Abstract
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase, initially discovered as part of the NPM-ALK fusion protein, resulting from the t(2;5) translocation that is frequently associated with anaplastic large-cell lymphomas. The native ALK protein is normally expressed in the developing and, at a weaker level, adult nervous system. We recently demonstrated that the oncogenic, constitutively kinase-activated NPM-ALK protein was antiapoptotic when expressed in Jurkat lymphoblastic cells treated with cytotoxic drugs. In contrast, we now show that Jurkat cells overexpressing the wild-type ALK receptor are more sensitive to doxorubicin-induced apoptosis than parental cells. Moreover, the ALK protein is cleaved during apoptosis in a caspase-dependent manner. Mutation of aspartic residues to asparagine allowed us to map the caspase cleavage site in the juxtamembrane region of ALK. In order to assess the role of ALK in neural cell-derived tissue, we transiently expressed ALK in the 13.S.1.24 rat neuroblast immortalized cell line. ALK expression led to apoptotic cell death of the neuroblasts. ALK ligation by specific activating antibodies decreased ALK-facilitated apoptosis in both lymphoid and neuronal cell lines. Moreover, ALK transfection reduced the survival of primary cultures of cortical neurons. Thus, ALK has a proapoptotic activity in the absence of ligand, whereas it is antiapoptotic in the presence of its ligand and when the kinase is intrinsically activated. These properties place ALK in the growing family of dependence receptors.
Anaplastic lymphoma kinase (ALK) is a 200-kDa receptor tyrosine kinase (RTK) encoded by the ALK gene on chromosome 2p23. ALK was first identified as part of the NPM-ALK oncogenic fusion protein, resulting from the (2;5)(p23;q35) translocation that is frequently associated with anaplastic large-cell lymphoma (ALCL) (30). This translocation produces a fusion gene that encodes a soluble chimeric transforming protein comprising the N-terminal portion of the phosphoprotein nucleophosmin (NPM) linked to the cytoplasmic portion of ALK. It has been demonstrated that the NPM portion is responsible for the dimerization of the fusion protein, leading to constitutive activation of the kinase and to oncogenicity (5). Phospholipase C-γ, PI3K, STATs, and Src appear to be important downstream targets of NPM-ALK that contribute to its mitogenic and antiapoptotic activities (2, 3, 10, 33, 46). ALK is also involved in different variant chromosomal translocations (see reference 35 for a review), all leading to the expression of fusion proteins with a constitutively active kinase.
Full-length ALK has the typical structure of an RTK, with a large extracellular domain, a lipophilic transmembrane segment, and a cytoplasmic tyrosine kinase domain (21, 31). ALK is highly homologous to leukocyte tyrosine kinase and belongs to the insulin receptor superfamily. Expression of the normal ALK gene in hematopoietic tissues has never been detected. It is, however, dominantly expressed in the neural system. In situ hybridization analysis performed with rodents showed that the ALK mRNA is essentially and transiently expressed in specific regions of the central and peripheral nervous systems, such as the thalamus, mid-brain, olfactory bulb, and peripheral ganglia, and that it is mainly localized in neuronal cells (21, 31). Since ALK expression is maintained, albeit at a lower level, in the adult brain, it might play an important role in both the normal development and function of the nervous system. Expression of the ALK protein has also been detected in tumors derived from the nervous system, such as neuroblastomas (23). Yet the function of ALK in adult normal tissue or in carcinogenesis is largely unknown. Several studies have recently indicated that pleiotrophin (PTN) and midkine, two heparin-binding growth factors with pleiotrophic activities involved in normal development and tumor growth (27, 45), may serve as possible ligands for ALK in mammals (38, 39). Although they appeared to induce the functional activation of ALK, it is still unclear whether these molecules are indeed the physiological ligands of ALK (11, 12, 28, 32).
Recent developments in cancer therapy are aimed at inactivating a key molecule in the mechanism of tumorigenesis, as demonstrated for Gleevec. This tyrosine kinase inhibitor is used in the treatment of chronic myeloid leukemia carrying t(9;22), responsible for the constitutive activation of another oncogenic chimeric tyrosine kinase, BCR-ABL (41). We have previously shown that ALK, expressed under its chimeric form NPM-ALK, has antiapoptotic effects in Jurkat human T-lymphoblastic leukemia cells treated with the chemotherapeutic drugs doxorubicin and etoposide. Moreover, the ALK kinase activity is essential for this antiapoptotic effect, as kinase-dead NPM-ALK-expressing cells were not protected against doxorubicin-induced apoptosis (19). One approach to treat ALK-positive tumors similarly aims at inactivating the kinase. It therefore seemed important to investigate the effect of ALK expression on apoptosis in the presence or absence of ALK kinase activation.
In this paper we used two cell lines of either lymphoid or neuronal origin as models to express the wild-type ALK receptor. Apoptosis was induced by doxorubicin in Jurkat T-lymphoblastic cells stably expressing ALK or triggered by serum deprivation in transiently ALK-transfected 13.S.1.24 murine immortalized olfactory neuronal cells. Our results show for the first time that ALK expression enhances apoptosis in both lymphoid and neuronal cells. In addition, we found that the ALK protein was cleaved intracellularly by caspases during apoptosis, exposing a potentially proapoptotic region within the juxtamembrane intracytoplasmic segment of ALK. Point mutation of the aspartic acid residue in position 1160, which appears to be the caspase cleavage site, reversed the proapoptotic effect of ALK. Activating ALK-specific antibodies, used as ALK ligands, also counteracted the proapoptotic effect of ALK. Finally, ALK expression also reduced the survival of primary cortical neurons.
MATERIALS AND METHODS
Reagents and antibodies.
Doxorubicin (Dakota Pharm) was obtained from Sanofi Winthrop (Gentilly, France), Geneticin (G418 sulfate) from GIBCO BRL (Life Technologies, Grand Island, NY), [32P-γ]ATP from Amersham (Les Ulis, France), and zVAD-fmk from R & D (Minneapolis, MN), and purified activated caspase-3 was a kind gift of G. S. Salvesen (The Burnham Institute, La Jolla, CA). All other chemicals were from Sigma Chemical Co. (St. Louis, MO).
Several anti-ALK antibodies were used: the ALK1 (34) and ALKc (15) mouse monoclonal antibodies (MAbs) recognize the intracellular, kinase-containing region of ALK. The REAB polyclonal rabbit antiserum, mouse MAbs 46 and 48 (28), and rat MAb 16-39 (32) are directed against the extracellular domain of ALK. The antiphosphotyrosine (clone 4G10) MAb was purchased from Upstate Biotechnology (Lake Placid, NY), and antiactin MAb (clone AC-10) from Sigma. Peroxidase-conjugated goat antimouse and goat antirabbit immunoglobulin (Ig) antisera were from Bio-Rad (Hercules, CA). Alexa Fluor 594 goat antimouse IgG was obtained from Molecular Probes (Eugene, Ore.).
Cell lines.
The Jurkat (clone E6.1; TIB 152) human T-lymphoblastic leukemia cell line was purchased from the ATCC (Rockville, MD). Cells were maintained in RPMI 1640 (GIBCO BRL) containing 10% fetal calf serum (FCS) (GIBCO BRL), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate (GIBCO BRL). Transfected Jurkat cells were cultured in the continuous presence of 2 mg/ml G418. The t(2;5)-positive ALCL cell line SU-DHL1 (29) was cultured in Iscove's medium supplemented with 15% FCS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate. The 13.S.1.24 immortalized olfactory neuronal rat cell line (9) was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 0.3 mg/ml gentamicin.
Culture and transfection of primary cortical neurons.
Primary cortical neurons were prepared from 18-day-old Sprague-Dawley rat embryos (Janvier). Briefly, cerebral cortices were dissected and dissociated by mechanical trituration and digestion in phosphate-buffered saline (PBS) with 0.3% Aspergillus protease (Sigma) for 10 min at room temperature. After addition of 35% FCS to stop the digestion, the suspension was filtered through a 70-μm cell strainer and centrifuged at 1,000 rpm for 10 min. After resuspension in neurone medium (Neurobasal medium supplemented with 0.5 mM glutamine and 2% B-27 supplement [Gibco]), the cell suspension was centrifuged at 1,000 rpm through a 4% bovine serum albumin cushion for 10 min. Neurons were used immediately for transfection. Transfections were performed with the rat Neuron Nucleofector kit (Amaxa), according to the manufacturer's protocol. We used 3 μg of ALK plasmid and 0.5 μg of a green fluorescent protein (GFP) expression vector per 5 × 106 cells in Dulbecco's modified Eagle's medium containing 10% FCS. Thereafter, cells were seeded on poly-d,l-ornithine (Sigma), laminin (Roche)-coated glass coverslips, or plastic petri dishes. The medium was replaced by neurone medium 4 h after transfection and plating. Culture medium was renewed (one-half of the volume) every other day, and transfected neurons were analyzed after 3 to 8 days. Neuronal cultures contained >80% neurons, as assessed by staining with neuron-specific markers (data not shown).
Site-directed mutagenesis and plasmid construction.
The pcDNA3 expression vector (Invitrogen, Groningen, The Netherlands), containing the cDNA for wild-type ALK (ALK-wt) and NPM-ALK, as well as the kinase-dead NPM-ALK (named K210R, corresponding to K1150R in the ALK complete protein sequence) mutant, have been previously described (5, 30, 31). Point mutations resulting from the substitution of asparagine to aspartic residues were done using the QuickChange site-directed mutagenesis system (Stratagene, Amsterdam, The Netherlands). The following mutants were constructed and inserted into the pcDNA3 vector using the following primers: D1141N (5′-CCGGAATGCCCAACAACCCAAGCCCCCTGC-3′ and 5′-GCAGGGGGCTTGGGTTGTTGGGCATTCCGG-3′), D1160N (5′-GTGCTCTGAACAGAACGAACTGGATT TCCTC-3′ and 5′-GAGGAAATCCAGTTCGTTCTGTTCAGAGCAC-3′), D1163N (5′-GAACAGGACGAACTGAATTTCCTCATGGAAG-3′ and 5′-CTTCCATGAGGAAATTCAGTTCGTCCTGTTC-3′), D1225N (5′-CTCCCTGGCCATGCTGAACCTTCTGCACGTG-3′ and 5′-CACGTGCAGAAGGTTCAGCATGGCCAGGGAG-3′), and the D1160-1163N double mutant (5′-GAACAGAACGAACTGAATTTCCTCATGGAAG-3′ and5′CTTCCATGAGGAAATTCAGTTCGTTCTGTTC-3′).
In addition, a stop codon was inserted to replace codon 1160 in order to generate a C-terminally truncated ALK protein (ALK-stop1160) using the following primers: 5′-TGCTCTGAACAGTAAGAACTGGATTTC-3′ and 5′-GAAATCCAGTTCTTACTGTTCAGAGCA-3′. Deletion of the 33 juxtamembrane amino acids 1057 to 1089 was further introduced into the ALK-stop1160 construct by digestion with BspHI to create the ALK-delta33-stop1160 construct. Another truncated form of ALK was generated by the insertion of a stop codon to replace the codon 1126 (ALK-stop1126) using the following primers: 5′-CTGGGCCATGGCTGATTTGGGGAGGTG-3′ and 5′-CACCTCCCCAAATCAGCCATGGCCCAG-3′. The cDNA coding for the ALK C-terminal fragment comprised between amino acids 1161 and 1620 (ALK-Cter) was inserted into the pcDNA3.1D/V5-His-TOPO vector (Invitrogen) using the following PCR primers: 5′-CACCATGGAACTGGATTTCCTCATGGAA-3′ and 5′-TCAGGGCCCAGGCTGGTTCAT-3′.
Inducible expression vectors were constructed by subcloning the full-length ALK-wt coding sequence or ALK-Cter (using HindIII and XbaI sites) from the pcDNA3 vectors into the pMTCB6+-derived expression vector (pMT) (8). Gene expression was under the control of the zinc-inducible sheep metallothionein promoter, resulting in the pMT-ALK and pMT-ALK-Cter vectors, respectively. All the mutant constructions were verified by sequencing.
DNA transfection of cell lines.
Stable transfection of ALK cDNA into Jurkat cells was performed by electroporation with a Bio-Rad (Ivry-sur-Seine, France) Gene Pulser apparatus at 270 V and 950 μF. After 48 h of culture, transfected cells were selected and further cultured with 2 mg/ml G418. Cells expressing the transgene were cloned by limiting dilution. Cells transfected with control vector (Jurkat/neo) or with NPM-ALK cDNA have been previously described (19). Transient transfection of ALK constructs into 13.S.1.24 cells was performed using the Fugene 6 reagent (Roche Diagnostics, Meylan, France) according to the manufacturer's instructions.
Immunodetection by immunocytochemistry and immunofluorescence.
Transfected Jurkat cells were cytospun onto glass slides coated with silane, fixed in acetone for 10 min, and stained using ALK1 MAb with a three-stage immunoperoxidase technique (4). For the detection of ALK at the cell surface, exponentially growing cells were centrifuged at 400 × g for 5 min, washed in PBS, and incubated for 30 min at 4°C with the REAB antibody. After two washes in PBS containing 1% bovine serum albumin and 0.1% sodium azide, fluorescein isothiocyanate (FITC)-coupled goat antirabbit Ig (Jackson Immunoresearch, West Grove, Pa) antiserum was added for 30 min at 4°C as the secondary reagent. Cells were then washed twice and fixed in 0.5% formaldehyde. For intracellular ALK detection, cells were fixed and permeabilized using the Intra-Prep reagent (Immunotech, Marseille, France) according to the manufacturer's protocol and then labeled with ALK1 MAb, followed by FITC-coupled goat antimouse Ig (Jackson). Detection of active caspase-3-expressing cells after apoptosis induction was performed with an FITC-coupled caspase-3-specific MAb from BD Pharmingen (BD Biosciences, San Jose, CA). Fluorescence was analyzed on an XL4C Coulter (Beckman Coulter, Hialeah, FL) flow cytometer (excitation wavelength at 488 nm and emission at 525 nm for FITC).
Transfected neurons grown on glass coverslips were fixed for 20 min at room temperature with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature, rinsed with PBS, and blocked overnight at 4°C in PBS with 2.5% normal goat serum. Incubation with ALKc MAb diluted in PBS with 1% bovine serum albumin for 1 h at room temperature was followed, after several washes in PBS, by a 1-h incubation at room temperature with Alexa Fluor 594-goat anti-mouse IgG. After extensive washing, coverslips were mounted using 4′,6′-diamidino-2-phenylindole-containing Prolong Gold (Invitrogen). Cells were examined using an LSM 510 confocal microscope (Zeiss axiovert 100) equipped with a ×63 immersion objective.
Western blot analysis.
Cells were washed once with PBS, pelleted, and extracted in passive lysis buffer (Promega, Madison, WI) containing 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM 4-2-aminoethyl-benzenesulfonyl fluoride, 1 mM sodium orthovanadate, and 4 mM sodium fluoride for 30 min on ice. Cell extracts were centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was recovered, and its protein content was quantified using the Bio-Rad protein assay. For Western blotting, 50 to 150 μg of total cell proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 7.5% or 10% gel under reducing (most cases) or nonreducing (for detection with the anti-ALK MAb 48) conditions and transferred for 1 h at 100 V onto a nitrocellulose membrane.
The ALK protein was detected using alternatively ALKc (1/50), ALK1 (1/200), and “48” (1/1,000) mouse MAbs, followed by horseradish peroxidase (HRP)-coupled goat anti-mouse Ig antiserum (1/6,000) or REAB rabbit antibody (1/4,000), followed by HRP-coupled goat anti-rabbit Ig antiserum (1/6,000). Tyrosine-phosphorylated proteins were detected using the 4G10 (1/1,000) mouse MAb and HRP-coupled anti-mouse Ig antiserum. Signal detection was performed with an enhanced chemoluminescence kit (ECL; Amersham).
Immunoprecipitation.
Cell pellets were suspended in lysis buffer (50 mM Tris HCl, 150 mM NaCl, 1% Triton X-100) containing 1 mM sodium orthovanadate, 4 mM sodium fluoride, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 2 μg/ml pepstatin A, and 1 mM aminoethyl-benzenesulfonyl fluoride at 4°C for 30 min. Samples were precleared at 4°C for 1 h using prewashed protein G-Sepharose beads. The supernatant was then incubated with ALK1-precoated protein G-Sepharose beads at 4°C for 1 h. After 5 washes in lysis buffer, the beads were heated at 95°C for 4 min. Protein separation was performed by SDS-PAGE, followed by Western blot analysis with the antiphosphotyrosine or ALKc MAb.
In vitro kinase assay.
Immunoprecipitation with ALK1 MAb was carried out as described above. Sepharose-bound immune complexes in lysis buffer were washed and resuspended in kinase buffer (20 mM HEPES, pH 7.4, 10 mM MnCl2, 10 mM sodium fluoride, 1 mM sodium orthovanadate) before incubation with 5 μCi [γ32-P]ATP (Redivue; Amersham) at 25°C for 15 min. Samples were boiled at 95°C for 4 min and separated on a 10% gel by SDS-PAGE prior to autoradiography.
Induction of apoptosis.
Exponentially growing cells were seeded at 4 × 105 cells/ml and incubated for various periods of time (up to 24 h) with doxorubicin (0.8 to 2.4 μM) as an inducer of apoptosis or with culture medium for controls.
Measure of cell viability.
Cell viability was determined by three methods, trypan blue dye exclusion, the “CellTiter 96 Aqueous one solution cell proliferation” colorimetric assay (Promega), based on enzyme conversion of the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium(MTS) dye by mitochondria of living cells, or the “CellTiter Glo” luminescence assay (Promega), to measure the ATP content in living cells.
Quantification of apoptosis by annexin V-FITC labeling and caspase activity.
Annexin V-FITC binding to phosphatidylserine, a membrane phospholipid exposed at the surface of apoptotic cells (22), was measured at the end of apoptosis induction. Cells were washed twice in PBS and resuspended in 100 μl annexin buffer (10 mM HEPES-NaOH, pH 7.4, 140 mM NaCl, 5 mM CaCl2) containing annexin V-FITC (1/100) (Roche Diagnostics, Meylan, France) and 1 μg/ml propidium iodide, a DNA-intercalating agent. Propidium iodide is used to discriminate between apoptotic and necrotic cells, which bind annexin due to loss of membrane integrity. After incubation for 15 min at room temperature, annexin buffer (400 μl) was added to samples and cell fluorescence was immediately analyzed on an XL4C Coulter (Beckman Coulter, Hialeah, FL) flow cytometer (excitation wavelength at 488 nm and emission at 525 and 640 nm for FITC and propidium iodide, respectively). Cells labeled with annexin V-FITC and negative for propidium iodide were scored as apoptotic cells.
Caspase-3 activity was determined using the “Caspase glo 3/7” luminescence assay (Promega) according to the manufacturer's instructions. Caspase activation is shown as the ratio between the caspase activity of the treated sample and the activity of the corresponding untreated cells (relative caspase activity index).
Statistical analysis.
A paired Student t test was used to compare apoptosis values in different cell populations. A P value of <0.05 was considered significant.
RESULTS
Characterization of ALK expression in transfected Jurkat cells.
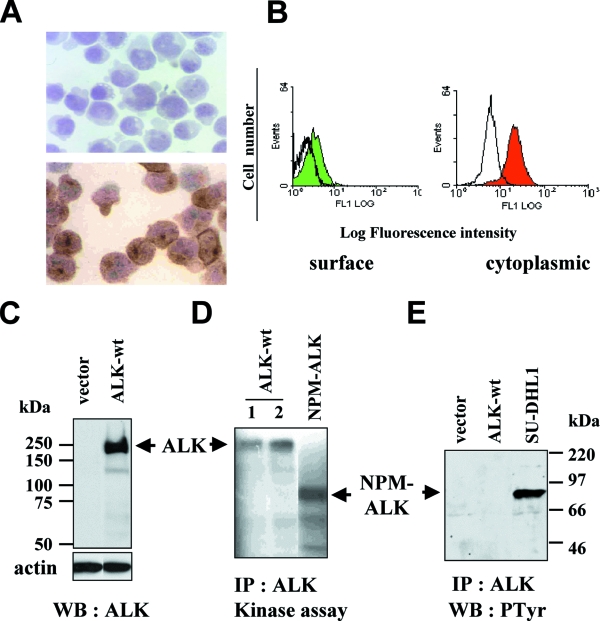
Jurkat T-lymphoid cells, transfected with ALK cDNA or with control vector (Jurkat/neo cells), were selected with Geneticin for stable gene expression and cloned by limiting dilution. Immunohistochemical and immunofluorescence analysis with ALK-specific antibodies directed against the intracytoplasmic (ALK1) and extracellular (REAB) portions of ALK revealed both cytoplasmic and membrane staining, respectively (Fig. 1A and B), confirming that ALK was expressed as a transmembrane receptor. To further characterize the protein expressed in Jurkat/ALK cells, Western blot analysis using the ALKc MAb, directed against the C-terminal domain of ALK, revealed a major protein band (sometimes seen as a doublet; see below) at approximately 200 kDa (Fig. 1C). Neither Jurkat (not shown) nor Jurkat/neo (Fig. 1C) cells expressed the ALK protein. In addition, immunoprecipitation of ALK followed by an in vitro kinase assay showed that ALK (200 kDa) expressed in Jurkat cells was capable of autophosphorylation (Fig. 1D), similarly to the NPM-ALK fusion protein (80 kDa), shown here as a positive control. Thus, Jurkat/ALK cells stably expressed a potentially functional full-size ALK protein. However, ALK immunoprecipitation followed by Western blotting with the 4G10 MAb revealed that in contrast to NPM-ALK (19), the ALK protein expressed in Jurkat was not phosphorylated on tyrosine (Fig. 1E).
FIG. 1.
Characterization of ALK expressed in stably transfected Jurkat cells. (A) Jurkat cells, transfected with ALK cDNA or with control vector (Jurkat/neo cells), were selected with Geneticin for stable gene expression and cloned by limiting dilution. Jurkat/neo cells (top) or an ALK-transfected Jurkat clone (bottom) were cytospun onto glass slides. Immunohistochemical analysis of the ALK protein was done using the ALK1 MAb with a three-stage immunoperoxidase technique. Magnification, ×800. (B) Intact (left panel) or permeabilized (right panel) Jurkat/ALK cells were stained with the REAB or ALK1 antibodies, followed, respectively, by FITC-coupled goat anti-rabbit or anti-mouse Ig. Immunofluorescence analysis of surface (left) and intracellular (right) ALK staining was analyzed by flow cytometry (colored histograms). Background cell fluorescence with an isotypic control antibody is shown as a black line histogram. (C) Fifty micrograms of protein from total cell lysates of Jurkat/neo (vector) or Jurkat/ALK (ALK-wt) cells was separated by 7.5% SDS-PAGE and analyzed by Western blotting using the ALKc MAb. ALK is detected as a 200-kDa band. Molecular mass markers are indicated on the left. The filter was stripped and reprobed using an antiactin MAb to assess comparable loading. (D) In vitro kinase assay of anti-ALK immunoprecipitates from total cell extracts of two ALK-expressing (labeled 1 and 2, ALK-wt) and one NPM-ALK-expressing clone, showing autophosphorylation of the proteins. (E) Cell lysates from vector- or ALK-wt-transfected Jurkat cells and from the NPM-ALK-expressing ALCL cell line SU-DHL1 were immunoprecipitated using the ALK1 MAb and then analyzed through Western blotting using the antiphosphotyrosine MAb 4G10. Tyrosine phosphorylation was detected in NPM-ALK-expressing but not in ALK-expressing cells. Molecular mass markers are indicated.
Doxorubicin-induced apoptosis is enhanced in ALK-expressing Jurkat cells.
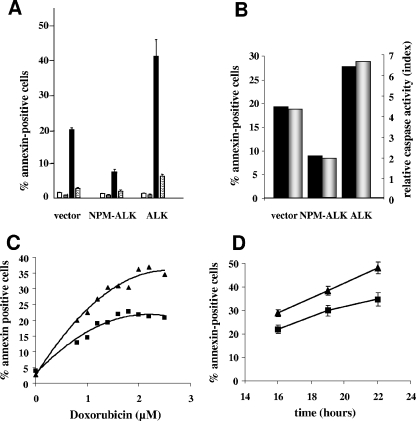
In a previous paper, we demonstrated that NPM-ALK expression in Jurkat cells led to a significant inhibition of doxorubicin-induced apoptosis, compared to the case with Jurkat/neo cells (19). In order to compare the respective effects of full-size ALK and NPM-ALK on apoptosis, we treated Jurkat/neo (vector), Jurkat/NPM-ALK, and Jurkat/ALK cells with 2 μM doxorubicin for 16 h. Apoptosis was measured using the annexin V-FITC labeling technique. Surprisingly, we found that the percentage of annexin-positive Jurkat/ALK cells was enhanced almost twofold compared to that for control Jurkat/neo cells and four- to fivefold compared to that for Jurkat/NPM-ALK cells (Fig. 2A). For each cell line, caspase-3 activity in doxorubicin-treated versus untreated cells closely paralleled the corresponding variations observed in annexin labeling (Fig. 2B). Thus, in the following experiments, we used the annexin technique to measure apoptosis unless otherwise indicated. Figure 2C and D show that the enhancement of doxorubicin-induced apoptosis in ALK-expressing cells was clearly dose and time dependent. Note that spontaneous apoptosis in untreated cells (incubated with culture medium alone) did not exceed 3% annexin-positive cells in all experiments (Fig. 2A).
FIG. 2.
Doxorubicin-induced apoptosis is enhanced in ALK-expressing Jurkat cells. (A) Jurkat cells stably transfected with vector or cDNA for NPM-ALK or ALK were seeded at 4 × 105 cells/ml and incubated for 16 h with culture medium (white bars) or 2 μM doxorubicin (black bars) as apoptosis inducer. The caspase inhibitor zVAD-fmk (10 μM) was added in the absence (gray bars) or presence (hatched bars) of doxorubicin. Apoptosis was measured using the annexin V-FITC labeling technique. The percentage of annexin-positive cells was significantly enhanced for Jurkat/ALK cells and decreased for Jurkat/NPM-ALK cells, respectively, compared to results for control vector-transfected cells (P < 0.001). Doxorubicin-induced apoptosis was also significantly inhibited by zVAD-fmk in all cases (P < 0.001). Histograms represent the means ± standard errors of the means for three to six independent experiments. (B) Correlation of apoptosis detection by two methods within a representative experiment. Cells were treated for 16 h with doxorubicin (2 μM) as in panel A, and the percentage of annexin-positive cells was determined (black histograms). Moreover, at the end of apoptosis induction, an aliquot of the culture was assayed for caspase-3/7 activity using the “Caspase glo 3/7” luminescence assay (Promega) according to the manufacturer's instructions. Caspase activation is shown as the ratio between the caspase activity of the treated sample and that of the corresponding untreated cells (relative caspase activity index; gray histograms, right side scale). (C) Dose-response curve and (D) time course analysis of doxorubicin-induced apoptosis in Jurkat/neo (black square symbols) and Jurkat/ALK (black triangles) cells. One representative experiment of two (C) and the means ± standard errors of the means for three independent experiments (D) are displayed.
We and others have previously shown that doxorubicin-induced apoptosis in Jurkat cells is a caspase-dependent process that involves the mitochondrial apoptosis signaling pathway (19). We show here that the pan-caspase inhibitor zVAD-fmk is able to inhibit approximately 85% of doxorubicin-induced apoptosis in Jurkat/ALK cells, indicating that it is a caspase-dependent process, as in Jurkat/neo and Jurkat/NPM-ALK cells (Fig. 2A).
Caspase-dependent ALK degradation during drug-induced apoptosis.
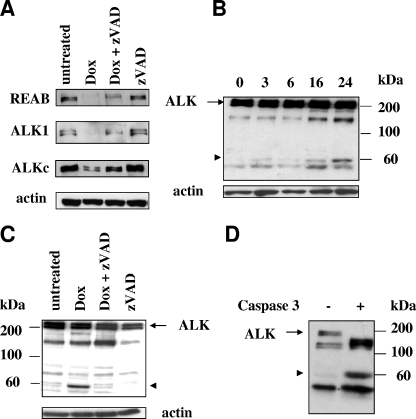
As many critical proteins are degraded in the course of caspase-dependent apoptosis, we followed ALK expression by Western blotting with different ALK-specific antibodies directed against the extracellular (REAB) or intracellular (ALK1 and ALKc) portion of ALK. The intensity of the 200- to 220-kDa doublet corresponding to full-size ALK decreased significantly after doxorubicin treatment of Jurkat/ALK cells when examined with all three antibodies (Fig. 3A), indicating that ALK was degraded. Moreover, ALK degradation was caspase dependent, as it was inhibited by the addition of the broad caspase inhibitor zVAD-fmk in association with doxorubicin (Fig. 3A). The ALKc MAb was used to study the kinetics of ALK degradation in drug-treated cells and to identify ALK-specific bands of lower molecular mass than the wild-type protein that could correspond to fragments resulting from caspase cleavage. As shown in Fig. 3B, a band of approximately 60 kDa appeared in a time-dependent manner and was maximal at 24 h of treatment with doxorubicin. A longer blot exposure was necessary to visualize this band, we were therefore unable to see on the same image the decrease in the full-size 200-kDa ALK protein. The 60-kDa product disappeared in the presence of 10 μM zVAD-fmk (Fig. 3C, lane “dox + zVAD”). Another ALK-specific band of 140 kDa was also frequently detected in untreated cells (Fig. 3B and C). This band most probably results from an extracellular cleavage or processing of ALK, as indicated by other groups (28, 32). A distinct band of 50 kDa (Fig. 3B) was inconsistently detected, did not vary in intensity, and was probably not specific, as it could sometimes be seen in overexposed blots of control Jurkat/neo cells (not shown).
FIG. 3.
Caspase-dependent ALK degradation during drug-induced apoptosis. (A) Jurkat/ALK cells were treated with 2 μM doxorubicin for 24 h in the presence or absence of 10 μM zVAD-fmk or incubated with culture medium alone. Cells were lysed, and 50 μg of protein from total cell extracts were separated by 7.5% SDS-PAGE and analyzed on replicate gels through Western blotting using antibodies directed against the extracellular (REAB), or intracellular (ALK1 and ALKc) domain of ALK. The filters were stripped and reprobed using an antiactin MAb to assess comparable loading. The intensity of the 200-kDa ALK band (seen as a doublet) decreases in doxorubicin-treated cells, a decrease partially inhibited by the caspase inhibitor zVAD-fmk. (B) Western blot analysis of time-dependent degradation of ALK using the ALKc MAb, showing the appearance of a 60-kDa band (arrowhead). The decrease of the 200-kDa band is not visible because of a longer exposure of the blot, necessary to visualize the 60-kDa band. Molecular mass markers are shown. (C) Jurkat/ALK cells were treated as in panel A, and ALK degradation was analyzed by Western blotting using the ALKc MAb. Appearance of the 60-kDa ALK fragment (arrowhead) is inhibited by zVAD-fmk. (D) Total protein cell lysates from untreated Jurkat/ALK cells were incubated in vitro with purified activated caspase-3 for 30 min at 37°C and analyzed by Western blotting using the ALKc MAb. The arrowhead indicates the 60-kDa ALK fragment. Lysates from equivalent numbers of cells were loaded onto a 10% SDS-PAGE gel.
We hypothesized that the 60-kDa fragment could result from a caspase cleavage within the intracellular domain of ALK. In order to see whether ALK cleavage could be due to the major effector caspase, caspase-3, we incubated protein extracts from Jurkat/ALK cells with purified activated caspase-3 for 30 min at 37°C. Western blotting with the ALKc MAb revealed that ALK was indeed cleaved in vitro by active caspase-3, giving rise to two major bands of 150 kDa and 60 kDa, respectively (Fig. 3D). As ALKc recognizes an epitope in the C-terminal region of ALK, it appears that the larger band results from a cleavage within the extracellular domain of ALK (irrelevant in vivo, as caspases are intracellular enzymes), whereas the smaller fragment of 60 kDa appears to be identical to the one detected after in vivo cell treatment with doxorubicin (seen in Fig. 3B and C, arrowhead).
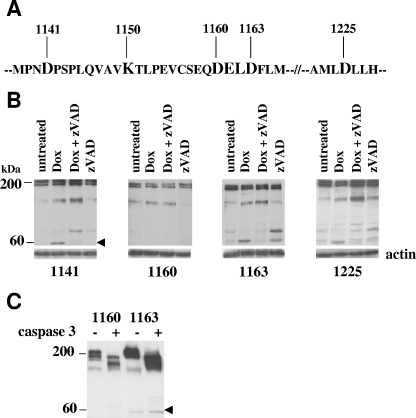
The caspase cleavage site of ALK is D1160.
We then examined the intracellular protein sequence of ALK in search of a potential caspase cleavage site. Amino acids 1160 to 1163 of ALK are “DELD,” a motif similar to the “DEVD” caspase-3 consensus cleavage site (Fig. 4A). Moreover, potential cleavage of ALK at this site would yield a protein fragment of 60 kDa, which matches our previous findings. In order to map the caspase cleavage site, we replaced aspartic acid residues with asparagine (D_N mutations) in positions 1141, 1160, 1163, and 1225 and also made a double mutant (1160-1163). Jurkat cells were stably transfected with these constructs and cloned by limiting dilution as described above. The pattern of ALK degradation after doxorubicin treatment in cells expressing the 1141, 1163, and 1225 D_N ALK mutant was the same as in the wild-type ALK-expressing cells, giving rise to the 60-kDa fragment (Fig. 4B). In contrast, this fragment was undetectable in cells expressing the D1160N ALK mutant (Fig. 4B), indicating that the 1,160-aspartic-acid residue is the site of ALK cleavage by caspase-3. In agreement with these findings, in vitro treatment of protein extracts from ALK-D1160N-expressing cells with active caspase-3 failed to release the 60-kDa fragment, whereas the D1163N ALK mutant was still cleaved (Fig. 4C). The 1160-1163 D_N double mutant responded like the D1160N mutant in vivo and in vitro (data not shown).
FIG. 4.
The caspase cleavage site of ALK is D1160. (A) Portion of the ALK intracellular sequence showing the caspase-3 consensus-like cleavage site (DELD) and the position of aspartic acids chosen for experimental D_N mutation, as well as the ATP-binding site (K1150). (B) Jurkat cells were permanently transfected with cDNAs coding for ALK mutants displaying a D_N mutation at position 1141, 1160, 1163, or 1225 and cloned by limiting dilution. One representative cell clone is shown for each mutant. Cells were treated with 2 μM doxorubicin for 24 h in the presence or absence of 10 μM zVAD-fmk or left untreated. The arrowhead indicates the 60-kDa ALK fragment, absent from the ALK D1160N mutant-expressing cells. The filters were stripped and reprobed using an antiactin MAb to assess comparable loading. (C) Total protein cell lysates from untreated Jurkat cells expressing the 1160 and 1163 D_N ALK mutants were incubated in vitro with purified activated caspase-3 for 30 min at 37°C and analyzed by Western blotting using the ALKc MAb. The arrowhead indicates the 60-kDa ALK fragment. Lysates from equivalent numbers of cells were loaded onto a 7.5% SDS-PAGE gel.
Kinase activation impairs NPM-ALK cleavage by caspase-3.
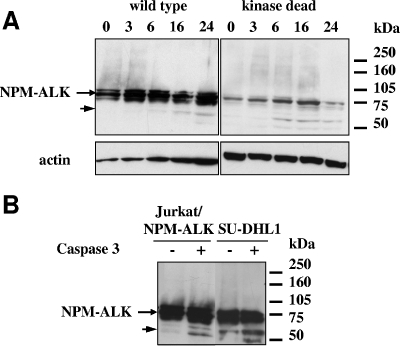
Since the DELD motif belongs to the intracellular region of ALK, which is conserved in the NPM-ALK hybrid protein, we expected NPM-ALK to undergo caspase cleavage after doxorubicin treatment of Jurkat/NPM-ALK cells. Indeed, Western blot analysis revealed a weak 60-kDa band after 16 h of cell incubation with the drug (Fig. 5A, left panel), instead of 3 h for Jurkat/ALK cells (Fig. 3B). Therefore, the generation of the 60-kDa fragment was both impaired and delayed in Jurkat/NPM-ALK versus Jurkat/ALK cells. In contrast, the kinase-dead (K210R) NPM-ALK, mutated on the ATP fixation site (lysine [K] 1150 of the full-length ALK sequence [Fig. 4A]), was cleaved as early as 6 h after the onset of treatment (Fig. 5A, right panel). Moreover, our published (19) and present (Fig. 5A) results show that the kinase-dead mutant was expressed at a lower level than wild-type NPM-ALK. Therefore, the relative ratio between the 60-kDa cleavage fragment and the full-size 80-kDa protein appears more important in the kinase-dead mutant than in the wild-type NPM-ALK. Active caspase-3 in vitro treatment of protein extracts from Jurkat/NPM-ALK cells and the SU-DHL1 ALCL cell line, confirmed that NPM-ALK was cleavable, generating a 60-kDa fragment, as expected (Fig. 5B). Taken together, our results suggest that activation/phosphorylation of the ALK kinase protects the protein from caspase-dependent degradation. It should be noted that ALK cleavage on residue 1160 disrupts the kinase domain of ALK, which spans between residues 1122 and 1376, but preserves lysine 1150, the ATP-binding site (but not necessarily the secondary structure). Thus, caspase cleavage of ALK probably inactivates the kinase.
FIG. 5.
Kinase activation impairs NPM-ALK cleavage by caspase-3. (A) Jurkat cells expressing the wild-type or kinase-dead NPM-ALK mutant were treated for the indicated times (hours) with 2 μM doxorubicin. Cells were lysed, and 50 μg of protein from total cell extracts was separated by 10% SDS-PAGE and analyzed by Western blotting using ALKc. The filters were stripped and reprobed using an antiactin MAb to assess protein loading. The arrow indicates the 60-kDa ALK fragment. (B) Total protein cell lysates from untreated Jurkat/NPM-ALK cells and from the ALCL line SU-DHL1 were incubated in vitro with purified activated caspase-3 for 30 min at 37°C and analyzed by Western blotting using the ALKc MAb. The arrow indicates the 60-kDa ALK fragment. Lysates from equivalent numbers of cells were loaded onto a 10% SDS-PAGE gel.
The D1160N mutation abrogates ALK-mediated enhancement of apoptosis.
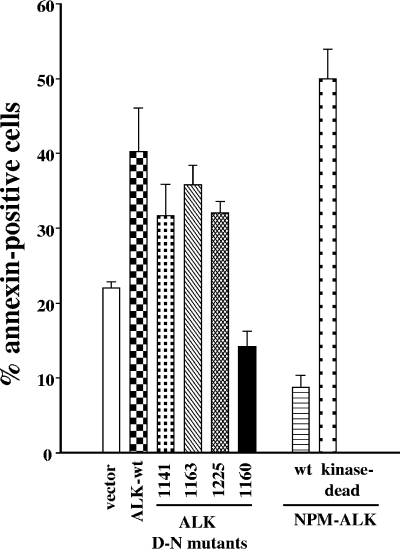
We next examined the effect of the various D_N mutations of ALK on doxorubicin-induced apoptosis to see whether a relationship existed between caspase-dependent ALK cleavage and the extent of cell death. The 1141, 1163, and 1225 ALK mutants transfected into Jurkat cells responded like wild-type ALK, i.e., enhanced doxorubicin-induced apoptosis by 1.5- to twofold compared to that for Jurkat/neo cells (Fig. 6). In contrast, the D1160N mutant conferred resistance to apoptosis similarly to NPM-ALK, reducing the percentage of annexin-positive cells to half that observed in Jurkat/neo cells (Fig. 6). In addition, the 1160-1163 double mutant also protected cells from drug-induced apoptosis (not shown). As observed in ALK-wt-expressing cells, we could not detect tyrosine phosphorylation in the noncleavable D1160N ALK mutant, either before or after cell treatment with doxorubicin (data not shown), suggesting that the abrogation of the proapoptotic activity of this mutant was not due to kinase activation. Notably, doxorubicin-induced apoptosis was enhanced in kinase-dead NPM-ALK-expressing cells (Fig. 6) to the same extent as in Jurkat/ALK cells, and we have shown that this mutant was relatively sensitive to caspase cleavage (Fig. 5A, right panel). Altogether these data strongly suggest that ALK cleavage by caspase at D1160 confers an active signal for enhancement of drug-induced apoptosis.
FIG. 6.
The D1160N mutation abrogates ALK-mediated enhancement of apoptosis. Jurkat cells expressing wild-type or mutant ALK displaying a D_N mutation at position 1141, 1160, 1163, or 1225 and wild-type or kinase-dead NPM-ALK were treated with 2 μM doxorubicin for 16 h. Apoptosis was measured using the annexin V-FITC labeling technique. One representative cell clone is shown for each cell line. The percentage of annexin-positive cells in all groups was statistically different (P < 0.05) from that in control vector-transfected cells (white bar). Apoptosis was increased in all groups (with no significant difference between them) except for the ALK D1160N mutant and the NPM-ALK-wt-expressing cells, in which apoptosis was inhibited in comparison to results with control vector-transfected cells. Bars represent the means ± standard errors of the means for three independent experiments.
Mapping of a proapoptotic segment of ALK to the juxtamembrane region.
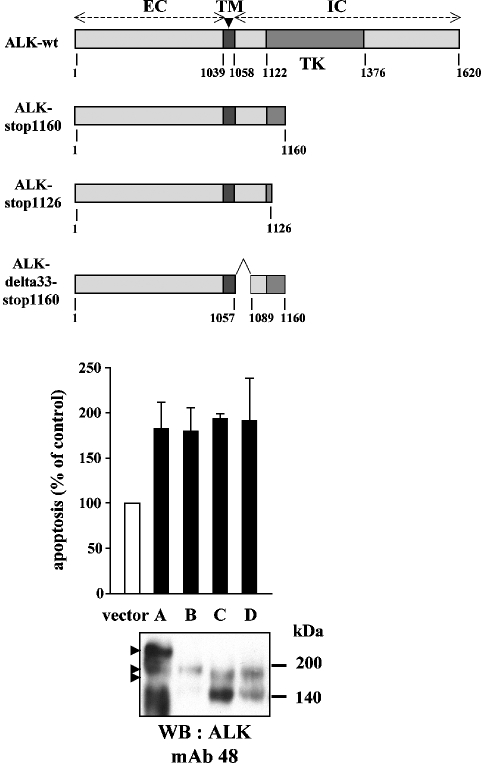
We have found that ALK-mediated facilitation of drug-induced apoptosis depended on its intracellular cleavage by caspase-3 at D1160. These data suggest that either the 60-kDa fragment released is proapoptotic or the intracellular segment upstream of D1160 contains a structure or sequence with proapoptotic properties. In a first approach, we constructed a pcDNA3 vector containing the coding sequence of the 1161-to-1620 ALK C-terminal fragment. However, we repeatedly failed to obtain clones in permanent transfection of Jurkat cells or to detect the peptide in transient transfections (data not shown). At this point in the study, this result could be due either to a potent proapoptotic effect of the fragment or to unresolved technical problems. To investigate the presence of a potential proapoptotic sequence within the N-terminal juxtamembrane part of the ALK intracellular region, we constructed a mutant with a stop codon in position 1160 (ALK-stop1160). Doxorubicin-induced apoptosis in these cells was enhanced to the same extent as in Jurkat/ALK cells (Fig. 7, middle panel). These data indicate that ALK might contain a proapoptotic domain in its intracytoplasmic juxtamembrane region between amino acids 1058 and 1160. In order to map this putative domain, we generated two additional ALK mutants, deleting the N- or C-terminal third of this 103-amino-acid peptide segment: ALK delta33_stop1160 (deleting amino acids 1057 to 1089) and ALK-stop1126, respectively. All the C-terminally truncated ALK proteins expressed in Jurkat cells were detected through Western blotting using the MAb 48 directed against the extracellular domain of ALK (Fig. 7, lower panel). Doxorubicin-induced apoptosis was also increased in cells expressing these mutant ALK proteins (Fig. 7), strongly suggesting that a proapoptotic region lies between amino acids 1090 and 1125 of the ALK intracellular domain.
FIG. 7.
Mapping of a proapoptotic segment of ALK to the juxtamembrane region. Upper panel: schematic representation of wild-type and C-terminally truncated ALK proteins; the extracellular (EC), transmembrane (TM), tyrosine kinase (TK), and intracellular (IC) domains are indicated. Middle panel: Jurkat cells, stably transfected with pcDNA3 vector (white bar) or cDNA coding for the wild-type (A) or C-terminally truncated ALK protein (black bars) ALK-stop1160 (B), ALK-delta33-stop1160 (C), or ALK-stop1126 (D), were treated with 2 μM doxorubicin for 16 h. Apoptosis was measured using the annexin V-FITC labeling technique. Results are represented as an index of the percentage of annexin-positive control vector-transfected cells (mean ± standard error of the mean for three independent experiments). Lower panel: Western blot detection of wt and truncated ALK proteins (indicated by arrowheads) using MAb 48 directed against the extracellular domain of ALK. SDS-PAGE (7.5%) was run under nonreducing conditions.
ALK ligation by activating antibodies inhibits ALK-mediated enhancement of apoptosis.
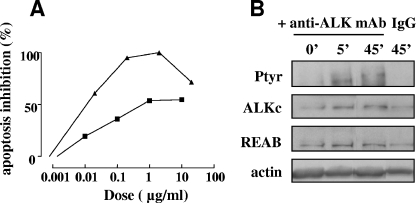
The activation of RTKs typically requires ligand-induced receptor oligomerization, which results in tyrosine autophosphorylation of the receptors (37). Since our in vivo and in vitro experiments suggest that activation/phosphorylation of the ALK kinase protects the protein from caspase-dependent degradation and the cells from doxorubicin-induced apoptosis, we tried to induce ALK receptor activation using a specific ligand. In our hands, commercially available recombinant PTN (Sigma) failed to activate the receptor. In addition, although cotransfection of Jurkat cells with ALK and PTN decreased drug-induced apoptosis, this effect was not ALK specific, since it was also observed in PTN-only-expressing cells (unpublished results). The use of ALK-specific activating MAbs, recently developed by two independent research groups (28, 32), was therefore a potent tool to activate the ALK receptor. Coincubation of Jurkat/ALK cells with doxorubicin and either the 16-39 or 46 MAb reversed the proapoptotic effect of ALK in a dose-dependent manner, with a maximal effect at 1 to 2 μg/ml of purified antibody (Fig. 8A). Note that MAb 16-39 decreased the level of apoptosis (measured by annexin labeling) of Jurkat/ALK cells to that of control Jurkat/neo cells (100% apoptosis inhibition) (Fig. 8A). In addition, the activating effect of MAb 46 on ALK kinase was confirmed, since cell incubation with this antibody induced tyrosine phosphorylation of ALK (Fig. 8B).
FIG. 8.
ALK ligation by activating antibodies inhibits ALK-mediated enhancement of apoptosis. (A) Jurkat/ALK cells were treated with 2 μM doxorubicin for 16 h in the presence of increasing doses of the ALK-specific activating MAb 46 (black square symbols) or 16-39 (black triangles). Apoptosis was measured using the annexin V-FITC labeling technique. Each results is expressed as the percentage of apoptosis inhibition in Jurkat/ALK cells relative to that for the control Jurkat/neo (vector) cells treated under the same conditions. One representative experiment of three is shown. (B) Jurkat/ALK cells were serum starved for 24 h and incubated for various times with 10 μg/ml MAb 46 or control isotypic mouse IgG. Lysates from equivalent numbers of cells were loaded onto a 7.5% SDS-PAGE gel. ALK phosphorylation was analyzed by Western blotting with the 4G10 antiphosphotyrosine MAb (Ptyr). The blot was stripped and sequentially reprobed with ALKc and REAB antibodies to visualize total ALK expression and with antiactin to assess comparable protein loading.
ALK induces cell death in a neuroblast cell line.
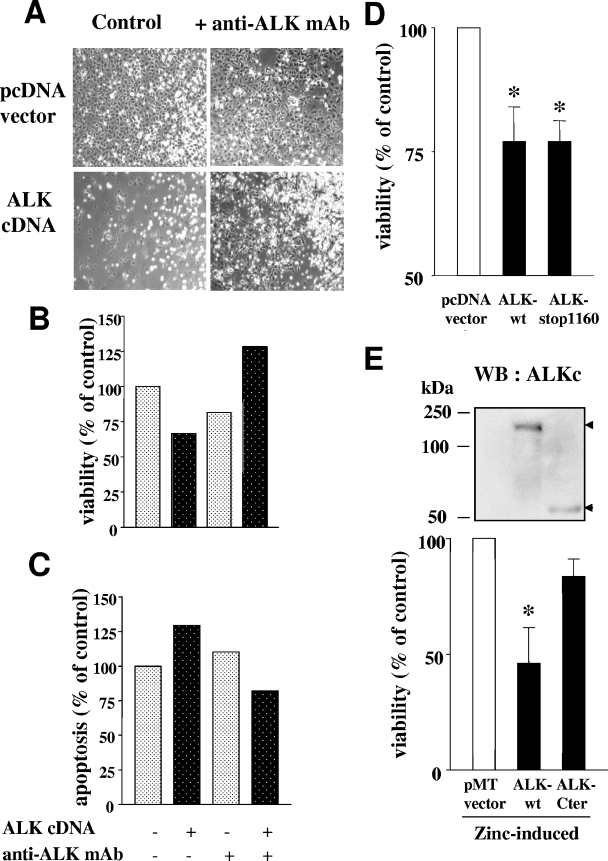
The ALK receptor is expressed during the development of the nervous system (21, 31). In order to determine whether ALK could have a proapoptotic effect in a more physiological context, we transiently expressed ALK in an immortalized murine neuroblast cell line, 13.S.1.24, which does not express endogenous ALK (not shown). Twenty-four hours after transfection, cells were deprived of serum (a stimulus which per se does not induce cell death in this cell line) and further incubated for 24 h in the absence or presence of 20 μg/ml anti-ALK MAb 16-39. The transfection efficiency was approximately 50% in all experiments (evaluated by transfection with a green fluorescent protein expression vector and flow cytometry analysis of cell fluorescence). As shown in Fig. 9A, ALK transfection (determined by Western blotting; not shown) induced significant cell death (lower left panel) compared to control vector-transfected cells (upper left panel). In contrast, incubation of ALK-transfected cells with MAb 16-39 rescued the cells from apoptosis triggered by serum deprivation (Fig. 9A, lower right panel). A normal rat IgG, used as a negative control for MAb 16-39, had no effect. Cell viability was measured using an MTS assay (Fig. 9B), and apoptosis was measured by assessing the percentage of active caspase-3-positive cells in the different culture conditions (Fig. 9C), confirming that the activating anti-ALK MAb 16-39 decreased apoptosis triggered by ALK in serum-deprived neuroblasts.
FIG. 9.
ALK induces cell death in neuroblasts. (A) ALK cDNA or empty pcDNA3 vector was transiently transfected into the 13.S.1.24 immortalized murine neuroblast cell line. Twenty-four hours after transfection, cells were serum starved and incubated in the presence or absence of 20 μg/ml ALK-specific activating MAb 16-39 for a further 24 h. Cell morphology was observed by phase-contrast microscopy under an inverted microscope. ALK-transfected cells exhibited significant cell death (lower left panel) but could be rescued by the activating anti-ALK MAb (lower right panel). (B) Cells from cultures shown in panel A were assessed for viability by using the MTS assay (CellTiter 96 Aqueous cell proliferation assay from Promega) and apoptosis (C), measuring the percentage of active caspase-3-expressing cells by flow cytometry. The control (set arbitrarily at 100%) in both graphs is the vector-transfected neuroblast cell line. Results represent the means of triplicate measures within one representative experiment of three. (D) In addition to vector and ALK-wt, the truncated ALK-stop1160 mutant cDNA was transfected into 13.S.1.24 cells. Cell viability was measured as in panel B. Bars represent the means ± standard errors of the means for three independent experiments. The difference between ALK-wt or ALK-stop1160 and the vector-transfected cells was statistically significant (*, P < 0.05, Student's t test). (E) The zinc-inducible metallothionein promoter-containing pMTCB6+-derived vector (pMT vector) was used to express ALK-wt or the ALK C-terminal peptide 1161 to 1620 (ALK-Cter) in 13.S.1.24 neuroblasts, respectively. Cells were transfected in the absence of serum and treated with 100 μM ZnSO4 5 h later. Both proteins were detected in transfected neuroblasts by Western blotting (upper panel), and cell viability was measured by trypan blue exclusion 48 h postinduction (lower panel). Bars represent the means ± standard errors of the means for four independent experiments. Zinc-induced ALK-wt expression significantly decreased the number of viable cells in the culture (*, P < 0.05), compared to the control pMT vector, whereas the Cter peptide did not have a significant effect.
Since the ALK-stop1160 mutant was proapoptotic in Jurkat cells, it was important to assess that it had the same effect in 13.S.1.24 neuroblasts. Indeed, cell viability was decreased to the same extent after ALK-stop1160 and ALK-wt transfection in neuroblasts (Fig. 9D). Protein expression was checked by Western blotting (not shown).
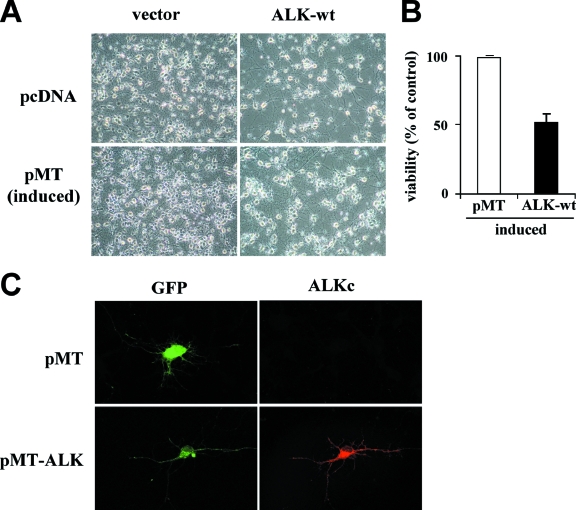
As in the Jurkat cell model, transfection of 13.S.1.24 neuroblasts with a pcDNA3-ALK-Cter vector did not lead to detectable expression of the C-terminal ALK 60-kDa fragment (data not shown). We then tried another strategy and inserted the coding sequence for ALK-Cter (1161 to 1620) or for ALK-wt into a pMTCB6+-derived expression vector (8) under the control of the zinc-inducible sheep metallothionein promoter. Cells were transfected with pMT vector, pMT-ALK-wt, or pMT-ALK-Cter in the absence of serum, induced or not with 100 μM ZnSO4 (an optimal dose to minimize toxicity for cells and obtain gene expression) 5 h later. Both proteins were detected in transfected neuroblasts by Western blotting in the presence (Fig. 9E, top), but not in the absence (not shown) of zinc induction. Cell viability was measured by trypan blue exclusion 48 h postinduction. Zinc-induced ALK-wt expression significantly decreased the number of viable cells in the culture (P < 0.05) compared to results with the control pMT vector. The slight decrease of cell viability observed with ALK-Cter did not appear to be statistically significant (Fig. 9E, lower part).
ALK induces cell death in primary cortical neurons.
We finally addressed the issue of the potential proapoptotic effect of ALK on primary cortical neurons, which do not express endogenous ALK ((31) and unpublished results). Primary cortical neurons were prepared from 18-day-old rat embryos (note that these cells are postmitotic and do not proliferate in culture) and were transfected with ALK-wt inserted either in the pcDNA3 or the zinc-inducible pMT vector. In both cases, ALK transfection decreased the number of viable cells observed after 6 to 8 days of culture, compared to the corresponding empty vectors (Fig. 10A and 10B). The expression of ALK was determined by indirect immunofluorescence on permeabilized neurons using the ALKc MAb (Fig. 10C).
FIG. 10.
ALK induces cell death in primary cortical neurons. Primary cortical neurons prepared from 18-day-old embryonic Sprague-Dawley rats were transfected with ALK cDNA inserted into the pcDNA3 or the zinc-inducible pMT vector, together with a GFP expression vector as a control, as described in Material and Methods. Zinc (100 μM Zn SO4) was added at days 2, 3, and 6 posttransfection for pMT vectors. Transfected neurons were analyzed after 6 to 8 days. (A) Phase-contrast microscopy of cultures at day 6, showing a lower density of neurons in ALK-transfected cultures. (B) Cell viability was measured at day 7 posttransfection using the CellTiterGlo luminescence assay. Results represent the means ± standard deviations of quadruplicate measures within one representative experiment of 2. (C) Immunofluorescence analysis of transfected neurons (day 8) grown on glass coverslips, permeabilized, stained with ALKc MAb followed by an Alexa Fluor 594 goat antimouse IgG (right panels). Left panels show the analysis of GFP expression in transfected neurons.
DISCUSSION
We present here data supporting that in the absence of a ligand, the ALK receptor kinase is not tyrosine phosphorylated and actively enhances or triggers apoptosis, whereas kinase activation, induced by a ligand or constitutive as with NPM-ALK, decreases apoptosis. Unligated/nonactivated ALK receptor facilitates apoptosis via its own cleavage by caspases at D1160, a phenomenon allowing the exposure of a proapoptotic juxtamembrane intracellular domain (located between residues 1058 and 1160), most likely through the association with proapoptotic effectors. Caspases have been shown to associate with death receptors, such as Fas/CD95, TNFR1, or TRAIL, via intracellular adaptor proteins through different motifs within the death-like domain superfamily: death domain, death-effector domain, or caspase recruitment domain (18, 36). We searched databases but were unable to find any such domain within the juxtamembrane intracytoplasmic sequence (1058 to 1160) of ALK. Finer mapping allowed us to restrict the proapoptotic domain to a sequence lying between amino acids 1090 and 1125 of ALK. The direct or indirect interaction of ALK with proapoptotic effector molecules will be the object of further studies. Yet preliminary data show that expression of several Bcl-2 family members is modulated in ALK-expressing Jurkat cells (data not shown).
This dual effect on apoptosis has been described for several receptors involved in development and tumorigenesis, composing the so-called functional family of “dependence receptors.” Members include p75NTR, the low-affinity nerve growth factor receptor (7), the DCC (deleted in colorectal cancer) and UNC5H netrin receptors (16, 17, 24, 25), the integrin αvβ3 (40), and the RET (rearranged during transfection) RTK, a receptor for glial cell-derived neurotrophic factor (6). Such receptors create states of dependence on their respective ligands by inducing apoptosis when the ligand is absent and inhibiting apoptosis in the presence of the ligand. Moreover, these receptors appear to be cleaved by caspases during apoptosis (7), as is the case for ALK. Recently, another RTK was identified as a receptor displaying several common traits with dependence receptors: MET is cleaved by caspases, this cleavage is inhibited by the presence of its ligand, hepatocyte growth factor/scatter factor, and the cleaved fragment was shown to be proapoptotic (43). However, the authors failed to detect a proapoptotic activity of the full-length receptor, while both RET and ALK are clearly proapoptotic in the absence of their respective ligands. Whether this is related to the experimental system or to the fact that MET is not a dependence receptor remains to be determined. However, it is intriguing to see that various kinase receptors could potentially be considered dependence receptors. The physiological relevance of this phenomenon has yet to be discovered.
Multiple oncogenes have the ability to trigger apoptosis when expressed in an inappropriate manner, and this is thought to restrict tumor formation by eliminating potentially malignant cells that may have acquired a mutation that stimulates proliferation. For example, deregulated expression of c-myc promotes proliferation but can also either induce or sensitize cells to apoptosis (14). A parallel could be made between c-myc and ALK. Inappropriate expression of c-myc under conditions which inhibit growth and down-regulate endogenous c-myc expression, including serum deprivation and exposure to cytotoxic agents, usually results in programmed cell death in many different cell types (reviewed in reference 13). We show here that inappropriate expression of ALK, in the absence of a ligand and/or in the wrong cellular context, also triggers or facilitates apoptosis. On the other hand, mechanisms that inhibit apoptosis should enhance or promote tumorigenesis. Indeed, when ALK is in its oncogenic form, NPM-ALK, the propapoptotic effect of ALK due to its cleavage by caspases is counteracted by the proliferative and prosurvival effect of the constitutively activated tyrosine kinase. We propose that apoptosis associated with inappropriate ALK expression could limit the tumorigenic effect of the ALK proto-oncogene.
It is possible that ALK does not trigger apoptosis directly but that it acts as a sensitizer to another existing trigger. Two signals would thus be necessary to enhance apoptosis. In the lymphoid Jurkat cells, we have observed that a certain degree of caspase activation is present in both parental and transfected cells (data not shown). Moreover, other authors have shown that lymphocytes can survive limited caspase activation (1) and that active caspase-3 was detected in ALK-positive ALCL tumors (42). If the level of caspase activation reaches a certain threshold, we propose a model, which may vary according to the cell type and the cell environment, in which ALK is cleaved by caspase-3 (or possibly a related subfamily caspase member), exposing a proapoptotic region and creating a positive feedback loop for apoptosis enhancement. This model could also apply to the neuronal cell, where serum deprivation, a stimulus that per se does not induce cell death in the 13.S.1.24 immortalized neuroblasts, “pushes” ALK to induce cell death.
Like several dependence receptors (26), ALK is involved in neural development (21, 31) and possibly morphogenesis, in spite of the lack of a lethal or abnormal phenotype in ALK-knockout mice (12; S. W. Morris, personal communication). Interestingly, two recent papers analyzed the temporal spatial expression of the ALK receptor during the development of mice (44) and chickens (20), showing that ALK is dynamically expressed on subsets of neurons of both the central and peripheral nervous system. It is noteworthy that the expression of the ALK transcript in spinal motoneurons overlaps temporally with the period of programmed cell death (which affects 50% of these neurons) in the chicken embryo, suggesting that ALK could play a role in this phenomenon (20). Our results showing a prodeath effect of ALK in both murine immortalized neuroblasts and ex vivo primary neurons support the potential proapoptotic role of ALK under certain physiological conditions that remain to be determined.
In conclusion, ALK is a receptor involved in both neural development and oncogenesis, with dual effects on apoptosis depending on the availability of the ligand. Therefore, this is the first demonstration that ALK could be a new member of the dependence receptor family.
Acknowledgments
We are grateful to Fatima L'Faqihi and Sophie Allart from the Services Communs de Cytométrie et d'Imagerie de l'IFR Purpan, respectively, Catherine Guix for excellent technical help, Marie-Claire Bordeaux for precious help for initial constructions, and Karen Pulford, Bruno Falini, and Tadashi Yamamoto for the gift of anti-ALK MAbs.
This work was partly funded by grants from the Conseil Régional Midi-Pyrénées, the Cancéropole Grand Sud-Ouest, and the Association pour la Recherche sur le Cancer (ARC). Jaouhar Mourali and Filipe Calheiros Lourenço are the recipients of Ph.D. fellowships from the French Embassy in Tunisia (Institut Français de Coopération) and from the Portuguese Ministery of Science (FCT), respectively. Céline Monnet has a postdoctoral fellowship from the INSERM. Catherine Greenland was supported in part by an ARC fellowship.
REFERENCES
- 1.Alam, A., L. Y. Cohen, S. Aouad, and R. P. Sekaly. 1999. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, R. Y., P. Dieter, C. Peschel, S. W. Morris, and J. Duyster. 1998. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-γ to mediate its mitogenicity. Mol. Cell. Biol. 18:6951-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, R. Y., T. Ouyang, C. Miething, S. W. Morris, C. Peschel, and J. Duyster. 2000. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 96:4319-4327. [PubMed] [Google Scholar]
- 4.Benharroch, D., Z. Meguerian-Bedoyan, L. Lamant, C. Amin, L. Brugieres, M. J. Terrier-Lacombe, E. Haralambieva, K. Pulford, S. Pileri, S. W. Morris, D. Y. Mason, and G. Delsol. 1998. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 91:2076-2084. [PubMed] [Google Scholar]
- 5.Bischof, D., K. Pulford, D. Y. Mason, and S. W. Morris. 1997. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol. Cell. Biol. 17:2312-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordeaux, M. C., C. Forcet, L. Granger, V. Corset, C. Bidaud, M. Billaud, D. E. Bredesen, P. Edery, and P. Mehlen. 2000. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19:4056-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredesen, D. E., P. Mehlen, and S. Rabizadeh. 2004. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol. Rev. 84:411-430. [DOI] [PubMed] [Google Scholar]
- 8.Cook, D. M., M. T. Hinkes, M. Bernfield, and F. J. Rauscher III. 1996. Transcriptional activation of the syndecan-1 promoter by the Wilms' tumor protein WT1. Oncogene 13:1789-1799. [PubMed] [Google Scholar]
- 9.Coronas, V., F. Feron, R. Hen, G. Sicard, F. Jourdan, and E. Moyse. 1997. In vitro induction of apoptosis or differentiation by dopamine in an immortalized olfactory neuronal cell line. J. Neurochem. 69:1870-1881. [DOI] [PubMed] [Google Scholar]
- 10.Cussac, D., C. Greenland, S. Roche, R. Y. Bai, J. Duyster, S. W. Morris, G. Delsol, M. Allouche, and B. Payrastre. 2004. Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-src to mediate its mitogenicity. Blood 103:1464-1471. [DOI] [PubMed] [Google Scholar]
- 11.Dirks, W. G., S. Fahnrich, Y. Lis, E. Becker, R. A. MacLeod, and H. G. Drexler. 2002. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int. J. Cancer 100:49-56. [DOI] [PubMed] [Google Scholar]
- 12.Duyster, J., R. Y. Bai, and S. W. Morris. 2001. Translocations involving anaplastic lymphoma kinase (ALK). Oncogene 20:5623-5637. [DOI] [PubMed] [Google Scholar]
- 13.Evan, G., and T. Littlewood. 1998. A matter of life and cell death. Science 281:1317-1322. [DOI] [PubMed] [Google Scholar]
- 14.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 15.Falini, B., B. Bigerna, M. Fizzotti, K. Pulford, S. A. Pileri, G. Delsol, A. Carbone, M. Paulli, U. Magrini, F. Menestrina, R. Giardini, S. Pilotti, A. Mezzelani, B. Ugolini, M. Billi, A. Pucciarini, R. Pacini, P. G. Pelicci, and L. Flenghi. 1998. ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am. J. Pathol. 153:875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forcet, C., E. Stein, L. Pays, V. Corset, F. Llambi, M. Tessier-Lavigne, and P. Mehlen. 2002. Netrin-1-mediated axon outgrowth requires Deleted in colorectal cancer-dependent MAPK activation. Nature 417:443-447. [DOI] [PubMed] [Google Scholar]
- 17.Forcet, C., X. Ye, L. Granger, V. Corset, H. Shin, D. E. Bredesen, and P. Mehlen. 2001. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc. Natl. Acad. Sci. USA 98:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, D. R., and G. I. Evan. 2002. A matter of life and death. Cancer Cell 1:19-30. [DOI] [PubMed] [Google Scholar]
- 19.Greenland, C., C. Touriol, G. Chevillard, S. W. Morris, R. Bai, J. Duyster, G. Delsol, and M. Allouche. 2001. Expression of the oncogenic NPM-ALK chimeric protein in human lymphoid T-cells inhibits drug-induced, but not Fas-induced apoptosis. Oncogene 20:7386-7397. [DOI] [PubMed] [Google Scholar]
- 20.Hurley, S. P., D. O. Clary, V. Copie, and F. Lefcort. 2006. Anaplastic lymphoma kinase is dynamically expressed on subsets of motor neurons and in the peripheral nervous system. J. Comp. Neurol. 495:202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwahara, T., J. Fujimoto, D. Wen, R. Cupples, N. Bucay, T. Arakawa, S. Mori, B. Ratzkin, and T. Yamamoto. 1997. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14:439-449. [DOI] [PubMed] [Google Scholar]
- 22.Koopman, G., C. P. Reutelingsperger, G. A. Kuijten, R. M. Keehnen, S. T. Pals, and M. H. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415-1420. [PubMed] [Google Scholar]
- 23.Lamant, L., K. Pulford, D. Bischof, S. W. Morris, D. Y. Mason, G. Delsol, and B. Mariame. 2000. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am. J. Pathol. 156:1711-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llambi, F., F. Causeret, E. Bloch-Gallego, and P. Mehlen. 2001. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20:2715-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehlen, P., and L. Mazelin. 2003. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol. Cell 95:425-436. [DOI] [PubMed] [Google Scholar]
- 26.Mehlen, P., F. Mille, and C. Thibert. 2005. Morphogens and cell survival during development. J. Neurobiol. 64:357-366. [DOI] [PubMed] [Google Scholar]
- 27.Mitsiadis, T. A., M. Salmivirta, T. Muramatsu, H. Muramatsu, H. Rauvala, E. Lehtonen, M. Jalkanen, and I. Thesleff. 1995. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121:37-51. [DOI] [PubMed] [Google Scholar]
- 28.Moog-Lutz, C. H., J. L. Degoutin, J. Y. Gouzi, Y. B. Frobert, N. C. Brunet-De Carvalho, J. F. Bureau, C. E. Creminon, and M. R. Vigny. 2005. Activation and inhibition of ALK receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Biol. Chem. 280:26039-26048. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, R., S. D. Smith, B. K. Hecht, V. Christy, J. D. Mellentin, R. Warnke, and M. L. Cleary. 1989. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis. Blood 73:2155-2164. [PubMed] [Google Scholar]
- 30.Morris, S. W., M. N. Kirstein, M. B. Valentine, K. G. Dittmer, D. N. Shapiro, D. L. Saltman, and A. T. Look. 1994. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263:1281-1284. [DOI] [PubMed] [Google Scholar]
- 31.Morris, S. W., C. Naeve, P. Mathew, P. L. James, M. N. Kirstein, X. Cui, and D. P. Witte. 1997. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 14:2175-2188. (Erratum, 15:2883.) [DOI] [PubMed] [Google Scholar]
- 32.Motegi, A., J. Fujimoto, M. Kotani, H. Sakuraba, and T. Yamamoto. 2004. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J. Cell Sci. 117:3319-3329. [DOI] [PubMed] [Google Scholar]
- 33.Nieborowska-Skorska, M., A. Slupianek, L. Xue, Q. Zhang, P. N. Raghunath, G. Hoser, M. A. Wasik, S. W. Morris, and T. Skorski. 2001. Role of signal transducer and activator of transcription 5 in nucleophosmin/anaplastic lymphoma kinase-mediated malignant transformation of lymphoid cells. Cancer Res. 61:6517-6523. [PubMed] [Google Scholar]
- 34.Pulford, K., L. Lamant, S. W. Morris, L. H. Butler, K. M. Wood, D. Stroud, G. Delsol, and D. Y. Mason. 1997. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 89:1394-1404. [PubMed] [Google Scholar]
- 35.Pulford, K., S. W. Morris, and F. Turturro. 2004. Anaplastic lymphoma kinase proteins in growth control and cancer. J. Cell Physiol. 199:330-358. [DOI] [PubMed] [Google Scholar]
- 36.Reed, J. C., K. S. Doctor, and A. Godzik. 2004. The domains of apoptosis: a genomics perspective. Sci. STKE 2004:re9. [DOI] [PubMed] [Google Scholar]
- 37.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 38.Stoica, G. E., A. Kuo, A. Aigner, I. Sunitha, B. Souttou, C. Malerczyk, D. J. Caughey, D. Wen, A. Karavanov, A. T. Riegel, and A. Wellstein. 2001. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 276:16772-16779. [DOI] [PubMed] [Google Scholar]
- 39.Stoica, G. E., A. Kuo, C. Powers, E. T. Bowden, E. B. Sale, A. T. Riegel, and A. Wellstein. 2002. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277:35990-35998. [DOI] [PubMed] [Google Scholar]
- 40.Stupack, D. G., X. S. Puente, S. Boutsaboualoy, C. M. Storgard, and D. A. Cheresh. 2001. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talpaz, M., R. T. Silver, B. J. Druker, J. M. Goldman, C. Gambacorti-Passerini, F. Guilhot, C. A. Schiffer, T. Fischer, M. W. Deininger, A. L. Lennard, A. Hochhaus, O. G. Ottmann, A. Gratwohl, M. Baccarani, R. Stone, S. Tura, F. X. Mahon, S. Fernandes-Reese, I. Gathmann, R. Capdeville, H. M. Kantarjian, and C. L. Sawyers. 2002. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99:1928-1937. [DOI] [PubMed] [Google Scholar]
- 42.ten Berge, R. L., C. J. Meijer, D. F. Dukers, J. A. Kummer, B. A. Bladergroen, W. Vos, C. E. Hack, G. J. Ossenkoppele, and J. J. Oudejans. 2002. Expression levels of apoptosis-related proteins predict clinical outcome in anaplastic large cell lymphoma. Blood 99:4540-4546. [DOI] [PubMed] [Google Scholar]
- 43.Tulasne, D., J. Deheuninck, F. C. Lourenco, F. Lamballe, Z. Ji, C. Leroy, E. Puchois, A. Moumen, F. Maina, P. Mehlen, and V. Fafeur. 2004. Proapoptotic function of the MET tyrosine kinase receptor through caspase cleavage. Mol. Cell. Biol. 24:10328-10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernersson, E., N. K. S. Khoo, M. L. Henriksson, G. Roos, R. H. Palmer, and B. Hallberg. 2006. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr. Patterns 6:448-451. (First published 31 January 2006; doi: 10.1016/j.modgep.2005.11.006.) [DOI] [PubMed] [Google Scholar]
- 45.Zhang, N., and T. F. Deuel. 1999. Pleiotrophin and midkine, a family of mitogenic and angiogenic heparin-binding growth and differentiation factors. Curr. Opin. Hematol. 6:44-50. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Q., P. N. Raghunath, L. Xue, M. Majewski, D. F. Carpentieri, N. Odum, S. Morris, T. Skorski, and M. A. Wasik. 2002. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immunol. 168:466-474. [DOI] [PubMed] [Google Scholar]