Abstract
The ongoing outbreak of avian influenza A virus (subtype H5N1) infection in Asia is of great concern because of the high human case fatality rate and the threat of a new influenza pandemic. Case reports in humans and felids suggest that this virus may have a different tissue tropism from other influenza viruses, which are normally restricted to the respiratory tract in mammals. To study its pathogenesis in a mammalian host, domestic cats were inoculated with H5N1 virus intratracheally (n = 3), by feeding on virus-infected chicks (n = 3), or by horizontal transmission (n = 2) and examined by virological and pathological assays. In all cats, virus replicated not only in the respiratory tract but also in multiple extra-respiratory tissues. Virus antigen expression in these tissues was associated with severe necrosis and inflammation 7 days after inoculation. In cats fed on virus-infected chicks only, virus-associated ganglioneuritis also occurred in the submucosal and myenteric plexi of the small intestine, suggesting direct infection from the intestinal lumen. All cats excreted virus not only via the respiratory tract but also via the digestive tract. This study in cats demonstrates that H5N1 virus infection causes systemic disease and spreads by potentially novel routes within and between mammalian hosts.
Related Commentary on page 6
Avian influenza A viruses of the H5N1 subtype, which are responsible for the ongoing outbreak of fowl plague in South East Asia, have caused fatal infections in humans and other mammals.1–3 Of 122 laboratory-confirmed cases, 62 patients have died of H5N1 virus infection as of 1 November 2005 (World Health Organization: http://www.who.int/csr/disease/avian_influenza/country/en/). In addition, H5N1 virus has caused fatal infection in domestic cats, leopards, and tigers, species previously considered to be resistant to disease from influenza A virus infection.4,5 The transmission of avian influenza A virus to mammalian species is of great concern because this may allow the virus to adapt to mammalian hosts and acquire pandemic potential. So far, however, there is only evidence for limited human spread.6 The pathogenesis of H5N1 virus infection in humans and other mammalian hosts is poorly understood, including how the virus spreads within the host and from one host to another.
When bird-to-human transmission of H5N1 virus was first recorded in 1997, with 6 deaths of 18 hospitalized patients,7–9 the question of why this virus was so pathogenic was raised. One hypothesis was that H5N1 virus had expanded its tissue tropism in humans beyond its normal location in the respiratory tract, resulting in systemic infection,10 as is the usual situation in poultry infected with highly pathogenic avian influenza virus.11 Although support for this hypothesis was provided neither by studies of patients who died in 199712 nor by experimental infections in cynomolgus macaques,13,14 the recent isolation of H5N1 virus from a patient with severe neurological symptoms15 once again raises the question of extra-respiratory tissue tropism of H5N1 virus in humans and other mammalian hosts.
Related to the question of tissue tropism is the question whether H5N1 virus can spread from one mammalian host to another and, if so, how. Although most human H5N1 virus infections have been due to contact with infected poultry or poultry products, probable person-to-person transmission has been recorded for two patients.6 The possibility of mammal-to-mammal transmission of this virus was confirmed by studies in cats and tigers.5,16 However, the routes by which H5N1 virus can spread from human to human is not known. The accepted transmission route of human influenza virus infection—by inhalation of virus-infected aerosol17—is based on the infection being restricted to the respiratory tract. However, fecal-oral transmission is considered the most important route of transmission of influenza A virus in birds.11 The isolation of H5N1 virus from a rectal swab of a human patient presenting with diarrhea suggests that transmission by infected feces in humans also could occur.15
To investigate the pathogenesis of H5N1 virus infection in a mammalian host, we performed detailed virological and pathological studies of domestic cats experimentally infected with H5N1 virus by different routes of inoculation. We have previously used this model to show that cats can be infected with H5N1 virus both by horizontal transmission and by feeding on virus-infected birds and that infected cats develop severe virus-associated pneumonia.5 In the present study we determined whether extra-respiratory spread of H5N1 virus occurred, and, if so, with which lesions this was associated. The results are discussed in the light of viral spread within the host and between hosts.
Materials and Methods
Virus Preparation
A virus stock was prepared of influenza virus A/Vietnam/1194/2004 (H5N1), which was isolated from a fatal human case. The virus (passage xE1) was kindly provided by Dr. W. Lim, Queen Mary Hospital, Hong Kong, and propagated once in Madin-Darby canine kidney (MDCK) cells (xE1MDCK1). It was titrated according to standard methods18 and reached an infectious virus titer of 106.2 median tissue culture infectious dose (TCID50) per ml.
Experimental Protocol
Four- to six-month-old specified pathogen-free European shorthair cats were purchased from a commercial breeder (Harlan, Indianapolis, IN). During the entire experiment the animals were housed in negatively pressurized isolator units. Three cats (nos.1 to 3) were infected intratracheally by use of a catheter with 2.5 × 104 TCID50 of H5N1 virus under anesthesia with ketamine. Two days after cats 1 to 3 were inoculated intratracheally, two sentinel cats (nos. 4 and 5) were placed in the same enclosure to investigate whether cats could be infected via horizontal transmission. A third group of three cats (nos. 6 to 8) were fed with infected birds. To this end, 1-day-old chicks were infected intratracheally with 2.5 × 104 TCID50 of H5N1 virus. At 1 day after infection, the chicks were euthanized and were fed to cats 6 to 8 (one chick per cat). Liver and lung homogenates obtained from infected chicks contained >109 TCID50 per g tissue and cloaca swabs reached titers of 104 to 107.5 TCID50/ml. Two cats (nos. 9 and 10) fed with chicks sham-infected with phosphate-buffered saline served as negative controls. Before and at 1, 3, 5, and 7 days after infection, pharyngeal, nasal, and rectal swabs were collected from cats under anesthesia and submersed in 3 ml of virus transport medium (minimal essential medium with antibiotics). All cats were euthanized at 7 days after infection by exsanguination under ketamine anesthesia, except for one cat that died on day 6 after infection. Experimental procedures were approved by an independent Animal Care and Use Committee.
Pathological Examination and Immunohistochemistry
Necropsies and tissue sampling were performed according to a standard protocol. After fixation in 10% neutral-buffered formalin and embedding in paraffin, tissue sections were stained with hematoxylin and eosin for histological evaluation or with an immunohistological method using a monoclonal antibody against the nucleoprotein of influenza A virus as a primary antibody for detection of influenza viral antigen.13 Lung tissue of an experimentally infected cynomolgus macaque (Macaca fascicularis) experimentally infected with influenza virus A/Hong Kong/156/1997 (H5N1) was included as a positive control. Isotype-matched and omission controls were included as negative controls. The following tissues were examined by these two methods: conjunctiva, nasal concha, trachea, lung (nine specimens), tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, tonsil, tracheo-bronchial lymph node, mesenteric lymph node, spleen, thymus, heart, liver, pancreas, kidney, adrenal gland, urinary bladder, olfactory bulb, cerebrum (at level of hippocampus), cerebellum, and brain stem.
Virus Titrations
Tissue samples were weighed and homogenized in 3 ml of transport medium with a homogenizer (Kinematica Polytron, Lucerne, Switzerland). Ten-fold serial dilutions of the tissue suspensions and swabs were inoculated in MDCK cells in quadruplicate as described previously.18 All experiments were performed under biosafety level 3+ conditions.
Bacteriology
Lung swabs were tested by routine bacteriological methods (with blood agar, chocolate agar, and MacConkey agar) under aerobic and anaerobic conditions for bacterial pathogens by the Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands.
Results
Clinical Signs
All intratracheally inoculated cats and cats fed on virus-infected chicks showed clinical signs, including raised body temperature, decreased activity, protrusion of the third eyelid, conjunctivitis, and labored breathing, from 2 days after infection onwards. Clinical signs in sentinel cats were similar, except that they developed symptoms later (from 5 days after infection onwards) and protrusion of the third eyelid was not observed. Negative control cats showed no clinical signs.
Virology and Bacteriology
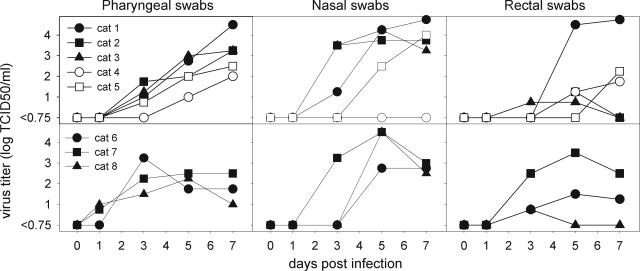
Virus was isolated from pharyngeal, nasal, and rectal swabs of all intratracheally inoculated cats, cats fed on virus-infected chicks, and sentinel cats (Figure 1). The only exception was the failure to isolate virus from nasal swabs of one sentinel cat (no. 4). In general, swabs first tested positive at 3 days after infection and remained positive until the end of the experiment at 7 days after infection. The titers in the pharyngeal swabs of the intratracheally inoculated cats continued to increase up to 104.5 TCID50/ml during the course of the experiment. Peak titers in nasal swabs ranged from 102.5 to 105.0 TCID50/ml. The virus titers in the rectal swabs varied widely. No virus was isolated from any of the swabs collected from the negative control cats.
Figure 1-6703.
Infectious virus titers in pharyngeal, nasal, and rectal swabs obtained from cats infected with influenza A virus (H5N1) by the intratracheal route (top, filled symbols), by horizontal transmission (top, open symbols), or by feeding on virus-infected chicks (bottom, filled symbols) at various time points after infection. No virus was isolated from any swabs of negative control cats (data not shown).
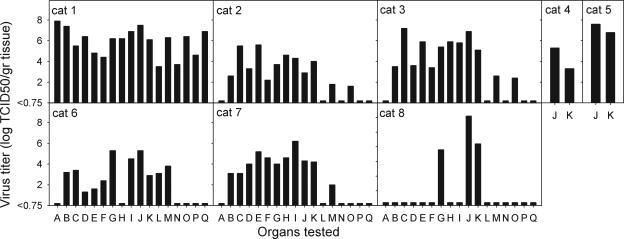
Seventeen different organs were tested for the presence of virus by virus-isolation procedures (Figure 2). Both in intratracheally inoculated cats and cats fed on virus-infected chicks, virus replication was demonstrated in extra-respiratory tissues, most often brain, liver, kidney, and heart (Figure 2). There was no clear difference in virus distribution between these two groups, except that the tracheo-bronchial lymph node was positive in all three intratracheally inoculated cats and negative in all three cats fed on virus-infected chicks. The stomach, eyelid, mesenteric lymph node, and spleen specimens were negative in all cats, except for cat 1, in which all tissues were positive. The respiratory tract specimens of the two sentinel cats (nos. 4 and 5) also tested positive, indicating that they became productively infected after contact with the intratracheally inoculated cats. No virus was isolated from any of the tissues collected from the negative control cats. No bacteria were isolated from lung specimens by bacteriological culture.
Figure 2-6703.
Infectious virus titers in organ tissue homogenates obtained from cats infected with influenza A virus (H5N1) by the intratracheal route (cats 1 to 3), by horizontal transmission (cats 4 and 5), or by feeding on virus-infected chicks (cats 6 to 8) 7 days after infection. Organs that were tested included stomach (A), liver (B), kidney (C), heart (D), cerebrum (E), cerebellum (F), brain stem (G), olfactory bulb (H), nasal concha (I), lung (J), trachea (K), jejunum (L), tonsil (M), eyelid (N), tracheo-broncheal lymph node (O), mesenteric lymph node (P), and spleen (Q).
Gross Pathology
All intratracheally inoculated cats, cats fed on virus-infected chicks, and sentinel cats had multifocal or coalescing pulmonary lesions, which were red-purple, slightly raised, and firmer than normal. The estimated percentage of lung tissue involved per cat varied: 95% (cat no. 1), 80% (no. 2), 66% (no. 3), 5% (no. 4), 33% (no. 5), 33% (no. 6), 95% (no. 7), and 33% (no. 8). Negative control cats (nos. 9 and 10) had no pulmonary lesions.
The only gross lesions in extra-respiratory organs were seen in cats fed on virus-infected chicks. All three cats had enlargement of and multifocal petechial hemorrhages in the tonsils, mandibular lymph nodes, retropharyngeal lymph nodes, or a combination of these tissues. Two of three cats (nos. 6 and 7) had multiple petechial hemorrhages in the liver. In one of these animals (no. 6), petechial hemorrhage was associated with generalized icterus, characterized by yellow staining of multiple tissues.
Histopathology
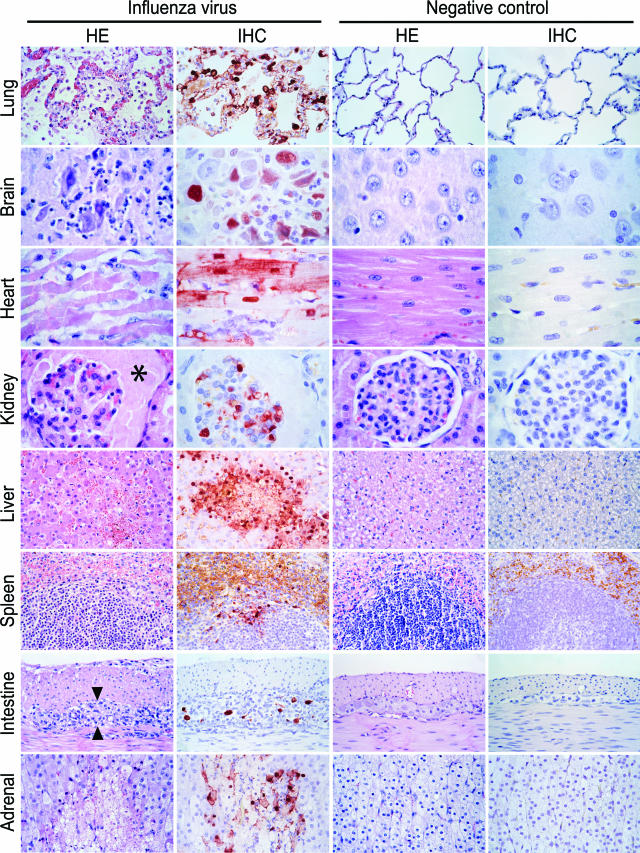
Intratracheally inoculated cats had histological lesions in the lung, brain, heart, kidney, liver, and adrenal gland (Figure 3). In the lung, all three cats had multiple or coalescing foci of inflammation and necrosis that centered on the bronchioles. In these foci, the alveolar and bronchiolar lumina were filled with alveolar macrophages, neutrophils, and erythrocytes, mixed with fibrin, edema fluid, and cellular debris. Some alveoli were covered by hyaline membranes. The epithelium of bronchiolar and alveolar walls had evidence of both necrosis and hyperplasia. The bronchiolar and alveolar walls were moderately thickened. Some foci in the lung parenchyma showed coagulation necrosis, whereas others had evidence of beginning alveolar fibroplasia and bronchiolitis obliterans. The bronchi were much less severely affected. There was edema and moderate accumulation of mononuclear cells around pulmonary artery branches.
Figure 3-6703.
Cats infected with influenza A virus (H5N1) (cats 1 to 8) have lesions associated with virus replication in multiple tissues. Necrotizing and inflammatory changes are seen in multiple tissues, except the spleen, of cats infected with H5N1 virus (first column). See the text for a more detailed description of these lesions. The asterisk indicates protein exudate in the Bowman’s capsule of a renal glomerulus. The two arrowheads point to the inflamed myenteric plexus in the intestinal wall. Serial sections of these tissues (second column) show that these lesions are closely associated with the expression of influenza virus antigen. Tissues of negative control cats (cats 9 and 10) show neither lesions (third column) nor influenza virus antigen expression (fourth column). Tissues were stained either with H&E or by immunohistochemistry using a monoclonal antibody against the nucleoprotein of influenza A virus as a primary antibody.
In the brain, all three cats had multiple randomly distributed foci of necrosis and inflammation, characterized by aggregates of neutrophils and glial cells, interstitial edema, neuronal necrosis, and perivascular mononuclear infiltrates. The leptomeninges had multifocal to diffuse mononuclear infiltrates. One cat (no. 1) had segmental loss of ependymal cells lining the lateral ventricle, with increased cellularity and edema in the underlying parenchyma. Another cat (no. 3) had mononuclear infiltrates in the choroid plexi of lateral and fourth ventricles. The lesions were generally more marked in the cerebrum than in the brain stem, and mild or absent in the cerebellum.
In the heart, two of three cats (nos. 2 and 3) had multiple foci of necrosis and inflammation in the myocardium, mainly near to the lumen of left or right ventricle. These foci were characterized by myocyte necrosis and accumulation of mononuclear cells. In the kidney, one of three cats (no. 1) had multiple foci of tubular epithelial necrosis and presence of protein exudate in the Bowman’s capsule of many glomeruli. In the liver, one of three cats (no. 1) had multiple randomly located foci of necrosis and inflammation, characterized by hepatocytic necrosis, accumulation of erythrocytes, and infiltration by macrophages and neutrophils. In the adrenal gland, all three cats had multiple foci of necrosis and inflammation in the cortex, with necrosis of adrenal cortical cells, accumulation of erythrocytes, and infiltration by neutrophils. The other tissues examined had no significant lesions.
Both sentinel cats had similar histological lesions compared to the intratracheally inoculated cats, with the following differences. In the lung, there were relatively more neutrophils and fewer macrophages in alveoli and bronchioles. There was no evidence of regeneration of alveolar and bronchiolar epithelium, alveolar fibroplasia, bronchiolitis obliterans, or perivascular cuffing. In the liver, the necrotic foci were infiltrated with relatively more neutrophils and fewer macrophages. Only one of two cats (no. 4) had lesions in the adrenal gland similar to those in the intratracheally inoculated cats, while neither had significant lesions in the heart, kidney, spleen, intestine, or other tissues examined. The brain of contact cats was not examined.
All three cats fed on virus-infected chicks had similar histological lesions compared to the intratracheally inoculated cats, with the following differences. In the brain, the character and distribution of lesions were similar, except that lesions were not observed in ependyma and choroid plexus. Only two of three cats (nos. 6 and 7) had lesions in the heart. Small intestine lesions were found only in cats fed on virus-infected chicks (and of these, only in cats 6 and 7). These consisted of multifocal to diffuse necrotic and inflammatory changes in the submucosal (Meissner’s) and myenteric (Auerbach’s) plexi. Affected plexi had loss and necrosis of ganglion cells and infiltration by neutrophils and mononuclear cells. In the adrenal gland, two of three cats (nos. 7 and 8) had multiple lesions in the medulla similar in character to those in the cortex. None of the cats had significant lesions in kidney, spleen, or other tissues examined. Negative control cats had no significant lesions in the lung, brain, heart, kidney, liver, spleen, intestine, adrenal gland, or other tissues examined.
Immunohistochemistry
Influenza virus antigen expression was visible as diffuse to granular red staining, which was usually darker in the nucleus than in the cytoplasm (Figure 3). Influenza virus antigen expression was seen most frequently in liver, heart, brain, lung, and adrenal gland (Table 1). In the lung (Figure 3), virus antigen expression was seen in many type 1 pneumocytes, type 2 pneumocytes, bronchiolar and bronchial epithelial cells, in occasional alveolar macrophages, and in rare endothelial cells and smooth muscle cells of pulmonary vein branches. In the brain, virus antigen expression was seen in many neurons and glial cells, and in occasional choroid epithelial cells, ependymal cells, and leptomeningeal epithelial cells. Virus antigen expression was seen in many cardiac myocytes and in occasional endocardial endothelial cells in the heart; in many glomerular cells (visceral epithelial and/or mesangial cells; cat no. 1 only) and in occasional distal tubular epithelial cells (cat no. 6 only) in the kidney; in many hepatocytes in the liver; in occasional large mononuclear cells (mainly at the border between red pulp and follicles) in the spleen; in occasional ganglion cells and Schwann cells in submucosal and myenteric plexi in duodenum and ileum; and in many cortical cells and in occasional pheochromocytes in the adrenal gland. Additionally, virus antigen expression was seen in a few medullary epithelial cells in the thymus and in a few large mononuclear cells in the germinal center of secondary follicles of the mesenteric lymph node in cat 1 only.
Table 1-6703.
Influenza Virus Antigen Expression in Tissues of Cats Infected with Influenza A Virus (H5N1) by Different Inoculation Routes
| Tissue* | Number of animals positive per method of inoculation
|
||
|---|---|---|---|
| Intratracheal (n = 3) | Sentinel (n = 2) | Fed on virus-infected chicks (n = 3) | |
| Liver | 3 | 2 | 1 |
| Kidney | 1 | 0 | 1 |
| Heart | 3 | 0 | 2 |
| Cerebrum | 3 | n.d.† | 3 |
| Cerebellum | 2 | n.d. | 1 |
| Brain stem | 3 | n.d. | 3 |
| Olfactory bulb | 3 | n.d. | 2 |
| Lung | 3 | 2 | 3 |
| Duodenum | 0 | n.d. | 2 |
| Ileum | 0 | n.d. | 1 |
| Mesenteric lymph node | 1 | 0 | 0 |
| Spleen | 2 | 2 | 0 |
| Thymus | 1 | n.d. | 0 |
| Adrenal gland | 3 | 1 | 3 |
No virus antigen expression was seen in the following tissues of the above animals: tongue, nasal concha, nasal septum, trachea, eyelid, third eyelid, thyroid, salivary gland, tonsil, tracheo-bronchial lymph node, retropharyngeal lymph node, mandibular lymph node, bone marrow, esophagus, stomach, pancreas, jejunum, cecum, colon, and urinary bladder.
Not done.
In all tissues except spleen, thymus, and mesenteric lymph node, influenza virus expression was closely associated with the presence of histological lesions. In general, influenza virus antigen expression was strongest at the transition of normal and necrotic tissue. In larger lesions, this resulted in a ring of antigen expression at the edge of the lesion and lack of antigen expression in the center (liver in Figure 3). Influenza virus expression was present in positive control tissues and absent in both isotype and omission controls. Erythrocytes showed nonspecific light brown staining, probably because of pseudoperoxidase,19 most prominent in the red pulp of the spleen.
Discussion
Our study in experimentally inoculated cats shows that there is extensive extra-respiratory spread of H5N1 virus, including tissues of the nervous, cardiovascular, urinary, digestive, lymphoid, and endocrine systems. In these tissues, we demonstrated both active virus replication and severe pathological changes, consisting of necrosis and inflammation. Our study also shows that infected cats excrete H5N1 virus via the rectum, suggesting that cat-to-cat transmission of H5N1 virus could occur through infected feces.
Although it has been shown previously that cats can be experimentally infected with influenza A viruses,5,20–22 to our knowledge this is the first time that systemic replication has been demonstrated in this species. The extensive extra-respiratory virus replication and its association with severe necrosis and inflammation in these cats suggest that the high pathogenicity of this H5N1 virus for mammalian species, including humans, may be related to its wide tissue tropism. Our findings in cats correspond with previous findings from experimentally infected ferrets23,24 and mice.25–27 Evidence for extra-respiratory replication of H5N1 virus in humans is the isolation of H5N1 virus from the cerebrospinal fluid of a human patient diagnosed clinically with acute encephalitis.15 The extensive virus-associated liver necrosis and inflammation we observed in the cats is compatible with the increased serum levels of alanine aminotransferase and aspartate aminotransferase, indicative of liver damage,28 seen in six of seven human patients.6,29 The virus-associated ganglioneuritis of the intestinal nervous plexi we observed in the cats is noteworthy considering that diarrhea was diagnosed in 9 of 12 human patients.15,29 Although ganglioneuritis from influenza virus infection has never been reported to our knowledge, ganglioneuritis from other causes, including virus infections, may result in diarrhea.30 Another example of extra-respiratory infection by avian influenza viruses in mammalian hosts involves the infection of human conjunctivae by viruses of the H7N7 subtype.31–34
There was evidence for at least two routes of virus entry, from blood and from the intestinal lumen, into extra-respiratory tissues of the cats. Evidence for virus entry from blood comes first from the pattern of virus infection in renal glomeruli and randomly located foci in liver and adrenal gland. This pattern closely resembles that seen in blood-borne spread of bacteria, eg, Salmonella septicemia.35 Second, the predominance of virus-associated myocardial lesions adjacent to the lumen of the heart ventricles and, third, the expression of virus antigen by endothelial cells of pulmonary veins and endocardium also point toward blood-borne spread of H5N1 virus. This route of virus spread corresponds to the detection of H5N1 virus in the serum of a human patient.15 In contrast to the above tissues, the submucosal and myenteric plexi in the intestine may have been infected directly from the intestinal lumen. We only observed virus expression by immunohistochemistry and associated lesions in the intestine of cats fed on virus-infected chicks. Although virus entry by this route has not been demonstrated before for influenza virus, herpes simplex virus has been shown to enter the submucosal and myenteric plexi from the intestinal lumen via nerve fibers projecting through the mucosa and interacting directly with surface epithelial cells.36 Entry of influenza virus into host tissues directly from the intestinal lumen remains to be conclusively demonstrated because we cannot exclude the possibility that the cats fed on virus-inoculated chicks received some inoculum through the respiratory tract while feeding. Virus entry via the intestinal lumen is of interest for humans because of reports of fatal H5N1 virus infection after consuming raw poultry products (Avian influenza, human–East Asia (64) Viet Nam. ProMED-mail 2005; 4 April: 20050404.0971 http://www.promedmail.org) or after using the mouth to suck exudate from the upper respiratory tract of fighting cocks (Avian influenza, human–Thailand (06) ProMED-mail 2004; 9 Sept: 20040909.2513 http://www.promedmail.org). Park and colleagues37 demonstrated a third route of influenza virus entry in extra-respiratory tissues of experimentally infected mice. They showed that influenza virus could enter the brain from the nasal cavity by way of the olfactory nerves—which penetrate the cribriform plate—and olfactory bulb. We have no evidence for this route of entry in the cats because the virus-associated lesions in the olfactory bulb and adjacent cerebral tissue did not appear more chronic or more frequent than those elsewhere in the brain. However, more detailed studies at earlier time points after infection would be needed to exclude this possibility.
Cat-to-cat spread of H5N1 virus may have occurred because of virus excretion from a number of organ systems. The first and most obvious possibility is excretion from the respiratory tract, based on virus isolation from nasal and pharyngeal swabs as well as from trachea and lung samples. This also has been found for H5N1 virus infection in human patients6,15,29 and in experimentally infected ferrets.23,24 The second possibility, previously considered important only for influenza virus infection in birds,11 is excretion from the digestive tract. This is based on virus isolation from rectal swabs as well as from jejunum samples. Surprisingly, we did not find immunohistochemical evidence for virus replication in the epithelium of the digestive tract at any of the seven levels (esophagus, stomach, duodenum, jejunum, ileum, cecum, colon) tested, even though this is an important site of influenza virus replication in birds.11 Transmission of H5N1 virus by infected feces should be considered in humans. The virus has been isolated from a rectal swab of a human patient.15 Also, a high proportion of H5N1 influenza patients presented with severe and watery diarrhea, which increases the risk of transmission via feces. H5N1 virus also has been isolated from the feces of experimentally infected ferrets.23 The third and previously not considered possibility of virus excretion is from the urinary tract. This is based on virus isolation from kidney samples of five of six cats and virus antigen detection in the glomeruli or renal tubular epithelial cells of two of eight cats. Virological examination of urine samples of H5N1 virus-infected hosts is necessary to determine whether urine could form a source of H5N1 virus. It cannot be excluded that there were other sources of excreted virus, like blood and saliva.
Our results on the pathogenesis and replication of H5N1 virus in experimentally infected cats has several implications for the clinical and epidemiological management of the ongoing H5N1 virus outbreak. First, the severity of the lesions associated with H5N1 virus replication outside the respiratory tract could partly explain the increased pathogenicity of this virus for mammalian hosts, including humans. If so, supportive measures in patients with severe disease should be directed not only to the respiratory tract but also to other organ systems. Second, the potential for H5N1 virus to enter mammalian hosts via the intestine provides a possible explanation for how this virus could present clinically in absence of respiratory tract disease, as suggested by the recent findings in two patients with encephalitis and diarrhea.15 Therefore, H5N1 virus infection needs to be included in the differential diagnosis of a broader range of clinical presentations than is currently done. Finally, the possibility for the spread of H5N1 virus via feces in mammals needs to be taken into account in the epidemiological control of H5N1 virus infections. The above measures may limit the risk of H5N1 virus developing into a pandemic influenza virus.
Acknowledgments
We thank F. van der Panne for help with microphotographs, R. Dias D’Ullois with animal experiments, and A. van Belkum and A. van Vliet with bacteriology testing.
Footnotes
Address reprint requests to Thijs Kuiken, Department of Virology, Erasmus MC, P.O. Box 1738, 3000 DR Rotterdam, The Netherlands. E-mail: t.kuiken@erasmusmc.nl.
Supported by the Novaflu EU grant QLRT 2001-01034.
R.F. is a fellow of the Royal Dutch Academy of Arts and Sciences.
References
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsan R, Arya N, Ratanakorn P, Osterhaus DM, Poovorawan Y. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan G, Van Riel D, Van Amerongen G, Baars M, Fouchier R, Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306:241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R, Cheng AF. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. Orthomyxovirus infection. McFerran JB, McNulty MS, editors. Amsterdam: Elsevier Science Publishers,; Virus Infections of Birds. 1993:pp 287–316. [Google Scholar]
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Kuiken T, van Amerongen G, Bestebroer TM, Fouchier RA, Osterhaus AD. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J Virol. 2001;75:6687–6691. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan GF, Van Amerongen G, Osterhaus AD. Pathology of human influenza A (H5N1) virus infection in cynomolgus macaques (Macaca fascicularis). Vet Pathol. 2003;40:304–310. doi: 10.1354/vp.40-3-304. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R, Amonsin A, Tantilertcharoen R, Damrongwatanapokin S, Theamboonlers A, Payungporn S, Nanthapornphipat K, Ratanamungklanon S, Tunak E, Songserm T, Vivatthanavanich V, Lekdumrongsak T, Kesdangsakonwut S, Tunhikorn S, Poovorawan Y. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg Infect Dis. 2005;11:699–701. doi: 10.3201/eid1105.050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Webster RG. Orthomyxoviruses. Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman R, Straus SE, editors. New York: Raven Press,; Fields Virology. (ed 3) 1996:pp 1397–1445. [Google Scholar]
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998;74:57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Vacca LL, Hewett D, Woodson G. A comparison of methods using diaminobenzidine (DAB) to localize peroxidases in erythrocytes, neutrophils, and peroxidase-antiperoxidase complex. Stain Technol. 1978;53:331–336. doi: 10.3109/10520297809111955. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Easterday BC, Bean WJ., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniker CKJ, Nair CMG. Experimental infection of animals with influenza virus types A and B. Bull WHO. 1972;47:461–463. [PMC free article] [PubMed] [Google Scholar]
- Paniker CKJ, Nair CMG. Infection with A2 Hong Kong influenza virus in domestic cats. Bull WHO. 1970;43:859–862. [PMC free article] [PubMed] [Google Scholar]
- Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol. 2005;79:2191–2198. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov AS, Krauss S, Guan Y, Peiris M, Rehg JE, Perez DR, Webster RG. Neurovirulence in mice of H5N1 influenza virus genotypes isolated from Hong Kong poultry in 2001. J Virol. 2003;77:3816–3823. doi: 10.1128/JVI.77.6.3816-3823.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Cho DS, Rowe T, Katz JM. Mechanisms of pathogenicity of influenza A (H5N1) viruses in mice. Avian Dis. 2003;47:1131–1134. doi: 10.1637/0005-2086-47.s3.1131. [DOI] [PubMed] [Google Scholar]
- Davern TJ, II, Scharschmidt BF. Feldman M, Scharschmidt BF, Sleisenger MH, editors. Philadelphia: W.B. Saunders Company,; Biochemical liver tests. Gastrointestinal and Liver Disease, Pathophysiology/Diagnosis/Management. 1998 [Google Scholar]
- Hien TT, de Jong M, Farrar J. Avian influenza—a challenge to global health care structures. N Engl J Med. 2004;351:2363–2365. doi: 10.1056/NEJMp048267. [DOI] [PubMed] [Google Scholar]
- Debinski HS, Kamm MA, Talbot IC, Khan G, Kangro HO, Jeffries DJ. DNA viruses in the pathogenesis of sporadic chronic idiopathic intestinal pseudo-obstruction. Gut. 1997;41:100–106. doi: 10.1136/gut.41.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G, Gagnon A, Geraci JR. Isolation of an influenza A virus from seals. Arch Virol. 1981;68:189–195. doi: 10.1007/BF01314571. [DOI] [PubMed] [Google Scholar]
- Banks J, Speidel E, Alexander DJ. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch Virol. 1998;143:781–787. doi: 10.1007/s007050050329. [DOI] [PubMed] [Google Scholar]
- Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, Meijer A, van Steenbergen J, Fouchier R, Osterhaus A, Bosman A. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker IK, van Dreumel AA, Palmer N. The alimentary system. Jubb KVF, Kennedy PC, Palmer N, editors. San Diego: Academic Press,; 1993:pp 1–318. [Google Scholar]
- Gesser RM, Koo SC. Oral inoculation with herpes simplex virus type 1 infects enteric neuron and mucosal nerve fibers within the gastrointestinal tract in mice. J Virol. 1996;70:4097–4102. doi: 10.1128/jvi.70.6.4097-4102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, Umemura T. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol. 2002;147:1425–1436. doi: 10.1007/s00705-001-0750-x. [DOI] [PubMed] [Google Scholar]