Abstract
AMP-activated protein kinase (AMPK) is a sensor of cellular energy state in response to metabolic stress and other regulatory signals. AMPK is controlled by upstream kinases which have recently been identified as LKB1 or Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ). Our study of human endothelial cells shows that AMPK is activated by thrombin through a Ca2+-dependent mechanism involving the thrombin receptor protease-activated receptor 1 and Gq-protein-mediated phospholipase C activation. Inhibition of CaMKK with STO-609 or downregulation of CaMKKβ using RNA interference decreased thrombin-induced AMPK activation significantly, indicating that CaMKKβ was the responsible AMPK kinase. In contrast, downregulation of LKB1 did not affect thrombin-induced AMPK activation but abolished phosphorylation of AMPK with 5-aminoimidazole-4-carboxamide ribonucleoside. Thrombin stimulation led to phosphorylation of acetyl coenzyme A carboxylase (ACC) and endothelial nitric oxide synthase (eNOS), two downstream targets of AMPK. Inhibition or downregulation of CaMKKβ or AMPK abolished phosphorylation of ACC in response to thrombin but had no effect on eNOS phosphorylation, indicating that thrombin-stimulated phosphorylation of eNOS is not mediated by AMPK. Our results underline the role of Ca2+ as a regulator of AMPK activation in response to a physiologic stimulation. We also demonstrate that endothelial cells possess two pathways to activate AMPK, one Ca2+/CaMKKβ dependent and one AMP/LKB1 dependent.
AMP-activated protein kinase (AMPK) is a heterotrimeric serine/threonine kinase composed of a catalytic α subunit and regulatory β and γ subunits (6, 16). AMPK has been shown to function as a sensor of the energy state of the cell. It is activated by a rise in the AMP/ATP ratio following a fall of intracellular ATP and initiates a series of changes aimed at regulating energy balance at the cellular level. These processes include the inhibition of ATP-requiring anabolic pathways and the stimulation of ATP-generating catabolic pathways as well as changes in gene and protein expression (6, 16). Additionally, AMPK acts as a regulator of the whole-body energy metabolism by mediating the effects of hormones such as adiponectin, leptin, or ghrelin (28).
AMPK activation requires phosphorylation of threonine 172 in the activation loop of the α subunit (18). Two AMPK-activating kinases have been identified recently. LKB1, a tumor suppressor kinase, in complex with two accessory subunits, STRAD and MO25, has been shown to phosphorylate AMPK (19, 23, 38, 46). Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) has also been identified as an AMPK kinase (20, 25, 45). In addition to phosphorylation, AMPK is allosterically activated by binding of AMP, and this can also promote phosphorylation of threonine 172 (21). However, AMPK can be activated in an AMP-independent manner as shown with hyperosmotic stress or with the antidiabetic drug metformin (14). The finding that CaMKKβ acts upstream of AMPK suggests that in addition to changes of the AMP/ATP ratio an increase of intracellular Ca2+ may act as a second pathway to activate AMPK.
AMPK is ubiquitously expressed and has also been reported in endothelial cells of different origins. Endothelial AMPK has been shown to be activated by hypoxic stress (33), peroxynitrite (49, 50), hormones including adiponectin (7, 35) and estradiol (37), metformin (51), and vascular mediators such as bradykinin (32) and histamine or thrombin (40). Several studies have suggested that AMPK may play a role in endothelial cell energy supply (10); protection from apoptosis (26); and regulation of inflammation, angiogenesis, and maintenance of perfusion (5, 33, 35, 36). Recently, a link between AMPK and endothelial nitric oxide synthase (eNOS) activation has garnered considerable interest, and NO formation has been thought to be implicated in angiogenic and anti-inflammatory effects of AMPK (7, 31, 33, 35, 37, 40, 49, 50, 51). Accordingly, AMPK has been reported to phosphorylate eNOS at its serine residue 1177 (7, 8, 31, 33, 35, 40, 49, 50) or to enhance the interaction between eNOS and heat shock protein 90 (37).
Thrombin, one of the stimuli shown to induce AMPK phosphorylation, is a multifunctional serine protease that is generated at the sites of vascular injury. It mediates the final step of the coagulation cascade and additionally activates vascular cells via G-protein-coupled protease-activated receptors (PARs) (9). Endothelial cell responses to thrombin include shape and permeability changes and expression of adhesion molecules as well as synthesis of cytokines and autocoids such as NO and are often related to an increase of intracellular Ca2+ (3, 15, 30, 42). So far, the upstream kinase that is mediating AMPK activation upon thrombin stimulation of endothelial cells is not known. Moreover, it is not fully understood under which conditions AMPK mediates the phosphorylation of its putative downstream target eNOS and whether AMPK and eNOS activation are linked in thrombin-treated cells.
The aim of our study was to investigate the signaling mechanisms leading to AMPK activation and the functional relevance of AMPK in endothelial cells stimulated with thrombin. The results presented here demonstrate that thrombin activates AMPK in endothelial cells via its receptor PAR-1 in a Gq-protein/phospholipase C- and Ca2+-dependent and ATP-independent manner. Our study identifies CaMKKβ as the upstream kinase responsible for AMPK phosphorylation upon thrombin stimulation. Thrombin also induced the phosphorylation of acetyl coenzyme A carboxylase (ACC), a well-known downstream target of AMPK, and of eNOS at serine 1177. Inhibition of either CaMKKβ or AMPK by two different approaches (pharmacological inhibition or protein downregulation using RNA interference) showed, however, that only thrombin-induced ACC but not eNOS phosphorylation was mediated by the CaMKKβ/AMPK pathway.
MATERIALS AND METHODS
Materials.
Antibodies reacting with AMPKα (rabbit polyclonal), phospho-AMPKα (threonine 172, rabbit monoclonal), phospho-ACC (rabbit polyclonal), and phospho-eNOS (serine 1177, rabbit polyclonal) were acquired from Cell Signaling Technologies (Frankfurt, Germany). The antibody against human eNOS (monoclonal, clone 3) was obtained from Transduction Laboratories (Lexington, KY). Rabbit polyclonal antibody directed against ACC and biotinylated anti-rabbit immunoglobulin G (IgG) were from Upstate Biotechnology (Dundee, United Kingdom). Goat anti-CaMKKβ antibody as well as peroxidase-labeled bovine anti-goat IgG was from Santa Cruz Biotechnology (Santa Cruz, California). Mouse monoclonal antibody against LKB1 was from Abcam Ltd. (Cambridge, United Kingdom). Peroxidase-labeled anti-mouse and anti-rabbit IgG and peroxidase-labeled streptavidin were purchased from Kirkegaard & Perry Laboratories Inc. (Gaithersburg, Maryland). The protease inhibitor cocktail Complete, EDTA free, was from Roche Diagnostics GmbH (Mannheim, Germany), and stock solution was prepared as described by the manufacturer. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR), calmodulin, the Gq-protein antagonist 2A, and inhibitors for phospholipase C (U-73122) and AMPK (compound C) as well as the phospholipase C activator m-3M3FBS were obtained from Merck Bioscience/Calbiochem (Darmstadt, Germany). STO-609 was from Tocris (Ellisville, Missouri), and the PAR-1-activating peptide TFLLR was a generous gift from E. Glusa (University of Jena, Jena, Germany). The [3H]cGMP Biotrak radioimmunoassay was purchased from Amersham Pharmacia Biotech (Freiburg, Germany). Thrombin, ionomycin, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), and other reagents were purchased from Sigma Chemical Co. (Deisenhofen, Germany).
Cell culture.
Human umbilical cord vein endothelial cells (HUVEC) were cultured in M199 containing 15% fetal calf serum, 5% human serum, and 7.5 μg/ml endothelial cell growth supplement as described previously (22). After the first or second passage cells were plated on 30-mm-diameter wells (transfection experiments and nucleotide measurements) or 60-mm-diameter dishes. Experiments were started after 6 h of incubation in serum-free M199 containing 0.25% human serum albumin (HSA). Preincubation with inhibitors and cell stimulation was performed in HEPES-HSA buffer (10 mM HEPES [pH 7.4], 145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, 1.5 mM CaCl2, 0.25% HSA). Ionomycin, compound C, STO-609, and m-3M3FBS were dissolved in dimethyl sulfoxide and stored at −20°C until use. U-73122 stock solution was prepared immediately before use. The final concentration of dimethyl sulfoxide during experimental incubations and cell stimulation did not exceed 0.1%, and control cells received the same volume addition of solvent. Experiments involving administration of the Gq-protein antagonist 2A were performed in cells that were transiently permeabilized as described previously (24, 27). Briefly, HUVEC were gradually cooled and incubated on ice for 10 min in permeabilization buffer (20 mM HEPES [pH 7.4], 10 mM EGTA, 140 mM KCl, 5 mM oxalic acid dipotassium salt) containing the peptide antagonist and 0.005% saponin. Subsequently, cells were rinsed several times and gradually warmed. The original medium was replaced, and cells were allowed to recover for 30 min before stimulation.
siRNA-mediated knockdown of AMPK, CaMKKβ, and LKB1.
Small interfering RNA (siRNA) duplex oligonucleotides used in this study are based on the human cDNAs encoding AMPKα1, CaMKKβ, and LKB1. AMPKα1-specific siRNA duplexes were purchased from Sigma-Proligo (Hamburg, Germany). CaMKKβ and LKB1 siRNAs as well as a nonsilencing control siRNA were obtained from QIAGEN GmbH (Hilden, Germany). The siRNA sequences applied to target AMPKα1 were 5′-UGCCUACCAUCUCAUAAUAdTdT-3′ (sense) and 5′-UAUUAUGAGAUGGUAGGCAdTdT-3′ (antisense). For CaMKKβ, 5′-CGAUCGUCAUCUCUGGUUAdTdT-3′ (sense) and 5′-UAACCAGAGAUGACGAUCG-3′ (antisense) and, for LKB1, 5′-GGCUCUUACGGCAAGGUGAdTdT-3′ (sense) and 5′-UCACCUUGCCGUAAGAGCCdTdT-3′ (antisense) were used. The siRNA sequences employed as negative controls were 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAAdTdT-3′ (antisense). HUVEC (1.8 × 105) were plated on 30-mm-diameter dishes 24 h prior to transfection and were 50% confluent when siRNA was added. The amount of siRNA duplexes applied was 1 μg/dish for CaMKKβ or LKB1 and 2 μg/dish for AMPKα1. Transfection was performed using the amphiphilic delivery system SAINT-RED (Synvolux Therapeutics B.V., Groningen, The Netherlands) according to the manufacturer's instructions. Briefly, siRNA was complexed with 15 nmol of transfection reagent, diluted with M199-HSA to 1 ml, and added to the cells for 4 h. Subsequently, 2 ml of culture medium was added and incubation proceeded for 72 h.
Western blot analysis.
HUVEC were lysed in ice-cold Tris buffer (50 mM Tris [pH 7.4], 2 mM EDTA, 1 mM EGTA) containing 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 50 mM NaF, 10 mM Na4P2O7, 1 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 μl/ml of the protease inhibitor cocktail stock solution. For the detection of CaMKKβ cell lysates were subjected to immunoprecipitation with a polyclonal anti-CaMKKβ antibody (4 μg/mg protein, overnight) and protein G-Sepharose (1 h). Lysate proteins and immune complexes were solubilized in Laemmli buffer and separated by SDS-polyacrylamide gel electrophoresis (50 μg/lane, 7.5% gels). Blots were subjected to immunostaining with primary antibodies overnight and peroxidase-conjugated secondary antibodies for 1 hour, and proteins were visualized using the ECL technique. ACC staining was achieved by using a biotinylated secondary antibody and peroxidase-labeled streptavidin. Protein bands were evaluated by densitometry, and phosphospecific signals were normalized against the amount of total protein. Changes in phosphorylation were calculated by comparing the differences between basal and stimulated values of specifically treated samples and their respective controls.
Sample preparation for AMPK, CaMKKβ, and LKB1 assays.
Following experimental treatments HUVEC were rinsed with HEPES buffer (50 mM HEPES [pH 7.4] including 50 mM NaF, 1 mM EDTA, 1 mM dithiothreitol, 5 mM Na4P2O7) and lysed in HEPES buffer containing 0.1 mM phenylmethylsulfonyl fluoride, 0.157 mg/ml benzamidine, 4 mg/ml trypsin inhibitor, 1% Triton X-100, and 10% glycerol.
AMPK immunoprecipitation and assay.
AMPK was immunoprecipitated from 50 to 100 μg cell lysate protein using a rabbit anti-pan-β antibody (44) and protein A-Sepharose, or sheep anti-α1 antibodies (47) and protein G-Sepharose. AMPK activity was determined by phosphorylation of the synthetic substrate HMRSAMSGLHLVKRR (SAMS) peptide (11) in the presence of 5 mM MgCl2, 0.2 mM [γ-32P]ATP (specific radioactivity of approximately 200 cpm/pmol), and 0.2 mM AMP.
CaMKKβ immunoprecipitation and assay.
CaMKKβ was immunoprecipitated from 100 μg of cell lysate protein using 2 μl rabbit sera raised to bacterially expressed CaMKKβ bound to protein A-Sepharose. Activity was measured by activation of purified recombinant AMPK complexes in the presence of 2 mM CaCl2, 2 μM bovine brain calmodulin, and 0.2 mM AMP (45).
LKB1 immunoprecipitation and assay.
LKB1 was immunoprecipitated from 100 μg of cell lysate protein using 2 μl rabbit sera raised to bacterially expressed LKB1 bound to protein A-Sepharose. LKB1 activity was measured in the same way as CaMKK activity by activation of AMPK but in the absence of CaCl2 and calmodulin.
Adenine nucleotide measurement.
HUVEC monolayers were stimulated with thrombin (1 U/ml), and the reaction was stopped by rinsing the cells with phosphate-buffered saline and adding perchloric acid to a final concentration of 5% (wt/vol). Acid-insoluble material was removed by centrifugation, and perchloric acid was extracted from the supernatant by three washes with 10% excess (by volume) of a 1:1 mixture of tri-n-octylamine and 1,1,2-trichlorotrifluoroethane. Nucleotides were separated by ion-exchange chromatography on a Mono Q PC1.6/5 column run on a SMART System (Amersham Biosciences) as described elsewhere (14). Nucleotides were detected by their absorbance at 254 nm and compared with the elution position of standards. Areas under the AMP and ATP peaks were quantified by integration using SMART System software and used to calculate the AMP/ATP ratios.
Determination of cGMP.
HUVEC monolayers were incubated for 30 min in M199-HSA containing 0.5 mM isobutylmethylxanthine and stimulated with thrombin for 15 min. The reaction was stopped with 96% ethanol, and after evaporation 50 mM Tris-4 mM EDTA (pH 7.5) was added. The cGMP content of the cellular extracts was measured by radioimmunoassay as described previously (22). Cells from parallel dishes were lysed with 100 mM NaOH, 2% Na2CO3, and 1% SDS, and protein quantities were determined according to the method of Lowry. The intracellular cGMP concentration was expressed in pmol/mg cell protein.
Statistical analysis.
All data are given as means ± standard errors of the means (SEMs) of three to four independent experiments. To determine the statistical significance of the described results, analysis of variance with Bonferroni's correction for multiple comparisons or t tests were performed. A P value of <0.05 was accepted as statistically significant.
RESULTS
Thrombin activates AMPK in endothelial cells.
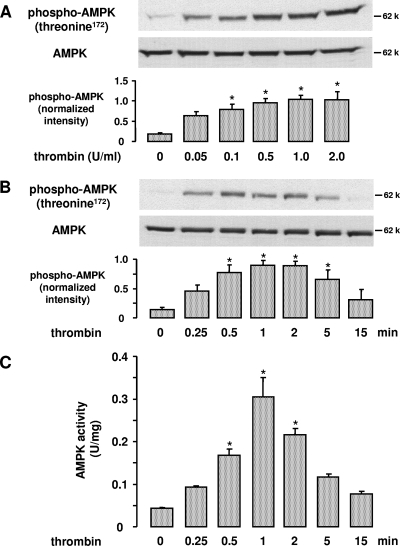
Treatment of HUVEC with thrombin (0.05 to 2 U/ml, 0.25 to 15 min) resulted in a dose- and time-dependent phosphorylation of AMPK at threonine residue 172 (Fig. 1A and B). This phosphorylation is essential for AMPK activity and reflects AMPK activation. Similar results were obtained when AMPK was immunoprecipitated and its activity measured by 32P incorporation into the AMPK-specific SAMS peptide (Fig. 1C). AMPK activation was transient, detectable at 15 s, peaking at 1 min, and returning to control levels at 15 min.
FIG. 1.
Effect of thrombin on AMPK activation in endothelial cells. HUVEC were stimulated with different thrombin concentrations for 1 min (A) or with 1 U/ml thrombin for the indicated times (B and C). A and B. Cell lysates were subjected to Western blot analysis using an antibody against AMPKα phosphorylated at threonine 172 or an anti-AMPKα antibody for counterstaining. Representative blots and densitometric analyses are shown (mean ± SEM, n = 3). C. AMPK was immunoprecipitated from 100 μg total protein using an anti-AMPKβ antibody, and the activity of the immune complexes was measured using the SAMS peptide assay. AMPK activity is shown as U/mg lysate protein (mean ± SEM, n = 3), where 1 unit equals 1 nmol 32PO4 incorporated into SAMS peptide per min.  , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.
Thrombin activation of AMPK is independent of the cellular AMP/ATP ratio.
Because the cellular AMP/ATP ratio is one of the major determinants of AMPK activity, the effect of thrombin on this ratio was measured at time points where AMPK was maximally stimulated by thrombin. Stimulation of HUVEC by thrombin for 0 (control), 0.5, 1, and 2 min resulted in AMP/ATP ratios of 0.037 ± 0.003, 0.034 ± 0.002, 0.037 ± 0.003, and 0.041 ± 0.002, respectively (data are means ± SEMs of three experiments). As can be seen, there was no significant change in the AMP/ATP ratio upon thrombin stimulation, indicating that AMPK activation occurred independently of changes in the levels of adenine nucleotides. In contrast, dinitrophenol (500 μM, 15 min), an uncoupler of the mitochondrial electron transport known to activate AMPK, clearly increased the AMP/ATP ratio from 0.033 ± 0.001 to 0.119 ± 0.006 (n = 2).
Thrombin activates AMPK via PAR-1 and Gq-protein-mediated phospholipase C activation.
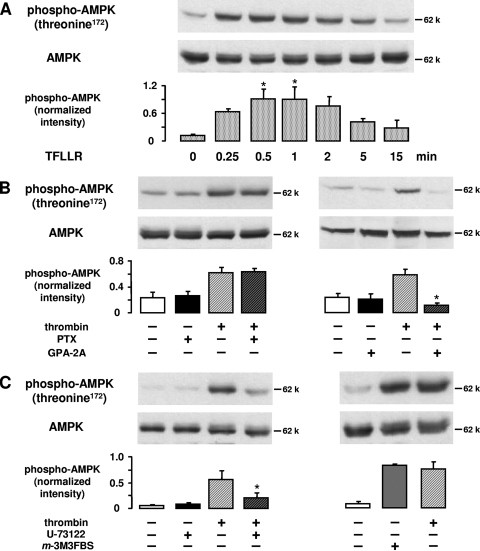
Thrombin exerts its effects on endothelial cells through G-protein-coupled PARs via a tethered ligand mechanism. This includes proteolytic cleavage at the amino terminus of the receptor and an intramolecular binding of the newly exposed tethered ligand to the activation site of the receptor (9). We used TFLLR, a peptide analogue of the new N terminus of PAR-1, to examine whether PAR-1, the predominant thrombin receptor expressed in HUVEC (34), was involved in AMPK activation. TFLLR (10 μM, 0.25 to 15 min) mimicked the effect of thrombin and induced a time-dependent increase of AMPK phosphorylation at threonine 172 (Fig. 2A).
FIG. 2.
Thrombin-induced AMPK activation is mediated via PAR-1, Gq coupling, and phospholipase C activation. A. HUVEC were stimulated with the PAR-1 agonist peptide TFLLR (10 μM) for the indicated times. B. HUVEC were pretreated with pertussis toxin (PTX) (500 ng/ml, 3 h) or with Gq-protein antagonist 2A (GPA-2A) (5 μM, 10-min preincubation in transiently permeabilized cells) and subsequently stimulated with thrombin (1 U/ml, 1 min). C. HUVEC were pretreated with U-73122 (10 μM, 30 min) and stimulated with thrombin (1 U/ml, 1 min) or alternatively treated with the phospholipase C activator m-3M3FBS (100 μM, 2 min). For panels A to C, cells were lysed and subjected to immunoblotting using antibodies against phosphorylated AMPKα (threonine 172) and total AMPKα for counterstaining. Representative figures and densitometry data (means ± SEMs) of three independent experiments for each treatment are shown. Phosphospecific signals from TFLLR-stimulated and nonstimulated cells (A) or from cells stimulated with thrombin in the absence or presence of inhibitors (B and C) were compared.  , P < 0.05.
, P < 0.05.
PAR-1 is known to exert its effects on endothelium by concomitant activation of the Gαi/o, Gαq, and Gα12/13 families of G proteins (9). To determine which G proteins mediated AMPK activation, thrombin-induced AMPK phosphorylation was measured in cells pretreated with G-protein inhibitors. Figure 2B shows that pertussis toxin (500 ng/ml, 3 h), which inhibits Gαi/o subunits by ADP ribosylation, had no effect on AMPK activation. In contrast, thrombin-stimulated AMPK phosphorylation was abolished when cells were pretreated with the Gq-protein antagonist 2A (5 μM, 10 min). To verify that Gq activation of phospholipase C was requisite for thrombin-induced AMPK activation, we performed experiments with U-73122, an inhibitor of phospholipase C. Pretreatment of cells with U-73122 (10 μM, 30 min) prevented thrombin-induced AMPK phosphorylation significantly (78% inhibition), and conversely, stimulation of phospholipase C by m-3M3FBS (100 μM, 2 min) mimicked the effect of thrombin on AMPK (Fig. 2C).
Thrombin-induced Ca2+ mobilization triggers AMPK activation.
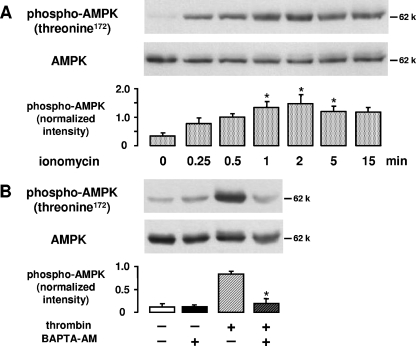
When HUVEC were stimulated with the Ca2+ ionophore ionomycin (2 μM, 0.25 to 15 min) AMPK phosphorylation was similar to that following thrombin stimulation (Fig. 3A), suggesting that Ca2+ may be a signal for AMPK activation in endothelial cells. Furthermore, thrombin is known to induce an increase of intracellular Ca2+ via Gαq-coupled stimulation of phospholipase C-β (43). Consequently, we investigated whether a Ca2+ signal may be involved in thrombin-induced AMPK activation. Our data show that chelation of intracellular Ca2+ by BAPTA (20 μM, 30 min) led to a complete inhibition of thrombin-stimulated AMPK phosphorylation (Fig. 3B).
FIG. 3.
Role of Ca2+ in thrombin-induced AMPK activation. A. HUVEC were stimulated with ionomycin (2 μM) for the indicated times. B. HUVEC were pretreated with BAPTA-AM (20 μM, 30 min) and subsequently stimulated with thrombin (1 U/ml, 1 min). For panels A and B, the degree of threonine 172 phosphorylation of AMPK was determined by Western blot analysis with an anti-phosphospecific threonine 172 antibody. AMPK was stained with an antibody against AMPKα. Representative figures and densitometry data (means ± SEMs) of three independent experiments for each treatment are shown. Phosphospecific signals from ionomycin-stimulated and nonstimulated cells (A) or from cells stimulated with thrombin in the absence or presence of BAPTA-AM (B) were compared.  , P < 0.05.
, P < 0.05.
CaMKKβ mediates AMPK activation upon thrombin stimulation.
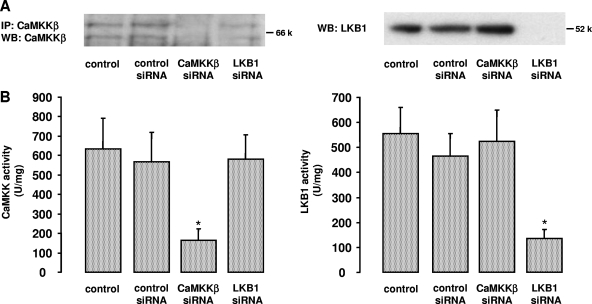
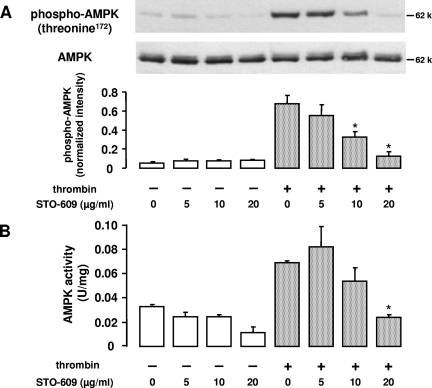
Both LKB1 and CaMKKβ are expressed in HUVEC, as shown in Fig. 4. Since thrombin-stimulated AMPK activation was Ca2+ sensitive, we reasoned that CaMKKβ was likely to be the AMPK kinase involved. As a first attempt to address this question, we performed experiments with STO-609, a relatively selective inhibitor of CaMKKα and CaMKKβ (20, 41). STO-609 (5, 10, and 20 μg/ml; 30 min) caused a dose-dependent reduction of thrombin-induced AMPK phosphorylation at threonine 172 (Fig. 5A). Correspondingly, AMPK activity as monitored by the SAMS peptide assay was inhibited by up to 66% (Fig. 5B).
FIG. 4.
Downregulation of CaMKKβ and LKB1 by RNA interference. HUVEC were incubated in the presence of synthetic RNA duplexes targeted to human CaMKKβ or human LKB1 (1 μg/30-mm dish, 72 h). Incubations with transfection reagent only (control) or with a control RNA duplex containing an unrelated sequence (control siRNA) were run in parallel. A. Protein expression was examined in anti-CaMKKβ immune complexes (left panel) or cell lysates (right panel) probed with either anti-CaMKKβ antibody or anti-LKB1 antibody, respectively. Representative Western blots out of three independent experiments for each treatment are shown. B. CaMKKβ or LKB1 activities were determined in anti-CaMKKβ or anti-LKB1 immune complexes isolated from 100 μg cell lysate. Results are plotted as AMPK activity stimulated by the immune complex and measured using the SAMS peptide (U/mg recombinant AMPK, where 1 unit equals 1 nmol 32PO4 incorporated into SAMS peptide per min). Means ± SEMs for three independent experiments are shown.  , P < 0.05 versus control siRNA.
, P < 0.05 versus control siRNA.
FIG. 5.
Effect of CaMKKβ inhibition on thrombin-induced AMPK activation. HUVEC were preincubated for 30 min with STO-609 at the indicated concentrations. A. Cells were lysed and subjected to immunoblotting using antibodies against phosphorylated AMPKα (threonine 172) and total AMPKα for counterstaining. A typical experiment and the densitometric analysis of four experiments are shown (means ± SEMs). B. AMPK activity was examined in anti-AMPKα1 immune complexes isolated from 100 μg total protein by phosphorylation of the SAMS peptide. AMPK activity is shown as U/mg lysate protein (mean ± SEM, n = 3), where 1 unit equals 1 nmol 32PO4 incorporated into SAMS peptide per min.  , P < 0.05 versus untreated thrombin-stimulated controls.
, P < 0.05 versus untreated thrombin-stimulated controls.
To further assess whether CaMKKβ or LKB1 acts as an AMPK kinase in thrombin-stimulated cells, we used an siRNA approach to knock down the expression of LKB1 and CaMKKβ. Downregulation of proteins was verified by Western blot analysis of cell lysates (LKB1) or immunoprecipitates (CaMKKβ) and by enzyme activity measurements. Figure 4 demonstrates a selective downregulation of CaMKKβ or LKB1 by the respective specific siRNA with both methods. Compared to transfections with control siRNA, CaMKKβ activity was decreased by 72% ± 8.1% (n = 3) and LKB1 activity by 71% ± 3.21% (n = 3), and protein expression in immunoblots was no more detectable.
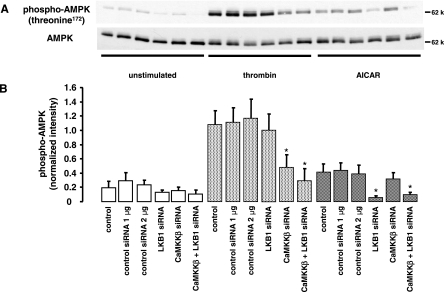
In parallel experiments, we investigated the effect of LKB1 or CaMKKβ downregulation on AMPK activation induced by thrombin (1 U/ml, 1 min). As shown in Fig. 6, AMPK phosphorylation with thrombin was not significantly changed when cells were pretreated with LKB1 siRNA. Downregulation of CaMKKβ, however, caused a significant reduction of thrombin-induced AMPK phosphorylation (66% ± 15.5%, n = 4), suggesting that CaMKKβ was the major AMPK kinase under these conditions. In contrast, AMPK phosphorylation induced by AICAR (2 mM, 2 h), an activator of AMPK mimicking the action of AMP, was completely inhibited in cells with reduced LKB1 expression but not affected by downregulation of CaMKKβ. Basal phosphorylation of AMPK was very low and appeared to be reduced by depletion of either LKB1 or CaMKKβ. Interestingly, when cells were treated with a combination of siRNA duplexes to target both CaMKKβ and LKB1, the effects of the individual siRNAs on thrombin- or AICAR-induced AMPK activation, respectively, were not amplified.
FIG. 6.
Effect of CaMKKβ and LKB1 downregulation on thrombin-induced AMPK activation. Synthetic RNA duplexes (1 μg/30-mm dish) targeted to human CaMKKβ or to human LKB1 were added to HUVEC alone or in combination, and incubations were carried out for 72 h. Treatments with transfection reagent only (control) or with a control RNA duplex containing an unrelated sequence (control siRNA, 1 or 2 μg) were run in parallel. Following siRNA treatment, cells were stimulated with thrombin (1 U/ml, 1 min) or AICAR (2 mM, 2 h) and AMPK activation was monitored by phosphorylation of threonine 172 with a phosphospecific antibody. AMPK was stained with an antibody against AMPKα. A typical blot (A) and the densitometric analysis results (means ± SEMs) of four experiments (B) are presented.  , P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.
, P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.
Taken together, the data from two independent approaches, i.e., pharmacological enzyme inhibition and protein downregulation, reveal the role of CaMKK, particularly CaMKKβ, as a thrombin-activated AMPK kinase whereas LKB1 does not seem to contribute to thrombin-induced AMPK activation.
Thrombin-stimulated AMPK phosphorylates ACC but not eNOS.
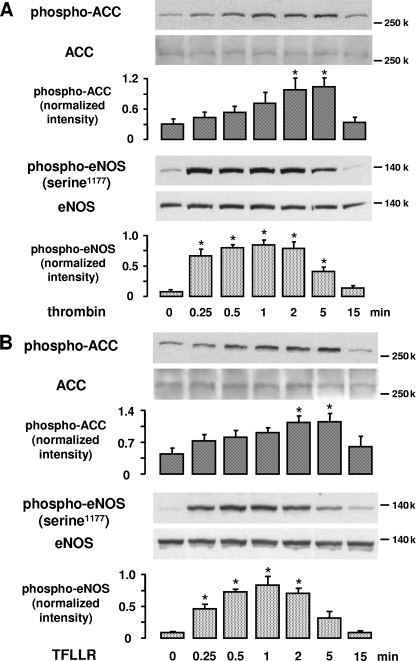
To understand whether AMPK activation by thrombin is of functional relevance, we investigated the phosphorylation of ACC, a well-characterized substrate of AMPK (16), and of eNOS, which is thought to be a potential downstream target of AMPK in endothelial cells (7, 31, 33, 35, 40, 49, 50). Figure 7A demonstrates that thrombin (1 U/ml, 0.25 to 15 min) stimulates both ACC and eNOS phosphorylation in a time-dependent manner with a kinetics comparable to those of AMPK activation. Similar results were obtained when HUVEC were stimulated with TFLLR (10 μM, 0.25 to 15 min) (Fig. 7B). To determine the role of AMPK in thrombin-induced ACC or eNOS phosphorylation, we used several approaches. Firstly, we prevented AMPK activation by targeting its upstream kinase CaMKKβ as described in the paragraph above. Secondly, we directly inhibited AMPK activity using compound C, a pharmacological inhibitor of AMPK (48), and finally, we downregulated AMPKα1, which has been reported to be the predominant AMPK isoform in endothelial cells (reference 31 and our data [not shown]), by a specific siRNA. The latter treatment caused a 66% ± 7.0% reduction of AMPK expression as demonstrated by Western blotting (n = 3, P < 0.05) (Fig. 8C).
FIG. 7.
Effect of thrombin and TFLLR on ACC and eNOS phosphorylation. HUVEC were stimulated with 1 U/ml thrombin (A) or 10 μM TFLLR (B) for the indicated times. ACC and eNOS phosphorylation was determined using specific antibodies against phosphorylated ACC or eNOS phosphorylated at serine 1177. Anti-ACC and anti-eNOS antibodies were used for the respective counterstainings. Representative experiments and densitometry data (means ± SEMs) of three independent experiments for each treatment are shown.  , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.
FIG. 8.
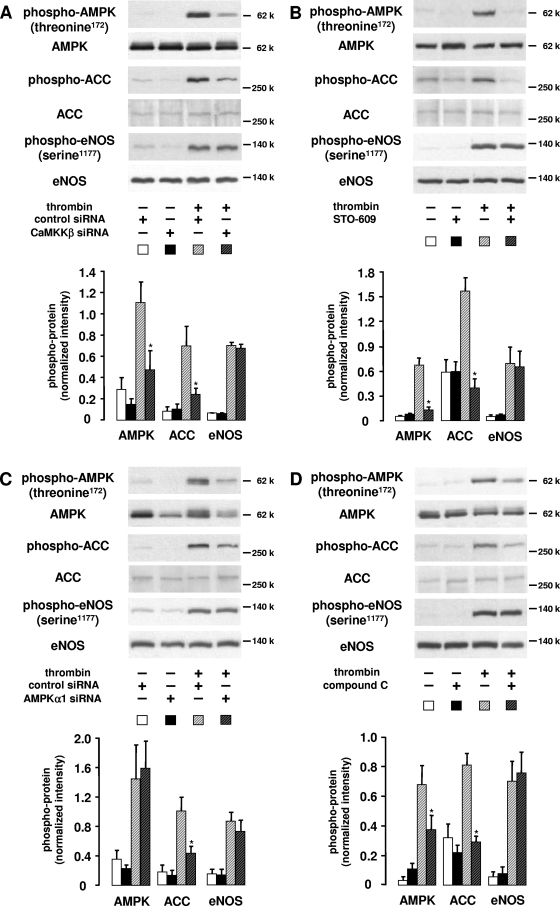
Effect of AMPK inhibition or downregulation on thrombin-induced ACC and eNOS phosphorylation. HUVEC were incubated for 72 h with synthetic siRNA duplexes targeted to CaΜΚΚβ or AMPKα1 or pretreated with inhibitors against either enzyme (STO-609 for CaΜΚΚβ and compound C for AMPK). Subsequently, cells were stimulated with thrombin (1 U/ml, 1 min) and processed for immunoblotting. AMPK, ACC, or eNOS phosphorylation was determined using specific antibodies against phosphorylated AMPK (threonine 172), ACC, or eNOS (serine 1177). Expression of proteins was monitored with anti-AMPKα, anti-ACC, or anti-eNOS antibodies. Representative figures and densitometry data (means ± SEMs) of three (C) or four (A, B, and D) independent experiments for each treatment are shown. A. Cells were incubated for 72 h in the presence of a synthetic siRNA targeted to human CaMKKβ or a nonsilencing control siRNA (1 μg/30-mm dish). Β. HUVEC were pretreated with STO-609 (20 μg/ml, 30 min). C. HUVEC were transfected with an siRNA targeted to human AMPKα1 or a nontargeting control siRNA (2 μg/30-mm dish). D. Cells were pretreated with compound C (20 μM, 30 min).  , P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).
, P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).
CaMKKβ depletion by siRNA as well as inhibition of CaMKK by STO-609 (20 μg/ml, 30 min) led to a significant inhibition of ACC phosphorylation by 76% and 98%, respectively (Fig. 8A and B). Furthermore, downregulation of AMPK expression or direct inhibition of AMPK by compound C (20 μM, 30 min) caused a significant decrease of thrombin-induced ACC phosphorylation by 69 or 86%, respectively (Fig. 8C and D). These data confirm that ACC is phosphorylated by AMPK in thrombin-stimulated endothelial cells. In contrast to ACC, however, eNOS could not be identified as an AMPK downstream target under these conditions. None of the applied treatments intended to inhibit AMPK activation or to reduce AMPK expression was able to affect thrombin-induced eNOS phosphorylation at serine 1177 (Fig. 8A to D).
To examine whether AMPK influenced eNOS activation independently of phosphorylation of eNOS, we evaluated NO formation in thrombin-stimulated HUVEC by measuring cGMP. An increase of cellular cGMP upon cell stimulation with thrombin reflects an activation of the soluble guanylate cyclase by intracellularly generated NO and is completely prevented by inhibiting eNOS with l-nitroarginine methylester (1 mM, 30 min; data not shown). Our results show that neither compound C nor downregulation of AMPK significantly affected thrombin-stimulated cGMP formation (Table 1), suggesting that thrombin-induced AMPK activation and eNOS activation are not linked.
TABLE 1.
Effect of AMPK inhibition or downregulation on thrombin-induced cGMP generationa
| Expt and treatment | cGMP (pmol/mg protein) |
|---|---|
| A | |
| Control | 1.03 ± 0.69 |
| Compound C | 1.03 ± 0.39 |
| Control + thrombin | 12.99 ± 4.51 |
| Compound C + thrombin | 13.62 ± 4.20 |
| B | |
| Control siRNA | 0.85 ± 1.44 |
| AMPKα1 siRNA | 0.60 ± 0.25 |
| Control siRNA + thrombin | 10.06 ± 5.76 |
| AMPKα1 siRNA + thrombin | 10.95 ± 2.74 |
HUVEC were pretreated with compound C (20 μM, 30 min) to inhibit AMPK activity (experiment A) or transfected with specific targeted AMPKα1 siRNA (2 μg/30-mm dish, 72 h) to downregulate AMPK (experiment B). Stimulation of HUVEC with thrombin (1 U/ml, 15 min) was performed in the presence of isobutylmethylxanthine, and the cGMP concentration was determined by means of radioimmunoassay. Data are given as means ± standard deviations, n = 4 (experiment A) or n = 2 (experiment B).
DISCUSSION
Our results show that thrombin is able to activate AMPK in endothelial cells via a Ca2+-dependent pathway and identify CaMKKβ as the responsible upstream kinase. Ca2+ mobilization appears to be mediated via binding of thrombin to its receptor PAR-1, activation of Gq protein, and subsequent stimulation of phospholipase C. The data presented here support a role of Ca2+ as a signal for AMPK activation and suggest a physiological function of the CaMKKβ/AMPK pathway in endothelial cells.
Our study is the first that describes the expression of CaMKKβ and its functional significance in AMPK activation in human primary endothelial cells. Previous studies reported that AMPK was activated via thrombin, histamine, or Gq-protein-coupled pathways (29, 40), although the upstream signaling pathway was not identified. Here we have characterized the upstream signaling events in AMPK activation by thrombin. We confirmed that thrombin activates AMPK in endothelial cells by measuring AMPK phosphorylation at its critical residue threonine 172 and by determining AMPK activity via phosphorylation of a specific substrate. We found that AMPK activation by thrombin was almost completely abolished by a Gq-protein antagonist, an inhibitor of phospholipase C, or a Ca2+-chelating agent, suggesting that the Ca2+ increase mediated via Gq-activated phospholipase C was an absolute requirement. Accordingly, two separate lines of evidence, pharmacological inhibition of CaMKK by STO-609 and downregulation of CaMKKβ with specific targeted siRNA, verified that CaMKKβ, a Ca2+-dependent AMPK kinase, was mediating AMPK activation with thrombin. In contrast, we did not find any evidence for a contribution by LKB1. We were able to downregulate LKB1 activity by about 71% but did not observe a significant effect of LKB1 siRNA alone or in combination with CaMKKβ siRNA on AMPK activation with thrombin. Neither STO-609 nor CaMKKβ siRNA was, however, able to block thrombin-induced AMPK activation completely. This may reflect incomplete inhibition or downregulation of CaMKKβ, since endothelial cells treated with specific siRNA contained about 28% residual CaMKKβ activity. However, the contribution of another, as yet unidentified, AMPK kinase cannot be excluded.
Interestingly, our results reveal that a functional LKB1/AMPK pathway also exists in endothelial cells. Although LKB1 did not contribute to thrombin-stimulated AMPK activation, it was able to mediate AICAR-induced AMPK activation. AICAR is phosphorylated to 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP) within the cell, which mimics the effect of AMP on AMPK. AICAR has previously been shown to act through LKB1 (19, 25, 38). Our data are in agreement with these observations. Downregulation of LKB1 by a specific targeted siRNA was able to prevent AICAR-induced AMPK phosphorylation whereas CaMKKβ siRNA, added either alone or in combination with LKB1 siRNA, had no effect on AICAR-induced responses. Taken together, these findings demonstrate that AMPK may be activated in endothelial cells via two separate mechanisms, i.e., a Ca2+/CaMKKβ-dependent and an AMP/LKB1-dependent pathway. These pathways may be stimulated individually, as shown here for thrombin and AICAR, or in parallel when both Ca2+ and ATP are altered under certain circumstances such as hypoxia (1). Interestingly, activation of AMPK following intracellular Ca2+ increase by thrombin appeared to be very rapid and transient and may be important to fulfill requirements during cell stimulation whereas the response to AICAR and to ATP depletion induced by dinitrophenol was delayed and long-lasting (data not shown). Thus, Ca2+- and AMP-dependent pathways may act together to ensure immediate and long-term responses when necessary.
To explore the functional relevance of thrombin-stimulated AMPK activation, we performed experiments to investigate downstream targets of AMPK. One of the substrates suggested to be phosphorylated by AMPK in endothelial cells is eNOS (7, 31, 33, 35, 40, 49, 50). It has recently been demonstrated that eNOS is regulated by reversible phosphorylation and that phosphorylation of serine 1177 promotes eNOS activation by lowering its sensitivity to Ca2+ (8, 12). This process seems to be active in various signaling pathways leading to NO formation, and different kinases besides AMPK (Akt, protein kinases A and G, and Ca2+/calmodulin-dependent kinase II) may be involved (12). AMPK has been shown to mediate serine 1177 phosphorylation in response to hypoxia, peroxynitrite, and adiponectin (7, 33, 35, 49, 50). Additionally, activation of AMPK by AICAR has been reported to induce eNOS phosphorylation and endothelial NO generation (31). Our results show that neither inhibition nor depletion of CaMKKβ, which largely prevented thrombin-induced AMPK activation, nor inhibition or downregulation of AMPK itself had any effect on thrombin-stimulated eNOS phosphorylation. Thus, upon thrombin stimulation of endothelial cells eNOS does not appear to be a downstream target of AMPK.
Our findings are in contrast to a previous study which suggested that AMPK was involved in thrombin-mediated eNOS phosphorylation (40). In that study, it was shown that H89, a relatively nonspecific protein kinase inhibitor, which inhibits AMPK, was able to abolish thrombin-induced eNOS phosphorylation. In contrast to this indirect approach, our study shows that thrombin-induced eNOS phosphorylation is not affected when AMPK activation by its upstream kinase is prevented or AMPK protein is downregulated. We suggest that thrombin may stimulate eNOS phosphorylation via activation of another kinase known to target eNOS serine 1177. We found that eNOS phosphorylation induced by thrombin was essentially dependent on Ca2+ since it was completely prevented by inclusion of BAPTA (data not shown). Thus, Ca2+/calmodulin-dependent kinase II is likely to be responsible for thrombin-mediated eNOS phosphorylation. Thrombin has already been shown to activate this kinase (4), and bradykinin, another Gq-protein-coupled stimulus, has been shown to phosphorylate eNOS at serine 1177 via Ca2+/calmodulin-dependent kinase II (13).
We cannot account for the difference between our study and other studies that indicated a role of AMPK in eNOS phosphorylation with hypoxia, peroxynitrite, adiponectin, or AICAR (31, 33, 35, 49, 50). It may be, however, that upon an intracellular Ca2+ increase induced by thrombin the rapid and direct phosphorylation of eNOS by a Ca2+-dependent kinase predominates in the AMPK pathway. Accordingly, a dominant-negative AMPK mutant had no effect on NO formation stimulated by ionomycin whereas AICAR-induced NO generation was inhibited (31). Furthermore, eNOS phosphorylation via AMPK may require phosphoinositide 3-kinase-sensitive pathways as had been suggested for peroxynitrite or adiponectin (7, 49) or may additionally involve changes in the AMP/ATP ratio as could be expected in hypoxic cells. Nagata et al. (33) have found, for example, that serine 1177 phosphorylation of eNOS by AMPK occurred only in hypoxic but not in normoxic conditions. On the other hand, recent studies have reported that AMPK activation and eNOS phosphorylation may also dissociate (32, 37) or that AMPK may increase NO formation independently of serine 1177 phosphorylation by enhancing the interaction between eNOS and heat shock protein 90 (37). Under our conditions, however, AMPK inhibition or downregulation had no effect on thrombin-induced cGMP formation, suggesting that it also did not affect eNOS activation by phosphorylation-independent mechanisms.
Although eNOS could not be verified as a downstream target of AMPK, our findings show that the CaMKKβ/AMPK pathway was functionally relevant in endothelial cells. ACC, a well-known substrate of AMPK, was phosphorylated upon thrombin stimulation, and this phosphorylation was inhibited when either CaMKKβ or AMPK was pharmacologically inhibited or downregulated. There are two isoforms of ACC (ACC1 and ACC2), and both are inhibited by phosphorylation by AMPK. The phosphospecific ACC antibody used in our study recognizes the phosphorylated form of both isoforms. ACC1 is a key enzyme in the regulation of fatty acid synthesis (17) whereas ACC2 is a key enzyme in the regulation of fatty acid oxidation (16). Phosphorylation of ACC1 would decrease fatty acid synthesis and lower ATP consumption while phosphorylation of ACC2 would increase fatty acid oxidation, increasing ATP supply (16). AMPK activation has already been shown to be involved in endothelial cell energy metabolism as shown by elevation in fatty acid oxidation and ATP increase following AICAR stimulation (10). Accordingly, incubation with AICAR has been reported to protect endothelial cells from a loss of intracellular ATP and a decrease of fatty acid oxidation induced by hyperglycemia (26). Thrombin is known to initiate processes in endothelial cells that require ATP such as shape change and expression of adhesion molecules as well as synthesis of vascular mediators and cytokines. Thus, our results suggest that thrombin-induced stimulation of AMPK and subsequent ACC phosphorylation may be important to balance energy metabolism after cell stimulation and to provide ATP for energy-requiring processes.
In summary, the present study demonstrates that both LKB1 and CaMKKβ exist in endothelial cells to mediate AMPK activation under different conditions. One possibility is that the LKB1-dependent pathway is activated following an increase in the AMP/ATP ratio, whereas the CaMKKβ-dependent pathway is activated following an increase in intracellular Ca2+. Thrombin, a potent vascular cell stimulus generated during blood coagulation, activates AMPK via a Ca2+-dependent pathway involving CaMKKβ. AMPK activation induced by thrombin leads to phosphorylation of ACC whereas thrombin-stimulated phosphorylation of eNOS is not mediated by AMPK. Our study suggests that according to the applied stimulus AMP- and Ca2+-dependent pathways of endothelial AMPK activation may act separately or in concert to maintain the energy balance of the cell.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (Graduiertenkolleg 768) and Interdisziplinäres Zentrum für Klinische Forschung, Klinikum der Friedrich-Schiller-Universität Jena, to R.H. as well as by an Integrated Project (LSHM-CT-2004-005272) from the European Commission and the Medical Research Council (UK) to D.C.
We thank Gunda Guhr and Elke Teuscher for their excellent technical assistance.
REFERENCES
- 1.Aley, P. K., K. E. Porter, J. P. Boyle, P. J. Kemp, and C. Peers. 2005. Hypoxic modulation of Ca2+ signaling in human venous endothelial cells. Multiple roles for reactive oxygen species. J. Biol. Chem. 280:13349-13354. [DOI] [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Bogatcheva, N. V., J. G. Garcia, and A. D. Verin. 2002. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Moscow) 67:75-84. [DOI] [PubMed] [Google Scholar]
- 4.Borbiev, T., A. D. Verin, S. Shi, F. Liu, and J. G. Garcia. 2001. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L983-L990. [DOI] [PubMed] [Google Scholar]
- 5.Cacicedo, J. M., N. Yagihashi, J. F. Keaney, Jr., N. B. Ruderman, and Y. Ido. 2004. AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 324:1204-1209. [DOI] [PubMed] [Google Scholar]
- 6.Carling, D. 2004. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem. Sci. 29:18-24. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., M. Montagnani, T. Funahashi, I. Shimomura, and M. J. Quon. 2003. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 278:45021-45026. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z.-P., K. I. Mitchelhill, B. J. Michell, D. Stapleton, I. Rodriguez-Crespo, L. A. Witters, D. A. Power, P. R. Ortiz de Montellano, and B. E. Kemp. 1999. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 443:285-289. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin, S. R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3:1800-1814. [DOI] [PubMed] [Google Scholar]
- 10.Dagher, Z., N. Ruderman, K. Tornheim, and Y. Ido. 1999. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 265:112-115. [DOI] [PubMed] [Google Scholar]
- 11.Davies, S. P., D. Carling, and D. G. Hardie. 1989. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur. J. Biochem. 186:123-128. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, I., and R. Busse. 2003. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284:R1-R12. [DOI] [PubMed] [Google Scholar]
- 13.Fleming, I., B. Fisslthaler, S. Dimmeler, B. E. Kemp, and R. Busse. 2001. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 88:E68-E75. [DOI] [PubMed] [Google Scholar]
- 14.Fryer, L. G., A. Parbu-Patel, and D. Carling. 2002. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277:25226-25232. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton, J. R., J. D. Moffatt, A. G. Frauman, and T. M. Cocks. 2001. Protease-activated receptor (PAR) 1 but not PAR2 or PAR4 mediates endothelium-dependent relaxation to thrombin and trypsin in human pulmonary arteries. J. Cardiovasc. Pharmacol. 38:108-119. [DOI] [PubMed] [Google Scholar]
- 16.Hardie, D. G. 2004. AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev. Endocr. Metab. Disord. 5:119-125. [DOI] [PubMed] [Google Scholar]
- 17.Hardie, D. G., and D. A. Pan. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30:1064-1070. [DOI] [PubMed] [Google Scholar]
- 18.Hardie, D. G., I. P. Salt, S. A. Hawley, and S. P. Davies. 1999. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem. J. 338:717-722. [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley, S. A., J. Boudeau, J. L. Reid, K. J. Mustard, L. Udd, T. P. Mäkelä, D. R. Alessi, and D. G. Hardie. 2003. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley, S. A., D. A. Pan, K. J. Mustard, L. Ross, J. Bain, A. M. Edelman, B. G. Frenguelli, and D. G. Hardie. 2005. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2:9-19. [DOI] [PubMed] [Google Scholar]
- 21.Hawley, S. A., M. A. Selbert, E. G. Goldstein, A. M. Edelman, D. Carling, and D. G. Hardie. 1995. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 270:27186-27191. [DOI] [PubMed] [Google Scholar]
- 22.Heller, R., A. Unbehaun, B. Schellenberg, B. Mayer, G. Werner-Felmayer, and E. R. Werner. 2001. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 276:40-47. [DOI] [PubMed] [Google Scholar]
- 23.Hong, S.-P., F. C. Leiper, A. Woods, D. Carling, and M. Carlson. 2003. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. USA 100:8839-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt, R. A., G. J. Bhat, and K. M. Baker. 1999. Angiotensin II-stimulated induction of sis-inducing factor is mediated by pertussis toxin-insensitive Gq proteins in cardiac myocytes. Hypertension 34:603-608. [DOI] [PubMed] [Google Scholar]
- 25.Hurley, R. L., K. A. Anderson, J. M. Franzone, B. E. Kemp, A. R. Means, and L. A. Witters. 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280:29060-29066. [DOI] [PubMed] [Google Scholar]
- 26.Ido, Y., D. Carling, and N. Ruderman. 2002. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells. Inhibition by the AMP-activated protein kinase activation. Diabetes 51:159-167. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, J. A., M. O. Gray, J. S. Karliner, C.-H. Chen, and D. Mochly-Rosen. 1996. An improved permeabilization protocol for the introduction of peptides into cardiac myocytes. Application to protein kinase C research. Circ. Res. 79:1086-1099. [DOI] [PubMed] [Google Scholar]
- 28.Kahn, B. B., T. Alquier, D. Carling, and D. G. Hardie. 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1:15-25. [DOI] [PubMed] [Google Scholar]
- 29.Kishi, K., T. Yuasa, A. Minami, M. Yamada, A. Hagi, H. Hayashi, B. E. Kemp, L. A. Witters, and Y. Ebina. 2000. AMP-activated protein kinase is activated by the stimulations of Gq-coupled receptors. Biochem. Biophys. Res. Commun. 276:16-22. [DOI] [PubMed] [Google Scholar]
- 30.Minami, T., A. Sugiyama, S.-Q. Wu, R. Abid, T. Kodama, and W. C. Aird. 2004. Thrombin and phenotypic modulation of the endothelium. Arterioscler. Thromb. Vasc. Biol. 24:41-53. [DOI] [PubMed] [Google Scholar]
- 31.Morrow, V. A., F. Foufelle, J. M. Connell, J. R. Petrie, G. W. Gould, and I. P. Salt. 2003. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J. Biol. Chem. 278:31629-31639. [DOI] [PubMed] [Google Scholar]
- 32.Mount, P. F., R. E. Hill, S. A. Fraser, V. Levidiotis, F. Katsis, B. E. Kemp, and D. A. Power. 2005. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am. J. Physiol. Renal Physiol. 289:F1103-F1115. [DOI] [PubMed] [Google Scholar]
- 33.Nagata, D., M. Mogi, and K. Walsh. 2003. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278:31000-31006. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, P. J., N. Prevost, M. Molino, M. K. Hollinger, M. J. Woolkalis, D. S. Woulfe, and L. F. Brass. 2000. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J. Biol. Chem. 275:13502-13509. [DOI] [PubMed] [Google Scholar]
- 35.Ouchi, N., H. Kobayashi, S. Kihara, M. Kumada, K. Sato, T. Inoue, T. Funahashi, and K. Walsh. 2004. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279:1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouchi, N., R. Shibata, and K. Walsh. 2005. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 96:838-846. [DOI] [PubMed] [Google Scholar]
- 37.Schulz, E., E. Anter, M.-H. Zou, and J. F. Keaney, Jr. 2005. Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation 111:3473-3480. [DOI] [PubMed] [Google Scholar]
- 38.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 101:3329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Thors, B., H. Halldórsson, and G. Thorgeirsson. 2004. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 573:175-180. [DOI] [PubMed] [Google Scholar]
- 41.Tokumitsu, H., H. Inuzuka, Y. Ishikawa, M. Ikeda, I. Saji, and R. Kobayashi. 2002. STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277:15813-15818. [DOI] [PubMed] [Google Scholar]
- 42.Tsopanoglou, N. E., and M. E. Maragoudakis. 2004. Role of thrombin in angiogenesis and tumor progression. Semin. Thromb. Hemost. 30:63-69. [DOI] [PubMed] [Google Scholar]
- 43.Vaidyula, V. R., and A. K. Rao. 2003. Role of Gαq and phospholipase C-β2 in human platelets activation by thrombin receptors PAR1 and PAR4: studies in human platelets deficient in Gαq and phospholipase C-β2. Br. J. Haematol. 121:491-496. [DOI] [PubMed] [Google Scholar]
- 44.Woods, A., P. C. Cheung, F. C. Smith, M. D. Davison, J. Scott, R. K. Beri, and D. Carling. 1996. Characterization of AMP-activated protein kinase β and γ subunits. Assembly of the heterotrimeric complex in vitro. J. Biol. Chem. 271:10282-10290. [DOI] [PubMed] [Google Scholar]
- 45.Woods, A., K. Dickerson, R. Heath, S.-P. Hong, M. Momcilovic, S. R. Johnstone, M. Carlson, and D. Carling. 2005. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2:21-33. [DOI] [PubMed] [Google Scholar]
- 46.Woods, A., S. R. Johnstone, K. Dickerson, F. C. Leiper, L. G. Fryer, D. Neumann, U. Schlattner, T. Wallimann, M. Carlson, and D. Carling. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13:2004-2008. [DOI] [PubMed] [Google Scholar]
- 47.Woods, A., I. Salt, J. Scott, D. G. Hardie, and D. Carling. 1996. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 397:347-351. [DOI] [PubMed] [Google Scholar]
- 48.Zhou, G., R. Myers, Y. Li, Y. Chen, X. Shen, J. Fenyk-Melody, M. Wu, J. Ventre, T. Doebber, N. Fujii, N. Musi, M. F. Hirshman, L. J. Goodyear, and D. E. Moller. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 108:1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou, M.-H., X.-Y. Hou, C.-M. Shi, S. Kirkpatick, F. Liu, M. H. Goldman, and R. A. Cohen. 2003. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J. Biol. Chem. 278:34003-34010. [DOI] [PubMed] [Google Scholar]
- 50.Zou, M.-H., X.-Y. Hou, C.-M. Shi, D. Nagata, K. Walsh, and R. A. Cohen. 2002. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J. Biol. Chem. 277:32552-32557. [DOI] [PubMed] [Google Scholar]
- 51.Zou, M.-H., S. S. Kirkpatrick, B. J. Davis, J. S. Nelson, W. G. Wiles, IV, U. Schlattner, D. Neumann, M. Brownlee, M. B. Freeman, and M. H. Goldman. 2004. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 279:43940-43951. [DOI] [PubMed] [Google Scholar]