Abstract
The Kit receptor tyrosine kinase functions in hematopoiesis, melanogenesis, and gametogenesis and in interstitial cells of Cajal. We previously identified two upstream hypersensitive site (HS) clusters in mast cells and melanocytes. Here we investigated the roles of these 5′ HS sequences in Kit expression using transgenic mice carrying Kit-GFP reporter constructs. In these mice there is close correspondence between Kit-GFP reporter and endogenous Kit gene expression in most tissues analyzed. Deletion analysis defined the 5′ upstream HS cluster region as critical for Kit expression in mast cells. Furthermore, chromatin immunoprecipitation analysis in mast cells showed that H3 and H4 histone hyperacetylation and RNA polymerase II recruitment within the Kit promoter and in the 5′ HS region were associated with Kit expression. Therefore, the 5′ upstream hypersensitivity sites appear to be critical components of locus control region-mediated Kit gene activation in mast cells.
Differential control of gene expression during embryonic development and in the adult organism is mediated by the interaction of the transcription machinery with cis regulatory elements located at the promoter, upstream, or in intronic sequences of a gene. The mechanism by which tissue-specific gene expression is achieved involves the action of transcription factors together with changes in chromatin structure. Chromatin structure is a major determinant of gene expression, and mechanisms of chromatin remodeling are critical components of its regulation. Whereas SWI/SNF-like chromatin-remodeling ATPases disrupt histone-DNA interactions, covalent N-terminal histone modifications, including acetylation, methylation, and phosphorylation, also have important roles in the remodeling of chromatin structure and the regulation of transcription (36). In this way histone modification patterns have been proposed to constitute a histone “code” which specifies downstream functions (45). Hyperacetylation and hypoacetylation of histones H3 and H4 correlate with open/accessible or closed/inaccessible chromatin structures, respectively, and transcriptional activation or repression (22, 31). Thus, the characterization of histone modification patterns has become an important tool in studies of gene expression.
The Kit receptor tyrosine kinase (RTK) functions in distinct cell populations during embryonic development and in the postnatal animal (5, 6). During gametogenesis, Kit is expressed in primordial germ cells as they migrate from the allantois to the genital ridge (10, 35). Subsequently, during postnatal gametogenesis, Kit is expressed in spermatogonia and oocytes and in endocrine Leydig and thecal cells (2). In hematopoiesis during embryogenesis and in postnatal animals, Kit is expressed in hematopoietic stem cells and lineage progenitors, as well as in mast cells and eosinophils (5, 18, 48). In melanogenesis, Kit is expressed in migrating melanoblasts during embryonic development, in differentiated melanocytes in hair follicles, and in the gastrointestinal tract in interstitial cells of Cajal (7, 34, 41). Kit loss-of-function mutations affect hematopoiesis, melanogenesis, and gametogenesis, as well as the autonomous movement of the gastrointestinal tract. In summary, Kit expression is restricted to distinct cell types in which the Kit receptor functions. Studies of the mechanisms that control cell type-specific Kit expression are therefore of great significance.
The Kit RTK is encoded at the white spotting (W) locus on mouse chromosome 5 in the vicinity of the RTKs PDGFRα and flk1 and comprises 21 exons contained in 70 kb (13, 19, 20, 39, 40). Many W mutations affecting Kit structure and function have been identified and characterized; the analysis of expression mutations has provided some insight into the mechanism of tissue-specific Kit expression. Wsh and Wbd mutant mice exhibit a pigmentation defect and lack tissue mast cells, but they are fertile and not anemic; furthermore, Wbd mice lack a functional network of interstitial cells of Cajal and intestinal pacemaker activity (15, 28, 29). In Wsh and Wbd mutant mice, Kit expression is diminished in hematopoietic progenitors in the bone marrow (BM) and lost in bone marrow-derived mast cells (BMMC). The W57 mutation is less severe, affecting melanogenesis and reducing mast cell numbers, and Kit expression is diminished in BM progenitors and BMMC, but like Wsh, this mutation does not affect erythropoiesis and gametogenesis (29). Our previous analysis of these mutations showed that the Wsh mutation arises from a 2-cM inversion on mouse chromosome 5 sequences 75 kb upstream of the Kit transcription start site, while W57 is a 110-kb deletion from approximately kb −147 to −34 from the Kit transcriptional start site (4, 15, 29). These observations suggested that the Wsh and W57 mutations affect 5′ upstream elements controlling Kit gene expression.
Locus control regions (LCRs) are cis-acting elements that determine normal levels of copy number-dependent and integration site-independent tissue-specific expression of a linked transgene in mice (21, 33). LCRs are composed of DNase I-hypersensitive sites containing binding sites for tissue-specific and ubiquitous transcription factors. The best-known example of an LCR is in the β-globin gene cluster, where the LCR regulates the expression of the different globin genes in a developmentally controlled order (11, 17). It would be of great interest to learn whether tissue-specific expression of the Kit gene is in part controlled by an LCR.
Previously, we identified two hypersensitive site clusters in bone marrow mast cells and melanocytes located at −147 to −154 kb and −21 to −28 kb upstream of the Kit transcription start site, respectively (4). DNase I-hypersensitive sites were also observed in the vicinity of the Kit transcription start site in the chromatin of Kit-expressing hematopoietic, melanocytic, and embryonic stem cells. In addition, a cluster of four hypersensitive sites has been detected in the middle of intron 1 in hematopoietic cells (12). In order to investigate the roles of the distant upstream sequences in Kit receptor expression in various cell systems, we have made transgenic mice carrying bacterial artificial chromosome (BAC) reporter constructs containing 200 kb upstream and 60-kb Kit coding sequences. In these mice, the reporter gene reflects the expression of the endogenous Kit gene in the brain, testis, oocytes, mast cells, and melanocytes, although no expression was observed in hematopoietic progenitors in bone marrow. We show that sequences within the 5′ upstream hypersensitive site cluster are critical for Kit expression in mast cells. We have also investigated molecular events associated with Kit activation in mast cells and determined the pattern of histone H3 and H4 acetylation and RNA polymerase II (Pol II) recruitment to the 5′ HS region and the Kit promoter. We propose that the 5′ upstream hypersensitivity sites are critical components of LCR-mediated Kit gene activation in mast cells.
MATERIALS AND METHODS
BACs, transgenes, and transgenic mice.
All BACs used in this study were derived from the mouse BAC RP23-232H18 (BACPAC Resources), which includes 60 kb of Kit coding sequences and 200 kb of Kit 5′ upstream sequences. BAC RP23-232H18 was modified by using homologous recombination in bacteria (51) to include an enhanced green fluorescent protein (EGFP) (GFP) expression cassette at the Kit translation start site. A 500-bp EcoRI fragment and a 800-bp XbaI fragment corresponding to sequences 5′ and 3′, respectively, of the Kit translation start site and including the GFP reporter gene, was cloned into the SalI site of the pSV1-RecA shuttle vector and was used to transform bacteria containing the target BAC clone. Cointegrant and resolved clones were detected by Southern blot analysis. Clones with the expected restriction pattern were further analyzed by a panel of restriction enzymes, followed by Southern blotting and hybridization with probes corresponding to the homology arms and the GFP reporter gene. Furthermore, homologous recombination was used to generate targeted deletions within the initial BAC200-Kit-GFP construct (37). Regions of 500 bp on either side of the deletions were amplified from the modified BAC200-Kit-GFP clone. The PCR products were cloned in the pSV1-RecA shuttle vector to generate the targeting vectors. Clones containing the deletion were identified using PCR. Three constructs, BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP containing, respectively, 30 kb, 3 kb, and 200 kb, including the 5′ HS deletion (7-kb SacI fragment) and 60 kb of Kit coding sequence were generated. BAC DNA was prepared using the PSIΨCLONE BAC DNA isolation kit (Princeton Separations) following the manufacturer's instructions. After purification with Ultrafree-MC filter (Millipore 30-kilodalton molecular mass cutoff; catalog no. UFC3LTK25), circular DNA was diluted in injection buffer (0.1 M NaCl, 10 mM Tris, pH 7.5, 0.1 M EDTA) and microinjected into the pronuclei of C57BL/6 mouse zygotes. Microinjection was done at Sloan-Kettering Transgenic Core Facility. Transgenic founder animals were typed by using PCR with the following primers from the EGFP reporter gene: forward (5′-CAGAAGAACGGCATCAAGGT-3′) and reverse (5′-GGCGGCGGTCACGAACTCCA3′-). Transgenic founders were bred to C57BL/6 mice, and offspring were typed by PCR using GFP primers.
DNA analysis.
Transgenic mouse lines were characterized by Southern blotting of tail DNA. Southern blotting and hybridization were carried out by standard procedures. Blots were scanned with a phosphorimager, and the results were used to calculate the transgene copy number. The integrity of transgenes was verified by pulsed-field gel electrophoresis. The preparation of samples for pulsed-field gel electrophoresis, enzyme digestions, and electrophoresis were performed as described previously (4, 15).
Cell culture.
Mast cells from bone marrow of adult mice and 32D myeloid cells were grown in RPMI 1640 supplemented with 10% fetal calf serum, 10% conditioned medium from X63 interleukin 3 (IL-3)-producing cells, nonessential amino acids, and sodium pyruvate.
RNase protection assays.
Total cellular RNA was extracted from tissues by RNAwiz (Ambion). T7 or T3 polymerase was used to prepare antisense mRNA transcripts from linearized plasmids (MAXIscript in vitro transcription kit; Ambion). GFP and Kit probes were fragments of 255 bp (GFP) and 370 bp (Kit exons 3 to 5), respectively. The transcripts were gel purified (5% acrylamide, 8 M urea denaturing gels) to isolate sense probes. The RNase protection experiments were performed using the RPA III RNase protection assay kit (Ambion). Protected RNA fragments were visualized with a phosphorimager.
Immunohistochemistry and microscopy.
Paraffin sections (8 μm) from 4% paraformaldehyde-fixed tissues were processed for immunohistochemistry at the Molecular Cytology Core Facility of the Memorial Sloan-Kettering Cancer Center using Discovery staining module (Ventana Medical Systems). The incubation with the anti-GFP antibody (rabbit polyclonal antibody; Molecular Probes catalog no. A11122) was followed by biotinylated goat anti-rabbit immunoglobulin G (Vectasin ABC kit, catalog no. PK-6101). Diaminobenzidine was detected with a kit containing blocker D, copper D, inhibitor D, streptavidin-horseradish peroxidase D, and diaminobenzidine D (Ventana Medical Systems) and used according to the manufacturer's instructions. Imaging was carried with a Zeiss Axioplan 2 microscope, equipped with Qimaging Retiga EX charge-coupled device camera and Improvision image acquisition software. The dorsal trunk region from E11.5 (embryonic day 11.5) embryos was optically sectioned with a Leica TCS SP2 confocal microscope, using a 488-nm laser line.
Flow cytometry analysis.
Monoclonal antibodies were from BD Pharmingen. Appropriately labeled isotype controls and single/double-color-stained cells were always used to define the specific gates. A FACScalibur or FACScan (BD Biosciences) was used for analysis.
BMMC, peritoneal mast cell, and melanocyte analyses.
Bone marrow-derived mast cells were derived from total BM cells as previously described (1). Peritoneal mast cells were obtained by postmortem peritoneal lavage with 5 ml of phosphate-buffered saline (PBS). A total of 3 × 105 cells resuspended in 200 μl staining buffer (PBS without Ca2+ and Mg2+, 3% fetal calf serum, 0.02% NaN3) were incubated for 20 min at 4°C with 0.5 μg of murine Fc block (anti-mouse CD16/CD32 monoclonal antibody), followed by a 30-min incubation with 1 μg of allophycocyanin (APC)-conjugated anti-Kit monoclonal antibody. The cells were then washed, resuspended in 400 μl of staining buffer, and analyzed.
(i) Analysis of bone marrow cells.
Bone marrow was flushed from femora with PBS, and a single-cell suspension was obtained by gentle pipetting and passage through a nylon strainer (Falcon). A total of 2.5 × 106 cells were resuspended in staining buffer and incubated for 15 min at 4°C with 1 μg of murine Fc block and then labeled with a mix of lineage-specific monoclonal antibodies (anti-mouse Ter119, B220, Mac-1, Gr-1, CD4, CD8, and CD3) conjugated to phycoerythrin (PE) and anti-Kit monoclonal antibody conjugated to APC. If necessary after incubation, mature red cells were depleted by hypotonic lysis (PharM Lyse; BD Pharmingen).
(ii) Sorting of lin− Kit+ cells.
A total of 1 × 108 bone marrow cells from femora and tibias were stained with lineage-specific PE-conjugated monoclonal antibodies and APC-conjugated anti-Kit monoclonal antibody. Gated lineage-negative, Kit-positive (lin− Kit+) bone marrow cells were sorted by a FACSVantage flow cytometer (BD Biosciences) or MoFlow sorter (Cytomation). Purification of the skin cells was performed as described previously (8). Briefly, skin from 4- to 5-day-old mice was incubated with collagenase (Sigma) following trypsinization. Neutralized cell suspension was obtained by passing the cells throughout 70-μm and 40-μm strainers (Falcon). A total of 5 × 106 cells resuspended in staining buffer were labeled with a specific monoclonal antibody, biotin-conjugated rat anti-mouse CD117 (c-kit) and APC-conjugated anti-CD45 monoclonal antibody (BD Pharmingen). Cells were gated for single events and viability and then sorted according to their expression of CD45+/Kit+/GFP and CD45−/Kit+/GFP. Fluorescence-activated cell sorting (FACS) analyses were performed either on FACSort or BD LSR (BD Biosciences).
ChIP and DNA analyses.
Chromatin immunoprecipitation (ChIP) experiments were performed according to a protocol provided by Upstate Biotechnology. For ChIP with BMMC and 32D cells, 106 cells were used per immunoprecipitation. Briefly, the cells were cross-linked with 1% formaldehyde, collected, and washed with PBS containing protease inhibitors. The cells were resuspended in 200 μl sodium dodecyl sulfate lysis buffer on ice for 10 min and then sonicated with 8 sets of 12-second pulses by a Branson Sonifier 250 cell sonicator to an average DNA size of 600 to 1,000 bp. Antibodies against acetyl-histone H4 and acetyl-histone H3 were purchased from Upstate Biotechnology, and rabbit anti-RNA polymerase II was obtained from Santa Cruz Biotechnology. DNAs were phenol-chloroform extracted and ethanol precipitated. Five percent (by volume) of the immunoprecipitated material was used as a template for semiquantitative radiolabeled PCR; 25 cycles of amplification were followed by the addition of 0.125 mCi [α-32P]dCTP. PCR products were resolved on 4.5% acrylamide gels, dried, and exposed on a phosphor screen, and images were quantitated by ImageQuant.
Primers.
Oligonucleotides for the constructs were as follows: for BAC200-Kit-GFP, HomologyA (5′-GGAATTCTTACTGAGGTCAGGGGTG-3′ and 5′-GGAATTCCATCGCGGTGGCTGCGCTAG-3′) and HomologyB (5′-GCTCTAGAGCGCCTGGGATCTGCTCTGC-3′ and 5′-GCTCTAGAGCTGCAGAGAGGGGCGAGCC-3′); for BAC30-Kit-EGFP, HomologyA (5′-GACATCAGAGTCGACGGCATTTTTGATACATAA-3′ and 5′-TCTCATTTAGTTGACACTCTTCTCTTTTTG-3′) and HomologyB (5′-GAGAAGAGTGTCAACTAAATGAGACCTTGCTT-3′ and 5′-GACATCAGAGTCGACATGCCCTGTGAGAACTTGAC-3′); for BAC3-Kit-GFP, HomologyA (5′-GACATCAGAGTCGACGGCATTTTTGATACATAA-3′ and 5′-TGTGAATAGGTTGACACTCTTCTCTTTTTG-3′) and HomologyB (5′-GAGAAGAGTGTCAACCTATTCACATCCAACAC-3′ and 5′-GACATCAGAGTCGACACCCACCTCACATCTCCTTA-3′); and for BAC200Δ5"HS-Kit-GFP, HomologyA (5′-GACATCAGAGTCGACCACCTATCCTGACCATCC-3′ and 5′-TTGCCTGCTATTCCAACAACTAAAACACAT-3′) and HomologyB (5′-TTTAGTTGTTGGAATAGCAGGCAAAACAGG-3′ and 5′-GACATCAGAGTCGACTCATCAAGGTGGAAGCAT-3′).
Primers to identify the deletion constructs were as follows: for BAC30-Kit-GFP, 5′-GGCATTTTTGATACATAA-3′ and 5′-GTGGGGGAGAAACTACAAAC-3′; for BAC3-Kit-GFP, 5′-GGCATTTTTGATACATAA-3′ and 5′-CACCCCCTAAGCACATTCCT; and for BAC200Δ5"HS-Kit-GFP, 5′-TAATCCAAGGTTCATGCCC-3′ and 5′-AGGTTCAGAGGTTCGGTCCA-3′.
Oligonucleotides for ChIP were as follows: P1, 5′-TCCCCAGACTTTACAATA-3′ and 5′-TGCCAACCTCATCATACT-3′; P2, 5′-AAACAGCACAAGCCAAGC-3′ and 5′-GGGATTCGGAGATTACAG-3′; P3, 5′-GTCAGCAGGCGGTTCATC-3′ and 5′-GAGGTGGGGAGTGGAGTG-3′; P4, 5′-TGTCATTCACTCTTCCTG-3′ and 5′-TTGCTTGTTTACTGTTTG-3′; P5, 5′-CACAAAGGACAAAAACAT-3′ and 5′-ATCAGGTATCAGCAAGGT-3′; P6, 5′-TTTCTTCAGTGGTGTAGC-3′ and 5′-TCTCCCGTTTCCTCGTTA-3′; P7, 5′-GAGTGAGCATCCCTACCA-3′ and 5′-TGTGAACCTTATCCTTAT-3′; P8, 5′-CTGGCGATTCATTTGGTA-3′ and 5′-CTGGGGTCTTTCACACAT-3′; P9, 5′-CCAAAGAAACTAAACTGC-3′ and 5′-ATCATCCAACGAAGAATC-3′; Promoter 1, 5′-AACACCTCCACCATAAGC-3′ and 5′-TAGCACTCTCCCTCCATC-3′; Promoter 2, 5′-AGGCAGCGGGAGGGAGTG-3′ and 5′-TTGTGGCCGTTTACGTCG-3′; P3 EGFP, 5′-CAGAAGAACGGCATCAAGGT-3′ and 5′-GGCGGCGGTCACGAACTCCA-3′; Promoter 4, 5′-AGGCAGCGGGAGGGAGTG-3′ and 5′-GAGCAGGACCAACAGGAC-3′; and Promoter 5, 5′-AGGCAGCGGGAGGGAGTG-3′ and 5′ CCATCGCGGTGGCTGCGCTAG-3′.
RESULTS
Generation of BAC transgenic mice carrying Kit-GFP reporter constructs.
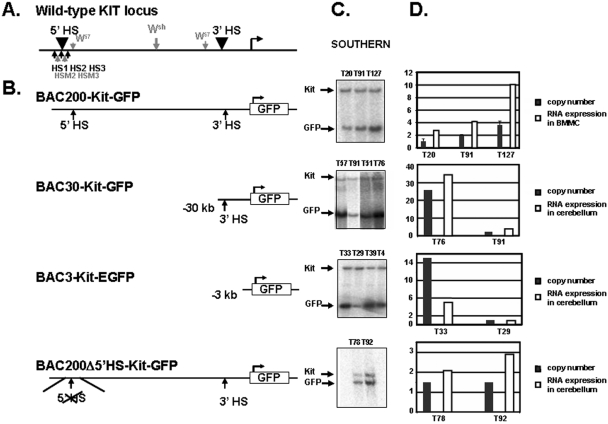
In order to identify cis-acting control elements involved in the transcriptional regulation of the Kit gene, we carried out a functional analysis using BAC transgenic mice. We identified a clone from a mouse BAC library (RP23-232H18) containing 60 kb of coding sequences and 200 kb of 5′ upstream sequences of the Kit gene (27). Subsequently, by using bacterial homologous recombination methodology (50), the BAC clone was modified by inserting an EGFP reporter gene (GFP) at the Kit translation start site in exon 1, as shown in Fig. 1. The resulting recombinant BAC was characterized extensively by restriction mapping using standard and pulsed-field gel electrophoresis. These analyses confirmed the presence of the targeted modification and failed to reveal any rearrangements or deletions in the modified BAC200-Kit-GFP clone. DNA from the BAC200-Kit-GFP clone was analyzed for integrity by pulsed-field gel electrophoresis and then microinjected into fertilized mouse oocytes to produce transgenic founder animals. Tail DNA prepared from founder offspring (Fo) was screened by PCR for the presence of the GFP gene, and transgene copy numbers were determined by Southern blot analysis (Fig. 1; Table 1). To confirm the integrity of the transgene in the three transgenic lines carrying the BAC200-Kit-GFP construct (lines T20, T91, and T127), high-molecular-weight DNA recovered from the spleen of F1 animals was embedded in agarose plugs and then analyzed by pulsed-field gel electrophoresis and blot hybridization. The GFP gene and a 500-bp fragment corresponding to the 5′ end of the BAC clone were used as probes to detect the NotI restriction fragment predicted from integration of the intact BAC200-Kit-GFP clone. The two probes detected the 200-kb NotI band in all three lines, indicating that each transgenic mouse line carried nonfragmented copies of the transgene.
FIG. 1.
Derivation of transgenic mice carrying BAC-Kit-GFP reporter constructs. (A) Schematic representation of the mouse Kit locus. Black arrowheads above the locus indicate the positions of the mast cell HS clusters. Arrows below the locus show the localization of the mast cell-specific HSs (black) and melanocyte-specific HSs (gray). (B) A GFP reporter was placed at the Kit translation start site in exon 1 of the BAC RP23-232H18 (BAC200-Kit-GFP). The BAC200-Kit-GFP construct includes 200 kb 5′ upstream sequences and 60-kb Kit coding sequences. Arrows indicate the positions of the 5′ and 3′ HS clusters. The BAC30-Kit-GFP and BAC3-Kit-GFP constructs include 30 and 3 kb of 5′ upstream sequences of the Kit gene, respectively. In BAC200-Δ5′HS-Kit-GFP, the 5′ HS cluster sequences (7 kb) were deleted. (C) Determination of transgene copy numbers by Southern blot analysis in transgenic mice carrying the four different constructs. DNA from cerebellum was digested with SpeI and hybridized with a probe located at the 5′ end of the BAC-RP23-232H18. The transgenic lines carrying the different constructs are indicated. (D) Copy number-dependent expression of the GFP transgene. The ratios of the DNA signals of the GFP transgene versus endogenous Kit gene were determined with a phosphorimager and are represented in the histogram by black bars. Error bars represent the standard deviations from three independent Southern analyses. The ratios of GFP RNA versus endogenous Kit RNA expression levels in BMMC (BAC200-Kit-GFP mice) and in cerebellum (BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP) were determined with a phosphorimager. GFP expression levels are shown as white bars.
TABLE 1.
Transgene copy numbers in BAC200-Kit-GFP, BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP transgenic micea
| BAC construct | Transgenic mouse line | No. of transgene copies |
|---|---|---|
| BAC200-Kit-GFP | T20 | 2 |
| T91 | 4 | |
| T127 | 8 | |
| BAC30-Kit-GFP | T51 | 11 |
| T57 | 50 | |
| T76 | 50 | |
| T93 | 4 | |
| BAC3-Kit-GFP | T4 | 27 |
| T29 | 2 | |
| T33 | 30 | |
| T39 | 14 | |
| T71 | 1 | |
| BAC200-Δ5′HS-Kit-GFP | T78 | 3 |
| T92 | 3 |
Transgene copy numbers were determined by comparing Kit-GFP and endogenous Kit signals from Southern blot analyses of genomic DNA using a phosphorimager (Bio-Rad).
Expression analysis of BAC200-Kit-GFP transgenic mice.
To determine whether the BAC200-Kit-GFP transgene mirrored endogenous Kit mRNA expression, we analyzed GFP reporter gene expression in different tissues using RNase protection assays. Kit-GFP reporter expression was evident in the cerebellum, testis, bone marrow-derived mast cells, and ovary, but not in the liver in the three lines analyzed. Analysis of these tissues from one line, T20, carrying two copies of the transgene is shown in Fig. 2A. Close correspondence between the expression of Kit-GFP reporter genes and endogenous Kit genes was observed in these tissues, except in the ovary where the level of transgene expression was lower than expected. Furthermore, Kit-GFP transgene expression was analyzed in BMMC of the three BAC200-Kit-GFP lines relative to the levels of endogenous Kit expression. By RNase protection assay and by FACS analysis, all three lines were positive for Kit-GFP expression. In addition, FACS analysis showed that 80% or more of the Kit-positive BMMC were also GFP positive (Fig. 4). Thus, the transgene was expressed in all lines irrespective of its site of integration, and the levels of expression were closely related to transgene copy number (Fig. 1 and 2A).
FIG. 2.
GFP reporter expression in BAC-Kit-GFP transgenic mice. (A) RNase protection assay. Total RNA from cerebellum, testis, ovary, and liver (as indicated) of BAC200-Kit-GFP, BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP transgenic mice was processed for RNase protection assay using 32P-labeled riboprobes specific for GFP and endogenous Kit RNA. Analysis of Kit-GFP and endogenous Kit expression in BMMC from T20, T91, and T127 BAC200-Kit-GFP mice by RNase protection and FACS analysis is shown. Mean values of fluorescence are indicated. Max, maximum. (B) Immunohistochemical detection of Kit-GFP transgene expression with anti-GFP antibody (signal in brown; hematoxylin counterstain in blue). Oocytes in the control ovary have no signal, while in the BAC200-Kit-GFP transgenic ovary, oocytes are stained in brown (red arrows). Representative sections of testis and cerebellum from nontransgenic and BAC200-Kit-GFP, BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP transgenic mice are shown. In the testis, Kit-GFP expression is found in spermatogonia (red arrow), and white stars show GFP expression in Leydig cells. The molecular layer (m) of the cerebellum shows staining in basket and stellate cell bodies as well as axons and dendrites. In addition, the molecular layer neurons form brown baskets around Purkinje cell bodies (p). Confocal microscopy identifies primordial germ cells in the mesentery and the gonadal ridge of E11.5 BAC200-Kit-GFP mouse embryos (red arrow).
FIG. 4.
Representative flow cytometric analysis of GFP expression in bone marrow progenitor cells (BM) and in bone marrow-derived mast cells of BAC200-Kit-GFP (T20), BAC30-Kit-GFP (T93), BAC3-Kit-GFP (T29), and BAC200-Δ5′HS-Kit-GFP transgenic mice (T92). (A) BM cells were stained with PE-conjugated anti-Lin and APC-conjugated anti-Kit antibodies, and cells within the lin− gate were analyzed on the basis of Kit and GFP expression. Only a small fraction (13%) of the Kit+ cells expressed the GFP in the BAC200-Kit-GFP transgenic mice. No GFP expression was found in lin− Kit+ BM cells of BAC30-Kit-GFP, BAC3-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP transgenic mice in all the transgenic lines analyzed. BMMC were stained with APC-conjugated anti-Kit antibody and analyzed for Kit and GFP expression. Expression of the GFP reporter was detected only in the BMMC of the BAC200-Kit-GFP transgenic mice. (B) Kit+ GFP+ and Kit+ GFP− lin− Kit+ BM cells from BAC200-Kit-GFP transgenic mice were sorted by FACS. After 3 weeks of culture in IL-3-containing medium, only the Kit+ GFP− cells gave rise to BMMC expressing the GFP transgene. Kit+ GFP+ cells die after 3 days in culture.
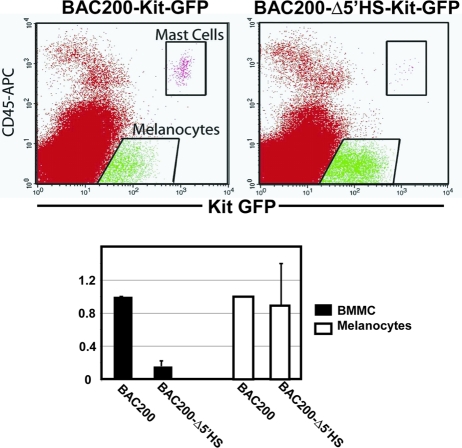
To further evaluate Kit-GFP reporter gene expression in tissues of transgenic mice, we performed immunohistochemical analysis on sections of adult organs using an anti-GFP antibody (Fig. 2B). Consistent with the known pattern of the endogenous Kit expression, Kit-GFP transgene expression was detected in the cerebellar stellate and basket neurons and their axons. In the adult testis, transgene expression was confined to spermatogonial cells and Leydig cells. In the ovary, Kit-GFP expression was observed in oocytes at all stages of follicle development but was absent in thecal cells (Fig. 2B). In E11.5 embryos, BAC200-Kit-GFP transgene expression was observed in primordial germ cells in the mesentery and gonadal ridges (Fig. 2B) and in melanoblasts (not shown) by using confocal microscopy, again in agreement with endogenous Kit expression. Expression was highly tissue specific and did not extend to surrounding tissues. In addition, Kit-GFP reporter expression was examined in mast cells and melanocytes from dorsal skin of the T20 transgenic mouse line. Single-cell suspensions from back skin of 4-day-old transgenic mice were prepared and analyzed by FACS (Fig. 3). Two positive populations were detected: GFPhigh cells expressing CD45 and Kit, presumptive mast cells, and GFPlow, Kit+, CD45− cells corresponding to melanocytes.
FIG. 3.
Kit-GFP reporter expression in mast cells and melanocytes in P4 back skin of BAC200-Kit-GFP (T20) and BAC200-Δ5′HS-Kit-GFP transgenic mice (T92). Cell suspensions of dorsal skin were stained with APC-conjugated anti-Kit and anti-CD45 antibodies, and cells were analyzed by FACS on the basis of Kit, CD45, and GFP expression. In the bar graph, the numbers of Kit+ CD45+ (mast cell subset) and Kit+ CD45− (melanocyte subset) cells expressing Kit-GFP from BAC200-Δ5′HS-Kit-GFP mice relative to the numbers of Kit-GFP expressing Kit+ CD45+ and Kit+ CD45− cells from BAC200-Kit-GFP mice are shown. The standard deviations are indicated by the error bars (n = 3).
GFP reporter gene expression was also analyzed in hematopoietic progenitors in the bone marrow by FACS in the three BAC200-Kit-GFP transgenic lines. Kit-positive cells were present in the lin− subset of cells in the adult BM (5, 9, 30). As expected, no GFP-positive cells were detected in the Kit-negative fraction; however, only a small fraction (13%) of the lin− Kit+ population expressed the GFP reporter (Fig. 4A). To further investigate the characteristics of the Kit+ GFP+ and Kit+ GFP− cells, both populations were sorted by FACS. The two fractions were then grown in medium supplemented with IL-3 to produce BMMC. After 4 weeks in culture, only the Kit+ GFP− cells gave rise to BMMC. Significantly, the BMMC derived from the GFP-negative subpopulation expressed high levels of the GFP transgene, demonstrating that the Kit-GFP reporter is turned on upon differentiation into mast cells (Fig. 4B). Taken together, these results suggest that the BAC200-Kit-GFP transgene includes most of the regulatory sequences required for cell type-specific Kit expression in primordial germ cells and melanoblasts during embryonic development, in oogenesis, and in the testis, in the cerebellum's basket and stellate neurons, in mast cells, and in melanocytes, but not in hematopoietic progenitors in the bone marrow and the fetal liver (not shown) and in thecal cells of the ovary.
Distant upstream sequences are required for Kit expression in mast cells.
To further define the roles of upstream regulatory sequences in cell type-specific Kit expression, we used bacterial homologous recombination to generate three Kit-GFP reporter constructs with 30 kb (BAC30-Kit-GFP) and 3 kb (BAC3-Kit-GFP) of 5′ upstream sequences, respectively (37). In addition, a construct, BAC200Δ5′HS-Kit-GFP, was made in which the 5′ HS cluster region, a 7-kb (kb −147 to −154) SacI fragment, was deleted (Fig. 1B). Transgenic mice carrying these constructs were obtained, the integrity of the transgenes was characterized, and the copy number was determined as described above (Table 1). We obtained the following transgenic mouse lines: four lines with BAC30-Kit-GFP (T51, T57, T76, and T93), five lines with BAC3-Kit-GFP (T4, T29, T33, T39, and T71), and two lines with BAC200Δ5′HS-Kit-GFP (T78 and T92).
Analysis of GFP reporter gene expression in these mice was again determined by RNase protection, FACS, and immunohistochemistry. In the BAC30-Kit-GFP mice, Kit-GFP reporter expression in cerebellum, testis, and ovary was comparable to that in mice carrying the intact BAC200-Kit-GFP transgene and expression remained copy number dependent (Fig. 1 and Fig. 2A and B). Therefore, the regulatory sequences controlling Kit expression in these tissues are located within the BAC30-Kit-GFP construct. However, none of the transgenic lines (T51, T57, T76, and T93) expressed the Kit-GFP reporter in BMMC and in hematopoietic progenitors in the bone marrow (Fig. 4A). These observations indicate that additional 5′ upstream sequences not present in the BAC30-Kit-GFP transgene are necessary for Kit expression in mast cells. Analysis of Kit-GFP reporter expression in the cerebellum and testis of BAC3-Kit-GFP transgenic mice showed that expression levels were lower than expected in mice with multicopy transgenes (Fig. 1 and Fig. 2A and B). Evidence for ectopic Kit expression was observed in the ovary of one of the lines. None of these transgenic lines (T4, T29, T33, T39, and T71) expressed the transgene in mast cells (Fig. 4A). Importantly, neither one of the two BAC3-Kit-GFP mice carrying only one or two copies of the transgene expressed the Kit-GFP reporter in any tissue analyzed (Fig. 2A). Thus, proximal regulatory elements appear to be insufficient to drive Kit-GFP reporter expression in a reproducible fashion and at wild-type (WT) levels.
We had previously identified a hypersensitive site cluster located between kb −147 and −154 (HS1, -2, and -3 and HSm2 and -m3) upstream of the Kit transcription start site in mast cells and melanocytes. FACS analysis of BMMC, peritoneal, and skin mast cells and melanocytes from BAC200-Δ5′HS-Kit-GFP mice, which lack these sequences, showed no GFP expression in BMMC and severely reduced reporter gene expression in skin (Fig. 3 and 4A) and peritoneal mast cells (not shown). However, the number of melanocytes expressing the GFP reporter in 4-day-old T92 BAC200-Δ5′HS-Kit-GFP mice was comparable to that in T20 BAC200-Kit-GFP mice (Fig. 3). Taken together, these results provide evidence that the 5′ HS cluster sequences are essential for Kit expression in mast cells but not in melanocytes. Analysis of Kit-GFP reporter expression in the cerebellum of BAC200-Δ5′HS-Kit-GFP mice was analogous to that in mice carrying the intact BAC200-Kit-GFP transgene, although specific staining in the molecular layer appeared to be somewhat reduced compared to that in the BAC200-Kit-GFP mice. However, in the testis only, Leydig cells were stained, while spermatogonia lacked signal in the two different lines analyzed (Fig. 2B).
Histone H3 and H4 hyperacetylation in the 5′ HS cluster region correlates with an open chromatin structure.
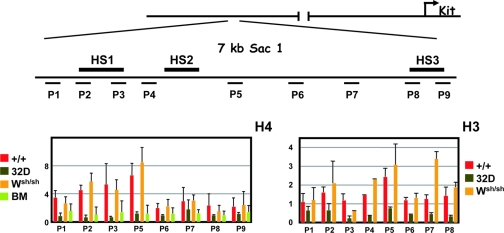
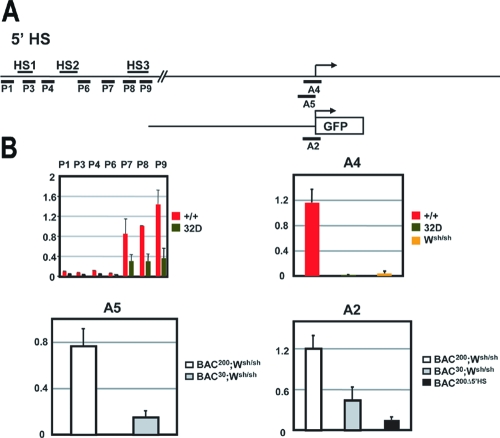
Enhancers and promoters of transcriptionally active genes are associated with open chromosomal regions, which are sensitive to nuclease digestion of DNase I-hypersensitive sites (49). In addition, modification of histones H3 and H4 is associated with gene activation in a number of systems and tends to be maximal at known regulatory sequences. To further define the chromatin structure in the 5′ HS cluster of the Kit gene, we characterized histone acetylation using chromatin immunoprecipitation. Formaldehyde-cross-linked chromatin from BMMC was immunoprecipitated with antibodies specific for the acetylated forms of histones H3 and H4. The amount of immunoprecipitated DNA was quantitated by semiquantitative PCR, using primers spanning the regions of interest, comparing the PCR products of the immunoprecipitated DNA and input DNA. The overall levels of H3 and H4 acetylation were assessed in the 5′ HS cluster of BMMC from WT and Wsh/sh mice, in Kit-negative myeloid 32D cells and in Kit-expressing lin− Kit+ BM progenitor cells. In mast cells from WT and Wsh/sh mice, H3 and H4 acetylation levels were shown to be two- and eightfold higher than in 32D cells, indicating a correlation between H3 and H4 hyperacetylation and an open chromatin structure in the 5′ HS cluster (Fig. 5). The mast cell specificity of the acetylation pattern was established by comparing these results with those obtained from analysis of lin− Kit+ hematopoietic progenitors isolated from BM. ChIP assays of lin− Kit+ hematopoietic progenitors revealed no acetylation enrichment in the 5′ HS cluster (Fig. 5).
FIG. 5.
Analysis of histone H3 and H4 acetylation in the chromatin of the 5′ HS cluster region. Formaldehyde-cross-linked chromatin obtained from WT (+/+) BMMC, Wsh/Wsh BMMC, 32D cells, and lin− Kit+ BM cells was immunoprecipitated with antibodies directed against acetylated forms of histones H3 and H4, and the amount of the immunoprecipitated and input DNA was assayed by semiquantitative PCR. A schematic representation of the Kit locus is shown at the top. The positions of the different PCR amplification units (P1 to P9) on the 7-kb SacI fragment are indicated by horizontal bars. The ratios of signals of bound versus input chromatin were determined with a phosphorimager and are represented in the histogram. The error bars represent the standard deviations from three independent ChIP experiments.
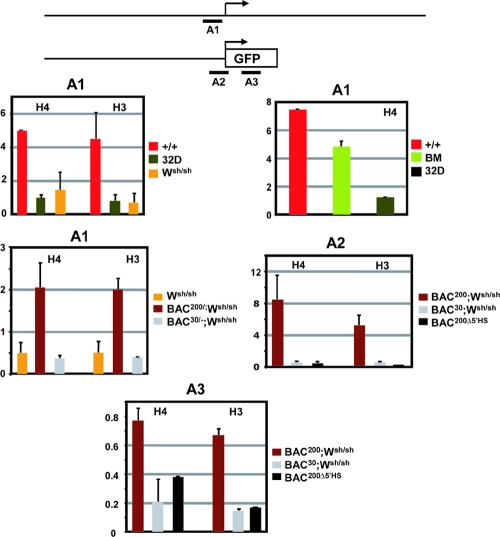
To evaluate the relation between histone acetylation and transcriptional activation, we also characterized the histone acetylation status of the proximal Kit promoter. High levels of H3 and H4 acetylation were detected in Kit-expressing BMMC and in lin− Kit+ BM cells. In contrast, in Kit-negative mast cells from Wsh/sh mice and in 32D cells, histone H3 and H4 acetylation levels were not increased (Fig. 6). Taken together, these data indicate that whereas acetylation in the 5′ HS cluster region correlates with Kit expression in mast cells, hyperacetylation in the promoter region correlates with Kit gene expression in mast cells and in hematopoietic progenitor cells.
FIG. 6.
Analysis of histone H3 and H4 acetylation in the chromatin of the proximal Kit promoter and the GFP reporter gene. A schematic representation of the endogenous Kit promoter and the transgene constructs is shown at the top. The positions of the different PCR amplification units A1 (−449 to −289), A2 (−247 to +63 of GFP), and A3 (GFP) are indicated by horizontal bars. Formaldehyde-cross-linked chromatin obtained from WT (+/+) BMMC, Wsh/Wsh BMMC, 32D cells, lin− Kit+ BM cells, and BMMC derived from BAC200-Kit-GFP (T20)/Wsh/Wsh, BAC30-Kit-GFP (T51)/Wsh/Wsh, and BAC200-Δ5′HS-Kit-GFP (T78) mice was immunoprecipitated with antibodies against acetylated forms of histones H3 and H4, and the amount of the immunoprecipitated and input DNA was assayed by semiquantitative PCR. The ratios of signals of bound versus input chromatin were determined with a phosphorimager and are represented in the histogram. The error bars represent the standard deviations from three independent ChIP experiments.
Deletion of the 5′ HS region abolishes histone H3 and H4 hyperacetylation at the Kit promoter.
We also investigated the consequences of deletion of the upstream regulatory sequences on chromatin structure in the Kit promoter and determined the pattern of acetylation of histones H3 and H4 in the Kit promoter of BMMC isolated from Wsh/sh mice, which lack endogenous Kit expression, carrying either the BAC200-Kit-GFP, BAC30-Kit-GFP, or BAC200-Δ5′HS-Kit-GFP transgene. Strong hyperacetylation was observed in the Kit promoter sequences and in the GFP-coding sequences in BMMC of Wsh/sh mice carrying the BAC200-Kit-GFP transgene. In contrast, no H3 and H4 hyperacetylation was apparent in BMMC isolated from Wsh/sh carrying the BAC30-Kit-GFP or BAC200-Δ5′HS-Kit-GFP transgene (Fig. 6). The loss of H3 and H4 hyperacetylation in these BMMC was consistent with a lack of GFP expression in the mast cells of both transgenic lines. Therefore, these results imply that, in mast cells, both histone acetylation in the promoter region and transcriptional activation of the Kit gene require a functional 5′ HS cluster.
Recruitment of RNA Pol II to the 5′ HS cluster and promoter region in mast cells.
RNA polymerase II has been reported to associate with distal upstream transcriptional control elements in several model systems (25, 42, 47). A long-range transfer mechanism in which Pol II is first recruited to the upstream regulatory element and then transferred to the promoter has been proposed. To further characterize the 5′ HS cluster and its role in the transcriptional regulation of the Kit gene, we sought to determine whether Pol II is recruited to this region. Using ChIP analysis and semiquantitative PCR with primers spanning this region, we measured Pol II binding in WT BMMC and myeloid 32D cells. Significant association of Pol II was observed with the HS3 region (Fig. 7) in the 5′ HS cluster region of WT mast cells. Next we investigated the association of Pol II with the Kit promoter in WT and Wsh/sh BMMC and 32D myeloid cells. Strong Pol II association was detected in WT BMMC but not in BMMC from Wsh/sh mice and in the 32D myeloid cells (Fig. 7). Thus, Pol II recruitment to the HS3 and to the Kit promoter regions correlates with Kit expression in BMMC. In addition, we wanted to determine whether the 5′ HS cluster induces Pol II recruitment to the Kit promoter. To investigate this question, we analyzed Pol II association with the promoter sequences in BMMC obtained from Wsh/sh mice carrying the BAC200-Kit-GFP and BAC30-Kit-GFP transgenes and in BAC200Δ5′HS-Kit-GFP transgenic mice. ChIP analysis revealed a significant recruitment of Pol II to the promoter sequences in BMMC carrying the BAC200-Kit-GFP transgene but not in cells carrying the BAC30-Kit-GFP and the BAC200-Δ5′HS-Kit-GFP transgenes (Fig. 7). Therefore, these results indicate that in mast cells, association of Pol II with the HS3 region in the 5′ HS cluster correlates with RNA polymerase II recruitment to the promoter and transcriptional activation.
FIG. 7.
Recruitment of RNA polymerase II to the chromatin of the 5′ HS cluster and in the Kit promoter region. A schematic representation of the Kit locus and the transgene constructs is shown at the top. The positions of the different PCR amplification units A2 (−247 to +63 of GFP), A4 (−247 to +37), and A5 (−247 to +3) and P1, P3, P4, P6, P7, P8, and P9 used for quantitation are indicated by horizontal bars. Formaldehyde-cross-linked chromatin obtained from WT (+/+) BMMC, Wsh/Wsh BMMC, 32D cells, and BMMC derived from BAC200-Kit-GFP (T20)/Wsh/Wsh, BAC30-Kit-GFP (T51)/Wsh/Wsh, and BAC200-Δ5′HS-Kit-GFP (T78) mice was immunoprecipitated with antibodies against RNA polymerase II, and the amount of immunoprecipitated and input DNA was assayed by semiquantitative PCR. The ratios of signals of bound versus input chromatin were determined with a phosphorimager and are represented in the histogram. The error bars represent the standard deviations from three independent ChIP experiments.
DISCUSSION
The Kit gene is expressed in hemato- and lymphopoietic progenitor cell populations, mast cells, and eosinophils and in gametogenesis and melanogenesis and in the pacemaker cells of the intestinal tract. In order to investigate the mechanisms controlling tissue-specific Kit gene expression, we previously characterized two different Kit expression mutations: Wsh and W57 (4, 14, 15). Molecular characterization of these mutations identified a far upstream 3′ breakpoint for the Wsh inversion at kb −72 and for W57 deletion endpoints at kb −34 to −38 and kb −146 to −147 upstream of the kit transcription start site. Since these mutations diminish or abolish Kit expression in hematopoietic BM progenitors and BMMC, they were hypothesized to affect distant upstream control sequences of the Kit gene. To identify these remote cis-acting elements, we have generated BAC-Kit-GFP transgenic reporter mice carrying 200 kb of Kit upstream and 60-kb Kit coding sequences. Analysis of Kit-GFP reporter expression in these mice revealed expression in the brain, testis, oocytes, skin, peritoneal and bone marrow mast cells, and melanocytes at levels and in a pattern comparable to those of the endogenous Kit gene. These results suggested that, in these tissues, the BAC200-Kit-GFP clone could establish its own stable chromatin environment irrespective of the transgene insertion site and was able to direct tissue-specific Kit-GFP expression.
In the bone marrow, the Kit gene is expressed in hematopoietic stem cells and hematopoietic lineage progenitors, but in more differentiated hematopoietic and lymphoid cell types, Kit expression is abolished, except in mast cells and eosinophils. However, in the BAC200-Kit-GFP transgenic mice described here, no full Kit-GFP reporter expression was evident in the lineage-negative fetal liver and BM progenitor compartment. In a recent study, Cairns et al. (12) utilized transgenic mice carrying a Kit-GFP reporter construct consisting of 6.7 kb 5′ upstream and 4.5-kb Kit downstream sequences to investigate regulatory mechanisms of Kit gene expression in vivo. In these mice, hematopoietic progenitors in the fetal liver and in the BM were found to express the GFP reporter, suggesting that this fragment is sufficient to drive expression in early hematopoietic cells. Lineage commitment in hematopoiesis is regulated mainly at the transcriptional level involving positive and negative regulatory mechanisms (24, 38, 44, 46). It is possible that the BAC200-Kit-GFP transgene includes upstream and/or downstream negative regulatory elements that abrogate Kit gene expression in the hematopoietic progenitors. Furthermore, sequences not present in the BAC200-Kit-GFP transgene may be required for faithful Kit gene expression in hematopoietic BM progenitors. Significantly, the sorted Kit+ GFP− BM cells gave rise to GFP+ BMMC, indicating that this population contains the mast cell progenitors. Thus, positive and negative regulatory sequences are critical in the regulation of Kit gene expression, likely involving different transcription factors and silencing mechanisms.
Deletion analysis of the upstream sequences in the BAC200-Kit-GFP transgene indicated that 3 kb of 5′ upstream sequences was insufficient to establish consistent GFP reporter expression in the transgenic mice. In contrast, inclusion of 30-kb upstream sequences gave reproducible and copy number-dependent expression of the GFP reporter in brain, testis, and oocytes, although there was no expression of the reporter in BMMC. Therefore, the lack of GFP expression in mast cells in these mice appears to recapitulate the Kit-negative mast cell phenotype in the Wsh and W57 mutant mice. It has been argued previously that the Wsh and W57 mutations result from position effects, but our observations here imply that the phenotypes of these mutations result from deletion of cis-acting control elements contained within the upstream BAC200-Kit-GFP sequences and not from position effects (3).
DNase I-hypersensitive regions in chromatin are often associated with gene regulatory sequences, such as enhancers, promoters, and locus control regions (16, 17). On the basis of our previous identification of a DNase I-hypersensitive site cluster in mast cells and melanocytes 147 to 154 kb upstream of the Kit transcription start site, we investigated the roles of these sequences in mediating Kit gene expression in vivo by generating BAC transgenic mice lacking these sequences. The lack of GFP reporter expression in BMMC implies that the 5′ HS cluster region includes cis regulatory sequences critical for Kit expression in mast cells. Furthermore, the copy number-dependent and position-independent expression of the GFP reporter gene in the BAC200-Kit-GFP transgenic mice suggests that these sequences constitute a locus control region, essential for Kit expression in mast cells.
A critical step in transcriptional activation of a gene is the remodeling of the chromatin from a condensed structure into an open structure. For a long time, DNase I hypersensitivity had been used to identify open chromatin structures. More recent work has identified modifications of the core histones H3 and H4 as indicators of chromatin remodeling. These modifications include histone H3 and H4 hyperacetylation and H3 K4 methylation. Our analysis of histone H3 and H4 hyperacetylation patterns in the 5′ HS cluster region and the proximal promoter of transcriptionally active WT BMMC showed a broad hyperacetylation pattern in the 5′ HS cluster region and strong hyperacetylation in the proximal promoter, whereas no hyperacetylation in the 5′ HS cluster region and in the proximal promoter of the inactive myeloid 32D cells was observed. Importantly, in Kit+ lin− BM cells, the proximal promoter was hyperacetylated in agreement with Kit promoter activity in these cells, but the 5′ HS cluster region was not hyperacetylated. In this regard, it is of interest to note that the 5′ HS cluster in BMMC from Wsh/sh mutant mice, in which more than 2 cM (far distal) of the Kit transcription start site was removed as a result of the chromosomal inversion, is hyperacetylated, even though Kit expression in these cells is abolished. Therefore, chromatin acetylation of the 5′ HS cluster region may be independent of its position relative to the Kit transcription start site and represent an epigenetic modification preventing chromatin from adopting a closed conformation. However, these results may also imply that chromatin remodeling in the 5′ HS cluster in mast cells precedes transcriptional activation of the Kit gene.
Analysis of acetylation of histones H3 and H4 in BMMC isolated from Wsh/sh and BAC200-Kit-GFP transgenic mice indicated strong hyperacetylation in the Kit promoter of the reporter constructs, which is in agreement with the strong hyperacetylation pattern of the Kit promoter observed in the WT BMMC. In contrast, no hyperacetylation was observed in Wsh/sh, BAC200-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP BMMC that lack GFP reporter gene expression. These results clearly indicate that the 5′ HS sequences are critical for driving mast cell Kit expression and mediating the chromatin remodeling in the Kit promoter region.
The impact of LCR deletions on promoter acetylation has been investigated in different systems. Deletion of the murine β-globin gene LCR does not affect hyperacetylation of the globin promoter, implying that acetylation of the β-globin gene promoter is independent of LCR function (42, 43). In contrast, at the human growth locus, hGH, deletion of HS1 in the LCR resulted in a loss of acetylation of the hGH-N promoter (23). Thus, similar to the hGH gene, hyperacetylation of the Kit promoter may reflect transcription and indicate that promoter activation follows LCR hyperacetylation. Furthermore, our results suggest that interaction between the 5′ HS sequences and the Kit promoter are necessary for acetylation in the promoter region, facilitating the recruitment of the basal transcriptional machinery and the binding of transcriptional activators.
LCRs may recruit transcription factors and components of the transcription preinitiation complex, including RNA polymerase II, that are subsequently transferred to the promoter region (25, 32, 47). Our finding that Pol II is bound to HS3 in the 5′ HS cluster and the promoter region in WT mast cells and BMMC isolated from Wsh/sh and BAC200-Kit-GFP transgenic mice, but not in the promoter of the GFP-negative BMMC isolated from Wsh/sh, BAC30-Kit-GFP, and BAC200-Δ5′HS-Kit-GFP mice, is consistent with such a mechanism. In the β-globin locus, deletion of the LCR results in a loss of expression, but hyperacetylation and assembly of the transcription preinitiation complex at the promoter are not affected (42). In contrast, in the Kit gene, the 5′ HS cluster sequences are essential for both hyperacetylation of the Kit promoter and Pol II recruitment.
Chromatin remodeling is likely a prerequisite for recruitment of Pol II; therefore, we compared the patterns of H3 and H4 acetylation with the pattern of Pol II recruitment in the 5′ HS cluster region (Fig. 7B). Interestingly, the sites of Pol II association did not necessarily correlate with sites enriched for H3 and H4 acetylation, and this is in agreement with findings in the β-globin locus (26). Pol II binding was detected exclusively in the HS3 region, whereas histone acetylation was more broadly distributed throughout the 5′ HS cluster region (Fig. 5 and 7). On the basis of these findings, it had been proposed that Pol II recruitment at hypersensitive sites in the LCR is mediated by trans-acting factors, rather than by interaction with acetylated lysine residues of H3 and H4.
In summary, our studies of the mechanism of Kit receptor expression have identified a mast cell-specific LCR located 147 to 154 kb upstream of the Kit transcription start site. The demonstration of mast cell-specific chromatin remodeling and histone hyperacetylation in the LCR implies a transcriptionally competent chromatin state. Consequently, these modifications could make the LCR accessible to the transcription factors and components of the transcription preinitiation complex, including RNA polymerase II. Furthermore, activation of the Kit promoter might be a consequence of direct or indirect interaction between the LCR and the promoter region. Many different models have been described to explain distant effects, including looping, linking, and tracking. In any case, enhancer-promoter communication appears to be required for the assembly of promoter initiation complexes, which then initiate Kit transcription in mast cells. The identification and characterization of factors that mediate cell type-specific transcriptional activation of the Kit gene are of great interest and will be a challenge of the future.
Acknowledgments
We thank Elizabeth Lacy and Willie Mark and their staff for their help in generating BAC transgenic mice, Antoinette Rookard for her help maintaining the mouse colony, Yasemine Yozgat and Prathima Nandivada for technical assistance. We thank Jan Hendrix for help with FACS analysis and Sandra Gonzales and Zsolt Lasar of the Molecular Cytology Facility for help with histological analysis. Furthermore, we thank Nat Heintz for advice and reagents for the generation of BAC constructs. We thank members of the laboratory of Elaine Fuchs for helpful discussions and Daniel Besser, Ines Ibanez-Talon, Imke Ehlers, and Eva Besmer for many useful discussions and comments on the manuscript.
This work was supported by grants from the National Institutes of Health, HL/DK55748 and DH38908 (to P.B.). C.B. is a NATO Postdoctoral Fellow.
REFERENCES
- 1.Agosti, V., S. Corbacioglu, I. Ehlers, C. Waskow, G. Sommer, G. Berrozpe, H. Kissel, C. M. Tucker, K. Manova, M. A. Moore, H. R. Rodewald, and P. Besmer. 2004. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. J. Exp. Med. 199:867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachvarova, R. F., K. Manova, and P. Besmer. 1993. Role in gametogenesis of c-kit encoded at the W locus of mice. Wiley-Liss, New York, N.Y.
- 3.Bedell, M. A., N. A. Jenkins, and N. G. Copeland. 1996. Good genes in bad neighbourhoods. Nat. Genet. 12:229-232. [DOI] [PubMed] [Google Scholar]
- 4.Berrozpe, G., I. Timokhina, S. Yukl, Y. Tajima, M. Ono, A. D. Zelenetz, and P. Besmer. 1999. The W(sh), W(57), and Ph Kit expression mutations define tissue-specific control elements located between −23 and −154 kb upstream of Kit. Blood 94:2658-2666. [PubMed] [Google Scholar]
- 5.Besmer, P. 1997. Kit-ligand-stem cell factor. Marcel Dekker, New York, N.Y.
- 6.Besmer, P. 1991. The kit ligand encoded at the murine Steel locus: a pleiotropic growth and differentiation factor. Curr. Opin. Cell Biol. 3:939-946. [DOI] [PubMed] [Google Scholar]
- 7.Besmer, P., K. Manova, R. Duttlinger, E. J. Huang, A. Packer, C. Gyssler, and R. F. Bachvarova. 1993. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. Suppl. 1993:125-137. [PubMed] [Google Scholar]
- 8.Blanpain, C., W. E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118:635-648. [DOI] [PubMed] [Google Scholar]
- 9.Broudy, V. C. 1997. Stem cell factor and hematopoiesis. Blood 90:1345-1364. [PubMed] [Google Scholar]
- 10.Buehr, M., A. McLaren, A. Bartley, and S. Darling. 1993. Proliferation and migration of primordial germ cells in We/We mouse embryos. Dev. Dyn. 198:182-189. [DOI] [PubMed] [Google Scholar]
- 11.Bulger, M., and M. Groudine. 1999. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 13:2465-2477. [DOI] [PubMed] [Google Scholar]
- 12.Cairns, L. A., E. Moroni, E. Levantini, A. Giorgetti, F. G. Klinger, S. Ronzoni, L. Tatangelo, C. Tiveron, M. De Felici, S. Dolci, M. C. Magli, B. Giglioni, and S. Ottolenghi. 2003. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood 102:3954-3962. [DOI] [PubMed] [Google Scholar]
- 13.Chabot, B., D. A. Stephenson, V. M. Chapman, P. Besmer, and A. Bernstein. 1988. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 335:88-89. [DOI] [PubMed] [Google Scholar]
- 14.Duttlinger, R., K. Manova, G. Berrozpe, T. Y. Chu, V. DeLeon, I. Timokhina, R. S. Chaganti, A. D. Zelenetz, R. F. Bachvarova, and P. Besmer. 1995. The Wsh and Ph mutations affect the c-kit expression profile: c-kit misexpression in embryogenesis impairs melanogenesis in Wsh and Ph mutant mice. Proc. Natl. Acad. Sci. USA 92:3754-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duttlinger, R., K. Manova, T. Y. Chu, C. Gyssler, A. D. Zelenetz, R. F. Bachvarova, and P. Besmer. 1993. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development 118:705-717. [DOI] [PubMed] [Google Scholar]
- 16.Felsenfeld, G., J. Boyes, J. Chung, D. Clark, and V. Studitsky. 1996. Chromatin structure and gene expression. Proc. Natl. Acad. Sci. USA 93:9384-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser, P., and F. Grosveld. 1998. Locus control regions, chromatin activation and transcription. Curr. Opin. Cell Biol. 10:361-365. [DOI] [PubMed] [Google Scholar]
- 18.Galli, S. J., K. M. Zsebo, and E. N. Geissler. 1994. The kit ligand, stem cell factor. Adv. Immunol. 55:1-96. [DOI] [PubMed] [Google Scholar]
- 19.Geissler, E. N., M. A. Ryan, and D. E. Housman. 1988. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 55:185-192. [DOI] [PubMed] [Google Scholar]
- 20.Gokkel, E., Z. Grossman, B. Ramot, Y. Yarden, G. Rechavi, and D. Givol. 1992. Structural organization of the murine c-kit proto-oncogene. Oncogene 7:1423-1429. [PubMed] [Google Scholar]
- 21.Grosveld, F., G. B. van Assendelft, D. R. Greaves, and G. Kollias. 1987. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 51:975-985. [DOI] [PubMed] [Google Scholar]
- 22.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 23.Ho, Y., F. Elefant, N. Cooke, and S. Liebhaber. 2002. A defined locus control region determinant links chromatin domain acetylation with long-range gene activation. Mol. Cell 9:291-302. [DOI] [PubMed] [Google Scholar]
- 24.Hu, M., D. Krause, M. Greaves, S. Sharkis, M. Dexter, C. Heyworth, and T. Enver. 1997. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11:774-785. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, K. D., H. M. Christensen, B. Zhao, and E. H. Bresnick. 2001. Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol. Cell 8:465-471. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, K. D., J. A. Grass, C. Park, H. Im, K. Choi, and E. H. Bresnick. 2003. Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain. Mol. Cell. Biol. 23:6484-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent, W. J., C. W. Sugnet, T. S. Furey, K. M. Roskin, T. H. Pringle, A. M. Zahler, and D. Haussler. 2002. The human genome browser at UCSC. Genome Res. 12:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluppel, M., J. D. Huizinga, J. Malysz, and A. Bernstein. 1998. Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev. Dyn. 211:60-71. [DOI] [PubMed] [Google Scholar]
- 29.Kluppel, M., D. L. Nagle, M. Bucan, and A. Bernstein. 1997. Long-range genomic rearrangements upstream of Kit dysregulate the developmental pattern of Kit expression in W57 and Wbanded mice and interfere with distinct steps in melanocyte development. Development 124:65-77. [DOI] [PubMed] [Google Scholar]
- 30.Kondo, M., A. J. Wagers, M. G. Manz, S. S. Prohaska, D. C. Scherer, G. F. Beilhack, J. A. Shizuru, and I. L. Weissman. 2003. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21:759-806. [DOI] [PubMed] [Google Scholar]
- 31.Kuo, M. H., J. Zhou, P. Jambeck, M. E. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach, K. M., K. Nightingale, K. Igarashi, P. P. Levings, J. D. Engel, P. B. Becker, and J. Bungert. 2001. Reconstitution of human beta-globin locus control region-hypersensitive sites in the absence of chromatin assembly. Mol. Cell. Biol. 21:2629-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Q., K. R. Peterson, X. Fang, and G. Stamatoyannopoulos. 2002. Locus control regions. Blood 100:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda, H., A. Yamagata, S. Nishikawa, K. Yoshinaga, S. Kobayashi, and K. Nishi. 1992. Requirement of c-kit for development of intestinal pacemaker system. Development 116:369-375. [DOI] [PubMed] [Google Scholar]
- 35.Manova, K., and R. F. Bachvarova. 1991. Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev. Biol. 146:312-324. [DOI] [PubMed] [Google Scholar]
- 36.Mellor, J. 2005. The dynamics of chromatin remodeling at promoters. Mol. Cell 19:147-157. [DOI] [PubMed] [Google Scholar]
- 37.Misulovin, Z., X. W. Yang, W. Yu, N. Heintz, and E. Meffre. 2001. A rapid method for targeted modification and screening of recombinant bacterial artificial chromosome. J. Immunol. Methods 257:99-105. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto, T., H. Iwasaki, B. Reizis, M. Ye, T. Graf, I. L. Weissman, and K. Akashi. 2002. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell 3:137-147. [DOI] [PubMed] [Google Scholar]
- 39.Nagle, D. L., C. A. Kozak, H. Mano, V. M. Chapman, and M. Bucan. 1995. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum. Mol. Genet. 4:2073-2079. [DOI] [PubMed] [Google Scholar]
- 40.Nagle, D. L., P. Martin-DeLeon, R. B. Hough, and M. Bucan. 1994. Structural analysis of chromosomal rearrangements associated with the developmental mutations Ph, W19H, and Rw on mouse chromosome 5. Proc. Natl. Acad. Sci. USA 91:7237-7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders, K. M., T. Ordog, S. D. Koh, S. Torihashi, and S. M. Ward. 1999. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol. Motil. 11:311-338. [DOI] [PubMed] [Google Scholar]
- 42.Sawado, T., J. Halow, M. A. Bender, and M. Groudine. 2003. The beta-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 17:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubeler, D., C. Francastel, D. M. Cimbora, A. Reik, D. I. Martin, and M. Groudine. 2000. Nuclear localization and histone acetylation: a pathway for chromatin opening and transcriptional activation of the human beta-globin locus. Genes Dev. 14:940-950. [PMC free article] [PubMed] [Google Scholar]
- 44.Smale, S. T. 2003. The establishment and maintenance of lymphocyte identity through gene silencing. Nat. Immunol. 4:607-615. [DOI] [PubMed] [Google Scholar]
- 45.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 46.Tagoh, H., A. Schebesta, P. Lefevre, N. Wilson, D. Hume, M. Busslinger, and C. Bonifer. 2004. Epigenetic silencing of the c-fms locus during B-lymphopoiesis occurs in discrete steps and is reversible. EMBO J. 23:4275-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira, K. F., P. P. Levings, M. A. Hill, V. J. Crusselle, S. H. Kang, J. D. Engel, and J. Bungert. 2004. Recruitment of transcription complexes to the beta-globin gene locus in vivo and in vitro. J. Biol. Chem. 279:50350-50357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waskow, C., S. Paul, C. Haller, M. Gassmann, and H. Rodewald. 2002. Viable c-Kit(W/W) mutants reveal pivotal role for c-Kit in the maintenance of lymphopoiesis. Immunity 17:277-288. [DOI] [PubMed] [Google Scholar]
- 49.Weintraub, H., and M. Groudine. 1976. Chromosomal subunits in active genes have an altered conformation. Science 193:848-856. [DOI] [PubMed] [Google Scholar]
- 50.Yang, D., C. Tournier, M. Wysk, H. T. Lu, J. Xu, R. J. Davis, and R. A. Flavell. 1997. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc. Natl. Acad. Sci. USA 94:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, X. W., P. Model, and N. Heintz. 1997. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat. Biotechnol. 15:859-865. [DOI] [PubMed] [Google Scholar]