Abstract
The classical mechanism by which prolactin transduces its signal in mammary epithelial cells is by activation of cytosolic signal transducer and activator of transcription 5 (Stat5) via a plasma membrane-associated prolactin receptor-Janus kinase 2 (Jak2) complex. Here we describe an alternative pathway through which prolactin via Jak2 localized in the nucleus activates the transcription factor nuclear factor 1-C2 (NF1-C2). Previous reports have demonstrated a nuclear localization of Jak2, but the physiologic importance of nuclear Jak2 has not been clear. We demonstrate that nuclear Jak2 regulates the amount of active NF1-C2 through tyrosine phosphorylation and proteasomal degradation. Our data also demonstrate a link between prolactin and p53 as well as the milk gene carboxyl ester lipase through nuclear Jak2 and NF1-C2. Hence, we describe a novel pathway through which nuclear Jak2 is subject to regulation by prolactin in mammary epithelial cells.
The mammary gland is a complex organ that undergoes development and differentiation under the control of a number of hormones and growth factors, their receptors, and transcription factors. The polypeptide hormone prolactin has an essential role both in the differentiation of the gland during pregnancy and in the regulation of milk protein gene expression (13). Prolactin receptor signaling can be mediated through several different signaling pathways (2, 4, 9, 10), the principal of which in mammary epithelial cells is that through the receptor-associated tyrosine kinase Janus kinase 2 (Jak2) (21). Binding of prolactin to its receptor activates Jak2, which phosphorylates the transcription factor signal transducer and activator of transcription 5 (Stat5). Phosphorylated Stat5 dimerizes, translocates to the nucleus, and binds to its response elements (35). Both Jak2 and Stat5 have been demonstrated to be essential for mammary gland development and milk protein gene expression (23, 29). Apart from the Jak2-Stat5 pathway, the target genes and factors that mediate the action of prolactin in the mammary gland under normal conditions are poorly understood.
One important mechanism regulating signal transduction is that through proteolysis (6, 11). The proteasome pathway in particular plays an important role in the degradation of a number of cellular proteins (26, 31). Earlier studies have shown that proteasome inhibitors prolong the activity of the Jak-Stat signaling pathway by protecting tyrosine-phosphorylated Jak2 proteins from degradation (33, 38).
We have previously identified the transcription factor nuclear factor 1-C2 (NF1-C2) as an important activator of milk genes as well as the p53 tumor suppressor gene in the mouse mammary gland during pregnancy (14, 16). This activity indicates that NF1-C2 might participate both in the establishment of a functional gland and in the protection of the gland against tumorigenesis during proliferation. Further, using studies with mouse mammary epithelial NMuMG cells and mammary tissue from heterozygous prolactin receptor knockout mice as a basis, we have demonstrated that the prolactin signaling pathway has a role in the maintenance of the NF1-C2 protein in mammary epithelial nuclei. Since treatment with prolactin resulted in a net increase of NF1-C2 protein in the whole cell and not simply in a nuclear translocation of already present cytoplasmic proteins, we suggested that prolactin regulates NF1-C2 by a mechanism distinct from its regulation of Stat5 (15).
In the present paper, we demonstrate (i) that prolactin-Jak2 can act through a novel pathway which involves NF1-C2 but not Stat5 in mammary epithelial cells, (ii) that active Jak2 is present in the nucleus, (iii) that Jak2 tyrosine phosphorylation of NF1-C2 proteins is restricted to the nucleus, and (iv) that an interaction between Jak2 and tyrosine-phosphorylated NF1-C2 prevents NF1-C2 association with and subsequent degradation by the proteasome.
MATERIALS AND METHODS
Reagents.
Tyrphostin AG490, wortmannin, PD 98059, z-LLL-H (MG-132), and thymidine were purchased from Sigma.
Two-hybrid screening.
A cDNA library from HC11 cells was made according to the manufacturer's instructions (Clontech). Full-length NF1-C2 cDNA was made by PCR using primers with overlapping sequences to the yeast (Saccharomyces cerevisiae) pGBT9 vector. The NF1-C2 fragment was transformed into the yeast strain PJ69-4a together with the open pGBT9 vector. The colonies growing on the selective plates hosted the correct vector where homologous recombination had occurred. The sequence of the vector was controlled just to assure that the NF1-C2 gene fragment was inserted in the correct reading frame. The two-hybrid screen was performed as described by the manufacturer (Clontech).
Cell cultures and mice.
The mouse mammary epithelial cell line HC11, kindly provided by R. Ball, Friedrich Miescher-Institute, Basel, Switzerland, was grown at 37°C in a 5% CO2-95% air atmosphere in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% penicillin-streptomycin, 5 μg/ml insulin, and 10 ng/ml epidermal growth factor.
The HC11 cells were blocked at the beginning of the S phase by 2 mM thymidine treatment for 18 h followed by a 9-h release in fresh medium and then another 2 mM thymidine treatment for 17 h. Synchronized as well as unsynchronized HC11 cells were treated with the specific inhibitors tyrphostin AG490 (10 μM), wortmannin (200 nM), and PD 98059 (100 μM) for 4 h and/or 15 min. Where indicated, prolactin (5 μg/ml) was added to the cells.
The human breast cell lines MDA-MB 436, T47D, SK-BR-3, and HBL100 (all obtained from ATCC) were grown at 37°C in a 5% CO2-95% air atmosphere in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% penicillin-streptomycin, and 5 μg/ml insulin.
The inguinal mammary glands from different stages of development were dissected from (C57BL/6 × CBA)F1 mice.
Protein preparations.
For whole-cell extract preparation, the adherent cells were treated with lysis buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 50 mM Tris-HCl [pH 8], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1× Complete [Roche]) for 30 min at 4°C. Preparations of nuclear and cytoplasmic extracts from HC11 cells and from glands from different stages of development were made as described previously (3). Protein concentrations of the extracts were determined by the method of Bradford (5), and the extracts were stored in aliquots in liquid nitrogen before use.
Western blot analysis.
The different extracts were electrophoresed through a NuPAGE 4 to 12% Bis-Tris sodium dodecyl sulfate-polyacrylamide gel (Invitrogen) and subsequently electroblotted onto a Hybond-P filter (Amersham Bioscience). The amounts of loaded extracts were adjusted to represent extracts from equal amounts of cells. NF1-C2 proteins were specifically detected with a 1:2,000 dilution of rabbit NF1-C2 antiserum (directed against amino acids 424 to 439 in the NF1-C2 protein) (15) or a 1:1,000 dilution of a rabbit polyclonal NF1-C-specific antibody (8199) (16). Jak2 proteins were detected with a 1:1,000 dilution of anti-Jak2 (Upstate), tyrosine-phosphorylated proteins were detected with the antiphosphotyrosine (anti-pY) clone 4G10 mouse monoclonal antibody (1:1,000 dilution) (Upstate), histone deacetylase-1 (HDAC-1) proteins were detected with a 1:1,000 dilution of anti-HDAC-1 (Santa Cruz), β-actin was detected with a 1:5,000 dilution of the mouse monoclonal β-actin antibody (Sigma), and α-tubulin was detected with a 1:1,000 dilution of the mouse monoclonal α-tubulin antibody (Sigma). The primary antibodies were detected with peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G by use of the ECL Western blotting detection reagents and ECL films (Amersham Bioscience).
In vitro degradation assays.
The different extracts used were nuclear or cytoplasmic extracts from HC11 or nuclear extract from MDA-MB 436 cells transfected with a plasmid encoding full-length NF1-C2. NF1-C2 was also generated with the in vitro transcription/translation TNT-coupled reticulocyte lysate system (Promega).
Each protein extract or protein was incubated at 37°C with or without 20S proteasome from erythrocytes (isolated from human blood) (Biomol) (1 μg/time point) for different lengths of time in phosphate-buffered saline (PBS). Where indicated, the proteasomal inhibitor MG-132 was added at 200 μM (final concentration) to inhibit 20S proteasome proteolytic activity.
Plasmids and cell transfections.
The NF1-C2 cDNA was cloned into the green fluorescent protein (GFP) vector (pEGFP-C1; BD Bioscience) in the EcoRI and KspI restriction enzyme sites, creating the GFP-NF1-C2 construct. HC11 cells were transiently transfected using Lipofectin in Optimem (Invitrogen) with 5 μg of the GFP-NF1-C2 construct.
The SOCS-1 construct was obtained by subcloning the SOCS-1 open reading frame into pcDNA3.1(+) (Invitrogen). Reverse transcriptase PCR (RT-PCR) experiments were carried out using the Titan One Tube RT-PCR system (Roche) and RNA from the HC11 cells. The primers used were SOCS-1 Fwd (5′-GAATTCATGGTAGCACGCAACCAG-3′) and SOCS-1 Rev (5′-CTCGAGTCAGATCTGGAAGGGGAAG-3′). The reaction mixtures were incubated at 50°C for 30 min and 97°C for 2 min followed by 40 cycles of 30 s at 97°C, 30 s at 56°C, and 1 min at 68°C. From cycle 11 onward, the 68°C step was extended by 5 s every cycle. Finally, the reaction mixtures were incubated at 68°C for 7 min. HC11 cells were stably transfected using Lipofectin with 5 μg of the SOCS-1 expression plasmid per 6-cm culture dish. Two days after transfection, the antibiotic G418 (350 μg/ml) was added to the cells. G418-resistant colonies were pooled and expanded in G418-containing medium and finally frozen.
Immunoprecipitation.
Nuclear and cytoplasmic extracts from HC11 cells or nuclear extracts from mammary glands taken at day 16 of pregnancy (P16) and day 6 of lactation (L6) were immunoprecipitated by rotation for 3 h at 4°C with the appropriate antibodies. Antibodies were captured by incubation overnight with protein A-Sepharose beads (Sigma) and washed three times in 1 ml lysis buffer. Immunoprecipitated proteins were dissolved in 2× loading buffer containing reducing agent. The proteins were electrophoresed through a NuPAGE 10% Bis-Tris sodium dodecyl sulfate-polyacrylamide gel (Invitrogen) and subsequently Western blotted as described previously.
RNA interference.
A 21-nucleotide small interfering RNA (siRNA) duplex targeting mouse Jak2 was custom synthesized by Dharmacon. The sequence used was GGAGAGUAUCUGAAGUUUC with 3′UU overhangs and corresponded to nucleotides 247 to 265 and the N-terminal region of the Jak2 protein (amino acids 52 to 58) (12). The control, nontargeting siRNA (UAGCGACUAAACACAUCAA) with 3′UU overhangs was obtained from Dharmacon. Transfection of siRNA duplexes was carried out using Oligofectamine (Invitrogen) according to the manufacturer's instructions. Two days after transfection, the cells were harvested and subcellular extracts were prepared.
Cell extracts and reporter gene assays.
HC11 cells, stably transfected with carboxyl ester lipase (CEL) promoter-luciferase constructs with an intact (mCEL-1831Luc) or mutated (mCEL-1831NF1:1mutLuc) NF1 site (previously described in reference 17) were grown to different degrees of confluence and then harvested as described previously (19). Luciferase assays were performed using a Promega kit with 50 μl of cell lysate, and results were assayed by use of a luminometer (Berthold, Pforzheim, Germany). The luciferase activity was normalized to the protein concentration of each extract, determined by the method of Bradford (5).
RNA analysis.
Total RNA was extracted from HC11 cells grown to different degrees of confluence by use of TRIzol (Invitrogen) according to the manufacturer's instructions. RT-PCR experiments were carried out using the Titan One Tube RT-PCR system (Roche). The primers used for mouse p53 amplification were 5′-GGAGGAGTCACAGTCGGATATCAGC-3′ and 5′-TCCTTCCACCCGGATAAGATGCTGG-3′, yielding a 577-bp fragment. For mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase) amplification, the primers used were 5′-CACCACCATGGAGAAGGCCGGGGCC-3′ and 5′-TTGAAGTCGCAGGAGACAACCTGGT-3′, yielding a 554-bp fragment. For each reaction, 500 ng total RNA was used, and the reaction mixtures were incubated at 50°C for 30 min and 97°C for 2 min followed by 10 cycles of 1 min at 97°C, 1 min at 57°C, and 1 min at 68°C and 20 cycles of 30 s at 97°C, 30 s at 57°C, and 1 min at 68°C. From cycle 11 onward, the 68°C step was extended by 5 s every cycle. Finally, the reaction mixtures were incubated at 68°C for 7 min.
RESULTS
The NF1-C2 protein interacts with and is degraded by the 20S proteasome in vitro.
To identify proteins that interact with the NF1-C2 protein in mammary cells, we constructed a cDNA library from the mouse mammary epithelial cell line HC11 and used the full-length NF1-C2 (amino acids 1 to 439) as bait in a yeast two-hybrid screen. We identified several clones encoding the α7 subunit of the 20S proteasome (accession no. AF055983) (data not shown).
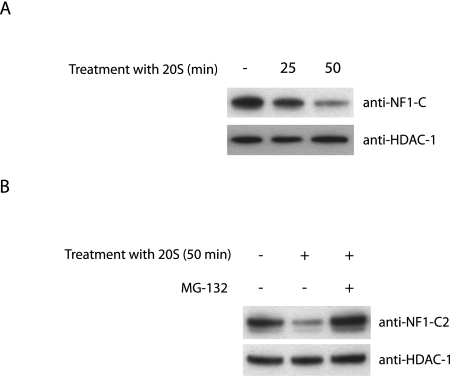
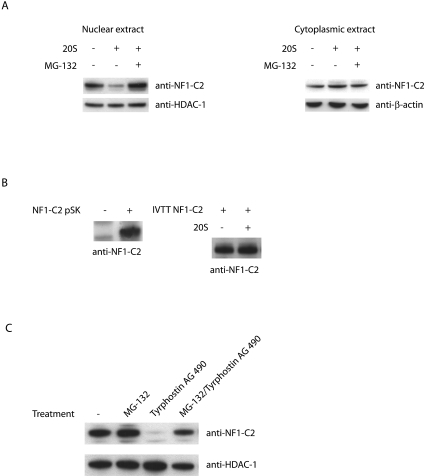
In order to test whether NF1-C2 can be degraded by 20S proteasomes, experiments with purified 20S proteasomes were performed. We isolated nuclear extracts from the HC11 cells, incubated the extracts with 20S proteasomes for different lengths of time as indicated, and analyzed the effect with Western blotting. The results revealed that in the absence of 20S proteasomes, the NF1-C2 protein was stable for at least 50 min at 37°C (Fig. 1A). However, in the sample to which 20S proteasomes were added, most of the NF1-C2 protein was degraded after 50 min (Fig. 1A). This proteolysis was blocked by the proteasome inhibitor MG-132 (Fig. 1B). As a control, we analyzed the amount of HDAC-1 under the same conditions and found no significant change in the amount of this protein (Fig. 1A and B). The results from these assays demonstrate that NF1-C2 is degraded by purified 20S proteasomes in vitro.
FIG. 1.
NF1-C2 is degraded by purified 20S proteasomes in vitro. (A) Nuclear extracts from MDA-MB 436 cells transfected with plasmids encoding full-length NF1-C2 were incubated at 37°C without 20S proteasome (−) or with 20S proteasome for different time periods in PBS. The treated extracts were then subjected to Western blot analysis. The blot was incubated with the NF1-C antibody (8199). The blot was then stripped and reincubated with an anti-HDAC-1 antibody as indicated. (B) Nuclear extracts from HC11 cells were incubated at 37°C with (+) or without 20S proteasome for 50 min in PBS. Where indicated, the proteasomal inhibitor MG-132 was added to inhibit the 20S proteasome proteolytic activity. The extracts were subjected to Western blot analysis. The blot was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with an anti-HDAC-1 antibody as indicated.
NF1-C2 undergoes proteasomal degradation in vivo in mammary epithelial cells.
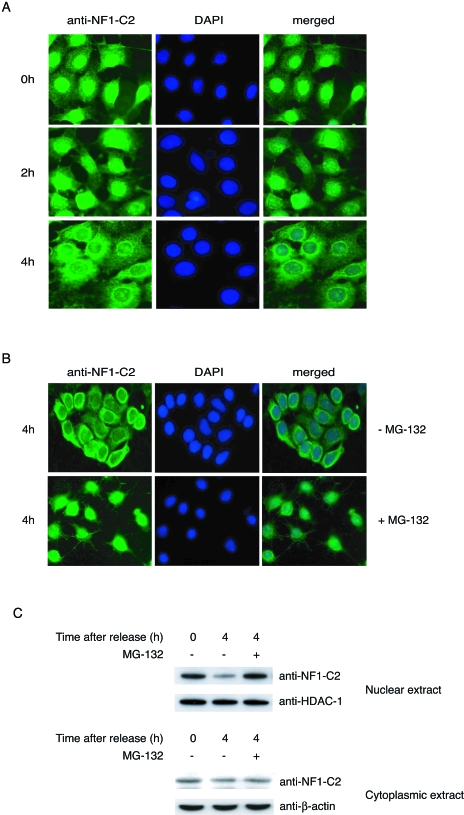
Recently the cyclin-dependent kinase inhibitor p21WAF/CIP1 was shown to be degraded by the 20S proteasome, through binding to the α7 subunit, in a ubiquitin-independent manner (32). Degradation of this protein plays a critical role in cell cycle control at the G1/S border, and we wanted to determine if the level of NF1-C2 protein also changes at this stage. HC11 cells were arrested at the G1/S border, as confirmed by fluorescence-activated cell sorter analysis, by a double thymidine block. After the HC11 cells were released from the thymidine block, they progressed through the S phase synchronously, and the level of NF1-C2 protein was analyzed by use of immunofluorescent staining of the cells. As can be seen in Fig. 2A, HC11 cells arrested at the G1/S border have NF1-C2 protein accumulated in the nucleus. However, 4 h after release from the arrest, when the cells are in mid-S to late S phase, the amount of NF1-C2 protein in the nucleus is reduced (Fig. 2A). This reduction was verified by Western blotting on nuclear extracts (Fig. 2C, upper panel). As a control, we analyzed the amount of HDAC-1 under the same conditions and found that there was no significant change in the amount of this protein. Further, since the amount of NF1-C2 proteins in the cytoplasmic fraction is unaffected by the treatment, the possibility that the decrease of nuclear NF1-C2 proteins due to nuclear cytoplasmic shuttling is excluded (Fig. 2C, lower panel). Since we had in vitro data showing that the NF1-C2 protein can be degraded by the 20S proteasome, we wanted to investigate whether the smaller amount of NF1-C2 4 h after release of the cell cycle block was due to protein degradation by the proteasome. As can be seen in Fig. 2B and C, treatment of the synchronized cells with the proteasome inhibitor MG-132 for 4 h prevented the decrease of nuclear NF1-C2 protein, indicating that the decrease of NF1-C2 proteins observed after 4 h was due to protein degradation by the proteasome.
FIG. 2.
NF1-C2 is degraded by the proteasome in vivo in mammary epithelial cells. (A) HC11 cells grown on small object glasses were arrested at the G1/S border by a double thymidine block. The object glasses containing cells were taken out after 0, 2, and 4 h after release in fresh medium. The subcellular localization of NF1-C2 was then analyzed by immunohistochemistry using the NF1-C2-specific antibody (1:100 dilution). After being incubated with the secondary antibody, the cells were stained with fluorescein isothiocyanate. Nuclei were visualized with DAPI (4′,6′- diamidino-2-phenylindole). (B) The double-thymidine-blocked cells were treated or not treated with MG-132 for 4 h as indicated. NF1-C2 proteins were visualized as described above. (C) Nuclear and cytoplasmic extracts were prepared from double-thymidine-blocked cells treated with (+) or without (−) MG-132 for 4 h and subjected to Western blot analysis. The filter containing the nuclear extracts was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with an HDAC-1 antibody. The filter containing the cytoplasmic extracts was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with a β-actin antibody. The HDAC-1 and β-actin blots served as loading controls.
Prolactin affects the stability of NF1-C2 proteins through Jak2.
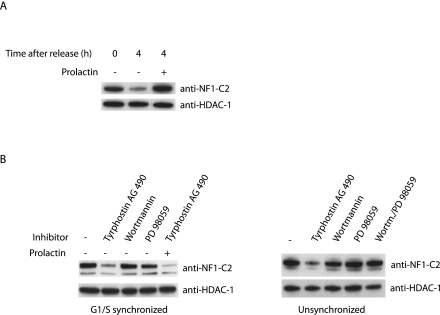
Previously we have reported that prolactin is involved in maintaining the basal levels of NF1-C2 proteins in the nuclei of mammary epithelial cells in pregnant mice as well as in cells derived from a virgin mouse (15). To investigate the hypothesis that prolactin prevents NF1-C2 degradation, we incubated the double-thymidine-blocked cells with prolactin for 4 h. Western blot analysis of extracts from these cells revealed that prolactin indeed prevents degradation of NF1-C2 (Fig. 3A).
FIG. 3.
Prolactin affects the stability of NF1-C2 proteins through Jak2. (A) Nuclear extracts were prepared from double-thymidine-blocked cells treated with (+) or without (−) prolactin for 4 h and subjected to Western blot analysis. The filter was first incubated with the NF1-C2-specific antibody and then stripped and reincubated with an HDAC-1 antibody. (B) Nuclear extracts were prepared from G1/S-synchronized as well as unsynchronized HC11 cells treated with different inhibitors for 20 min as indicated in the figure. Where indicated, prolactin was added to the cells for 20 min. The different blots were first incubated with the NF1-C2-specific antibody and then stripped and reincubated with the HDAC-1 antibody.
Prolactin receptor signaling can be mediated through several different pathways, such as the Jak, mitogen-activated protein (MAP) kinase, and phosphatidylinositol 3 (PI 3)-kinase pathways (18). To determine through which of these pathways the effect of prolactin on NF1-C2 is mediated, specific inhibitors were added to G1/S-synchronized as well as unsynchronized HC11 cells for 20 min. We used the inhibitors tyrphostin AG490 (for Jak), wortmannin (for PI 3-kinase), and PD98059 (for MAP kinase), and the reason for the short time of treatment is that earlier findings demonstrated that the prolactin response is fast (15). As can be seen in the left panel of Fig. 3B, treatment of the G1/S-synchronized cells with tyrphostin AG490 for 20 min decreased the amount of NF1-C2, while wortmannin and PD98059 had no effect. Similar results were observed for the unsynchronized HC11 cells (Fig. 3B, right panel). Treatment of the G1/S-synchronized HC11 cells with prolactin together with tyrphostin AG490 did not overcome the effect observed for the Jak inhibitor (Fig. 3B, left panel), indicating that prolactin affects NF1-C2 only through Jak. It has previously been shown that Jak2 is the only Jak family member expressed at significant levels in HC11 cells, a finding implying that prolactin affects NF1-C2 through Jak2 (36). These findings demonstrate that prolactin affects the stability of the NF1-C2 proteins through catalytically active Jak2.
Active Jak2 is present in the nuclei of mammary epithelial cells.
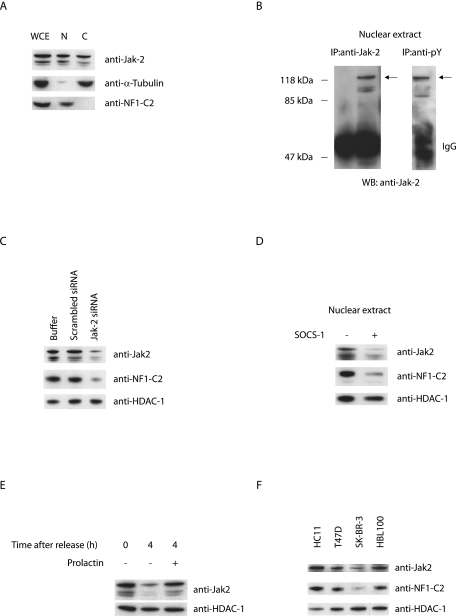
All functional features of Jak2 proteins found to date point to their involvement in events that take place at the level of complexes associated with the cell surface membrane. Previous reports demonstrated a nuclear localization of Jak2, but its functional significance remained elusive (22, 27, 30). Western blot analysis of whole-cell extract as well as of subcellular extracts prepared from HC11 cells revealed that Jak2 is present in the nuclear as well as the cytoplasmic fractions from these cells (Fig. 4A). Since Jak2 is widely believed to be primarily cytosolic, we wanted to examine the possibility that our detection of Jak2 in nuclear extracts might be a consequence of incomplete separation of the nuclear and cytosolic fractions during preparation. As can be seen in Fig. 4A, the cytosolic protein α-tubulin was almost completely restricted to the cytoplasmic fraction, whereas the nuclear NF1-C2 protein was restricted to the nuclear fraction, revealing that the presence of Jak2 in the nuclear extract is not due to contamination of the nuclear fraction.
FIG. 4.
Active Jak2 is localized in the nuclei of mammary epithelial cells, and its presence correlates with the amount of nuclear NF1-C2. (A) Subcellular extracts from HC11 cells were prepared and subjected to Western blot analysis. The amounts of loaded extracts were adjusted to represent extracts from an equal amount of cells. The blots were incubated with anti-Jak2, anti-α-tubulin, or anti-NF1-C2 as indicated. WCE, whole-cell extract; N, nuclear extract; C, cytoplasmic extract. (B) One hundred fifty micrograms of nuclear extract was immunoprecipitated (IP) with anti-Jak2 antibody or anti-pY antibody and subsequently Western blotted (WB) with anti-Jak2 antibody. The arrows indicate the tyrosine-phosphorylated Jak2 protein. IgG, immunoglobulin G. (C) Western blot analysis of nuclear extracts from untransfected HC11 cells or HC11 cells transiently transfected with scrambled siRNA or Jak2 siRNA as indicated. The blots were incubated with anti-Jak2, anti-NF1-C2, or anti-HDAC-1. (D) Nuclear extracts were prepared from HC11 cells stably transfected with a SOCS-1 expression plasmid (+) or not transfected (−) and subjected to Western blot analysis. The filter was first incubated with the Jak2 antibody and then stripped twice and incubated with the NF1-C2-specific antibody or the HDAC-1 antibody. (E) Nuclear proteins were prepared from double-thymidine-blocked cells treated with or without prolactin for 4 h and subjected to Western blot analysis. The filter was first incubated with anti-Jak2 antibody and then stripped and reincubated with anti-HDAC-1 antibody. (F) Nuclear extracts from four different mammary epithelial cell lines, HC11, T47D, SK-BR-3, and HBL100, were subjected to Western blot analysis. The filters were incubated with anti-Jak2, anti-NF1-C2, or anti-HDAC-1 as indicated.
Since Jak2 kinase activity seems to be important for the stability of the NF1-C2 proteins, we examined whether the nuclear Jak2 observed in the HC11 cells is tyrosine phosphorylated. Western blot analysis of nuclear extracts immunoprecipitated with anti-Jak2 or an antiphosphotyrosine antibody revealed that the nuclear Jak2 is tyrosine phosphorylated and hence active (Fig. 4B).
The conclusion that prolactin affects the stability of the NF1-C2 proteins through catalytically active Jak2 was based on the treatment of HC11 cells with the Jak inhibitor AG490. Since the specificity of AG490 has been questioned, we decided to use RNA interference to investigate the impact of nuclear Jak2 on NF1-C2 abundance. We used the Jak2 siRNA duplex reported by He et al. (12) in the RNA interference analysis, and by comparing wild-type cells with cells containing a reduced level of Jak2 we could conclude that there is indeed a direct correlation between the presence of nuclear Jak2 and that of NF1-C2 in the cells (Fig. 4C). Further, the catalytic activity of Jak2 has been found to be subject to negative regulation through various mechanisms, including association with SOCS-1 proteins (1). The inhibitory role of SOCS-1 is in part due to the fact that Jak2-SOCS-1 interaction blocks substrate access to Jak2 kinase. Another critical role for SOCS-1 in downregulating signaling is the targeting of associated proteins to proteasomal degradation. In order to investigate if SOCS-1 affects the amount of nuclear Jak2 and thereby affects the amount of NF1-C2 as well, we overexpressed SOCS-1 in the HC11 cells. A Western blot analysis of nuclear extracts from these cells revealed reduced levels of nuclear Jak2 and of NF1-C2 in the transfected cells compared to those seen for the untransfected cells (Fig. 4D).
Since we had shown in several ways that activated Jak2 in the nucleus is important for the presence of NF1-C2, we wanted to investigate whether the decrease of nuclear NF1-C2 proteins in the double-thymidine-blocked cells was associated with a decrease of nuclear Jak2. The results from Western blot analysis demonstrated that the reduction of NF1-C2 proteins correlates well with a reduction of Jak2 proteins (compare Fig. 3A and 4E).
In order to evaluate whether nuclear Jak2 is a feature of HC11 cells alone, we investigated the amounts of nuclear Jak2 in three human breast cancer cell lines, T47D, SK-BR-3, and HBL100. A Western blot analysis of nuclear extract from these cells revealed that Jak2 is located in the nucleus in these cells as well (Fig. 4F). Figure 4F also demonstrates that the amount of Jak2 in the nucleus affects the amount of NF1-C2. The level of nuclear Jak2 is lowest in the SK-BR-3 cells, and these cells also have the smallest amount of NF1-C2.
All these results together demonstrate that there is a correlation between nuclear Jak2 and the presence of NF1-C2 in the nuclei of mammary epithelial cells.
NF1-C2 is tyrosine phosphorylated by and associated with Jak2 in the nucleus.
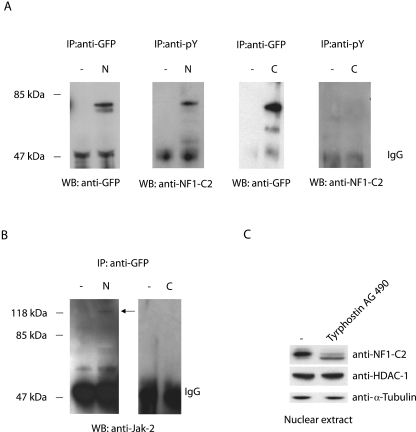
Previously, we have reported that the nuclear NF1-C2 protein is a phosphoprotein (16). Since nuclear Jak2 seems to be involved in the regulation of NF1-C2, we wanted to investigate whether NF1-C2 in the nucleus is tyrosine phosphorylated. Subcellular extracts from HC11 cells transfected with a GFP-NF1-C2 construct were prepared and immunoprecipitated with either an antiphosphotyrosine antibody or an anti-GFP antibody. As can be seen in Fig. 5A, only the low-mobility band, which was present only in the nucleus, was precipitated by the anti-pY antibody, demonstrating that the NF1-C2 protein in the nucleus is tyrosine phosphorylated (Fig. 5A). The reason for using GFP fusion proteins in the immunoprecipitation experiments is strictly technical, since the mobility of the NF1-C2 proteins is almost the same as that for the heavy chain of the antibodies.
FIG. 5.
Jak2 interacts with and tyrosine phosphorylates NF1-C2 in the nucleus. (A) Subcellular extracts from HC11 cells transfected with a plasmid encoding GFP-NF1-C2 fusion protein were immunoprecipitated (IP) with either anti-GFP or anti-pY antibody and subsequently Western blotted (WB) with an anti-GFP antibody or an anti-NF1-C2 antibody as indicated. N, nuclear extract; C, cytoplasmic extract. (B) The blots containing extracts immunoprecipitated with anti-GFP were stripped and reincubated with anti-Jak2 antibody. (C) Nuclear extract from HC11 cells treated with tyrphostin AG490 for 4 h was subjected to Western blot analysis. The blot was incubated with the NF1-C2-specific antibody as well as with anti-HDAC-1 and anti-α-tubulin as indicated. The amount of α-tubulin was controlled to guarantee that the difference observed was not due to differences in nuclear/cytoplasmic contamination levels. IgG, immunoglobulin G.
Further, in order to investigate whether there is any protein-protein interaction between NF1-C2 and Jak2 in mammary epithelial cells, the filter containing the anti-GFP-immunoprecipitated extracts was stripped and reincubated with the anti-Jak2 antibody. The anti-Jak2 blotting revealed that Jak2 interacts with NF1-C2 in the nucleus and not with the NF1-C2 protein located in the cytoplasm (Fig. 5B). In order to determine whether Jak2 tyrosine phosphorylates NF1-C2 in the nucleus, the HC11 cells were treated with tyrphostin AG490 for 4 h. As illustrated in Fig. 5C, most of the tyrosine-phosphorylated NF1-C2 proteins in the nucleus were shifted to the unphosphorylated form after 4 h of treatment, consistent with nuclear Jak2 being responsible for the phosphorylation of NF1-C2. Taken together, these results demonstrate that only nuclear Jak2 interacts with and tyrosine phosphorylates NF1-C2.
Interaction between Jak2 and tyrosine-phosphorylated NF1-C2 prevents NF1-C2 association with and subsequent degradation by the proteasome in vivo.
It has previously been shown that tyrosine phosphorylation is a requirement for the proteasomal degradation of some proteins (33). Since we had observed that the main NF1-C2 protein in the nucleus is tyrosine phosphorylated, we wanted to investigate whether tyrosine phosphorylation is a requirement for proteasomal degradation of NF1-C2 as well. We isolated protein extracts from the nuclei and the cytoplasms of HC11 cells and mixed the different extracts with purified 20S. The result from this study revealed that 20S was affecting only the tyrosine-phosphorylated form of NF1-C2 (Fig. 6A). In accordance with this finding, NF1-C2 produced by in vitro transcription/translation was unaffected by 20S treatment (Fig. 6B).
FIG. 6.
Interaction between Jak2 and tyrosine-phosphorylated NF1-C2 prevents NF1-C2 association with and subsequent degradation by the proteasome in vivo. (A) Western blot analysis of subcellular extracts incubated at 37°C for 50 min in the absence (−) or presence (+) of purified 20S proteasome. Where indicated, the proteasome inhibitor MG-132 was added. The blot was incubated with the anti-NF1-C2-specific antibody. HDAC-1 and β-actin were used as loading controls. (B) Western blot on NF1-C2 protein generated with the in vitro transcription/translation (IVTT) TNT-coupled reticulocyte lysate system (left panel). The right panel shows a Western blot analysis of the in vitro transcription/translation NF1-C2 protein treated with or without 20S as indicated. (C) Nuclear proteins were prepared from HC11 cells treated with MG-132 and/or tyrphostin AG490 as indicated and subjected to Western blot analysis. The filter was first incubated with anti-NF1-C2 antibody and then stripped and reincubated with anti-HDAC-1 antibody.
The results we had obtained up to that point demonstrated that Jak2 is responsible for the tyrosine phosphorylation of NF1-C2. To determine if a decreased amount of active Jak2 affects the association of tyrosine-phosphorylated NF1-C2 proteins with the proteasome and thereby their proteasomal degradation, we treated HC11 cells with tyrphostin AG490 and/or MG-132 for 20 min. As shown in Fig. 6C, treatment of the cells with tyrphostin AG490 alone caused a dramatic decrease of the tyrosine-phosphorylated NF1-C2 protein, whereas treatment of the cells with MG-132 together with tyrphostin AG490 did not affect the amount of NF1-C2 protein. Taken together with the coimmunoprecipitation of Jak2 with nuclear NF1-C2, these results demonstrate that nuclear Jak2 tyrosine phosphorylates NF1-C2 and prevents it from proteasomal degradation.
Nuclear Jak2 is important for the expression of the p53 gene as well as the gene encoding the milk protein carboxyl ester lipase in mammary epithelial cells.
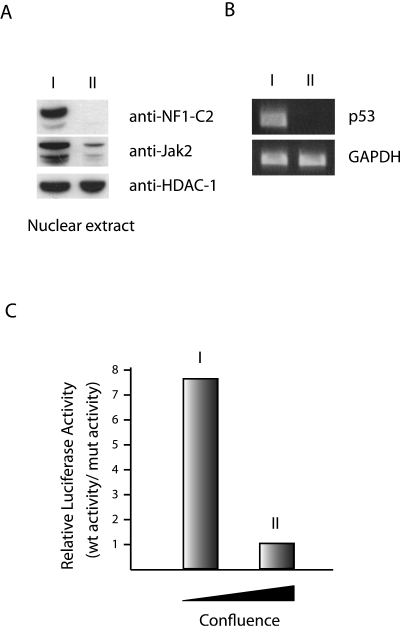
Previously, we have reported that the amount of NF1-C2 protein in HC11 cells is affected by the degree of confluence of the cells (16). The amount of NF1-C2 proteins is decreased as the cells became more confluent. In order to investigate whether this decrease is a consequence of a smaller amount of Jak2 proteins in the nucleus, a Western blot analysis was performed. As can be seen in Fig. 7A, the amount of Jak2 proteins is strongly reduced in cells grown to the highest degree of confluence. We have previously identified different targets for NF1-C2 in mouse mammary epithelial cells. We showed by using promoter studies and the in vivo methods chromatin immunoprecipitation and oligonucleotide decoy that binding of NF-C2 to the mouse p53 promoter is important for the expression of p53 at midpregnancy. Furthermore, we have shown that during the development of the mouse mammary epithelial cells, NF1-C2 activates the CEL and whey acidic protein gene promoters (14, 16). Since we had shown by siRNA experiments and several other methods that there is a correlation between nuclear Jak2 and the level of NF1-C2, we wanted to investigate if the decrease of Jak2/NF1-C2 had any effect on the expression of the p53 gene and the CEL gene. The amount of p53 mRNA was investigated by RT-PCR using RNA from HC11 cells grown to different degrees of confluence. As can be seen in Fig. 7B, the amount of p53 is reduced in the absence of nuclear Jak2/NF1-C2. Further, the expression of the mouse CEL gene was analyzed for HC11 cells stably transfected with the mouse CEL gene promoter construct −1831, referred to as mCEL-1831Luc, and for those transfected with the mCEL-1831NF1:1mutLuc construct containing a mutated NF1 binding site. The cells were grown to different degrees of confluence, and the result from this luciferase reporter gene assay revealed that the proportional difference between the luciferase activities of the two constructs decreased as the cells became more confluent (Fig. 7C). These data together with our earlier results suggest that both the p53 and CEL genes are downstream targets for nuclear Jak2/NF1-C2 signaling in mammary epithelial cells.
FIG. 7.
Nuclear Jak2 affects the expression of p53 and CEL genes through NF1-C2. (A) Western blot analysis with nuclear extracts from HC11 cells grown to different degrees of confluence (stages I and II). Stage I is less confluent than stage II. (B) The endogenous mRNA expression levels of the p53 gene in HC11 cells grown to different degrees of confluence (stages I and II) were assayed. (C) Luciferase activity levels were measured in HC11 cells stably transfected with either the mCEL-1831Luc or the mCEL-1831NF1:1mutLuc construct and grown to different degrees of confluence as indicated. The relative luciferase activity levels were normalized to the total protein concentration of each extract. Data represent means from three independent experiments. To illustrate the proportional difference between the two promoter constructs, the average luciferase activity level of the wild-type (wt) construct from each stage was divided by the average activity level of the mutant (mut) construct from each stage.
The reduction of tyrosine-phosphorylated NF1-C2 proteins in vivo in the mammary epithelial cells at lactation is due to the lack of activated Jak2 in the nucleus.
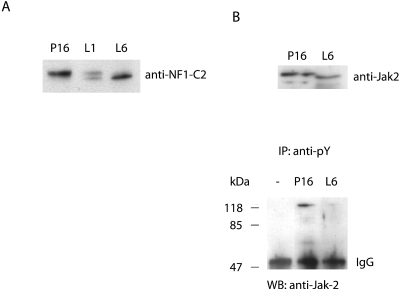
An interesting finding from our previous work is the decreased levels of NF1-C2 proteins in terminally differentiated mammary epithelial cells at lactation (15). We now have data indicating not only that there is a decrease in the level of NF1-C2 proteins at lactation but also that the remaining NF1-C2 proteins are also differentially modified. As illustrated in Fig. 8A, the amount of the tyrosine-phosphorylated NF1-C2 proteins was decreased between P16 and L1. At L6, the tyrosine-phosphorylated form is absent and the unphosphorylated form dominates.
FIG. 8.
The decreased level of tyrosine-phosphorylated NF1-C2 proteins in the nucleus at lactation correlates with a decrease of active Jak2 in the nucleus at this stage. (A) Nuclear extracts from mammary glands at P16, L1, and L6 were subjected to Western blot analysis. The filter was incubated with the anti-NF1-C2 antibody. (B) Upper panel: Western blot analysis of nuclear extract from P16 and L6. The blot was incubated with anti-Jak2 antibody. Lower panel: 150-μg portions of nuclear extracts from P16 and L6 were immunoprecipitated (IP) with anti-pY antibody and subsequently analyzed by Western blotting (WB) with anti-Jak2 antibody.
It is clear from earlier studies that the distribution of NF1-C2 resembles that of activated Stat5 in the mouse mammary gland during pregnancy (15, 24). However, at lactation, when the NF1-C2 protein levels are decreased, the Stat5 protein levels are maintained. Hence, the regulation diverges after pregnancy, despite the fact that Jak2 phosphorylates both these proteins. It is well established that prolactin activates Stat5 through Jak2 in the cytoplasm, which results in a translocation of the Stat5 proteins into the nucleus (reviewed in reference 34). Nevertheless, we are now able to show that phosphorylation of NF1-C2 by Jak2 takes place in the nucleus. In order to investigate whether the decreased level of tyrosine-phosphorylated NF1-C2 proteins in the nucleus at lactation correlates with a decrease of active Jak2 in the nucleus at this stage, extracts from P16 and L6 were immunoprecipitated with an antiphosphotyrosine antibody. Western blot analysis of these extracts with an anti-Jak2 antibody indicated that the amount of active Jak2 is indeed lower in the nucleus at lactation (Fig. 8B, lower panel). This further confirms our earlier conclusion that the phosphorylation of NF1-C2 is restricted to the nucleus.
DISCUSSION
Mammary gland development is orchestrated by changes in hormone levels, and specific hormones direct specific mechanisms at specific stages. Our studies have led to the identification of prolactin as a hormone directly regulating NF1-C2 proteins in the mouse mammary gland during pregnancy (15). Mammary tissue from heterozygous prolactin receptor knockout mice demonstrated that prolactin has a direct effect in the maintenance of the NF1-C2 protein levels in mammary epithelial nuclei. The importance of prolactin in the proliferation and differentiation of the mammary gland is well established (18). The demonstration of NF1-C2 as a target transcription factor activated by prolactin provided new insight into the mechanism of prolactin action in the mammary gland.
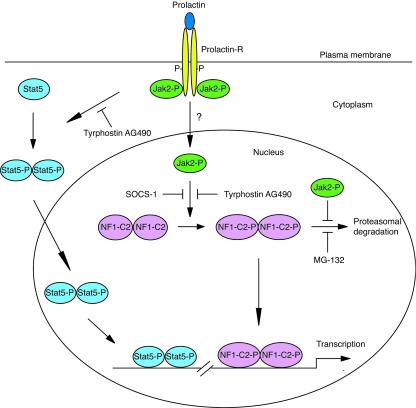
Prolactin receptor signaling can be mediated through several different pathways besides that of Jak-Stat, such as the MAP kinase and PI 3-kinase pathways (18). In this paper we show that prolactin affects the NF1-C2 protein through Jak2. Furthermore, we found that the prolactin-activated Jak2 acts in the nuclei of the mammary epithelial cells, where it tyrosine phosphorylates NF1-C2. This is to our knowledge the first demonstration that nuclear Jak2 provides a prolactin response. Hence, we also demonstrate a novel non-Stat-including pathway by which prolactin-Jak2 acts in the mammary gland (Fig. 9).
FIG. 9.
Mechanisms for prolactin action in the mouse mammary gland. The novel mechanism proposed by us is that prolactin/prolactin receptor (Prolactin-R) activates Jak2, which translocates to the nucleus by an as-yet-unknown mechanism. In the nucleus, Jak2 tyrosine phosphorylates (P) the transcription factor NF1-C2 and protects it from proteasomal degradation. The classical way by which prolactin transduces its signal in mammary gland is through the depicted Jak2-Stat5 pathway. When prolactin binds to its receptor, the receptor dimerizes and Jak2 is activated by transphosphorylation. Jak2 mediates phosphorylation of Stat5, which dimerizes and translocates to the nucleus, where it activates target genes.
Another important finding in the present study is that nuclear Jak2 protects the NF1-C2 protein from degradation. An inhibition of Jak2 activity by the addition of tyrphostin AG490 or a reduction in the amount of Jak2 by RNA interference resulted in a dramatic reduction of the tyrosine-phosphorylated NF1-C2 proteins in the nucleus. Since the proteasome inhibitor MG-132 could prevent the decrease of NF1-C2 proteins, we concluded that the presence of Jak2 in the nucleus prevents NF1-C2 association with and subsequent degradation by the proteasome (Fig. 9). Proteasome inhibitors have been shown to prolong the activity of the Jak-Stat signaling pathway by preventing tyrosine-phosphorylated Jak2 protein degradation (38). This could indicate that the effect obtained with NF1-C2 when the proteasomal inhibitor is added could be indirect. However, inactivating Jak2 by adding tyrphostin AG490, and thereby preventing its interaction with NF1-C2, resulted in an immediate reduction in the level of NF1-C2 proteins. This result suggests that the proteasome is acting directly on NF1-C2. That Jak2 can protect proteins from proteasomal degradation has recently been shown in a paper by Royer et al. (28). In their work, they demonstrated that Jak2, besides signaling downstream of the activated thrombopoietin receptor, strongly promotes cell surface localization and enhances total protein levels of the receptor by protecting the receptor from proteasomal degradation (28).
It is clear from earlier studies that the distribution of NF1-C2 resembles that of activated Stat5 in the mouse mammary gland during pregnancy (15, 24). However, at lactation, the regulation of NF1-C2 and that of activated Stat5 diverge. At this stage, the NF1-C2 protein level is decreased, whereas the level of activated Stat5 protein is maintained. This finding was somewhat surprising, since the level of prolactin, which affects them both, is peaking at this stage. However, in the present paper we demonstrate that the level of activated Jak2 in the nucleus at lactation is decreased. Thus, the different mechanisms, which involve either prolactin activation of nuclear NF1-C2 via a direct Jak2 kinase interaction in the nucleus or prolactin activation of cytosolic Stat5 via a plasma membrane-associated prolactin receptor-Jak2 kinase complex (Fig. 9), could explain the observed difference in prolactin effects on the NF1-C2 and Stat5 proteins at lactation (15). The divergence in the regulation of NF1-C2 and that of Stat5 at lactation suggests specific roles for each of them for mammary gland function and remodeling. For example, both factors might play important roles for the establishment of the gland, but while Stat5 acts as a survival factor preventing apoptosis of terminally differentiated epithelial cells (34), NF1-C2 might have opposing effects at these mature stages. Besides being a proliferative stimulus (21), prolactin might also signal through antiproliferative pathways. This might be an intricate means by which prolactin enforces an appropriate fine balance between mitotic and growth-suppressive signals while regulating functional differentiation of the gland.
p53 is a central component in the protection of the mammary epithelium against tumor progression, and transcriptional control of p53 expression participates in the generation of appropriate levels of active p53 in response to mitogenic stimulation. We have previously provided data showing that NF1-C2 is one of the factors responsible for stimulating p53 gene expression in the mouse mammary gland (14). In the present paper, we extend this information by showing that nuclear Jak2 is important for the expression of p53. That there is a relationship between the amount of nuclear Jak2/NF1-C2 and p53 mRNA expression is further supported by comparing the amounts of nuclear Jak2/NF1-C2 in three human breast cancer cell lines, T47D, HBL100, and SK-BR-3, which are depicted in Fig. 4F with the corresponding levels of p53 mRNA expression in these same cell lines as described by Concin et al. (7). As can be seen in Fig. 4F, the level of nuclear Jak2/NF1-C2 in the SK-BR-3 cells is considerably lower than the levels detected for the other cell lines. This decrease in nuclear Jak2/NF1-C2 correlates with the lower level of p53 mRNA expression (7). Hence, nuclear Jak2/NF1-C2 signaling participates in the tight control of p53. Since the p53 protein has no apparent role for the growth and differentiation of the mammary gland, the role of nuclear Jak2 and NF1-C2 in p53 regulation is most probably to keep sufficient levels of latent p53 mRNA to ensure rapid accumulation of p53 proteins in response to cellular stress.
Although NF1-C2 has an important role in milk gene activation, milk genes are most highly expressed at lactation, when the NF1-C2 protein levels are reduced. This observation suggests that NF1-C2 is important for the initiation and not the maintenance of milk gene expression. In contrast, since Stat5 is important for the maintenance of the differentiated mammary epithelium, it has been proposed that Stat5 not only establishes transcription in these cells but also maintains an active transcription complex. Rapid loss of Stat5 will hence result in a loss of transcription of target genes (8). Hence, these two transcription factors might provide distinct functions in milk gene regulation. Unless NF1-C2 can bind during pregnancy, the expression of the CEL and whey acidic protein milk genes might never be initiated. However, after the initiation of these genes, NF1-C2 might not be needed. How NF1-C2 proteins accomplish initiation of milk gene transcription is not known, but one mechanism could be by bringing together other transcriptional regulators into a complex important for gene regulation. Another mechanism by which NF1-C2 could initiate milk gene expression could be by opening up the chromatin to increase the accessibility for other factors.
Even though the intracellular signaling pathways activated by prolactin are relatively well understood, the mechanisms by which signaling is attenuated are yet to be defined. However, there are indications that SOCS-1 has a biological role in the developing mammary gland, where it acts as a negative regulator of prolactin signaling and may play a negative regulatory role in the induction of lactation after parturition (20, 25). Recruitment of SOCS-1 to tyrosine-phosphorylated Jak2 leads to inhibition of the catalytic activity of Jak2 (37). In mammary glands from SOCS-1-deficient mice, there are significantly higher levels of milk proteins, indicating that factors activated by Jak2, such as Stat5 and NF1-C2, are important for the expression of the corresponding genes (20). Since this could not be coupled to elevated levels of Stat5 phosphorylation, the higher level of milk proteins could be due to a higher level of NF1-C2. This suggestion is in fact supported by the observation that overexpression of SOCS-1 in HC11 cells resulted in decreased levels of nuclear Jak2 protein as well as of NF1-C2 proteins, indicating that NF1-C2 is involved in a SOCS-1-regulated pathway through Jak2.
Earlier findings demonstrated nuclear localization of Jak2, but the significance of nuclear Jak2 had not been clear (22, 27, 30). However, we demonstrate here that Jak2 possesses tyrosine kinase activity in the nucleus and that its interaction with NF1-C2 prevents the protein from degradation by the proteasome. Thus, we show that Jak2 has at least two functions in the nucleus and also that prolactin can exert an effect in the nucleus by a novel mechanism, i.e., by acting through nuclear Jak2/NF1-C2. Our discovery of a new signaling pathway in the developmental process of the mammary gland seems to give prolactin an important function for balancing proliferation and differentiation. Future studies in our laboratory will focus on the elucidation of molecular mechanisms involved in this regulation and on the question of whether a deviation from a balanced situation might be involved in the oncogenic process.
. . . . . . . .
Acknowledgments
We are grateful to Kerstin Dahlenborg for technical assistance. We also thank N. Tanese, NYU Medical Center, New York, for the NF1-C-specific antibody (8199).
This work was supported by grants from the Swedish Cancer Society, the Assar Gabrielsson Foundation, the Lars Hierta Foundation, and the Magnus Bergvall Foundation.
REFERENCES
- 1.Alexander, W. S., R. Starr, D. Metcalf, S. E. Nicholson, A. Farley, A. G. Elefanty, M. Brysha, B. T. Kile, R. Richardson, M. Baca, J. G. Zhang, T. A. Willson, E. M. Viney, N. S. Sprigg, S. Rakar, J. Corbin, S. Mifsud, L. DiRago, D. Cary, N. A. Nicola, and D. J. Hilton. 1999. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J. Leukoc. Biol. 66:588-592. [DOI] [PubMed] [Google Scholar]
- 2.al-Sakkaf, K. A., P. R. Dobson, and B. L. Brown. 1997. Prolactin induced tyrosine phosphorylation of p59fyn may mediate phosphatidylinositol 3-kinase activation in Nb2 cells. J. Mol. Endocrinol. 19:347-350. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. G. Seidman, J. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
- 4.Berlanga, J. J., J. A. Fresno Vara, J. Martin-Perez, and J. P. Garcia-Ruiz. 1995. Prolactin receptor is associated with c-src kinase in rat liver. Mol. Endocrinol. 9:1461-1467. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanover, A. 1994. The ubiquitin-proteasome proteolytic pathway. Cell 79:13-21. [DOI] [PubMed] [Google Scholar]
- 7.Concin, N., C. Zeillinger, D. Tong, M. Stimpfl, M. Konig, D. Printz, F. Stonek, C. Schneeberger, L. Hefler, C. Kainz, S. Leodolter, O. A. Haas, and R. Zeillinger. 2003. Comparison of p53 mutational status with mRNA and protein expression in a panel of 24 human breast carcinoma cell lines. Breast Cancer Res. Treat. 79:37-46. [DOI] [PubMed] [Google Scholar]
- 8.Cui, Y., G. Riedlinger, K. Miyoshi, W. Tang, C. Li, C. X. Deng, G. W. Robinson, and L. Hennighausen. 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24:8037-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, R., and B. K. Vonderhaar. 1996. Activation of raf-1, MEK, and MAP kinase in prolactin responsive mammary cells. Breast Cancer Res. Treat. 40:141-149. [DOI] [PubMed] [Google Scholar]
- 10.Das, R., and B. K. Vonderhaar. 1996. Involvement of SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary epithelial cells. Oncogene 13:1139-1145. [PubMed] [Google Scholar]
- 11.Goldberg, A. L., and K. L. Rock. 1992. Proteolysis, proteasomes and antigen presentation. Nature 357:375-379. [DOI] [PubMed] [Google Scholar]
- 12.He, K., K. Loesch, J. W. Cowan, X. Li, L. Deng, X. Wang, J. Jiang, and S. J. Frank. 2005. Janus kinase 2 enhances the stability of the mature growth hormone receptor. Endocrinology 146:4755-4765. [DOI] [PubMed] [Google Scholar]
- 13.Houdebine, L. M., J. Djiane, I. Dusanter-Fourt, P. Martel, P. A. Kelly, E. Devinoy, and J. L. Servely. 1985. Hormonal action controlling mammary activity. J. Dairy Sci. 68:489-500. [DOI] [PubMed] [Google Scholar]
- 14.Johansson, E. M., M. Kannius-Janson, G. Bjursell, and J. Nilsson. 2003. The p53 tumor suppressor gene is regulated in vivo by nuclear factor 1-C2 in the mouse mammary gland during pregnancy. Oncogene 22:6061-6070. [DOI] [PubMed] [Google Scholar]
- 15.Johansson, E. M., M. Kannius-Janson, A. Gritli-Linde, G. Bjursell, and J. Nilsson. 2005. Nuclear factor 1-C2 is regulated by prolactin and shows a distinct expression pattern in the mouse mammary epithelial cells during development. Mol. Endocrinol. 19:992-1003. [DOI] [PubMed] [Google Scholar]
- 16.Kannius-Janson, M., E. M. Johansson, G. Bjursell, and J. Nilsson. 2002. Nuclear factor 1-C2 contributes to the tissue-specific activation of a milk protein gene in the differentiating mammary gland. J. Biol. Chem. 277:17589-17596. [DOI] [PubMed] [Google Scholar]
- 17.Kannius-Janson, M., U. Lidberg, K. Hulten, A. Gritli-Linde, G. Bjursell, and J. Nilsson. 1998. Studies of the regulation of the mouse carboxyl ester lipase gene in mammary gland. Biochem. J. 336:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly, P. A., A. Bachelot, C. Kedzia, L. Hennighausen, C. J. Ormandy, J. J. Kopchick, and N. Binart. 2002. The role of prolactin and growth hormone in mammary gland development. Mol. Cell. Endocrinol. 197:127-131. [DOI] [PubMed] [Google Scholar]
- 19.Lidberg, U., M. Kannius-Janson, J. Nilsson, and G. Bjursell. 1998. Transcriptional regulation of the human carboxyl ester lipase gene in exocrine pancreas. Evidence for a unique tissue-specific enhancer. J. Biol. Chem. 273:31417-31426. [DOI] [PubMed] [Google Scholar]
- 20.Lindeman, G. J., S. Wittlin, H. Lada, M. J. Naylor, M. Santamaria, J. G. Zhang, R. Starr, D. J. Hilton, W. S. Alexander, C. J. Ormandy, and J. Visvader. 2001. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 15:1631-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovera, M., P. Touraine, P. A. Kelly, and V. Goffin. 2000. Involvement of prolactin in breast cancer: redefining the molecular targets. Exp. Gerontol. 35:41-51. [DOI] [PubMed] [Google Scholar]
- 22.Lobie, P. E., B. Ronsin, O. Silvennoinen, L. A. Haldosen, G. Norstedt, and G. Morel. 1996. Constitutive nuclear localization of Janus kinases 1 and 2. Endocrinology 137:4037-4045. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi, K., J. M. Shillingford, G. H. Smith, S. L. Grimm, K. U. Wagner, T. Oka, J. M. Rosen, G. W. Robinson, and L. Hennighausen. 2001. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 155:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevalainen, M. T., J. Xie, L. Bubendorf, K. U. Wagner, and H. Rui. 2002. Basal activation of transcription factor signal transducer and activator of transcription (Stat5) in nonpregnant mouse and human breast epithelium. Mol. Endocrinol. 16:1108-1124. [DOI] [PubMed] [Google Scholar]
- 25.Ormandy, C. J., A. Camus, J. Barra, D. Damotte, B. Lucas, H. Buteau, M. Edery, N. Brousse, C. Babinet, N. Binart, and P. A. Kelly. 1997. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11:167-178. [DOI] [PubMed] [Google Scholar]
- 26.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 27.Ram, P. A., and D. J. Waxman. 1997. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J. Biol. Chem. 272:17694-17702. [DOI] [PubMed] [Google Scholar]
- 28.Royer, Y., J. Staerk, M. Costuleanu, P. J. Courtoy, and S. N. Constantinescu. 2005. Janus kinases affect thrombopoietin receptor cell surface localization and stability. J. Biol. Chem. 280:27251-27261. [DOI] [PubMed] [Google Scholar]
- 29.Shillingford, J. M., K. Miyoshi, G. W. Robinson, S. L. Grimm, J. M. Rosen, H. Neubauer, K. Pfeffer, and L. Hennighausen. 2002. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 16:563-570. [DOI] [PubMed] [Google Scholar]
- 30.Sorenson, R. L., and L. E. Stout. 1995. Prolactin receptors and JAK2 in islets of Langerhans: an immunohistochemical analysis. Endocrinology 136:4092-4098. [DOI] [PubMed] [Google Scholar]
- 31.Stancovski, I., H. Gonen, A. Orian, A. L. Schwartz, and A. Ciechanover. 1995. Degradation of the proto-oncogene product c-Fos by the ubiquitin proteolytic system in vivo and in vitro: identification and characterization of the conjugating enzymes. Mol. Cell. Biol. 15:7106-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touitou, R., J. Richardson, S. Bose, M. Nakanishi, J. Rivett, and M. J. Allday. 2001. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. EMBO J. 20:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ungureanu, D., P. Saharinen, I. Junttila, D. J. Hilton, and O. Silvennoinen. 2002. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol. Cell. Biol. 22:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson, C. J. 2001. Stat transcription factors in mammary gland development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 6:115-127. [DOI] [PubMed] [Google Scholar]
- 35.Watson, C. J., and T. G. Burdon. 1996. Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway. Rev. Reprod. 1:1-5. [DOI] [PubMed] [Google Scholar]
- 36.Xie, J., M. J. LeBaron, M. T. Nevalainen, and H. Rui. 2002. Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J. Biol. Chem. 277:14020-14030. [DOI] [PubMed] [Google Scholar]
- 37.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu, C. L., and S. J. Burakoff. 1997. Involvement of proteasomes in regulating Jak-STAT pathways upon interleukin-2 stimulation. J. Biol. Chem. 272:14017-14020. [DOI] [PubMed] [Google Scholar]