Abstract
Inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene is linked to the development of tumors of the eyes, kidneys, and central nervous system. VHL encodes two gene products, pVHL30 and pVHL19, of which one, pVHL30, associates functionally with microtubules (MTs) to regulate their stability. Here we report that pVHL30 is a novel substrate of glycogen synthase kinase 3 (GSK3) in vitro and in vivo. Phosphorylation of pVHL on serine 68 (S68) by GSK3 requires a priming phosphorylation event at serine 72 (S72) mediated in vitro by casein kinase I. Functional analysis of pVHL species carrying nonphosphorylatable or phosphomimicking mutations at S68 and/or S72 reveals a central role for these phosphorylation events in the regulation of pVHL's MT stabilization (but not binding) activity. Taken together, our results identify pVHL as a novel priming-dependent substrate of GSK3 and suggest a dual-kinase mechanism in the control of pVHL's MT stabilization function. Since GSK3 is a component of multiple signaling pathways that are altered in human cancer, our results further imply that normal operation of the GSK3-pVHL axis may be a critical aspect of pVHL's tumor suppressor mechanism through the regulation of MT dynamics.
von Hippel-Lindau disease is a hereditary cancer syndrome that displays an autosomal dominant pattern of inheritance (2, 21). The hallmark feature of this disorder is the development of blood vessel tumors (hemangioblastomas) of the central nervous system and retina, often in association with other tumors such as renal clear cell carcinomas and pheochromocytomas. Biallelic VHL inactivation due to somatic mutations is also a common feature of nonhereditary renal clear cell carcinomas and hemangioblastomas.
VHL disease demonstrates a complex genotype-phenotype relationship suggesting the operation of distinct tumor suppressor mechanisms. Indeed, pVHL, through its oxygen-dependent polyubiquitylation of HIFα, has been shown to play a central role in the mammalian oxygen-sensing pathway (9, 16, 18, 19, 31). However, a distinct aspect of pVHL's tumor suppressor function has previously been revealed through studies demonstrating a HIF (hypoxia-inducible factor)-independent functional association of pVHL with the microtubule (MT) apparatus (14). The form of pVHL most prominently associated with MTs in vivo appears to be the long form of pVHL, pVHL30, and not its short form, pVHL19 (14). pVHL19 is mostly found in the nucleus; however, cytoplasmic pVHL19 can bind to and stabilize MTs (14). Functional analysis of naturally occurring pVHL mutants revealed a link between altered MT stabilization function and pVHL-associated tumor-suppressing activity. In keeping with these findings, the MT-stabilizing activity of pVHL has been shown to be localized specifically to the cell periphery (29). Thus, apart from its role in oxygen sensing, pVHL also participates in the control of MT dynamics.
Here we analyzed the regulation of pVHL's MT-stabilizing activity to gain further insight into this potential tumor suppressor activity. Our data show that the functional association of pVHL30 with MTs is dynamically regulated by a dual kinase mechanism. A priming phosphorylation of pVHL30 on S72 allows phosphorylation at S68 by glycogen synthase kinase 3 (GSK3), thereby negatively regulating pVHL-mediated MT stabilization. We also provide data suggesting that phosphorylation of pVHL on S68 and S72 affects not only pVHL's MT-stabilizing activity but also the interaction of pVHL with HIFα.
MATERIALS AND METHODS
Cell culture, generation of cell lines, chemicals, and drug treatments.
COS-7, U2-OS, 786-O, and IMCD-3 cells (obtained from ATCC) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (FCS). Renal proximal tubule epithelial cells (RPTECs) were obtained from Cambrex Bioscience Inc. (Walkersville, MD) and cultured as described by the manufacturer. Insect Sf9 cells were grown in Grace's medium containing 10% FCS. COS-7 cells were transfected using Fugene 6 (Roche) according to the manufacturer's instructions. VHL knockdown and control retrovirus pools were generated as described by Open Biosystems. Briefly, 48 h after infection, RPTECs were selected with 1 μg/ml puromycin for 2 weeks before processing for immunoblotting or immunofluorescence. Procedures for expression of proteins in Sf9 cells have been described previously (41). The procedure for the generation of retrovirus pools of 786-O cells is described elsewhere (14). Nocodazole and the GSK3 inhibitor 361535 [3-(3-carboxy-4-chloroanilino)-4-(3-nitrophenyl)maleimide] were from Sigma and Calbiochem, respectively. Cells were incubated with 20 mM LiCl for 4 h to block GSK activity and supplemented with 5 mM myo-inositol to compensate for the hypothetical depletion of the intracellular inositol pools caused by the lithium-mediated inhibition of inositol monophosphatase (23). As an osmotic control, cells were incubated with 20 mM NaCl and 5 mM myo-inositol.
Construction of plasmids.
pcDNA3-HA-VHL deletion constructs have been described earlier (14), except for pcDNA3-HA-VHL(Δ54-73) and pcDNA3-HA-VHL1-195. All mutants of pVHL were generated by a two-step PCR-based mutagenesis procedure using pcDNA3-HA-VHL30 as a template. Individual PCR products were subsequently digested with BamHI and EcoRI and cloned into a pcDNA3 derivative containing a hemagglutinin (HA) epitope. To obtain pGST-VHL30 mutants, the corresponding fragments were subcloned into pGEX-4T-1 (for bacterial expression) or pAc-GST (for expression in Sf9 cells) using BamHI and EcoRI. GSK3β constructs were also generated by a two-step PCR-based mutagenesis procedure using pMT-GSK3β as a template. For the generation of retrovirus expression plasmids pCMV(neo-retro)-VHL30 and the indicated mutants thereof, the corresponding fragments were subcloned into pCMV(neo-retro) using BamHI and XhoI restriction sites. The VHL targeting and nonsilencing microRNA 30-based short-hairpin RNA (shRNAmir) were obtained from Open Biosystems (Huntsville, AL). Clones V2HS_201603 and RHS1703 were cloned into pLMP (7) as EcoRI/XhoI fragments. All constructs were confirmed by sequence analysis. Details on the generation of constructs are available upon request.
Generation of antibodies.
Generation and purification of anti-pVHL and anti-Cul2 antibodies have been described previously (14, 25). Phosphospecific antibodies were raised against the synthetic peptides VLRSVNSpREPSpQVIF and SVNSREPSpQVIFCNR, corresponding to phosphorylated S68 or S72 of human pVHL, respectively. Before injection into rabbits, the peptides were coupled to keyhole limpet hemocyanin (Pierce) by glutaraldehyde coupling. Polyclonal rabbit sera were either preabsorbed with unphosphorylated pVHL peptide (VLRSVNSREPSQVIFCNR) to obtain an anti-pVHL(S72-P) antibody or preincubated with 20 μM unphosphorylated pVHL peptide and S72-phosphorylated peptide (SVNSREPSpQVIFCNR) to obtain an anti-pVHL(S68-P) antibody. A rat monoclonal anti-α-tubulin (YL1/2)-producing cell line was from ATCC. Anti-HA antibodies were from Santa Cruz (Y-11), Babco (12CA5), and Roche (3F10). Anti-GSK3β, anti-Cdk2, anti-pVHL (monoclonal antibody Ig32), anti-glutathione S-transferase (anti-GST) (G-7781), and anti-acetyl-tubulin (6-11B-1) were from Transduction Laboratories, Santa Cruz Biotechnology, Inc., Pharmingen, and Sigma, respectively. Anti-HIF2α and anti-Glut-1 were obtained from Novus Biologicals.
Immunoblotting, immunoprecipitation, immunofluorescence microscopy, and in vitro HIF2α/pVHL binding assay.
Immunoblotting, immunoprecipitation, and immunofluorescence experiments were performed essentially as described previously (14, 26). Immunoblots were processed by ECL (Amersham) according to the manufacturer's instructions. To test the interaction of pVHL with HIF2α in vitro, GST-pVHL30 was purified from programmed Sf9 lysates. Purified protein was incubated in the absence or presence of 50 U of recombinant GSK3 (New England Biolabs) in 30 μl of GSK3 kinase buffer (20 mM Tris, 10 mM MgCl2, 5 mM dithiothreitol [DTT] at pH 7.5) plus ATP. Kinase reactions were performed for 30 min at 30°C. Then beads were washed four times with 1 ml of TNN buffer (50 mM Tris, 250 mM sodium chloride, 5 mM EDTA, 0.5% NP-40, 50 mM sodium fluoride, 0.2 mM ortho-vanadate, 1 mM DTT, 10 mM phenylmethylsulfonyl fluoride, and 1 μg ml−1 aprotinin at pH 7.5), before GST-pVHL30 was incubated with cold in vitro-translated, HA-tagged HIF2α in 0.5 ml of TNN buffer for 2 h. Finally, beads were washed four times with 1 ml of TNN buffer, and bound proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting. To produce HA-tagged HIF2α, plasmid DNA was translated in vitro using the TNT Quick Coupled transcription/translation system (Promega).
In vitro phosphorylation of recombinant pVHL.
GST-pVHL30 was expressed in Escherichia coli and purified using glutathione-Sepharose affinity resin (Sigma). Alternatively, GST-pVHL30 was purified from programmed Sf9 lysates. Purified protein was incubated with either 100 U of recombinant casein kinase I (CKI) (New England Biolabs) in 30 μl of CKI kinase buffer (50 mM Tris, 10 mM MgCl2, 5 mM DTT at pH 7.5) or 50 U of recombinant GSK3 (New England Biolabs) in 30 μl of GSK3 kinase buffer plus 10 μCi of [γ-32P]ATP (2,000 Ci/mmol; Amersham). Kinase reactions were performed for 30 min at 30°C. To test whether pVHL is a primed substrate, GST-pVHL30 was first incubated with λ-phosphatase (100 U; New England Biolabs) for 30 min at 30°C and then washed with TNN buffer before the kinase assay was carried out. For sequential phosphorylation, purified proteins were incubated with recombinant CKI using unlabeled ATP. Then beads were washed four times with 1 ml of TNN buffer and twice with 1 ml of GSK3 kinase buffer before a kinase experiment with GSK3 was performed using radiolabeled ATP. Kinase reaction mixtures with protein kinase A (PKA) (500 U; New England Biolabs) were incubated as described above. Phosphorylated proteins were separated by SDS-PAGE, and gels were dried and visualized by autoradiography.
GSK3 peptide kinase assay.
In a total volume of 30 μl kinase buffer (20 mM Tris, 10 mM MgCl2, 5 mM DTT at pH 7.5) containing 100 μM [γ-32P]ATP (∼1,000 cpm/pmol), 1 mM substrate peptide (VLRSVNSREPSQVIFCNR or SVNSREPSpQVIFCNR) and GSK3 (50 U; New England Biolabs) were incubated at 30°C for the indicated times. Reactions were stopped by addition of EDTA, pH 8.0, and 20 μl of supernatants was spotted onto squares of P-81 phosphocellulose paper (Whatman). These were then washed four times, for 10 min each time, in 1% phosphoric acid and once in acetone before being counted in a liquid scintillation counter. Experiments were performed in triplicate.
Microtubule cosedimentation and stability assays.
The cosedimentation assay has been described elsewhere (14). The MT stabilization assay was performed as described previously (14). Briefly, exponentially growing COS-7 cells were plated on coverslips and transfected the next day with the indicated constructs. Eighteen to 24 h posttransfection, cells were treated with 10 μM nocodazole for 20 min before being processed for immunofluorescence using anti-α-tubulin (YL1/2), anti-HA (Y11), and anti-GSK3β antibodies. At least 300 cells per experiment were analyzed for an intact MT network. Only cells with intact nuclei and a clearly detectable cytoplasmic signal of HA-pVHL were included in the evaluation. Experiments were repeated as blind assays.
RESULTS
Endogenous pVHL contributes to the regulation of MT stability in primary kidney cells.
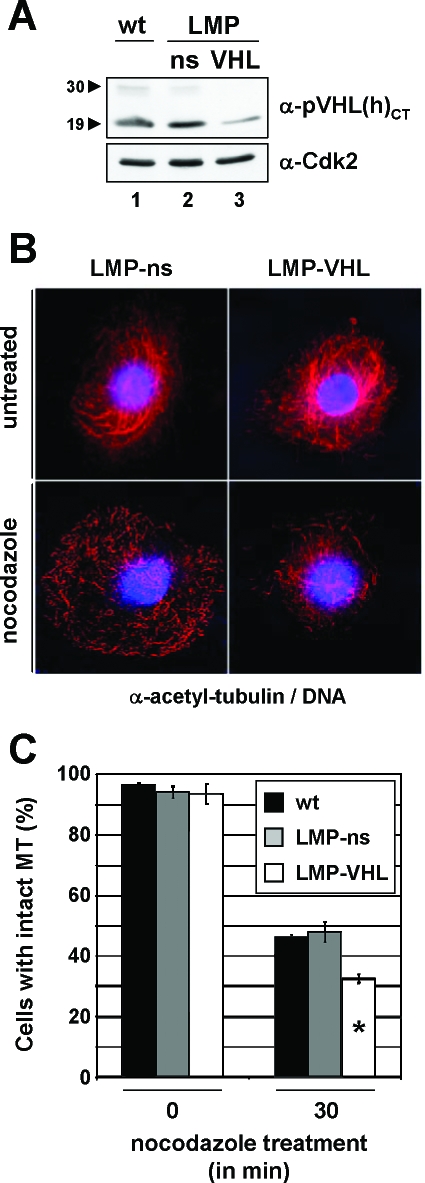
Since pVHL's MT-stabilizing activity has thus far been documented only in cell systems expressing exogenous pVHL (14, 29), we asked whether endogenous pVHL would be critical for normal MT stability. To this end, human primary RPTECs were depleted of pVHL species by using retrovirus-delivered shRNAmir directed against both isoforms of pVHL (Fig. 1A). After efficient knockdown of pVHL levels (Fig. 1A, lane 3), cells were treated with nocodazole, a potent MT-depolymerizing agent, and analyzed for the presence of stable MTs by using an anti-acetyl-tubulin antibody (Fig. 1B). Strikingly, the total number of cells displaying stable MTs was significantly reduced for pVHL-depleted cells compared to control cells (Fig. 1C). Therefore, endogenous pVHL is required to maintain stable MTs in primary cells of renal origin, in agreement with the original proposal that linked pVHL function to MT stability (14, 29).
FIG. 1.
Endogenous pVHL regulates MT stability in a primary cell line. (A) Infected RPTEC cells were selected for 2 weeks before analysis of cell lysates by Western blotting with anti-pVHL(h)CT (top panel) and anti-Cdk2 (bottom panel) antibody. The positions of pVHL30 and pVHL19 species are highlighted by arrowheads. LMP, LTRmiR30-PIG; ns, nonsilencing; wt, wild type (untreated). (B) RPTECs expressing control (left panels) or miR30-based shRNA directed against pVHL (right panels) were processed for immunofluorescence using an anti-acetyl-tubulin antibody (red) before (top panels) or after (bottom panels) treatment with 10 μM nocodazole for 30 min. DNA is stained in blue. (C) Transfected cells were scored for the presence of stable MTs before and after treatment of cells with nocodazole. The experiment was done in triplicate; each bar represents a total of at least 400 cells analyzed. Error bars, standard deviations of the triplicates. pVHL-depleted cells displayed a significantly lower number of cells with intact MTs (white bar with asterisk).
pVHL is a GSK3 target in vitro.
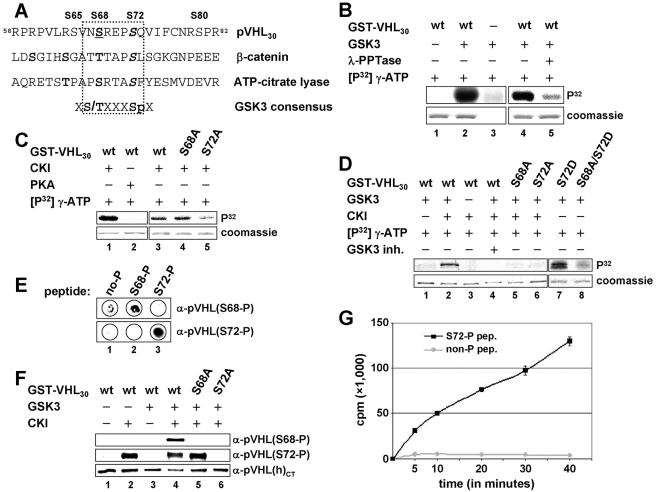
To identify potential regulators of pVHL's MT stabilization function, we searched the primary sequence of pVHL for potential phosphorylation sites in silico. This analysis revealed one consensus phosphorylation site for GSK3 [X(S/T)XXXSp] encompassing amino acid residues S68 and S72 of pVHL30 (Fig. 2A). When tested, GSK3 indeed phosphorylated GST-pVHL30 in vitro (Fig. 2B, lane 2) but not GST protein (data not shown). Since most GSK3 substrates must first be phosphorylated by another kinase, a so-called priming kinase (6), Sf9-expressed GST-pVHL30 was pretreated with λ-phosphatase prior to being tested as a GSK3 substrate (Fig. 2B). Dephosphorylated GST-pVHL30 served as a weak in vitro substrate for GSK3 in comparison to untreated protein (Fig. 2B, lanes 4 and 5). In agreement with this finding, bacterially expressed GST-pVHL30 did not serve as a GSK3 substrate (Fig. 2D, lane 1). These data suggests that phosphorylation of pVHL by GSK3 in vitro requires priming phosphorylation on one or more sites.
FIG. 2.
Primed phosphorylation on serine 72 allows GSK3 phosphorylation of pVHL on serine 68 in vitro. (A) Alignment of amino acids 58 to 82 of pVHL30 with known GSK3 substrates. Serines 68 and 72 of pVHL30 are consistent with the GSK3 motif [(S/T)XXXSp]. Boldface indicates predicted phospho-residues. Underlining shows a putative GSK3 site, and italics indicate predicted priming sites. Serines 65, 68, 72, and 80 are indicated and exist in pVHL30 and pVHL19. (B) Purified GST-pVHL30 protein (Sf9 expressed) was incubated with kinase buffer alone (lane 1) or GSK3 (lanes 2 and 4). As a control, GSK3 was incubated in kinase buffer alone (lane 3). GST-pVHL30 was incubated with λ-phosphatase (λ-PPTase) before the phosphatase was washed out and the kinase assay was carried out (lane 5). wt, wild type. (C) Purified bacterially expressed GST-pVHL30 was incubated with CKI (lanes 1 and 3) or PKA (lane 2). GST-pVHL30(S68A) or -(S72A) was also incubated with CKI (lanes 4 and 5). (D) Bacterially expressed GST-pVHL30 was either incubated with GSK3 alone (lanes 1, 7, and 8) or sequentially incubated with first CKI and then GSK3 (lanes 2 to 6). The GSK3 inhibitor (inh.) 361535 was added at a concentration of 100 μM (lane 4). GST-pVHL30(S68A) or -(S72A) was not phosphorylated by GSK3 (lanes 5 and 6). GST-pVHL30(S72D) was a good (lane 7) and GST-pVHL30(S68A/S72D) a poor (lane 8) GSK3 substrate without prior CKI phosphorylation. (E) S68-phosphorylated, S72-phosphorylated, or nonphosphorylated peptides were spotted onto a membrane before immunoblotting using an anti-pVHL(S68-P) (top) or an anti-pVHL(S72-P) antibody (bottom). (F) Bacterially expressed GST-pVHL30 was either incubated with CKI (lane 2) or GSK3 (lane 3) or sequentially incubated with first CKI and then GSK3 (lanes 3 to 6). Proteins were analyzed by immunoblotting using an anti-pVHL(S68-P) (top), anti-pVHL(S72-P) (center), or anti-pVHLCT (bottom) antibody. (G) Phospho-S72 (black) or nonphosphorylated (gray) peptides were incubated with GSK3 for the indicated times before incorporated radioactivity was analyzed in counts per minutes. In panels B, C, and D, proteins were separated by SDS-PAGE and visualized either by autoradiography (upper panel) or Coomassie staining (lower panel).
Priming phosphorylation sites of GSK3 substrates are generally located four residues carboxy-terminal to the GSK3 phosphorylation site (6), raising the possibility that phosphorylation of pVHL on S72 might represent such a priming phosphorylation event. Therefore, two known priming kinases for GSK3, PKA (3, 20, 34) and CKI (1, 27, 42), were assayed for their abilities to phosphorylate bacterially expressed GST-pVHL30 on S72 in vitro (Fig. 2C). CKI phosphorylated GST-pVHL30(wt) and GST-pVHL30(S68A) efficiently, but PKA did not (Fig. 2C, lanes 1 to 4). CKI phosphorylated GST-pVHL30(S72A) much less efficiently than wild-type pVHL (Fig. 2C, lane 5). To determine whether this phosphorylation of pVHL by CKI would prime pVHL for phosphorylation by GSK3, bacterially expressed GST-pVHL30 was phosphorylated first with CKI using unlabeled ATP, followed by a separate kinase reaction using radiolabeled ATP with GSK3 (Fig. 2D). Importantly, prephosphorylated GST-pVHL30 was efficiently phosphorylated by GSK3 in vitro, while nonphosphorylated protein was not (Fig. 2D, lanes 1 and 2). This phosphorylation was dependent on the presence of active GSK3 (Fig. 2D, lanes 3 and 4), demonstrating that phosphorylation of pVHL was carried out by GSK3. Substitutions at either S68 or S72 of pVHL with nonphosphorylatable alanine residues prevented phosphorylation by GSK3 (Fig. 2D, lanes 5 and 6), whereas GST-pVHL30 protein with an aspartic acid residue at S72 (to mimic phosphorylation at that site) was targeted by GSK3 without any prephosphorylation (Fig. 2D, lane 7). Notably, phosphorylation was blocked in the GST-pVHL30(S68A/S72D) double mutant (Fig. 2D, lane 8). Altogether, these results suggest that phosphorylation of pVHL at S72 (mediated by CKI) is required for phosphorylation of S68 by GSK3, therefore establishing pVHL as a priming-dependent GSK3 substrate, at least in vitro.
To examine pVHL phosphorylation further, we raised antibodies that recognize specifically pVHL phosphorylated on S68 [anti-pVHL(S68-P)] or S72 [anti-pVHL(S72-P)] (Fig. 2E). Anti-pVHL(S68-P) antibody displayed a specific signal only when CKI-prephosphorylated GST-pVHL30 was incubated with GSK3 (Fig. 2F, lane 4), whereas anti-pVHL(S72-P) antibody detected selectively species phosphorylated by CKI (Fig. 2F, lanes 2, 4, and 5). Mutations at S68 or S72 of pVHL blocked the corresponding signals (Fig. 2F, lanes 5 and 6), suggesting that these antibodies recognize the relevant phosphorylated sites. In addition, a peptide encompassing serine residues 68 and 72 of pVHL was phosphorylated by GSK3 only when it was phosphorylated at S72 (Fig. 2G). These data fully support the above-noted observation that S68 phosphorylation of pVHL by GSK3 is dependent on phosphorylation at S72.
GSK3 phosphorylates pVHL30 on serine 68 in vivo.
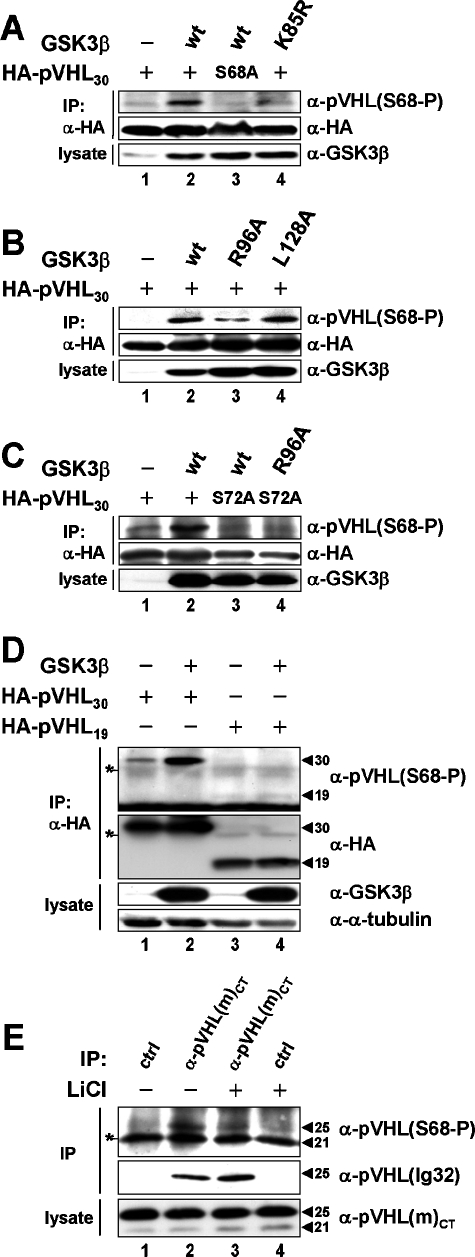
Given the specificity of our anti-phospho-antibodies in vitro (Fig. 2E and F), we next addressed their specificity in vivo (Fig. 3). Anti-pVHL(S68-P) antibody readily detected pVHL upon coexpression with functional GSK3β and did not detect a specific signal when catalytically inactive GSK3β(K85R) was coexpressed or pVHL30(S68A) was used (Fig. 3A, lanes 2 to 4). Anti-pVHL(S72-P) antibody did not detect a specific signal even when CKI and/or GSK3β were coexpressed with pVHL (data not shown). These results imply that the anti-pVHL(S68-P) antibody signal is specific and a consequence of pVHL phosphorylation by GSK3 in vivo. Similar experiments carried out with anti-pVHL(S72-P) revealed that this antibody is not suitable for in vivo studies due to limitations in affinity and specificity.
FIG. 3.
Phosphorylation of pVHL by GSK3 in vivo. (A, B, and C) Lysates of COS-7 cells expressing the indicated cDNAs were processed for immunoprecipitation (IP) using an anti-HA antibody before analysis by Western blotting with anti-pVHL(S68-P) (top) or an anti-HA (center) antibody. Input lysates were immunoblotted using an anti-GSK3β antibody (bottom). (D) Cell lysates were processed and analyzed as described above, but input lysates were further immunoblotted with an anti-α-tubulin antibody. The positions of pVHL30 and pVHL19 species are highlighted by arrowheads. The anti-HA antibody light chain is indicated by an asterisk. (E) IMCD-3 cells were grown to confluence, then serum starved (no FCS) for 48 h and incubated in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of 20 mM LiCl for 4 h before processing for immunoprecipitation using an anti-pVHL(m)CT (lanes 2 and 3) or a control (lanes 1 and 4) antibody. Immunoprecipitates were analyzed by Western blotting with an anti-pVHL(S68-P) (top) and an anti-pVHL (Ig32) (center) antibody. Input lysates were immunoblotted using an anti-pVHL(m)CT antibody (bottom). The positions of mouse pVHL25 and pVHL21 species are highlighted by arrowheads. A nonspecific band is indicated by an asterisk.
To address next whether pVHL is also a primed GSK3 substrate in cells, pVHL was coexpressed with either GSK3β(R96A), a mutant allele unable to target primed substrates but still able to support unprimed substrate phosphorylation, or GSK3β(L128A), a mutant allele with intact priming phosphorylation activity but impaired activity for unprimed phosphorylation (8, 11). While GSK3β(L128A) phosphorylated pVHL comparably to wild-type protein, GSK3β(R96A) was much less efficient (Fig. 3B, lanes 2 to 4). Importantly, pVHL30(S72A) was also not phosphorylated on S68 by GSK3β or GSK3β(R96A) (Fig. 3C, lanes 3 and 4). Thus, in agreement with our in vitro results, pVHL serves also as a priming-dependent GSK3 substrate in vivo.
Human cells express two pVHL isoforms, pVHL30 and pVHL19 (4, 17, 36), but the function of only one, namely, pVHL30, has been linked to pVHL's MT stability control (14). Strikingly, pVHL30 was phosphorylated on S68 by GSK3β and pVHL19 was not, although both pVHL species and GSK3β were expressed to similar levels (Fig. 3D, lanes 2 and 4). This suggests that only pVHL30, the MT-associated pVHL species (14), is targeted for phosphorylation by GSK3β in vivo. Notably, this finding is in full agreement with a recent report showing that pVHL30, but not pVHL19, is phosphorylated in mammalian cells (28). Of note, GSK3 also failed to phosphorylate recombinant GST-pVHL19 in vitro (data not shown), most likely because CKI could not prephosphorylate GST-pVHL19 (see Fig. S1B in the supplemental material).
Human primary or cancer cells express pVHL19 abundantly, but little pVHL30 (14), thus making it difficult to determine whether native pVHL30 is phosphorylated on S68 in vivo. To circumvent this limitation, we used the mouse renal cancer cell line IMCD-3, which expresses higher levels of pVHL25 than of pVHL21, the long and short isoforms in mice (Fig. 3E, bottom). Therefore, taking into account that the primary sequence of mouse and human pVHL is 100% identical in the region encompassing S68 and S72 (whereas S34 and S38 of mouse pVHL correspond to S68 and S72 of human pVHL), this mouse cell line allowed us to address the phosphorylation of endogenous pVHL by GSK3 (Fig. 3E). As shown in Fig. 3E, immunoblotting of anti-pVHL(m)CT immunoprecipitates derived from IMCD-3 cells subjected to serum deprivation, a condition known to activate endogenous GSK3, with anti-pVHL(S68-P) antibody revealed a specific phospho-signal. LiCl treatment reduced the signal significantly (Fig. 3E; compare lanes 3 and 2), implying that the anti-pVHL(S68-P) antibody immune reactivity is dependent on active GSK3. No such signal was seen in control immunoprecpitates (Fig. 3E, lanes 1 and 4). Moreover, reblotting of the same membrane with a monoclonal antibody against pVHL demonstrated the identity of the phospho-signal as phosphorylated pVHL25 (Fig. 3E, center). These results would suggest that mouse pVHL25 is phosphorylated on S34 (the residue corresponding to human S68) in a GSK3-dependent manner in vivo. Since the band corresponding to the pVHL21 isoform comigrated with an unspecific band that appeared in these immunoprecipitation/immunoblotting experiments, we were unable to assess whether pVHL21 is phosphorylated on this site.
GSK3 negatively affects pVHL's MT stabilization activity.
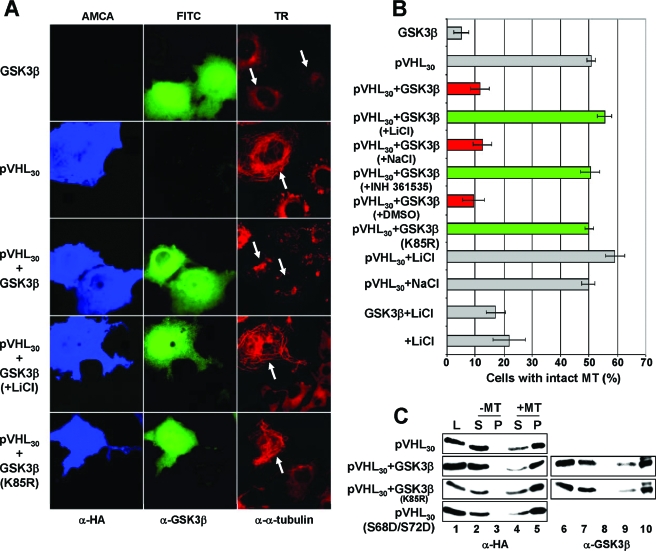
GSK3β has been shown previously to regulate MT dynamics via phosphorylation of MT-associated proteins (5, 12, 13, 30, 35, 39, 43), including the tumor suppressor protein adenoma polyposis coli (APC) (10, 44). Therefore, we tested whether GSK3β would affect pVHL30's MT stabilization function. To this end, we adapted a previously developed assay (14). COS-7 cells expressing either HA-tagged pVHL30 alone or HA-pVHL30 and GSK3β were treated with nocodazole, and transfected cells were assessed for the presence of stable MTs by using triple immunofluorescence microscopy (Fig. 4A). As shown previously (14), expression of pVHL30 protected MTs from depolymerization. However, coexpression of GSK3β with pVHL30 inhibited this activity (Fig. 4A). Addition of LiCl, a known GSK3 inhibitor (23, 37), blocked the negative effect of GSK3β, and coexpression of GSK3β(K85R) failed to inhibit pVHL's MT stabilization function (Fig. 4A). Of note, addition of 20 mM LiCl alone did not lead to a significant increase of cells displaying stabilized MTs, which is in full agreement with previous findings (24). These results, together with the quantification of GSK3β's effect on pVHL's MT stabilization function (Fig. 4B), imply a role for GSK3β in the negative regulation of pVHL30's MT stabilization function.
FIG. 4.
GSK3β negatively affects pVHL's microtubule stabilization function without altering pVHL's microtubule association. (A) COS-7 cells transiently expressing HA-tagged pVHL30 and the indicated GSK3β constructs were treated with nocodazole before triple staining with anti-HA (blue), anti-GSK3β (green), and anti-α-tubulin (red) antibodies. White arrows indicate transfected cells. (B) Transfected cells were scored for intact MTs after treatment of cells with nocodazole. The percentage of transfected cells with intact MTs is plotted. Error bars, standard deviations for three independent experiments. Active GSK3β downregulated pVHL's MT stabilization function (red bars), in contrast to inactive GSK3β (green bars). (C) Whole-cell extracts (L) of COS-7 cells transfected with the indicated HA-tagged pVHL30 and GSK3β constructs were prepared and incubated with (lanes 4, 5, 9, and 10) or without (lanes 2, 3, 7, and 8) taxol-stabilized MTs. Supernatant (S) and pellet (P) fractions were processed for Western blotting with an anti-HA (left panels) and an anti-GSK3β (right panels) antibody.
To determine whether these effects are a result of changes in the ability of pVHL30 to bind to MTs, whole-cell extracts of transfected cells were subjected to a previously described MT cosedimentation assay (14). As shown in Fig. 4C, irrespective whether GSK3β was coexpressed or not, the majority of HA-pVHL30 bound to polymerized MTs in vitro (Fig. 4C, lane 5). Strikingly, pVHL30(S68D/S72D) also associated with MTs comparably to wild-type pVHL (Fig. 4C, bottom). Thus, GSK3β and modifications of pVHL on S68 and S72 do not affect pVHL's MT binding capacity in this experimental setting. Therefore, it is unlikely that GSK3-mediated inhibition of pVHL30's MT stabilization function is linked to changes in pVHL30's MT binding.
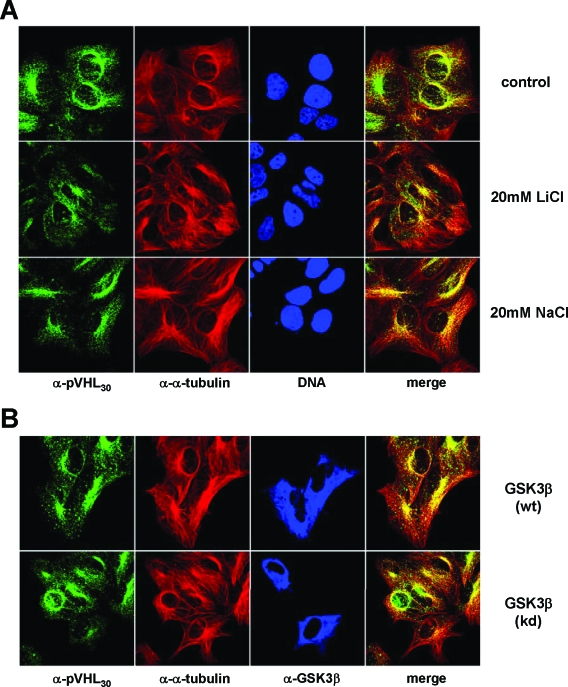
To address whether GSK3 might alter the subcellular localization of pVHL species, we either added LiCl to cells to inhibit endogenous GSK3 or overexpressed GSK3β and analyzed the localization of endogenous pVHL in transfected cells by immunofluorescence microscopy (Fig. 5). Regardless of whether chemical inhibitors were used or not, the majority of pVHL30 was concentrated on the MT organizing center in close proximity to the nucleus (Fig. 5A). Upon LiCl treatment, a subfraction of pVHL30 was enriched in the cell periphery at sites of MT stabilization as a consequence of GSK3 inhibition. However, this result is not surprising, considering that chemically induced MT stabilization is known to affect the localization of pVHL30 (14). The overexpression of GSK3β also did not obviously change the subcellular localization of native pVHL30, since the expression of wild-type or kinase-dead GSK3β did not affect the accumulation of pVHL on the MT organizing center (Fig. 5B). Altogether, it appears that GSK3 does not grossly alter the localization of endogenous pVHL30.
FIG. 5.
The subcellular localization of pVHL30 is not affected by GSK3. (A) U2-OS cells were either left untreated or incubated with 20 mM LiCl or NaCl before processing for immunofluorescence with an anti-pVHL30 (green) and an anti-α-tubulin (red) antibody. DNA stainings are in blue. (B) U2-OS cells expressing empty vector, wild-type GSK3β [GSK3β(wt)], or kinase-dead GSK3β [GSK3β(kd)] were processed for immunofluorescence using an anti-pVHL30 (green), an anti-α-tubulin (red), and an anti-GSK3β (blue) antibody. Colocalization of pVHL30 with MTs appears yellow in the merged pictures.
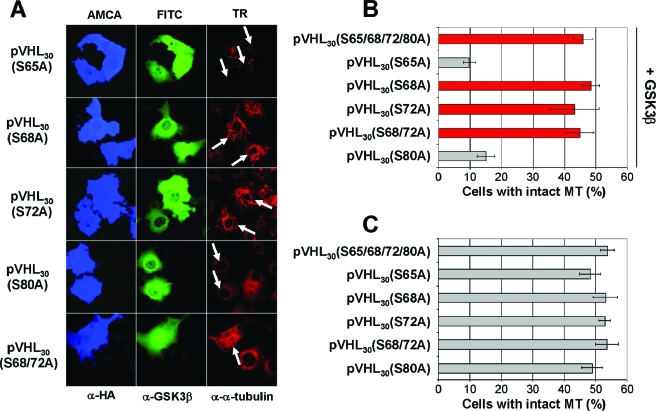
S68 and S72 of pVHL are essential for regulation by GSK3.
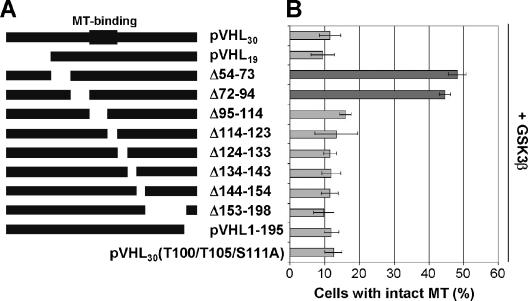
Next, we tested pVHL mutants containing alanine substitutions at potential phosphorylation sites between residues 54 and 94 (including S65, S68, S72, and S80) for their abilities to stabilize MTs in the presence of GSK3β. As shown in Fig. 6A and B, only pVHL mutant species containing alanine substitutions at S68 and/or S72 stabilized MTs in the presence of GSK3β, underscoring the notion that GSK3-mediated phosphorylation of pVHL at these specific sites is critical for pVHL's MT-regulatory function. Of note, all pVHL species tested promoted MT stabilization in the absence of GSK3β (Fig. 6C). A further indication that GSK3 mediates its negative effect on pVHL's MT stabilization function via promotion of the phosphorylation of S68 is given by the failure of a series of deletion constructs of pVHL to resist the inhibitory action of GSK3, with the notable exception of mutant species lacking amino acid residues encompassing S68 and S72 of pVHL (Fig. 7), while all deletion mutants (except for MT binding-deficient pVHL) were able to stabilize MTs in the absence of exogenous GSK3 (14). To exclude the possibility that potential phosphorylation sites are located in the MT binding domain of pVHL (amino acids 95 to 123), a pVHL mutant harboring nonphosphorylatable alanine residues at T100, T105, and S111 was also analyzed. While pVHL30(T100/T105/S111A) stabilized MTs alone (data not shown), this mutant failed to resist GSK3β-mediated inhibition of pVHL's MT stabilization function (Fig. 7B). These results strongly support a model in which GSK3 phosphorylates pVHL30 at S68 to regulate its MT stabilization function.
FIG. 6.
Serines 68 and 72 of pVHL are required for the regulation of pVHL's microtubule function by GSK3β. (A) COS-7 cells coexpressing the indicated cDNAs were treated with nocodazole before triple staining with anti-HA (blue), anti-GSK3β (green), and anti-α-tubulin (red) antibodies. White arrows indicate transfected cells. (B) The percentage of cells coexpressing pVHL and GSK3 with intact MTs is plotted. Error bars, standard deviations for three independent experiments. (C) The percentage of cells expressing pVHL derivatives alone with intact MTs is plotted. Error bars, standard deviations for two independent experiments.
FIG. 7.
Residues 54 to 94 of pVHL are required for the regulation of pVHL's microtubule function by GSK3β. (A) Schematic representation of pVHL and mutant derivatives. The domain involved in MT binding (residues 95 to 123) is indicated. (B) COS-7 cells were first double transfected with the indicated HA-tagged deletion constructs/point mutants of pVHL30 and untagged wild-type GSK3β cDNAs and then scored for intact MTs after nocodazole treatment. The percentage of transfected cells with intact MTs is plotted. Error bars, standard deviations from at least two independent experiments. Dark gray bars indicate mutants unaffected by GSK3β.
Regulation of pVHL30 by GSK3 requires priming on S72.
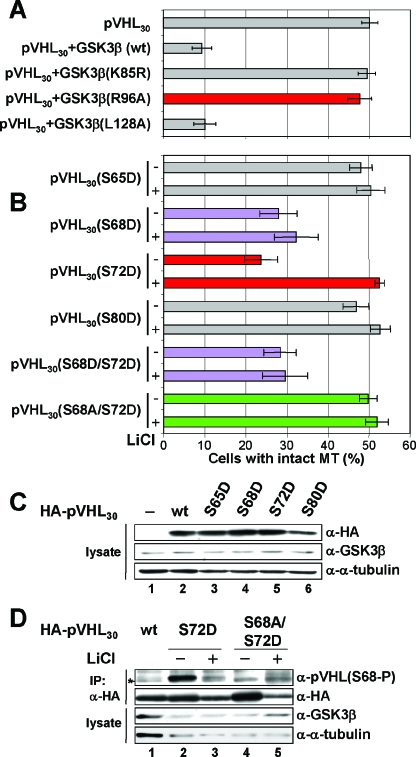
Since pVHL30(S72A) also escaped GSK3β regulation (which implied that the regulation of pVHL by GSK3β is priming dependent in vivo), we subsequently compared the effects of GSK3β(wt), GSK3β(K85R), GSK3β(R96A), and GSK3β(L128A) species on pVHL30 function (Fig. 8A). While the GSK3β(R96A) mutant failed to affect pVHL's MT-stabilizing activity (Fig. 8A), GSK3β(L128A) inhibited pVHL's activity. When expressed alone, none of the GSK3β species were able to stabilize MTs (data not shown). Therefore, regulation of pVHL's MT stabilization activity by GSK3β occurs through a priming-dependent phosphorylation event.
FIG. 8.
pVHL's microtubule function is regulated by the priming-dependent kinase activity of GSK3. (A) COS-7 cells coexpressing the indicated cDNAs {wild-type GSK3β [GSK3β(wt)], kinase-dead GSK3β [GSK3β(K85R)], priming-dependent activity-deficient GSK3β [GSK3β(R96A)], and unprimed activity-deficient GSK3β [GSK3β(L128A)]} were treated with nocodazole before processing for triple staining with anti-HA, anti-GSK3β, and anti-α-tubulin antibodies. The percentage of transfected cells with intact MTs is plotted. Error bars, standard deviations for three independent experiments. (B) Cells were either left untreated (−) or preincubated with LiCl (+) before nocodazole was added. Selected mutants are shown in purple (S68D and S68D S72D), red (S72D), or green (S68A S72D). (C) In parallel, lysates of cells expressing the indicated HA-pVHL30 species were processed for immunoblotting using an anti-HA (α-HA) (top), anti-GSK3β (center), or anti-α-tubulin (bottom) antibody. (D) Lysates of cells expressing the indicated HA-pVHL30 species were processed for immunoprecipitation (IP) using an anti-HA antibody, followed by immunoblotting with an anti-pVHL(S68-P) (top panel) or anti-HA (second panel) antibody. Input lysates were immunoblotted using an anti-GSK3β antibody (third panel) or an anti-α-tubulin antibody (bottom panel). Cells were either left untreated (−) or preincubated with LiCl (+).
To determine whether the phosphorylation of pVHL on S68 and S72 is sufficient to affect pVHL's MT-stabilizing activity, we generated pVHL mutants that harbor aspartic acid residues at S68 and/or S72 to mimic phosphorylation events. These mutants were then tested for their abilities to stabilize MTs in the presence or absence of a GSK3 inhibitor (Fig. 8B) in order to score for a possible involvement of endogenous GSK3 in this process. As shown in Fig. 8B, pVHL30(S65D) and pVHL30(S80D) mutants stabilized MTs, while pVHL30(S68D) and pVHL30(S68D/S72D) species were defective in this regard, irrespective of whether GSK3 inhibitor was present. Notably, pVHL30(S72D) lacked potent MT stabilization activity in the absence of GSK3 inhibitor but displayed that function when endogenous GSK3 was inhibited (Fig. 8B). In agreement with this finding, pVHL30(S68A/S72D) stabilized MTs independently of GSK3 inactivation (Fig. 8B). The specificity and significance of these results are further underscored by the fact that application of a distinct inhibitor of GSK3 (the chemical compound 361535) or transfection of cells with the known GSK3-inactivating kinase PKCζ (10) resulted in similar effects on the MT stabilization function of pVHL30(S72D) (data not shown). Of note, pVHL mutants were expressed at similar levels (Fig. 8C), thus excluding the possibility that our results are a consequence of unequal expression.
Finally, to test whether the pVHL30(S72D) mutation is actually mimicking phosphorylation at this site in vivo, as suggested by the data above, cells expressing pVHL30(S72D) were analyzed by immunoblotting (Fig. 8D). pVHL30(wt) or the S68A S72D mutant showed no obvious increase in phosphorylation of S68 (Fig. 8D, lanes 1, 4, and 5). However, pVHL30(S72D) was phosphorylated on S68 in a GSK3-dependent manner (Fig. 8D, lanes 2 and 3). Inactivation of GSK3 by a second inhibitor had similar effects (data not shown), further emphasizing the point that S72D substitution renders pVHL a GSK3 substrate in vivo. These data provide a potential explanation for why pVHL30(S72D) was altered in its MT stabilization function only under conditions where endogenous GSK was active, namely, because the S72D mutation allows for the efficient phosphorylation of pVHL30 by endogenous GSK3.
Considering that only a certain subset of reported pVHL mutations affect pVHL's MT-stabilizing activity (14), we speculated that these mutants do not stabilize MTs due to increased phosphorylation by GSK3. However, we did not observe any significant difference with regard to S68 phosphorylation when comparing these mutants with wild-type protein (see Fig. S2 in the supplemental material), ruling out the possibility that the reported decrease in MT-stabilizing activity by these pVHL mutants is a result of altered phosphorylation on S68.
Phosphomimicking mutations on S68 and S72 of pVHL30 negatively affect HIF2α binding.
Since Etienne-Manneville and Hall reported that the MT-dependent migration of cells can be regulated by GSK3 (10), we generated cell lines that stably express various pVHL30 derivatives in VHL-deficient 786-O cells (Fig. 9A) and analyzed their cell migration abilities. However, we could not observe a statistically significant difference with respect to cell migration when testing these various cell lines (data not shown). This finding is not surprising, given the fact that the migration of parental 786-O cells was not affected by nocodazole (data not shown). Thus, the migration of 786-O cells appears to be independent of MT dynamics.
FIG. 9.
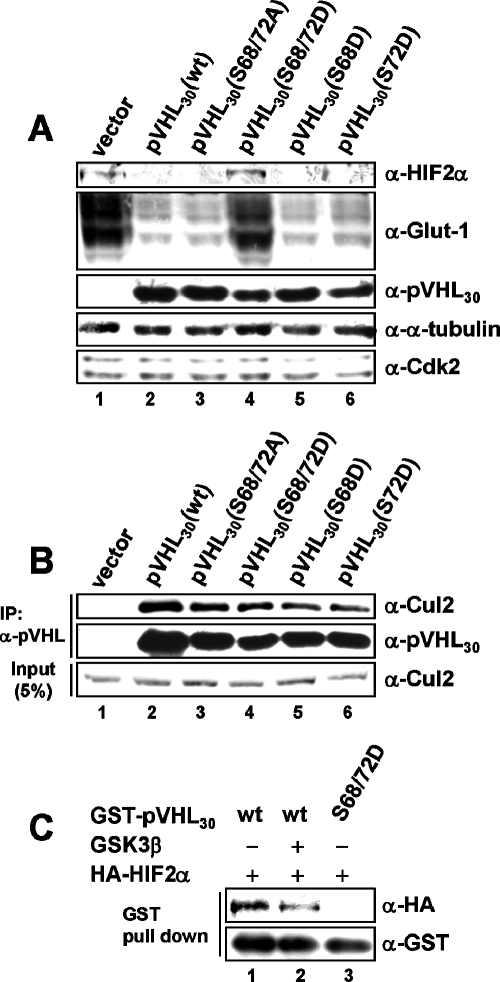
pVHL30(S68D/S72D) forms an E3 ligase complex but does not bind to HIF2α. (A) 786-O cell pools stably expressing empty vector, wild-type pVHL30, or pVHL30 harboring different amino acid substitutions at S68 and S72 were processed for immunoblotting using an anti-HIF2α (top panel), anti-Glut-1 (second panel), anti-pVHL30 (third panel), anti-α-tubulin (fourth panel), or anti-Cdk2 (bottom panel) antibody. (B) Whole-cell extracts of 786-O cell pools were subjected to immunoprecipitation with an anti-pVHL (Ig32) antibody, and immunoprecipitates were analyzed by immunoblotting using an anti-Cul2 (top) or anti-pVHL30 (center) antibody. In parallel, input lysates were processed for Western blotting with an anti-Cul2 antibody (bottom). (C) Sf9-expressed and purified GST-pVHL30 was incubated with kinase buffer alone (lane 1) or recombinant GSK3 (lane 2). After kinase reaction, glutathione-Sepharose-bound proteins were washed with lysis buffer before incubation with equal amounts of in vitro-translated, HA-tagged HIF2α. Finally, unbound HIF2α was washed out, and glutathione-bound proteins were analyzed by immunoblotting using an anti-HA (top) or anti-GST (bottom) antibody.
To assess potential effects of these phosphorylation site mutants of pVHL on HIFα levels, we determined HIF2α protein levels in these cell lines (Fig. 9A). Regulation of HIF1α protein levels could not be addressed, since 786-O cells do not express HIF1α (31). Interestingly, pVHL30(S68A/S72A) down-regulated HIF2α comparably to wild-type protein, while pVHL30(S68D/S72D) did not (Fig. 9A; compare lanes 3 and 4), implying that the latter mutant is defective in targeting HIF2α for degradation. Consistent with this finding, levels of Glut-1 protein, a known transcriptional target of HIF2α, remained high in cells expressing the pVHL30(S68D/S72D) mutant (Fig. 9A, lane 4). Cells expressing pVHL30(S65D) or pVHL30(S80D) regulated HIF2α levels like wild-type protein (data not shown).
Since pVHL30(S68D/S72D) failed to down-regulate HIF2α levels, we asked whether it is defective in pVHL/elongin C/elongin B (VCB)-cullin 2 (Cul2) E3 ligase complex formation. We subjected whole-cell extracts of the various cell lines to immunoprecipitation experiments using an anti-pVHL antibody (Fig. 9B). Anti-pVHL immunoprecipitates from 786-O vector cells failed to coimmunoprecipitate Cul2, a well-established E3 ligase partner protein of pVHL, while all pVHL species tested were able to interact with Cul2 comparably to wild-type protein (Fig. 9B). This suggests that E3 ligase complex formation is not altered in the pVHL30(S68D/S72D) mutant.
Next we tested whether the interaction of pVHL30(S68D/S72D) with HIF2α is affected. Thus, we analyzed the interaction of recombinant pVHL30(S68D/S72D) and HIF2α in vitro (Fig. 9C). In our experimental settings, wild-type pVHL30 bound HIF2α quite efficiently (about 20 to 30% of total input [data not shown]), while the binding of pVHL to HIF2α was reduced when pVHL that had previously been phosphorylated by recombinant GSK3 was used (Fig. 9C; compare lanes 1 and 2). Consistent with this observation, the pVHL30(S68D/S72D) mutant was completely defective in HIF2α binding (Fig. 9C, lane 3). These data would suggest that phospho-like modifications of pVHL on S68 and S72 negatively affect the direct interaction of pVHL with HIF2α.
An analysis of the crystal structures of the VCB complex either unbound (38) or bound to a 20-amino-acid HIF1α peptide (15, 32) fully supports our biochemical observation (see Fig. S3 in the supplemental material). Overall, the structure of pVHL does not appear to be affected by HIF1α binding (see Fig. S3A and B in the supplemental material), but a closer look at S68 of pVHL revealed a striking difference. The carbonyl group of S68 serves as hydrogen-bonding partner for a key water molecule, thereby blocking the interaction of Arg69 (R69) with this water molecule (see Fig. S3C in the supplemental material). Upon HIF1α binding, this carbonyl group turns away from the water molecule, allowing R69 to act as a hydrogen bond acceptor for the water molecule (see Fig. S3D in the supplemental material). Furthermore, S72 of pVHL stabilizes S111 by two hydrogen bonds, thereby supporting the direct interaction of S111 with the hydroxyproline of HIF1α (see Fig. S3E in the supplemental material). Therefore, the existing structural data on the pVHL-HIF1α interaction also suggest that S68 and S72 of pVHL could contribute to the interaction of pVHL with HIF1α that is hydroxylated on proline 564.
DISCUSSION
The data presented here establish pVHL as a priming-dependent substrate of GSK3 in vitro and in vivo. We further demonstrate that GSK3 phosphorylates pVHL on S68, thereby negatively regulating the MT-stabilizing function of pVHL. The fact that pVHL must be phosphorylated on S72 to be converted into an efficient GSK3 substrate points to the existence of an S72 kinase that precedes and thus initiates a pVHL phosphorylation cascade. While the identity of the kinase mediating the priming phosphorylation event at residue S72 of pVHL in vivo is not known at present, CKI was able to fulfill such a function in vitro. Therefore, our results suggest the existence of a dual-kinase mechanism that acts sequentially on the pVHL tumor suppressor to control its MT stabilization function.
MT binding and stabilization are properties of the VHL tumor suppressor protein that have been directly connected to the long and not the short form of pVHL (14). Notably, it is also the long form of pVHL that serves as an efficient substrate for GSK3 in vivo (Fig. 3D). In this regard, we have previously shown that pVHL30 localizes predominantly to the cytoplasm whereas pVHL19 is mostly nuclear (14). Thus, differential localization of these isoforms may contribute, at least in part, to the apparent selectivity of GSK3 for pVHL30.
Phosphorylation of pVHL30 by GSK3 influences pVHL30's MT stabilization and not MT binding activity (see Fig. 4). One possible outcome of these phosphorylation events might be recruitment/displacement of specific proteins to/from MT-bound pVHL30. In addition, phosphorylation of pVHL30 by GSK3 could regulate aspects of MT dynamics at specific subcellular sites, thereby contributing to the proper control of cellular processes such as cell polarization. Of particular interest in this regard is the fact that the MT-stabilizing activity of pVHL is restricted to sites in the cell periphery (29). Therefore, local inactivation of GSK3 could lead to a spatially restricted MT-stabilizing activity of pVHL in a fashion similar to that previously reported for the interplay of APC and GSK3 (10). Specifically, Hall and colleagues (10) have shown that Cdc42-mediated activation of PKCζ leads to inactivation of GSK3β, followed by the association of APC with MT ends at the leading edge and stabilization of selected MTs. Considering these findings, further work on the factors that associate with pVHL30 in the performance of its MT-stabilizing function and its role at the cell periphery is clearly warranted.
Strikingly, phosphomimicking mutations on S68 and S72 affected not only the MT stabilization activity of pVHL but also the interaction of pVHL with HIFα (Fig. 9). Taking this finding at face value, one is tempted to argue that the dual-kinase regulatory mechanism described here adds a new level to the regulation of the HIF pathway through negatively affecting pVHL-HIFα interactions. An intriguing alternative interpretation is that phosphorylation of pVHL30 on S68 and S72 contributes to proper partitioning of total cellular pVHL into distinct pools that participate in the HIF pathway and MT dynamic control, respectively. However, further work will be required to distinguish between these possibilities.
The findings that a dual-kinase mechanism is involved in the regulation of pVHL's MT function and that at least one upstream kinase, GSK3, is part of signaling networks that play an important role in various aspects of tumor development (22, 33) raise the intriguing possibility that inactivation of this specific function of pVHL could be achieved through mechanisms other than genetic alterations of the VHL locus itself. In this regard, it is interesting that the incidence of retinal angioma cannot be correlated with specific germ line mutations in patients suffering from VHL disease (40). In fact, Maher and colleagues speculated that other genetic factors (and not VHL itself), may contribute to disease development, although such factors have not been identified as yet. Therefore, altered GSK3 signaling might represent such an alternative mechanism for pVHL tumor suppressor inactivation at the posttranslational level.
. . . . . . . . .
Supplementary Material
Acknowledgments
We thank all members of our laboratory for helpful discussions. We also thank M. Pietrzak for sequencing, P. Mueller for synthesis of oligonucleotides, R. Goold for providing the pMT-GSK3β plasmid; A. Jaeschke for pcDNA3-HA-PKCζ (wild-type and kinase-dead) constructs, and S. W. Lowe for the pLMP retrovirus vector. We are thankful to M. Cabrita and I. Frew for critical comments on the manuscript.
This work was supported by the Steiner Foundation, the Novartis Research Foundation, and a grant from the Swiss National Science Foundation to W.K.
REFERENCES
- 1.Amit, S., A. Hatzubai, Y. Birman, J. S. Andersen, E. Ben-Shushan, M. Mann, Y. Ben-Neriah, and I. Alkalay. 2002. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, R. E., and W. Krek. 2004. The von Hippel-Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol. Med. 10:466-472. [DOI] [PubMed] [Google Scholar]
- 3.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 4.Blankenship, C., J. G. Naglich, J. M. Whaley, B. Seizinger, and N. Kley. 1999. Alternate choice of initiation codon produces a biologically active product of the von Hippel Lindau gene with tumor suppressor activity. Oncogene 18:1529-1535. [DOI] [PubMed] [Google Scholar]
- 5.Ciani, L., O. Krylova, M. J. Smalley, T. C. Dale, and P. C. Salinas. 2004. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J. Cell Biol. 164:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, P., and S. Frame. 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2:769-776. [DOI] [PubMed] [Google Scholar]
- 7.Dickins, R. A., M. T. Hemann, J. T. Zilfou, D. R. Simpson, I. Ibarra, G. J. Hannon, and S. W. Lowe. 2005. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37:1289-1295. [DOI] [PubMed] [Google Scholar]
- 8.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. [DOI] [PubMed] [Google Scholar]
- 10.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421:753-756. [DOI] [PubMed] [Google Scholar]
- 11.Frame, S., P. Cohen, and R. M. Biondi. 2001. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7:1321-1327. [DOI] [PubMed] [Google Scholar]
- 12.Goold, R. G., and P. R. Gordon-Weeks. 2001. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3β is induced during PC12 cell differentiation. J. Cell Sci. 114:4273-4284. [DOI] [PubMed] [Google Scholar]
- 13.Goold, R. G., R. Owen, and P. R. Gordon-Weeks. 1999. Glycogen synthase kinase 3β phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci. 112:3373-3384. [DOI] [PubMed] [Google Scholar]
- 14.Hergovich, A., J. Lisztwan, R. Barry, P. Ballschmieter, and W. Krek. 2003. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 5:64-70. [DOI] [PubMed] [Google Scholar]
- 15.Hon, W. C., M. I. Wilson, K. Harlos, T. D. Claridge, C. J. Schofield, C. W. Pugh, P. H. Maxwell, P. J. Ratcliffe, D. I. Stuart, and E. Y. Jones. 2002. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature 417:975-978. [DOI] [PubMed] [Google Scholar]
- 16.Iliopoulos, O., A. P. Levy, C. Jiang, W. G. Kaelin, Jr., and M. A. Goldberg. 1996. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc. Natl. Acad. Sci. USA 93:10595-10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliopoulos, O., M. Ohh, and W. G. Kaelin, Jr. 1998. pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc. Natl. Acad. Sci. USA 95:11661-11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 19.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 20.Jia, J., K. Amanai, G. Wang, J. Tang, B. Wang, and J. Jiang. 2002. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416:548-552. [DOI] [PubMed] [Google Scholar]
- 21.Kaelin, W. G., Jr. 2002. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2:673-682. [DOI] [PubMed] [Google Scholar]
- 22.Kim, L., and A. R. Kimmel. 2000. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr. Opin. Genet. Dev. 10:508-514. [DOI] [PubMed] [Google Scholar]
- 23.Klein, P. S., and D. A. Melton. 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93:8455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krylova, O., M. J. Messenger, and P. C. Salinas. 2000. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3β. J. Cell Biol. 151:83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisztwan, J., G. Imbert, C. Wirbelauer, M. Gstaiger, and W. Krek. 1999. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 13:1822-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisztwan, J., A. Marti, H. Sutterluty, M. Gstaiger, C. Wirbelauer, and W. Krek. 1998. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 17:368-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 28.Lolkema, M. P., M. L. Gervais, C. M. Snijckers, R. P. Hill, R. H. Giles, E. E. Voest, and M. Ohh. 2005. Tumor suppression by the von Hippel-Lindau protein requires phosphorylation of the acidic domain. J. Biol. Chem. 280:22205-22211. [DOI] [PubMed] [Google Scholar]
- 29.Lolkema, M. P., N. Mehra, A. S. Jorna, M. van Beest, R. H. Giles, and E. E. Voest. 2004. The von Hippel-Lindau tumor suppressor protein influences microtubule dynamics at the cell periphery. Exp. Cell Res. 301:139-146. [DOI] [PubMed] [Google Scholar]
- 30.Lucas, F. R., R. G. Goold, P. R. Gordon-Weeks, and P. C. Salinas. 1998. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 111:1351-1361. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 32.Min, J. H., H. Yang, M. Ivan, F. Gertler, W. G. Kaelin, Jr., and N. P. Pavletich. 2002. Structure of an HIF-1α-pVHL complex: hydroxyproline recognition in signaling. Science 296:1886-1889. [DOI] [PubMed] [Google Scholar]
- 33.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 34.Price, M. A., and D. Kalderon. 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108:823-835. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez, C., M. Perez, and J. Avila. 2000. GSK3β-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur. J. Cell Biol. 79:252-260. [DOI] [PubMed] [Google Scholar]
- 36.Schoenfeld, A., E. J. Davidowitz, and R. D. Burk. 1998. A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc. Natl. Acad. Sci. USA 95:8817-8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stambolic, V., L. Ruel, and J. R. Woodgett. 1996. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6:1664-1668. [DOI] [PubMed] [Google Scholar]
- 38.Stebbins, C. E., W. G. Kaelin, Jr., and N. P. Pavletich. 1999. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284:455-461. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, U., M. Utton, J. M. Gallo, and C. C. Miller. 1996. Cellular phosphorylation of tau by GSK-3β influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 109:1537-1543. [DOI] [PubMed] [Google Scholar]
- 40.Webster, A. R., F. M. Richards, F. E. MacRonald, A. T. Moore, and E. R. Maher. 1998. An analysis of phenotypic variation in the familial cancer syndrome von Hippel-Lindau disease: evidence for modifier effects. Am. J. Hum. Genet. 63:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirbelauer, C., H. Sutterluty, M. Blondel, M. Gstaiger, M. Peter, F. Reymond, and W. Krek. 2000. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 19:5362-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagawa, S., Y. Matsuda, J. S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura, T., Y. Kawano, N. Arimura, S. Kawabata, A. Kikuchi, and K. Kaibuchi. 2005. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120:137-149. [DOI] [PubMed] [Google Scholar]
- 44.Zumbrunn, J., K. Kinoshita, A. A. Hyman, and I. S. Nathke. 2001. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3β phosphorylation. Curr. Biol. 11:44-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.