Abstract
Over 40 nonhuman primate (NHP) species harbor species-specific simian immunodeficiency viruses (SIVs). Similarly, more than 20 species of nondomestic felids and African hyenids demonstrate seroreactivity against feline immunodeficiency virus (FIV) antigens. While it has been challenging to study the biological implications of nonfatal infections in natural populations, epidemiologic and clinical studies performed thus far have only rarely detected increased morbidity or impaired fecundity/survival of naturally infected SIV- or FIV-seropositive versus -seronegative animals. Cross-species transmissions of these agents are rare in nature but have been used to develop experimental systems to evaluate mechanisms of pathogenicity and to develop animal models of HIV/AIDS. Given that felids and primates are substantially evolutionarily removed yet demonstrate the same pattern of apparently nonpathogenic lentiviral infections, comparison of the biological behaviors of these viruses can yield important implications for host-lentiviral adaptation which are relevant to human HIV/AIDS infection. This review therefore evaluates similarities in epidemiology, lentiviral genotyping, pathogenicity, host immune responses, and cross-species transmission of FIVs and factors associated with the establishment of lentiviral infections in new species. This comparison of consistent patterns in lentivirus biology will expose new directions for scientific inquiry for understanding the basis for virulence versus avirulence.
INTRODUCTION
In ancient times, citizens sought answers to their most important inquiries by consulting oracles. Myths were part of daily life, and mythological creatures were feared or defeated. Oedipus deciphered the riddle of the Sphinx, a creature that was part primate (human) and part feline (lion). In modern times, many astute scientists have worked diligently to decipher a modern riddle, i.e., understanding human immunodeficiency virus (HIV) and using this knowledge to halt the AIDS pandemic. Although we endeavor to solve this riddle with the tools of science, we should keep in mind that, like Oedipus, we may find clues hidden in the intricate biology of primate and feline lentiviruses.
This review provides an overview of comparative aspects of feline and primate lentiviral infections, highlighting the striking similarities in both natural and cross-species transmission and pathogenicity of these agents. This side-by-side comparison of attributes of lentiviral infections conserved across divergent mammalian families is relevant for understanding the natural course of lentiviral disease. By taking a broader view of viral-host interactions, novel observations can be revealed, framing unanswered questions about HIV/AIDS pathogenesis and lentiviral/host adaptation and suggesting new avenues for mechanistic studies and therapeutic interventions for HIV.
SIV AND FIV POSITIONS WITHIN THE LENTIVIRUS GENUS
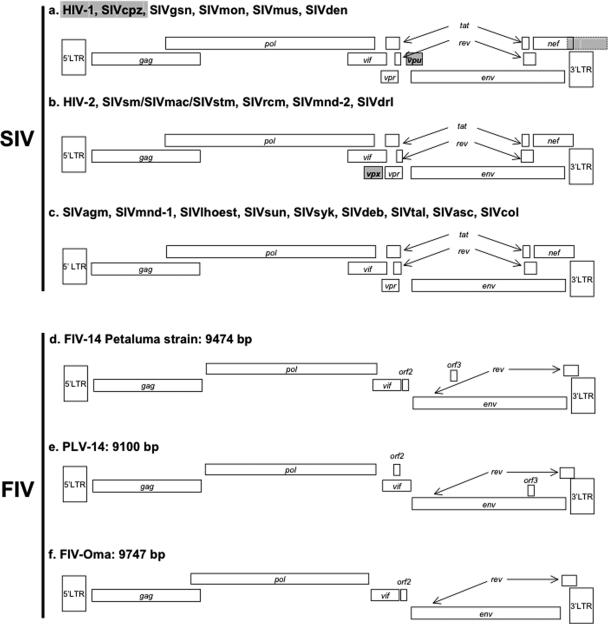
Simian immunodeficiency virus (SIV) and Feline immunodeficiency virus (FIV) belong to the Lentivirus genus of the Retroviridae. These viruses are morphologically distinct from other retroviruses in that they have a bar- or cone-shaped core (nucleoid). Lentiviruses have a complex structure with numerous accessory genes in addition to gag-, pol-, and env-encoded structural elements. The number of accessory genes (open reading frames [ORFs]) varies according to the strain of virus. All known lentiviruses are exogenous. At least 41 species of primates and 11 felid species have been diagnosed with species-specific lentiviral strains (see below). Besides primates and feline species, lentiviral infections have been identified in horses, goats, sheep, and cattle (253). Infection of certain felid and primate species results in immunodeficiency; however, the majority of these infections appear to be clinically silent. Ovine and caprine lentiviral infections may result in neurological disorders, arthritis, and pneumonia, while equine lentiviral infections result in recurrent fever and blood dyscrasias (253). Pathology has not been reported for cattle infected with bovine immunodeficiency virus (253). All lentiviruses include the accessory genes vif (virus infectivity factor) and rev (regulator of virus gene expression) (231, 253, 413), both of which have been implicated as facilitators of viral transcription and activation. FIVs also carry one or two small open reading frames; ORF 2 has been associated with a vpr-like function in domestic cat FIV (133). Three accessory genes are specific for primate lentiviruses, namely, vpr, vpx, and vpu; SIVs also include a nef gene. Figure 1 illustrates comparative genomic sequence organizations for three families of primate and feline lentiviruses.
FIG. 1.
Genomic organization of SIV and FIV strains belonging to different lineages. The SIV classification demonstrated is based on genomic structure relationships, which are not superimposable on phylogenetic relationships. For references, see Table 1.
Interestingly, despite the fact that differences in ORF structure exist between FIVs and primate lentiviruses, at least domestic cat FIV has retained similar immunodeficiency-inducing properties, suggesting that immunopathogenicity is either unrelated to these structural components or that FIV has evolved distinct but phenotypically convergent properties resulting in similar biological consequences for its host.
DETECTION AND NOMENCLATURE
FIV
Several rapid, sensitive, and specific enzyme-linked immunosorbent assays (ELISAs) are widely available for detection of FIV antibodies from domestic cats (159). Several laboratories offer PCR-based assays for FIV detection, although these are less reliable (221). Serosurveys of nondomestic felids have generally evaluated seroreactivities in captive or free-ranging animal sera versus domestic cat FIV antigens (53, 58, 116, 176, 281, 356). Subsequent studies have demonstrated that using species-specific lentiviral antigen preparations in a Western blot format significantly enhances the sensitivity. Currently, only puma and lion virus-based Western blot assays are available (286, 376, 383, 384); for detection of antibodies from other species, comparison using a multistrain-antigen Western blot approach has provided greater sensitivity (288, 376; S. Franklin et al., Abstr. 7th Int. Feline Retrovir. Res. Symp., p. 69, 2004). The use of a conserved-epitope peptide-based ELISA has been evaluated for rapid screening of puma and lion sera (201, 389), and a flow cytometry-based antibody detection assay has also been reported (40). Despite the fact that available tests often lack sensitivity, 27 of 35 felid species evaluated demonstrated seroreactivity; FIV infection has been confirmed in 11 species by PCR amplification of lentiviral sequences from peripheral blood mononuclear cells (PBMC). Sequence analysis has confirmed nine unique species-specific highly heterologous strains (53, 58, 235, 281, 376). “FIV” is typically used to refer to lentiviruses arising from domestic cats; when more than one feline species of lentivirus is being discussed, the standard nomenclature for designation of strains originating from different species is to append genus and species identifiers for the feline species as a subscript to FIV; for example, domestic cat (Felis cattus) FIV becomes FIVfca, African lion (Panthera leo) FIV is designated FIVple, and puma (also cougar, mountain lion, and panther; Puma concolor) isolates are referred to as FIVpco. As an exception, lion and puma lentiviruses have also been referred to as LLV and PLV to facilitate recognition by readers unfamiliar with felid species designations (281, 386). Five clades (A to E) of FIVfca have been defined based upon env sequence phylogeny (19, 177, 269, 351), and a recent report identified a sixth apparently unique isolate (107). Six infectious FIVfca clones have been characterized, including FIV-GL8 (180), FIV-PPR (355), FIV-PET (365), FIV-C36 (355), FIV-NCSU (409), and FIV-19K1 (341).
Clade designations have also been determined for lion (FIVple-A, -B, and -C) and puma (FIVpco-A and -B) lentiviruses, although these classifications were based on phylogenetic differentiation within pol, not env. Divergence among FIV strains is significant. In conserved regions of pol, FIVfca, FIVple, and FIVpco differ by as much as 30%, and differences in env and gag are even more pronounced (53, 59, 214, 376). FIVpco and FIVple intraspecies differences are also large; i.e., 10 to 20% divergence in pol occurs among isolates from the same species (53, 59, 376). In contrast, domestic cat FIVfca clades only differ in their nucleotide sequences by 5 to 10% across the entire genome (59, 351). FIV clades tend to be geographically oriented; for example, domestic cats in California are predominantly infected with the A clade, and infected pumas in the upper Midwest cluster significantly with respect to home ranges (41, 351). This pattern is not as strictly observed for lions. For example, lions of the Serengeti may be infected with A, B, or C clade viruses (53, 375). Partial genomic sequences are available for 11 nondomestic FIVs (376). Three nondomestic FIVs (FIVple, FIVpco, and Pallas cat-derived FIVoma) have been cultured and extensively sequenced (23, 24, 41, 53, 58, 214, 375).
SIV
Serology is the “gold standard” for studying the prevalence of SIVs in nonhuman primates (NHPs). In the past, most laboratories screened NHPs for anti-SIV antibodies by using commercial ELISA and Western blot kits (31, 68, 109, 123, 134, 258). ELISA screening tests are based on antigens consisting of viral lysates, recombinant proteins, or synthetic peptides corresponding to immunodominant epitopes of the two HIV type 1 (HIV-1) subtype B variants (strains LAI and MN) and HIV-2 group A (ROD strain). These “mixed” tests are therefore able to detect anti-HIV-1 and anti-HIV-2 antibodies. Cross-reactivity with other lentiviral lineages enables the use of these tests for screening nonhuman primate samples. For more sensitive detection of SIVs in NHPs, two strategies have been developed. The first uses a highly sensitive line assay (INNO-LIA HIV; Innogenetics, Ghent, Belgium) as a screening test. Using this strategy, more than 10 different new SIV types have been identified in NHPs (301). A second strategy uses synthetic peptides based on the gp41/36 and V3 peptides, allowing for increased sensitivity (gp41/36) and specificity (V3 peptides) (2, 346). This technique has also led to the discovery of several SIVs.
In the vast majority of cases, the infected NHP species represents the reservoir of that virus type, which is designated by a three-letter abbreviation of the host primate species name (with numerous exceptions). When related species of the same genus are infected, the name of the subspecies is included in the virus designation. Thus, the four species of African green monkeys (AGMs), i.e., vervet, grivet, tantalus, and sabaeus, are infected by SIVagm.Ver, SIVagm.Gri, SIVagm.Tan, and SIVagm.Sab, respectively (3, 120, 172, 190, 193, 258). For chimpanzee subspecies infected by SIVs, there is an exception to this rule; each SIVcpz isolate is named from the known or last known country of origin of the chimpanzee. Thus, the Pan troglodytes troglodytes subspecies is infected by SIVcpzGAB (Gabon), SIVcpzCAM (Cameroon), and SIVcpzUS (U.S. captivity), whereas Pan troglodytes schweinfurthii is infected by SIVcpzANT (Democratic Republic of Congo [DRC] via Antwerpen), SIVcpzTAN (Tanzania), and SIVcpzDRC1 (DRC) (75, 128, 268, 302, 303, 324, 405). Some authors have adopted the abbreviations SIVcpz.Ptt and SIVcpz.Pts to differentiate between the SIV strains infecting these two species of chimpanzees (165, 339).
For individual isolates of different SIV types, the current nomenclature rules include the country of origin in the name of the isolate; thus, SIVmnd-1GB1 and SIVrcmGAB1 are viruses isolated from a mandrill (MND) and a red-capped mangabey (RCM), respectively, from Gabon (134, 378), whereas SIVrcmNG409 originates from Nigeria (31). Some authors also include the year of sampling. Thus, SIVsmmSL92 is a sooty mangabey (SM) virus isolated from a sample collected in Sierra Leone in 1992 (67). This is a useful feature for tracing the origins of viruses, allowing for a better understanding of their evolution. In a recent paper, an attempt was made to rename SIVs using three-letter names. This method would introduce modifications to the current nomenclature, i.e., SIVsm would become SIVsmm, while SIVlhoest would become SIVlho (37).
Currently, there are 41 fully sequenced SIV genomes representing 23 different species types. Partial genomic sequences are available for 11 additional SIVs, and serologic evidence of SIV infection has been obtained for seven primate species for which no sequence information has been obtained (see Table 2). Ten infectious molecular SIV clones have been derived from different nonhuman primate species (rhesus macaques [Rh macaques], SMs, and AGMs).
TABLE 2.
African NHPs infected with SIV
| Common name (species) | Virus strain | Geographic location | Seroprevalence (%) | Available sequence(s) | Pathogenicity | Cross-species transmission | Reference(s) |
|---|---|---|---|---|---|---|---|
| Common chimp (Pan troglodytes troglodytes) | SIVcpz.Ptt | Central Africa (Cameroon, Gabon, Congo) | <10 | Complete genomes and partial sequences | Not reported | Humans (HIV-1) | 39, 75, 128, 205, 268, 303 |
| Eastern chimp (Pan troglodytes schwenfurthi) | SIVcpz.Pts | East Africa (Tanzania, Democratic Republic of Congo) | <10 | Complete genomes and partial sequences | Thrombocytopenia | Not reported | 302, 323, 325, 327, 405 |
| Pan troglodytes velorosus | SIVcpz.Ptta | Zoo in Cameroon | Complete sequence | Not reported | 75 | ||
| Sooty mangabey (Cercocebus atys) | SIVsmm | West Africa (Sierra Leone, Liberia, Ivory Coast) | 20-58 | Complete genomes and partial sequences | AIDS | Humans (HIV-2), macaques (SIVmac, SIVptm, SIVstm) | 9, 14, 67, 68, 173, 243, 326 |
| Red-capped mangabey (Cercocebus torquatus) | SIVrcm | West-Central Africa (Gabon, Cameroon, Nigeria) | 10-20 | Complete genomes and partial sequences | Not reported | Agile mangabey infection experimentally transmitted to Macaca mulatta and M. fascicularis (no AIDS) | 31, 134 |
| Agile mangabey (Cercocebus agilis) | SIVagi | West-Central Africa (Cameroon) | 0-10 | Partial sequences | Not reported | Not reported | Apetrei, unpublished data |
| White-crowned mangabey (Cercocebus lunulatus) | SIVagm.vera | Zoo in Tanzania | Partial sequence | Not reported | 368 | ||
| Gray-crested mangabey (Lophocebus albigena) | Unknown | Central Africa | Unknown | Serological evidence | Unknown | Unknown | 301 |
| Black mangabey (Lophocebus aterrimus) | SIVbkm | Central Africa (Democratic Republic of Congo) | Unknown | Partial sequence | Not reported | Not reported | 364 |
| Mandrill (Mandrillus sphinx) | SIVmnd-1 | Central Africa (Gabon) | 50 | Complete genomes and partial sequences | AIDS in captivity | Not reported | 353, 377, 378 |
| SIVmnd-2 | West-Central Africa (Cameroon, Gabon) | 50 | Complete genomes and partial sequences | AIDS in captivity | Transient infection in Rh macaque upon experimental transmission | 181, 353, 363 | |
| Drill (Mandrillus leucophaeus) | SIVdrl | West-Central Africa (Nigeria, Cameroon, Bioko) | Unknown | Complete genomes and partial sequences | Not reported | Not reported | 73, 181 |
| Yellow baboon (Papio cynocephalus) | SIVagm.verb | Tanzania | Unknown | Partial sequence | Not reported | Not reported | 191 |
| Chacma baboon (Papio ursinus) | SIVagm.verb | South Africa | Unknown | Partial sequence | Not reported | Not reported | 388 |
| Allen's monkey (Allenopithecus nigroviridis) | Unknown | Central Africa | Unknown | Serological evidence | Not reported | Not reported | 233 |
| Talapoin (Miophitecus talapoin, M. ougouensis) | SIVtal | Central Africa (Gabon, Angola, Cameroon) | 11 | Complete genomes and partial sequences | Not reported | Transient infection in Rh macaques upon experimental transmission | 223, 287 |
| Patas (Erythrocebus patas) | SIVagm.verb | West Africa | Unknown | Partial sequence | Not reported | Not reported | 39 |
| Grivet (Chlorocebus aethiops) | SIVagm.gri | East Africa | >50 | Complete genomes and partial sequences | Not reported | Not reported | 120 |
| Vervet (Chlorocebus pygerythrus) | SIVagm.ver | East and South Africa | >50 | Complete genomes and partial sequences | AIDS in a monkey coinfected with STLV | Naturally transmitted to baboons in the wild and to white-crowned mangabeys in captivity; experimentally transmitted to pig-tailed macaques (AIDS) and Rh macaques (no AIDS) | 86, 193 |
| Tantalus (Chlorocebus tantalus) | SIVagm.tan | Central Africa | >50 | Complete genomes and partial sequences | Not reported | Not reported | 172, 258 |
| Sabaeus (Chlorocebus sabaeus) | SIVagm.sab | West Africa | >60 | Complete genomes and partial sequences | Not reported | Naturally transmitted to patas monkeys (no AIDS); experimentally transmitted to Rh macaques (no AIDS) | 3, 190, 258 |
| Diana (Cercopithecus diana) | Unknown | West-Central Africa | Unknown | Serological evidence | Unknown | Unknown | 233 |
| Greater spot-nosed monkey (Cercopithecus nictitans) | SIVgsn | Central Africa | 4-20 | Complete genomes and partial sequences | Not reported | Potential source virus for SIVcpz | 76, 80 |
| Blue monkey (Cercopithecus mitis) | SIVblu | Central-East Africa | >60 | Partial sequences | Not reported | Not reported | 37 |
| Syke's monkey (Cercopithecus albogularis) | SIVsyk | East Africa | 30-60 | Complete genomes and partial sequences | Not reported | Transient infection in Rh macaques upon experimental transmission | 109, 169 |
| Mona (Cercopithecus mona) | SIVmon | West-Central Africa (Cameroon, Nigeria) | Unknown | Complete genome | Not reported | Not reported | 21, 76 |
| Dent's mona (Cercopithecus denti) | SIVden | Central Africa | 10 | 88 | |||
| Crested mona (Cercopithecus pogonias) | Unknown | West Africa | Unknown | Seroogical evidence | Unknown | Unknown | 301 |
| Campbell's mona (Cercopithecus campbelli) | Unknown | West Africa | Unknown | Serological evidence | Unknown | Unknown | 28 |
| Lowe's mona (Cercopithecus lowei) | Unknown | Unknown | Unknown | Serological evidence | Unknown | Unknown | 280 |
| Mustached monkey (Cercopithecus cephus) | SIVmus | Central Africa | 3 | Complete genomes and partial sequences | Not reported | Potential source virus for SIVcpz | 76 |
| Red-tailed monkey (Cercopithecus ascanius) | SIVasc/SIVschm | Central Africa (Democratic Republic of Congo) | Unknown | Complete genome and partial sequence | Not reported | Not reported | 392 |
| Red-eared monkey (Cercopithecus erythrotis) | SIVery | Central Africa (Bioko) | Unknown | Partial sequences | Not reported | Not reported | P. A. Marx, unpublished data |
| De Brazza's monkey (Cercopithecus neglectus) | SIVdeb | West-Central and Central Africa | 40 | Complete genomes and partial sequences | Not reported | Not reported | 37 |
| Owl-faced monkey (Cercopithecus hamlyni) | Unknown | Unknown | Unknown | Serological evidence | Unknown | Unknown | 280 |
| L'Hoest monkey (Cercopithecus lhoesti) | SIVlhoest/SIVlho | East Africa | 50 | Complete genomes and partial sequences | Not reported | Experimentally transmitted to pig-tailed macaques (AIDS) | 27, 166, 324 |
| Sun-tailed monkey (Cercopithecus solatus) | SIVsun | Central Africa | Unknown | Complete genomes and partial sequences | Not reported | Source virus for SIVmnd-1; experimentally transmitted to pig-tailed macaques (AIDS) | 28 |
| Preuss's monkey (Cercopithecus preussi) | SIVpre | Central Africa (Bioko) | Unknown | Partial sequence | Not reported | Not reported | Marx, unpublished data |
| Mantled colobus (Colobus guereza) | SIVcol | Central Africa | 28 | Complete genomes and partial sequences | Not reported | Not reported | 79 |
| Western Red colobus (Piliocolobus badius) | SIVwrc | West Africa | 40 | Partial sequence | Not reported | Not reported | 77 |
| Olive colobus (Procolobus verus) | SIVolc | West Africa | 40 | Partial sequence | Not reported | Not reported | 77 |
Cross-species transmission in captivity.
Cross-species transmission in the wild.
In conclusion, serologic surveillance is the most useful tool for detection of infection in individuals and in new species, whereas genetic analysis allows classification of viruses and viral subtypes (Tables 1 and 2). FIVs and SIVs occur naturally in many feline and primate species and are characterized genetically as species specific. While intra- and interspecies heterogeneity may be high, FIVs and SIVs almost uniformly cluster by species and by family.
TABLE 1.
Felids and hyaenids infected with FIV
| Common name (species) | Virus type | Geographic location | Seroprevalence (%) | Sequence | Pathogenicity | Cross-species transmission | Reference(s) |
|---|---|---|---|---|---|---|---|
| Domestic cat (Felis cattus) | FIV, FIVfca, various subtypes | Worldwide | 1-35 | Six complete genomes | AIDS | One puma and one Tsushima cat infected with domestic cat FIV | 59, 101, 216, 226, 234, 241, 305, 360, 376, 403 |
| Lion (Panthera leo) | FIVple (LLV) | Africa (Serengeti, Ngorongor Crater, Lake Manyara, and Kruger Park) | 80-90 | pol region | Possible CD4+ cell depletion with AIDS in captive animals; no association with increased morbidity in Serengeti lions | One snow leopard and one tiger from an Asian zoo infected with FIVple | 53, 58, 176, 281, 356, 375 |
| Namibia | 0 | 53, 235 | |||||
| Botswana | 26 | pol region | 286 | ||||
| European zoo | 57 | 235 | |||||
| Asia (free ranging) | 0 | 53, 235, 356 | |||||
| Asian origin; U.S. zoo | 73 | 218 | |||||
| Japan (captive) | 40 | pol region | 110 | ||||
| Puma, mountain lion, or cougar (Puma concolor) | FIVpco (PLV) | North, Central, and South America | PLV-14 published, PLV-1695 | Possible CD4+ cell depletion, no association with increased morbidity in Midwestern cougars | One puma infected with FIVfca | 58, 214, 281, 376 | |
| Wyoming, Montana | 58 | Multiple gag/pol/env sequences | 40, 41 | ||||
| Washington | 25 | 116 | |||||
| Florida (Florida panther) | PLV-14 full length published | 252 | |||||
| Pallas cat (Oocolobus manul) | FIVoma | Mongolia | >80 | Complete genome | None detected | None reported | 24, 376 |
| Bobcat (Lynx rufus) | FIVlru | California | 0-30 | pol region | None detected | Possible transmission to puma (S. Franklin, J. Troyer, and S. VandeWoude, unpublished data) | 317, 376; S. Franklin et al., Abstr. 7th Int. FRS, 2004 |
| Jaguarundi (Herpailurus yagouaroundi) | FIVhya | Central/South America | ∼20 | pol | None detected | None reported | 214, 376 |
| Cheetah (Acinonyx jubatus) | FIVaju | Africa | <10 | pol region | None detected | None reported | 261, 281, 286, 376 |
| Leopard (Panthera pardus) | FIVppa | Botswana, Africa | 16, ∼50 | None detected | None reported | 286, 376 | |
| Asia | 0 | 376 | |||||
| Ocelot (Leopardus pardalis) | FIVlpa | Central/South America | 10 | pol region | None detected | None reported | 214, 376 |
| Tiger (Panthera tigris) | European zoo | <10 | None detected | 235 | |||
| Asia | <10 | pol (FIV-Ple) | None detected | One tiger from an Asian zoo infected with FIVple | 376 | ||
| Sand cat/desert cat (Felis margarita) | Saudi Arabia | <10 | None detected | None reported | 286, 376 | ||
| European wildcat (Felis sylvestris) | Europe (Scotland, France, Switzerland, and Germany), Saudi Arabia | 0-10 | pol region | Non detected | None reported | 87, 122, 220, 288, 376 | |
| Margay (Leopardus wiedii) | Central/South America | <10 | None detected | None reported | 214, 376 | ||
| Geoffroy's cat (Leopardus geoffroyi) | Central/South America | <10 | None detected | None reported | 214, 376 | ||
| Tigrina (Leopardus tigrinis) | Central/South America | <10 | None detected | None reported | 214, 376 | ||
| Jaguar (Panthera onca) | Central/South America | <10 | None detected | None reported | 376 | ||
| Leopard cat (Prionailurus bengalensis) | Vietnam, Asia | 0 | 184, 255, 376 | ||||
| Tsushima cat (Felis bengalensis euptilura) | Japan | None reported | One free-ranging animal infected with FIVfca | 270 | |||
| Spotted hyena (Crocuta crocuta) | FIVccr | Masai Mara Naitonal Reserve (Serengeti) | 10-30 | pol region | None detected | None reported | 158, 376 |
| Striped hyena (Hyeana hyeana) | Africa | ∼40 | None reported | None reported | 376 |
SEROPREVALENCE OF FIV AND SIV
Feline Species with FIV
The seroprevalence of FIVs varies dramatically by species and geographic locale. For example, Serengeti African lions are nearly 100% seropositive (53, 375), as are pumas in Wyoming that are over 4 years of age (41). Pumas in Montana have maintained an approximate seroprevalence rate of 20% over time (40), similar to seroprevalence rates detected in Florida panthers and in cougars from Washington State (116, 252). In contrast, significant numbers of free-ranging Asiatic lions or those found in Etosha Pan (Namibia) were all seronegative (53, 356, 375). Asian lions held in captivity at the Lincoln Park Zoo in Chicago were noted to be 75% FIV seropositive, demonstrating that lions of Asian origin are not intrinsically resistant to infection (218). Interestingly, a similar geographic dispersal of seropositivity was noted for Asian versus African leopards (Panthera pardus), i.e., free-ranging African populations demonstrate seropositivity of >25%, whereas Asian-born animals are seronegative (286, 376). Greater than 50% of Pallas cats tested harbored anti-FIV antibodies (376); this is the only species of Asian origin as yet to be defined by a unique FIV strain. Six of 12 adult ocelots on Barro Colorado Island, Panama, were seropositive, which is a higher prevalence than those reported in other serosurveys (53, 281, 376; Franklin et al., unpublished data). While a recent report did not detect anti-FIV antibodies in a large cohort of bobcats from Northern California (317), close to 40% prevalence has been noted in a Southern California population. This divergence in prevalence may result from isolation of certain populations when wildlife corridors are disrupted, leading to geographically limited habitats and increased conspecific interactions (similar to island populations) (376). Interestingly, 22 of 51 spotted hyenas (Crocuta crocuta) from the Serengeti harbored anti-FIV antibodies (158, 376), confirming the findings of an earlier serosurvey of a separate population (158). FIV-like pol sequences were amplified from hyena PBMC, confirming infection with an FIV-related agent (158, 376). Serum antibodies have been detected in 8 of 17 striped hyenas (Hyeana hyeana); however, infection of these animals has not been confirmed by viral isolation or PCR amplification (376).
Captive nondomestic felid seroprevalence rates range from 3 to 75%, depending on the species (110, 201). The seroprevalence of domestic cat FIV varies between 1 and 15%, with significantly higher incidences occurring in certain demographics, such as feral animals, those presenting as clinically ill, or intact males allowed to roam outdoors (59, 101, 216, 226, 234, 241, 305, 360, 376, 403). Two reports describing the prevalence of infection in feral domestic cats in England (59) and Vietnam (184) demonstrated that FIVfca can be diagnosed in >25% of certain populations.
Serosurveys have not detected FIV antibodies in cheetahs (261), wildcats (87, 220), five species of South American felids (119), leopard cats (184, 255), civets (184), or sandcats (288), whereas evaluations of wildcats from France (122) and Saudi Arabia (288) demonstrated low levels (<10%) of FIV seropositivity.
In summary, a wide range of feline species demonstrate antibodies which recognize FIV antigens, and nine of these species (lion, cheetah, leopard, Pallas cat, jaguarundi, ocelot, domestic cat, puma, and bobcat) have been shown to harbor species-specific FIVs by evaluation of partial viral genomic sequences. African lion, puma, African leopard, and Pallas cat populations demonstrate very high rates of seropositivity. Other species, including the domestic cat, cheetah, and South American neotropical free-ranging populations, tend to demonstrate seroprevalence rates of 10% or less. Asian species other than the Pallas cat are apparently not infected with an endemic FIV, although when exposed to other species harboring FIVs, particularly during captivity, these animals may become infected, as evidenced by seroconversion (Table 1).
Primate Species with SIV
SIV infections are described below for the major phylogenetic primate radiations, namely, anthromorphic primates (great apes), cercopithecines (guenons), Papionini, and Colobinae (Table 2). A description of the relatedness of these families is discussed later.
Studies of SIV seroprevalence in great apes have revealed SIV in only two subspecies of chimpanzees, i.e., Pan troglodytes troglodytes and P. t. schweinfurthii (75, 128, 268, 302, 303, 323-325, 405). The current view is that HIV-1 originates from SIVcpz.Ptt, which naturally infects the common chimpanzee (P. t. troglodytes), whose range is in West-Central Africa. Studies of hundreds of captive wild-born common chimps from Gabon, Cameroon, and Democratic Republic of Congo resulted in the isolation of five SIVcpz strains (named SIVcpzGAB-1, SIVcpzGAB-2, SIVcpzCAM-3, SIVcpzCAM-5, and SIVcpzCAM) (39, 75, 268, 303). Another case of SIVcpz infection has been identified in a captive chimpanzee in the United States (SIVcpzUS) (128). Therefore, the prevalence of SIVcpz was considered to be very low, although given that primarily juveniles were tested in these surveys, these rates may be underestimates. However, an extensive seroepidemiological study recently conducted in Cameroon, using a noninvasive sampling approach (205), reported a higher seroprevalence (10%). This finding is consistent with the emergence of three HIV-1 groups (M, N, and O) in this region (91, 150, 345, 381). Indeed, by sequencing of these new SIVcpz strains, the circulation of group M- and N-like SIVcpz strains in Southern Cameroon was demonstrated (205).
Until recently, only one isolate had been characterized from eastern chimpanzees (P. t. schweinfurthii), namely, SIVcpzANT (302, 382). Three more cases of SIVcpz infection were recently identified in chimps from a single familial group from National Kibale Park (Uganda) and Gombe National Park (Tanzania) (323-325), indicating that geographical foci of SIVcpz infections can be defined within the area of endemicity (325). Recently, an SIVcpz sequence from an eastern chimp originating from the Kisangani region of Democratic Republic of Congo was shown to cluster together with the other SIVcpz.Pts strains (405).
Despite extensive testing, naturally occurring lentiviruses have not been detected in West African chimpanzees (P. t. verus) or the P. t. vellorosus subspecies, although one P. t. vellorosus chimpanzee contracted SIV from a P. t. troglodytes chimpanzee in captivity (75, 314, 362). Seroepidemiological studies failed to produce evidence of SIV infection in the two other African ape species, gorillas (Gorilla gorilla) (233, 346) and bonobos (Pan paniscus) (387). Given that both of these species are frugivorous, they do not experience interspecies aggression and predation, suggesting that this behavior might be protective against lentiviral infection.
In conclusion, epidemiological data revealed that the prevalence rates of SIVcpz infection in wild chimpanzees are significantly lower than those reported for other species of nonhuman primates and that other great ape species are not infected with lentiviruses.
Guenons with SIV
Due to their number, genetic diversity, and large distribution in sub-Saharan Africa, guenons (tribe Cercopitecini) are the largest reservoir species for SIV. Studies of hundreds of wild-born AGMs (genus Chlorocebus) belonging to different subspecies revealed prevalence rates of SIVagm infection of 40 to 50% (163, 233, 280). These prevalence levels are similar for all four AGM subspecies, i.e., vervet (Chlorocebus pygerythrus), grivet (Chlorocebus aethiops), tantalus (Chlorocebus tantalus), and sabaeus (Chlorocebus sabaeus), and are independent of geographic origin (258). Interestingly, seroepidemiological studies showed that AGMs from Caribbean Islands, which were extensively imported from Africa in the 17th and 18th centuries (92), are not infected with SIV (85, 163). This lack of exposure has been attributed to the capture and movement of these animals as unexposed juveniles; an alternative, less probable explanation is that SIVagm was not yet present in the AGM population 3 centuries ago.
Eight of 14 (57%) l'Hoest monkeys (Cercopithecus lhoesti) originating from the Haut-Congo and Kiwu regions of the DRC were infected with SIVlhoest (27). Prevalence rates of SIV infection in Syke's monkeys (Cercopithecus albogularis) have been reported to be between 28 and 59% for wild-caught or colony-born Syke's monkeys (109, 169, 368). Nine of 14 (64%) blue monkeys (Cercopithecus mitis) tested positive for SIV, and SIVblu has been characterized genetically (154). The prevalence of SIVdeb, which naturally infects de Brazza's monkeys (Cercopithecus neglectus), is relatively high (39%) (2). No prevalence study exists thus far for the SIVs infecting different species of mona monkeys (Cercopithecus mona); however, viruses have been isolated from naturally infected monas (C. mona mona) from Nigeria and Cameroon (21, 76) and from a Dent's monkey (C. mona denti) from Democratic Republic of Congo (88). Of 302 tested mustached monkeys (Cercopithecus cephus), 56 (19%) had cross-reactions with HIV antigens (76). In Cameroon, the prevalence of SIVgsn, the virus that naturally infects greater spot-nosed monkeys (Cercopithecus nictitans), was established at 16% (27/165) (80). However, when a significant number of samples were tested using specific SIVgsn and SIVmus peptides, prevalence rates of 5% and 4%, respectively, were reported (2).
Papionini Tribe Members with SIV
Several studies produced compelling evidence that SMs are infected at high prevalence rates in the wild (25 to 55%) and also suggested that the major route of transmission in this species is sexual (14, 67, 326). Initially, a study of SIVsmm prevalence in feral SMs in Sierra Leone reported a 25% (4/16) prevalence of SIVsmm infection. Three of the four seropositive animals were adults, once again demonstrating an age-related increase in SIV seroprevalence (67). Pet SMs in Sierra Leone and Liberia have a 4 to 8% seroprevalence, probably as a consequence of being separated from feral populations while juvenile (14, 67, 243).
Testing of nine RCMs (Cercocebus torquatus) in Gabon revealed a SIVrcm infection in one (134). A more recent report established a higher SIVrcm prevalence level of 22% (4/18) (346). For a related species, the agile mangabey (Cercocebus agilis), two independent studies produced conflicting results. We characterized two SIVagi strains as being closely related to SIVrcm and concluded that SIVagi may have resulted from cross-species transmission (267). Other authors failed to identify any SIVagi strain following testing of 92 agile mangabeys (2).
Other mangabey species have not been reported to carry species-specific SIVs. A limited number of samples from white-crowned mangabeys (Cercocebus lunulatus) were repeatedly negative for SIV by PCR, despite serological cross-reactivity with both HIV-1 and HIV-2 antigens (C. Apetrei, unpublished data). Sera from three dozen gray-cheeked mangabeys (Lophocebus albigena) did not reveal any seropositivity. No data are available concerning SIV prevalence in Eastern mangabeys, Tana river mangabeys (Cercocebus galeritus), Sanje mangabeys (Cercocebus sanjei), or the newly described highland mangabey (Lophocebus kipunji) (196).
The prevalence of SIVmnd-1 infection in wild MNDs (Mandrillus sphinx) originating from the Lope Reserve in Gabon was 76% (16/21) (353). Age-related differences in prevalence levels have been reported for SIVmnd-1-infected MNDs (353). Moreover, earlier studies reported lower prevalence rates in juvenile MNDs of 9 to 17% (135, 233, 377). A recent study found a high SIVmnd-2 prevalence in MNDs (52%) from Cameroon (2).
To date, there is no proof that baboons (genus Papio) are naturally infected with SIVs. Several studies reported relatively frequent serological reactivities in both ELISA and Western blotting with baboon sera (34, 208, 346). However, no species-specific virus could be recovered from different baboon species. Two baboon species (Papio anubis and Papio hamadryas) have been shown to carry SIVagm from sympatric green monkeys (191).
Colobinae Members with SIV
Seven of 25 (28%) wild-born black and white colobus (Colobus guereza) monkeys from Cameroon were infected with SIVcol (79). Three species of West African colobines have been evaluated for the presence of SIV seroreactivity; 46% (6/13) of animals tested seropositive. Two isolates were characterized from this population, namely, SIVolc from the olive colobus (Procolobus verus) and SIVwrc from the Western red colobus (Piliocolobus badius) (77). To date, there is no study reporting the presence of SIV in Asian species of colobus.
Seroepidemiological surveys of Cercopithecus hamlyni, Allenopithecus nigroviridis, Lophocebus albigena, Cercopithecus pogonias, and Cercopithecus lowei have detected seroantibodies reactive against SIV, but no genetic evaluations have been performed (155, 233, 301).
Comparison of FIV and SIV Distributions
A comparative analysis of feline and simian lentiviral distributions provides interesting parallels. It appears that, like the recent emergence of HIV in humans, domestic cat and chimpanzee lentiviral infections are relatively new diseases, with more limited distribution and lower seroprevalence than infections noted in lions, pumas, AGMs, and SMs. Interestingly, SIV infection has been confined to African species, whereas FIV infection has a worldwide distribution, although the Mongolian Pallas cat is the only Asian species with a unique and endemic strain of FIV (Tables 1 and 2). The reasons underlying this observation are unknown but could include factors such as different migration patterns and home range territories for feline versus primate species, different levels of interspecies aggression, an earlier emergence of FIV than of SIV, or different population dynamics and speciation patterns in the two families. Because the dynamics of lentiviral evolution occurs on a shorter timeline than that of species evolution, these infections may be useful for tracking species dispersion, radiations, and population declines or expansions (40). In populations with high seroprevalence rates, these viruses may drive species evolution, as suggested to occur in regions with high HIV seroprevalence (P. J. Goulder, Abstr. Keystone Symp., Keystone, Colo., abstr. 11, 2006).
PHYLOGENY OF FIV AND SIV
Phylogenetic Clusters of FIVs
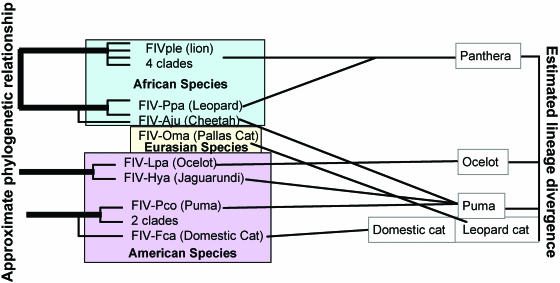
FIV isolates are highly divergent within and among strains but are monophyletic. Partial FIV genome sequences have been identified from 12 different species, which correlate with 10 distinct genotypes. While strains are highly divergent (up to 25% heterogeneity in pol), monophyly within species and clustering within felid families have been demonstrated conclusively (58, 281, 376). Large portions of puma FIVpco genomes have been analyzed comparatively and found to form largely phylogeographic patterns of evolution. However, comparative phylogenetic studies of most other nondomestic cat FIVs have been limited to relatively short sequence comparisons for pol, often with small numbers of animals. This has limited the ability to form deep nodes conclusively relating divisions between FIV subfamilies (40, 41, 53, 58, 376). Thus, while FIVs cluster distinctly from all other lentiviruses, with the closest associations being bovine and equine lentiviruses (281), the exact relationship and origin of primordial FIVs have not been determined. In the most comprehensive phylogenetic study performed to date, nucleotide and amino acid sequence phylogenetic trees were constructed using sequences amplified from seven nondomestic feline species, the spotted hyena, and domestic cats (376). These analyses found that species lineages tended to be most closely related and roughly followed a pattern of phylogeography, particularly when amino acid sequences were compared (Fig. 2).
FIG. 2.
FIVs appear to cluster phylogeographically rather than ancestrally. FIV sequences were analyzed phylogenetically from eight feline species (376) representing five of eight feline lineages (195). Comparison of FIV relationships with ancestral lineages does not demonstrate consistent relationships, as represented by lines drawn between FIVs and their corresponding familial groups. However, FIVs are roughly divided between New and Old World species, with relatively high bootstrap associations, suggesting that recent geography is more highly related to FIV strain relationships than are lineage radiations.
Phylogenetic Clusters of SIVs
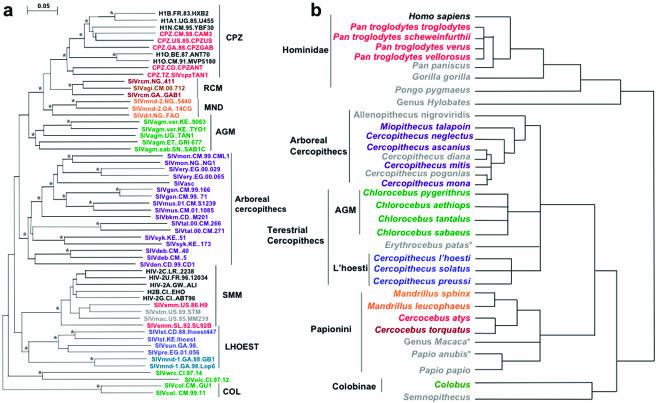
Partial or full-length nucleic acid sequences are available for 34 SIV types, whereas for 23 types there is at least one complete genome sequence. Phylogenetic analyses of the available strains have shown a high level of variation in SIVs and a starburst phylogenetic pattern suggesting evolution from a single ancestor (Fig. 3). The approximate equidistances among the major SIV lineages do not always match the relationships among their hosts. Asian species of Old World monkeys (colobines and macaques), as well as some African species (such as baboons), do not carry species-specific SIVs, which also suggests that the last common ancestor of the catarrhines (Old World monkeys and apes) was not infected by SIV 25 million years ago (29, 143). This suggests that the emergence of SIV followed infection after radiation by these species, possibly from a nonprimate source (334).
FIG. 3.
Comparison between SIV phylogeny (a) and primate phylogeny (b). (a) Neighbor-joining tree constructed from available SIV sequences; (b) primate phylogeny is shown by a schematic using relationships cited in the text. While general alignment of hosts and viruses can be observed, cross-species transmissions and viral recombination events make this correlation less than absolute. Asterisks indicate significant bootstrap values.
Phylogenetic analysis of the available SIV strains is complicated due to sequence diversity and recombination between divergent lineages resulting in different patterns of clustering when different genomic regions are analyzed. Originally, the different clusters were reported as lineages. Classically, the following six such lineages have been described: SIVcpz/HIV-1, SIVsmm/HIV-2, SIVagm, SIVlhoest, SIVsyk, and SIVcol (29, 79, 338). Recent studies have shown that the definition of “pure” versus “recombinant” lineages is primarily a matter of chronology and have suggested that each of the “classical” lineages might in fact be recombinant (322). With the recent discovery of new SIVs, some of the classical lineages were indeed shown to be formed by recombinant strains, with the most notable being the SIVcpz/HIV-1 lineage. The remaining “nonrecombinant” strains cluster into six lineages.
These six phylogenetic lineages are approximately equidistant, with genetic distances of up to 40% in Pol proteins. All six lineages have two or more strains. The l'Hoest lineage is unique in being formed by SIVs circulating in distantly related species. The relationship between these SIV lineages and newly characterized SIVs is complex, such that the characterization of recombinants is limited to the identification of the most obvious mosaic genomes. Table 3 and Fig. 3 demonstrate SIV clusters based on genomic identity. These phylogenetic clusters are partially superimposable on primate phylogenetic trees.
TABLE 3.
SIV clusters based upon phylogenetic relationships
| Cluster | Organism(s) (species) | SIV strain(s) | Comments | Reference(s) |
|---|---|---|---|---|
| 1 | Arboreal guenons (Cercopithecus) | SIVsyk, SIVblu, SIVgsn, SIVdeb, SIVmon, SIVden, SIVmus, SIVasc, SIVtal, SIVery | Ancestral source of SIVcpz/HIV-1 (SIVgsn, SIVmon, SIVmus, and SIVden harbor a vpu gene); lineage formed by all arboreal guenons; partial sequences from SIVbkm from the black mangabey cluster in this lineage | 20, 21, 37, 76, 80, 88, 169, 223, 287, 301, 339, 364, 392 |
| 2 | Sooty mangabey | SIVsmm | Ancestral virus of SIVmac/HIV-2; SMs from Ivory Coast harbor SIVsmm strains related to the epidemic HIV-2 groups A and B; those from Sierra Leone are the sources of HIV-2 groups C to H | 9, 14, 61, 67, 68, 82, 123, 129, 130, 155, 173, 207, 228, 238, 243, 262, 326 |
| 3 | African green monkey | SIVagm (SIVagm.ver, SIVagm.tan, SIVrcm.gri, SIVagm.sab) | Four different SIV subtypes are described for each species in the Chlorocebus genus, suggesting host-dependent evolution; SIVagm.sab is a recombinant between an SIVagm ancestor and a SIVrcm-like virus | 3, 31, 38, 86, 120, 172, 193, 190, 191, 258, 368, 388 |
| 4 | L'Hoest supergroup, mandrill | SIVlhoest, SIVsun, SIVmnd-1, SIVpre | Host-dependent evolution for monkeys in the C. l'hoesti supergroup; cross-species transmission from solatus guenons to mandrills | 27, 28, 166, 353, 377, 378 |
| 5 | Red-capped mangabeys | SIVrcm, SIVagi | Originally considered recombinants, now appear to be pure viruses; SIVagi is cross-species transmitted from RCMs | 31, 135, 143, 339 |
| 6 | Mantled colobus | SIVcol | First virus isolated from Colbinae; other viruses from Western colobus species do not cluster with SIVcol | 79 |
SIV Diversity Relative to Viral Genomic Structure
Using gene and ORF structures as a second method for determining relatedness, three groupings of SIVs can be identified (Fig. 1). All primate lentiviruses harbor five regulatory genes (vif, rev, tat, vpr, and nef) that generally fall in the same regions of the SIV/HIV genome. tat and rev each consists of two exons. The presence of two other regulatory genes (vpx and vpu) is variable and thus defines three patterns of genomic organization, as follows. (i) SIVsyk, SIVasc, SIVdeb, SIVblu, SIVtal, SIVagm, SIVmnd-1, SIVlhoest, SIVsun, and SIVcol contain only five accessory genes (tat, rev, nef, vif, and vpr) (27, 28, 37, 79, 86, 166, 169, 172, 223). (ii) HIV-1, SIVcpz, SIVgsn, SIVmus, SIVmon, and SIVden genomes also include a supplementary gene, vpu (76, 88, 182, 395). (iii) HIV-2, SIVsmm, SIVmac, SIVrcm, SIVmnd-2, and SIVdrl form the third genomic group, which is characterized by the presence of the vpx gene (31, 61, 151, 173, 181, 353). Thus far, vpx appears to be specific for SIVs infecting the Papionini group of monkeys and was acquired following a nonhomologous recombination which resulted in a duplication of the vpr gene (336). SIVblu, SIVolc (77), SIVwrc (77), SIVasc (392), SIVbkm (364), SIVery (P. A. Marx, personal communication), and SIVagi (Apetrei, unpublished results) have not been sequenced completely, so it is not possible to characterize these viruses by gene organization at this time.
In conclusion, SIVs that infect apes contain vpu, whereas Papionini-infecting SIVs contain vpx (11, 15). Three of eight guenon species have been shown to harbor vpu-containing viruses (C. mona, C. mitis, and C. cephus groups). Chlorocebus, C. lhoesti supergroup, and Miopithecus SIVs have an eight-gene organization, whereas Allenopithecus and Erythrocebus have no specific SIVs. This points to the Cercopithecini as the origin of SIVs or at least as the major reservoir of the viruses. Since vpu first appeared in SIVs from cercopithecines, these also appear to be a significant reservoir for viruses in the SIVcpz/HIV-1 lineage (20).
Feline and Primate Speciation and Relationship to Virus Phylogeny
FIV and feline species radiation.
A recent study elegantly demonstrated feline species origins based on an assortment of host genetic markers (195). This analysis distributed the 37 living species into eight major lineages that have radiated over the past 11 million years. A comparison of the feline tree with available FIV phylogeny demonstrates a closer association of recent home territory locations than of species relatedness with respect to FIV similarities (Fig. 2). While significant numbers of partial sequences have been evaluated only from lions, pumas, and domestic cats, limiting the conclusions that can be drawn, it appears that proximate recent geography may be more closely linked to FIV phylogeny than feline ancestry (195, 376). This analysis is complicated by the fact that members of only four of the eight lineages still inhabit the regions of their original establishment; the other lineages (three of which [domestic cat, puma, and panther] contain multiple FIV-positive species) have undergone significant migrations since species radiations occurred (195). Further studies using FIV-versus-host genomic analysis will be useful in determining when these viruses may have appeared relative to species radiations and the relationship between ancestral migrations and FIV evolution.
SIV and primate species radiation.
The Old World monkeys (family Cercopithecidae) are divided into two subfamilies (Cercopithecinae and Colobinae), which also separated 11 million years ago (Fig. 3) (149). Colobus monkeys are a large subfamily common in both Africa and Asia. SIVs have been described only from African colobines. The Cercopithecinae subfamily is further divided into two tribes, the Papionini and Cercopithecini. The Papionini tribe includes the two mangabey genera (Chlorocebus and Lophocebus), baboons (Papio), mandrills and drills (Mandrillus), and gelada (Theropithecus) as well as the Asian genus, Macaca (156). Only African Papionini members have been shown to be natural hosts of SIVs. Finally, there are over 25 guenon species. These include three arboreal genera, Allenopithecus, Miopithecus, and Cercopithecus, and three terrestrial genera, Erythrocebus, Chlorocebus, and the Cercopithecus lhoesti supergroup. Cercopithecini are terrestrial only when circumstances do not favor arboreal survival. Recent studies have defined a clade of terrestrial monkeys exclusive of all other guenons (369-371), indicating that the evolutionary transition between arboreality and terrestriality has occurred only once among the extant lineages (369-371).
This primate classification can be partially correlated with SIV phylogenetic classification (Fig. 1 and 3). Arboreal guenons are infected with viruses sharing biological properties and structural features and forming a single cluster. Conversely, each of the terrestrial genera is infected with specific viral lineages, with the exception of Erythrocebus, which carries a cross-species-transmitted SIVagm (38). Papionini monkeys are infected with related viruses, although a larger proportion of recombinant viruses can be observed in these monkeys (181, 353).
Humans are infected by two different lentiviral types, HIV-1 and HIV-2, belonging to two lineages harboring two different genomic types with different simian origins (see below). In phylogenetic trees, HIV-1 and HIV-2 are dispersed among related SIVs and show no species-specific pattern. Thus, from a phylogenetic point of view, the differentiation between HIVs and SIVs is irrelevant, which is the basis for the argument supporting the simian origin of HIV (333, 335, 337).
SIV phylogenetic lineages continue to become more difficult to superimpose on primate phylogeny as new strains are defined. It may ultimately be more effective to consider the three types of primate lentiviruses based on their genomic organization (i.e., accessory gene structure) (Fig. 1) versus their phylogenetic relationships with other viruses (Fig. 3).
RECOMBINATION BETWEEN VIRUS SPECIES
FIV and SIV Recombinants
Recombination events have been documented to occur during both FIV and SIV infections. This has been recorded during cross-species transmission as well as between different viral subtypes within the same species. Several reports have demonstrated that recombination between FIVfca env variable regions is relatively frequent during natural infection and may give rise to new clade designations. Individuals infected with multiple clade types of FIV (i.e., domestic cat [59, 212] and puma [58]) have been described, implying that infected individuals are not immune to reinfection with different strains of species-specific virus. In one large cohort of African lions, over 40% of FIVple-seropositive individuals were coinfected with two or more distinct and highly divergent FIV clades. In the latter study, evidence of recombination between coinfecting viruses was detected (375).
The most critical recombination of SIVs appears to be that involving SIVgsn and SIVrcm and resulting in the origin of the chimpanzee SIVcpz (20, 339). Subsequent cross-species transmission from chimpanzees to humans created the HIV/AIDS pandemic (75, 128, 182, 188, 302, 303, 323, 325, 326, 381, 405). Other SIVs originating from recombination events include SIVagm.Sab (containing SIVrcm-like fragments) (20, 190) and SIVmnd-2/SIVdrl (mosaic between SIVrcm and SIVmnd-1) (73, 181, 353).
SIVcpz Arose following Recombination between SIVrcm and SIVgsn
Although chimpanzees have been shown to be a reservoir for SIV (327), available data fail to demonstrate that SIVcpz coevolved with its host (314, 327, 362). Therefore, it has been postulated that chimpanzees acquired SIVcpz after their divergence into different subspecies about 1.5 million years ago (339).
Genomic analysis offers strong support that SIVcpz is a recombinant virus. In the 5′ region of the genome, SIVcpz's closest relative is SIVrcm (31), whereas in the 3′ half of its genome, it aligns most closely with SIVgsn (80). SIVgsn was the first monkey virus identified to contain a vpu gene, an accessory gene also found in SIVcpz (80, 182). Initially, SIVrcm and SIVgsn were believed to be recombinant viruses resulting from SIVcpz and unidentified SIV lineages (31, 80); however, recent characterizations of SIVs from other primates (mustached monkeys and mona monkeys) provide evidence of other SIV types similar to SIVgsn (21, 76, 88). Therefore, it appears that the SIVgsn lineage predates SIVcpz, which arose via interstrain recombination events (discussed more completely below).
Thus, in both feline and simian lentiviral infections, virus substrain recombination appears to be a relatively common occurrence and, at least in the case of SIV, may represent an important intrinsic mechanism for the development of strains which may infect new species. The potential for recombination to occur with a relatively high frequency in lentiviral infections provides a mechanism other than rapidly accumulating point mutations for the viruses to adapt to evade host defense mechanisms.
ROUTES OF TRANSMISSION
FIV
FIV seroprevalence rates for domestic cats, lions, and pumas increase as animals reach sexual maturity, suggesting that horizontal transmission is by far the most prevalent mode of transmission (32, 41, 58, 234, 376). The correlation between seropositivity and sexual maturity suggests that mating behavior is associated with exposure, either during the mating act itself or during conspecific interactions occurring around the time of mating. Long-term direct contact appears to be required for efficient transfer of FIV among domestic cats (83). Experimentally, domestic cat FIV can be transmitted via the oronasal, rectal, vaginal, intravenous, intraperitoneal, and subcutaneous routes (32, 56). Domestic cat FIV intrauterine or milk transmission has been documented experimentally, with a frequency that varies with the viral strain (6, 7, 284, 320). Obviously, such conditions are much more difficult to observe or simulate in nondomestic cat lentiviral infections. However, closely related virus sequences have been isolated from mother-cub lion and puma pairs, suggesting that infection can occur via maternal contact (41, 58). The frequency of this observation compared to age-related seroconversion suggests that vertical or maternal lentiviral transmission is the exception rather than the rule.
SIV
Seroepidemiologic surveys of AGMs, SMs, and MNDs revealed higher prevalence levels for adult monkeys than for juveniles, indicating a horizontal route of transmission. A retrospective analysis conducted at Pasteur Institute of Dakar on sera collected between 1967 and 2000 demonstrated that the SIVagm prevalence in adult AGMs was 80%, which is fourfold higher than that in juveniles aged 1 to 3 years (99). This is consistent with an earlier study on vervet monkeys in Awash National Park in Ethiopia, East Africa, in which all animals identified as juveniles by dentition were SIV seronegative (308).
However, in a semifree colony of MNDs at the International Medical Research Center of Franceville, no sexual transmission was found after 16 years of follow-up (74, 113, 135, 353). Two of the founders (one female and one male) had been infected with two different viral types, i.e., SIVmnd-1 and SIVmnd-2, respectively (353). SIVmnd-1 had been transmitted to four offspring (males and females) of the SIVmnd-1-infected female founder. SIVmnd-2 had been transmitted from the infected male founder to four other males following aggressive contacts for dominance (266, 353). Interestingly, two of the dominant males became SIVmnd-2 infected, with no evidence of sexual transmission of this virus being observed. In wild MNDs from the Lope Reserve in Central Gabon, cases of SIVmnd-1 infection could be diagnosed in both sexes (353).
Several cases of horizontal transmission occurring by biting have been described for captive monkeys, including AGMs (99, 308), SMs (315), and chimpanzees belonging to two different subspecies (75). SIVsmm has been reported to be transmitted among macaques by biting (232).
SIV vertical transmission seems less frequent than horizontal transmission, and if it does occur, the point of transmission (in utero, perinatally, or via breast milk) has not been identified. In a recent prospective study, experimental mother-to-offspring transmission by breast-feeding was not observed in MNDs (I. Pandrea, unpublished results), while another study did not demonstrate vertical transmission in AGMs (289). Some recent phylogenetic studies also suggested vertical transmission as a potential mechanism of SIVsmm transmission (14, 326).
Epidemiologic patterns of seroconversion in wild felid and primate populations endemically infected with lentiviruses suggest that these agents are most efficiently transmitted during adult contacts. Considering that it is well established that HIV is spread by sexual contact via primarily mucosal exposure, it is likely that naturally occurring viruses are spread via this route as well. While mother-to-offspring transmissions have been reported for felids and primates, they are relatively rare compared to horizontal transmissions.
CROSS-SPECIES LENTIVIRAL TRANSMISSION
Cases of FIV and SIV infections crossing species barriers have been documented; however, these events appear to be rare, despite ample opportunities for cross-species transmission to occur.
Naturally Occurring Cross-Species Transmission of FIVs
Cross-species transmission of FIVs has been documented primarily for captive nondomestic cats, suggesting that artificially close contact contributed to the opportunity for cross-species transmission. For example, a captive puma in an Argentinian zoo was infected with a domestic cat FIVfca isolate (58), and a captive-born snow leopard and tiger were both infected with a FIVple (lion lentivirus) isolate (376). Interestingly, the latter report represents the only documented infection of either of these species with FIV, suggesting that while these species are not infected in the wild, they are clearly susceptible to FIV infection. One additional report demonstrated that a wild Asian leopard cat became infected with a domestic cat FIV isolate (270). Although these reports document the potential for cross-species transmission of FIV to occur, the overwhelming pattern of infection is for each species to have its own strain or strains of FIV, which map nearly exclusively to that species.
Naturally Occurring Cross-Species Transmission of SIVs
Clear evidence of the cross-species transmission potential of SIVagm has been observed in the wild, where this virus has been isolated from a yellow baboon (Papio cynocephalus) (191), a chacma baboon (Papio ursinus) (388), and a patas monkey (Erythrocebus patas) (38). In Kenya, SIVagm.Ver was transmitted to a white-crowned mangabey (Cercocebus lunulatus) in captivity housed at the same primate center (368). Systematic prevalence studies have not been carried out yet to determine if SIVagm is established as a virus endemic to these species or if the isolation of these strains is the result of unique, accidental transmissions. No long-term follow-up is available for these cases to conclude that SIVagm is pathogenic in African NHP species following cross-species transmission. It is noteworthy that none of these species were reported to date to carry a specific SIV, which may explain their higher susceptibilities to cross-species-transmitted infection and, as described above for FIV, document the susceptibilities of these species to lentiviral infection.
Origins of HIV-1 and HIV-2
Following the discovery in 1986 of HIV-2 (71), a virus which was shown to be closely related to SIVsmm (72), it was rapidly established that this human AIDS virus had a simian origin (66, 129). The discovery of SIVcpz in the area of HIV-1 epidemic emergence pointed to a simian source for HIV-1 as well (128, 303). In 1998, HIV-1 group N was discovered in a Cameroonian patient with AIDS (345). This virus clearly clusters with SIVcpz from Cameroon in parts of the genome, which reinforced the hypothesis that SIVcpz was the ancestor of HIV-1 (259, 321, 345). Altogether, these seminal studies have solved the origins of HIV-1 and HIV-2. However, the mechanism(s) of HIV-1 and HIV-2 emergence in the human population as pathogenic agents is still under debate. Based on results showing the simian origin of HIV, AIDS was postulated to be a zoonosis (reviewed in reference 155). This hypothesis was based on data showing cross-species transmission (128), such as (i) similarities in viral genome organization, (ii) phylogenetic relatedness, (iii) prevalence in the natural host, (iv) geographic coincidence, and (v) plausible routes of transmission. Both SIVsmm/HIV-2 and SIVcpz/HIV-1 fulfill these criteria (66, 128, 155); however, it seems more likely that the emergence of AIDS constitutes a rare occurrence of cross-species transmission versus a readily transmissible zoonosis (12).
In spite of the frequent contact between humans and SIV-infected monkeys in Central and West Africa (301), extensive molecular epidemiologic studies have documented only 10 cross-species transmission events to humans during the last century (11, 15, 155, 333-335). Four of these events resulted in the following epidemic strains: HIV-1 group M, the major group of viruses of the AIDS pandemic (13, 187, 248, 304); group O, which is responsible for approximately 5% of HIV cases in Cameroon (17); and epidemic groups A and B of HIV-2 (81, 129). HIV-1 group N and HIV-2 groups C through G are nearly identical to SIVcpz and SIVsmm, respectively, and are extremely rare in humans, with only seven known HIV-1 group N-infected patients (18, 44, 321) and only single individuals infected by HIV-2 groups C to G (66, 129, 407). Group C to H (66, 82, 129, 407) nonepidemic strains are weakly pathogenic, replicate poorly in infected humans, and are found only within the range of SMs or in persons who emigrated from Western Africa (66, 129). It thus appears that cross-species transmission is not the only requirement for the emergence of HIV and that additional events, such as viral adaptation through serial passages or a lack of host defenses against specific viral strains, may contribute to successful lentiviral adaptation to new host species (12, 106, 242).
Origin of SIVcpz
As described previously, there is strong evidence that recombination between ancestral SIVs found in greater spot-nosed, mustached, and mona monkeys was the source of SIVcpz (21, 76). Four scenarios describing SIVcpz occurrence and emergence are as follows (339): (i) chimpanzees acquired ancestral viruses through hunting, resulting in exposure to more than one SIV type (254, 397), which then recombined in the new host; (ii) the two SIVs that generated SIVcpz were transmitted independently to different chimpanzees and then spread separately in the new host population until coinfection occurred, resulting in SIVcpz; (iii) an anscestral SIV established itself as a chimpanzee virus and, following superinfection with a new SIV, evolved into the SIVcpz present today; and (iv) the recombinant virus was generated in another monkey host species which has yet to be identified and was subsequently transmitted to chimpanzees.
Comparison of naturally occurring cross-species transmissions of either SIV or FIV demonstrates that successful infection is a rare event. In FIV infections, direct and “unnatural” contact, such as that occurring in captivity, seems to be a prerequisite for cross-species transmission events. While evidence points to cross-species transmission events leading to emerging lentiviral diseases in primates, including humans, the reasons for the success of these infections and the factors necessary for disease to result in pathogenicity have not been delineated clearly. Further studies of natural SIV or FIV infections may provide answers to these questions.
PATHOGENICITY OF NATURALLY OCCURRING FIV AND SIV
Pathogenicity of Nondomestic Cat FIV
It is difficult to determine whether FIV or SIV infection of free-ranging species causes significant changes in hematological parameters or other subclinical disease. The stress associated with sample collection can preclude meaningful interpretation of hematological tests, and field samples are often suboptimal for all but the most resilient assays. Epidemiological studies evaluating survival success and reproduction or coinfection/seroconversion rates among lentiviruses and other intercurrent diseases have not revealed statistically significant disadvantages associated with FIV seroconversion (40, 176). Studies of lentiviral pathogenicity in nondomestic felids have been hampered by the fact that for the two most highly studied populations, African lions and North American pumas, age-matched seronegative cohorts are not readily available for comparisons. While it appears that the trend is for naturally occurring infections to be apathogenic for native hosts, several reports implicating both FIV and SIV diseases have been published, typically from captive settings where animals have outlived their “normal” life span. For example, an aged FIVple-positive captive lion developed lymphoma (312), and three aged FIVple-infected lions in the Columbus zoo developed neurological disease reminiscent of HIV-induced encephalitis (51). There is also a trend for FIV-infected lions and pumas to experience CD4+ T-cell depletion, similar to that noted in domestic cats infected with virulent FIV (55, 319). However, functionally measurable negative impacts of infection, such as fecundity and intercurrent disease rates, have not been detected in seropositive lions (176) or pumas (40) in the wild. The trend for seropositive status to increase with age also suggests that lentiviral infection does not hamper survival rates.
FIV Infection of Domestic Cats Causes AIDS
FIV was discovered in 1987 in a cattery in California that had been experiencing high morbidity and mortality of unknown origin (299). Since the initial report, serosurveys of contemporary and banked cat serum samples have revealed that the virus has been present in the domestic cat population since at least 1968 (32), with the current prevalence in the United States ranging from 1 to 15% and approaching 50% in at least one population (59, 279, 379, 408). Feral cats, animals with outdoor access, and animals presented for undiagnosed illness are more likely to be infected than are indoor neutered house cats (32, 234, 265).
Strain variation correlates with the pathogenicity of virus for natural hosts in domestic cat FIV infections (300). Natural and experimental FIV infections in domestic cats result in classical immunodeficiency disease at 3 months to 10 years postinfection. Clinical findings include CD4+ T-cell depletion, weight loss, neurological disease, and opportunistic infections (32, 56). The range of the disease spectrum has been related to different strains of virus; predictably, tissue culture passage tends to result in strains with attenuated infectivity. Several naturally occurring cloned and uncloned isolates used in pathogenesis and vaccine studies are highly to moderately pathogenic, resulting in disease in as little as 4 to 8 weeks postinoculation (97, 98). Strain virulence is generally related to the env-defined clade classification; however, it is likely that other genomic features besides this region are influential in modulating disease outcome. For example, an FIV vif-null mutant was replication defective in vivo relative to wild-type FIV (231).
Pathogenicity of African Primate SIV Infection
For 20 years, it was believed that SIV infections are nonpathogenic in their natural hosts (52, 139, 140, 203, 260, 282, 283, 291, 292, 294, 295, 315, 342-344, 393, 394, 396). This was a major paradox in the context of active viral replication and high prevalence levels. Recent reports have demonstrated that SIV infection in natural hosts can eventually lead to the development of immunodeficiency. However, clinical disease seems to occur in the minority of cases, and only when animals have been infected over long periods of time, as follows. (i) AIDS cases were reported for MNDs infected with SIVmnd-1 and SIVmnd-2 after 17 years of infection (296). In both cases, the clinical diagnosis of AIDS was supported by biological signs, an increase in viral replication, weight loss, and opportunistic infections (296). (ii) An SM naturally infected with SIVsmm progressed to AIDS after an incubation period of 18 years (227). The AIDS diagnosis was supported by biological signs, an increase in viral replication, and lymphoma. (iii) An AGM coinfected with SIVagm and simian T-cell leukemia virus (STLV) was also reported to progress to AIDS, as defined clinically by the presence of opportunistic infections (374).
Moreover, AIDS was reported to develop in African NHPs after infection with heterologous virus, as follows. (i) During leprosy experiments at the Tulane National Primate Research Center (TNPRC), three black mangabeys (BkMs) (Lophocebus aterrimus) were inoculated with lepromatous tissue that had been serially passaged in SIV-positive SMs. Retrospectively, all three BkMs subsequently became SIVsmm infected (8). Virological and histopathological data confirmed that SIV was cleared in two BkMs, whereas the third progressed to AIDS after 5 years. This was the first compelling evidence that direct cross-species transmission of SIV may induce AIDS in heterologous African nonhuman primate species, although clearance of infection also occurred in two of three animals. (ii) Baboons, which are not known to be infected naturally by SIV, developed AIDS following infection with HIV-2 (22). Moreover, an increase in pathogenicity following serial passages of HIV-2 in baboons was recently reported (230). (iii) HIV-1 in chimpanzees is generally asymptomatic, but AIDS has been reported for a subset of chimpanzees (274, 285).
Cases of naturally occurring FIV and SIV infections that progress to AIDS are rare, possibly because host-virus adaptation has occurred, resulting in persistent infections with incubation periods that exceed the normal life spans of naturally infected animals (296). This is supported by the fact that all AIDS cases reported have occurred in monkeys and lions significantly older than the mean life spans of the species.
One of the most interesting parallels between African monkey SIV and nondomestic cat FIV infections is the seeming lack of pathogenicity observed in the vast majority of naturally occurring infections. The mechanisms underlying this apparent host-lentivirus adaptation are numerous and may include (i) effective host control of viral replication, (ii) effective host control of viral pathogenicity, and (iii) failure of the virus to induce immunodeficiency, despite successful persistent infection. Studies defining which of these mechanisms are relevant have been lacking, and conclusive results remain elusive. Research to date into this intriguing observation is discussed below.
VL AS AN INDICATOR OF PATHOGENICITY
For HIV-infected patients, SIVmac/smm-infected macaques, and FIVfca-infected domestic cats, plasma viral loads (VLs) are the best predictors of disease progression (98, 145, 170, 224, 236, 250, 251, 278, 300). Asymptomatic patients show low VLs, while the progression to AIDS, resulting from failure of the immune system to control virus replication or failure of antiretroviral treatments, is always associated with significant increases in the VL (236). The corollary of this conclusion is that immune or therapeutic control of virus should result in low VLs (175, 250), and thus the study of this parameter is of interest for species which have apparently apathogenic lentiviral infections.
FIV VLs during Naturally Occurring Infections
Once established, naturally occurring viral infections of nondomestic cats appear to persist for the life of the host, as evidenced by the ability to reproducibly detect provirus in seropositive individuals over multiple time points. Two studies have evaluated VLs of nondomestic FIVs in their native hosts. The most comprehensive of these (43) demonstrated a range of 30 to 6.7 × 104 copies of provirus/106 PBMC, similar to the proviral burden detected in peripheral lymph nodes (LNs). The proviral load in this cohort was correlated with both the viral strain and host age. Plasma viremia was detected in 21 of 32 samples and averaged 5.7 × 105 virions/ml. Plasma viremia did not correlate with host age, viral strain, or proviral load. In a second study evaluating the tissue distribution of FIVple in a lion that apparently died of FIV-associated encephalitis, proviral loads were extremely low in all tissues evaluated, ranging from undetectable in the cerebellum to approximately 1,000 copies/106 cells in lymphoid organs (51).
SIV VLs during Naturally Occurring Infections
In captive African NHPs naturally infected with SIVs (SIVsmm, SIVagm, SIVmnd-1, and SIVmnd-2), VLs quantified during the chronic phase of infection were higher than those in chronically HIV-1-infected asymptomatic patients (52, 63, 100, 139, 140, 178, 228, 282, 283, 291, 294-297, 315, 342, 343). The only investigation of SIV VLs in wild animals showed similarly high levels of viral replication (295). Longitudinal analyses of the dynamics of plasma viremia in naturally SIV-infected animals suggested that the level of viral replication is relatively constant over time (227, 295, 296). Interestingly, a SIVsmm-infected SM and SIVmnd-1-infected mandrill progressed to AIDS when the set-point level of viral replication was higher than average (227, 296; Apetrei, unpublished data).
Although studies have repeatedly reported that the levels of viral replication are high in African species, some species-specific differences can be observed in comparative studies (297). AGMs naturally infected with SIVagm display a considerably wider range of VLs than those observed in SMs (140, 343, 344). Proviral loads in LN mononuclear cells from AGMs are 100-fold lower than the viral DNA loads observed in naturally infected SM or MND LNs (26, 282, 283). Altogether, these data suggest that VLs in SIVagm-infected AGMs are generally lower than those observed in other African NHP species and that viral replication kinetics may differ among African NHP natural hosts of SIV without significant pathogenic consequences.
A series of recent studies investigated the dynamics of early SIV replication by performing experimental SIV infections in natural hosts, such as SMs, AGMs, and MNDs (100, 139, 210, 282, 283, 291, 294, 343). These studies showed a consistent pattern of SIV VL dynamics consisting of a peak of viremia (106 to 109 copies/ml of plasma) occurring around days 9 to 11 postinfection (100, 139, 210, 282, 283, 291, 294, 343). Peak viremia was followed by a sharp decline (1 to 2 logs) and attainment of a stable level of viral replication (set point), which was maintained during the chronic phase of infection (100, 139, 210, 282, 283, 291, 294, 343). Lower levels of peak viremia (104 to 106 copies/ml) but not of chronic set-point viremia were found after experimental infection of vervets with SIVagm.ver644 (294, 297).
To determine whether viral replication during acute SIV infection of natural hosts is dependent upon the virus or host, cross infections of vervets and sabeus monkeys with SIVagm.sab92018 and SIVagm.ver644 were performed. In this experimental system, the profile of viral replication during primary infection appeared to be dependent on the viral strain, not the host subspecies (294).
In conclusion, while limited studies have been conducted with naturally infected nondomestic cats to measure VLs, evidence from one study suggests that proviral and plasma VLs in pumas are consistent with the high VLs observed in naturally or experimentally infected African monkeys. Thus, it does not appear that the lack of disease in naturally infected animals is associated with effective host containment of viral replication in these species.
RECEPTOR USE AND TISSUE TROPISM RELATING TO PATHOGENICITY
FIV Receptor Use and Tropism
FIV preferentially infects activated T cells in lymphoid organs, with a strain-dependent tropism for target organs. For example, FIVfca strain C-PG appears to preferentially target bone marrow, whereas strain PPR is neurotropic (306, 307). Domestic cat FIV uses the chemokine receptor CXCR4 as an entry receptor and the tumor necrosis factor receptor CD134 (OX40) as a binding receptor. While FIVfca does not use CD4 as a receptor, conformational changes in the V3 region of Env are induced following binding to CD134, allowing entry via CXCR4. This mechanism is similar to the cellular entry binding and internalization described for HIV (93, 94, 340, 401, 402). Also analogous to primate lentivirus receptor usage, the predominant FIVfca quasispecies changes during the course of FIV infection, in that isolates from terminally infected animals have been reported to be CD134 independent (95). The receptors for nondomestic cat FIVs have not been characterized completely but appear to be different from those for domestic cat FIVs (349). Interestingly, a puma FIV isolate targeted gastrointestinal peripheral lymphoid tissues or other sites in a domestic cat infection model (367). Differences in receptor usage and target tissues have obvious implications for the comparative pathology of these viruses.
SIV Receptor Use and Tropism
Most of the SIVs naturally infecting NHPs in Africa have been reported to use the same repertoire of receptors used by HIV-1, i.e., CD4 as a binding receptor and chemokine coreceptors (69, 412). For HIV-1 replication in vivo, the most relevant coreceptors are CCR5 and CXCR4 (256); in 50% of cases, the progression to AIDS is characterized by a switch in viral tropism from R5 (“macrophage” tropic) to X4 (“lymphocyte” tropic) viruses (256). Most of the SIVs naturally infecting African NHPs use CCR5 as the main coreceptor (69, 412). However, for NHPs, no correlation can be established between coreceptor usage and pathogenesis in vivo. Thus, SIVmac strains are R5 viruses, despite the fact that SIVmac is more virulent than HIV-1 (412). SIVmnd-1, SIVagm.sab, and some strains of SIVsmm have been reported to use CXCR4, but no pathological correlation has been described for the host monkey species (290, 294, 331). Moreover, experimental infection of sabeus AGMs with SIVagm.sab does not show a different pattern of viral replication or disease progression from that of other SIV infections in NHP natural hosts (210, 291, 294). SIVrcm uses CCR2b instead of CCR5 or CXCR4 as the coreceptor for viral entry (31, 65). This is a consequence of the fact that the CCR5 gene of RCMs contains a 24-bp deletion in the env binding region (65). As such, this may constitute an example of convergent evolution, similar to the cases of humans who possess the delta-32 mutation in the CCR5 gene and FIV usage of CD134 versus CD4 to gain cellular entry to the analogous subset of cells to those infected by HIV. SIVagi from the agile mangabey also uses CCR2, despite the fact that all agile mangabeys tested thus far do not have a CCR5 gene deletion (267). The close phylogenetic relationship between SIVrcm and SIVagi, which is corroborated by these biological properties, suggests that SIVagi was derived from cross-species transmission of SIVrcm (Apetrei, unpublished observation).
Natural hosts for SIV infection, such as SMs, AGMs, MNDs, and chimpanzees, express lower levels of CCR5 on memory CD4+ T cells in PBMC and mucosal tissues than do immunodeficiency-susceptible hosts, such as macaques, baboons, and humans (292). Moreover, chimpanzees, which are considered a more recent host of SIVcpz, show intermediate levels of CD4+ CCR5+ T cells (292). A hallmark of pathogenic primate lentiviral infections is early and persistent depletion of mucosal memory/activated CD4+ CCR5+ T cells (50, 222, 245, 249, 309, 391), and virus coreceptor usage is a key factor in determining which CD4+ T-cell subsets are depleted. Since CCR5 has been shown to be the main coreceptor used by SIVs in natural hosts (412), African species with endemic naturally occurring SIVs may be less susceptible to pathogenic disease because they have fewer receptor targets for infection (292). This hypothesis would provide an elegant evolutionary mechanism of “pacific coexistence” between SIV replication and natural host immune system function.
While this possibility presents an attractive explanation for species differences in SIV disease expression, the fact that SIV VLs in apathogenic infections are equivalent to pathogenic levels does not support a simple association between the number of susceptible target cells and disease. Moreover, dramatic CD4+ T-cell depletion in the intestine was recently reported for SMs and AGMs during acute SIV infection (142, 293). More studies of the in vivo dynamics of SIVagm and SIVsmm replication are still needed to investigate if the major target cells of these viruses differ from those of SIVmac and HIV.
To summarize, the following parallels can be drawn between FIV and SIV infections with respect to virologic patterns in apathogenic naturally occurring infections. (i) Viral infections persist during the life of the host and may, in fact, cause disease in captive animals that outlive their typical life span. (ii) Although studies with nondomestic cats infected with FIV are lacking, viral replication is chronically detected in animals, despite the fact that the infection is avirulent. (iii) Differences in receptor usage or target cell receptor displays may have evolved over time to favor a more balanced host-virus relationship. It is also apparent that domestic cat FIV infections do not resemble the same epidemiologic, virologic, or clinical pattern noted for SIV and FIV infections in African monkeys and nondomestic cats; in contrast, FIV infections of domestic cats more closely resemble pathogenic HIV/SIVmac infections of humans and macaques.
VIRAL DETERMINANTS OF PATHOGENICITY
As reviewed above and shown in Fig. 1, the structures of the genomes of three FIV species are similar and do not reveal obvious patterns correlating with pathogenicity. The function of FIV vif and its role in viral replication are essential for productive infection and are discussed in further detail below. FIV carries two other accessory genes that have been studied in some detail. FIV, like other nonprimate lentiviruses, carries a dUTPase gene positioned in frame within the pol gene (298). In vitro studies indicated that this gene is responsible for preventing uracil misincorporations during viral replication, and its inactivation results in both in vitro and in vivo attenuation, although tissue infections and pathology are not totally abrogated in dUTPase-negative FIVfca infections (185, 298, 307). A second open reading frame designated orf-A (also called orf-2) encodes a 77-amino-acid accessory protein previously thought to be similar to HIV Tat (132, 133). However, closer characterization has determined that this protein lacks trans-activating properties but is critical in early infection and late particle formation and localizes to the nucleus, which is more reminiscent of Vpr (132, 133, 185). Disruption of Orf-A function results in decreased viral replication and pathogenicity, although productive infection and antibody responses still occur following in vivo infection (132, 133, 185, 186). An Orf-A-defective mutant has been studied as a candidate vaccine for FIV (311).
Several viral factors have been reported to be related to a lack of virulence in naturally occurring SIV infections. SIVmac nef gene deletion mutants were reported to replicate poorly in vivo and to be nonpathogenic in Rh macaques (206). This observation was corroborated by the description of HIV-1-infected long-term progressor nef gene mutations (240). Studies have shown that Nef functions include the ability to downregulate CD4, CD28, and the class I major histocompatibility complex (398), which will result in virus immune evasion (195). Nef may also enhance the responsiveness of T cells to activation (118, 121), but this effect is not uniformly observed among primate lentiviruses (398). However, all SIVs from African monkeys have open reading frames corresponding to a functional Nef, and therefore the nef structure cannot account entirely for differences in pathogenicity. It was recently suggested that Nef proteins from the great majority of primate lentiviruses, including HIV-2, down-modulate T-cell receptor-CD3 in infected T cells in natural hosts, which subsequently blocks T-cell activation. In contrast, Nef proteins derived from HIV-1 and a subset of closely related SIVs do not induce CD3 downregulation, which may have predisposed the simian precursor of HIV-1 to have greater pathogenicity in humans (328). However, simian counterparts of HIV-1, such as SIVcpz or the viruses from the SIVgsn/SIVmon/SIVmus lineage, which do not induce CD3 downregulation, do not typically induce AIDS in their natural hosts. Further, SIVmac, which induces CD3 downregulation, is even more pathogenic in Rh macaques than HIV-1 is in humans. Moreover, FIVfca is immunopathogenic despite the fact that its genome lacks nef, again suggesting that this accessory gene does not solely account for virulence. The accumulation of glycosylation sites in the SIVmac V1 loop or an increase in cellular activation of SIVmac nef allowed viral replication in nonstimulated cells (78, 174, 276, 277, 366). However, these mutations only generate an increase in virulence if all of the other mutations are present, and therefore point mutations themselves cannot be virulence determinants. In summary, while it is clear that Nef plays an important role in HIV/SIV infection and pathogenesis, it alone does not explain the pathogenicities of different lentiviruses for their hosts.
EXPERIMENTAL CROSS-SPECIES TRANSMISSION
The following sections review the literature associated with experimental cross-species transmission of FIV and SIV, as these experimental models have been used to develop animal models for the study of HIV pathogenesis, vaccinology, and interventional strategies and have more recently been used to compare differences in host and virus dynamics in avirulent versus virulent infections. This is followed by an overview of adaptive and innate immune responses associated with disease or protection from disease in naturally occurring and experimentally induced lentiviral infections.
Experimental FIV Cross-Species Transmission
Puma FIV (FIVpco) has been shown in several studies to experimentally infect domestic cats via both the intravenous and oronasal routes (367, 384-386). Following infection, the virus replicates to relatively high proviral titers. The only clinical symptom noted is a transient but marked peripheral lymphadenopathy. The T-cell subsets and immunophenotypic markers evaluated were not altered compared to those in uninfected controls, and proliferation following mitogen stimulation or exposure to an irrelevant antigen was not impaired (367). In oronasally exposed animals, VLs diminished by several logs within 3 months of infection, and in three of four animals the virus was not detectable peripherally after 16 weeks. Rechallenge did not result in exacerbation of the proviral FIVpco load, nor did immunosuppressive doses of corticosteroids. In addition to these findings, FIVpco infection of domestic cats appears to be more concentrated in the gastrointestinal tract than domestic cat FIV infection, which is centered in the thymus and bone marrow (367).
In other studies, prior infection with puma FIV partly abrogated subsequent virulent domestic cat FIV infection; in vitro studies have verified diminished domestic cat FIV replication rates in puma FIV-infected cells (383). This finding is not closely correlated with traditional indicators of immunity, as discussed in more detail below. Lion and Pallas cat FIVs (FIVple and FIVoma, respectively) have been shown to infect domestic cats apathogenically, although the characterization of these infections has not been as extensive as that of FIVpco (23, 385).
Experimental SIV Cross-Species Transmission
Although SIV infection in macaques presently represents the predominant animal model for the study of AIDS, consideration of several factors suggests that this model is not ideal. The clinical course of SIV infection in macaques is significantly more aggressive than that of HIV-1 infection in humans, with a significantly larger proportion of rapid progressors (400). This has hampered the identification of immune correlates of protection and resulted in vaccine study failures. Therefore, an understanding of the circumstances of the emergence of virulent SIV in Asian macaques is needed in order to improve current animal models.
It is a widely held belief that SIVsmm is highly intrinsically pathogenic for several macaque species (246, 262, 275). However, the high pathogenicity of SIVsmm for Rh macaques would be unique to SIVsmm, since other strains of SIV (i.e., SIVcpz, SIVrcm, SIVagm, SIVmnd-2, and SIVsyk) replicate transiently and at relatively low VLs following inoculation of Rh macaques (25, 109, 194, 287, 350, 363). This observation of low intrinsic pathogenicity of direct cross-species-transmitted viruses raises critical questions as to whether the high pathogenic potential of SIVsmm in Rh macaques is a reflection of intrinsic virulence or whether external factors were involved in the adaptation of SIVsmm to different species of macaques, resulting in the pathogenic viruses used in many studies.
SIV Infection of Asian Macaques
The circumstances of SIVsmm epizootic occurrences in macaques are now well characterized, and sources have been traced to SMs in different colonies in U.S. Primate Centers; however, the discovery of SIVs in macaques only occurred when these viruses showed clear pathogenic potential (9, 207, 232, 238). As discussed below, five recent observations strongly support the hypothesis that pathogenic viruses developed as a result of serial passage in macaques rather than from single-exposure cross-species transmission events. Further evidence presented below suggests that following infection of macaques with African SIVs, AIDS may be the exception rather than the rule.
(i) In the early 1980s, leprosy experiments were carried out at TNPRC with SMs. Blood and tissues from a leprous SM were passaged in four Rh macaques; two of these animals subsequently developed AIDS. Blood from one Rh macaque with AIDS was inoculated into six Rh macaques, five of which developed AIDS following a shorter incubation period (6 to 18 months versus 18 to 24 months). A virulent SIVsmmB670 strain was isolated from one animal (RhB670) (144). We recently characterized the natural history of SIVsmm infection in two Rh macaques inoculated with lepromatous SIV-positive SM brain that did not develop AIDS. This analysis demonstrated transient low levels of SIVsmm in one animal and a high plasma VL, which was controlled, in the second (Apetrei, unpublished observations).
(ii) Full-length SIVmac/mne sequences form a tight cluster in the HIV-2/SIVsmm/SIVmac phylogenetic trees which matches viral strains from infected captive SMs from the California National Primate Research Center (CNPRC) (9, 33, 238). SIVstm could also be traced to stump-tailed macaques and SMs from the CNPRC (207, 232). These isolates define the original source of SIVmac251/mne and SIVstm (9).
SIVmac251, the widely used pathogenic virus associated with simian AIDS, was isolated in 1984 at the New England Primate Research Center (NEPRC) (84, 219). The history and derivation of the various SIVmac isolates at the NEPRC revealed that SIVmac251, SIVmac239, and their descendants are all derived from a single SIV-infected Rh macaque shipped from CNPRC in 1970 (238), concurrent with an epizootic of lymphomas in Rh macaques (130). SIVmac infection has been documented retrospectively for a Rh macaque from CNPRC (238). At the NEPRC, the virus circulated undetected in the 1970s, as shown by a retrospective analysis that identified 10 SIV-infected Rh macaques housed at NEPRC between 1975 and 1987, all of which were “naturally” infected while housed in the colony (183, 238). At least five serial passages could be documented for SIVmac251, whereas SIVmac239 resulted from four additional serial passages of SIVmac251 (238).
(iii) SIVsmmF236 originated from a Rh macaque inoculated with a SIVsmm strain derived directly from an SM infected during the leprosy study at TNPRC. The SIVsmmF236 strain induces AIDS, but with a latency period of more than 3 years (171). Molecular clones of SIVsmm236 (168) mirror the pathogenic effects of the parental SIVsmm236 strain (194). More virulent strains (SIVsmm660 and SIVsmm543-3) were derived by further passage in macaques (164, 171).
(iv) SIVsmmPBj14 is perhaps the most acutely pathogenic strain of SIV for pig-tailed macaques (PTMs); this isolate was selected during intentional serial passaging to develop a highly virulent SIV. A PTM was inoculated with tissues from a naturally SIVsmm-infected animal and demonstrated symptoms of AIDS after 14 months of incubation (126). Reinoculation of PBMC from this animal into PTMs induced lethal disease in 1 to 2 weeks. Interestingly, SIVsmmPBj14 is also pathogenic in SMs (124, 126), which is not typically a feature of other macaque-passaged SIVsmm strains (203).
(v) SIVmne also originated at the CNPRC and is highly related to SIVmac251 and SIVmac239, but it is significantly less pathogenic (33). The fact that SIVmne was passaged through Rh macaques fewer times than was SIVmac further supports the serial-passage pathogenicity hypothesis (257).
There is evidence that the emergence of SIVmac at CNPRC may have occurred following kuru experiments carried out in the 1960s (10), as follows. (i) Rh macaques were used as tissue recipients in kuru transmission experiments starting in 1964 (127), 4 years prior to the appearance of Rh macaque AIDS cases. Stump-tailed macaques were included in tissue passage studies starting in 1971 (111), followed in 1975 by an outbreak of lymphomas and opportunistic infections. (ii) We have demonstrated that kuru experiments successfully transmitted SIVsmm between SMs at CNPRC and SMs housed at the New Iberia Primate Center. The SIVsmm lineages were segregated based on their use in kuru research in these analyses, strongly correlating with inadvertent passage of SIV-containing tissues (10). (iii) Animals that received tissues from kuru donors seroconverted to SIV during these experiments. Altogether, these arguments support the hypothesis that serial passage of SIVmac-like strains as a result of in vivo kuru tissue passage experiments preceded the emergence of the highly pathogenic SIVmac strain (10) and that this represents a variation of the normal outcome of SIV cross-species transmissions, namely, aborted or controlled, nonpathogenic infections.
As an additional example of infections of Rh macaques with SIVsmm that do not result in virulent disease, we observed 22 animals following exposure to plasma sampled from SMs chronically infected (infectious dose equivalent to 100,000 copies/ml) with different clades of SIVsmm. These infections were characterized by (i) the absence of rapid progressors, (ii) lower VLs than those noted for SIVsmm infections progressing to AIDS, (iii) a gradual loss of CD4+ T cells, and (iv) initial destruction of the intestinal cells, followed by partial recovery (Apetrei, unpublished observations). Therefore, Rh macaque infections with these primary SIVsmm isolates more faithfully recapitulate the immunopathobiology of HIV-1 in humans and also more closely parallel the infection of domestic cats with unpassaged isolates of puma FIV, as outlined above.
Experimental cross-species transmission of SIVagm to different species of Asian macaques demonstrates various pathogenic outcomes. SIVagm is cleared by the Rh macaque host after replicating to high levels during primary infection (194, 293), whereas SIVagm infection of PTMs was reported to progress to AIDS (141, 167). Overall, PTMs seem to be more susceptible to virulent cross-species SIV infections, as illustrated by the progression to AIDS of PTMs infected with SIVlhoest and SIVsun (30, 166).
Summary: Evidence against Intrinsic Cross-Species Pathogenicity of SIVs
Altogether, these examples illustrate the different outcomes of SIV infection in primates, i.e., (i) a chronic endemic infection which is generally asymptomatic and (ii) a persistent infection with a variable incubation period which progresses to AIDS in those species recently exposed to cross-species-transmitted lentiviral infections after viral adaptation to the new host. This evidence suggests that the pattern of infection seen in domestic cats infected with nondomestic viruses, namely, apathogenic infection which may be cleared, may be the most likely outcome of lentiviral cross-species transmission in NHPs as well. Conversely, the observations made regarding virulent SIV emergence also suggest that serial passage of nondomestic cat lentiviruses in domestic cats may ultimately result in an AIDS-like syndrome.
CD4+ T-CELL DYNAMICS RELATING TO PATHOGENICITY
As noted above, puma virus infection of domestic cats does not result in CD4+ T-cell depletion. Similarly, SIV infection of natural hosts may be associated with normal or even increased CD4+ T-cell regeneration, which eventually plays a key role in determining the lack of disease progression in naturally SIV-infected monkeys. This possibility is intriguing because, for HIV infection, a failure of the lymphoid regenerative capacity has been proposed to be an important factor in the pathogenesis of the immunodeficiency (49, 147, 152, 247, 309, 310). In particular, bone marrow suppression, reduced thymic output, and the loss of naive T cells have all been observed in both FIV- and HIV-infected individuals (103, 104, 162, 239, 318). In marked contrast with this picture, studies of SIV-infected SMs and AGMs showed that the regenerative capacity of the CD4+ T-cell compartment is fully preserved (293, 344). Recent observations illustrate the critical role for interleukin-7 (IL-7)-dependent preserved T-cell regeneration in avoiding CD4+ T-cell depletion and disease progression in SIV-infected SMs (263, 264).
ADAPTIVE IMMUNE RESPONSE AND RELATIONSHIP TO DISEASE
Immune Response during FIV Infection
The antibody response following nondomestic cat FIV infection is generally vigorous, although approximately 20% of animals exhibit an indeterminant antibody response, as evaluated by Western blot analysis (252, 376). This is likely at least partially due to poor cross-reactivity between viral antigens prepared in vitro and naturally occurring viruses, particularly when an antigen from one species is used to detect antibodies to another. Antibody titers in three captive lions naturally infected with FIVple were found to be >1:800 (235). Serum neutralizing titers against lion, puma, and domestic cat FIVs have been observed to be low (in the range of 1:50) and relatively non-cross-reactive against other FIV strains (Fig. 4).
FIG. 4.
FIV neutralizing antibodies are FIV strain specific. Serially diluted sera from domestic cats (cat 1, naïve; cat 2, FIVfca infected, cat 3, FIVfca infected; cat 4, FIVpco infected), pumas (pumas 1 and 2, FIVpco infected), and lions (lion 1, FIVple clade A infected; lion 2, FIVple clade C infected) were tested for the ability to neutralize FIVfca (FIV-C clone) (97), FIVpco (PLV-1695) (367), and FIVple (LLV458) (376). The highest dilutions of sera that neutralized viral growth are indicated. Sera neutralized virus strain-matched FIVs most demonstrably.
Domestic cats infected with FIVfca typically develop measurable T- and B-cell immune responses against viral epitopes, but these responses are ultimately ineffective at controlling viral replication and immunodeficiency (32, 56). Proliferation indices against T-cell mitogens are impaired in domestic cat FIV infection, correlating with marked CD4+ T-cell depletion in infected animals (42). Two studies have noted CD4+ T-cell depletion in captive lions infected with FIVple (55), and this trend has also been noted for free-ranging Florida pumas and African lions (319). Unfortunately, age-matched control animals have not been readily available for these comparative studies, due to the high rate of seroprevalence which occurs in aged populations (41, 43, 58). It has also been difficult thus far to study functional immunological deficits and antiviral immune responses in nondomestic cats infected with FIVs due to the difficulty in collecting appropriate samples, a lack of species-specific diagnostic assays, and a lack of funding to support such studies.
Compensatory expansion of the CD8+βlow T-cell subset has been noted for domestic cats infected with FIVfca and lions naturally infected with FIVple but not for FIVpco-infected pumas (54, 131). Thus, the changes in circulating CD8+ T-cell fractions following FIV infection are not correlated with disease state and/or virulence.
In the puma FIV cross-species transmission model discussed above, neutralizing antibody and cellular responses directed against puma FIV were detected in infected cats, but these responses were relatively meager and were detectable following apparent viral control by the host, suggesting that VL diminution was not correlated with measurable host adaptive immune responses (367).
Immune Response during SIV Infection
The high level of viral replication during steady-state SIV infection in natural hosts is associated with low immunologic pressure (260, 315), i.e., less T-cell proliferation and apoptosis are observed (64, 112, 293, 344), resulting in a limited bystander pathology (295, 344, 390). In nonpathogenic infections, high VLs result mainly from active viral replication, since immunological destruction is ineffective. Thus, natural hosts of SIV are not confronted with the consequence of chronic immune activation, namely, the destruction of infected tissues. This equilibrium may be disrupted following cross-species transmission of viruses, when the virus penetrates a new ecological niche, inducing different immune characters.
Both HIV and SIVmac infections induce immune responses characterized by robust neutralizing and cellular immune responses (48, 96, 316). However, analogous to domestic cat FIV infection, continuous immune escape is the hallmark of HIV/SIV infection in pathogenic models (57). Conversely, for natural infections of African NHPs, the general consensus is that de novo immune responses are muted compared to those in pathogenic models (108, 202). This has led to the hypothesis that the natural host species develop tolerance to the viruses or to specific antigens or epitopes (117, 271, 273) or develop immune responses that differ qualitatively and quantitatively from those observed in pathogenic infections. AGMs, SMs and l'Hoest monkeys generate antibody responses to their respective SIVs; however, the predominant responses are directed to Env rather than Gag, which differs from what is typically noted for Rh macaque SIVmac infection (165, 166, 273). This observation was reproduced experimentally in AGMs and macaques infected with a SIVagm molecular clone (272). While it is unlikely that Gag-specific antibodies per se are harmful, the lack of such antibody responses in naturally infected species suggests differences in the host immune responses that may explain the lack of disease progression in these animals. Another difference lies in the intensity of the response, which appears to be lower in the natural host; for equivalent VLs, SMs have antibody titers that are about 1 log lower than those of Rh macaques (62).
Relatively few studies have investigated the neutralizing antibody activities in African natural hosts of SIVs, and results are generally conflicting. It was initially reported that SIVagm is not neutralized by sera from infected AGMs (273). However, in a more recent study, it was found that specific SIVagm isolates are susceptible to neutralization, depending on the cell line used in the assay (136). In contrast to that of HIV-1, SIVagm infectivity is enhanced by the addition of soluble CD4 (4, 399), and this enhanced infectivity can easily be abrogated by SIVagm-specific antibodies (4), similar to the results of studies performed with FIV utilizing soluble CD134 (95). Replacing the portion of the Env glycoprotein responsible for the coreceptor tropism with the corresponding region of a CXCR4-tropic HIV isolate not only conferred tropism for CXCR4 but also rendered the variant SIVagm strain susceptible to neutralization (209). Neutralizing antibodies are rarely detected in SIV-infected SMs (125), although we recently identified an SIVsmm strain which is highly susceptible to neutralization by both autologous and heterologous sera from SIVsmm-infected SMs (Apetrei, unpublished data). The failure of very large amounts of passively transferred specific immunoglobulin to prevent SIVagm infection suggests that the humoral immune response in AGMs is largely ineffective (272).
In pathogenic HIV and SIV infections, the generation of HIV/SIV-specific cellular immune responses, in particular those mediated by CD8+ T cells, protects hosts from disease progression (146, 329, 330). This hypothesis is supported by several lines of evidence, as follows: (i) experimental CD8 depletion in SIV-infected macaques results in increased viral replication and rapid disease progression (192, 225, 244, 329), (ii) the postpeak decline of viremia in acute HIV/SIV infection is coincidental with the expansion of HIV/SIV-specific cytotoxic T lymphocytes (CTLs) (46, 211), and (iii) immunologic pressure exerted by CTLs results in viral escape mutations (5, 47, 114, 115, 146, 217). Therefore, initial studies postulated that natural hosts for SIV do not progress to AIDS because they are able to exert better immune control of the virus, most probably through CTLs. The main focus of cytotoxic T-cell response characterization was performed with SMs. Clearly, SIVsmm-infected SMs develop CTL responses (108, 204, 396), and CTL escape can also be documented (202), which shows that the CTL responses are functional to some degree in controlling viral replication. It is difficult to compare the strengths of CTL responses in macaques and SMs. However, while SIV-specific T-cell responses can be detected in the majority of naturally SIVsmm-infected SMs, their magnitude is generally lower than what has been described, using the same technique, for HIV-infected patients (36). In addition, no correlation was found between the breadth or magnitude of SIV-specific T-cell responses and either VLs or CD4+ T-cell counts (108). Moreover, the magnitude of the SIV-specific cellular responses did not appear to determine the level of T-cell activation and proliferation in SIVsmm-infected SMs (108). Therefore, it was concluded that the presence of a strong and broadly reactive T-cell response to SIV antigens is not a requirement for the lack of disease progression in SIVsmm-infected SMs; conversely, the complete suppression of SIV-specific T-cell responses (i.e., immunologic tolerance and/or ignorance) is not required for the low levels of T-cell activation that are likely instrumental in the avoidance of AIDS in these animals (108).
Studies to dissect the role of CD8+ T cells in controlling viral replication in SMs and AGMs are in progress. Two independent experiments of CD8+ T-cell depletion in SIVsmm-infected SMs generated controversial results, with one experiment showing a significant increase in viral replication after depletion (Z. Wang, N. Kassis, S. Staprans, M. Elliott, S. P. O'Neil, J. E. Schmitz, K. A. Reimann, H. McClure, P. R. Johnson, and A. Kaur, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 121, 2003), while another showed only minor changes in the pattern of viral replication (A. P. Barry, B. Sumpter, G. Silvestri, S. Staprans, and M. Feinberg, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 120, 2003). Note that in both studies, the anti-CD8 monoclonal antibody treatment used also had the potential to deplete other cell populations, most notably CD8+ NK cells, which may also be involved in the control of viral replication. It is also conceivable that the increase in SIV VLs after anti-CD8 treatment may be partially related to an increase in the availability of activated CD4+ T cells residing in lymphoid or mucosal tissues, as CD4+ T cells activated by exposure to nonself immunoglobulin (i.e., anti-CD8) would serve as highly fertile targets for SIV infection. The proinflammatory signal resulting from the loss of large numbers of CD8+ T cells (in lymphoid tissues), the reactivation of latent viruses (e.g., cytomegalovirus) resulting from the loss of CD8+ T cells, or increased CD4+ T-cell proliferation resulting from an attempt of the T-lymphocyte compartment to restore the overall T-cell homeostasis could also contribute to the rise in VLs observed in these experiments (357).
Efforts to measure SIVagm-specific CTLs using traditional assays have been unsuccessful, due either to reduced activity or to a lack of reagent specificity to detect responses in AGMs. However, there is evidence of massive expansion of CD8+ T cells in infected AGMs (179, 210, 291, 294) and SIVmnd-infected mandrills (282, 283). Although flow cytometric analysis is not specific for the functional specificity of these cells, this observation provides a strong indication that the immune systems of African NHPs are influenced by SIV infection. It is not yet clear if this CD8+ T-cell expansion results from active stimulation of the specific immune response or from a nonspecific stimulation of the immune system in general (272).
The conflicting findings of the levels and characters of neutralizing antibody responses and T-cell subset depletion or expansion in pathogenic versus apathogenic FIV and SIV have not elucidated an obvious adaptive immune response correlating with disease. A more targeted dissection of the roles of antibodies and CTLs in controlling SIV and FIV infection in natural hosts is warranted to clarify this issue (342).
CYTOKINE INDUCTION AND IMMUNE ACTIVATION DURING INFECTION
FIV
Studies investigating domestic cat FIV cytokine profiles have detected an initial increase in IL-10/IL-12 suggestive of a type 2 versus type 1 immune response (16, 89, 90, 404). Cats infected with puma FIV demonstrate persistently high gamma interferon (IFN-γ) mRNA production compared to uninfected controls or domestic cats infected with native FIV, suggesting that puma lentivirus is more likely to direct a cellular versus humoral immune response in domestic cats (J. Terwee and S. VandeWoude, Keystone Symposium on HIV Pathogenesis, Keystone, Colo., 27 March to 2 April 2006). Recent studies have documented preferential FIVfca infection and viral replication in CD4+ CD25+ T cells in vitro and in vivo. This appears to be due to receptor expression as well as cell activation (197-199, 380).
SIV
It was recently reported (210) that the low immune activation levels in AGMs infected with SIVagm are due to a strong induction of transforming growth factor β1 and FOXP3, followed by a significant increase in IL-10 expression, which occurs early in SIVagm infection (210). For HIV-infected humans and SIV-infected Rh macaques, significant increases in beta-chemokine expression have been reported (60, 70) to contribute to nonspecific inflammation and immune activation as well as to high VLs in lymphoid tissues (213). In sharp contrast to pathogenic lentiviral infections, only a transient increase of IFN-γ expression and no changes in the levels of tumor necrosis factor alpha and macrophage inflammatory protein 1α/β expression were observed in SIVagm-infected AGMs (210). These results, combined with the finding of an early increase in the level of CD4+ CD25+ T cells, suggest that SIVagm infection of AGMs is associated with the rapid establishment of an anti-inflammatory environment, which may prevent the host from developing the aberrant chronic T-cell hyperactivation that is correlated with the progression to AIDS during HIV-1 infection (210). CD4+ CD25+ T cells are maintained in chronically infected AGMs and SMs (361).
Taken together, these data further support the hypothesis of a protective role for the downregulation of T-cell activation in natural hosts infected with species-specific SIVs. In particular, it is becoming clear that the establishment of anti-inflammatory profiles of gene expression early in the immune response to SIV antigens, and possibly to puma FIV antigens, is associated with protection against AIDS. These early studies suggest that cytokine and chemokine signaling mechanisms may be key elements differentiating apathogenic from pathogenic FIV and SIV disease and point to another area where study of the natural course of these infections may be informative for pathogenesis and interventional strategy development.
Immune Activation in SIV Infection
Several recent studies performed with SMs, AGMs, and mandrills indicated that both acute and chronic SIV infections of natural hosts are associated with lower levels of T-cell activation, proinflammatory responses, immunopathology, and bystander apoptosis than those for pathogenic HIV/SIV infection (63, 64, 112, 210, 283, 293, 295, 296, 343, 344, 390). The major differences reported for SIVsmm infection in SMs compared to SIVmac infection in Rh macaques were (i) only mild increases in the fraction of proliferating CD4+ T cells in the blood, with normal levels of proliferating CD8+ T cells; (ii) normal levels of proliferating CD4+ and CD8+ T cells in the LNs; (iii) normal production of proinflammatory cytokines by T cells; (iv) a low frequency of apoptotic T cells in the LNs; and (v) normal in vitro susceptibility to apoptosis (344). In addition, SIVsmm-infected SMs seem to maintain a preserved T-cell regenerative capacity, with normal bone marrow morphology and function, normal levels of T-cell receptor excision circle-expressing T cells, and preserved LN architecture (344). A significant inverse correlation between the CD4+ T-cell count and the level of T-cell activation was described for SIVsmm-infected SMs (344).
The kinetics of immune cell activation was investigated in LNs and blood of SIVsmm-infected SMs, SIVagm-infected AGMs, and SIVmnd-2-infected mandrills (203, 210, 283, 293, 343). In all three species, increased numbers of activated CD4+ and CD8+ T cells were detected in blood and LNs at the time of peak viral replication (203, 210, 283, 293, 343). The levels of activated T cells then returned to preinfection values, despite continuously high viremia in the chronic phase of SIV infection. In chronic SIVmnd-1 and SIVsmm infections, there is only a minor increase in CD8+ T-cell activation and no increase in CD4+ T-cell activation (203, 210, 283, 293, 343).
Functional immune assays would be valuable to perform for nondomestic cat FIV infections if acceptable control groups and useful samples can be obtained in order to examine whether T-cell function is diminished during FIV infections.
Comparative studies on SIV pathogenesis in both SMs and Rh macaques showed that when SMs and Rh macaques are infected with the same viral strain (SIVsmm/SIVmac239), high VLs are observed in both species. However, Rh macaques developed chronic immune activation and increased T-cell apoptosis (203, 343), whereas minimal immune activation and T-cell apoptosis were observed in SMs, with these animals remaining clinically normal (203, 343), consistent with the hypothesis that chronic immune activation is a main determinant of disease progression during HIV infection (137, 161, 354). SIVagm-infected macaques develop lymphadenopathy with paracortical and follicular hyperplasia, the progression to AIDS, CD4+ T-cell depletion, and an increase in the number of T cells expressing activation and proliferation markers (165). In contrast, SIVagm-infected AGMs display normal LN morphology without evidence of either hyperplasia or depletion. Activation and proliferation markers are generally not upregulated in natural infections (64, 293, 295, 344, 390). Altogether, these data offer strong proof that the major difference in pathology between natural hosts of SIVs and Asian macaques results indirectly from hyperimmune activation in the latter, which drives excessive activation-induced apoptosis. These observations led to the hypothesis that impaired regeneration of T cells and the steady loss of CD4+ T cells by direct virus-induced cytopathic effects, along with the chronic generalized immune activation seen in HIV infection, contribute to AIDS-associated T-cell depletion (147, 148, 152, 153, 160, 247, 344), disease progression (137, 161, 354), and ultimately the collapse of the immune system and AIDS (49, 102, 105).
Conversely, in nonpathogenic infection of naturally SIV-infected monkeys, downregulation of the immune response favors the preservation of CD4+ T-cell homeostasis.
This scenario would predict that in the few SIV-positive African monkeys that ultimately progress to AIDS, the level of immune activation is higher than that in animals that retain CD4+ T cells. Studies are currently ongoing to test this hypothesis in several models of SIV infection of natural hosts. Ultimately, as low levels of immune activation may also be a consequence, rather than a cause, of a lack of pathogenicity due to other factors, more direct approaches should determine whether artificial enhancement of immune activation results in disease progression in natural hosts for SIV infection.
SUMMARY: IMMUNE RESPONSES AND LENTIVIRAL DISEASE
In summary, there is no evidence that the lack of disease associated with naturally occurring lentiviral infections can be ascribed to an effective adaptive immune response to infection; in contrast, the evidence to date suggests that these apathogenic infections in fact elicit an attenuated immune response, potentially allowing the host to avoid chronic immune stimulation and the eventual exhaustion noted for FIV and HIV infections. One may speculate that host-virus adaptation has resulted in low levels of lentivirus-specific cellular immune responses, resulting in a chronic yet apathogenic infection. This observation emphasizes the tremendous challenge of artificially inducing, with an AIDS vaccine, a type of protective immunity that has not been selected for in many thousands of years of evolutionary pressure posed by retroviruses on the human immune system. Furthermore, studies of the cytokine and chemokine innate protective mechanism and mechanisms for preserving CD4+ T-cell populations suggest that innate immune parameters are critical elements of host-lentiviral adaptation.
INTRACELLULAR RESTRICTION FACTORS LIMITING CROSS-SPECIES LENTIVIRAL TRANSMISSON
Lentiviral species specificity has typically been ascribed to factors such as virus-host receptor compatibility and cellular machinery needed to direct viral replication. Newly classified host factors have been identified that prevent cross-species SIV infections in vitro. Although this field is only in its initial phase of development, data are rapidly accumulating demonstrating new mechanisms by which cross-species transmission of viruses can be blocked effectively in the new host. The viral adaptation required for replicative survival in a new species is highly relevant to pathogenesis and therapeutic intervention in lentiviral disease.
Cytidine Deaminase and vif
Initial studies postulated the role of vif in the species specificity of SIVs, and this accessory gene was also determined to be essential for efficient FIVfca replication (347, 348). The identification of an SIV vif cellular target, a member of the cytidine deaminase APOBEC family, has permitted a better understanding of species restrictions on the emergence of lentiviral infections. The cellular deaminase is incorporated into the lentiviral virion during reverse transcription to direct the deamination of cytidine to uridine on the minus strand of viral DNA (200). This deamination results in catastrophic G-to-A mutations in the viral genome, determining inactivation (157, 215, 237, 411) and/or degradation (348) of the viral genome. Two primate APOBEC family members (APOBEC3G and APOBEC3F) are believed to play a central role in antagonizing viral replication because they are expressed in natural targets of HIV-1 infection, including lymphocytes and macrophages. However, lentiviruses are able to successfully infect and replicate in host target cells containing APOBEC when host-adapted viral Vif interferes with this mechanism. This Vif activity is species specific, i.e., human APOBEC3G is inhibited by HIV-1 Vif but not by SIVagm Vif, whereas AGM APOBEC3G is inhibited by SIVagm Vif but not by HIV-1 Vif. Several recent studies identified the mechanism of this specificity and showed that a single amino acid change can alter the ability of Vif to interfere with APOBEC activity (45, 332, 406). The mechanism of this reaction is complex and is still being investigated intensively.
While the aforementioned studies were dissected using in vitro methodology, recent studies have demonstrated that when domestic cats are infected with puma FIV, the proviral genome progressively accumulates a large error burden with a large proportion of G-to-A mutations, suggestive of enhanced in vivo cytidine deaminase activity, which would be predicted for an infecting virus harboring a nonadapted Vif protein (313). The domestic cat analogue of APOBEC3G has been identified as fe-3 (229); this protein has been shown to exhibit cytidine deaminase properties. Thus, it is conceivable that cross-species transmission of FIVpco to domestic cats is limited by the capacity of puma lentiviral Vif to overcome cytidine deaminase activity. Interestingly, in the same study, selective pressure on specific residues in Pol, but not in other viral structural proteins, was noted. This was unanticipated, since immunological pressures would have been predicted to result in positive selection of Env or Gag residues thought to be important for neutralization of CTL epitopes, and suggests that intracellular lentiviral replication restriction exerts greater selection pressure than host immune surveillance in the establishment of lentiviral infection in a new host species (313).
TRIM5-α
A second cellular restriction factor is represented by the cytoplasmic body component TRIM5-α, which restricts HIV-1 infection of monkey cells (358). TRIM5-α is now considered to be the cellular factor previously referred to as REF-1 or LV-1 and mediates antiretroviral restriction of infection (35, 372, 373). Although the mechanism of antiviral action is not fully understood, it is believed that TRIM5-α interferes with the viral uncoating step that is required to liberate viral nucleic acids into the cytoplasm upon viral binding and fusion with the target cell (138). Sensitivity to TRIM5-α restriction is dictated by a small region in the viral capsid gene which was previously shown to be involved in cyclophilin A binding (372). Therefore, subtle amino acid differences in this region of the capsid influence the strength of binding to TRIM5-α and, hence, the relative sensitivity to its restriction (189, 359). HIV-2, but not the closely related SIVmac strain, is highly susceptible to Rh macaque TRIM5-α (410). Furthermore, HIV-2 was weakly restricted by human TRIM5-α, which may contribute to the lower pathogenic potential of HIV-2 than of HIV-1 in human hosts (410). Such experiments will build strategies to exploit TRIM5-α restriction for intervention with HIV-1 replication (352).
CONCLUSIONS
This extensive review of naturally occurring SIVs and FIVs and their behavior following introduction into nonnative hosts provides the following conclusions: (i) naturally occurring T-tropic lentiviral infections are highly prevalent, productive, and yet apparently avirulent for native hosts; and (ii) judging by the degree of inter- and intrastrain phylogenetic variation, nondomestic FIVs and SIVs are apparently ancient viruses and may have undergone a prolonged period of host-virus adaptation.
Crossing species barriers may result in more virulent strains; however, (i) variants emerging within the primary host may also result in virulence, albeit with an initially longer incubation period; (ii) some cross-species transmissions can result in abortive infections (in which the role of sterilizing immunity is unclear); and (iii) cross-species transmissions and recombinations appear to be rare events but provide lentiviruses with a mechanism to regulate virulence and/or successfully infect new species.
Lentiviral infections of free-ranging African monkeys and many species of nondomestic felids are very successful infections in terms of persistence and prevalence. Ecological and epidemiologic considerations, including high seroprevalence (even in solitary species), viral genomes which are highly genetically divergent (suggesting long-standing infections), and the large number of species which harbor these naturally occurring infections, suggest that infection may confer an evolutionary advantage to the host. It is possible that an altered expression phenotype of susceptible target cells, the immune activation profile, or another effect of chronic viral infection prevents coinfection with pathogens with the capacity to diminish fecundity or successful offspring rearing/survival.
FUTURE DIRECTIONS
This review suggests many avenues for continued research on host-lentivirus determinants of pathogenicity, including (i) evaluation of the implications of receptor tropisms on pathogenicity, infected target cells, and outcomes of infection; (ii) determination of mechanisms underlying peripheral CD4+ immune cell exhaustion during pathogenic infection; (iii) evaluation of the role of intracellular factors in control of viral transcription; (iv) determination of characteristics of nonspecific immune activation/immune dysregulation that contribute to the disease phenotype; (v) determination of the vaccine potential of ancient, apathogenic viruses; (vi) development of therapeutic interventions via enhanced intracellular enzymatic activities, usurping mechanisms developed by hosts over time to avoid highly pathogenic disease; and (vii) evaluation of positive effects of long-term infection on survival/fecundity/resistance to coinfections.
Pathogenic patterns of lentiviral infections in feline species and nonhuman primates are remarkably similar—recalling remarkable similarities observed elsewhere—as illustrated in the following quote by Douglas Adams (1): “It is a curious fact, and one to which no one knows quite how much importance to attach that something like 85% of all known worlds in the Galaxy, be they primitive or highly advanced, have invented a drink called: Jynnan tonnyx, Gel-N-N-T'N-ix, Jinond-o-nicks, Chinanto/mnings, Tzjin-anthony-ks.” Research in the last 20 years has allowed us to discover the lentivirus cocktail. Future research should allow us to discover the recipe.
Acknowledgments
We thank Jennifer Troyer for contributing data used to construct Fig. 4 and Beatrice H. Hahn, Vanessa M. Hirsch, Amitinder Kaur, Michaela Muller-Trutwin, Ivona Pandrea, Mary L. Poss, Julie A. Terwee, Daniel Douek, John Elder, Samuel P. Franklin, Andrew A. Lackner, Preston A. Marx, Richard Onanga, Louis Picker, Sentob Saragosti, Guido Silvestri, Francois Simon, and Donald Sodora for sharing unpublished information and sequences and for helpful discussions of data and hypotheses. We also give thanks to Robin Rodriguez for help with figures and to Helle Bielefeldt-Ohmann for a critical editorial review.
This work was supported by grants R01, AI065325, P20 RR020159 (C.A.), P51 RR000164 (TNPRC), and R01 AI52055 (CSU), from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, from the National Center for Research Resources, and from the Colorado State University College of Veterinary Medicine and Biomedical Sciences Research Council.
REFERENCES
- 1.Adams, D. 2005. The restaurant at the end of the universe, p. 266. In The ultimate hitchhiker's guide. Gramercy Books, New York, N.Y.
- 2.Aghokeng, A. F., W. Liu, F. Bibollet-Ruche, S. Loul, E. Mpoudi-Ngole, C. Laurent, J. M. Mwenda, D. K. Langat, G. K. Chege, H. M. McClure, E. Delaporte, G. M. Shaw, B. H. Hahn, and M. Peeters. 2006. Widely varying SIV prevalence rates in naturally infected primate species from Cameroon. Virology 345:174-189. [DOI] [PubMed] [Google Scholar]
- 3.Allan, J. S., M. Short, M. E. Taylor, S. Su, V. M. Hirsch, P. R. Johnson, G. M. Shaw, and B. H. Hahn. 1991. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J. Virol. 65:2816-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan, J. S., J. Strauss, and D. W. Buck. 1990. Enhancement of SIV infection with soluble receptor molecules. Science 247:1084-1088. [DOI] [PubMed] [Google Scholar]
- 5.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 6.Allison, R. W., and E. A. Hoover. 2003. Covert vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 19:421-434. [DOI] [PubMed] [Google Scholar]
- 7.Allison, R. W., and E. A. Hoover. 2003. Feline immunodeficiency virus is concentrated in milk early in lactation. AIDS Res. Hum. Retrovir. 19:245-253. [DOI] [PubMed] [Google Scholar]
- 8.Apetrei, C., B. Gormus, I. Pandrea, M. Metzger, P. ten Haaft, L. N. Martin, R. Bohm, X. Alvarez, G. Koopman, M. Murphey-Corb, R. S. Veazey, A. A. Lackner, G. Baskin, J. Heeney, and P. A. Marx. 2004. Direct inoculation of simian immunodeficiency virus from sooty mangabeys in black mangabeys (Lophocebus aterrimus): first evidence of AIDS in a heterologous African species and different pathologic outcomes of experimental infection. J. Virol. 78:11506-11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetrei, C., A. Kaur, N. W. Lerche, M. Metzger, I. Pandrea, J. Hardcastle, S. Falkenstein, R. Bohm, J. Koehler, V. Traina-Dorge, T. Williams, S. Staprans, G. Plauche, R. S. Veazey, H. McClure, A. A. Lackner, B. Gormus, D. L. Robertson, and P. A. Marx. 2005. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J. Virol. 79:8991-9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apetrei, C., N. W. Lerche, I. Pandrea, B. Gormus, M. Metzger, G. Silvestri, A. Kaur, R. Bohm, D. L. Robertson, J. Hardcastle, A. A. Lackner, and P. A. Marx. 2006. Kuru experiments triggered the emergence of pathogenic SIVmac. AIDS 20:317-321. [DOI] [PubMed] [Google Scholar]
- 11.Apetrei, C., and P. A. Marx. 2005. African lentiviruses related to HIV. J. Neurovirol. 11(Suppl. 1):33-49. [PubMed] [Google Scholar]
- 12.Apetrei, C., and P. A. Marx. 2004. Simian retroviral infections in human beings. Lancet 364:137-138. [DOI] [PubMed] [Google Scholar]
- 13.Apetrei, C., P. A. Marx, and S. M. Smith. 2004. The evolution of HIV and its consequences. Infect. Dis. Clin. N. Am. 18:369-394. [DOI] [PubMed] [Google Scholar]
- 14.Apetrei, C., M. J. Metzger, D. Robinson, B. Ling, P. T. Telfer, P. Reed, D. L. Robertson, and P. A. Marx. 2005. Detection and partial characterization of new simian immunodeficiency virus (SIVsm) strains from bush meat samples from rural Sierra Leone. J. Virol. 79:2631-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apetrei, C., D. L. Robertson, and P. A. Marx. 2004. The history of SIVs and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 9:225-254. [DOI] [PubMed] [Google Scholar]
- 16.Avery, P. R., and E. A. Hoover. 2004. Gamma interferon/interleukin 10 balance in tissue lymphocytes correlates with down modulation of mucosal feline immunodeficiency virus infection. J. Virol. 78:4011-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayouba, A., P. Mauclere, P. M. Martin, P. Cunin, J. Mfoupouendoun, B. Njinku, S. Souquieres, and F. Simon. 2001. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg. Infect. Dis. 7:466-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayouba, A., S. Souquieres, B. Njinku, P. M. Martin, M. C. Muller-Trutwin, P. Roques, F. Barre-Sinoussi, P. Mauclere, F. Simon, and E. Nerrienet. 2000. HIV-1 group N among HIV-1-seropositive individuals in Cameroon. AIDS 14:2623-2625. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann, M. H., C. Mathiason-Dubard, G. H. Learn, A. G. Rodrigo, D. L. Sodora, P. Mazzetti, E. A. Hoover, and J. I. Mullins. 1997. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 71:4241-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. [DOI] [PubMed] [Google Scholar]
- 21.Barlow, K. L., A. O. Ajao, and J. P. Clewley. 2003. Characterization of a novel simian immunodeficiency virus (SIVmonNG1) genome sequence from a mona monkey (Cercopithecus mona). J. Virol. 77:6879-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett, S. W., K. K. Murthy, B. G. Herndier, and J. A. Levy. 1994. An AIDS-like condition induced in baboons by HIV-2. Science 266:642-646. [DOI] [PubMed] [Google Scholar]
- 23.Barr, M. C., L. Zou, D. L. Holzschu, L. Phillips, F. W. Scott, J. W. Casey, and R. J. Avery. 1995. Isolation of a highly cytopathic lentivirus from a nondomestic cat. J. Virol. 69:7371-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr, M. C., L. Zou, F. Long, W. A. Hoose, and R. J. Avery. 1997. Proviral organization and sequence analysis of feline immunodeficiency virus isolated from a Pallas' cat. Virology 228:84-91. [DOI] [PubMed] [Google Scholar]
- 25.Beer, B., M. Peeters, S. G. Norley, G. van der Groen, L. K. Fransen, H. Willkommen, and R. Kurth. 1995. Failure to infect lower primate species with SIVcpz. AIDS 9:527-528. [PubMed] [Google Scholar]
- 26.Beer, B., J. Scherer, J. zur Megede, S. Norley, M. Baier, and R. Kurth. 1996. Lack of dichotomy between virus load of peripheral blood and lymph nodes during long-term simian immunodeficiency virus infection of African green monkeys. Virology 219:367-375. [DOI] [PubMed] [Google Scholar]
- 27.Beer, B. E., E. Bailes, G. Dapolito, B. J. Campbell, R. M. Goeken, M. K. Axthelm, P. D. Markham, J. Bernard, D. Zagury, G. Franchini, P. M. Sharp, and V. M. Hirsch. 2000. Patterns of genomic sequence diversity among their simian immunodeficiency viruses suggest that L'Hoest monkeys (Cercopithecus lhoesti) are a natural lentivirus reservoir. J. Virol. 74:3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beer, B. E., E. Bailes, R. Goeken, G. Dapolito, C. Coulibaly, S. G. Norley, R. Kurth, J. P. Gautier, A. Gautier-Hion, D. Vallet, P. M. Sharp, and V. M. Hirsch. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734-7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beer, B. E., E. Bailes, P. M. Sharp, and V. M. Hirsch. 1999. Diversity and evolution of primate lentiviruses, p. 460-474. In C. L. Kuiken, B. Foley, B. Hahn, B. Korber, F. McCutchan, P. A. Marx, J. W. Mellors, J. I. Mullins, J. Sodroski, S. Wolinsky, and B. Karber (ed.), Human retroviruses and AIDS 1999. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 30.Beer, B. E., C. R. Brown, S. Whitted, S. Goldstein, R. Goeken, R. Plishka, A. Buckler-White, and V. M. Hirsch. 2005. Immunodeficiency in the absence of high viral load in pig-tailed macaques infected with simian immunodeficiency viruses SIVsun and SIVlhoest. J. Virol. 79:14044-14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beer, B. E., B. T. Foley, C. L. Kuiken, Z. Tooze, R. M. Goeken, C. R. Brown, J. Hu, M. St. Claire, B. T. Korber, and V. M. Hirsch. 2001. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and -NG411). J. Virol. 75:12014-12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendinelli, M., M. Pistello, S. Lombardi, A. Poli, C. Garzelli, D. Matteucci, L. Ceccherini-Nelli, G. Malvaldi, and F. Tozzini. 1995. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 8:87-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benveniste, R. E., L. O. Arthur, C. C. Tsai, R. Sowder, T. D. Copeland, L. E. Henderson, and S. Oroszlan. 1986. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J. Virol. 60:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benveniste, R. E., R. W. Hill, W. B. Knott, C. C. Tsai, L. Kuller, and W. R. Morton. 1993. Detection of serum antibodies in Ethiopian baboons that cross-react with SIV, HTLV-I, and type D retroviral antigens. J. Med. Primatol. 22:124-128. [PubMed] [Google Scholar]
- 35.Berthoux, L., G. J. Towers, C. Gurer, P. Salomoni, P. P. Pandolfi, and J. Luban. 2003. As2O3 enhances retroviral reverse transcription and counteracts Ref1 antiviral activity. J. Virol. 77:3167-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bibollet-Ruche, F., E. Bailes, F. Gao, X. Pourrut, K. L. Barlow, J. Clewley, J. M. Mwenda, D. K. Langat, G. K. Chege, H. M. McClure, E. Mpoudi-Ngole, E. Delaporte, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2004. A new simian immunodeficiency virus lineage (SIVdeb) infecting de Brazza's monkeys (Cercopithecus neglectus): evidence for a Cercopithecus monkey virus clade. J. Virol. 78:7748-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bibollet-Ruche, F., A. Galat-Luong, G. Cuny, P. Sarni-Manchado, G. Galat, J. P. Durand, X. Pourrut, and F. Veas. 1996. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J. Gen. Virol. 77:773-781. [DOI] [PubMed] [Google Scholar]
- 39.Bibollet-Ruche, F., F. Gao, E. Bailes, S. Saragosti, E. Delaporte, M. Peeters, G. M. Shaw, B. H. Hahn, and P. M. Sharp. 2004. Complete genome analysis of one of the earliest SIVcpzPtt strains from Gabon (SIVcpzGAB2). AIDS Res. Hum. Retrovir. 20:1377-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biek, R., A. J. Drummond, and M. Poss. 2006. A virus reveals population structure and recent demographic history of its carnivore host. Science 311:538-541. [DOI] [PubMed] [Google Scholar]
- 41.Biek, R., A. G. Rodrigo, D. Holley, A. Drummond, C. R. Anderson, Jr., H. A. Ross, and M. Poss. 2003. Epidemiology, genetic diversity, and evolution of endemic feline immunodeficiency virus in a population of wild cougars. J. Virol. 77:9578-9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop, S. A., N. A. Williams, T. J. Gruffydd-Jones, D. A. Harbour, and C. R. Stokes. 1992. Impaired T-cell priming and proliferation in cats infected with feline immunodeficiency virus. AIDS 6:287-293. [DOI] [PubMed] [Google Scholar]
- 43.Blake, D. J., J. Graham, and M. L. Poss. 2006. Quantification of feline immunodeficiency virus (FIVpco) in peripheral blood mononuclear cells, lymph nodes and plasma of naturally infected cougars. J. Gen. Virol. 87:967-975. [DOI] [PubMed] [Google Scholar]
- 44.Bodelle, P., A. Vallari, R. Coffey, C. P. McArthur, M. Beyeme, S. G. Devare, G. Schochetman, and C. A. Brennan. 2004. Identification and genomic sequence of an HIV type 1 group N isolate from Cameroon. AIDS Res. Hum. Retrovir. 20:902-908. [DOI] [PubMed] [Google Scholar]
- 45.Bogerd, H. P., B. P. Doehle, H. L. Wiegand, and B. R. Cullen. 2004. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. USA 101:3770-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 48.Brander, C., and B. D. Walker. 2003. Gradual adaptation of HIV to human host populations: good or bad news? Nat. Med. 9:1359-1362. [DOI] [PubMed] [Google Scholar]
- 49.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7:235-239. [DOI] [PubMed] [Google Scholar]
- 50.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brennan, G., M. Podell, R. Wack, S. Kraft, H. Bielefeldt-Ohmann, and S. VandeWoude. Neurologic disease in captive lions (Panthera leo) with low titer lion lentivirus infection. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 52.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 75:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown, E. W., N. Yuhki, C. Packer, and S. J. O'Brien. 1994. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 68:5953-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bucci, J. G., R. V. English, H. L. Jordan, T. A. Childers, M. B. Tompkins, and W. A. Tompkins. 1998. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. J. Infect. Dis. 177:18-25. [DOI] [PubMed] [Google Scholar]
- 55.Bull, M. E., S. Kennedy-Stoskopf, J. F. Levine, M. Loomis, D. G. Gebhard, and W. A. Tompkins. 2003. Evaluation of T lymphocytes in captive african lions (Panthera leo) infected with feline immunodeficiency virus. Am. J. Vet. Res. 64:1293-1300. [DOI] [PubMed] [Google Scholar]
- 56.Burkhard, M. J., and G. A. Dean. 2003. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr. HIV Res. 1:15-29. [DOI] [PubMed] [Google Scholar]
- 57.Burns, D. P., and R. C. Desrosiers. 1994. Envelope sequence variation, neutralizing antibodies, and primate lentivirus persistence. Curr. Top. Microbiol. Immunol. 188:185-219. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter, M. A., E. W. Brown, M. Culver, W. E. Johnson, J. Pecon-Slattery, D. Brousset, and S. J. O'Brien. 1996. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor). J. Virol. 70:6682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carpenter, M. A., E. W. Brown, D. W. MacDonald, and S. J. O'Brien. 1998. Phylogeographic patterns of feline immunodeficiency virus genetic diversity in the domestic cat. Virology 251:234-243. [DOI] [PubMed] [Google Scholar]
- 60.Caufour, P., R. Le Grand, A. Cheret, O. Neildez, F. Theodoro, B. Boson, B. Vaslin, and D. Dormont. 1999. Secretion of beta-chemokines by bronchoalveolar lavage cells during primary infection of macaques inoculated with attenuated nef-deleted or pathogenic simian immunodeficiency virus strain mac251. J. Gen. Virol. 80:767-776. [DOI] [PubMed] [Google Scholar]
- 61.Chakrabarti, L., M. Guyader, M. Alizon, M. D. Daniel, R. C. Desrosiers, P. Tiollais, and P. Sonigo. 1987. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature 328:543-547. [DOI] [PubMed] [Google Scholar]
- 62.Chakrabarti, L. A. 2004. The paradox of simian immunodeficiency virus infection in sooty mangabeys: active viral replication without disease progression. Front. Biosci. 9:521-539. [DOI] [PubMed] [Google Scholar]
- 63.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Age-dependent changes in T cell homeostasis and SIV load in sooty mangabeys. J. Med. Primatol. 29:158-165. [DOI] [PubMed] [Google Scholar]
- 64.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen, Z., D. Kwon, Z. Jin, S. Monard, P. Telfer, M. S. Jones, C. Y. Lu, R. F. Aguilar, D. D. Ho, and P. A. Marx. 1998. Natural infection of a homozygous delta24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J. Exp. Med. 188:2057-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen, Z., A. Luckay, D. L. Sodora, P. Telfer, P. Reed, A. Gettie, J. M. Kanu, R. F. Sadek, J. Yee, D. D. Ho, L. Zhang, and P. A. Marx. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen, Z., P. Telfer, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen, Z., P. Telfer, P. Reed, L. Zhang, A. Getti, D. D. Ho, and P. A. Marx. 1995. Isolation and characterization of the first simian immunodeficiency virus from a feral sooty mangabey (Cercocebus atys) in West Africa. J. Med. Primatol. 24:108-115. [DOI] [PubMed] [Google Scholar]
- 69.Chen, Z., P. Zhou, D. D. Ho, N. R. Landau, and P. A. Marx. 1997. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J. Virol. 71:2705-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheret, A., R. Le Grand, P. Caufour, O. Neildez, F. Matheux, F. Theodoro, B. Vaslin, and D. Dormont. 1999. RANTES, IFN-gamma, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology 255:285-293. [DOI] [PubMed] [Google Scholar]
- 71.Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, et al. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233:343-346. [DOI] [PubMed] [Google Scholar]
- 72.Clavel, F., M. Guyader, D. Guetard, M. Salle, L. Montagnier, and M. Alizon. 1986. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature 324:691-695. [DOI] [PubMed] [Google Scholar]
- 73.Clewley, J. P., J. C. Lewis, D. W. Brown, and E. L. Gadsby. 1998. A novel simian immunodeficiency virus (SIVdrl) pol sequence from the drill monkey, Mandrillus leucophaeus. J. Virol. 72:10305-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper, R., A. Feistner, S. Evans, H. Tsujimoto, and M. Hayami. 1989. A lack of evidence of sexual transmission of a simian immunodeficiency agent in a semifree-ranging group of mandrills. AIDS 3:764. [PubMed] [Google Scholar]
- 75.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Courgnaud, V., B. Abela, X. Pourrut, E. Mpoudi-Ngole, S. Loul, E. Delaporte, and M. Peeters. 2003. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different Cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 77:12523-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Courgnaud, V., P. Formenty, C. Akoua-Koffi, R. Noe, C. Boesch, E. Delaporte, and M. Peeters. 2003. Partial molecular characterization of two simian immunodeficiency viruses (SIV) from African colobids: SIVwrc from Western red colobus (Piliocolobus badius) and SIVolc from olive colobus (Procolobus verus). J. Virol. 77:744-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Courgnaud, V., F. Laure, P. N. Fultz, L. Montagnier, C. Brechot, and P. Sonigo. 1992. Genetic differences accounting for evolution and pathogenicity of simian immunodeficiency virus from a sooty mangabey monkey after cross-species transmission to a pig-tailed macaque. J. Virol. 66:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Courgnaud, V., X. Pourrut, F. Bibollet-Ruche, E. Mpoudi-Ngole, A. Bourgeois, E. Delaporte, and M. Peeters. 2001. Characterization of a novel simian immunodeficiency virus from guereza colobus monkeys (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 75:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Courgnaud, V., M. Salemi, X. Pourrut, E. Mpoudi-Ngole, B. Abela, P. Auzel, F. Bibollet-Ruche, B. Hahn, A. M. Vandamme, E. Delaporte, and M. Peeters. 2002. Characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into simian/human immunodeficiency virus phylogeny. J. Virol. 76:8298-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damond, F., C. Apetrei, D. L. Robertson, S. Souquiere, A. Lepretre, S. Matheron, J. C. Plantier, F. Brun-Vezinet, and F. Simon. 2001. Variability of human immunodeficiency virus type 2 (HIV-2) infecting patients living in France. Virology 280:19-30. [DOI] [PubMed] [Google Scholar]
- 82.Damond, F., M. Worobey, P. Campa, I. Farfara, G. Colin, S. Matheron, F. Brun-Vézinet, D. L. Robertson, and F. Simon. 2004. Identification of a highly divergent HIV-2 and proposal for a change in HIV-2 classification. AIDS Res. Hum. Retrovir. 20:666-672. [DOI] [PubMed] [Google Scholar]
- 83.Dandekar, S., A. M. Beebe, J. Barlough, T. Phillips, J. Elder, M. Torten, and N. Pedersen. 1992. Detection of feline immunodeficiency virus (FIV) nucleic acids in FIV-seronegative cats. J. Virol. 66:4040-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniel, M. D., N. L. Letvin, N. W. King, M. Kannagi, P. K. Sehgal, R. D. Hunt, P. J. Kanki, M. Essex, and R. C. Desrosiers. 1985. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228:1201-1204. [DOI] [PubMed] [Google Scholar]
- 85.Daniel, M. D., N. L. Letvin, P. K. Sehgal, D. K. Schmidt, D. P. Silva, K. R. Solomon, F. S. Hodi, Jr., D. J. Ringler, R. D. Hunt, N. W. King, et al. 1988. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int. J. Cancer 41:601-608. [DOI] [PubMed] [Google Scholar]
- 86.Daniel, M. D., Y. Li, Y. M. Naidu, P. J. Durda, D. K. Schmidt, C. D. Troup, D. P. Silva, J. J. MacKey, H. W. Kestler III, P. K. Sehgal, et al. 1988. Simian immunodeficiency virus from African green monkeys. J. Virol. 62:4123-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daniels, M. J., M. C. Golder, O. Jarrett, and D. W. MacDonald. 1999. Feline viruses in wildcats from Scotland. J. Wildl. Dis. 35:121-124. [DOI] [PubMed] [Google Scholar]
- 88.Dazza, M. C., M. Ekwalanga, M. Nende, K. B. Shamamba, P. Bitshi, D. Paraskevis, and S. Saragosti. 2005. Characterization of a novel vpu-harboring simian immunodeficiency virus from a Dent's mona monkey (Cercopithecus mona denti). J. Virol. 79:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dean, G. A., A. LaVoy, and M. J. Burkhard. 2004. Peptide mapping of feline immunodeficiency virus by IFN-gamma ELISPOT. Vet. Immunol. Immunopathol. 100:49-59. [DOI] [PubMed] [Google Scholar]
- 90.Dean, G. A., and N. C. Pedersen. 1998. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. J. Virol. 72:9436-9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Leys, R., B. Vanderborght, M. Vanden Haesevelde, L. Heyndrickx, A. van Geel, C. Wauters, R. Bernaerts, E. Saman, P. Nijs, B. Willems, et al. 1990. Isolation and partial characterization of an unusual human immunodeficiency retrovirus from two persons of west-central African origin. J. Virol. 64:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Denham, W. W. 1981. History of green monkeys in West Indies: migration from Africa. J. Barbados Museum Historic. Soc. 36:210-229. [Google Scholar]
- 93.de Parseval, A., U. Chatterji, G. Morris, P. Sun, A. J. Olson, and J. H. Elder. 2005. Structural mapping of CD134 residues critical for interaction with feline immunodeficiency virus. Nat. Struct. Mol. Biol. 12:60-66. [DOI] [PubMed] [Google Scholar]
- 94.de Parseval, A., U. Chatterji, P. Sun, and J. H. Elder. 2004. Feline immunodeficiency virus targets activated CD4+ T cells by using CD134 as a binding receptor. Proc. Natl. Acad. Sci. USA 101:13044-13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Parseval, A., C. K. Grant, K. J. Sastry, and J. H. Elder. 2006. Sequential CD134-CXCR4 interactions in feline immunodeficiency virus (FIV): soluble CD134 activates FIV Env for CXCR4-dependent entry and reveals a cryptic neutralization epitope. J. Virol. 80:3088-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Derdeyn, C. A., J. M. Decker, F. Bibollet-Ruche, J. L. Mokili, M. Muldoon, S. A. Denham, M. L. Heil, F. Kasolo, R. Musonda, B. H. Hahn, G. M. Shaw, B. T. Korber, S. Allen, and E. Hunter. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019-2022. [DOI] [PubMed] [Google Scholar]
- 97.de Rozieres, S., C. K. Mathiason, M. R. Rolston, U. Chatterji, E. A. Hoover, and J. H. Elder. 2004. Characterization of a highly pathogenic molecular clone of feline immunodeficiency virus clade C. J. Virol. 78:8971-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diehl, L. J., C. K. Mathiason-Dubard, L. L. O'Neil, L. A. Obert, and E. A. Hoover. 1995. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 69:6149-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diop, O. M., A. Gueye, A. Ayouba, E. Nerrienet, S. Corbet, P. Mauclere, F. Simon, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2002. Simian immunodeficiency viruses and the origin of HIVs, 2nd ed. Kluwer Academic/Plenum Publishers, Dordrecht, The Netherlands.
- 100.Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 74:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dorny, P., N. Speybroeck, S. Verstraete, M. Baeke, A. De Becker, D. Berkvens, and J. Vercruysse. 2002. Serological survey of Toxoplasma gondii, feline immunodeficiency virus and feline leukaemia virus in urban stray cats in Belgium. Vet. Rec. 151:626-629. [DOI] [PubMed] [Google Scholar]
- 102.Douek, D. C. 2003. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 5:172-177. [PubMed] [Google Scholar]
- 103.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167:6663-6668. [DOI] [PubMed] [Google Scholar]
- 104.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 105.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 106.Drucker, E., P. G. Alcabes, and P. A. Marx. 2001. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet 358:1989-1992. [DOI] [PubMed] [Google Scholar]
- 107.Duarte, A., and L. Tavares. 2006. Phylogenetic analysis of Portuguese feline immunodeficiency virus sequences reveals high genetic diversity. Vet. Microbiol. 114:25-33. [DOI] [PubMed] [Google Scholar]
- 108.Dunham, R., P. Pagliardini, S. Gordon, B. Sumpter, J. Engram, A. Moanna, M. Paiardini, J. N. Mandl, B. Lawson, S. Garg, H. M. McClure, H. Xian-Xu, C. Ibegbu, K. Easley, N. Katz, I. Pandrea, C. Apetrei, D. L. Sodora, S. Staprans, M. B. Feinberg, and G. Silvestri. 2006. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emau, P., H. M. McClure, M. Isahakia, J. G. Else, and P. N. Fultz. 1991. Isolation from African Sykes' monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J. Virol. 65:2135-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Endo, Y., M. Uema, R. Miura, K. Tsukiyama-Kohara, H. Tsujimoto, K. Yoneda, and C. Kai. 2004. Prevalence of canine distemper virus, feline immunodeficiency virus and feline leukemia virus in captive African lions (Panthera leo) in Japan. J. Vet. Med. Sci. 66:1587-1589. [DOI] [PubMed] [Google Scholar]
- 111.Espana, C., D. C. Gajdusek, C. J. Gibbs, Jr., B. I. Osburn, D. H. Gribble, G. H. Cardinet, and R. M. Chanock. 1975. Transmission of Creutzfeldt-Jakob disease to the stumptail macaque (Macaca arctoides). Proc. Soc. Exp. Biol. Med. 149:723-724. [DOI] [PubMed] [Google Scholar]
- 112.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Estaquier, J., M. Peeters, L. Bedjabaga, C. Honore, P. Bussi, A. Dixson, and E. Delaporte. 1991. Prevalence and transmission of simian immunodeficiency virus and simian T-cell leukemia virus in a semi-free-range breeding colony of mandrills in Gabon. AIDS 5:1385-1386. [DOI] [PubMed] [Google Scholar]
- 114.Evans, D. T., and R. C. Desrosiers. 2001. Immune evasion strategies of the primate lentiviruses. Immunol. Rev. 183:141-158. [DOI] [PubMed] [Google Scholar]
- 115.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 116.Evermann, J. F., W. J. Foreyt, B. Hall, and A. J. McKeirnan. 1997. Occurrence of puma lentivirus infection in cougars from Washington. J. Wildl. Dis. 33:316-320. [DOI] [PubMed] [Google Scholar]
- 117.Feinberg, M. 2002. Ignorance is bliss: how natural hosts for SIV remain healthy despite long-term high level virus replication. J. Hum. Virol. 5:564. [Google Scholar]
- 118.Fenard, D., W. Yonemoto, C. de Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J. Immunol. 175:6050-6057. [DOI] [PubMed] [Google Scholar]
- 119.Filoni, C., C. H. Adania, E. L. Durigon, and J. L. Catao-Dias. 2003. Serosurvey for feline leukemia virus and lentiviruses in captive small neotropic felids in Sao Paulo state, Brazil. J. Zoo Wildl. Med. 34:65-68. [DOI] [PubMed] [Google Scholar]
- 120.Fomsgaard, A., V. M. Hirsch, J. S. Allan, and P. R. Johnson. 1991. A highly divergent proviral DNA clone of SIV from a distinct species of African green monkey. Virology 182:397-402. [DOI] [PubMed] [Google Scholar]
- 121.Fortin, J. F., C. Barat, Y. Beausejour, B. Barbeau, and M. J. Tremblay. 2004. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-kappaB, and AP-1 induction. J. Biol. Chem. 279:39520-39531. [DOI] [PubMed] [Google Scholar]
- 122.Fromont, E., A. Sager, F. Leger, F. Bourguemestre, E. Jouquelet, P. Stahl, D. Pontier, and M. Artois. 2000. Prevalence and pathogenicity of retroviruses in wildcats in France. Vet. Rec. 146:317-319. [DOI] [PubMed] [Google Scholar]
- 123.Fultz, P. N., H. M. McClure, D. C. Anderson, R. B. Swenson, R. Anand, and A. Srinivasan. 1986. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc. Natl. Acad. Sci. USA 83:5286-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fultz, P. N., H. M. McClure, D. C. Anderson, and W. M. Switzer. 1989. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res. Hum. Retrovir. 5:397-409. [DOI] [PubMed] [Google Scholar]
- 125.Fultz, P. N., R. B. Stricker, H. M. McClure, D. C. Anderson, W. M. Switzer, and C. Horaist. 1990. Humoral response to SIV/SMM infection in macaque and mangabey monkeys. J. Acquir. Immune Defic. Syndr. 3:319-329. [PubMed] [Google Scholar]
- 126.Fultz, P. N., and P. M. Zack. 1994. Unique lentivirus-host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 32:205-225. [DOI] [PubMed] [Google Scholar]
- 127.Gajdusek, D. C., and C. J. Gibbs, Jr. 1972. Transmission of kuru from man to rhesus monkey (Macaca mulatta) 8 and one-half years after inoculation. Nature 240:351. [DOI] [PubMed] [Google Scholar]
- 128.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 129.Gao, F., L. Yue, D. L. Robertson, S. C. Hill, H. Hui, R. J. Biggar, A. E. Neequaye, T. M. Whelan, D. D. Ho, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433-7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gardner, M. B. 1996. The history of simian AIDS. J. Med. Primatol. 25:148-157. [DOI] [PubMed] [Google Scholar]
- 131.Gebhard, D. H., J. L. Dow, T. A. Childers, J. I. Alvelo, M. B. Tompkins, and W. A. Tompkins. 1999. Progressive expansion of an L-selectin-negative CD8 cell with anti-feline immunodeficiency virus (FIV) suppressor function in the circulation of FIV-infected cats. J. Infect. Dis. 180:1503-1513. [DOI] [PubMed] [Google Scholar]
- 132.Gemeniano, M. C., E. T. Sawai, C. M. Leutenegger, and E. E. Sparger. 2003. Feline immunodeficiency virus ORF-A is required for virus particle formation and virus infectivity. J. Virol. 77:8819-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gemeniano, M. C., E. T. Sawai, and E. E. Sparger. 2004. Feline immunodeficiency virus Orf-A localizes to the nucleus and induces cell cycle arrest. Virology 325:167-174. [DOI] [PubMed] [Google Scholar]
- 134.Georges-Courbot, M. C., C. Y. Lu, M. Makuwa, P. Telfer, R. Onanga, G. Dubreuil, Z. Chen, S. M. Smith, A. Georges, F. Gao, B. H. Hahn, and P. A. Marx. 1998. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J. Virol. 72:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Georges-Courbot, M. C., P. Moisson, E. Leroy, A. M. Pingard, E. Nerrienet, G. Dubreuil, E. J. Wickings, F. Debels, I. Bedjabaga, V. Poaty-Mavoungou, N. T. Hahn, and A. J. Georges. 1996. Occurrence and frequency of transmission of naturally occurring simian retroviral infections (SIV, STLV, and SRV) at the CIRMF Primate Center, Gabon. J. Med. Primatol. 25:313-326. [DOI] [PubMed] [Google Scholar]
- 136.Gicheru, M. M., M. Otsyula, P. Spearman, B. S. Graham, C. J. Miller, H. L. Robinson, N. L. Haigwood, and D. C. Montefiori. 1999. Neutralizing antibody responses in Africa green monkeys naturally infected with simian immunodeficiency virus (SIVagm). J. Med. Primatol. 28:97-104. [DOI] [PubMed] [Google Scholar]
- 137.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 138.Goff, S. P. 2004. HIV: replication trimmed back. Nature 427:791-793. [DOI] [PubMed] [Google Scholar]
- 139.Goldstein, S., C. R. Brown, I. Ourmanov, I. Pandrea, A. Buckler-White, C. Erb, J. S. Nandi, G. J. Foster, P. Autissier, J. E. Schmitz, and V. M. Hirsch. 2006. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J. Virol. 80:4868-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldstein, S., I. Ourmanov, C. R. Brown, B. E. Beer, W. R. Elkins, R. Plishka, A. Buckler-White, and V. M. Hirsch. 2000. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J. Virol. 74:11744-11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goldstein, S., I. Ourmanov, C. R. Brown, R. Plishka, A. Buckler-White, R. Byrum, and V. M. Hirsch. 2005. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J. Virol. 79:5153-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gordon, S., N. R. Klatt, J. M. Milush, J. Engram, R. M. Dunham, M. Paiardini, E. A. Strobert, C. Apetrei, I. Pandrea, S. I. Staprans, D. L. Sodora, and G. Silvestri. Severe depletion of mucosal CD4+ T cells in AIDS-free SIV-infected sooty mangabeys. Submitted for publication.
- 143.Gordon, S., I. Pandrea, R. Dunham, C. Apetrei, and G. Silvestri. 2005. The call of the wild: what can be learned from studies of SIV infection of natural hosts?, p. 2-29. In T. Leitner, B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. Korber (ed.), HIV sequence compendium 2004. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 144.Gormus, B. J., L. N. Martin, and G. B. Baskin. 2004. A brief history of the discovery of natural simian immunodeficiency virus (SIV) infections in captive sooty mangabey monkeys. Front. Biosci. 9:216-224. [DOI] [PubMed] [Google Scholar]
- 145.Goto, Y., Y. Nishimura, K. Baba, T. Mizuno, Y. Endo, K. Masuda, K. Ohno, and H. Tsujimoto. 2002. Association of plasma viral RNA load with prognosis in cats naturally infected with feline immunodeficiency virus. J. Virol. 76:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630-640. [DOI] [PubMed] [Google Scholar]
- 147.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 148.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 149.Groves, C. 2001. Primate taxonomy. Smithsonian Institution Press, Washington, D.C.
- 150.Gurtler, L. G., P. H. Hauser, J. Eberle, A. von Brunn, S. Knapp, L. Zekeng, J. M. Tsague, and L. Kaptue. 1994. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J. Virol. 68:1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guyader, M., M. Emerman, P. Sonigo, F. Clavel, L. Montagnier, and M. Alizon. 1987. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature 326:662-669. [DOI] [PubMed] [Google Scholar]
- 152.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783-792. [DOI] [PubMed] [Google Scholar]
- 153.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 154.Hahn, B. 2002. Bernard Fields Memorial Lecture: SIV reservoirs and human zoonotic risk, abstr. L1. Presented at the 9th Conference on Retroviruses and Opportunistic Infections, Seattle, Wash.
- 155.Hahn, B. H., G. M. Shaw, K. M. De Cock, and P. M. Sharp. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607-614. [DOI] [PubMed] [Google Scholar]
- 156.Harris, E. E., and T. R. Disotell. 1998. Nuclear gene trees and the phylogenetic relationship of the mangabeys (Primates: Papionini). Mol. Biol. Evol. 15:892-900. [DOI] [PubMed] [Google Scholar]
- 157.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 158.Harrison, T. M., J. K. Mazet, K. E. Holekamp, E. Dubovi, A. L. Engh, K. Nelson, R. C. Van Horn, and L. Munson. 2004. Antibodies to canine and feline viruses in spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve. J. Wildl. Dis. 40:1-10. [DOI] [PubMed] [Google Scholar]
- 159.Hartmann, K., R. M. Werner, H. Egberink, and O. Jarrett. 2001. Comparison of six in-house tests for the rapid diagnosis of feline immunodeficiency and feline leukaemia virus infections. Vet. Rec. 149:317-320. [DOI] [PubMed] [Google Scholar]
- 160.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 161.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 162.Hellerstein, M., M. B. Hanley, D. Cesar, S. Siler, C. Papageorgopoulos, E. Wieder, D. Schmidt, R. Hoh, R. Neese, D. Macallan, S. Deeks, and J. M. McCune. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5:83-89. [DOI] [PubMed] [Google Scholar]
- 163.Hendry, R. M., M. A. Wells, M. A. Phelan, A. L. Schneider, J. S. Epstein, and G. V. Quinnan. 1986. Antibodies to simian immunodeficiency virus in African green monkeys in Africa in 1957-62. Lancet ii:455. [DOI] [PubMed] [Google Scholar]
- 164.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hirsch, V. M. 2004. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 6:40-53. [PubMed] [Google Scholar]
- 166.Hirsch, V. M., B. J. Campbell, E. Bailes, R. Goeken, C. Brown, W. R. Elkins, M. Axthelm, M. Murphey-Corb, and P. M. Sharp. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J. Virol. 73:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hirsch, V. M., G. Dapolito, P. R. Johnson, W. R. Elkins, W. T. London, R. J. Montali, S. Goldstein, and C. Brown. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hirsch, V. M., G. Dapolito, C. McGann, R. A. Olmsted, R. H. Purcell, and P. R. Johnson. 1989. Molecular cloning of SIV from sooty mangabey monkeys. J. Med. Primatol. 18:279-285. [PubMed] [Google Scholar]
- 169.Hirsch, V. M., G. A. Dapolito, S. Goldstein, H. McClure, P. Emau, P. N. Fultz, M. Isahakia, R. Lenroot, G. Myers, and P. R. Johnson. 1993. A distinct African lentivirus from Sykes' monkeys. J. Virol. 67:1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32:183-203. [DOI] [PubMed] [Google Scholar]
- 172.Hirsch, V. M., C. McGann, G. Dapolito, S. Goldstein, A. Ogen-Odoi, B. Biryawaho, T. Lakwo, and P. R. Johnson. 1993. Identification of a new subgroup of SIVagm in tantalus monkeys. Virology 197:426-430. [DOI] [PubMed] [Google Scholar]
- 173.Hirsch, V. M., R. A. Olmsted, M. Murphey-Corb, R. H. Purcell, and P. R. Johnson. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389-392. [DOI] [PubMed] [Google Scholar]
- 174.Hirsch, V. M., M. E. Sharkey, C. R. Brown, B. Brichacek, S. Goldstein, J. Wakefield, R. Byrum, W. R. Elkins, B. H. Hahn, J. D. Lifson, and M. Stevenson. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ho, D. D. 1995. Time to hit HIV, early and hard. N. Engl. J. Med. 333:450-451. [DOI] [PubMed] [Google Scholar]
- 176.Hofmann-Lehmann, R., D. Fehr, M. Grob, M. Elgizoli, C. Packer, J. S. Martenson, S. J. O'Brien, and H. Lutz. 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 3:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hohdatsu, T., H. Hirabayashi, K. Motokawa, and H. Koyama. 1996. Comparative study of the cell tropism of feline immunodeficiency virus isolates of subtypes A, B and D classified on the basis of the env gene V3-V5 sequence. J. Gen. Virol. 77:93-100. [DOI] [PubMed] [Google Scholar]
- 178.Holzammer, S., E. Holznagel, A. Kaul, R. Kurth, and S. Norley. 2001. High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology 283:324-331. [DOI] [PubMed] [Google Scholar]
- 179.Holznagel, E., S. Norley, S. Holzammer, C. Coulibaly, and R. Kurth. 2002. Immunological changes in simian immunodeficiency virus (SIVagm)-infected African green monkeys (AGM): expanded cytotoxic T lymphocyte, natural killer and B cell subsets in the natural host of SIV(agm). J. Gen. Virol. 83:631-640. [DOI] [PubMed] [Google Scholar]
- 180.Hosie, M. J., T. Dunsford, D. Klein, B. J. Willett, C. Cannon, R. Osborne, J. Macdonald, N. Spibey, N. Mackay, O. Jarrett, and J. C. Neil. 2000. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus. J. Virol. 74:9403-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu, J., W. M. Switzer, B. T. Foley, D. L. Robertson, R. M. Goeken, B. T. Korber, V. M. Hirsch, and B. E. Beer. 2003. Characterization and comparison of recombinant simian immunodeficiency virus from drill (Mandrillus leucophaeus) and mandrill (Mandrillus sphinx) isolates. J. Virol. 77:4867-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huet, T., R. Cheynier, A. Meyerhans, G. Roelants, and S. Wain-Hobson. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345:356-359. [DOI] [PubMed] [Google Scholar]
- 183.Hunt, R. D., B. J. Blake, L. V. Chalifoux, P. K. Sehgal, N. W. King, and N. L. Letvin. 1983. Transmission of naturally occurring lymphoma in macaque monkeys. Proc. Natl. Acad. Sci. USA 80:5085-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ikeda, Y., T. Miyazawa, K. Nakamura, R. Naito, Y. Inoshima, K. C. Tung, W. M. Lee, M. C. Chen, T. F. Kuo, J. A. Lin, and T. Mikami. 1999. Serosurvey for selected virus infections of wild carnivores in Taiwan and Vietnam. J. Wildl. Dis. 35:578-581. [DOI] [PubMed] [Google Scholar]
- 185.Inoshima, Y., T. Miyazawa, and T. Mikami. 1998. In vivo functions of the auxiliary genes and regulatory elements of feline immunodeficiency virus. Vet. Microbiol. 60:141-153. [DOI] [PubMed] [Google Scholar]
- 186.Inoshima, Y., T. Miyazawa, and T. Mikami. 1998. The roles of vif and ORF-A genes and AP-1 binding site in in vivo replication of feline immunodeficiency virus. Arch. Virol. 143:789-795. [DOI] [PubMed] [Google Scholar]
- 187.Janssens, W., A. Buve, and J. N. Nkengasong. 1997. The puzzle of HIV-1 subtypes in Africa. AIDS 11:705-712. [DOI] [PubMed] [Google Scholar]
- 188.Janssens, W., K. Fransen, M. Peeters, L. Heyndrickx, J. Motte, L. Bedjabaga, E. Delaporte, P. Piot, and G. van der Groen. 1994. Phylogenetic analysis of a new chimpanzee lentivirus SIVcpz-gab2 from a wild-captured chimpanzee from Gabon. AIDS Res. Hum. Retrovir. 10:1191-1192. [DOI] [PubMed] [Google Scholar]
- 189.Javanbakht, H., F. Diaz-Griffero, M. Stremlau, Z. Si, and J. Sodroski. 2005. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J. Biol. Chem. 280:26933-26940. [DOI] [PubMed] [Google Scholar]
- 190.Jin, M. J., H. Hui, D. L. Robertson, M. C. Muller, F. Barre-Sinoussi, V. M. Hirsch, J. S. Allan, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Mosaic genome structure of simian immunodeficiency virus from west African green monkeys. EMBO J. 13:2935-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin, M. J., J. Rogers, J. E. Phillips-Conroy, J. S. Allan, R. C. Desrosiers, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J. Virol. 68:8454-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnson, P. R., A. Fomsgaard, J. Allan, M. Gravell, W. T. London, R. A. Olmsted, and V. M. Hirsch. 1990. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J. Virol. 64:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johnson, P. R., S. Goldstein, W. T. London, A. Fomsgaard, and V. M. Hirsch. 1990. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and African green monkeys. J. Med. Primatol. 19:279-286. [PubMed] [Google Scholar]
- 195.Johnson, W. E., E. Eizirik, J. Pecon-Slattery, W. J. Murphy, A. Antunes, E. Teeling, and S. J. O'Brien. 2006. The late Miocene radiation of modern Felidae: a genetic assessment. Science 311:73-77. [DOI] [PubMed] [Google Scholar]
- 196.Jones, T., C. L. Ehardt, T. M. Butynski, T. R. Davenport, N. E. Mpunga, S. J. Machaga, and D. W. De Luca. 2005. The highland mangabey Lophocebus kipunji: a new species of African monkey. Science 308:1161-1164. [DOI] [PubMed] [Google Scholar]
- 197.Joshi, A., H. Garg, M. B. Tompkins, and W. A. Tompkins. 2005. Different thresholds of T cell activation regulate FIV infection of CD4+CD25+ and CD4+CD25− cells. Virology 335:212-221. [DOI] [PubMed] [Google Scholar]
- 198.Joshi, A., H. Garg, M. B. Tompkins, and W. A. Tompkins. 2005. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates with both surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J. Virol. 79:4965-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Joshi, A., T. W. Vahlenkamp, H. Garg, W. A. Tompkins, and M. B. Tompkins. 2004. Preferential replication of FIV in activated CD4+CD25+ T cells independent of cellular proliferation. Virology 321:307-322. [DOI] [PubMed] [Google Scholar]
- 200.Kaiser, S. M., and M. Emerman. 2004. Controlling lentiviruses: single amino acid changes can determine specificity. Proc. Natl. Acad. Sci. USA 101:3725-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kania, S. A., M. A. Kennedy, and L. N. Potgieter. 1997. Serologic reactivity using conserved envelope epitopes in feline lentivirus-infected felids. J. Vet. Diagn. Investig. 9:125-129. [DOI] [PubMed] [Google Scholar]
- 202.Kaur, A., L. Alexander, S. I. Staprans, L. Denekamp, C. L. Hale, H. M. McClure, M. B. Feinberg, R. C. Desrosiers, and R. P. Johnson. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur. J. Immunol. 31:3207-3217. [DOI] [PubMed] [Google Scholar]
- 203.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J. Virol. 72:9597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kaur, A., J. Yang, D. Hempel, L. Gritz, G. P. Mazzara, H. McClure, and R. P. Johnson. 2000. Identification of multiple simian immunodeficiency virus (SIV)-specific CTL epitopes in sooty mangabeys with natural and experimentally acquired SIV infection. J. Immunol. 164:934-943. [DOI] [PubMed] [Google Scholar]
- 205.Keele, B. F., F. Van Heuverswyn, Y. Li, E. Bailes, J. Takehisa, M. L. Santiago, F. Bibollet-Ruche, Y. Chen, L. V. Wain, F. Liegeois, S. Loul, E. Mpoudi Ngole, Y. Bienvenue, E. Delaporte, J. F. Brookfield, P. M. Sharp, G. M. Shaw, M. Peeters, and B. H. Hahn. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 206.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 207.Khan, A. S., T. A. Galvin, L. J. Lowenstine, M. B. Jennings, M. B. Gardner, and C. E. Buckler. 1991. A highly divergent simian immunodeficiency virus (SIVstm) recovered from stored stump-tailed macaque tissues. J. Virol. 65:7061-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kodama, T., D. P. Silva, M. D. Daniel, J. E. Phillips-Conroy, C. J. Jolly, J. Rogers, and R. C. Desrosiers. 1989. Prevalence of antibodies to SIV in baboons in their native habitat. AIDS Res. Hum. Retrovir. 5:337-343. [DOI] [PubMed] [Google Scholar]
- 209.Konig, R. R., E. Flory, S. Steidl, J. Neumann, C. Coulibaly, E. Holznagel, S. Holzammer, S. Norley, and K. Cichutek. 2002. Engineered CD4- and CXCR4-using simian immunodeficiency virus from African green monkeys is neutralization sensitive and replicates in nonstimulated lymphocytes. J. Virol. 76:10627-10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 115:1082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kyaw-Tanner, M. T., and W. F. Robinson. 1996. Quasispecies and naturally occurring superinfection in feline immunodeficiency virus infection. Arch. Virol. 141:1703-1713. [DOI] [PubMed] [Google Scholar]
- 213.LaFranco-Scheuch, L., K. Abel, N. Makori, K. Rothaeusler, and C. J. Miller. 2004. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J. Virol. 78:6399-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Langley, R. J., V. M. Hirsch, S. J. O'Brien, D. Adger-Johnson, R. M. Goeken, and R. A. Olmsted. 1994. Nucleotide sequence analysis of puma lentivirus (PLV-14): genomic organization and relationship to other lentiviruses. Virology 202:853-864. [DOI] [PubMed] [Google Scholar]
- 215.Lecossier, D., F. Bouchonnet, F. Clavel, and A. J. Hance. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. [DOI] [PubMed] [Google Scholar]
- 216.Lee, I. T., J. K. Levy, S. P. Gorman, P. C. Crawford, and M. R. Slater. 2002. Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in unowned free-roaming cats. J. Am. Vet. Med. Assoc. 220:620-622. [DOI] [PubMed] [Google Scholar]
- 217.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 218.Letcher, J. D., and T. P. O'Conner. 1991. Incidence of antibodies reacting to feline immunodeficiency virus in a population of Asian lions. J. Zoo Wildl. Med. 22:324-329. [Google Scholar]
- 219.Letvin, N. L., M. D. Daniel, P. K. Sehgal, R. C. Desrosiers, R. D. Hunt, L. M. Waldron, J. J. MacKey, D. K. Schmidt, L. V. Chalifoux, and N. W. King. 1985. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 230:71-73. [DOI] [PubMed] [Google Scholar]
- 220.Leutenegger, C. M., R. Hofmann-Lehmann, C. Riols, M. Liberek, G. Worel, P. Lups, D. Fehr, M. Hartmann, P. Weilenmann, and H. Lutz. 1999. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 35:678-686. [DOI] [PubMed] [Google Scholar]
- 221.Levy, J., J. Richards, D. Edwards, T. Elston, K. Hartmann, I. Rodan, V. Thayer, M. Tompkins, and A. Wolf. 2003. 2001 Report of the American Association of Feline Practitioners and Academy of Feline Medicine advisory panel on feline retrovirus testing and management. J. Feline Med. Surg. 5:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 223.Liegeois, F., V. Courgnaud, W. M. Switzer, H. W. Murphy, S. Loul, A. Aghokeng, X. Pourrut, E. Mpoudi-Ngole, E. Delaporte, and M. Peeters. 2006. Molecular characterization of a novel simian immunodeficiency virus lineage (SIVtal) from northern talapoins (Miopithecus ogouensis). Virology 349:55-65. [DOI] [PubMed] [Google Scholar]
- 224.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin, J. A., M. C. Cheng, Y. Inoshima, K. Tomonaga, T. Miyazawa, Y. Tohya, K. Toh, Y. S. Lu, and T. Mikami. 1995. Seroepidemiological survey of feline retrovirus infections in cats in Taiwan in 1993 and 1994. J. Vet. Med. Sci. 57:161-163. [DOI] [PubMed] [Google Scholar]
- 227.Ling, B., C. Apetrei, I. Pandrea, R. S. Veazey, A. A. Lackner, B. Gormus, and P. A. Marx. 2004. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 78:8902-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ling, B., M. L. Santiago, S. Meleth, B. Gormus, H. M. McClure, C. Apetrei, B. H. Hahn, and P. A. Marx. 2003. Noninvasive detection of new simian immunodeficiency virus lineages in captive sooty mangabeys: ability to amplify virion RNA from fecal samples correlates with viral load in plasma. J. Virol. 77:2214-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lochelt, M., F. Romen, P. Bastone, H. Muckenfuss, N. Kirchner, Y. B. Kim, U. Truyen, U. Rosler, M. Battenberg, A. Saib, E. Flory, K. Cichutek, and C. Munk. 2005. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc. Natl. Acad. Sci. USA 102:7982-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Locher, C. P., S. A. Witt, B. G. Herndier, N. W. Abbey, K. Tenner-Racz, P. Racz, N. B. Kiviat, K. K. Murthy, K. Brasky, M. Leland, and J. A. Levy. 2003. Increased virus replication and virulence after serial passage of human immunodeficiency virus type 2 in baboons. J. Virol. 77:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lockridge, K. M., S. Himathongkham, E. T. Sawai, M. Chienand, and E. E. Sparger. 1999. The feline immunodeficiency virus vif gene is required for productive infection of feline peripheral blood mononuclear cells and monocyte-derived macrophages. Virology 261:25-30. [DOI] [PubMed] [Google Scholar]
- 232.Lowenstine, L. J., N. W. Lerche, J. L. Yee, A. Uyeda, M. B. Jennings, R. J. Munn, H. M. McClure, D. C. Anderson, P. N. Fultz, and M. B. Gardner. 1992. Evidence for a lentiviral etiology in an epizootic of immune deficiency and lymphoma in stump-tailed macaques (Macaca arctoides). J. Med. Primatol. 21:1-14. [PubMed] [Google Scholar]
- 233.Lowenstine, L. J., N. C. Pedersen, J. Higgins, K. C. Pallis, A. Uyeda, P. Marx, N. W. Lerche, R. J. Munn, and M. B. Gardner. 1986. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int. J. Cancer 38:563-574. [DOI] [PubMed] [Google Scholar]
- 234.Luria, B. J., J. K. Levy, M. R. Lappin, E. B. Breitschwerdt, A. M. Legendre, J. A. Hernandez, S. P. Gorman, and I. T. Lee. 2004. Prevalence of infectious diseases in feral cats in northern Florida. J. Feline Med. Surg. 6:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lutz, H., E. Isenbugel, R. Lehmann, R. H. Sabapara, and C. Wolfensberger. 1992. Retrovirus infections in non-domestic felids: serological studies and attempts to isolate a lentivirus. Vet. Immunol. Immunopathol. 35:215-224. [DOI] [PubMed] [Google Scholar]
- 236.Lyles, R. H., A. Munoz, T. E. Yamashita, H. Bazmi, R. Detels, C. R. Rinaldo, J. B. Margolick, J. P. Phair, and J. W. Mellors. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. J. Infect. Dis. 181:872-880. [DOI] [PubMed] [Google Scholar]
- 237.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 238.Mansfield, K. G., N. W. Lerche, M. B. Gardner, and A. A. Lackner. 1995. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 24:116-122. [DOI] [PubMed] [Google Scholar]
- 239.Marandin, A., A. Katz, E. Oksenhendler, M. Tulliez, F. Picard, W. Vainchenker, and F. Louache. 1996. Loss of primitive hematopoietic progenitors in patients with human immunodeficiency virus infection. Blood 88:4568-4578. [PubMed] [Google Scholar]
- 240.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Maruyama, S., H. Kabeya, R. Nakao, S. Tanaka, T. Sakai, X. Xuan, Y. Katsube, and T. Mikami. 2003. Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiol. Immunol. 47:147-153. [DOI] [PubMed] [Google Scholar]
- 242.Marx, P. A., C. Apetrei, and E. Drucker. 2004. AIDS as a zoonosis? Confusion over the origin of the virus and the origin of the epidemics. J. Med. Primatol. 33:220-226. [DOI] [PubMed] [Google Scholar]
- 243.Marx, P. A., Y. Li, N. W. Lerche, S. Sutjipto, A. Gettie, J. A. Yee, B. H. Brotman, A. M. Prince, A. Hanson, R. G. Webster, and R. C. Desrosiers. 1991. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J. Virol. 65:4480-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 246.McClure, H. M., D. C. Anderson, P. N. Fultz, A. A. Ansari, E. Lockwood, and A. Brodie. 1989. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 21:13-24. [DOI] [PubMed] [Google Scholar]
- 247.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 248.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14(Suppl. 3):S31-S44. [PubMed] [Google Scholar]
- 249.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mellors, J. W. 1998. Viral-load tests provide valuable answers. Sci. Am. 279:90-93. [DOI] [PubMed] [Google Scholar]
- 251.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 252.Miller, D. L., S. K. Taylor, D. S. Rotstein, M. B. Pough, M. C. Barr, C. A. Baldwin, M. Cunningham, M. Roelke, and D. Ingram. 2006. Feline immunodeficiency virus and puma lentivirus in Florida panthers (Puma concolor coryi): epidemiology and diagnostic issues. Vet. Res. Commun. 30:307-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Miller, R. J., J. S. Cairns, S. Bridges, and N. Sarver. 2000. Human immunodeficiency virus and AIDS: insights from animal lentiviruses. J. Virol. 74:7187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mitani, J. C., and D. P. Watts. 1999. Demographic influences on the hunting behavior of chimpanzees. Am. J. Phys. Anthropol. 109:439-454. [DOI] [PubMed] [Google Scholar]
- 255.Miyazawa, T., Y. Ikeda, K. Maeda, T. Horimoto, Y. Tohya, M. Mochizuki, D. Vu, G. D. Vu, D. X. Cu, K. Ono, E. Takahashi, and T. Mikami. 1998. Seroepidemiological survey of feline retrovirus infections in domestic and leopard cats in northern Vietnam in 1997. J. Vet. Med. Sci. 60:1273-1275. [DOI] [PubMed] [Google Scholar]
- 256.Moore, J. P., S. G. Kitchen, P. Pugach, and J. A. Zack. 2004. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 20:111-126. [DOI] [PubMed] [Google Scholar]
- 257.Morton, W. R., L. Kuller, R. E. Benveniste, E. A. Clark, C. C. Tsai, M. J. Gale, M. E. Thouless, J. Overbaugh, and M. G. Katze. 1989. Transmission of the simian immunodeficiency virus SIVmne in macaques and baboons. J. Med. Primatol. 18:237-245. [PubMed] [Google Scholar]
- 258.Muller, M. C., N. K. Saksena, E. Nerrienet, C. Chappey, V. M. Herve, J. P. Durand, P. Legal-Campodonico, M. C. Lang, J. P. Digoutte, A. J. Georges, et al. 1993. Simian immunodeficiency viruses from central and western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J. Virol. 67:1227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Muller-Trutwin, M. C., S. Corbet, S. Souquiere, P. Roques, P. Versmisse, A. Ayouba, S. Delarue, E. Nerrienet, J. Lewis, P. Martin, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. SIVcpz from a naturally infected Cameroonian chimpanzee: biological and genetic comparison with HIV-1 N. J. Med. Primatol. 29:166-172. [DOI] [PubMed] [Google Scholar]
- 260.Muller-Trutwin, M. C., S. Corbet, M. D. Tavares, V. M. Herve, E. Nerrienet, M. C. Georges-Courbot, W. Saurin, P. Sonigo, and F. Barre-Sinoussi. 1996. The evolutionary rate of nonpathogenic simian immunodeficiency virus (SIVagm) is in agreement with a rapid and continuous replication in vivo. Virology 223:89-102. [DOI] [PubMed] [Google Scholar]
- 261.Munson, L., L. Marker, E. Dubovi, J. A. Spencer, J. F. Evermann, and S. J. O'Brien. 2004. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus). J. Wildl. Dis. 40:23-31. [DOI] [PubMed] [Google Scholar]
- 262.Murphey-Corb, M., L. N. Martin, S. R. Rangan, G. B. Baskin, B. J. Gormus, R. H. Wolf, W. A. Andes, M. West, and R. C. Montelaro. 1986. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature 321:435-437. [DOI] [PubMed] [Google Scholar]
- 263.Muthukumar, A., A. Wozniakowski, M. C. Gauduin, M. Paiardini, H. M. McClure, R. P. Johnson, G. Silvestri, and D. L. Sodora. 2004. Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood 103:973-979. [DOI] [PubMed] [Google Scholar]
- 264.Muthukumar, A., D. Zhou, M. Paiardini, A. P. Barry, K. S. Cole, H. M. McClure, S. I. Staprans, G. Silvestri, and D. L. Sodora. 2005. Timely triggering of homeostatic mechanisms involved in the regulation of T-cell levels in SIVsm-infected sooty mangabeys. Blood 106:3839-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Natoli, E., L. Say, S. Cafazzo, R. Bonanni, M. Schmid, and D. Pontier. 2005. Bold attitude makes male urban feral domestic cats more vulnerable to feline immunodeficiency virus. Neurosci. Biobehav. Rev. 29:151-157. [DOI] [PubMed] [Google Scholar]
- 266.Nerrienet, E., X. Amouretti, M. C. Muller-Trutwin, V. Poaty-Mavoungou, I. Bedjebaga, H. T. Nguyen, G. Dubreuil, S. Corbet, E. J. Wickings, F. Barre-Sinoussi, A. J. Georges, and M. C. Georges-Courbot. 1998. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res. Hum. Retrovir. 14:785-796. [DOI] [PubMed] [Google Scholar]
- 267.Nerrienet, E., C. Apetrei, Y. Foupouapouognigni, B. Ling, A. Luckay, L. Chakrabarti, A. Ayouba, and P. A. Marx. 2002. Presented at the XIV International Conference on AIDS, Barcelona, Spain.
- 268.Nerrienet, E., M. L. Santiago, Y. Foupouapouognigni, E. Bailes, N. I. Mundy, B. Njinku, A. Kfutwah, M. C. Muller-Trutwin, F. Barre-Sinoussi, G. M. Shaw, P. M. Sharp, B. H. Hahn, and A. Ayouba. 2005. Simian immunodeficiency virus infection in wild-caught chimpanzees from Cameroon. J. Virol. 79:1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nishimura, Y., Y. Goto, H. Pang, Y. Endo, T. Mizuno, Y. Momoi, T. Watari, H. Tsujimoto, and A. Hasegawa. 1998. Genetic heterogeneity of env gene of feline immunodeficiency virus obtained from multiple districts in Japan. Virus Res. 57:101-112. [DOI] [PubMed] [Google Scholar]
- 270.Nishimura, Y., Y. Goto, K. Yoneda, Y. Endo, T. Mizuno, M. Hamachi, H. Maruyama, H. Kinoshita, S. Koga, M. Komori, S. Fushuku, K. Ushinohama, M. Akuzawa, T. Watari, A. Hasegawa, and H. Tsujimoto. 1999. Interspecies transmission of feline immunodeficiency virus from the domestic cat to the Tsushima cat (Felis bengalensis euptilura) in the wild. J. Virol. 73:7916-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Norley, S., B. Beer, S. Holzammer, J. zur Megede, and R. Kurth. 1999. Why are the natural hosts of SIV resistant to AIDS? Immunol. Lett. 66:47-52. [DOI] [PubMed] [Google Scholar]
- 272.Norley, S., and R. Kurth. 2004. The role of the immune response during SIVagm infection of the African green monkey natural host. Front. Biosci. 9:550-564. [DOI] [PubMed] [Google Scholar]
- 273.Norley, S. G., G. Kraus, J. Ennen, J. Bonilla, H. Konig, and R. Kurth. 1990. Immunological studies of the basis for the apathogenicity of simian immunodeficiency virus from African green monkeys. Proc. Natl. Acad. Sci. USA 87:9067-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Novembre, F. J., J. de Rosayro, S. Nidtha, S. P. O'Neil, T. R. Gibson, T. Evans-Strickfaden, C. E. Hart, and H. M. McClure. 2001. Rapid CD4+ T-cell loss induced by human immunodeficiency virus type 1NC in uninfected and previously infected chimpanzees. J. Virol. 75:1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Novembre, F. J., V. M. Hirsch, H. M. McClure, P. N. Fultz, and P. R. Johnson. 1992. SIV from stump-tailed macaques: molecular characterization of a highly transmissible primate lentivirus. Virology 186:783-787. [DOI] [PubMed] [Google Scholar]
- 276.Novembre, F. J., P. R. Johnson, M. G. Lewis, D. C. Anderson, S. Klumpp, H. M. McClure, and V. M. Hirsch. 1993. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J. Virol. 67:2466-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Novembre, F. J., M. M. Saucier, V. M. Hirsch, P. R. Johnson, and H. M. McClure. 1994. Viral genetic determinants in SIVsmmPBj pathogenesis. J. Med. Primatol. 23:136-145. [DOI] [PubMed] [Google Scholar]
- 278.Nowak, M. A., A. L. Lloyd, G. M. Vasquez, T. A. Wiltrout, L. M. Wahl, N. Bischofberger, J. Williams, A. Kinter, A. S. Fauci, V. M. Hirsch, and J. D. Lifson. 1997. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J. Virol. 71:7518-7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.O'Connor, T. P., Jr., Q. J. Tonelli, and J. M. Scarlett. 1991. Report of the National FeLV/FIV Awareness Project. J. Am. Vet. Med. Assoc. 199:1348-1353. [PubMed] [Google Scholar]
- 280.Ohta, Y., T. Masuda, H. Tsujimoto, K. Ishikawa, T. Kodama, S. Morikawa, M. Nakai, S. Honjo, and M. Hayami. 1988. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int. J. Cancer 41:115-122. [DOI] [PubMed] [Google Scholar]
- 281.Olmsted, R. A., R. Langley, M. E. Roelke, R. M. Goeken, D. Adger-Johnson, J. P. Goff, J. P. Albert, C. Packer, M. K. Laurenson, and T. M. Caro. 1992. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J. Virol. 66:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Onanga, R., C. Kornfeld, I. Pandrea, J. Estaquier, S. Souquiere, P. Rouquet, V. P. Mavoungou, O. Bourry, S. M'Boup, F. Barre-Sinoussi, F. Simon, C. Apetrei, P. Roques, and M. C. Muller-Trutwin. 2002. High levels of viral replication contrast with only transient changes in CD4+ and CD8+ cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 76:10256-10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Onanga, R., S. Souquiere, M. Makuwa, A. Mouinga-Ondeme, F. Simon, C. Apetrei, and P. Roques. 2006. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx). J. Virol. 80:3301-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.O'Neil, L. L., M. J. Burkhard, L. J. Diehl, and E. A. Hoover. 1995. Vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 11:171-182. [DOI] [PubMed] [Google Scholar]
- 285.O'Neil, S. P., F. J. Novembre, A. B. Hill, C. Suwyn, C. E. Hart, T. Evans-Strickfaden, D. C. Anderson, J. deRosayro, J. G. Herndon, M. Saucier, and H. M. McClure. 2000. Progressive infection in a subset of HIV-1-positive chimpanzees. J. Infect. Dis. 182:1051-1062. [DOI] [PubMed] [Google Scholar]
- 286.Osofsky, S. A., K. J. Hirsch, E. E. Zuckerman, and W. D. Hardy. 1996. Feline lentivirus and feline oncovirus status of free-reanging lions (Panthera leo), leopards (Panthera pardus), and cheetahs (Acinonyx jubatus) in Botswana: a regional perspective. J. Zoo Wildl. Med. 27:453-467. [Google Scholar]
- 287.Osterhaus, A. D., N. Pedersen, G. van Amerongen, M. T. Frankenhuis, M. Marthas, E. Reay, T. M. Rose, J. Pamungkas, and M. L. Bosch. 1999. Isolation and partial characterization of a lentivirus from talapoin monkeys (Myopithecus talapoin). Virology 260:116-124. [DOI] [PubMed] [Google Scholar]
- 288.Ostrowski, S., M. Van Vuuren, D. M. Lenain, and A. Durand. 2003. A serologic survey of wild felids from central west Saudi Arabia. J. Wildl. Dis. 39:696-701. [DOI] [PubMed] [Google Scholar]
- 289.Otsyula, M., J. Yee, M. Jennings, M. Suleman, A. Gettie, R. Tarara, M. Isahakia, P. Marx, and N. Lerche. 1996. Prevalence of antibodies against simian immunodeficiency virus (SIV) and simian T-lymphotropic virus (STLV) in a colony of non-human primates in Kenya, East Africa. Ann. Trop. Med. Parasitol. 90:65-70. [DOI] [PubMed] [Google Scholar]
- 290.Owen, S. M., S. Masciotra, F. Novembre, J. Yee, W. M. Switzer, M. Ostyula, and R. B. Lal. 2000. Simian immunodeficiency viruses of diverse origin can use CXCR4 as a coreceptor for entry into human cells. J. Virol. 74:5702-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pandrea, I., C. Apetrei, J. Dufour, N. Dillon, N. Katz, J. Barbercheck, B. Jacquelin, R. Bohm, P. A. Marx, F. Barre-Sinoussi, M. C. Muller-Trutwin, V. M. Hirsch, A. A. Lackner, and R. Veazey. 2006. SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 80:4858-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pandrea, I., C. Apetrei, S. Gordon, J. Dufour, R. Bohm, B. Sumpter, P. Roques, A. Kaur, P. A. Marx, A. A. Lackner, R. S. Veazey, and G. Silvestri. Paucity of CD4+CCR5+ T-cells is a typical feature of natural SIV hosts. Blood, in press. [DOI] [PMC free article] [PubMed]
- 293.Pandrea, I., R. Gautam, J. Barbercheck, I. F. Butler, T. Rasmussen, P. A. Marx, G. Silvestri, A. A. Lackner, R. S. Veazey, and C. Apetrei. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 294.Pandrea, I., C. Kornfeld, M. J. Ploquin, C. Apetrei, A. Faye, P. Rouquet, P. Roques, F. Simon, F. Barre-Sinoussi, M. C. Muller-Trutwin, and O. M. Diop. 2005. Impact of viral factors on very early in vivo replication profiles in simian immunodeficiency virus SIVagm-infected African green monkeys. J. Virol. 79:6249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pandrea, I., R. Onanga, C. Kornfeld, P. Rouquet, O. Bourry, S. Clifford, P. T. Telfer, K. Abernethy, L. T. White, P. Ngari, M. Muller-Trutwin, P. Roques, P. A. Marx, F. Simon, and C. Apetrei. 2003. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology 317:119-127. [DOI] [PubMed] [Google Scholar]
- 296.Pandrea, I., R. Onanga, P. Rouquet, O. Bourry, P. Ngari, E. J. Wickings, P. Roques, and C. Apetrei. 2001. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS 15:2461-2462. [DOI] [PubMed] [Google Scholar]
- 297.Pandrea, I., G. Silvestri, R. Onanga, R. S. Veazey, P. A. Marx, V. Hirsch, and C. Apetrei. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 35:194-201. [DOI] [PubMed] [Google Scholar]
- 298.Payne, S. L., and J. H. Elder. 2001. The role of retroviral dUTPases in replication and virulence. Curr. Protein Pept. Sci. 2:381-388. [DOI] [PubMed] [Google Scholar]
- 299.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 300.Pedersen, N. C., C. M. Leutenegger, J. Woo, and J. Higgins. 2001. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 79:53-67. [DOI] [PubMed] [Google Scholar]
- 301.Peeters, M., V. Courgnaud, B. Abela, P. Auzel, X. Pourrut, F. Bibollet-Ruche, S. Loul, F. Liegeois, C. Butel, D. Koulagna, E. Mpoudi-Ngole, G. M. Shaw, B. H. Hahn, and E. Delaporte. 2002. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg. Infect. Dis. 8:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Peeters, M., K. Fransen, E. Delaporte, M. Van den Haesevelde, G. M. Gershy-Damet, L. Kestens, G. van der Groen, and P. Piot. 1992. Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild-captured chimpanzee. AIDS 6:447-451. [DOI] [PubMed] [Google Scholar]
- 303.Peeters, M., C. Honore, T. Huet, L. Bedjabaga, S. Ossari, P. Bussi, R. W. Cooper, and E. Delaporte. 1989. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS 3:625-630. [DOI] [PubMed] [Google Scholar]
- 304.Peeters, M., and P. M. Sharp. 2000. Genetic diversity of HIV-1: the moving target. AIDS 14(Suppl. 3):S129-S140. [PubMed] [Google Scholar]
- 305.Peri, E. V., W. Ponti, P. Dall'ara, M. Rocchi, A. Zecconi, and L. Bonizzi. 1994. Seroepidemiological and clinical survey of feline immunodeficiency virus infection in northern Italy. Vet. Immunol. Immunopathol. 40:285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Phillips, T. R., J. N. Billaud, and S. J. Henriksen. 2000. Methamphetamine and HIV-1: potential interactions and the use of the FIV/cat model. J. Psychopharmacol. 14:244-250. [DOI] [PubMed] [Google Scholar]
- 307.Phillips, T. R., O. Prospero-Garcia, D. W. Wheeler, P. C. Wagaman, D. L. Lerner, H. S. Fox, L. R. Whalen, F. E. Bloom, J. H. Elder, and S. J. Henriksen. 1996. Neurologic dysfunctions caused by a molecular clone of feline immunodeficiency virus, FIV-PPR. J. Neurovirol. 2:388-396. [DOI] [PubMed] [Google Scholar]
- 308.Phillips-Conroy, J. E., C. J. Jolly, B. Petros, J. S. Allan, and R. C. Desrosiers. 1994. Sexual transmission of SIVagm in wild grivet monkeys. J. Med. Primatol. 23:1-7. [DOI] [PubMed] [Google Scholar]
- 309.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Picker, L. J., and D. I. Watkins. 2005. HIV pathogenesis: the first cut is the deepest. Nat. Immunol. 6:430-432. [DOI] [PubMed] [Google Scholar]
- 311.Pistello, M., F. Bonci, P. Isola, P. Mazzetti, A. Merico, L. Zaccaro, D. Matteucci, and M. Bendinelli. 2005. Evaluation of feline immunodeficiency virus ORF-A mutants as candidate attenuated vaccine. Virology 332:676-690. [DOI] [PubMed] [Google Scholar]
- 312.Poli, A., F. Abramo, P. Cavicchio, P. Bandecchi, E. Ghelardi, and M. Pistello. 1995. Lentivirus infection in an African lion: a clinical, pathologic and virologic study. J. Wildl. Dis. 31:70-74. [DOI] [PubMed] [Google Scholar]
- 313.Poss, M. L., H. A. Ross, S. L. Painter, D. C. Holley, J. A. Terwee, S. VandeWoude, and A. Rodrigo. 2006. Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerases. J. Virol. 80:2728-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Prince, A. M., B. Brotman, D. H. Lee, L. Andrus, J. Valinsky, and P. Marx. 2002. Lack of evidence for HIV type 1-related SIVcpz infection in captive and wild chimpanzees (Pan troglodytes verus) in West Africa. AIDS Res. Hum. Retrovir. 18:657-660. [DOI] [PubMed] [Google Scholar]
- 315.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Riley, S. P., J. Foley, and B. Chomel. 2004. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a national park in California. J. Wildl. Dis. 40:11-22. [DOI] [PubMed] [Google Scholar]
- 318.Roederer, M., J. G. Dubs, M. T. Anderson, P. A. Raju, L. A. Herzenberg, and L. A. Herzenberg. 1995. CD8 naive T cell counts decrease progressively in HIV-infected adults. J. Clin. Investig. 95:2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Roelke, M., J. Pecon-Slattery, S. Taylor, S. Citino, E. Brown, C. Packer, S. VandeWoude, and S. J. O'Brien. 2006. T-lymphocyte profiles in FIV infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 42:234-248. [DOI] [PubMed] [Google Scholar]
- 320.Rogers, A. B., and E. A. Hoover. 1998. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J. Infect. Dis. 178:960-967. [DOI] [PubMed] [Google Scholar]
- 321.Roques, P., D. L. Robertson, S. Souquiere, C. Apetrei, E. Nerrienet, F. Barre-Sinoussi, M. Muller-Trutwin, and F. Simon. 2004. Phylogenetic characteristics of three new HIV-1 N strains and implications for the origin of group N. AIDS 18:1371-1381. [DOI] [PubMed] [Google Scholar]
- 322.Salemi, M., T. De Oliveira, V. Courgnaud, V. Moulton, B. Holland, S. Cassol, W. M. Switzer, and A. M. Vandamme. 2003. Mosaic genomes of the six major primate lentivirus lineages revealed by phylogenetic analyses. J. Virol. 77:7202-7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Santiago, M. L., F. Bibollet-Ruche, E. Bailes, S. Kamenya, M. N. Muller, M. Lukasik, A. E. Pusey, D. A. Collins, R. W. Wrangham, J. Goodall, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2003. Amplification of a complete simian immunodeficiency virus genome from fecal RNA of a wild chimpanzee. J. Virol. 77:2233-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Santiago, M. L., F. Bibollet-Ruche, N. Gross-Camp, A. C. Majewski, M. Masozera, I. Munanura, B. A. Kaplin, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2003. Noninvasive detection of simian immunodeficiency virus infection in a wild-living L'Hoest's monkey (Cercopithecus lhoesti). AIDS Res. Hum. Retrovir. 19:1163-1166. [DOI] [PubMed] [Google Scholar]
- 325.Santiago, M. L., M. Lukasik, S. Kamenya, Y. Li, F. Bibollet-Ruche, E. Bailes, M. N. Muller, M. Emery, D. A. Goldenberg, J. S. Lwanga, A. Ayouba, E. Nerrienet, H. M. McClure, J. L. Heeney, D. P. Watts, A. E. Pusey, D. A. Collins, R. W. Wrangham, J. Goodall, J. F. Brookfield, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2003. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan troglodytes schweinfurthii). J. Virol. 77:7545-7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Santiago, M. L., F. Range, B. F. Keele, Y. Li, E. Bailes, F. Bibollet-Ruche, C. Fruteau, R. Noe, M. Peeters, J. F. Brookfield, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2005. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 79:12515-12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Santiago, M. L., C. M. Rodenburg, S. Kamenya, F. Bibollet-Ruche, F. Gao, E. Bailes, S. Meleth, S. J. Soong, J. M. Kilby, Z. Moldoveanu, B. Fahey, M. N. Muller, A. Ayouba, E. Nerrienet, H. M. McClure, J. L. Heeney, A. E. Pusey, D. A. Collins, C. Boesch, R. W. Wrangham, J. Goodall, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2002. SIVcpz in wild chimpanzees. Science 295:465. [DOI] [PubMed] [Google Scholar]
- 328.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 125:1055-1067. [DOI] [PubMed] [Google Scholar]
- 329.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 330.Schmitz, J. E., M. A. Simon, M. J. Kuroda, M. A. Lifton, M. W. Ollert, C. W. Vogel, P. Racz, K. Tenner-Racz, B. J. Scallon, M. Dalesandro, J. Ghrayeb, E. P. Rieber, V. G. Sasseville, and K. A. Reimann. 1999. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am. J. Pathol. 154:1923-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schols, D., and E. De Clercq. 1998. The simian immunodeficiency virus mnd(GB-1) strain uses CXCR4, not CCR5, as coreceptor for entry in human cells. J. Gen. Virol. 79:2203-2205. [DOI] [PubMed] [Google Scholar]
- 332.Schrofelbauer, B., D. Chen, and N. R. Landau. 2004. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc. Natl. Acad. Sci. USA 101:3927-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sharp, P. M., E. Bailes, R. R. Chaudhuri, C. M. Rodenburg, M. O. Santiago, and B. H. Hahn. 2001. The origins of acquired immune deficiency syndrome viruses: where and when? Philos. Trans. R. Soc. Lond. B 356:867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sharp, P. M., E. Bailes, F. Gao, B. E. Beer, V. M. Hirsch, and B. H. Hahn. 2000. Origins and evolution of AIDS viruses: estimating the time-scale. Biochem. Soc. Trans. 28:275-282. [DOI] [PubMed] [Google Scholar]
- 335.Sharp, P. M., E. Bailes, D. L. Robertson, F. Gao, and B. H. Hahn. 1999. Origins and evolution of AIDS viruses. Biol. Bull. 196:338-342. [DOI] [PubMed] [Google Scholar]
- 336.Sharp, P. M., E. Bailes, M. Stevenson, M. Emerman, and B. H. Hahn. 1996. Gene acquisition in HIV and SIV. Nature 383:586-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sharp, P. M., and W. H. Li. 1988. Understanding the origins of AIDS viruses. Nature 336:315. [DOI] [PubMed] [Google Scholar]
- 338.Sharp, P. M., D. L. Robertson, F. Gao, and B. H. Hahn. 1994. Origins and diversity of human immunodeficiency viruses. AIDS 8(Suppl. 1):S27-S42. [Google Scholar]
- 339.Sharp, P. M., G. M. Shaw, and B. H. Hahn. 2005. Simian immunodeficiency virus infection of chimpanzees. J. Virol. 79:3891-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shimojima, M., T. Miyazawa, Y. Ikeda, E. L. McMonagle, H. Haining, H. Akashi, Y. Takeuchi, M. J. Hosie, and B. J. Willett. 2004. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 303:1192-1195. [DOI] [PubMed] [Google Scholar]
- 341.Siebelink, K. H., I. H. Chu, G. F. Rimmelzwaan, K. Weijer, A. D. Osterhaus, and M. L. Bosch. 1992. Isolation and partial characterization of infectious molecular clones of feline immunodeficiency virus obtained directly from bone marrow DNA of a naturally infected cat. J. Virol. 66:1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Silvestri, G. 2005. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 34:243-252. [DOI] [PubMed] [Google Scholar]
- 343.Silvestri, G., A. Fedanov, S. Germon, N. Kozyr, W. J. Kaiser, D. A. Garber, H. McClure, M. B. Feinberg, and S. I. Staprans. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 79:4043-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 345.Simon, F., P. Mauclere, P. Roques, I. Loussert-Ajaka, M. C. Muller-Trutwin, S. Saragosti, M. C. Georges-Courbot, F. Barre-Sinoussi, and F. Brun-Vezinet. 1998. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 4:1032-1037. [DOI] [PubMed] [Google Scholar]
- 346.Simon, F., S. Souquiere, F. Damond, A. Kfutwah, M. Makuwa, E. Leroy, P. Rouquet, J. L. Berthier, J. Rigoulet, A. Lecu, P. T. Telfer, I. Pandrea, J. C. Plantier, F. Barre-Sinoussi, P. Roques, M. C. Muller-Trutwin, and C. Apetrei. 2001. Synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res. Hum. Retrovir. 17:937-952. [DOI] [PubMed] [Google Scholar]
- 347.Simon, J. H., and M. H. Malim. 1996. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J. Virol. 70:5297-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Simon, J. H., D. L. Miller, R. A. Fouchier, M. A. Soares, K. W. Peden, and M. H. Malim. 1998. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 17:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Smirnova, N., J. L. Troyer, J. Schissler, J. Terwee, M. Poss, and S. Vandewoude. 2005. Feline lentiviruses demonstrate differences in receptor repertoire and envelope structural elements. Virology 342:60-76. [DOI] [PubMed] [Google Scholar]
- 350.Smith, S. M., M. Makuwa, F. Lee, A. Gettie, C. Russo, and P. A. Marx. 1998. SIVrcm infection of macaques. J. Med. Primatol. 27:94-98. [DOI] [PubMed] [Google Scholar]
- 351.Sodora, D. L., E. G. Shpaer, B. E. Kitchell, S. W. Dow, E. A. Hoover, and J. I. Mullins. 1994. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J. Virol. 68:2230-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Song, B., H. Javanbakht, M. Perron, D. H. Park, M. Stremlau, and J. Sodroski. 2005. Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J. Virol. 79:3930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Souquiere, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J. C. Plantier, F. Gao, K. Abernethy, L. J. White, W. Karesh, P. Telfer, E. J. Wickings, P. Mauclere, P. A. Marx, F. Barre-Sinoussi, B. H. Hahn, M. C. Muller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 75:7086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sousa, A. E., J. Carneiro, M. Meier-Schellersheim, Z. Grossman, and R. M. Victorino. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 169:3400-3406. [DOI] [PubMed] [Google Scholar]
- 355.Sparger, E. E., A. M. Beebe, N. Dua, S. Himathongkam, J. H. Elder, M. Torten, and J. Higgins. 1994. Infection of cats with molecularly cloned and biological isolates of the feline immunodeficiency virus. Virology 205:546-553. [DOI] [PubMed] [Google Scholar]
- 356.Spencer, J. A., A. A. Van Dijk, M. C. Horzinek, H. F. Egberink, R. G. Bengis, D. F. Keet, S. Morikawa, and D. H. Bishop. 1992. Incidence of feline immunodeficiency virus reactive antibodies in free-ranging lions of the Kruger National Park and the Etosha National Park in southern Africa detected by recombinant FIV p24 antigen. Onderstepoort J. Vet. Res. 59:315-322. [PubMed] [Google Scholar]
- 357.Staprans, S. I., and M. B. Feinberg. 2004. The roles of nonhuman primates in the preclinical evaluation of candidate AIDS vaccines. Expert Rev. Vaccines 3(Suppl.):S5-S32. [DOI] [PubMed] [Google Scholar]
- 358.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 359.Stremlau, M., M. Perron, S. Welikala, and J. Sodroski. 2005. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J. Virol. 79:3139-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sukura, A., T. Salminen, and L. A. Lindberg. 1992. A survey of FIV antibodies and FeLV antigens in free-roaming cats in the capital area of Finland. Acta Vet. Scand. 33:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sumpter, B., R. Dunham, S. Gordon, J. Engram, M. Paiardini, B. Cervasi, H. McClure, S. Staprans, D. L. Sodora, and G. Silvestri. Correlates of decreased CD4+ T-cell counts during natural, non-pathogenic SIV infection of sooty mangabeys: implications for AIDS pathogenesis. J. Immunol., in press. [DOI] [PubMed]
- 362.Switzer, W. M., B. Parekh, V. Shanmugam, V. Bhullar, S. Phillips, J. J. Ely, and W. Heneine. 2005. The epidemiology of simian immunodeficiency virus infection in a large number of wild- and captive-born chimpanzees: evidence for a recent introduction following chimpanzee divergence. AIDS Res. Hum. Retrovir. 21:335-342. [DOI] [PubMed] [Google Scholar]
- 363.Takehisa, J., Y. Harada, N. Ndembi, I. Mboudjeka, Y. Taniguchi, C. Ngansop, S. Kuate, L. Zekeng, K. Ibuki, T. Shimada, B. Bikandou, Y. Yamaguchi-Kabata, T. Miura, M. Ikeda, H. Ichimura, L. Kaptue, and M. Hayami. 2001. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 17:1143-1154. [DOI] [PubMed] [Google Scholar]
- 364.Takemura, T., M. Ekwalanga, B. Bikandou, E. Ido, Y. Yamaguchi-Kabata, S. Ohkura, H. Harada, J. Takehisa, H. Ichimura, H. J. Parra, M. Nende, E. Mubwo, M. Sepole, M. Hayami, and T. Miura. 2005. A novel simian immunodeficiency virus from black mangabey (Lophocebus aterrimus) in the Democratic Republic of Congo. J. Gen. Virol. 86:1967-1971. [DOI] [PubMed] [Google Scholar]
- 365.Talbott, R. L., E. E. Sparger, K. M. Lovelace, W. M. Fitch, N. C. Pedersen, P. A. Luciw, and J. H. Elder. 1989. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA 86:5743-5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Tao, B., and P. N. Fultz. 1995. Molecular and biological analyses of quasispecies during evolution of a virulent simian immunodeficiency virus, SIVsmmPBj14. J. Virol. 69:2031-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Terwee, J. A., J. K. Yactor, K. S. Sondgeroth, and S. Vandewoude. 2005. Puma lentivirus is controlled in domestic cats after mucosal exposure in the absence of conventional indicators of immunity. J. Virol. 79:2797-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Tomonaga, K., J. Katahira, M. Fukasawa, M. A. Hassan, M. Kawamura, H. Akari, T. Miura, T. Goto, M. Nakai, M. Suleman, et al. 1993. Isolation and characterization of simian immunodeficiency virus from African white-crowned mangabey monkeys (Cercocebus torquatus lunulatus). Arch. Virol. 129:77-92. [DOI] [PubMed] [Google Scholar]
- 369.Tosi, A. J., K. M. Detwiler, and T. R. Disotell. 2005. X-chromosomal window into the evolutionary history of the guenons (Primates: Cercopithecini). Mol. Phylogenet. Evol. 36:58-66. [DOI] [PubMed] [Google Scholar]
- 370.Tosi, A. J., T. R. Disotell, J. C. Morales, and D. J. Melnick. 2003. Cercopithecine Y-chromosome data provide a test of competing morphological evolutionary hypotheses. Mol. Phylogenet. Evol. 27:510-521. [DOI] [PubMed] [Google Scholar]
- 371.Tosi, A. J., D. J. Melnick, and T. R. Disotell. 2004. Sex chromosome phylogenetics indicate a single transition to terrestriality in the guenons (tribe Cercopithecini). J. Hum. Evol. 46:223-237. [DOI] [PubMed] [Google Scholar]
- 372.Towers, G. J., and S. P. Goff. 2003. Post-entry restriction of retroviral infections. AIDS Rev. 5:156-164. [PubMed] [Google Scholar]
- 373.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 374.Traina-Dorge, V., J. Blanchard, L. Martin, and M. Murphey-Corb. 1992. Immunodeficiency and lymphoproliferative disease in an African green monkey dually infected with SIV and STLV-I. AIDS Res. Hum. Retrovir. 8:97-100. [DOI] [PubMed] [Google Scholar]
- 375.Troyer, J. L., J. Pecon-Slattery, M. E. Roelke, L. Black, C. Packer, and S. J. O'Brien. 2004. Patterns of feline immunodeficiency virus multiple infection and genome divergence in a free-ranging population of African lions. J. Virol. 78:3777-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Troyer, J. L., J. Pecon-Slattery, M. E. Roelke, W. Johnson, S. VandeWoude, N. Vazquez-Salat, M. Brown, L. Frank, R. Woodroffe, C. Winterbach, H. Winterbach, G. Hemson, M. Bush, K. A. Alexander, E. Revilla, and S. J. O'Brien. 2005. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 79:8282-8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tsujimoto, H., R. W. Cooper, T. Kodama, M. Fukasawa, T. Miura, Y. Ohta, K. Ishikawa, M. Nakai, E. Frost, and G. E. Roelants. 1988. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J. Virol. 62:4044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tsujimoto, H., A. Hasegawa, N. Maki, M. Fukasawa, T. Miura, S. Speidel, R. W. Cooper, E. N. Moriyama, T. Gojobori, and M. Hayami. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341:539-541. [DOI] [PubMed] [Google Scholar]
- 379.Uema, M., Y. Ikeda, T. Miyazawa, J. A. Lin, M. C. Chen, T. F. Kuo, C. Kai, T. Mikami, and E. Takahashi. 1999. Feline immunodeficiency virus subtype C is prevalent in northern part of Taiwan. J. Vet. Med. Sci. 61:197-199. [DOI] [PubMed] [Google Scholar]
- 380.Vahlenkamp, T. W., M. B. Tompkins, and W. A. Tompkins. 2005. The role of CD4+CD25+ regulatory T cells in viral infections. Vet. Immunol. Immunopathol. 108:219-225. [DOI] [PubMed] [Google Scholar]
- 381.Vanden Haesevelde, M., J. L. Decourt, R. J. De Leys, B. Vanderborght, G. van der Groen, H. van Heuverswijn, and E. Saman. 1994. Genomic cloning and complete sequence analysis of a highly divergent African human immunodeficiency virus isolate. J. Virol. 68:1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Vanden Haesevelde, M. M., M. Peeters, G. Jannes, W. Janssens, G. van der Groen, P. M. Sharp, and E. Saman. 1996. Sequence analysis of a highly divergent HIV-1-related lentivirus isolated from a wild captured chimpanzee. Virology 221:346-350. [DOI] [PubMed] [Google Scholar]
- 383.VandeWoude, S., C. A. Hageman, S. J. O'Brien, and E. A. Hoover. 2002. Nonpathogenic lion and puma lentiviruses impart resistance to superinfection by virulent feline immunodeficiency virus. J. Acquir. Immune. Defic. Syndr. 29:1-10. [DOI] [PubMed] [Google Scholar]
- 384.VandeWoude, S., C. L. Hageman, and E. A. Hoover. 2003. Domestic cats infected with lion or puma lentivirus develop anti-feline immunodeficiency virus immune responses. J. Acquir. Immune Defic. Syndr. 34:20-31. [DOI] [PubMed] [Google Scholar]
- 385.VandeWoude, S., S. J. O'Brien, and E. A. Hoover. 1997. Infectivity of lion and puma lentiviruses for domestic cats. J. Gen. Virol. 78:795-800. [DOI] [PubMed] [Google Scholar]
- 386.VandeWoude, S., S. J. O'Brien, K. Langelier, W. D. Hardy, J. P. Slattery, E. E. Zuckerman, and E. A. Hoover. 1997. Growth of lion and puma lentiviruses in domestic cat cells and comparisons with FIV. Virology 233:185-192. [DOI] [PubMed] [Google Scholar]
- 387.Van Dooren, S., W. M. Switzer, W. Heneine, P. Goubau, E. Verschoor, B. Parekh, W. De Meurichy, C. Furley, M. Van Ranst, and A. M. Vandamme. 2002. Lack of evidence for infection with simian immunodeficiency virus in bonobos. AIDS Res. Hum. Retrovir. 18:213-216. [DOI] [PubMed] [Google Scholar]
- 388.van Rensburg, E. J., S. Engelbrecht, J. Mwenda, J. D. Laten, B. A. Robson, T. Stander, and G. K. Chege. 1998. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J. Gen. Virol. 79:1809-1814. [DOI] [PubMed] [Google Scholar]
- 389.Van Vuuren, M., E. Stylianides, S. A. Kania, E. E. Zuckerman, and W. D. Hardy, Jr. 2003. Evaluation of an indirect enzyme-linked immunosorbent assay for the detection of feline lentivirus-reactive antibodies in wild felids, employing a puma lentivirus-derived synthetic peptide antigen. Onderstepoort J. Vet. Res. 70:1-6. [PubMed] [Google Scholar]
- 390.Veazey, R., B. Ling, I. Pandrea, H. McClure, A. Lackner, and P. Marx. 2003. Decreased CCR5 expression on CD4+ T cells of SIV-infected sooty mangabeys. AIDS Res. Hum. Retrovir. 19:227-233. [DOI] [PubMed] [Google Scholar]
- 391.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 392.Verschoor, E. J., Z. Fagrouch, I. Bontjer, H. Niphuis, and J. L. Heeney. 2004. A novel simian immunodeficiency virus isolated from a Schmidt's guenon (Cercopithecus ascanius schmidti). J. Gen. Virol. 85:21-24. [DOI] [PubMed] [Google Scholar]
- 393.Villinger, F., G. T. Brice, A. Mayne, P. Bostik, and A. A. Ansari. 1999. Control mechanisms of virus replication in naturally SIVsmm infected mangabeys and experimentally infected macaques. Immunol. Lett. 66:37-46. [DOI] [PubMed] [Google Scholar]
- 394.Villinger, F., T. M. Folks, S. Lauro, J. D. Powell, J. B. Sundstrom, A. Mayne, and A. A. Ansari. 1996. Immunological and virological studies of natural SIV infection of disease-resistant nonhuman primates. Immunol. Lett. 51:59-68. [DOI] [PubMed] [Google Scholar]
- 395.Wain-Hobson, S., M. Alizon, and L. Montagnier. 1985. Relationship of AIDS to other retroviruses. Nature 313:743. [DOI] [PubMed] [Google Scholar]
- 396.Wang, Z., B. Metcalf, R. M. Ribeiro, H. McClure, and A. Kaur. 2006. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J. Virol. 80:2771-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Watts, D. P., and J. C. Mitani. 2002. Hunting behaviour of chimpanzees at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 23:1-28. [Google Scholar]
- 398.Wei, B. L., V. K. Arora, J. L. Foster, D. L. Sodora, and J. V. Garcia. 2003. In vivo analysis of Nef function. Curr. HIV Res. 1:41-50. [DOI] [PubMed] [Google Scholar]
- 399.Werner, A., G. Winskowsky, and R. Kurth. 1990. Soluble CD4 enhances simian immunodeficiency virus SIVagm infection. J. Virol. 64:6252-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Westmoreland, S. V., E. Halpern, and A. A. Lackner. 1998. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J. Neurovirol. 4:260-268. [DOI] [PubMed] [Google Scholar]
- 401.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]
- 402.Willett, B. J., L. Picard, M. J. Hosie, J. D. Turner, K. Adema, and P. R. Clapham. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Winkler, I. G., M. Lochelt, and R. L. Flower. 1999. Epidemiology of feline foamy virus and feline immunodeficiency virus infections in domestic and feral cats: a seroepidemiological study. J. Clin. Microbiol. 37:2848-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Woo, J. C., G. A. Dean, N. C. Pedersen, and P. F. Moore. 1997. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J. Virol. 71:8632-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Worobey, M., M. L. Santiago, B. F. Keele, J. B. Ndjango, J. B. Joy, B. L. Labama, A. B. Dhed, A. Rambaut, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2004. Origin of AIDS: contaminated polio vaccine theory refuted. Nature 428:820. [DOI] [PubMed] [Google Scholar]
- 406.Xu, H., E. S. Svarovskaia, R. Barr, Y. Zhang, M. A. Khan, K. Strebel, and V. K. Pathak. 2004. A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion. Proc. Natl. Acad. Sci. USA 101:5652-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Yamaguchi, J., S. G. Devare, and C. A. Brennan. 2000. Identification of a new HIV-2 subtype based on phylogenetic analysis of full-length genomic sequence. AIDS Res. Hum. Retrovir. 16:925-930. [DOI] [PubMed] [Google Scholar]
- 408.Yamamoto, J. K., H. Hansen, E. W. Ho, T. Y. Morishita, T. Okuda, T. R. Sawa, R. M. Nakamura, and N. C. Pedersen. 1989. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 194:213-220. [PubMed] [Google Scholar]
- 409.Yang, J. S., R. V. English, J. W. Ritchey, M. G. Davidson, T. Wasmoen, J. K. Levy, D. H. Gebhard, M. B. Tompkins, and W. A. Tompkins. 1996. Molecularly cloned feline immunodeficiency virus NCSU1 JSY3 induces immunodeficiency in specific-pathogen-free cats. J. Virol. 70:3011-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ylinen, L. M., Z. Keckesova, S. J. Wilson, S. Ranasinghe, and G. J. Towers. 2005. Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5alpha alleles. J. Virol. 79:11580-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhang, Y., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Zou, L., M. C. Barr, W. A. Hoose, and R. J. Avery. 1997. Characterization of the transcription map and Rev activity of a highly cytopathic feline immunodeficiency virus. Virology 236:266-278. [DOI] [PubMed] [Google Scholar]