Abstract
The transmembrane peptidase prostate-specific membrane antigen (PSMA) is universally upregulated in the vasculature of solid tumors, but its functional role in tumor angiogenesis has not been investigated. Here we show that angiogenesis is severely impaired in PSMA-null animals and that this angiogenic defect occurs at the level of endothelial cell invasion through the extracellular matrix barrier. Because proteolytic degradation of the extracellular matrix is a critical component of endothelial invasion in angiogenesis, it is logical to assume that PSMA participates in matrix degradation. However, we demonstrate a novel and more complex role for PSMA in angiogenesis, where it is a principal component of a regulatory loop that is tightly modulating laminin-specific integrin signaling and GTPase-dependent, p21-activated kinase 1 (PAK-1) activity. We show that PSMA inhibition, knockdown, or deficiency decreases endothelial cell invasion in vitro via integrin and PAK, thus abrogating angiogenesis. Interestingly, the neutralization of β1 or the inactivation of PAK increases PSMA activity, suggesting that they negatively regulate PSMA. This negative regulation is mediated by the cytoskeleton as the disruption of interactions between the PSMA cytoplasmic tail and the anchor protein filamin A decreases PSMA activity, integrin function, and PAK activation. Finally, the inhibition of PAK activation enhances the PSMA/filamin A interaction and, thus, boosts PSMA activity. These data imply that PSMA participates in an autoregulatory loop, wherein active PSMA facilitates integrin signaling and PAK activation, leading to both productive invasion and downregulation of integrin β1 signaling via reduced PSMA activity. Therefore, we have identified a novel role for PSMA as a true molecular interface, integrating both extracellular and intracellular signals during angiogenesis.
Proteolysis plays an important role in numerous biological processes, including the regulation of cellular responses to external stimuli. Peptidases and peptidase cascades have recently emerged as important regulators of angiogenesis (7, 8, 20, 41, 54, 61, 68, 80, 81) where new blood vessels are formed from existing vessels (31). Angiogenesis occurs predominantly during development and is rare in adults (27) except for pathological states, where angiogenesis is a major contributor to several diseases, including rheumatoid arthritis (22, 33, 66), psoriasis (4), tumor growth and metastasis (28), diabetic retinopathy (23), cardiovascular disease (35), and bone repair (23). Accordingly, its widespread contribution to many disorders prompted early predictions that angiogenesis would be a particularly effective therapeutic target. However, the results of clinical trials evaluating the efficacy of modulators of angiogenesis in the treatment of cancer, macular degeneration, and cardiovascular disease suggest that more precisely targeted therapies are needed to improve the therapeutic regulation of angiogenesis in the treatment of angiogenesis-associated diseases (71). Therefore, elucidating the specific mechanisms governing angiogenesis will facilitate the identification of potential new targets for therapy.
We have extensively characterized the type II transmembrane metalloprotease, CD13/aminopeptidase N (APN), in angiogenic endothelial cells as one of several peptidases that play a role in angiogenesis (for a review, see reference 5). We have shown that endothelial CD13/APN expression is induced in response to environmental angiogenic signals and that it plays an important functional role in endothelial cell invasion and morphogenesis (7, 8, 63). Importantly, the inhibition of CD13/APN′s activity with antagonists or monoclonal antibodies inhibits tumor growth in xenograft-bearing animals, demonstrating its utility as a therapeutic angiogenic target (61). Recently, a second cell surface exopeptidase, prostate-specific membrane antigen (PSMA) or glutamate carboxypeptidase II, has been shown to display an expression pattern analogous to that of CD13/APN, where it is found in angiogenic but not normal tumor vasculature (17, 18, 49, 70). PSMA was originally cloned and characterized in the tissues of prostate tumors (34, 38), where the full-length, transmembrane form of the protein is dramatically upregulated in the majority of advanced prostate carcinomas (hence the name PSMA [72]). In these tumors, there is a strong correlation between a negative prognosis and cell surface expression of PSMA (24, 37) and its precise contribution to prostate tumorigenesis is currently under investigation. Two site-specific carboxypeptidase activities have been assigned to PSMA: N-acetylated α-linked acidic dipeptidase (NAALADase), which hydrolyzes the neuropeptide NAAG (15, 74) in the brain to regulate release of neurotransmitters (9), and folate hydrolase activity, which is characterized by the cleavage of terminal glutamates from poly- and gamma-glutamated folate which play a role in the cellular uptake of dietary folate (65). PSMA contains a small intracellular amino-terminal domain, a transmembrane domain, and a large extracellular domain, including the catalytic region (57). PSMA expression has also been identified in various nonprostatic tissues, including brain and intestine (46, 76). More recently, PSMA expression was specifically detected, in a pattern remarkably similar to that of CD13/APN, in the endothelial cells lining the vasculature of several human tumors (17, 47, 49, 70), suggesting the possibility that PSMA may also functionally contribute to tumor-associated vasculature formation.
In the present study, we demonstrate that PSMA is required for angiogenesis in vivo and is essential for endothelial cell invasion in vitro, where it appears to participate in laminin-specific integrin signaling and the regulation of cytoskeletal dynamics via the Rho GTPase effector molecule p21-activated kinase 1 (PAK-1). We show that the inhibition of PSMA impedes the activation of integrin β1 (the common β chain of the laminin binding integrins [16, 43]) and PAK-1, which is consistent with its role in endothelial invasion. Importantly, we also provide evidence that tethering PSMA to the cytoskeleton via the actin binding protein filamin A modulates its enzyme activity, which is reminiscent of the impact of the cytoskeleton on integrin function. Interestingly, manipulation of integrin β1 or PAK activity impacts PSMA/filamin A interactions and PSMA activity, suggesting that, while PSMA facilitates integrin activation, integrin β1 and PAK in turn negatively regulate PSMA function. In support of this notion, the transfection of endothelial cells with a constitutively active PAK mutant decreases PSMA activity, implying that PSMA participates in an autoregulatory loop wherein active PSMA increases integrin signaling, PAK activation, and endothelial adhesion and invasion. This mechanism leads to the dissociation of the PSMA/filamin complex and the downregulation of PSMA activity and, thus, holds β1 integrin signaling in check. Our results linking PSMA to the regulation of PAK implicate PSMA as an important regulator of endothelial cell invasion and angiogenesis and maybe a therapeutic target for angiogenesis-related diseases.
MATERIALS AND METHODS
Inhibitors and antibodies.
2-(Phosphonomethyl)-pentanedioic acid (PMPA) was obtained through Alexis Biochemicals (Lausanne, Switzerland [39]). The Rac inhibitor was purchased from Sigma. PSMA antibodies were purchased from Anogen (YPSMA-1) or Zymed (for coimmunoprecipitation, Western blotting, and immunohistochemistry). β1-Neutralizing antibody was purchased from Cell Sciences, and β1-activating antibodies were purchased from Pharmingen (HUTS-21) or BioLegend (TS2/16). Phospho-specific and total PAK and focal adhesion kinase (FAK) antibodies were purchased from Santa Cruz Biotechnologies.
Animals.
PSMA-null mice and wild-type littermates were described previously (3). C57Bl/6 mice were acquired from Jackson Labs (Bar Harbor, Maine).
Cell culture.
Primary human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics Corporation (San Diego, CA) and maintained according to the manufacturer's protocol. The human Kaposi's sarcoma endothelial cell line (KS1767) was the gift of Renata Pasqualini and maintained as described previously (7).
In vitro endothelial cell invasion assay.
In vitro invasion assays of endothelial cells were performed as described previously (8). Invasion was measured by staining invading cells with calcein-AM, and fluorescent pixels were quantitated using Photoshop software.
siRNA synthesis.
A Silencer short interfering RNA (siRNA) construction kit (Ambion) was used to design and construct PSMA-specific siRNA according to the manufacturer's recommendations. PSMA-specific oligonucleotides were designed. The sequence of the sense strand oligonucleotide used was 5′-AATCTCCTTCACGAAACCGACCCTGTCTC-3′, and the sequence for the antisense strand oligonucleotide was 5′-AAGTCGGTTTCGTGAAGGAGACCTGTCTC-3′. Filamin A siRNA sequences were as follows: 5′-AACATACTTATCTTGGTCAATCCTGTCTC-3′ and 5′-AAATTGACCAAGATAAGTATGCCTGTCTC-3′. The control siRNA sequence used in the PSMA siRNA experiment was designed against a transcription factor gene, but it did not knock down protein levels of its intended gene. The sequences for this siRNA were 5′-AATCAAGAATATGGACCAGGTCCTGTCTC-3′ and 5′-AAACCTGGTCCATATTCTTGACCTGTCTC-3′. For the filamin A siRNA experiment, the negative control scrambled siRNA was purchased from Ambion. KS1767 or HUVECs were transfected with the completed siRNA (30 nM) using Lipofectamine reagent (Invitrogen). Cells were harvested and assayed for protein expression and function 3 days after transfection.
In vivo Matrigel plug angiogenesis assay.
The Matrigel plug assay was utilized as previously described (62). Briefly, thawed Matrigel (Becton Dickinson) was mixed with heparin and β-FGF and, in some cases, with the PSMA inhibitor PMPA (100 μM) or monoclonal antibody YPSMA-1 (1:200) and injected subcutaneously into anesthetized animals. Seven days after injection, the animals were sacrificed and plugs were harvested, fixed, and paraffin embedded for staining with hematoxylin and eosin to identify functional microvessels. Microvessel density was determined by counting the number of functional microvessels (defined as erythrocyte-containing vessels) per field. The three highest microvessel density fields for each sample were recorded, and the average was taken for each sample. Alternatively, plugs were homogenized and hemoglobin content was assessed using Drabkin's reagent kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol.
Primary lung endothelial cell isolation.
Lungs were harvested from PSMA knockout and wild-type mice as described previously (44). Immunofluorescence staining with platelet/endothelial cell adhesion molecule antibody revealed 75 to 85% endothelial cell purity in the final cultures.
Cell transfections.
The transfection of PAK constructs was performed using Lipofectamine 2000 (Invitrogen) as described previously (45). PAK expression was verified by immunoblot analysis (wild type and constitutively active) or fluorescence imaging of the green fluorescent protein-expressing autoinhibitory domain of PAK. For peptide transfections, 1 mg of PSMA cytoplasmic tail domain (CTD) and scrambled peptides were transfected using Stratagene's Biotrek protein delivery system according to the manufacturer's recommendations. Cotransfection of a positive control fluorescein isothiocyanate (FITC)-containing protein ensured appropriate transfection efficiency.
Immunoprecipitation/immunoblots.
For immunoprecipitation, cells were lysed in Triton X-100 containing lysis buffer and precleared with protein A-agarose beads (Bio-Rad). Lysates were incubated with the indicated antibodies at 4°C before overnight precipitation with protein A-agarose beads. Immunoprecipitated proteins were lysed in sodium dodecyl sulfate-containing buffer, run on 4 to 15% Tris-HCl gels, and blotted with filamin A antibody (Chemicon) or the indicated control antibodies. For general immunoblotting, blots were incubated with a 1:200 (PAK and FAK) or 1:400 (all others) dilution of the indicated primary antibodies and with 1:2,500 of the appropriate horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences); detection was performed using the ECL reagent (Amersham Biosciences).
Rac activation assay.
HUVECs were plated on Matrigel-coated cell culture dishes in the presence or absence of 100 μM PMPA for 4 h. Cells were lysed with the Pierce lysis/binding/wash buffer containing protease inhibitors, sodium orthovanadate, and 10 μg of the PAK p21 binding domain-glutathione S-transferase construct. Lysates were centrifuged, and the supernatant was pulled down in the presence of 10 μg PAK p21 binding domain-glutathione S-transferase and glutathione-Sepharose 4B beads (Amersham Biosciences) overnight at 4°C. The beads were collected, washed three times, and boiled in sample buffer. Immunoblotting with Rac antibody (Pierce) allowed detection of activated and total Rac.
Immunofluorescence.
Cells were fixed with 100% methanol, permeabilized with 0.1% NP-40, blocked with 3% bovine serum albumin-phosphate-buffered saline (PBS), and stained with PSMA (Zymed) and filamin A (Cell Signaling) antibodies followed by fluorescently conjugated secondary antibodies. Cells were observed with a Zeiss Axioplan 2 (40× objective) attached to a Zeiss AxioCam HRc digital camera.
PSMA activity assay.
Cells grown in 24-well plates were washed with PBS and incubated with 3H[NAAG] in Krebs-Ringer buffer (1 μCi/ml) for 1 h at 37°C. PSMA activity was determined as described previously (74). Activity was normalized to protein concentration as determined by the Bradford Assay (Bio-Rad).
Phalloidin staining of cells.
Cells grown on coverslips or cell culture dishes were fixed with 4% paraformaldehyde-PBS for 20 min, permeabilized with 1% Triton X-100, and stained with tetramethyl rhodamine isocyanate-phalloidin (Chemicon; 1:500 in PBS) for 45 min at room temperature. Cells were washed three times and observed with a Zeiss Axioplan 2 (40× objective) attached to a Zeiss AxioCam HRc digital camera.
β1 integrin adhesion assay.
Twenty-four-well cell culture plates were coated with laminin or the indicated matrix proteins (10 μg/ml) overnight at 4°C. Cells were trypsinized and resuspended in complete medium with or without 100 μM PMPA (basal activation) or with or without β1-activating antibodies (1 μg HUTS-21 or TS2/16) and 100 μM PMPA (induced activation). Alternatively, cells transfected with control or PSMA siRNA were assessed for adhesion in the absence (basal activation) or presence (induced activation) of β1-activating antibody. After 20 min, floating cells were removed, and adherent cells were stained with calcein-AM and counted. Percent adhesion was calculated from the total number of cells (adherent and floating).
Statistical analysis.
All experiments in this study were repeated for a minimum of three independent experiments. Differences between means were analyzed using the two-tailed Student's t test, and significance was set at a P value of <0.05.
RESULTS
Angiogenesis is impaired in PSMA-null animals.
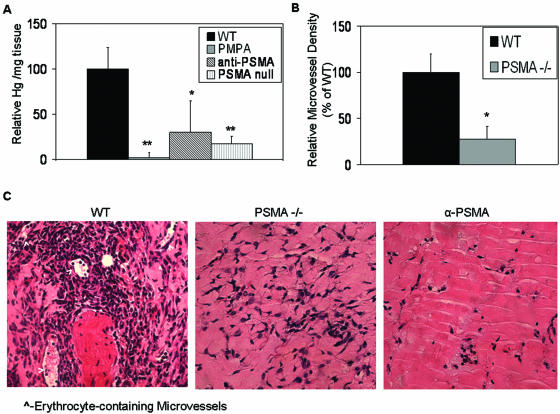
We have shown that the expression of the membrane-anchored peptidase CD13/APN is highly expressed on angiogenic but not normal vasculature, where it plays an important functional role in both endothelial cell invasion and morphogenesis and, thus, is essential for functional angiogenesis. Recent reports have demonstrated that similar to CD13, the majority of blood vessels in normal tissues express minor amounts of a second peptidase, PSMA, but levels of this protein are significantly upregulated in the vasculature of numerous human tumors. To investigate the possible angiogenic role of PSMA, we injected PSMA-null (3) and control mice with Matrigel, an acellular tumor-derived matrix consisting of many components of the tumor microenvironment, such as basement membrane proteins and growth factors (42) (62). Lack of PSMA resulted in a striking attenuation of neovessel formation in the Matrigel plug, as quantitated by hemoglobin content or formation of erythrocyte-containing capillaries (a >10-fold reduction relative to controls) (Fig. 1A and B). Similarly, wild-type animals injected with Matrigel containing either PMPA, a highly specific peptide inhibitor of PSMA activity (39), or YPSMA-1, a PSMA-neutralizing antibody, resulted in decreased hemoglobin content (Fig. 1A) and a clear lack of capillaries in the presence of either antagonist (Fig. 1C). Thus, PSMA is important for angiogenesis in vivo, where its specific and potent expression on angiogenic vasculature likely contributes to pathological angiogenesis.
FIG. 1.
Loss of PSMA in vivo abrogates angiogenesis. Results of in vivo Matrigel plug assay show that (A) hemoglobin (Hg) content is decreased in PSMA-inhibited (n = 6; P = 0.002) or PSMA-null plugs (n = 6; P = 0.0001), (B) microvessel density is decreased in PSMA-null plugs (n = 6; P = 0.014), and (C) hematoxylin and eosin staining of Matrigel plug sections reveals a significant decrease in erythrocyte-containing microvessels in PSMA-null or -inhibited plugs (indicated by arrowheads). WT, wild type; α-PSMA, PSMA-neutralizing antibody; PSMA −/−, PSMA null. *, 0.05 < P > 0.01; **, 0.01 < P > 0.001. Error bars indicate standard deviations.
PSMA participates in endothelial cell invasion in vitro.
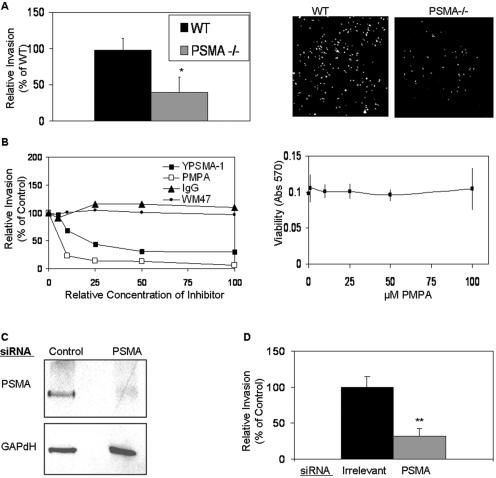
Angiogenesis is a complex process that includes the activation, proliferation, migration, invasion, and differentiation of endothelial cells (27, 32). Previous studies have demonstrated that the cell surface peptidases CD13 (7, 8), aminopeptidase A (54), and dipeptidyl peptidase IV (20) regulate angiogenesis by controlling endothelial cell invasion. To determine whether PSMA also participates in this process, we assayed purified primary pulmonary endothelial cells from PSMA-null or control animals for their abilities to penetrate Matrigel in vitro. The invasion of PSMA-null endothelial cells was severely impaired relative to wild-type endothelial cells (Fig. 2A). Consistent with this observation and our in vivo data, the invasion of primary HUVECs was markedly inhibited in the presence of either PMPA or YPSMA-1 (the neutralizing antibody) (75) in a dose-dependent manner (Fig. 2B), confirming that the effects on invasion are due to the inhibition of PSMA. Two additional PSMA-specific antibodies resulted in a similar degree of inhibition (data not shown). The loss of invasion in the presence of PSMA antibodies is not due to the interference of cell function by general antibody binding, as neither immunoglobulin G nor an anti-CD13 antibody that binds to CD13 (but does not inhibit its function) (WM4.7) affected invasion. Importantly, cell viability was not affected in the presence of a relatively high concentration of PMPA that significantly inhibits invasion (100 μm) (Fig. 2B) and endothelial morphogenesis was similarly unaffected (data not shown). Therefore, endothelial cell invasion is dependent on functional PSMA.
FIG. 2.
PSMA is required for endothelial cell invasion. (A) Lung endothelial cells isolated from PSMA-null (PSMA −/−) animals have decreased abilities to invade in vitro (n = 3; P = 0.02). Right panels show images of invading cells stained with calcein-AM. WT, wild type. Error bars indicate standard deviations. *, 0.05 < P > 0.01. (B) Dose dependency of inhibition of HUVEC invasion in response to YPSMA-1 monoclonal antibody (doses range from 0 to 0.1 mg/ml) or PMPA (doses range from 0 to 100 μM). The right panel shows HUVEC cell viability assay in the presence of 100 μM PMPA. IgG, immunoglobulin G; Abs, antibodies. (C) KS1767 cells transfected with PSMA siRNA have decreased PSMA protein levels. (D) Transfection of KS1767 cells with PSMA siRNA decreases invasion, (n = 3, P = 0.009). **, 0.01 < P > 0.001. Error bars indicate standard deviations.
While chemical inhibitors can often affect a group of proteins sharing similar functions, RNA interference is a strategy for specifically reducing the expression of a particular protein by sequence-specific degradation of its mRNA (13, 26, 36). Kaposi's sarcoma-derived KS1767 cells were transfected with siRNA specific for either PSMA or an irrelevant gene and assayed for PSMA protein expression. We have extensively characterized this cell line and found that it faithfully recapitulates many features of primary vascular endothelial cells (7, 8, 63). Western blot analysis confirmed that the transfection of PSMA siRNA but not irrelevant control siRNA preparations specifically reduced PSMA protein levels in the endothelial-derived cell line (Fig. 2C). In agreement with our results using inhibitors and antibodies, functional assessment of PSMA siRNA-transfected KS1767 cells showed that specific reduction of PSMA protein levels significantly hampered endothelial cell invasion by more than 50% relative to cells transfected with control preparations (Fig. 2D), thus confirming that PSMA is required for functional angiogenic processes.
PSMA participates in integrin signaling and FAK activation.
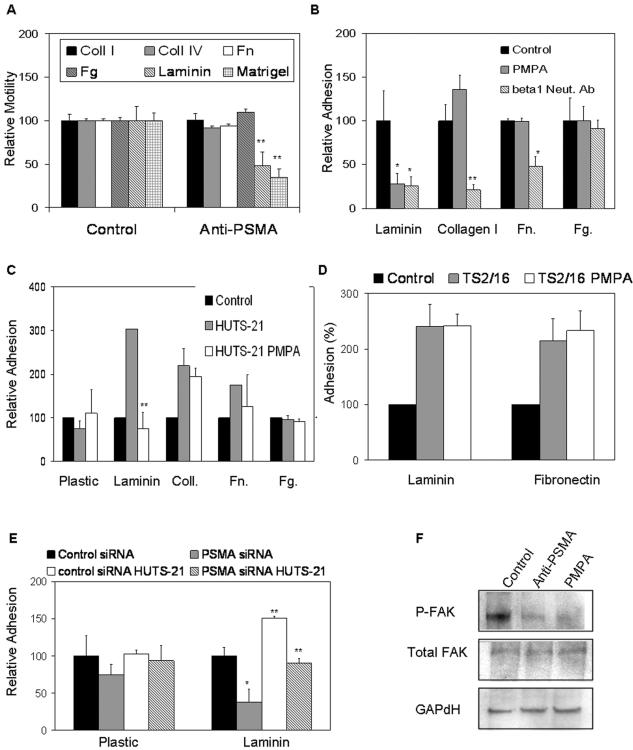
Cell motility in vivo is regulated by the cumulative interactions of specific integrins with individual extracellular matrix proteins that trigger the activation of signaling pathways critical for migration and invasion (for a review, see reference 67). To determine whether PSMA-dependent cell motility may be mediated through specific integrin signaling, we repeated our in vitro transwell assays using individual matrix proteins, including collagen I and IV, fibrinogen, fibronectin, and laminin in the presence or absence of PMPA. Interestingly, PMPA alters cell motility on laminin (the major component of Matrigel) but not on any of the other matrix proteins tested (Fig. 3A), suggesting that PSMA-dependent endothelial cell motility may be mediated specifically via laminin binding integrins. The specific integrin receptors (consisting of an α and a β subunit) that interact with laminin have been identified (6, 16, 43). While multiple α integrin subunits can be components of laminin binding integrins, integrins containing the β1 subunit are the predominant laminin receptors expressed on primary endothelial cells in culture (6, 16, 43). Therefore, we initially focused on this integrin subunit for our study of PSMA-dependent motility on laminin. To test whether PSMA regulation involves β1 integrin, we assayed the effects of manipulating PSMA on endothelial cell adhesion to laminin. Treatment of HUVECs with the PSMA antagonist impairs basal adhesion to laminin to the same extent as blocking integrin-specific interactions with a β1-neutralizing antibody (Fig. 3B). This interaction is specific for laminin, as demonstrated by the inability of PMPA to affect adhesion to other β1-dependent substrates collagen or fibronectin or to the β2 integrin substrate fibrinogen (Fig. 3B). Flow cytometric analysis of endothelial cells plated on laminin demonstrates that total cell surface levels of β1 integrin are unchanged after PMPA treatment, indicating that PSMA does not affect adhesion by regulating integrin expression (data not shown).
FIG. 3.
PSMA increases β1 integrin signaling in endothelial cells. (A) PSMA-dependent in vitro motility of HUVECs is specifically mediated through laminin (n = 3; P = 0.005) but not other matrix proteins. (B) PSMA antagonists inhibit basal adhesion of HUVECs to laminin equally as well as neutralizing β1 antibodies (Neut. Ab) do, but have no significant effect on binding to fibrinogen, fibronectin, or collagen. (C) Inhibition of PSMA interferes with activated β1 integrin (activated with HUTS-21 antibody) binding to laminin but not other substrates (n = 3; P = 0.002). (D) PSMA inhibition in the presence of the consitutively activating β1 antibody TS2/16 does not alter adhesion to laminin or fibronectin. (E) HUVECs transfected with siRNA targeted against PSMA show reduced basal (n = 3; P = 0.028) and β1-mediated (n = 3; P = 0.001) adhesion compared to that of cells transfected with control siRNA; (F) HUVECs treated with PMPA or anti-PSMA antibody have decreased levels of phosphorylated FAK. *, 0.05 < P > 0.01; **, 0.01 < P > 0.001. Error bars indicate standard deviations.
To further examine the connection between PSMA and β1 integrin, we specifically stimulated β1-mediated adhesion with two different β1-activating antibodies and measured cell adhesion in the presence of the PSMA inhibitor. The HUTS-21 antibody recognizes a β1 integrin epitope that is masked on inactive integrins but is exposed upon ligand binding or partial integrin activation (51), while the TS2/16 reagent binds to epitopes that are constitutively expressed regardless of the state of integrin activation (2). Upon binding, both of these antibodies are capable of inducing β1-dependent adhesion to its substrates (for a review, see reference 51) (Fig. 3B and C). However, while HUTS-21-stimulated endothelial cell adhesion to laminin but not other substrates is strikingly inhibited by the PSMA antagonist (Fig. 3C), adhesion to laminin following stimulation via the β1 constitutive epitope is not significantly affected (Fig. 3D). These results suggest that PSMA participates in the initial laminin binding, leading to HUTS-21 epitope exposure. Similarly, rescue of PMPA inhibition upon constitutive activation with TS2/16 implies that, once the integrin is activated, PSMA is no longer required. Parallel experiments measuring basal and induced adhesion of cells transfected with PSMA-directed siRNA confirmed results obtained with PSMA inhibition (Fig. 3E). Importantly, the fact that cells transfected with PSMA-directed siRNA were impaired in their abilities to adhere to laminin implies that the inhibitor or antibodies do not physically interfere with integrin-laminin interactions and that PSMA must be present for productive adhesion.
Finally, because integrin signal transduction typically results in the phosphorylation of the downstream effector FAK, we assessed phosphorylated FAK levels in cells treated with PSMA antagonists. FAK phosphorylation is significantly decreased upon inhibition of PSMA (Fig. 3F) or in cells treated with PSMA-directed siRNA (data not shown), again supporting our hypothesis that PSMA modulates laminin-specific β1 integrin function. These data distinguish PSMA from other cell-surface peptidases that have been implicated in regulating invasion but have not been shown to participate in integrin signaling (7, 8, 20, 54).
PSMA plays a role in PAK activation and endothelial cytoskeletal integrity.
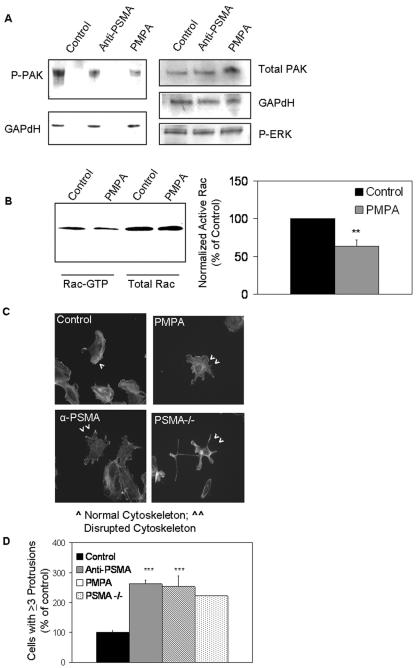
Because β1 integrin signaling has been shown to regulate endothelial cell motility, in part, by facilitating the activation of effector molecules such as PAK and Rac (73; for a review, see references 10 and 67), we hypothesized that PSMA may be involved in Rac or PAK activation. The members of the PAK family are well characterized effector molecules that mediate cell motility and the actin cytoskeleton (for a review, see reference 10). Treatment of HUVECs with either of the PSMA antagonists resulted in decreases in phosphorylated PAK protein levels (53, 55) but had no effect on activated extracellular signal-regulated kinase levels, indicating that PSMA contributes specifically to the activation of this pathway (Fig. 4A). Comparable results were obtained with cells treated with PSMA-directed siRNA (data not shown). The effect of PSMA on PAK activation appears to be mediated via small GTPases as the levels of activated Rac, a member of the Rho family of small GTPases that are upstream activators of PAK, are similarly decreased upon PSMA inhibition (Fig. 4B).
FIG. 4.
PSMA regulates PAK phosphorylation and cytoskeletal morphology. (A) HUVECs treated with PSMA inhibitor or neutralizing antibody have decreased levels of phosphorylated PAK, while total PAK and phosphorylated extracellular signal-regulated kinase levels are unaffected. P-ERK, phospho-extracellular signal-regulated kinase (ERK). (B) HUVECs treated with PMPA show reduced levels of activated Rac, while total Rac levels remain unchanged. Quantification of the average of three Rac activation experiments is shown by the graph (n = 3; P = 0.002). **, 0.01 < P > 0.001. (C) HUVECs treated with either the PMPA inhibitor, the PSMA neutralizing antibody (α-PSMA), or siRNA-treated endothelial cells isolated from PSMA-null (PSMA−/−) animals display actin-containing protrusions extending in multiple directions. (D) quantitation of cells with protrusions extending in at least three directions reveals an approximate twofold increase in PSMA-inhibited or PSMA-null (PSMA −/−) cells (P < 0.001). *** = 0.001< P > 0.0001. Error bars indicate standard deviations.
Dysregulated PAK activity in cells has been shown to result in morphologies typified by increased membrane protrusions and cells lacking a distinct leading edge, resulting in compromised directional migration and invasion (40, 67, 69). Similarly, we observed an increase in membrane protrusions in multiple directions (29) and an apparent loss of normal structural integrity of PSMA-null endothelial cells or in HUVECs incubated in the presence of PSMA antagonists (an average twofold increase in cells with multiple leading edges) (Fig. 4C and 4D). Although increased protrusions are generally associated with increased motility, cells lacking a distinct leading edge often exhibit compromised directional migration and invasion (40, 67, 69). Collectively, these observations suggest that PSMA is necessary for optimal PAK activation downstream of laminin binding β1 integrin and, thus, impacts cytoskeletal integrity and endothelial invasion.
Filamin A interacts with PSMA and regulates its activity in endothelial cells.
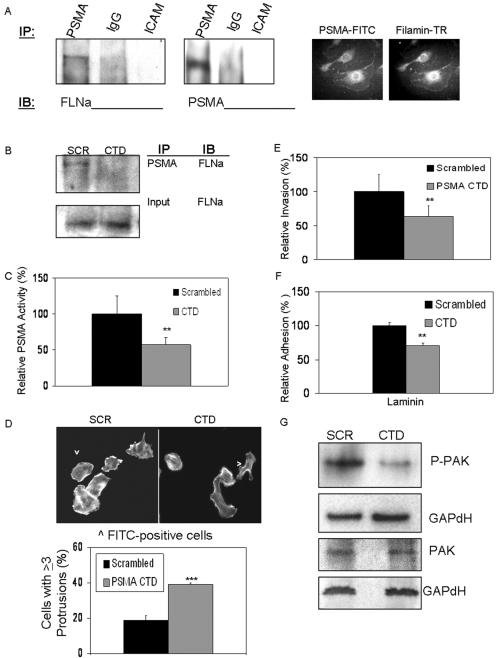
PAK regulates cytoskeletal integrity, in part, by its interaction with numerous substrates, such as myosin light chain kinase, caldesmon, and the actin binding protein ABP280/filamin A (10). Because the cytoplasmic domain of PSMA has also been shown to interact with filamin A both in vitro and in epithelial cells in vivo (1), we asked whether the PSMA/filamin A interaction could play a role in endothelial invasion. By coimmunoprecipitation of endogenous filamin A from HUVECs with anti-PSMA antibodies, we initially established that filamin A is indeed present in a complex with PSMA in endothelial cells (Fig. 5A). Importantly, filamin is not detected in complexes precipitated with antibodies recognizing ICAM, another transmembrane protein, demonstrating the specificity of this interaction. Importantly, PSMA does not associate with talin, a prominent cytoskeletal tether (data not shown). Additionally, filamin and PSMA exhibit similar localization patterns in migrating HUVECs labeled with PSMA-FITC and filamin-Texas Red antibodies (Fig. 5A), supporting the concept that PSMA and filamin may be associated in endothelial cells.
FIG. 5.
PSMA interaction with filamin A in endothelial cells regulates PSMA function. (A) Immunoprecipitation (IP) of PSMA but not immunoglobulin G (IgG) or ICAM-1 pulls down filamin A in HUVECs and filamin, and PSMA colocalizes in migrating HUVECs (PSMA-FITC and filamin-Texas Red [TR] antibodies). Control photobleaching data are provided in Fig. S1 of the supplemental material. (B) PSMA CTD peptide transfection in HUVECs disrupts PSMA/filamin A interaction. Disrupting PSMA/filamin interaction by transfecting the PSMA CTD peptide (C) decreases PSMA enzyme activity (P = 0.003), (D) disrupts endothelial cell morphology, resulting in an increase in membrane protrusions (P < 0.001), (E) decreases endothelial cell invasion (P = 0.002), (F) decreases endothelial cell adhesion to laminin (P = 0.001), and (G) decreases PAK activation as measured by phosphorylated-PAK levels. SCR, scrambled peptides; IB, immunoblot. **, 0.01 < P > 0.001; ***, 0.001 < P > 0.0001. Error bars indicate standard deviations.
To investigate the functional significance of the endothelial PSMA/filamin interaction, we designed a peptide corresponding to the 19-amino-acid CTD of PSMA (1) to compete with endogenous PSMA for binding to filamin A. Filamin no longer coimmunoprecipitated with anti-PSMA in endothelial cells transfected with the CTD peptide, confirming its ability to disrupt PSMA/filamin interactions (Fig. 5B). Because alterations in cytoskeletal attachments often affect the function of the coupled molecules (12, 56, 64), we investigated the effect of disruption of the PSMA/filamin A association on PSMA activity. CTD peptide transfection into cells (as determined by cotransfected FITC-labeled control protein) reduced PSMA activity (Fig. 5C), possibly due to destabilization of PSMA on the cell surface. Because we have shown that PSMA activity is important for a number of cell functions, we predicted that the decrease in PSMA activity generated by disruption of PSMA/filamin association would disrupt these processes as well. Indeed, normal actin cytoskeletal dynamics are predictably compromised (Fig. 5D) and endothelial cell invasion is decreased (Fig. 5E), as is β1 integrin-mediated adhesion (Fig. 5F), in cells transfected with the competing CTD peptide compared with cells transfected with scrambled control peptide. Finally, PAK phosphorylation is diminished when PSMA/filamin binding is abrogated (Fig. 5G), thus implicating filamin A as a regulator of PSMA activity and confirming the link between PSMA activity, integrin activation, PAK activity, and endothelial cell invasion.
PSMA is regulated by negative feedback mechanisms involving integrin β1 and PAK.
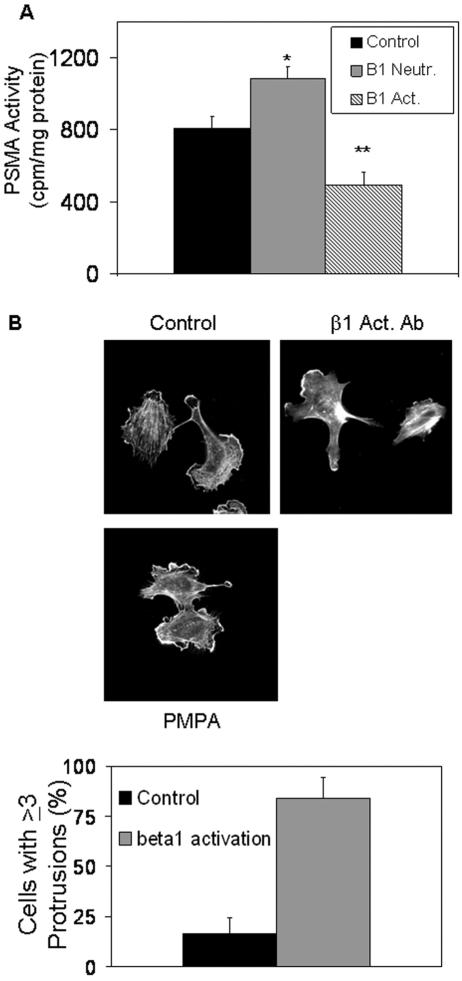
We have identified several key molecules that are important in PSMA-mediated endothelial cell invasion. All cellular processes contributing to invasion and migration events must be tightly regulated, as productive motility requires the precisely controlled activation of essential proteins (67). Based on our results, unregulated PSMA activity would be predicted to lead to constant β1 integrin signaling and constitutively activated PAK. However, since uncontrolled PAK activation is known to result in dysregulated motility (40, 67, 69), these positive signals must be modulated for invasion to proceed. Therefore, we asked whether PSMA activity is also regulated, allowing for precise modulation of downstream events leading to invasion. To initially test this possibility, we investigated the effects of β1 integrin activation and signal transduction on PSMA function. When β1 function is inhibited in endothelial cells with a neutralizing antibody, we observed an increase in PSMA activity, whereas β1 activation with an activating antibody decreased activity, suggesting that β1 regulates PSMA (Fig. 6A). Flow cytometric analysis indicates that PSMA expression levels are unchanged under conditions where β1 is activated (adhesion to laminin), arguing that the β1 effects are not likely due to alterations in PSMA expression (data not shown). Consistent with these results, HUVECs incubated on laminin-coated cell culture dishes in the presence of the β1-activating antibody display the characteristic morphology of protrusions in multiple directions, mimicking the effect mediated by PSMA inhibition and compatible with a mechanism where an overriding β1 integrin signal results in a decrease in PSMA activity (Fig. 6B). These results are in agreement with our model of interacting regulatory mechanisms linking PSMA and β1 integrin.
FIG. 6.
Integrin β1 regulates PSMA activity. (A) β1 integrin inhibition (Neutr.) increases PSMA activity (n = 3; P = 0.01), while β1 activation (Act.) decreases PSMA activity (n = 3; P = 0.001). *, 0.05 < P > 0.01; **, 0.01 < P > 0.001. (B) HUVECs incubated on laminin-coated cell culture dishes with a β1-activating antibody (β1-Act. Ab) display protrusions in multiple directions, similar to those of PSMA inhibited cells (PMPA treatment). Error bars indicate standard deviations.
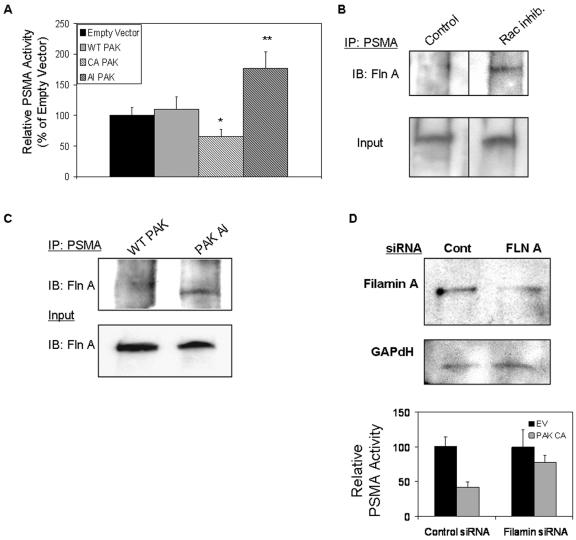
Having implicated PAK in PSMA-mediated invasion, we next determined the consequences of PAK manipulation on PSMA activity. We transiently transfected expression plasmids encoding either wild-type, constitutively active, or the autoinhibitory domain of PAK-1 that interrupts PAK activation (45) into primary endothelial cells (Fig. 7A; also see Fig. S2 in the supplemental material). PSMA activity is predictably upregulated by inhibition of PAK and downregulated by uncontrolled PAK activation. In addition, activated PAK has been shown to physically interact with filamin A, leading to the phosphorylation of filamin on Ser2152 and a boost in PAK activity (19). We hypothesized that the binding of activated PAK to filamin would disrupt PSMA/filamin interactions, and as we have shown, cause a decrease in PSMA activity, thus forming a mechanistic explanation for our observations. To examine this possibility, we evaluated the PSMA/filamin association in endothelial cells under conditions where PAK is inhibited. The immunoprecipitation of PSMA in cells treated with Rac inhibitor (Fig. 7B) or transfected with the autoinhibitory PAK expression construct (Fig. 7C) shows an increase in the association of filamin with PSMA, suggesting that the activation status of PAK impacts PSMA/filamin association and, thus, regulates PSMA enzymatic activity. According to this hypothesis, knock down of filamin A in HUVECs should rescue loss of PSMA activity in the presence of activated PAK. Therefore, we introduced filamin A-specific siRNA in HUVECs prior to transfection with the constitutively active PAK construct. As expected, cells with reduced filamin A protein levels were less sensitive to the effects of constitutively active PAK on PSMA activity (Fig. 7D), further implicating filamin A as an important link between PAK and PSMA.
FIG. 7.
PAK regulates PSMA activity. (A) PSMA activity is decreased in cells transfected with a construct encoding a constitutively active (CA) PAK mutant (P = 0.045) and increased in cells transfected with a plasmid encoding the PAK autoinhibitory (AI) domain (P = 0.001). Results are the average of three independent experiments performed in triplicate. WT, wild type. *, 0.05 < P > 0.01; **, 0.01 < P > 0.001. Error bars indicate standard deviations. (B) Filamin/PSMA interaction is increased in cells treated with the Rac inhibitor; a nonspecific band is shown as a loading control. Fln A, filamin A; IP, immunoprecipitate; IB, immunoblot. (C) HUVECs transfected with autoinhibitory (AI) PAK also show increased PSMA/filamin interaction. Experiments controlling for the expression of the PAK constructs transfected in HUVECs are provided in Fig. S2 of the supplemental material. Fln A, filamin A; IP, immunoprecipitate; IB, immunoblot; WT, wild type. (D) HUVECs transfected with filamin A (FLN A) siRNA show reduced levels of filamin A protein compared to cells transfected with control siRNA (Cont). Additionally, reducing filamin A levels in HUVECs rescues loss of PSMA activity caused by transfecting constitutively active (CA) PAK constructs into HUVECs (for control siRNA, P < 0.001; for filamin A siRNA, P = 0.149). EV, empty vector. Error bars indicate standard deviations.
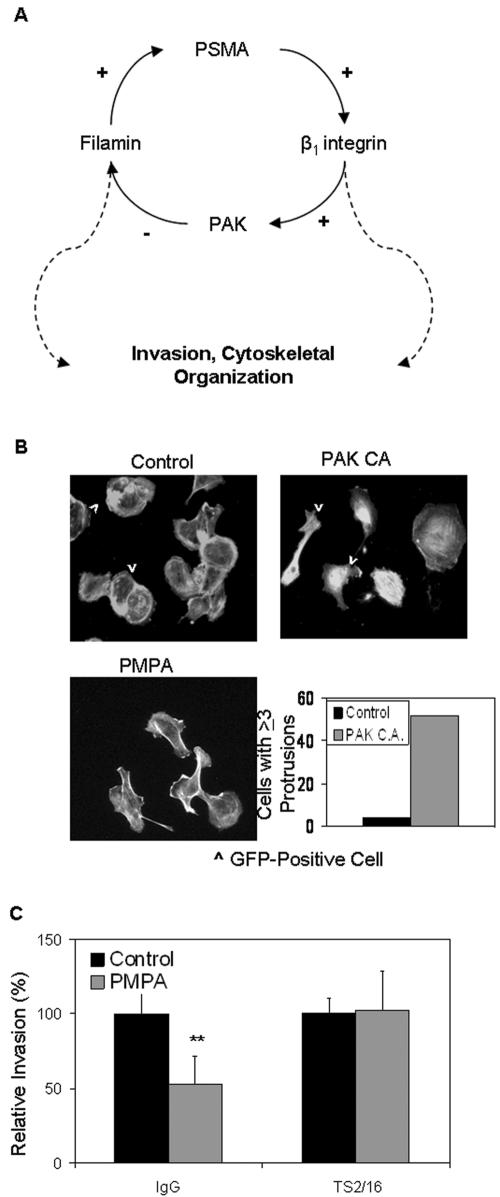
Taken together, our results are consistent with a negative feedback loop model of PSMA regulation in endothelial cells, where PAK activation leads to both productive invasion and a downregulation of laminin-specific integrin β1 signaling via reduction in PSMA activity (Fig. 8A). To relate this pathway to endothelial functions that are critical for angiogenesis, we determined the effect of dysregulated PAK on endothelial cell cytoskeletal integrity. The transfection of cells with the constitutively active PAK mutant expression plasmid, along with a plasmid encoding green fluorescent protein to identify cells containing transfected plasmids, showed a phenotype very similar to those of PMPA-treated cells with multiple protrusions extending in disparate directions (Fig. 8B), indicating that sustained activation of PAK-1 causes cytoskeletal alterations. Similarly, PMPA-inhibited endothelial invasion is rescued in the presence of the β1-activating antibody (TS2/16) but not a control antibody (Fig. 8C). These results demonstrate that PSMA-mediated regulation of integrin activation and PAK activity are critical for endothelial cell invasion and angiogenesis. Thus, this tight regulation of sequential protein activation/deactivation steps essential for motility and invasion identifies a novel role for PSMA as a functional contributor to tumor neovascularization.
FIG. 8.
PSMA-mediated PAK and integrin activation affect endothelial cell function. (A) Hypothetical mechanism for PSMA regulation of endothelial cell invasion. PSMA positively regulates β1 integrin signaling and PAK phosphorylation in endothelial cells. Because PAK activation/deactivation must be precisely regulated for productive motility, we predict that PAK activation negatively regulates PSMA activity. Therefore, these components constitute a regulatory loop of PSMA regulation in endothelial cells, where β1 integrin signal trans duction and subsequent PAK activation leads to both productive invasion and downregulation of integrin β1 signaling via reduction in PSMA activity. (B) Cells transfected with plasmids encoding constitutively active (CA) PAK display increased protrusions in multiple directions. GFP, green fluorescent protein. (C) β1 activation by the constitutively activating antibody TS2/16 rescues PSMA-dependent invasion in the presence of the PMPA inhibitor. IgG, immunoglobulin G. **, 0.01< P > 0.001. Error bars indicate standard deviations.
DISCUSSION
PSMA was originally described as a highly abundant protein in prostate tumors whose expression level correlates with poor prognosis. Subsequent histological observations demonstrated that PSMA is also highly expressed on the vasculature of virtually every solid tumor examined (19, 47). While its function in the prostate remains unclear, its specific expression on angiogenic vasculature suggests that it participates in neovessel growth in developing tumors, a role similar to those of other cell surface peptidases with analogous vascular expression patterns (for a review, see reference 5). This possibility was confirmed by our in vivo studies in PSMA-null animals and using PSMA antagonists where angiogenesis is significantly impaired in the Matrigel implant model. Because Matrigel is derived from a murine chondrosarcoma, the matrix proteins present in this model are physiologically relevant to the extracellular matrices surrounding tumors in vivo (42, 60). Further investigation into the mechanistic basis of PSMA activity in angiogenesis focused on endothelial function in the presence of PSMA inhibitors. Primary endothelial cells treated with the functional inhibitor or neutralizing antibodies suggested that PSMA enzymatic activity was required for endothelial cell invasion through the extracellular matrix in vitro but did not contribute significantly to endothelial viability, proliferation, or morphogenesis (Fig. 2B and data not shown). Finally, both cells containing PSMA-specific siRNA or purified primary endothelial cells from PSMA-null animals resulted in significant reductions in invasive capacity and laminin-specific adhesion, again confirming a role for PSMA in endothelial cell invasion and, thus, angiogenesis. Our data, coupled with the high expression of PSMA in angiogenic vasculature and the prominent presence of laminin in the tumor microenvironment, identify a novel role for PSMA as a functional contributor to tumor neovascularization.
Because extensive neovascularization occurs during development, the fact that PSMA-null animals are viable and fertile (3) suggests that PSMA is not essential for angiogenesis. However, examples of gene-deficient animals that develop normal vasculature yet fail to mount efficient angiogenic responses in pathological situations have been described previously (e.g., placental growth factor, PlGF [14], platelet/endothelial cell adhesion molecule 1 [25], and aminopeptidase A [54]). Of particular relevance to our work is the aminopeptidase A-deficient model that deletes a gene which also encodes a cell surface metallopeptidase (54). These animals show no overt phenotype, but they are severely impaired in their abilities to form new capillaries in response to ischemia or angiogenic growth factors. These results strongly support the notion that unique mechanisms exist to regulate developmental versus pathological angiogenesis, and it is intriguing to speculate that cell surface peptidases, such as aminopeptidase A and PSMA, may participate in solely the pathological response and not the developmental angiogenic response.
An initial clue to the mechanism of PSMA's role in angiogenesis was provided by our observation that PSMA-dependent endothelial invasion was strictly laminin specific, suggesting that distinct integrins might be involved in this process. This possibility was supported by our finding that the functional activity and activation of downstream effectors of the laminin binding β1 integrin are significantly decreased in the absence of functional PSMA. Integrin activation and its subsequent transduction into cellular processes such as adhesion, migration, and invasion are precisely controlled at numerous points that could provide possible sites for PSMA participation. For example, the binding of integrins to their extracellular ligands is profoundly impacted by mechanism including conformational changes in extracellular domains, alterations in integrin expression levels, changes in cytoskeletal tethers, integrin clustering, and receptor diffusion, all of which cooperate to facilitate productive integrin-ligand interactions. Alternately, increased integrin activation can result from the exposure of cryptic matrix epitopes following cleavage of extracellular ligands such as laminin (for a review, see reference 11). Therefore, PSMA may conceivably act at any of a number of regulatory steps. However, relevant insights may be found in results from our adhesion studies using the β1-activating antibodies TS2/16 and HUTS-21. While TS2/16 recognizes a constitutively exposed site, the HUTS-21 antibody binds to an epitope on the β1 chain that is exposed only after a conformational change that is triggered by the initial interaction with ligand. HUTS-21 binding to this liberated epitope then secures the integrin in a maximally high-affinity conformation and enhances adhesion (51). The fact that PSMA dependence persists in the presence of HUTS-21 suggests that the peptidase may contribute to the initial ligand binding that leads to HUTS-21 epitope exposure. Similarly, the rescue of PMPA inhibition upon constitutive activation by TS2/16 suggests that, once the integrin is fully activated, PSMA no longer plays a role. Since PSMA cleaves small peptides, it is intriguing to speculate that a novel regulatory PSMA peptide substrate (perhaps derived from laminin itself) that is found at angiogenic sites is then cleaved by PSMA to facilitate integrin activation. In support of this notion, the degradation of the extracellular matrix is known to produce smaller proteolytic fragments with pro- or antiangiogenic activity which affects integrin function (52, 58, 59) and also has been shown to create integrin binding sites (78) The identification of putative angiogenic peptidase substrates and their possible effects on angiogenesis are the subjects of active investigation in our laboratory.
The link between the extracellular space and the cytoskeleton is critically important to diverse cell functions, including motility and invasion. Many of these connections involve interactions between integrins and actin binding proteins, including filamin (12, 50, 64), which we find interacts with PSMA in endothelial cells. Similar to our findings, filamin A was also found to interact with PSMA using either purified bacterial proteins in vitro or in prostate epithelial cells in vivo (1) and, thus, may be a common transmembrane link to the cytoskeleton in various cell types. While filamin A regulates the actin cytoskeleton by facilitating the branching and polymerization of actin necessary for cell invasion (30), it has also been described to regulate cell motility by binding to transmembrane molecules and modulating their stability. For example, the tight interaction of the β7 integrin cytoplasmic tail with filamin A regulates integrin function by stabilizing the actin microfilaments at the site of integrin attachment, thus regulating cell protrusions (12). Similarly, exogenous expression of filamin in filamin A-deficient cells boosts cell surface levels of β1 integrin (56). Though PSMA levels are unchanged, we see that the enzyme activity of PSMA is also regulated by its interaction with filamin, perhaps by a similar stabilizing mechanism that would result in increased activity, enhanced integrin signal transduction, and cell invasion. Because the interactions of integrins with their cytoskeletal tethers are quite specific (48) and changes in these cytoskeletal tethers can both contribute to and result from integrin activation (11), it is logical that the activity of other transmembrane molecules may be similarly controlled by regulated interactions with the cytoskeleton.
Further investigation of signaling mechanisms downstream of integrin activation that may mediate PSMA-dependent invasion was prompted, in part, by the striking morphological changes observed in endothelial cells purified from PSMA-null animals or cultured in the presence of PSMA antagonists. These cells consistently showed an altered phenotype characterized by numerous lamellipodia-like protrusions extending in multiple directions, suggesting a role for PSMA in maintaining normal cytoskeletal dynamics, consistent with its role in invasion (21, 79). Because the effects on PSMA-inhibited cells resembled those observed in cells containing dysregulated PAK (40, 69), we investigated the phosphorylation status of PAK in these cells. Significantly, we found that levels of phosphorylated PAK are decreased in the presence of both PSMA antibodies and inhibitor, suggesting that PSMA's effects on the cytoskeleton may be mediated by influencing the activity of cytoskeletal effector proteins. In support of this concept, the expression of either constitutively active or dominant-negative PAK expression plasmids in microvascular endothelial cells has been shown to result in a reduction in growth factor-stimulated motility (40), illustrating the critical importance of precise modulation of PAK activity for polarized cell movement and the necessity for multiple regulatory mechanisms (for a review, see reference 10). PAK is often activated by members of the Rho family of small GTPases, and we find that the PSMA antagonists also affect Rac activation, which is similar to the results of studies where integrin activation results in small GTPase-dependent activation of PAK (for a review, see reference 10). Therefore, PSMA's influence on β1 integrin-dependent PAK activation and ensuing cytoskeletal consequences likely contributes to its regulation of endothelial cell invasion.
How does the activation of PAK regulate PSMA activity? It is well documented that alterations in the anchors linking the cytoplasmic domains of transmembrane proteins to the cytoskeleton can profoundly affect the activity of extracellular domains, as exemplified by integrin activation (11). As previously mentioned, both PSMA and activated PAK have been shown to physically interact with the actin binding protein filamin A in epithelial cells (1, 77). We find that PSMA and filamin are also associated in endothelial cells and that activation of PAK disrupts the PSMA/filamin complex and decreases PSMA activity, possibly by decreasing its surface stability. In epithelial cells, the PAK/filamin interaction is essential for motility, presumably by serving as a scaffold for PAK activation (77). Both the PSMA and PAK interactions have been mapped to the filamin dimerization domain contained in repeats 22 to 24 (1, 77). It is intriguing, therefore, to speculate that, upon endothelial integrin engagement, PAK is activated, thus promoting its interaction with filamin and supporting cell motility. However, the binding of activated PAK to filamin could disrupt PSMA/filamin interactions, thus affecting PSMA stability and inhibiting PSMA enzymatic activity, leading to diminished integrin function and subsequent downregulation of PAK activation. Elucidation of the precise mechanisms regulating intermediate steps in this negative regulatory loop is the subject of ongoing investigation in our labratory.
The results of the current study add PSMA to an increasing number of cell-surface peptidases that contribute to angiogenesis. It will be important to understand how these peptidases interact globally to promote neovascularization; for instance, if distinct subpopulations of endothelial cells are dependent on distinct peptidases or if multiple peptidases function collectively to promote endothelial cell function. In this regard, emerging results from our laboratory on the function of the CD13 cell surface peptidase suggest that, while both peptidases regulate angiogenesis by controlling endothelial invasion, CD13 functions via a mechanism distinct from that of PSMA (N. Petrovic, W. Schacke, X. Liu, P. Mina-Osorio, and L. H. Shapiro, submitted for publication). However, a more complete characterization of the functions of the individual peptidases in the regulation of neovascularization is necessary before potential cooperative roles can be definitively established.
Supplementary Material
Acknowledgments
We thank Timothy Hla, Kevin Claffey, Ann Cowan, Wolfgang Schacke, Kathleen Mahoney, and Xiufang Liu for technical assistance and for helpful discussions, Carol Pilbeam for use of photomicrographic equipment, Kelley Harsch for excellent technical assistance with the animals, and David Shapiro for critical reading of the manuscript.
This study was supported by NIH grants R01 CA 85714 and R01 HL 69442 to L.H.S. and NIH grant CA101069 to W.H.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anilkumar, G., S. A. Rajasekaran, S. Wang, O. Hankinson, N. H. Bander, and A. K. Rajasekaran. 2003. Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63:2645-2648. [PubMed] [Google Scholar]
- 2.Arroyo, A. G., A. Garcia-Pardo, and F. Sanchez-Madrid. 1993. A high affinity conformational state on VLA integrin heterodimers induced by an anti-β1 chain monoclonal antibody. J. Biol. Chem. 268:9863-9868. [PubMed] [Google Scholar]
- 3.Bacich, D. J., E. Ramadan, D. S. O'Keefe, N. Bukhari, I. Wegorzewska, O. Ojeifo, R. Olszewski, C. C. Wrenn, T. Bzdega, B. Wroblewska, W. D. Heston, and J. H. Neale. 2002. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J. Neurochem. 83:20-29. [DOI] [PubMed] [Google Scholar]
- 4.Barker, J. N. 1991. The pathophysiology of psoriasis. Lancet 338:227-230. [DOI] [PubMed] [Google Scholar]
- 5.Bauvois, B. 2004. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene 23:317-329. [DOI] [PubMed] [Google Scholar]
- 6.Belkin, A. M., and M. A. Stepp. 2000. Integrins as receptors for laminins. Microsc. Res. Tech. 51:280-301. [DOI] [PubMed] [Google Scholar]
- 7.Bhagwat, S. V., J. Lahdenranta, R. Giordano, W. Arap, R. Pasqualini, and L. H. Shapiro. 2001. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97:652-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhagwat, S. V., N. Petrovic, Y. Okamoto, and L. H. Shapiro. 2003. The angiogenic regulator CD13/APN is a transcriptional target of Ras signaling pathways in endothelial morphogenesis. Blood 101:1818-1826. [DOI] [PubMed] [Google Scholar]
- 9.Bischofberger, J., and D. Schild. 1996. Glutamate and N-acetylaspartylglutamate block HVA calcium currents in frog olfactory bulb interneurons via an mGluR2/3-like receptor. J. Neurophysiol. 76:2089-2092. [DOI] [PubMed] [Google Scholar]
- 10.Bokoch, G. M. 2003. Biology of the P21-activated kinases. Ann. Rev. Biochem. 72:743-781. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood, D. A. 2004. Integrin activation. J. Cell Sci. 117:657-666. [DOI] [PubMed] [Google Scholar]
- 12.Calderwood, D. A., A. Huttenlocher, W. B. Kiosses, D. M. Rose, D. G. Woodside, M. A. Schwartz, and M. H. Ginsberg. 2001. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat. Cell Biol. 3:1060-1068. [DOI] [PubMed] [Google Scholar]
- 13.Caplen, N. J., S. Parrish, F. Imani, A. Fire, and R. A. Morgan. 2001. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98:9742-9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet, P., L. Moons, A. Luttun, V. Vincenti, V. Compernolle, M. de Mol, Y. Wu, F. Bono, L. Devy, H. Beck, D. Scholz, T. Acker, T. DiPalma, M. Dewerchin, A. Noel, I. Stalmans, A. Barra, S. Blacher, T. Vandendriessche, A. Ponten, U. Eriksson, K. H. Plate, J. M. Foidart, W. Schaper, D. S. Charnock-Jones, D. J. Hicklin, J. M. Herbert, D. Collen, and M. G. Persico. 2001. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7:575-583. [DOI] [PubMed] [Google Scholar]
- 15.Carter, R. E., A. R. Feldman, and J. T. Coyle. 1996. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Natl. Acad. Sci. USA 93:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter, W., E. Wayner, T. Bouchard, and P. Kaur. 1990. The role of integrins α2β1 and α2β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J. Cell Biol. 110:1387-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang, S. S., and W. D. Heston. 2002. The clinical role of prostate-specific membrane antigen (PSMA). Urol. Oncol. 7:7-12. [DOI] [PubMed] [Google Scholar]
- 18.Chang, S. S., D. S. O'Keefe, D. J. Bacich, V. E. Reuter, W. D. Heston, and P. B. Gaudin. 1999. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 5:2674-2681. [PubMed] [Google Scholar]
- 19.Chang, S. S., V. E. Reuter, W. D. Heston, N. H. Bander, L. S. Grauer, and P. B. Gaudin. 1999. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59:3192-3198. [PubMed] [Google Scholar]
- 20.Chen, W. T. 2003. DPPIV and seprase in cancer invasion and angiogenesis. Adv. Exp. Med. Biol. 524:197-203. [DOI] [PubMed] [Google Scholar]
- 21.Chou, J., N. A. Burke, A. Iwabu, S. C. Watkins, and A. Wells. 2003. Directional motility induced by epidermal growth factor requires Cdc42. Exp. Cell Res. 287:47-56. [DOI] [PubMed] [Google Scholar]
- 22.Colville-Nash, P. R., and D. L. Scott. 1992. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann. Rheum. Dis. 51:919-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doshi, S. N., S. J. Moat, M. J. Lewis, I. F. McDowell, J. C. Giddings, and J. Goodfellow. 2004. Short-term high-dose folic acid does not alter markers of endothelial cell damage in patients with coronary heart disease. Int. J. Cardiol. 94:203-207. [DOI] [PubMed] [Google Scholar]
- 24.Drachenberg, D. E., A. A. Elgamal, R. Rowbotham, M. Peterson, and G. P. Murphy. 1999. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 41:127-133. [DOI] [PubMed] [Google Scholar]
- 25.Duncan, G. S., D. P. Andrew, H. Takimoto, S. A. Kaufman, H. Yoshida, J. Spellberg, J. Luis de la Pompa, A. Elia, A. Wakeham, B. Karan-Tamir, W. A. Muller, G. Senaldi, M. M. Zukowski, and T. W. Mak. 1999. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J. Immunol. 162:3022-3030. [PubMed] [Google Scholar]
- 26.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 27.Folkman, J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27-31. [DOI] [PubMed] [Google Scholar]
- 28.Folkman, J., and P. A. D'Amore. 1996. Blood vessel formation: what is its molecular basis? Cell 87:1153-1155. [DOI] [PubMed] [Google Scholar]
- 29.Fukata, M., T. Watanabe, J. Noritake, M. Nakagawa, M. Yamaga, S. Kuroda, Y. Matsuura, A. Iwamatsu, F. Perez, and K. Kaibuchi. 2002. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109:873-885. [DOI] [PubMed] [Google Scholar]
- 30.Gorlin, J. B., R. Yamin, S. Egan, M. Stewart, T. P. Stossel, D. J. Kwiatkowski, and J. H. Hartwig. 1990. Human endothelial actin binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 111:1089-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan, D., G. Christofori, P. Naik, and J. Arbeit. 1996. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur. J. Cancer 32A:2386-2393. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan, D., and J. Folkman. 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353-364. [DOI] [PubMed] [Google Scholar]
- 33.Harris, E. D., Jr. 1986. Pathogenesis of rheumatoid arthritis. Am. J. Med. 80:4-10. [DOI] [PubMed] [Google Scholar]
- 34.Horoszewicz, J. S., E. Kawinski, and G. P. Murphy. 1987. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 7:927-935. [PubMed] [Google Scholar]
- 35.Huang, X., M. Bennett, and P. E. Thorpe. 2004. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate 61:1-11. [DOI] [PubMed] [Google Scholar]
- 36.Hutvagner, G., and P. D. Zamore. 2002. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297:2056-2060. [DOI] [PubMed] [Google Scholar]
- 37.Israeli, R. S., W. H. Miller, Jr., S. L. Su, D. S. Samadi, C. T. Powell, W. D. Heston, G. J. Wise, and W. R. Fair. 1995. Sensitive detection of prostatic hematogenous tumor cell dissemination using prostate specific antigen and prostate specific membrane-derived primers in the polymerase chain reaction. J. Urol. 153:573-577. [DOI] [PubMed] [Google Scholar]
- 38.Israeli, R. S., C. T. Powell, W. R. Fair, and W. D. Heston. 1993. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 53:227-230. [PubMed] [Google Scholar]
- 39.Jackson, P. F., D. C. Cole, B. S. Slusher, S. L. Stetz, L. E. Ross, B. A. Donzanti, and D. A. Trainor. 1996. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J. Med. Chem. 39:619-622. [DOI] [PubMed] [Google Scholar]
- 40.Kiosses, W. B., R. H. Daniels, C. Otey, G. M. Bokoch, and M. A. Schwartz. 1999. A role for p21-activated kinase in endothelial cell migration. J. Cell Biol. 147:831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitlinska, J., E. W. Lee, S. Movafagh, J. Pons, and Z. Zukowska. 2002. Neuropeptide Y-induced angiogenesis in aging. Peptides 23:71-77. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman, H. K., and G. R. Martin. 2005. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 15:378-386. [DOI] [PubMed] [Google Scholar]
- 43.Kramer, R. H. 1994. Characterization of laminin binding integrins. Methods Enzymol. 245:129-147. [DOI] [PubMed] [Google Scholar]
- 44.Lee, M., S. Thangada, J. Paik, G. P. Sapkota, N. Ancellin, S. Chae, M. Wu, M. Morales-Ruiz, W. C. Sessa, D. R. Alessi, and T. Hla. 2001. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8:693-704. [DOI] [PubMed] [Google Scholar]
- 45.Li, Z., M. Hannigan, Z. Mo, B. Liu, W. Lu, Y. Wu, A. V. Smrcka, G. Wu, L. Li, M. Liu, C. K. Huang, and D. Wu. 2003. Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell 114:215-227. [DOI] [PubMed] [Google Scholar]
- 46.Lintula, S., and U. H. Stenman. 1997. The expression of prostate-specific membrane antigen in peripheral blood leukocytes. J. Urol. 157:1969-1972. [PubMed] [Google Scholar]
- 47.Liu, H., P. Moy, S. Kim, Y. Xia, A. Rajasekaran, V. Navarro, B. Knudsen, and N. H. Bander. 1997. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 57:3629-3634. [PubMed] [Google Scholar]
- 48.Liu, S., D. Calderwood, and M. Ginsberg. 2000. Integrin cytoplasmic domain binding proteins. J. Cell Sci. 113:3563-3571. [DOI] [PubMed] [Google Scholar]
- 49.Liu, Z. Y., R. K. Ganju, J. F. Wang, K. Schweitzer, B. Weksler, S. Avraham, and J. E. Groopman. 1997. Characterization of signal transduction pathways in human bone marrow endothelial cells. Blood 90:2253-2259. [PubMed] [Google Scholar]
- 50.Loo, D. T., S. B. Kanner, and A. Aruffo. 1998. Filamin binds to the cytoplasmic domain of the β1-integrin. Identification of amino acids responsible for this interaction. J. Biol. Chem. 273:23304-23312. [DOI] [PubMed] [Google Scholar]
- 51.Luque, A., M. Gomez, W. Puzon, Y. Takada, F. Sanchez-Madrid, and C. Cabanas. 1996. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J. Biol. Chem. 271:11067-11075. [DOI] [PubMed] [Google Scholar]
- 52.Maeshima, Y., P. C. Colorado, A. Torre, K. A. Holthaus, J. A. Grunkemeyer, M. B. Ericksen, H. Hopfer, Y. Xiao, I. E. Stillman, and R. Kalluri. 2000. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J. Biol. Chem. 275:21340-21348. [DOI] [PubMed] [Google Scholar]
- 53.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 54.Marchio, S., J. Lahdenranta, R. O. Schlingemann, D. Valdembri, P. Wesseling, M. A. Arap, A. Hajitou, M. G. Ozawa, M. Trepel, R. J. Giordano, D. M. Nanus, H. B. Dijkman, E. Oosterwijk, R. L. Sidman, M. D. Cooper, F. Bussolino, R. Pasqualini, and W. Arap. 2004. Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell 5:151-162. [DOI] [PubMed] [Google Scholar]
- 55.Martin, G. A., G. Bollag, F. McCormick, and A. Abo. 1995. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 14:4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer, S. C., D. A. Sanan, and J. E. Fox. 1998. Role of actin binding protein in insertion of adhesion receptors into the membrane. J. Biol. Chem. 273:3013-3020. [DOI] [PubMed] [Google Scholar]
- 57.O'Keefe, D. S., S. L. Su, D. J. Bacich, Y. Horiguchi, Y. Luo, C. T. Powell, D. Zandvliet, P. J. Russell, P. L. Molloy, N. J. Nowak, T. B. Shows, C. Mullins, R. A. Vonder Haar, W. R. Fair, and W. D. Heston. 1998. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim. Biophys. Acta 1443:113-127. [DOI] [PubMed] [Google Scholar]
- 58.O'Reilly, M. S., T. Boehm, Y. Shing, N. Fukai, G. Vasios, W. S. Lane, E. Flynn, J. R. Birkhead, B. R. Olsen, and J. Folkman. 1997. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277-285. [DOI] [PubMed] [Google Scholar]
- 59.O'Reilly, M. S., L. Holmgren, Y. Shing, C. Chen, R. A. Rosenthal, M. Moses, W. S. Lane, Y. Cao, E. H. Sage, and J. Folkman. 1994. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79:315-328. [DOI] [PubMed] [Google Scholar]
- 60.Orkin, R. W., P. Gehron, E. B. McGoodwin, G. R. Martin, T. Valentine, and R. Swarm. 1977. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 145:204-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasqualini, R., E. Koivunen, R. Kain, J. Lahdenranta, M. Sakamoto, A. Stryhn, R. A. Ashmun, L. H. Shapiro, W. Arap, and E. Ruoslahti. 2000. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 60:722-727. [PMC free article] [PubMed] [Google Scholar]
- 62.Passaniti, A., R. M. Taylor, R. Pili, Y. Guo, P. V. Long, J. A. Haney, R. R. Pauly, D. S. Grant, and G. R. Martin. 1992. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Investig. 67:519-528. [PubMed] [Google Scholar]
- 63.Petrovic, N., S. V. Bhagwat, W. J. Ratzan, M. C. Ostrowski, and L. H. Shapiro. 2003. CD13/APN transcription is induced by RAS/MAPK-mediated phosphorylation of Ets-2 in activated endothelial cells. J. Biol. Chem. 278:49358-49368. [DOI] [PubMed] [Google Scholar]
- 64.Pfaff, M., S. Liu, D. J. Erle, and M. H. Ginsberg. 1998. Integrin β cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 273:6104-6109. [DOI] [PubMed] [Google Scholar]
- 65.Pinto, J. T., B. P. Suffoletto, T. M. Berzin, C. H. Qiao, S. Lin, W. P. Tong, F. May, B. Mukherjee, and W. D. Heston. 1996. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 2:1445-1451. [PubMed] [Google Scholar]
- 66.Ribatti, D., A. Vacca, L. Roncali, and F. Dammacco. 1991. Angiogenesis under normal and pathological conditions. Haematologica 76:311-320. [PubMed] [Google Scholar]
- 67.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 302:1704-1709. [DOI] [PubMed] [Google Scholar]
- 68.Sarker, K. P., M. Nakata, T. Nakajirna, I. Kitajirna, and I. Maruyarna. 1999. Increased production of vascular endothelial growth factor (VEGF) by angiotensin II in neuro-2A cells. Neurosci. Res. Commun. 25:79-88. [Google Scholar]
- 69.Sells, M. A., J. T. Boyd, and J. Chernoff. 1999. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145:837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silver, D. A., I. Pellicer, W. R. Fair, W. D. Heston, and C. Cordon-Cardo. 1997. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3:81-85. [PubMed] [Google Scholar]
- 71.Sivakumar, B., L. E. Harry, and E. M. Paleolog. 2004. Modulating angiogenesis: more vs less. JAMA 292:972-977. [DOI] [PubMed] [Google Scholar]
- 72.Su, S. L., I. P. Huang, W. R. Fair, C. T. Powell, and W. D. Heston. 1995. Alternatively spliced variants of prostate-specific membrane antigen RNA: ratio of expression as a potential measurement of progression. Cancer Res. 55:1441-1443. [PubMed] [Google Scholar]
- 73.Suzuki-Inoue, K., Y. Yatomi, N. Asazuma, M. Kainoh, T. Tanaka, K. Satoh, and Y. Ozaki. 2001. Rac, a small guanosine triphosphate binding protein, and p21-activated kinase are activated during platelet spreading on collagen-coated surfaces: roles of integrin α2β1. Blood 98:3708-3716. [DOI] [PubMed] [Google Scholar]
- 74.Tiffany, C. W., R. G. Lapidus, A. Merion, D. C. Calvin, and B. S. Slusher. 1999. Characterization of the enzymatic activity of PSM: comparison with brain NAALADase. Prostate 39:28-35. [DOI] [PubMed] [Google Scholar]
- 75.Tino, W. T., M. J. Huber, T. P. Lake, T. G. Greene, G. P. Murphy, and E. H. Holmes. 2000. Isolation and characterization of monoclonal antibodies specific for protein conformational epitopes present in prostate-specific membrane antigen (PSMA). Hybridoma 19:249-257. [DOI] [PubMed] [Google Scholar]
- 76.Troyer, J. K., M. L. Beckett, and G. L. Wright, Jr. 1995. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 62:552-558. [DOI] [PubMed] [Google Scholar]
- 77.Vadlamudi, R. K., F. Li, L. Adam, D. Nguyen, Y. Ohta, T. P. Stossel, and R. Kumar. 2002. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat. Cell Biol. 4:681-690. [DOI] [PubMed] [Google Scholar]
- 78.Vorup-Jensen, T., C. V. Carman, M. Shimaoka, P. Schuck, J. Svitel, and T. A. Springer. 2005. Exposure of acidic residues as a danger signal for recognition of fibrinogen and other macromolecules by integrin αXβ2. Proc. Natl. Acad. Sci. USA 102:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worthylake, R. A., and K. Burridge. 2003. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278:13578-13584. [DOI] [PubMed] [Google Scholar]
- 80.Yoshiji, H., S. Kuriyama, and H. Fukui. 2002. Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumour Biol. 23:348-356. [DOI] [PubMed] [Google Scholar]
- 81.Yoshiji, H., S. Kuriyama, M. Kawata, J. Yoshii, Y. Ikenaka, R. Noguchi, T. Nakatani, H. Tsujinoue, and H. Fukui. 2001. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin. Cancer Res. 7:1073-1078. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.