Abstract
Exogenous delivery of carrier-linked phosphatidylinositol 3-phosphate [PtdIns(3)P] to adipocytes promotes the trafficking, but not the insertion, of the glucose transporter GLUT4 into the plasma membrane. However, it is yet to be demonstrated if endogenous PtdIns(3)P regulates GLUT4 trafficking and, in addition, the metabolic pathways mediating plasma membrane PtdIns(3)P synthesis are uncharacterized. In unstimulated 3T3-L1 adipocytes, conditions under which PtdIns(3,4,5)P3 was not synthesized, ectopic expression of wild-type, but not catalytically inactive 72-kDa inositol polyphosphate 5-phosphatase (72-5ptase), generated PtdIns(3)P at the plasma membrane. Immunoprecipitated 72-5ptase from adipocytes hydrolyzed PtdIns(3,5)P2, forming PtdIns(3)P. Overexpression of the 72-5ptase was used to functionally dissect the role of endogenous PtdIns(3)P in GLUT4 translocation and/or plasma membrane insertion. In unstimulated adipocytes wild type, but not catalytically inactive, 72-5ptase, promoted GLUT4 translocation and insertion into the plasma membrane but not glucose uptake. Overexpression of FLAG-2xFYVE/Hrs, which binds and sequesters PtdIns(3)P, blocked 72-5ptase-induced GLUT4 translocation. Actin monomer binding, using latrunculin A treatment, also blocked 72-5ptase-stimulated GLUT4 translocation. 72-5ptase expression promoted GLUT4 trafficking via a Rab11-dependent pathway but not by Rab5-mediated endocytosis. Therefore, endogenous PtdIns(3)P at the plasma membrane promotes GLUT4 translocation.
The glucose transporter GLUT4 facilitates glucose uptake in response to insulin stimulation in adipose tissue and striated muscle. In the basal state GLUT4 is sequestered in a specialized intracellular endosomal compartment. After insulin stimulation, GLUT4 storage vesicles traffic to and fuse with the plasma membrane (PM) facilitating increased cellular glucose uptake (11, 79). The signaling pathways that stimulate glucose uptake are complex; however, two major pathways have been identified: the first mediated by the phosphoinositide 3-kinase (PI3-kinase) and the second involving the small GTP-binding protein TC10 (56, 62, 66). After insulin stimulation, the class I PI3-kinase phosphorylates phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] transiently generating PtdIns(3,4,5)P3, which recruits to the PM and activates the cytosolic effectors Akt, PDK1, and atypical protein kinase C (11, 52). In the absence of agonist stimulation, although Vps34-mediated PtdIns(3)P synthesis on early endosomes is constitutive, PM PtdIns(3)P, PtdIns(3,4)P2, or PtdIns(3,4,5)P3 synthesis cannot be detected (52). Many studies using PI3-kinase inhibitors such as wortmannin, expression of PI3-kinase constructs, and microinjection of neutralizing antibodies to the catalytic subunit of PI3-kinase have revealed that PI3-kinase-catalyzed generation of PtdIns(3,4,5)P3 is required for insulin-stimulated glucose uptake (3, 13, 19, 22, 29, 34, 35, 39, 51, 72).
Recently, the insulin-stimulated generation of PtdIns(3)P at the PM has been demonstrated in adipocytes (41). Intracellular delivery of exogenous carrier-linked-PtdIns(3)P into adipocytes, in the absence of insulin stimulation, promotes the movement of GLUT4-containing vesicles to the PM, but not glucose transport, in insulin-responsive cells (41). Furthermore, studies using exogenously added carrier linked-PtdIns(3,4,5)P3 versus PtdIns(3)P suggests that PtdIns(3,4,5)P3 and PtdIns(3)P are required for distinct steps in the pathway of GLUT4 fusion and insertion into the PM (27). This model predicts PtdIns(3,4,5)P3 facilitates GLUT4 translocation and insertion into the PM, whereas exogenously added PtdIns(3)P stimulates translocation and unmasking of the C-terminal domain of GLUT4 but not insertion. However, recently it has been shown that in the absence of Munc18, exogenously added PtdIns(3)P can promote GLUT4 insertion into the PM (30). All of these studies by necessity were undertaken using exogenously added carrier-linked-phosphoinositides, which may not precisely reflect the activities of the endogenous lipids. In addition, whether these carrier-linked phosphoinositides localize to the same compartment as insulin-stimulated phosphoinositides has not been addressed. Delineation of the discrete roles of the endogenous PtdIns(3)P in intact adipocytes has not been possible given insulin stimulates the concomitant synthesis of both PtdIns(3,4,5)P3 and PtdIns(3)P (27).
The inositol polyphosphate 5-phosphatases (5-phosphatases)hydrolyze PtdIns(3,4,5)P3 and/or PtdIns(4,5)P2 forming PtdIns(3,4)P2 and PtdIns (4)P, respectively (42). The 5-phosphatases SHIP2 and SKIP, by hydrolyzing PtdIns(3,4,5)P3, are implicated in negatively regulating insulin-stimulated GLUT4 trafficking (17, 26). We and others have cloned and characterized a 72-kDa 5-phosphatase (72-5ptase) (mouse), also called pharbin (rat), or the type IV 5-phosphatase (human) (4, 37, 38) that share 74% amino acid sequence identity. This 72-5ptase contains an N-terminal proline-rich region, a central 5-phosphatase catalytic domain, and a C-terminal CAAX motif. In the study presented here we demonstrate the 72-5ptase hydrolyzes PtdIns(3,5)P2, generating PtdIns(3)P, and its overexpression in adipocytes generates PtdIns(3)P at the PM of unstimulated 3T3-L1 adipocytes. Therefore, ectopic overexpression of this enzyme was used in adipocytes as an experimental tool to generate PtdIns(3)P, in the absence of PtdIns(3,4,5)P3, to dissect the functional role of endogenous PtdIns(3)P in regulating GLUT4 trafficking and insertion into the PM. In the absence of insulin stimulation and PtdIns(3,4,5)P3 generation, the wild-type 72-5ptase, but not catalytically inactive 5-phosphatase, stimulated GLUT4 translocation and insertion into the PM, an effect blocked upon sequestration of PtdIns(3)P and/or actin monomers. Therefore, endogenous PtdIns(3)P can promote GLUT4 PM translocation and insertion under conditions of actin remodeling.
MATERIALS AND METHODS
The 3T3-L1 cell line was from the American Tissue Culture Collection. Monoclonal antibody to the hemagglutinin (HA) tag was from Babco, and monoclonal antibody to the FLAG epitope was from Sigma. Monoclonal antibodies to the Myc and Xpress tag epitopes were from Invitrogen. Polyclonal rabbit C-terminal antibody to GLUT4 was from Chemicon. Akt and phospho(Ser473)-Akt antibodies were from Cell Signaling Technologies. All other reagents were from Sigma unless otherwise stated. Plasmids for green fluorescent protein (GFP)-GLUT4 and FLAG-Ras and the GLUT1 antibody were kindly provided by Jeffrey Pessin. Exofacial Myc-GLUT4-GFP and TC10 constructs were gifts from Alan Saltiel. GFP-PH/ARNO and GFP-FYVE/EEA1 plasmids were gifts from Tamas Balla. GFP-2xFYVE/Hrs plasmid was from Harald Stenmark. pAdEasy-1 and pAdTrackCMV were gifts from Bert Vogelstein. HA-GLUT1 and Rab5 plasmids were kindly provided by Sam Cushman and Rob Parton, respectively.
Cell culture and differentiation of 3T3-L1 preadipocytes.
3T3-L1 fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM) (Gibco-BRL) containing 4.5 mg of glucose/ml and 10% fetal bovine serum (FBS) (CSL). Confluent 3T3-L1 preadipocytes were differentiated with DMEM containing 10% FBS, 4 μg of insulin/ml, 500 μM isobutyl 1-methylxanthine (IBMX), and 250 nM dexamethasone. Growth media was replaced with DMEM plus 10% FBS 4 days after the addition of differentiation media and incubated for a further 4 days.
Northern blot analysis.
Poly(A) RNA from undifferentiated 3T3-L1 preadipocytes and differentiated adipocytes was size fractionated in an agarose gel and transferred to nitrocellulose. The membrane was incubated with a 32P-labeled 2.0-kb 72-5ptase cDNA. The membrane was subsequently probed for GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Hybridization and washing conditions were as previously described (38).
Detection of 72-5ptase in 3T3-L1 adipocytes by immunoblot analysis.
Detergent-soluble and -insoluble cellular fractions for immunoblotting with 72-5ptase-specific antibodies were prepared by harvesting adipocytes in cold lysis buffer (50 mM Tris [pH 7], 450 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate supplemented with Complete Mini [with EDTA] protease inhibitor cocktail tablets [Roche]), followed by centrifugation (13,000 × g, 10 min, 4°C). Cytosolic fractions were prepared after homogenization in HES buffer (255 mM sucrose, 1 mM EDTA, 20 mM HEPES [pH 7.2]) and centrifugation at 100,000 × g for 60 min at 4°C. Immunoblots were developed by using enhanced chemiluminescence (NEN).
Generation of catalytic inactive 72-D480N5ptase mutant.
Sequential PCR-based site-directed mutagenesis was carried out to generate a catalytically inactive mutant of the 72-5ptase in which a catalytic Asp residue was substituted with Asn. cDNA sequences flanking the site to be mutated were amplified using the primers GCTCTAGAATGCCATCCAAGTCAGCT and CTGGTTTGGGAACTTCAACTTCCGC or CGTCTAGATCAGGACACGGTGCAAAC and GCGGAAGTTGAAGTTCCCAAACCAG (with nucleotide substitutions underlined). Purified PCR products were used as a template in a subsequent PCR using the end primers to amplify the full-length cDNA containing the desired mutation and XbaI sites at the ends. The resulting product was subcloned into pCR-Blunt and sequenced before ligation into the XbaI site of the pCGN expression vector.
Generation of FLAG-2xFYVE/Hrs construct.
cDNA sequence coding for 2xFYVE/Hrs was amplified and ligated into the MluI site of pEFBOS vector in-frame with the N-terminal FLAG tag.
Transfection of 3T3-L1 adipocytes and indirect immunofluorescence.
Adipocytes were transiently transfected 8 days after differentiation by electroporation as described previously (31). Electroporation cuvettes containing 107 cells and 50 μg of each plasmid DNA were pulsed at 0.17 kV and 975 μF. Cells were plated onto 0.01% poly-l-lysine-coated glass coverslips and incubated for 24 or 48 h. Cells on coverslips were washed twice with cold phosphate-buffered saline (PBS) supplemented with 1 mM CaCl2 and 0.5 mM MgCl2 and then fixed with 3% paraformaldehyde for 20 min, permeabilized with 0.2% Triton X-100 in PBS (5 min), incubated with blocking buffer (5% goat serum in PBS) (30 min), overlaid with primary antibody diluted in blocking buffer (1 h), washed, and incubated with secondary antibody-Alexa Fluor conjugate (Molecular Probes) diluted in blocking buffer (1 h). Coverslips were washed and mounted onto glass slides using Fluoromount-G mounting medium (Southern Biotech) and imaged by laser scanning confocal microscopy (Monash Micro Imaging Facility).
GLUT4 translocation assay.
At 24 or 48 h after adipocyte cotransfection with GFP-GLUT4 (50 μg) and pCGN constructs (50 μg), cells were serum starved (2 h at 37°C), treated with 100 nM insulin (30 min, 37°C) fixed and stained with HA antibodies, and scored for PM GFP-GLUT4 by fluorescence microscopy. Pharmacological agents, including 100 nM wortmannin (45 min, 37°C) and brefeldin A (10 μg/ml, 30 min, 37°C) were used after serum starvation and prior to insulin stimulation.
Subcellular fractionation of transfected 3T3-L1 adipocytes.
Cells cotransfected with GFP-GLUT4 and HA-vector or HA-72-5ptase were washed and homogenized in cold resuspension buffer (255 mM sucrose, 1 mM EDTA, 20 mM HEPES [pH 7.2], 2 μg of aprotinin/ml, 2 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride, 3 μg of pepstatin A/ml) before differential centrifugation (49). A 25-μg portion of each fraction was subjected to immunoblot analysis using monoclonal GFP antibodies (Roche).
PM sheets.
PM sheets were prepared by hypotonic swelling and sonication (54). Sheets were fixed in 3% paraformaldehyde for 20 min, incubated with quench buffer (70 mM KCl, 30 mM HEPES [pH 7.5], 5 mM MgCl2, 3 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride) for 15 min, followed by blocking buffer (5% goat serum in PBS, 30 min), and labeled for indirect immunofluorescence.
Adenoviral constructs and transduction of 3T3-L1 adipocytes.
HA-72-5ptase (GenBank accession no. AF226683) or HA-72-D480N5ptase cDNA were ligated into pAdTrack-CMV, which contains a cDNA encoding green fluorescent protein (GFP). Recombinant adenoviral plasmids were generated by homologous recombination with pAdEasy-1 in BJ5183 Escherichia coli according to the manufacturer's instructions (Stratagene) (23). PacI-linearized plasmids were transfected into HEK293T packaging cells, and lysates were collected. 3T3-L1 adipocytes in DMEM containing 2% FBS were transduced with adenovirus at a multiplicity of infection of 40 PFU/cell (16 h) and cultured for a further 24 h prior to analysis.
2-Deoxy-d-glucose (2-DOG) uptake assays.
At 48 h after transduction with empty vector, HA-72-5ptase, or HA-72-D480N5ptase adenovirus, adipocytes were assayed for 2-deoxy-d-[2,6-3H]glucose uptake (49). Serum-starved cells were treated with or without 100 nM insulin in 125 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 2.6 mM MgSO4, 25 mM HEPES (pH 7.4) containing 2 mM Na pyruvate, and 1% (wt/vol) bovine serum albumin (10 min, 37°C) prior to the addition of 100 μM 2-[3H]DOG (7.5 μCi/ml, 20 min, 37°C; Amersham). Cells were washed, and scintillation was counted for 3H and assayed in replicates of four per experiment.
PtdIns(3,4,5)P3 and PtdIns(3,5)P2 5-phosphatase assays.
PtdIns(3,4,5)P3 and PtdIns(3,5)P2 5-phosphatase assays were performed as previously described (38). 3T3-L1 adipocytes expressing HA-vector, HA-72-5ptase, or HA-72-D480N5ptase were immunoprecipitated with HA antibody and immunoprecipitates washed twice with lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% Na deoxycholate), twice in assay buffer (20 mM HEPES [pH 7.5], 5 mM MgCl2, 1 mM EGTA), and assayed for 5-phosphatase activity as described previously (38).
Transferrin uptake.
Untransfected adipocytes were serum starved and incubated with serum-free DMEM containing Texas Red-transferrin conjugate (Molecular Probes) at 20 μg/ml for 20 min at 37°C prior to fixation for indirect immunofluorescence. For transferrin uptake in transfected adipocytes, serum-starved cells were cooled (16°C, 20 min) and incubated with serum-free DMEM containing Texas Red-transferrin (20 μg/ml, 1 h, 16°C) and washed once with cold acid wash buffer (150 mM NaCl, 1 mM CaCl2, 20 mM Na acetate [pH 4.6]), followed by a PBS wash. Cells were incubated with chase medium containing holotransferrin (200 μg/ml, 5 min, 37°C) before fixation for indirect immunofluorescence (63, 83).
Image analysis.
Analysis was performed using the public domain Image J program (version 1.34 NIH) (1). The fluorescence intensity was measured in cells as the average pixel fluorescence intensity within an area of defined size drawn over three distinct areas of the plasma membrane (5 × 10 pixels) or the average of three boxes in the cytosol (10 × 10 pixels). Ratios of plasma membrane to cytosolic pixel fluorescence intensity were determined and subjected to statistical analysis.
Statistical analysis.
Statistical analyses of cell counts and fluorescence intensity measurements were all performed using unpaired Student t Test.
RESULTS
Expression of 72-5ptase in differentiated 3T3-L1 adipocytes.
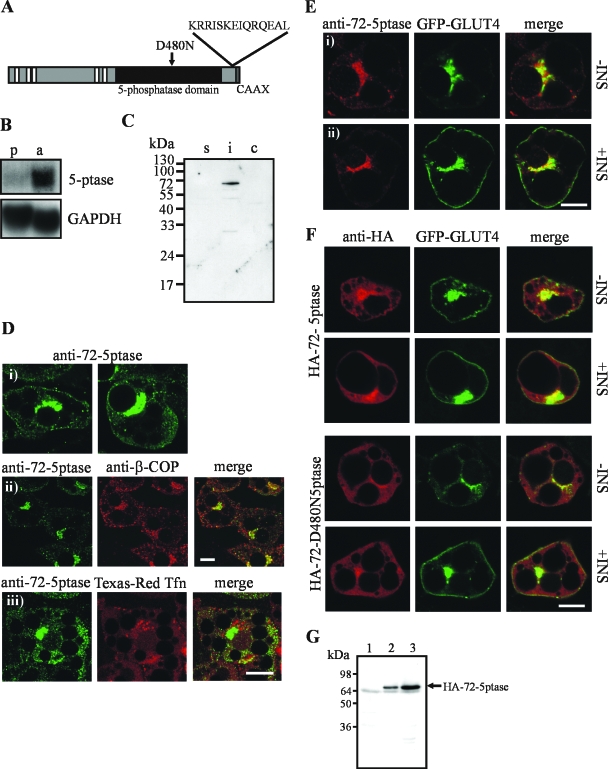
The 72-5ptase hydrolyzes the 5-position phosphate from both PtdIns(3,4,5)P3 and PtdIns(3,5)P2, forming PtdIns(3,4)P2 and PtdIns(3)P, respectively (38). We reasoned the overexpression of the wild-type enzyme may generate a functional pool of PtdIns(3)P, while maintaining low cellular levels of PtdIns(3,4,5)P3, enabling dissection of the role of endogenous PtdIns(3)P in GLUT4 trafficking and insertion into the PM. We first investigated whether the 72-5ptase was expressed in 3T3-L1 adipocytes. The 72-5ptase cDNA, encoding the open reading frame, was used to probe a Northern blot containing 3T3-L1 preadipocyte and differentiated adipocyte RNA (Fig. 1A and B). A faint 3.9-kb transcript was detected in RNA from preadipocytes, which increased in intensity upon adipocyte differentiation (Fig. 1B, upper panel), whereas GAPDH levels remained unchanged (Fig. 1B, lower panel). Immunoblot analysis with 72-5ptase C-terminal affinity-purified antibody demonstrated a 72-kDa immunoreactive polypeptide in the detergent-insoluble fraction of differentiated adipocytes (Fig. 1C).
FIG. 1.
Expression and intracellular localization of 72-5ptase in 3T3-L1 adipocytes. (A) 72-5ptase domain structure showing proline-rich (PxxP) motifs (white boxes), the catalytic domain (black box), the D480N mutation which renders the enzyme inactive, and the C-terminal peptide sequence used to raise polyclonal antibodies. (B) Northern blot containing mRNA (20 μg) from precursor 3T3-L1 fibroblasts (p) or differentiated 3T3-L1 adipocytes (a) was hybridized with 72-5ptase cDNA (nucleotides 595 to 2538) (upper panel). After exposure, the membrane was hybridized to a GAPDH probe (lower panel). (C) Detergent-soluble (s), detergent-insoluble (i), and cytosolic (c) lysates of differentiated 3T3-L1 adipocytes (50 μg) were immunoblotted with 72-5ptase antibodies. (D and E) Differentiated 3T3-L1 adipocytes were labeled with 72-5ptase-specific antibodies (i) and markers for Golgi (β-COP antibody) (ii), sorting and recycling endosomes (Texas Red-transferrin) (D), or GLUT4 (GFP-GLUT4) in resting (−INS) or insulin-stimulated (+INS) cells (E). (F) Resting (−INS) or insulin-stimulated (+INS) 3T3-L1 adipocytes coexpressing recombinant HA-72-5ptase or inactive HA-72-D480N5ptase and GFP-GLUT4 were labeled with HA-specific antibodies. Scale bar, 10 μm. (G) Cell lysates from vector (lane 1)-, HA-72-5ptase (lane 2)-, or HA-72-D480N5ptase (lane 3)-transfected adipocytes were immunoblotted with HA-specific antibodies.
The intracellular localization of endogenous 72-5ptase was determined by indirect immunofluorescence of 3T3-L1 adipocytes with 72-5ptase anti-peptide antibodies revealing cytosolic staining (Fig. 1Di) and concentrated perinuclear staining which colocalized with the Golgi marker, β-COP (Fig. 1Dii), but not endocytosed Texas Red-labeled transferrin (Fig. 1Diii). As reported previously, the localization of GFP-GLUT4 is indistinguishable from endogenous GLUT4 in resting and insulin-treated adipocytes (73). Partial colocalization of the 72-5ptase was shown with GFP-GLUT4 in unstimulated adipocytes (Fig. 1Ei). After insulin stimulation GFP-GLUT4 translocated to the PM, whereas 72-5ptase perinuclear and cytosolic localization persisted (Fig. 1Eii). Recombinant HA-72-5ptase and a catalytically inactive HA-72-5ptase that contains a point mutation of a critical aspartic acid residue (HA-72-D480N5ptase) displayed a cytosolic distribution with enrichment in the perinuclear region, colocalizing with β-COP (not shown) and partially with intracellular GFP-GLUT4 (Fig. 1F). HA-72-5ptase distribution did not change upon insulin stimulation (Fig. 1F). Immunoblot analysis demonstrated intact expression of HA-72-5ptase and HA-72-D480N5ptase (Fig. 1G).
The 72-5ptase regulates insulin-stimulated PtdIns(3,4,5)P3 levels.
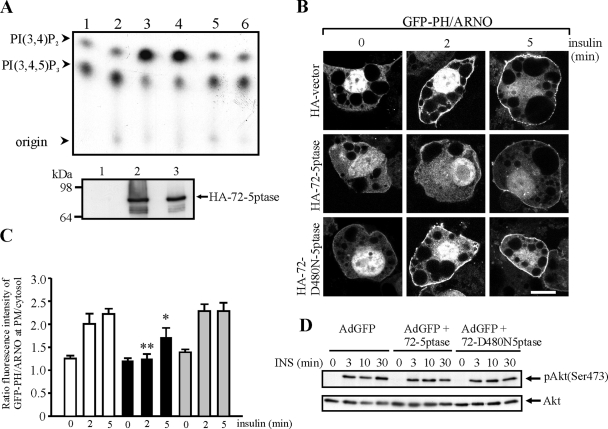
PtdIns(3,4,5)P3 is a critical signaling molecule facilitating GLUT4 exocytosis to the PM (11, 61). Previous studies have demonstrated the 72-5ptase shows the greatest catalytic activity toward PtdIns(3,4,5)P3 of any 5-phosphatase (37). We generated a catalytically inactive 72-5ptase that contains a point mutation of a critical catalytic aspartic acid residue (HA-72-D480N5ptase), based on the active site identified by crystallization studies of an archetypal 5-phosphatase (74). To demonstrate the wild-type HA-72-5ptase, but not HA-72-D480N5ptase, hydrolyzed the 5-position phosphate from PtdIns(3,4,5)P3, lysates derived from cells transiently transfected with these constructs were immunoprecipitated and PtdIns(32P-3,4,5)P3 5-phosphatase assays performed as described previously (38). Wild-type HA-72-5ptase, but not HA-72-D480N5ptase, hydrolyzed PtdIns(32P-3,4,5)P3 forming PtdIns(32P-3,4)P2 (Fig. 2A). Immunoblot analysis of parallel immunoprecipitates indicated equivalent immunoreactive levels of wild-type HA-72-5ptase and inactive HA-72-D480N5ptase (Fig. 2A, lower panel). To evaluate insulin-stimulated PtdIns(3,4,5)P3 accumulation at the PM in adipocytes expressing the 72-5ptase, we used the pleckstrin homology (PH) domain of the Arf-nucleotide binding site opener (ARNO) fused to GFP, which has been used extensively as a marker of PM PtdIns(3,4,5)P3 (6). In nonstimulated cells, cotransfected with HA-vector and GFP-PH/ARNO, GFP-PH/ARNO PM fluorescence was not detected, a finding consistent with many studies reporting that this phosphoinositide is undetectable in quiescent cells (Fig. 2B) (6, 18, 43, 77). Upon insulin treatment, GFP-PH/ARNO translocated rapidly to the PM in >85% of cells (200 cells scored for three transfections), maximally 2 to 5 min after stimulation. In contrast, <20% of the cells expressing the 72-5ptase demonstrated PtdIns(3,4,5)P3 at the PM after insulin stimulation or in unstimulated cells (200 cells scored for three transfections) (not shown). Adipocytes expressing the catalytically inactive 72-5ptase demonstrated GFP-PH/ARNO PM fluorescence similar to vector-transfected cells (Fig. 2B). In individual cells expressing GFP-PH/ARNO, we determined the ratio of PM to cytosolic GFP-PH/ARNO fluorescence as described previously (45). By this analysis after insulin stimulation a 1.6-fold increase in this ratio was noted in insulin-stimulated vector-expressing adipocytes at 2 and 5 min of stimulation, which was reduced significantly in cells expressing the wild type but not the inactive 72-5ptase (Fig. 2C). In unstimulated cells the GFP-PH/ARNO PM/cytosolic fluorescence ratio was similar for all constructs. Thus, the 72-5ptase hydrolyzes PtdIns(3,4,5)P3 at the PM in insulin-stimulated adipocytes. To further evaluate the regulation of the PI3-kinase-dependent signaling pathway, phosphorylation of Akt at Ser473 was assessed by using phosphorylation-specific antibodies; however, no change in the ratio of insulin-stimulated pSer473Akt to total Akt protein was noted upon the overexpression of the wild type versus the empty vector or inactive 72-5ptase (Fig. 2D). The failure of the 72-5ptase to regulate Akt Ser473 phosphorylation in 3T3-L1 adipocytes, in contrast to studies in fibroblasts (36), may relate to the observation that this enzyme only reduced PM PtdIns(3,4,5)P3 levels by ca. 50% in insulin-stimulated cells, perhaps as a consequence of its perinuclear localization in adipocytes.
FIG. 2.
The 72-5ptase regulates PtdIns(3,4,5)P3 levels in insulin-treated 3T3-L1 adipocytes. (A) HA immunoprecipitates from COS-7 cells transfected with HA-vector (lanes 1 and 2), HA-72-5ptase (lanes 3 and 4), or HA-72-D480N5ptase (lanes 5 and 6) were subjected to PtdIns(32P-3,4,5)P3 5-phosphatase assays. Lipids were extracted and analyzed by thin-layer chromatography. An autoradiogram, representative of three independent PtdIns(3,4,5)P3 5-phosphatase assays, is shown (upper panel). The migration of standards for PtdIns(3,4)P2, or PtdIns(3,4,5)P3 is indicated. Parallel HA immunoprecipitates immunoblotted with HA antibody (lower panel), HA-vector (lane 1), HA-72-5ptase (lane 2), and HA-72-D480N5ptase (lane 3). (B) 3T3-L1 adipocytes expressing GFP-PH/ARNO and the indicated constructs were stimulated with 100 nM insulin for 2 or 5 min. Representative cells are shown. Scale bar, 10 μm. (C) The ratio of fluorescence intensity of GFP-PH/ARNO at the PM (average fluorescence intensity of three defined boxes of 5 × 10 pixels of the plasma membrane per cell) to that of the cytosol (average fluorescence intensity of three boxes of defined area of 10 × 10 pixels per cell) was determined using Image J software. Bars (empty vector, white bars; wild-type 72-5ptase, black bars; inactive 72-5ptase, gray bars) represent the mean ± the standard error of the mean (SEM) of two independent experiments with >20 cells scored for each construct for each time point (*, P < 0.05; **, P < 0.01 compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase). (D) Adipocytes transduced with the indicated constructs were serum starved and treated with 10 nM insulin for 3, 10, or 30 min. Lysates were immunoblotted with Akt or phospho(Ser473)-Akt antibody. Representative blots of four independent experiments are shown.
72-5ptase hydrolyzes PtdIns(3,5)P2, forming PtdIns(3)P.
In response to insulin stimulation, PtdIns(3)P is produced at the PM, downstream of the small GTP binding protein TC10. Exogenous carrier-labeled delivery of PtdIns(3)P stimulates GLUT4 PM translocation, but not insertion into the PM, or glucose uptake (27, 41). The metabolic routes for the synthesis of PM PtdIns(3)P remain to be comprehensively delineated; however, this phosphoinositide is generated by a wortmannin-resistant PI3-kinase, possibly the class II PI3-kinase (14, 27, 41). In addition, another phosphoinositide, PtdIns(3,5)P2, is present in unstimulated cells (58). The PIKfyve kinase phosphorylates phosphatidylinositol (PtdIns) and PtdIns(3)P, forming PtdIns (5)P and PtdIns(3,5)P2, respectively, regulating actin dynamics and multivesicular body/late endocytic function (57, 65). Enzymatically inactive PIKfyve mutants induce dilation of late endocytic structures and inhibit insulin-stimulated GLUT4 translocation (66). We have previously reported, using a purified component enzyme assay, that the 72-5ptase hydrolyzes PtdIns(3,5)P2 in vitro, forming PtdIns(3)P (38). However, whether this occurs in intact adipocytes, the site at which PtdIns(3)P is generated, and the functional significance of this reaction in GLUT4 translocation are unknown. We investigated whether the 72-5ptase hydrolyzes the 5-position phosphate from PtdIns(3,5)P2, generating PtdIns(3)P in adipocytes, by using two experimental approaches: first, PtdIns(3,5)P2 5-phosphatase enzyme assays were performed on immunoprecipitated HA-72-5ptase from transfected 3T3-L1 adipocytes, and second, PtdIns(3)P production was examined in intact adipocytes by using a GFP-labeled phosphoinositide biosensor, GFP-FYVE, which specifically binds PtdIns(3)P (6).
Immunoprecipitated HA-72-5ptase, but not inactive HA-72-D480N5ptase, from adipocyte lysates, hydrolyzed PtdIns(32P-3,5)P2, forming PtdIns(32P-3)P (Fig. 3A). To detect in vivo PtdIns(3)P production, the FYVE domain of early endosomal antigen 1 (EEA1) fused to GFP (6), was coexpressed with the 72-5ptase. In quiescent adipocytes cotransfected with vector, GFP-FYVE/EEA1 was restricted to discrete vesicles, a finding consistent with PtdIns(3)P on early endosomes (Fig. 3B). After insulin stimulation, in cells coexpressing vector and GFP-FYVE/EEA1, PM fluorescence was detected, indicating PtdIns(3)P production at this site as previously reported using the biosensor GFP-2xFYVE/Hrs (41). Notably, in unstimulated cells expressing HA-72-5ptase GFP-FYVE/EEA1 was detected at the PM, as well as decorating cytosolic vesicles (Fig. 3B, see arrows) and after insulin stimulation no change in the distribution of this phosphoinositide biosensor was detected. GFP-FYVE/EEA1 distribution in cells expressing the inactive 72-5ptase resembled that detected in vector-expressing cells. PM GFP-FYVE/EEA1 fluorescence was quantified relative to that detected in the cytosol (excluding vesicular staining) in transfected adipocytes. By this analysis in nonstimulated adipocytes, expression of the wild-type 72-5ptase resulted in a >2.5-fold increase in PM/cytosol GFP-FYVE/EEA1 fluorescence that was significantly greater than that detected in insulin-stimulated vector-expressing cells, which demonstrated a 1.5-fold increase in this ratio relative to nonstimulated cells (Fig. 3C).
FIG. 3.
The 72-5ptase hydrolyzes PtdIns(3,5)P2, forming PtdIns(3)P. (A) PtdIns(32P-3,5)P2 5-phosphatase assays of HA immunoprecipitates from adipocytes transfected with HA-vector (lane 1), HA-72-5ptase (lane 2), or HA-72-D480N5ptase (lane 3). Extracted lipids were separated by thin-layer chromatography and visualized by autoradiography. An autoradiogram representative of three independent PtdIns(3,5)P2 5-phosphatase assays is shown (upper panel). The migration of labeled standards for PtdIns(32P-3)P or PtdIns(32P-3,5)P2 is indicated. The lower panel shows parallel HA immunoprecipitates immunoblotted with HA antibody. The migration of immunoglobulin G light (LC) and heavy chains (HC) are shown. (B) Localization of GFP-FYVE/EEA1 in cells cotransfected with GFP-FYVE/EEA1 and HA-vector, HA-72-5ptase, or HA-72-D480N5ptase, treated or not treated with 300 nM insulin (10 min) in the absence (−) or presence (+) of 100 nM wortmannin (w). Single confocal laser-scanning sections through the middle of representative cells shows PtdIns(3)P at the PM (arrows). Representative images from four independent transfections are shown. (C) The fluorescence intensity of GFP-FYVE/EEA1 at the PM relative to that in the cytosol (excluding vesicular fluorescence) was determined, in untreated cells or cells treated with 300 nM insulin for 10 min, using Image J software. The average fluorescence intensity of three defined boxes of 5 × 10 pixels of the plasma membrane per cell was determined relative to that of the cytosol (average fluorescence intensity of three boxes of defined area of 10 × 10 pixels per cell). Bars (empty vector, white bars; wild-type 72-5ptase, black bars; inactive 72-ptase, gray bars) represent mean ± the SEM of three independent experiments (>20 cells were scored for each construct for unstimulated and insulin-stimulated conditions) (*, P < 0.05; **, P < 0.01 compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase). (D) Localization of FLAG-2xFYVE/Hrs in serum-starved cells cotransfected with HA-vector, HA-72-5ptase, or HA-72-D480N5ptase as indicated. Representative images from three independent transfections are shown. (E and F) Localization of GFP-2xFYVE/Hrs (E) or GFP-FYVE/EEA1 (F) in serum-starved 3T3-L1 adipocytes coexpressing HA-72-5ptase, HA-(or Xpress-)OCRL, HA-SKIP, HA-SHIP2, or HA-vector. Arrows indicate the PM localization of PtdIns(3)P-binding proteins in HA-72-5ptase-expressing cells. Scale bar, 10 μm.
To verify PM PtdIns(3)P production, we also determined the localization of ectopically expressed FLAG-2xFYVE/Hrs which binds PtdIns(3)P with high specificity and affinity (41). Overexpression of the wild-type HA-72-5ptase, but not vector or the catalytically inactive 5-phosphatase, promoted PM association of FLAG-2xFYVE/Hrs in unstimulated cells (Fig. 3D). FLAG-2xFYVE/Hrs was also detected on cytosolic vesicles regardless of the construct expressed.
To evaluate the specificity of GFP-FYVE/EEA1-PtdIns(3)P binding, adipocytes were treated with wortmannin (100 nM), which inhibits class III PI3-kinase Vps34-mediated generation of PtdIns(3)P on early endosomes, resulting in endosomal dilation and partial or complete displacement of EEA1 from endosomes depending on the cell type (28, 67). Insulin-dependent PM PtdIns(3)P production is resistant to such wortmannin treatment (41). In unstimulated and insulin-treated vector-, 72-5ptase-, and 72-D480N5ptase-containing adipocytes, GFP-FYVE/EEA1 labeling of vesicles was decreased upon wortmannin treatment, correlating with enhanced cytosolic GFP fluorescence, a finding consistent with the loss of PtdIns(3)P on endosomes (Fig. 3B). However, in the presence of wortmannin, GFP-FYVE/EEA1 PM fluorescence was still evident in unstimulated HA-72-5ptase-expressing cells and after insulin stimulation GFP-FYVE/EEA1 PM fluorescence persisted despite wortmannin treatment regardless of the construct expressed. These studies indicate that the wild type, but not inactive 72-5ptase, generates PtdIns(3)P at the PM in nonstimulated adipocytes.
The 5-phosphatase domain of the Lowe's protein (OCRL) has been shown by using in vitro purified component enzyme assays to hydrolyze PtdIns(3,5)P2, forming PtdIns(3)P, and the isolated SHIP-2 catalytic domain has also been demonstrated to hydrolyze PtdIns(3,5)P2 in vitro. However, to our knowledge this has not been examined in intact cells (12, 59). We evaluated whether OCRL, SHIP-2, or SKIP, a 5-phosphatase that also functions in adipocytes, could generate PtdIns(3)P at the plasma membrane of unstimulated adipocytes. To this end, adipocytes were transfected with constructs encoding these 5-phosphatases, and the distribution of GFP-2xFVVE/Hrs (Fig. 3E), or GFP-FYVE/EEA1 (Fig. 3F) was examined in unstimulated adipocytes. Only the 72-5-ptase stimulated the association of both PtdIns(3)P-binding biosensors with the plasma membrane. Occasionally, in less than 20% of cells expressing OCRL, we noted faint plasma membrane localization of GFP-FYVE/EEA1, but this was never detected using GFP-2xFYVE/Hrs.
The 72-5ptase hydrolyzes PtdIns(4,5)P2.
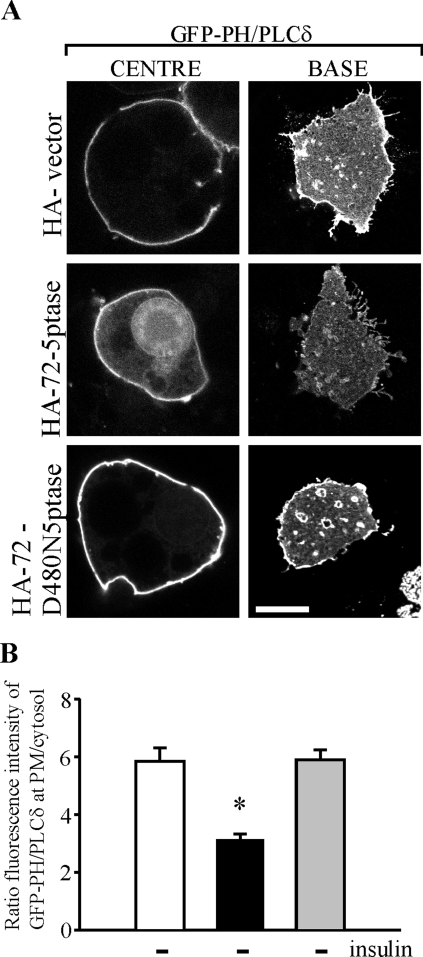
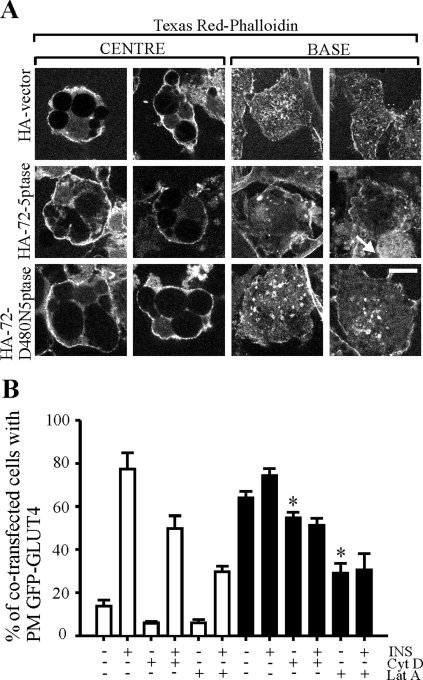
PtdIns(4,5)P2 regulates GLUT4 endocytosis from the PM and exocytosis from intracellular sites, via the regulation of actin dynamics (31). Since PtdIns(4,5)P2 is a predicted 72-5ptase substrate (4, 37), the effect of phosphatase expression on PtdIns(4,5)P2 levels was determined in intact adipocytes. The GFP-tagged pleckstrin homology (PH) domain of phospholipase Cδ (GFP-PH/PLCδ) acts as a sensitive in vivo marker of PM PtdIns(4,5)P2 (6). To enhance the visualization of GFP-PH/PLCδ in adipocytes, we imaged through the cell center and at the base. In vector- and HA-72-D480N5ptase-containing 3T3-L1 adipocytes, GFP-PH/PLCδ was expressed exclusively at the PM (Fig. 4A). At the cell base, which reflects the PM adherent to the substratum, GFP-PH/PLCδ was accentuated in tubulo-vesicle-like structures as described previously (9, 46). Adipocytes overexpressing HA-72-5ptase showed faint cytosolic and nuclear GFP-PH/PLCδ staining. At 46 kDa, the GFP-PH/PLCδ fusion protein can passively diffuse between the cytosol and nucleus, if not bound to PM PtdIns(4,5)P2 (6). The GFP-PH/PLCδ fluorescence intensity at the cell base was decreased upon expression of HA-72-5ptase, indicating decreased PM PtdIns(4,5)P2. To quantitate the PtdIns(4,5)P2 plasma membrane levels, the ratio of GFP-PH/PLCδ plasma membrane/cytosol fluorescence was examined in individual cells as described previously (45) (Fig. 4B). By this analysis, a >40% reduction in plasma membrane GFP-PH/PLCδ fluorescence was noted relative to the cytosolic fluorescence, in cells expressing the 72-5ptase consistent with the contention that ectopic expression of the 72-5ptase results in PtdIns(4,5)P2 hydrolysis in intact adipocytes.
FIG. 4.
The 72-kDa 5-phosphatase hydrolyzes PtdIns(4,5)P2 in adipocytes. (A) Localization of GFP-PH/PLCδ in the middle or at the base of 3T3-L1 adipocytes cotransfected with the indicated constructs. Representative images are shown. Scale bar, 10 μm. (B) The ratio of the fluorescence intensity of GFP-PH/PLCδ at the PM (average fluorescence intensity of three defined boxes of 5 × 10 pixels of the plasma membrane per cell) to that of the cytosol (average fluorescence intensity of three boxes of a defined area of 10 × 10 pixels per cell) was determined by using Image J software. Bars (empty vector, white bars; wild-type 72-5ptase, black bars; inactive 72-ptase, gray bars) represent mean ± the SEM of two independent experiments (>20 cells scored for each construct for each time point). *, P < 0.01 compared to cells expressing HA-vector or HA-72-D480N5ptase.
The formation of PtdIns(3)P at the PM correlates with insulin-independent GLUT4 PM trafficking.
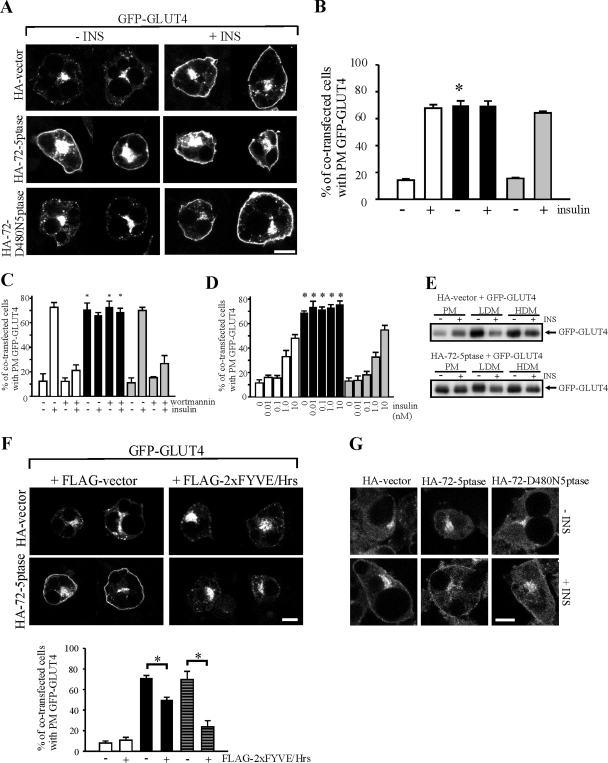
We investigated whether overexpression of the 72-5ptase promoted GLUT4 trafficking in the absence of insulin stimulation, given the active 5-phosphatase generated PM PtdIns(3)P. Differentiated 3T3-L1 adipocytes were cotransfected with GFP-GLUT4 and either HA-72-5ptase, HA-72-D480N-5ptase, or HA-empty vector and the percentage of adipocytes showing GFP-GLUT4 PM rim fluorescence was determined. In the absence of insulin stimulation, 68% of 72-5ptase-expressing adipocytes displayed GFP-GLUT4 PM rim fluorescence compared to 15% of empty vector-transfected cells (Fig. 5A and B). In contrast, HA-72-D480N5ptase did not promote GFP-GLUT4 translocation in unstimulated cells. After insulin treatment (100 nM, 30 min), 64 to 70% of HA-72-5ptase, HA-72-D480N5ptase, or vector-containing adipocytes demonstrated PM GFP-GLUT4 (Fig. 5B). The effect of HA-72-5ptase on GLUT4 translocation was equivalent whether the cells were examined 24 or 48 h after transfection (not shown).
FIG. 5.
The 72-5ptase promotes insulin-independent GLUT4 translocation. (A) 3T3-L1 adipocytes expressing GFP-GLUT4 and vector, HA-72-5ptase, or HA-72-D480N5ptase were serum starved or stimulated with 100 nM insulin (30 min). Fixed cells were labeled with HA antibody (not shown). GFP-GLUT4 localization is shown. Scale bar, 10 μm. (B) For each experiment 100 cells per transfection were scored for GFP-GLUT4 PM rim fluorescence. Bars (empty vector, white bars; wild-type 72-5ptase, black bars; inactive 72-ptase, gray bars) represent mean percentage ± the SEM from four experiments. *, P < 0.05 compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase. (C) After serum starvation, adipocytes coexpressing GFP-GLUT4 and HA-vector (white bars), HA-72-5ptase (black bars), or HA-72-D480N5ptase (gray bars) were incubated with (+) or without (−) 100 nM wortmannin (45 min) prior to insulin stimulation (100 nM, 30 min). GFP-GLUT4 rim fluorescence was scored for 100 cells per transfection. Mean values ± the SEM from four independent experiments are shown. *, P < 0.05, compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase. (D) Adipocytes expressing GFP-GLUT4 and either HA-vector (white bars), HA-72-5ptase (black bars), or HA-72-D480N5ptase (gray bars) were serum starved and treated with 0 to 10 nM insulin (30 min). Mean percentage values of GFP-GLUT4 rim fluorescence ± the SEM were determined for four independent experiments scoring 100 cells for each transfection. *, P < 0.05, compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase. (E) PM, low-density microsome (LDM), and high-density microsome (HDM) fractions from resting or insulin-stimulated adipocytes cotransfected with GFP-GLUT4 and HA-72-5ptase or HA-vector were immunoblotted with GFP antibody to detect GFP-GLUT4. (F) Unstimulated 3T3-L1 adipocytes coexpressing GFP-GLUT4 and HA-vector or HA-72-5ptase, and FLAG-2xFYVE/Hrs (or empty vector) were labeled with HA- and FLAG-specific antibodies (not shown). GFP-GLUT4 localization is shown. GFP-GLUT4 PM rim fluorescence in transfected adipocytes was scored for 50 cells per transfection. Bars represent mean percentage ± the SEM from at least three independent transfections expressing empty vector (white bars) or wild-type 72-5ptase at high levels (black bars), or cells coexpressing GFP-GLUT4 with moderate levels of HA-72-5ptase (striped gray bars), in the absence (−) or presence (+) of FLAG-2xFYVE/Hrs. *, P < 0.05 compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase. (G) Serum-starved adipocytes transfected with HA-vector, HA-72-5ptase, or HA-72-D480N5ptase were left untreated or stimulated with 100 nM insulin (30 min) and then labeled with polyclonal GLUT1-specific antibody. Representative images shown from two separate experiments. Scale bar, 10 μm.
Insulin-stimulated class IA PI3-kinase-mediated generation of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 at the PM and the constitutive synthesis of PtdIns(3)P by Vps34 on early endosomes is sensitive to the PI3-kinase inhibitor wortmannin (5, 62). However, wortmannin at these doses does not inhibit the insulin-stimulated PM synthesis of PtdIns(3)P (41). Interestingly, class II PI3-kinase C2α activity is stimulated by insulin but is wortmannin insensitive (10, 14, 70, 76). This latter PI3-kinase synthesizes both PtdIns(3)P and PtdIns(3,4)P2, and the PtdIns(3)P pool generated by this kinase may serve as a substrate for PIKfyve to generate PtdIns(3,5)P2. To determine whether 72-5ptase-stimulated PtdIns(3)P production, and thereby GLUT4 translocation, was in the same pathway as insulin-stimulated PtdIns(3)P production, adipocytes were treated with wortmannin, which inhibited insulin-stimulated GLUT4 translocation in cells expressing empty vector or inactive 72-D480N5ptase (Fig. 5C) but had no effect on GLUT4 translocation in cells expressing 72-5ptase under resting or insulin-stimulated conditions.
We also characterized GLUT4 translocation under conditions of low-dose insulin stimulation, revealing an insulin dose-dependent increase in GFP-GLUT4 PM rim fluorescence in empty vector- or inactive HA-72-D480N5ptase-expressing adipocytes. In contrast, upon 72-5ptase overexpression, >60% of cells showed PM GFP-GLUT4 in both unstimulated cells and at all of the insulin concentrations tested (Fig. 5D). To further verify that the 72-5ptase promotes insulin-independent GLUT4 translocation, we analyzed the distribution of GFP-GLUT4 in adipocyte subcellular fractions. In unstimulated cells coexpressing GFP-GLUT4 and HA-vector, GFP-GLUT4 was detected faintly in the PM fraction and intensely in the low-density microsome fraction (Fig. 5E). After insulin treatment the level of GFP-GLUT4 was increased in the PM fraction and reduced in the LDM fraction with no change in the high-density microsome fraction. However, in HA-72-5ptase-expressing adipocytes, GFP-GLUT4 was detected in equal amounts in the PM fraction of unstimulated and insulin-stimulated cells, a finding consistent with the contention that the 72-5ptase promotes insulin-independent GLUT4 translocation. The effect of the 72-5ptase on GLUT4 translocation in the nonstimulated adipocyte was not a general property of all 5-ptases. Expression of the Lowe's protein (OCRL), a PtdIns(4,5)P2 specific 5-ptase (82); SKIP, which hydrolyzes PtdIns(4,5)P2 and PtdIns(3,4,5)P3 (25); or SHIP-2, which hydrolyzes PtdIns(3,4,5)P3 (48) did not promote GLUT4 trafficking in nonstimulated adipocytes (not shown). After insulin stimulation, both SKIP and SHIP-2 inhibited insulin-stimulated GLUT4 translocation, as previously reported (26, 78), whereas the expression of the Lowe's protein had no effect on insulin-stimulated GLUT4 translocation (not shown).
To determine whether the 72-5ptase-induced GLUT4 translocation was a consequence of PtdIns(3)P production, we coexpressed FLAG-2xFYVE/Hrs at high levels with GFP-GLUT4 and analyzed cotransfected cells for GLUT4 translocation in the absence of insulin stimulation. Overexpression of 2xFYVE/Hrs at high levels binds and sequesters PtdIns(3)P (21). Cells were scored for high expression of FLAG-2xFYVE/Hrs and/or 72-5ptase. When both 2xFYVE/Hrs and the 72-5ptase constructs were expressed at high levels, a 40% reduction in the number of cells demonstrating GLUT4 translocation to the PM was noted, relative to cells expressing the 72-5ptase alone. In addition, at moderate levels expression of the 72-5ptase, a >80% reduction in the number of adipocytes demonstrating GLUT4 PM translocation in unstimulated cells was detected upon coexpression of 2xFYVE/Hrs (Fig. 5F). Moderate expression of the 72-5ptase alone promoted GLUT4 translocation in the majority of adipocytes, similar to high-level overexpression. In control studies the overexpression of GFP-PH/PLCδ had no effect (see below). These studies suggest that the overexpression of the 72-5ptase induces GLUT4 trafficking via PM PtdIns(3)P production.
The specificity of the effects of the 72-5ptase on GLUT4 trafficking were determined by analysis of GLUT1 trafficking. Both endogenous and transfected HA-GLUT1 (not shown) demonstrated similar localization in empty-vector-, wild-type 5ptase-, or inactive-5ptase-expressing cells, both in unstimulated and insulin-stimulated adipocytes (Fig. 5G).
Overexpression of the 72-5ptase promotes insertion of GLUT4 into the PM.
To examine the effects of 72-5ptase expression on endogenous GLUT4 distribution, the localization of GLUT4-containing vesicles in contact with the PM was determined by indirect immunofluorescence on isolated PM sheets. Recently, it has been proposed that the C-terminal antigenicity of endogenous or recombinant GLUT4 may be unmasked by insulin treatment, by removing bound proteins (27). These studies demonstrated that the delivery of exogenous PtdIns(3,4,5)P3 failed to be associated with a significant gain in C-terminal GLUT4 antibody immunoreactivity, while delivery of exogenous PtdIns(3)P results in detection of the C terminus of GLUT4 in isolated membrane sheets. To investigate whether in unstimulated adipocytes expression of the 72-5ptase resulted in the unmasking of the C-terminal epitope of GLUT4 at the PM, adipocytes were cotransfected with wild-type 72-5ptase and FLAG-Ras (a PM marker), and isolated PM sheets were examined for immunoreactivity for both FLAG and endogenous GLUT4. The GLUT4 antibody utilized for these studies recognizes only the C-terminal domain of the protein (amino acids 498 to 510) and therefore will only detect endogenous GLUT4 which has arrived but is not necessarily inserted into the PM. Only 5 to 8% of HA-vector- or inactive HA-72-D480N5ptase-transfected quiescent adipocytes (Fig. 6A and B) demonstrated GLUT4 immunoreactivity in isolated PM sheets, in contrast to 25% of PM sheets expressing HA-72-5ptase. Upon insulin stimulation, 25 to 30% of the PM sheets from vector- or inactive HA-72-D480N5ptase-expressing adipocytes demonstrated GLUT4 immunoreactivity (Fig. 6A and B).
FIG. 6.
The 72-5ptase promotes insertion of GLUT4 into the PM. (A) Plasma membrane sheets, prepared from serum starved or insulin-stimulated adipocytes cotransfected with the indicated constructs, were analyzed by indirect immunofluorescence using FLAG or GLUT4 antibodies. Scale bar, 10 μm. (B) The percentage of Ras-positive sheets demonstrating GLUT4 staining was determined, and the mean ± the SEM of four independent transfections scoring 100 sheets are shown (empty vector, white bars; wild-type 72-5ptase, black bars; inactive 72-5ptase, gray bars). *, P < 0.05 compared to same conditions for cells coexpressing empty vector or HA-72-D480N5ptase. (C) At 48 h posttransfection, cells were serum starved, before stimulation with 100 nM insulin (30 min). Fixed and unpermeabilized adipocytes expressing exofacial Myc-GLUT4-GFP and the indicated constructs were analyzed by indirect immunofluorescence using monoclonal Myc antibody. Scale bar, 10 μm. (D) The percentage of GFP-expressing cells displaying Myc PM staining ± the SEM, from three independent transfections, was determined for each construct, scoring 100 cells per transfection. *, P < 0.05 compared to same conditions for cells expressing empty vector or HA-72-D480N5ptase. (E) Lysates of adipocytes transduced with the indicated vector as for 2[3H]deoxyglucose uptake assays, were immunoblotted with GFP or HA antibodies. Indirect immunofluorescence demonstrated >60% of cells exposed to adenovirus were transduced with the desired construct (not shown). (F) Adipocytes transduced with vector containing GFP reporter alone (white bars), GFP+HA-72-5ptase (black bars), or GFP+HA-72-D480N5ptase (gray bars) were assayed for 2[3H]deoxyglucose uptake. Bars represent mean disintegrations per minute (dpm) ± the SEM calculated for each condition from four independent infections.
Recent studies in skeletal muscle cells have revealed that the addition of exogenous carrier-tagged PtdIns(3,4,5)P3, but not carrier-labeled PtdIns(3)P, promotes cell surface exposure of the N-terminal domain of GLUT4 on the extracellular face of the cell membrane, indicating that PtdIns(3)P does not promote GLUT4 membrane insertion (27, 71). Exofacial Myc-GLUT4-GFP, which contains a Myc-epitope tag in the large extracellular loop between transmembrane domains 1 and 2, was used to determine the cell surface exposure of GLUT4 in unpermeabilized cells, by Myc antibody indirect immunofluorescence. We noted 50% of unstimulated adipocytes expressing HA-72-5ptase showed Myc rim fluorescence, compared to vector (10%)- or HA-72-D480N5ptase (15%)-expressing cells (Fig. 6C and D). All insulin-stimulated cells showed Myc rim fluorescence to a similar extent, regardless of the construct expressed. The percentage of unstimulated cells exhibiting GLUT4 PM insertion after transfection of HA-72-5ptase, as indicated by Myc staining of unpermeabilized cells was less than the percentage of cells showing GFP-GLUT4 PM translocation. However, only 60 to 70% of Myc-GLUT4-GFP-expressing cells were cotransfected with HA-72-5ptase. After this was taken into account, the stimulatory effect of the 72-5ptase on Myc-GLUT4 insertion into the PM was found to be comparable to GLUT4 translocation. Therefore, expression of the 72-5ptase promotes PtdIns(3)P PM accumulation, correlating with GLUT4 translocation, unmasking of its C-terminal domain and insertion into the PM in unstimulated adipocytes.
To exclude the possibility that the hydrolysis of PtdIns(4,5)P2 by the 72-5ptase may contribute to GLUT4 translocation and/or insertion, we undertook two distinct approaches. First, we coexpressed exofacially tagged Myc-GLUT4-GFP with the wild-type 5-ptase and added exogenous carrier-labeled PtdIns(4,5)P2 as described previously (41). However, this did not inhibit or enhance 72-5ptase-stimulated GLUT4 translocation and/or insertion (results not shown). In addition, in an alternative approach, we coexpressed PH/PLCδ at high levels as described previously (24) to sequester PtdIns(4,5)P2. In cells overexpressing the wild-type 72-5ptase, however, this did not affect 72-5ptase-stimulated GLUT4 insertion into the plasma membrane, although as reported, evidence of PtdIns(4,5)P2 sequestration was shown by the inhibition of endocytosis (not shown). Collectively, this analysis is consistent with the contention that the formation of endogenous PtdIns(3)P at the plasma membrane contributes to both GLUT4 translocation and insertion.
Since 72-5ptase overexpression promoted the translocation, tethering, and PM insertion of GLUT4, we determined its effect on 2-deoxyglucose uptake in adipocytes transduced with adenovirus expressing this enzyme (Fig. 6E and F). In unstimulated cells expressing 72-5ptase, the 2-deoxyglucose uptake was similar to vector-expressing cells. After insulin stimulation, glucose uptake was equally stimulated (four- to fivefold) in cells expressing vector, 72-5ptase, or inactive 72-D480N5ptase (Fig. 6F), revealing that, despite evidence that the 72-5ptase promotes GLUT4 translocation and insertion into the PM, glucose uptake was not increased. These results are consistent with other reports that suggest PtdIns(3)P alone cannot stimulate glucose uptake (41).
It has been proposed that insulin-stimulated PtdIns(3)P production at the PM, and thereby GLUT4 translocation, occurs downstream of TC10 activation (41). To investigate whether 72-5ptase-mediated production of PtdIns(3)P and/or GLUT4 translocation to the PM is dependent on TC10 activation, adipocytes were cotransfected with 72-5ptase and either constitutively active (Q75L) or inactive (T31N) TC10. However, expression of either active or inactive TC10 had no effect on 72-5ptase-stimulated GLUT4 recruitment to the PM or PtdIns(3)P production, indicating the enzyme acts independent of TC10 (not shown).
PtdIns(4,5)P2, PtdIns(3,4,5)P3, and TC10-dependent signaling pathways contribute to actin dynamics in adipocytes (56, 80). The role of plasma membrane PtdIns(3)P in the regulation of actin dynamics is unclear but in other cells, it may increase actin polymerization via recruitment of CKIP-1, although this has not been demonstrated in adipocytes (55). To determine the effect of 72-5ptase on actin rearrangement in adipocytes, cells were stained with phalloidin and cortical actin (imaging through cell center), and actin stress fibers (imaging at the cell base) were assessed (Fig. 7A). At the cell base, small patches of punctate actin were detected, which represented the adipocyte counterpart of stress fibers (32). Imaging through the center identified subcortical actin as a thin rim around the cell. In cells expressing HA-72-5ptase, punctate actin staining at the cell base was markedly reduced, suggesting decreased actin polymerization, most evident in cells in which 72-5ptase-expressing cells localized adjacent to nontransfected cells (Fig. 7A, see arrow). In 72-5ptase-expressing cells the decreased actin polymerization observed in adipocytes overexpressing the 5-ptase is most likely a consequence of decreased PtdIns(4,5)P2 and/or PtdIns(3,5)P2, rather than due to plasma membrane PtdIns(3)P which has been predicted in one study to bind and recruit CKIP-1 which promotes actin polymerization, although this has not been demonstrated in adipocytes (55). To investigate whether 72-5ptase-stimulated GLUT4 PM translocation was dependent on actin, adipocytes were treated with either cytochalasin D, which depolymerizes actin, or latrunculin A, which binds actin monomers (32, 44). Cytochalasin D inhibited insulin-stimulated GFP-GLUT4 PM rim fluorescence from 78 to 48% in vector-expressing cells (Fig. 7B) and decreased cortical actin staining (not shown). Similar treatment inhibited GFP-GLUT4 PM trafficking in 72-5ptase-expressing cells, although to a lesser extent (65 to 55%). Latrunculin A disrupts actin filaments in adipocytes more effectively than cytochalasin D, correlating with the inhibition of GLUT4 translocation and glucose transport (44). Latrunculin A treatment significantly inhibited unstimulated and insulin-stimulated GFP-GLUT4 PM rim fluorescence (70 to 28%) in cells expressing the 72-5ptase. These studies reveal that 72-5ptase-stimulated GLUT4 translocation is facilitated by the regulation of actin dynamics.
FIG. 7.
Sequestration of actin monomers decreases 72-5ptase-mediated GLUT4 trafficking. (A) 3T3-L1 adipocytes were transfected with the indicated constructs and labeled with Texas Red-phalloidin. Representative images of single optical sections through the center or base of transfected cells are shown. Scale bar, 10 μm. (B) Serum-starved adipocytes transfected with GFP-GLUT4 and HA-empty vector (white bars), or wild-type HA-72-5ptase (black bars), were left untreated or pretreated with 50 μM cytochalasin D, or 25 μg of latrunculin A/ml prior to stimulation with insulin. Cotransfected cells were scored for GFP-GLUT4 plasma membrane fluorescence, with 100 cells examined per transfection. Mean percentage values ± the SEM from three independent experiments are shown. *, P < 0.05 compared to nonstimulated untreated cells expressing wild-type 72-5ptase.
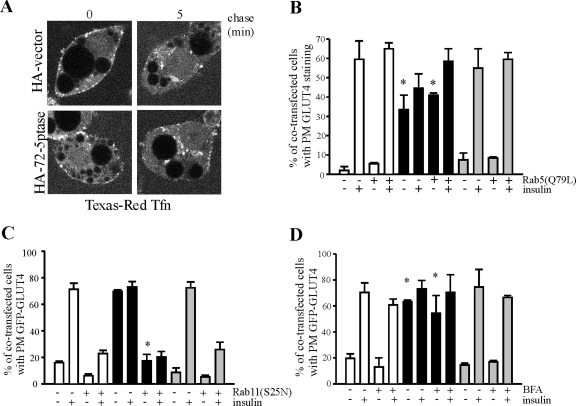
GLUT4 continuously recycles between the PM and its intracellular stores, and in the steady state the majority of GLUT4 is sequestered in intracellular vesicles. Insulin stimulates exocytosis and PM GLUT4 accumulation (11, 79). GLUT4 is internalized via clathrin- and caveola-dependent mechanisms (2, 33, 54). In nonstimulated cells PtdIns(3)P is localized to early endosomes and regulates endosomal morphology, endosomal membrane transport, and multivesicular endosome biogenesis (5, 68). The functional role of PM PtdIns(3)P in endocytic trafficking is unknown. To address this issue, we investigated whether 72-5ptase-generated PtdIns(3)P influenced endocytic trafficking, in addition to GLUT4 trafficking, and whether various key effectors in endocytic trafficking could inhibit 72-5ptase-induced GLUT4 PM translocation. To this end, we examined the effect of 72-5ptase expression on clathrin-mediated endocytosis of Texas Red-labeled transferrin (63, 83). Similar uptake of Texas Red-transferrin was observed in vector- and HA-72-5ptase-transfected adipocytes, with the majority of transferrin-containing endosomes detected at perinuclear sites 5 min after its internalization (Fig. 8A). Rab5 regulates clathrin-coated vesicle formation, endosome motility, and early endosome fusion (69). Overexpression of the GTPase-deficient Rab5 mutant, Rab5Q79L, causes enlargement of early endosomes, resulting from enhanced homo- and heterotypic fusion (7). Recently, it has been demonstrated that Rab5 regulates the production of PtdIns(3)P on early endosomes by the actions of both the PI3-kinase Vps34 and by a sequential growth factor-stimulated enzymatic cascade initiated by the PI3-kinaseβ (PI3Kβ) and in turn the actions of PtdIns(3,4,5)P3 5- and PtdIns(3,4)P2 4-phosphatases (64). However, coexpression of Rab5Q79L had no effect on GLUT4 trafficking in empty vector or 72-5ptase-expressing cells (Fig. 8B). The small GTPase Rab11 localizes to recycling endosomes, trans-Golgi networkand the PM, and regulates the passage of recycling proteins through these compartments (60). Rab11 is required for GLUT4 transport from endosomes to GLUT4 storage vesicles and for insulin-induced GLUT4 translocation to the cell surface (81). In addition, Rab11 complexes with GLUT4 and regulates endocytosis and GLUT4 trafficking in both skeletal and cardiac muscle (40, 75). A dominant-negative Rab11 mutant, Rab11 (S25N), which constitutively binds GDP, and blocks endosomal trafficking (53) was coexpressed with GFP-GLUT4 and HA-72-5ptase (Fig. 8C). Significantly, coexpression of Xpress-Rab11(S25N) with HA-72-5ptase inhibited GFP-GLUT4 translocation in both unstimulated and insulin-stimulated adipocytes.
FIG. 8.
Rab11 regulates 72-5ptase-mediated GLUT4 trafficking. (A) Serum-starved adipocytes transfected with HA-vector or HA-72-5ptase were incubated with Texas Red-transferrin (20 μg/ml) for 1 h at 16°C to facilitate uptake and accumulation in early endosomes. Chase was initiated by shift to 37°C for 5 min in the presence of holotransferrin (200 μg/ml). Scale bar, 10 μm. (B) Adipocytes coexpressing GFP-Rab5(Q79L) and HA-vector (white bars), HA-72-5ptase (black bars), or HA-D480N5ptase (gray bars) were serum starved (2 h) prior to insulin stimulation (100 nM, 30 min). Fixed cells were labeled with GLUT4-specific antibody and GFP-positive cells were scored for positive PM GLUT4 fluorescence. HA staining was performed separately to confirm transfection efficiency. *, P < 0.05 compared to the same conditions for cells expressing HA-vector or HA-72-D480N5ptase. (C) Adipocytes coexpressing Xpress-tagged Rab11(S25N) and GFP-GLUT4, and HA-vector (white bars), HA-72-5ptase (black bars), or HA-D480N5ptase (gray bars) were serum starved prior to insulin stimulation (100 nM, 30 min). For panels B and C, bars represent the mean percentages of cotransfected cells displaying PM GFP-GLUT4 or positive GLUT4 labeling ± the SEM from three independent transfections for each construct, in which 100 cells were scored are shown. *, P < 0.05 compared to unstimulated HA-72-5ptase-transfected cells not coexpressing Rab11(S25N). (D) Serum-starved adipocytes expressing GFP-GLUT4 and HA-vector (white bars), HA-72-5ptase (black bars), or HA-72-D480N5ptase (gray bars) were treated with brefeldin A (BFA) (10 mg/ml) prior to insulin stimulation. Cotransfected cells were scored for GFP-GLUT4 fluorescence with 100 cells examined per transfection. Mean percentages ± the SEM from three independent experiments are shown. *, P < 0.05 compared to same conditions for cells expressing HA-vector or HA-72-D480N5ptase.
We also determined whether the 72-5ptase stimulates GLUT4 exit from the Golgi complex and trafficking to the cell surface. To disrupt the Golgi complex, adipocytes were treated with brefeldin A, which leads to collapse of the Golgi complex via the inhibition of ARF-mediated COPI coat assembly (15), but this treatment did not affect basal or insulin-stimulated GLUT4 translocation in adipocytes expressing empty vector, HA-72-5ptase, or HA-72-D480N5ptase (Fig. 8D). In control studies brefeldin A-induced Golgi collapse was confirmed by labeling cells with β-COP antibodies (results not shown). Finally, expression of the 72-5ptase had no effect on constitutive exocytic trafficking from the Golgi complex, as shown by unaltered trafficking of GFP-VSV-G (50) (results not shown) in nonstimulated or insulin-stimulated adipocytes. Therefore, 72-5ptase-generated PtdIns(3)P specifically regulated GLUT4 trafficking rather than having a global effect on endocytic membrane trafficking.
DISCUSSION
The results of this study have revealed several significant findings with respect to the role of 3-position phosphoinositides and GLUT4 trafficking. First, although carrier-linked exogenous delivery of PtdIns(3)P promotes GLUT4 translocation to the PM, we have shown here the generation of endogenous PM PtdIns(3)P also promotes the arrival and insertion of GLUT4 at the PM, independent of PtdIns(3,4,5)P3 and independent of insulin stimulation. Second, our data provide evidence that an inositol polyphosphate 5-phosphatase may produce a functional pool of PtdIns(3)P at the PM.
Insulin stimulation results in PtdIns(3)P production at the PM. Evidence to support the contention that this phosphoinositide promotes GLUT4 trafficking has been shown by the temporal correlation of its insulin-stimulated accumulation at the PM with GLUT4 trafficking and with studies in which synthetic carrier-labeled PtdIns(3)P delivery into nonstimulated adipocytes promotes GLUT4 translocation (27, 41). However, it has been proposed that PtdIns(3)P, unlike PtdIns(3,4,5)P3, does not promote GLUT4 insertion; rather, it mediates the C-terminal unmasking of the GLUT4 at the PM (27). The results from the studies shown here demonstrate endogenous PtdIns(3)P production at the PM, in the absence of insulin stimulation, can promote both the translocation and the insertion of GLUT4 at the PM. Insertion of GLUT4 was demonstrated using exofacially tagged GLUT4 constructs, analogous to the recently reported studies using exogenously added carrier-linked PtdIns(3)P (27), which did not promote GLUT4 insertion. The reason for these apparently discrepant results may relate to the failure of correct targeting of exogenous lipids to the PM and/or that the turnover of the exogenous lipids may differ from that of the endogenous phosphoinositides. In addition, the levels of the exogenously added carrier-linked lipids such as PtdIns(3)P may not reach that of the endogenous lipids at the PM. In this regard, it is noteworthy that we demonstrated that the levels of PtdIns(3)P at the PM of 72-5ptase-expressing cells were significantly higher than those detected in empty vector-transfected insulin-stimulated cells. Interestingly, a recent model by Klip and coworkers proposed that remodeled actin sites may function to position GLUT4 near fusion sites on the PM (16, 47). In this regard it is of interest that we noted in 72-5ptase expressing cells significantly decreased polymerized actin. Furthermore, latrunculin A treatment inhibited 72-5ptase induced GLUT4 translocation. Therefore, it is possible that plasma membrane PtdIns(3)P, in the context of decreased submembraneous actin, facilitates GLUT4 translocation and insertion. Recently, it has been demonstrated that exogenous PtdIns(3)P can induce GLUT4 PM insertion in Munc18c−/− adipocytes. This study proposes that perhaps insulin-induced synthesis of PtdIns(3)P may be sufficient to induce GLUT4 translocation and insertion into the PM (30). Collectively, these studies imply that plasma membrane PtdIns(3)P may have the capacity to promote GLUT4 insertion under specific cellular contexts in nonstimulated cells, in which the normal cellular constraints which inhibit GLUT4 insertion, such as submembraneous actin and/or Munc18 are absent.
Enhanced glucose uptake was not exhibited in 72-5ptase-expressing cells, despite evidence of GLUT4 translocation, tethering, and insertion into the PM. These results are consistent with other studies indicating that glucose uptake requires both localization and increased intrinsic activity of GLUT4, which involves the activation of signaling pathways in addition to those generated by PtdIns(3)P and/or PtdIns(3,4,5)P3 (20, 41, 71).
An emerging role for PtdIns(3,4,5)P3, PtdIns(3,5)P2, and PtdIns(3)P in the regulation of insulin-stimulated GLUT4 translocation is established (8, 41, 66). However, to our knowledge the studies described here are the first to report insulin-independent endogenous PtdIns(3)P generation at the PM. We propose the effect of the 72-5ptase expression on GLUT4 trafficking in the unstimulated adipocytes was a consequence of the production of PtdIns(3)P at the PM, in the absence of PtdIns(3,4,5)P3. The following experimental findings supports this contention. First, we could detect no GFP-PH/ARNO, a PtdIns(3,4,5)P3-specific biosensor, at the PM of unstimulated cells and no evidence of phosphorylation of Akt at Ser473, indicating that in the unstimulated cell there was no activation of the class I PI3-kinase. Second, wortmannin treatment, which blocks class I PI 3-kinase activation and thereby PtdIns(3,4,5)P3 synthesis, did not inhibit either 72-5ptase-induced PM PtdIns(3)P accumulation or GLUT4 translocation. In unstimulated cells, overexpression of the wild type but not inactive 72-5ptase resulted in PM PtdIns(3)P accumulation, which correlated with GLUT4 translocation. Upon sequestration of PtdIns(3)P by overexpression of 2xFYVE/Hrs, 72-5ptase-stimulated GLUT4 translocation in unstimulated cells was significantly attenuated, a finding consistent with the contention that this was the molecular mechanism by which the 72-5ptase induced GLUT4 trafficking.
We propose that 72-5ptase hydrolysis of PtdIns(3,5)P2 produces PtdIns(3)P, since the 5-phosphatase hydrolyzes this phosphoinositide in vitro, forming PtdIns(3)P (38), and in intact cells overexpressing the 72-5ptase, PtdIns(3)P was generated at the PM, as shown by the recruitment of two distinct PtdIns(3)P biosensors to this site. Recent studies by our laboratory and others have highlighted that the lipid phosphatases may function as signal-terminating enzymes but can also generate functional pools of phosphoinositides at distinct subcellular sites (28, 64). PtdIns(3)P generation at the plasma membrane in cells overexpressing the 72-5ptase is most likely derived from 5-position phosphate dephosphorylation of PtdIns(3,5)P2 and/or PtdIns(3,4,5)P3 since 5-phosphatases exclusively dephosphorylate only the 5-position phosphate and hydrolysis of PtdIns(4,5)P2 would produce PtdIns(4)P (42, 74). PtdIns(3,4,5)P3, generated in response to agonist stimulation can be sequentially dephosphorylated by 5- and 4-phosphatases to form PtdIns(3)P, which is functional in the Rab5-regulated endocytic pathway (64). However, as shown here, PtdIns(3,4,5)P3 was not detected in unstimulated cells; therefore, PtdIns(3)P generated at the PM by the 72-5ptase must by exclusion be derived from the hydrolysis of PtdIns(3,5)P2.
Previous studies have not revealed the identity of the PI3-kinase responsible for insulin-stimulated PM PtdIns(3)P production. However, this is a wortmannin-resistant activity. Class II PI3-kinase enzyme activity is wortmannin resistant and is stimulated by insulin, and its principal substrate in vitro is PtdIns, generating PtdIns(3)P (10, 14). It is possible that this class II generated pool of PtdIns(3)P may be further phosphorylated by PIKfyve to generate PtdIns(3,5)P2, although this has yet to be shown. Interestingly, in unstimulated adipocytes significant levels of PtdIns(3,5)P2 can be detected (58, 65).
Although the PtdIns(3)P effectors on early endosomes have been identified, the effectors that are recruited and/or activated by PM-localized PtdIns(3)P in adipocytes remain to be identified. Interestingly, we found no evidence that 72-5ptase-generated PtdIns(3)P contributes to transferrin receptor endocytosis. In addition, GLUT4 trafficking to the PM in cells overexpressing the 72-5ptase was not regulated by expression of Rab5 mutants but was inhibited after the coexpression of dominant-negative Rab11. Recently, both Rab11 and Rab14 have been shown to be constitutively associated with GLUT4 vesicles (40). The precise physiological role of Rab11 in regulating GLUT4 trafficking remains to be fully delineated; however, Rab11 may promote GLUT4 trafficking from the Golgi/TGN to the PM, and/or targeting of GLUT4 into specialized endosomal compartments.
We have demonstrated here that overexpression of the 72-5ptase promotes GLUT4 translocation and insertion into the PM; however, we have no evidence yet that this 5-phosphatase is functionally significant in the insulin-stimulated GLUT4 translocation pathway in adipocytes. Rather, we have used the overexpression of this 5-phosphatase as an experimental tool to dissect the function of PtdIns(3)P at the PM in GLUT4 trafficking. In summary, we demonstrate here that the generation of PtdIns(3)P at the PM correlates with GLUT4 translocation and insertion into the plasma membrane.
Acknowledgments
This research was supported by grants from the NH and MRC of Australia.
We thank Cynthia Ng for outstanding technical assistance and David James, Jenny Stow, and Jennifer Dyson for advice and helpful discussions. We thank Judy Callaghan for 2xFYVE/Hrs cDNA, Xiang-Ming Zhang for the Rab11 plasmid, and Phil Majerus for the OCRL cDNA. GFP-VSV-G plasmid was a gift from Jennifer Lippincott-Schwartz. Imaging was performed at Monash Micro Imaging Facility.
REFERENCES
- 1.Abramoff, M., P. Magelhaes, and S. Ram. 2004. Image processing with Image J. Biophotonics Int. 11:36-42. [Google Scholar]
- 2.Al-Hasani, H., C. S. Hinck, and S. W. Cushman. 1998. Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J. Biol. Chem. 273:17504-17510. [DOI] [PubMed] [Google Scholar]
- 3.Asano, T., A. Kanda, H. Katagiri, M. Nawano, T. Ogihara, K. Inukai, M. Anai, Y. Fukushima, Y. Yazaki, M. Kikuchi, R. Hooshmand-Rad, C. H. Heldin, Y. Oka, and M. Funaki. 2000. p110beta is up-regulated during differentiation of 3T3-L1 cells and contributes to the highly insulin-responsive glucose transport activity. J. Biol. Chem. 275:17671-17676. [DOI] [PubMed] [Google Scholar]
- 4.Asano, T., Y. Mochizuki, K. Matsumoto, T. Takenawa, and T. Endo. 1999. Pharbin, a novel inositol polyphosphate 5-phosphatase, induces dendritic appearances in fibroblasts. Biochem. Biophys. Res. Commun. 261:188-195. [DOI] [PubMed] [Google Scholar]
- 5.Backer, J. M. 2000. Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol. Cell. Biol. Res. Commun. 3:193-204. [DOI] [PubMed] [Google Scholar]
- 6.Balla, T., T. Bondeva, and P. Varnai. 2000. How accurately can we image inositol lipids in living cells? Trends Pharmacol. Sci. 21:238-241. [DOI] [PubMed] [Google Scholar]
- 7.Barbieri, M. A., G. Li, L. S. Mayorga, and P. D. Stahl. 1996. Characterization of Rab5:Q79L-stimulated endosome fusion. Arch. Biochem. Biophys. 326:64-72. [DOI] [PubMed] [Google Scholar]
- 8.Berwick, D. C., G. C. Dell, G. I. Welsh, K. J. Heesom, I. Hers, L. M. Fletcher, F. T. Cooke, and J. M. Tavare. 2004. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J. Cell Sci. 117:5985-5993. [DOI] [PubMed] [Google Scholar]
- 9.Brown, F. D., A. L. Rozelle, H. L. Yin, T. Balla, and J. G. Donaldson. 2001. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154:1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, R. A., J. Domin, A. Arcaro, M. D. Waterfield, and P. R. Shepherd. 1999. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J. Biol. Chem. 274:14529-14532. [DOI] [PubMed] [Google Scholar]
- 11.Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 3:267-277. [DOI] [PubMed] [Google Scholar]
- 12.Chi, Y., B. Zhou, W. Q. Wang, S. K. Chung, Y. U. Kwon, Y. H. Ahn, Y. T. Chang, Y. Tsujishita, J. H. Hurley, and Z. Y. Zhang. 2004. Comparative mechanistic and substrate specificity study of inositol polyphosphate 5-phosphatase Schizosaccharomyces pombe Synaptojanin and SHIP2. J. Biol. Chem. 279:44987-44995. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, J. F., P. W. Young, K. Yonezawa, M. Kasuga, and G. D. Holman. 1994. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem. J. 300:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domin, J., F. Pages, S. Volinia, S. E. Rittenhouse, M. J. Zvelebil, R. C. Stein, and M. D. Waterfield. 1997. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 326:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson, J. G., D. Finazzi, and R. D. Klausner. 1992. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature 360:350-352. [DOI] [PubMed] [Google Scholar]
- 16.Dugani, C. B., and A. Klip. 2005. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 6:1137-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyson, J. M., A. M. Kong, F. Wiradjaja, M. V. Astle, R. Gurung, and C. A. Mitchell. 2005. The SH2 domain containing inositol polyphosphate 5-phosphatase-2: SHIP2. Int. J. Biochem. Cell Biol. 37:2260-2265. [DOI] [PubMed] [Google Scholar]
- 18.Dyson, J. M., C. J. O'Malley, J. Becanovic, A. D. Munday, M. C. Berndt, I. D. Coghill, H. H. Nandurkar, L. M. Ooms, and C. A. Mitchell. 2001. The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin. J. Cell Biol. 155:1065-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frevert, E. U., and B. B. Kahn. 1997. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol. Cell. Biol. 17:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funaki, M., P. Randhawa, and P. A. Janmey. 2004. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol. Cell. Biol. 24:7567-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillooly, D. J., I. C. Morrow, M. Lindsay, R. Gould, N. J. Bryant, J. M. Gaullier, R. G. Parton, and H. Stenmark. 2000. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19:4577-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausdorff, S. F., D. C. Fingar, K. Morioka, L. A. Garza, E. L. Whiteman, S. A. Summers, and M. J. Birnbaum. 1999. Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes: dissociation of insulin-stimulated glucose uptake and glut4 translocation. J. Biol. Chem. 274:24677-24684. [DOI] [PubMed] [Google Scholar]
- 23.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, S., L. Lifshitz, V. Patki-Kamath, R. Tuft, K. Fogarty, and M. P. Czech. 2004. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol. Cell. Biol. 24:9102-9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ijuin, T., Y. Mochizuki, K. Fukami, M. Funaki, T. Asano, and T. Takenawa. 2000. Identification and characterization of a novel inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275:10870-10875. [DOI] [PubMed] [Google Scholar]
- 26.Ijuin, T., and T. Takenawa. 2003. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol. Cell. Biol. 23:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishiki, M., V. K. Randhawa, V. Poon, L. Jebailey, and A. Klip. 2005. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J. Biol. Chem. 280:28792-28802. [DOI] [PubMed] [Google Scholar]
- 28.Ivetac, I., A. D. Munday, M. V. Kisseleva, X. M. Zhang, S. Luff, T. Tiganis, J. C. Whisstock, T. Rowe, P. W. Majerus, and C. A. Mitchell. 2005. The type Ialpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol. Biol. Cell 16:2218-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanai, F., K. Ito, M. Todaka, H. Hayashi, S. Kamohara, K. Ishii, T. Okada, O. Hazeki, M. Ui, and Y. Ebina. 1993. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem. Biophys. Res. Commun. 195:762-768. [DOI] [PubMed] [Google Scholar]
- 30.Kanda, H., Y. Tamori, H. Shinoda, M. Yoshikawa, M. Sakaue, J. Udagawa, H. Otani, F. Tashiro, J. Miyazaki, and M. Kasuga. 2005. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Investig. 115:291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanzaki, M., M. Furukawa, W. Raab, and J. E. Pessin. 2004. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 279:30622-30633. [DOI] [PubMed] [Google Scholar]
- 32.Kanzaki, M., and J. E. Pessin. 2001. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276:42436-42444. [DOI] [PubMed] [Google Scholar]
- 33.Kao, A. W., B. P. Ceresa, S. R. Santeler, and J. E. Pessin. 1998. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 273:25450-25457. [DOI] [PubMed] [Google Scholar]
- 34.Katagiri, H., T. Asano, K. Inukai, T. Ogihara, H. Ishihara, Y. Shibasaki, T. Murata, J. Terasaki, M. Kikuchi, Y. Yazaki, and Y. Oka. 1997. Roles of PI 3-kinase and Ras on insulin-stimulated glucose transport in 3T3-L1 adipocytes. Am. J. Physiol. 272:E326-E331. [DOI] [PubMed] [Google Scholar]
- 35.Katagiri, H., T. Asano, H. Ishihara, K. Inukai, Y. Shibasaki, M. Kikuchi, Y. Yazaki, and Y. Oka. 1996. Overexpression of catalytic subunit p110alpha of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. J. Biol. Chem. 271:16987-16990. [DOI] [PubMed] [Google Scholar]
- 36.Kisseleva, M. V., L. Cao, and P. W. Majerus. 2002. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 277:6266-6272. [DOI] [PubMed] [Google Scholar]
- 37.Kisseleva, M. V., M. P. Wilson, and P. W. Majerus. 2000. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275:20110-20116. [DOI] [PubMed] [Google Scholar]
- 38.Kong, A. M., C. J. Speed, C. J. O'Malley, M. J. Layton, T. Meehan, K. L. Loveland, S. Cheema, L. M. Ooms, and C. A. Mitchell. 2000. Cloning and characterization of a 72-kDa inositol-polyphosphate 5-phosphatase localized to the Golgi network. J. Biol. Chem. 275:24052-24064. [DOI] [PubMed] [Google Scholar]
- 39.Kotani, K., A. J. Carozzi, H. Sakaue, K. Hara, L. J. Robinson, S. F. Clark, K. Yonezawa, D. E. James, and M. Kasuga. 1995. Requirement for phosphoinositide 3-kinase in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 209:343-348. [DOI] [PubMed] [Google Scholar]
- 40.Larance, M., G. Ramm, J. Stockli, E. M. van Dam, S. Winata, V. Wasinger, F. Simpson, M. Graham, J. R. Junutula, M. Guilhaus, and D. E. James. 2005. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 280:37803-37813. [DOI] [PubMed] [Google Scholar]
- 41.Maffucci, T., A. Brancaccio, E. Piccolo, R. C. Stein, and M. Falasca. 2003. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J. 22:4178-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell, C. A., R. Gurung, A. M. Kong, J. M. Dyson, A. Tan, and L. M. Ooms. 2002. Inositol polyphosphate 5-phosphatases: lipid phosphatases with flair. IUBMB Life 53:25-36. [DOI] [PubMed] [Google Scholar]
- 43.Oatey, P. B., K. Venkateswarlu, A. G. Williams, L. M. Fletcher, E. J. Foulstone, P. J. Cullen, and J. M. Tavare. 1999. Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3-L1 adipocytes. Biochem. J. 344(Pt. 2):511-518. [PMC free article] [PubMed] [Google Scholar]
- 44.Omata, W., H. Shibata, L. Li, K. Takata, and I. Kojima. 2000. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem. J. 346:321-328. [PMC free article] [PubMed] [Google Scholar]
- 45.Ooms, L. M., C. G. Fedele, M. V. Astle, I. Ivetac, V. Cheung, R. B. Pearson, M. J. Layton, A. Forrai, H. H. Nandurkar, and C. A. Mitchell. 2006. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol. Biol. Cell 17:607-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parton, R. G., J. C. Molero, M. Floetenmeyer, K. M. Green, and D. E. James. 2002. Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J. Biol. Chem. 277:46769-46778. [DOI] [PubMed] [Google Scholar]
- 47.Patel, N., C. Huang, and A. Klip. 2006. Cellular location of insulin-triggered signals and implications for glucose uptake. Pflugers Arch. 451:499-510. [DOI] [PubMed] [Google Scholar]
- 48.Pesesse, X., C. Moreau, A. L. Drayer, R. Woscholski, P. Parker, and C. Erneux. 1998. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett. 437:301-303. [DOI] [PubMed] [Google Scholar]
- 49.Piper, R. C., L. J. Hess, and D. E. James. 1991. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 260:C570-C580. [DOI] [PubMed] [Google Scholar]
- 50.Presley, J. F., N. B. Cole, T. A. Schroer, K. Hirschberg, K. J. Zaal, and J. Lippincott-Schwartz. 1997. ER-to-Golgi transport visualized in living cells. Nature 389:81-85. [DOI] [PubMed] [Google Scholar]
- 51.Quon, M. J., H. Chen, B. L. Ing, M. L. Liu, M. J. Zarnowski, K. Yonezawa, M. Kasuga, S. W. Cushman, and S. I. Taylor. 1995. Roles of 1-phosphatidylinositol 3-kinase and ras in regulating translocation of GLUT4 in transfected rat adipose cells. Mol. Cell. Biol. 15:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347-8350. [DOI] [PubMed] [Google Scholar]
- 53.Ren, M., G. Xu, J. Zeng, C. De Lemos-Chiarandini, M. Adesnik, and D. D. Sabatini. 1998. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95:6187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson, L. J., S. Pang, D. S. Harris, J. Heuser, and D. E. James. 1992. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J. Cell Biol. 117:1181-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safi, A., M. Vandromme, S. Caussanel, L. Valdacci, D. Baas, M. Vidal, G. Brun, L. Schaeffer, and E. Goillot. 2004. Role for the pleckstrin homology domain-containing protein CKIP-1 in phosphatidylinositol 3-kinase-regulated muscle differentiation. Mol. Cell. Biol. 24:1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saltiel, A. R., and J. E. Pessin. 2003. Insulin signaling in microdomains of the plasma membrane. Traffic 4:711-716. [DOI] [PubMed] [Google Scholar]
- 57.Sbrissa, D., O. C. Ikonomov, J. Strakova, and A. Shisheva. 2004. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology 145:4853-4865. [DOI] [PubMed] [Google Scholar]
- 58.Sbrissa, D., and A. Shisheva. 2005. Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J. Biol. Chem. 280:7883-7889. [DOI] [PubMed] [Google Scholar]
- 59.Schmid, A. C., H. M. Wise, C. A. Mitchell, R. Nussbaum, and R. Woscholski. 2004. Type II phosphoinositide 5-phosphatases have unique sensitivities toward fatty acid composition and head group phosphorylation. FEBS Lett. 576:9-13. [DOI] [PubMed] [Google Scholar]
- 60.Seabra, M. C., E. H. Mules, and A. N. Hume. 2002. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 8:23-30. [DOI] [PubMed] [Google Scholar]
- 61.Shepherd, P. R. 2005. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol. Scand. 183:3-12. [DOI] [PubMed] [Google Scholar]
- 62.Shepherd, P. R., D. J. Withers, and K. Siddle. 1998. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 333:471-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shigematsu, S., R. T. Watson, A. H. Khan, and J. E. Pessin. 2003. The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. 278:10683-10690. [DOI] [PubMed] [Google Scholar]
- 64.Shin, H. W., M. Hayashi, S. Christoforidis, S. Lacas-Gervais, S. Hoepfner, M. R. Wenk, J. Modregger, S. Uttenweiler-Joseph, M. Wilm, A. Nystuen, W. N. Frankel, M. Solimena, P. De Camilli, and M. Zerial. 2005. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170:607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shisheva, A. 2001. PIKfyve: the road to PtdIns 5-P and PtdIns 3,5-P(2). Cell Biol. Int. 25:1201-1206. [DOI] [PubMed] [Google Scholar]
- 66.Shisheva, A. 2003. Regulating Glut4 vesicle dynamics by phosphoinositide kinases and phosphoinositide phosphatases. Front. Biosci. 8:s945-s946. [DOI] [PubMed] [Google Scholar]
- 67.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 68.Simonsen, A., A. E. Wurmser, S. D. Emr, and H. Stenmark. 2001. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13:485-492. [DOI] [PubMed] [Google Scholar]
- 69.Smythe, E. 2002. Direct interactions between rab GTPases and cargo. Mol. Cell 9:205-206. [DOI] [PubMed] [Google Scholar]
- 70.Soos, M. A., J. Jensen, R. A. Brown, S. O'Rahilly, P. R. Shepherd, and J. P. Whitehead. 2001. Class II phosphoinositide 3-kinase is activated by insulin but not by contraction in skeletal muscle. Arch. Biochem. Biophys. 396:244-248. [DOI] [PubMed] [Google Scholar]
- 71.Sweeney, G., R. R. Garg, R. B. Ceddia, D. Li, M. Ishiki, R. Somwar, L. J. Foster, P. O. Neilsen, G. D. Prestwich, A. Rudich, and A. Klip. 2004. Intracellular delivery of phosphatidylinositol (3,4,5)-trisphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J. Biol. Chem. 279:32233-32242. [DOI] [PubMed] [Google Scholar]
- 72.Tanti, J. F., T. Gremeaux, S. Grillo, V. Calleja, A. Klippel, L. T. Williams, E. Van Obberghen, and Y. Le Marchand-Brustel. 1996. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote Glut 4 translocation in adipocytes. J. Biol. Chem. 271:25227-25232. [DOI] [PubMed] [Google Scholar]
- 73.Thurmond, D. C., B. P. Ceresa, S. Okada, J. S. Elmendorf, K. Coker, and J. E. Pessin. 1998. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 273:33876-33883. [DOI] [PubMed] [Google Scholar]
- 74.Tsujishita, Y., S. Guo, L. E. Stolz, J. D. York, and J. H. Hurley. 2001. Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell 105:379-389. [DOI] [PubMed] [Google Scholar]
- 75.Uhlig, M., W. Passlack, and J. Eckel. 2005. Functional role of Rab11 in GLUT4 trafficking in cardiomyocytes. Mol. Cell Endocrinol. 235:1-9. [DOI] [PubMed] [Google Scholar]
- 76.Urso, B., R. A. Brown, S. O'Rahilly, P. R. Shepherd, and K. Siddle. 1999. The alpha-isoform of class II phosphoinositide 3-kinase is more effectively activated by insulin receptors than IGF receptors, and activation requires receptor NPEY motifs. FEBS Lett. 460:423-426. [DOI] [PubMed] [Google Scholar]
- 77.Venkateswarlu, K., P. B. Oatey, J. M. Tavare, and P. J. Cullen. 1998. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr. Biol. 8:463-466. [DOI] [PubMed] [Google Scholar]
- 78.Wada, T., T. Sasaoka, M. Funaki, H. Hori, S. Murakami, M. Ishiki, T. Haruta, T. Asano, W. Ogawa, H. Ishihara, and M. Kobayashi. 2001. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via Its 5′-phosphatase catalytic activity. Mol. Cell. Biol. 21:1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watson, R. T., M. Kanzaki, and J. E. Pessin. 2004. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocrinol. Rev. 25:177-204. [DOI] [PubMed] [Google Scholar]
- 80.Yin, H. L., and P. A. Janmey. 2003. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65:761-789. [DOI] [PubMed] [Google Scholar]
- 81.Zeigerer, A., M. A. Lampson, O. Karylowski, D. D. Sabatini, M. Adesnik, M. Ren, and T. E. McGraw. 2002. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13:2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, X., A. B. Jefferson, V. Auethavekiat, and P. W. Majerus. 1995. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc. Natl. Acad. Sci. USA 92:4853-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zobiack, N., U. Rescher, C. Ludwig, D. Zeuschner, and V. Gerke. 2003. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 14:4896-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]