreatic growth in rats. Digestion. 1989;43:98–112. doi: 10.1159/000199867. [DOI] [PubMed] [Google Scholar]
- Nitta T, Igarashi K, Yamamoto N. Polyamine depletion induces apoptosis through mitochondria-mediated pathway. Exp Cell Res. 2002;276:120–128. doi: 10.1006/excr.2002.5517. [DOI] [PubMed] [Google Scholar]
- Seiler N, Atanassov CL. The natural polyamines and the immune system. Prog Drug Res. 1994;43:87–141. doi: 10.1007/978-3-0348-7156-3_4. [DOI] [PubMed] [Google Scholar]
- Halangk W, Krüger B, Ruthenbürger M, Stürzebecher J, Albrecht E, Lippert H, Lerch MM. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol. 2002;282:G367–G374. doi: 10.1152/ajpgi.00315.2001. [DOI] [PubMed] [Google Scholar]
- Leviant MI, Bylinkina VS, Orekhovich VN. Inhibitors of cathepsin R (ribosomal proteinase). Polyamines as natural inhibitors of the proteinase. Biokhimiia. 1979;44:1454–1459. [PubMed] [Google Scholar]