Abstract
Myc is a transcription factor with pleiotropic effects on tumorigenesis which are likely to be mediated by its target genes. A known Myc transcriptional target is the catalytic subunit of telomerase, Tert. However, the contribution of Tert activation to Myc-induced tumorigenesis in vivo remains unknown. In this study, we addressed the role of telomerase in Myc-induced skin papillomatosis by using compound mice with a switchable Myc gene, Inv-MycERTAM mice, in combination with either telomerase deficiency (Terc−/−) or telomerase overexpression (K5-mTert) in the skin. We first demonstrated that Myc activates telomerase in the skin. With Inv-MycERTAM × Terc−/− mice, we further showed that this telomerase activation is partially required to elicit a full hyperplastic Myc-induced response. The presence of critically short telomeres in late-generation Inv-MycERTAM × Terc−/− mice further reduced the skin lesion induced by Myc. On the other hand, telomerase overexpression in the skin of K5-mTert mice augments Myc-induced hyperplasia in the absence of changes in telomere length, suggesting a direct role of telomerase in the Myc protumorigenic response. Taken together, these results highlight telomerase as a mediator of Myc-induced papillomatosis and suggest telomerase as a putative therapeutic target for Myc-dependent lesions.
A plethora of advantageous events for tumor formation and maintenance are triggered by Myc, such as enhanced proliferation, differentiation blockage, and angiogenesis (26). At the same time, Myc activation can be detrimental for tumor growth as it may sensitize cells to apoptosis (9). Both the pro- and antitumorigenic effects of Myc have been linked with its target genes (24, 25), but the contribution of each gene to Myc actions is only starting to be elucidated in vivo (22, 23).
One of the known transcriptional targets of Myc is the catalytic subunit of telomerase or Tert (7, 8, 18, 32, 33, 35). Tert, together with its partner, the telomerase RNA component or Terc, can reconstitute an active telomerase complex in vitro (34). Telomerase is absent form most adult somatic tissues, with the exception of certain stem cell populations and lymphoid tissues, resulting in a progressive loss of telomeric sequences with age (2). This progressive telomere loss has been proposed to suppress tumorigenesis by limiting the replicative potential of cells (1, 2, 16). In agreement with this notion, telomerase is aberrantly reactivated in the majority of human cancers, where it is thought to sustain tumor growth by elongating critically short telomeres and providing limitless replicative potential, which has highlighted telomerase as an attractive target for therapeutic strategies (19, 30). In addition, mounting evidence suggests that telomerase not only promotes tumorigenesis by its ability to elongate short telomeres but might also enhance cell proliferation and survival independently of telomere length (17, 31). In this regard, it has been recently shown that mouse Tert (mTert) overexpression in basal cells and stem cells of mouse skin results in increased epidermal stem cell mobilization and that promotes the anagen phase of hair growth independently of telomere length (12, 28). Interestingly, these telomere length-independent effects of mTert in the skin anticipate the skin tumor-prone phenotype previously described for these mice (17).
Similar to that of humans, mouse adult skin has very low or undetectable levels of telomerase activity, which is increased in late-stage skin tumors (5). To evaluate the contribution of telomerase to Myc actions in vivo, we have used a previously described Myc model of skin papillomatosis, the Inv-MycERTAM mouse (27). In this mouse model, expression of a switchable form of Myc has been targeted to the skin epidermis via the involucrin promoter. Activation of MycERTAM in adult mice by topical and daily application of the ERTAM-activating ligand 4-hydroxytamoxifen (4-OHT) induces keratinocyte proliferation, which in turn provokes an increase in the number of keratinocyte layers in the area treated (13, 27). Given that MycERTAM is driven by the involucrin promoter, the transgene is expressed in the epidermis at the basal-suprabasal transition, further increasing its expression as keratinocytes progress through their differentiation program (13).
To explore a possible role for Myc in telomerase regulation in vivo and a role for telomerase and telomere length in Myc-induced papillomatosis, we have generated various compound mice for Myc and telomerase in the skin. The results described here demonstrate that Myc regulates telomerase activity levels in the skin in vivo by upregulating mTert transcription and that this telomerase activation significantly contributes to Myc-elicited skin papillomatosis. Moreover, we show that critical telomere shortening in telomerase-deficient mice severely impairs the Myc response. Finally, we show that mice doubly transgenic for Myc and mTert show augmented skin papillomatosis, further suggesting a role for telomerase activation in Myc-induced papillomatosis.
MATERIALS AND METHODS
Generation of mutant mice and treatment regimens.
Inv-MycERTAM, Terc−/−, and K5-mTert mice have been described elsewhere (4, 17, 27). Telomerase-deficient Inv-MycERTAM mice were generated from a cross between Inv-MycERTAM mice and Terc+/− mice to obtain Inv-MycERTAM × Terc+/− animals. These mice were then crossed with Terc+/− mice to generate Inv-MycERTAM × first-generation (G1) Terc−/− mice or with second-generation (G2) Terc−/− mice to obtain Inv-MycERTAM × third-generation (G3) Terc−/− mice. Inv-MycERTAM mice overexpressing telomerase in the skin were generated by crossing Inv-MycERTAM mice with K5-mTert mice. All of the above-described double-mutant mice were generated in the same genetic background, C57BL6. MycERTAM protein was activated in the skin of four 8- to 10-week old mutant mice of each genotype by daily topical administration of 4-OHT (1 mg dissolved in 0.2 ml of ethanol [EtOH]; Sigma) to a shaved area (approximately 4 cm2) of the upper dorsal skin. For systemic labeling of S-phase cells, mice received an intraperitoneal injection of 400 μl of bromodeoxyuridine (BrdU; 10 mM in saline) 5 h prior to sacrifice.
Real-time quantitative PCR for mTert mRNA detection.
Three or four 8-week-old mice of the indicated genotypes were treated by daily topical application of 4-OHT or EtOH to the upper dorsal skin. At the indicated time points, treated skin was collected and total RNA was prepared with Trizol (Invitrogen). mTert mRNA detection was performed as previously described (15). Briefly, reverse transcription was conducted with 1 μg of total RNA, random hexamers as primers, and Superscript II reverse transcriptase (Invitrogen). Real-time PCR was done with two dilutions in duplicates of cDNA on an ABI Prism 7700 Sequence Detector (Applied Biosystems), with SYBR Green (Applied Biosystems), where each reaction contained 1× SYBR-Green mix, 3 mM MgCl2, 0.4 μM each primer, 0.5 mM deoxynucleoside triphosphates, and 5 μl of the sample. For each mRNA sample, mTert expression was corrected by the actin mRNA content in each sample. The PCRs employed a set of primers specific for the mTert gene (TERT-F, 5′ GGA TTG CCA CTG GCT CCG 3′; TERT-R, 5′ TGC CTG ACC TCC TCT TGT GAC 3′) and a set specific for actin (ACTIN-F, 5′ GGC ACC ACA CCT TCT ACA ATG 3′, ACTIN-R, 5′ GTG GTG GTG AAG CTG TAG 3′).
Telomerase assays.
Telomerase activity of skin was measured with a modified telomeric repeat protocol (TRAP) as previously described (4). As a control for PCR efficiency, a previously described internal control was used (4). TRAP assays were performed under linear conditions of product amplification, which allowed semiquantitative detection of telomerase activity levels. TRAP products were quantified with ImageJ (version 1.32) software.
IHC of mouse skin.
For BrdU detection in epidermis, samples from the upper back skin or from the tail skin were fixed overnight in neutral-buffered formalin at 4°C, dehydrated through graded alcohols and xylene, and embedded in paraffin. Five-micrometer sections were used for immunohistochemistry (IHC). Prior to IHC, slides were deparaffinized, rehydrated, and immersed in 10 mM citrate solution and epitopes were retrieved by three high-power 5-min microwave pulses. Slides were washed in water, blocked in a 1:10 dilution of normal goat serum (Vectors Labs), and incubated for 1 h at room temperature with a mouse monoclonal antibody against BrdU at 1:400 (clone BU-1; Amersham). Samples were then incubated at room temperature for 1 h with biotinylated secondary antibodies from Vector Labs (goat anti-rabbit at 1:200 or goat anti-mouse at 1:200), followed by signal development with an immunoperoxidase reagent (avidin-biotin-peroxidase-horseradish peroxidase; Vector Labs) and diaminobenzidine (Sigma) as the substrate. Eight different sections per genotype and treatment regimen were slightly counterstained with hematoxylin and analyzed by light microscopy.
Telomere Q-FISH analysis on skin.
For quantitative fluorescence in situ hybridization (Q-FISH) determinations, paraffin-embedded skin sections from the upper back and tail were hybridized with a PNA-telo probe and the fluorescence intensity of telomeres was determined as previously described (17). Slides were deparaffinized in three xylene washes and then treated with 100, 95, and 70% EtOH. Fifty nuclei per keratinocyte layer were captured for each mouse at a magnification of ×100, and the telomere fluorescence was integrated by spot integrated optical density analysis with the TFL-TELO program (17). To correct for daily variations in lamp alignment and intensity, images from fluorescent beads (Molecular Probes, Eugene, Oreg.) and from wild-type (WT) skin were captured and analyzed with the TFL-TELO program. The average telomere fluorescence of WT basal keratinocytes was normalized between different experiments.
Statistical analysis.
Statistical analysis of differences between different mouse cohorts was performed with the Student t test with one tail and two samples of equal variance. Microsoft Excel v. X was used for calculations. For Student's t test, the differences are considered not significant for P > 0.05, significant for P < 0.05, highly significant for P < 0.01, and extremely significant for P < 0.001.
RESULTS
Myc regulates mTert mRNA expression and telomerase activity in vivo.
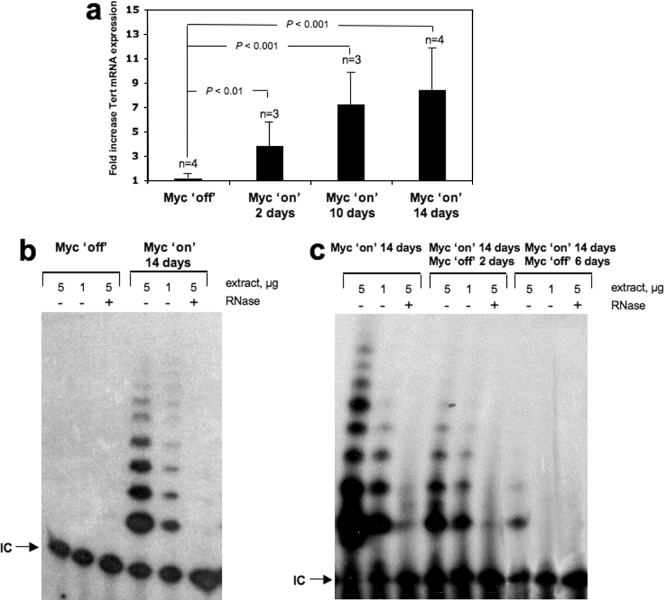
Studies with cultured cells indicated that Myc transcriptionally up-regulates Tert expression, resulting in telomerase activation (7, 8, 18, 33, 35). However, it has not yet been demonstrated whether Myc increases Tert mRNA expression and activates telomerase in the context of the organism. To address this, we examined mTert mRNA expression levels in the skin of Inv-MycERTAM mice treated daily for 2, 10, and 14 days with the activating ligand 4-OHT or with the EtOH carrier as a control (see Materials and Methods). This protocol has been previously demonstrated by us to activate c-MycERTAM in the skin of adult mice (13, 27). We observed that mTert mRNA levels were significantly elevated in 4-OHT-treated (Myc on) Inv-MycERTAM skin as early as 2 days after the start of treatment compared to the EtOH-treated controls (highly significant, P < 0.01 [Fig. 1a]), and they were further increased at days 10 and 14 after Myc induction (extremely significant in both cases, P < 0.001 [Fig. 1a]). Coincidental with augmented mTert mRNA expression, telomerase activity was increased in 4-OHT-treated animals compared to the controls at day 14 after Myc induction (Fig. 1b). A marked increase in telomerase activity was also observed when comparing untreated skin areas to 4-OHT-treated skin areas within the same mouse (data not shown). In agreement with previous findings, control untreated Inv-MycERTAM mice showed no detectable telomerase activity in the skin (Fig. 1b) (5). Similarly, WT skin treated with 4-OHT did not show increased mTert RNA expression or increased telomerase activity compared to untreated WT skin (data not shown). A decrease in telomerase activity was obtained when Myc was deactivated by stopping 4-OHT administration in previously treated Inv-MycERTAM skin (Fig. 1c); in particular, telomerase activity decreased 2 days after Myc was switched off, reaching undetectable levels at day 6 after deactivation. All together, these results indicate that Myc directly or indirectly regulates telomerase levels in vivo in the skin, in particular, that Myc activation leads to increased mTert mRNA levels and increased telomerase activity in the skin, and that this telomerase is progressively downregulated after Myc is switched off.
FIG. 1.
Myc activates telomerase in vivo. (a) mTert mRNA expression levels in Inv-MycERTAM skin treated topically and daily for 2, 10, and 14 days with either EtOH (Myc off) or 4-OHT (Myc on). Three or four (n) skin samples were collected in each treatment from three or four different mice. Averages (black bars) and standard deviations (error bars) are indicated. Differences were highly (P < 0.01) to extremely (P < 0.001) significant compared to the Myc-off controls. (b) Telomerase TRAP activity in representative Inv-MycERTAM skin (n = 4) treated for 14 days with either EtOH (Myc off) or 4-OHT (Myc on). +, treated with RNase; −, not treated with RNase; IC, internal PCR control. (c) Telomerase TRAP activity in Inv-MycERTAM skin. Skin was treated for 14 days with 4-OHT (Myc on), and 4-OHT administration was then stopped to deactivate Myc and skin was harvested immediately or 2 and 6 days thereafter. Skin collected from two different mice per time point was then used to measure telomerase activity with the TRAP assay. +, treated with RNase; −, not treated with RNase.
Roles of telomerase and telomere length in Myc-induced skin papillomatosis.
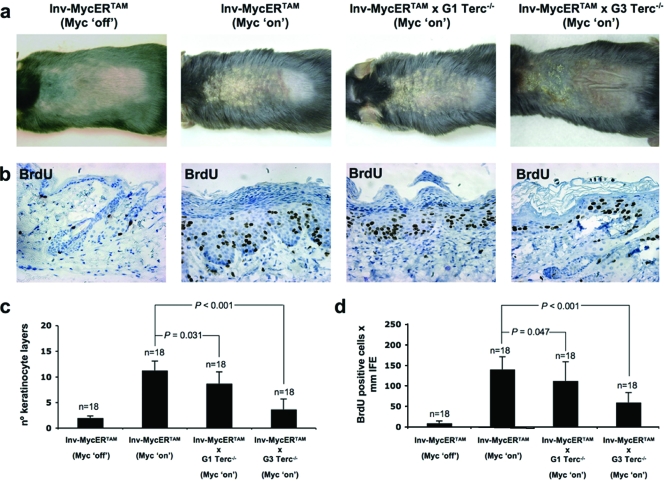
Since we have shown that Myc can upregulate mTert mRNA and activate telomerase in mouse skin in vivo, we next determined whether telomerase upregulation contributes to Myc-induced skin papillomatosis. To this end, we generated Inv-MycERTAM mice that simultaneously lack the telomerase RNA component Terc and therefore cannot reconstitute an active telomerase enzyme (Inv-MycERTAM × Terc−/− mice; see Materials and Methods). Terc-deficient mice have been previously shown to be telomerase deficient and to suffer progressive telomere attrition with increasing generations in the presence of normal mTert levels (4, 21). G1 Terc−/− mice in a C57BL6 background show slightly shorter telomeres than control mice, and G3 Terc−/− mice have critically short telomeres and show severe loss of organismal viability (20). First, 4-OHT treatment of Inv-MycERTAM mice (Myc on) for 14 days induced a marked skin hyperplasia compared to untreated controls (Myc off) (Fig. 2a), as previously described (13). Following Myc activation for 14 days, Inv-MycERTAM × G1 Terc−/− mice showed less-advanced skin lesions in the area of treatment (upper back skin) than similarly treated Inv-MycERTAM mice (Fig. 2a). IHC of 4-OHT-treated skin sections with anti-BrdU antibodies confirmed the macroscopic observations (Fig. 2b shows representative examples). In particular, an ∼24% reduction in the number of interfollicular keratinocyte layers (statistically significant, P = 0.031 [Fig. 2c]) and an ∼17% reduction in the abundance of total BrdU-proliferating cells within the interfollicular epidermis (IFE) (statistically significant, P = 0.047 [Fig. 2d]) were found in Inv-MycERTAM × G1 Terc−/− mouse skin compared to the skin of similarly treated Inv-MycERTAM mice. These findings indicate that the presence of Terc, and therefore of active Tert/Terc telomerase complexes, has a modest but significant effect in eliciting a full Myc-induced hyperplastic response.
FIG. 2.
Telomerase deficiency attenuates Myc-induced hyperplasia. (a) Representative images of EtOH-treated (Myc off) and 4-OHT-treated (Myc on) skin of Inv-MycERTAM, Inv-MycERTAM × G1 Terc−/−, and Inv-MycERTAM × G3 Terc−/− animals. 4-OHT was applied topically and daily for 2 weeks to the upper dorsal skin of three mice per genotype. (b) Representative images of BrdU incorporation in EtOH-treated (Myc off) and 4-OHT-treated (Myc on) skin from Inv-MycERTAM, Inv-MycERTAM × G1 Terc−/−, and Inv-MycERTAM × G3 Terc−/− animals. (c and d) Quantification of the average numbers of keratinocyte layers (black bars in part c) and BrdU-positive cells within the IFE (black bars in part d) from the above-described EtOH-treated and 4-OHT-treated animals. Error bars represent standard deviations. Eighteen skin sections (n = 18) of 1 mm each from a total of three mice per genotype were used for quantification. Differences in the numbers of keratinocyte layers and BrdU-positive cells between genotypes were significant (P = 0.031 and P = 0.047, respectively) for Inv-MycERTAM versus Inv-MycERTAM × G1 Terc−/− mice and extremely significant (P < 0.001 in both cases) for Inv-MycERTAM versus Inv-MycERTAM × G3 Terc−/− mice.
In addition to being telomerase deficient, G1 Terc−/− mice also show shorter telomeres than WT mice in basal skin keratinocytes (6, 16), in agreement with telomerase deficiency during one mouse generation. Given that telomere shortening has been proposed to limit tumor growth in the skin (3, 16), the observed attenuation in the hyperplastic response in Inv-MycERTAM × G1 Terc−/− mice could also be explained, at least in part, by the presence of shorter telomeres in these mice (6, 16). To directly address the effect of telomere length on Myc-induced papillomatosis, we generated transgenic Myc mice in a G3 telomerase-deficient background with critically short telomeres (Inv-MycERTAM × G3 Terc−/− mice; see Materials and Methods). Myc activation for 14 days in Inv-MycERTAM × G3 Terc−/− skin led to a stunted hyperplastic response compared to the controls (Fig. 2a and b show representative examples). In particular, upon 4-OHT treatment, the number of interfollicular keratinocyte layers only increased to 1 to 3 layers in Inv-MycERTAM × G3 Terc−/− mice, in contrast to 9 or 10 layers in the case of similarly treated control Inv-MycERTAM epidermis (Fig. 2b shows examples, and Fig. 2c shows quantification). This represented an ∼78% reduction in 4-OHT-treated MycERTAM × G3 Terc−/− mice compared to similarly treated Inv-MycERTAM controls (extremely significant, P < 0.001 [Fig. 2c]). Coincidentally, the number of total proliferating keratinocytes (BrdU-positive cells) in Inv-MycERTAM × G3 Terc−/− epidermis was lower compared to Inv-MycERTAM epidermis (Fig. 2b shows examples, and Fig. 2d shows quantification). This represented an ∼56% reduction in 4-OHT-treated MycERTAM × G3 Terc−/− mice compared to similarly treated Inv-MycERTAM controls (extremely significant, P < 0.001 [Fig. 2d]). Taken together, these findings demonstrate that short telomeres severely limit Myc-induced papillomatosis in the skin. Furthermore, they show a modest but significant decrease in Myc-induced papillomatosis in G1 Terc−/− mice, suggesting that this response is partially dependent on telomerase activity. In turn, the fact that the Myc-induced hyperplastic response was only partially inhibited in the absence of telomerase in G1 Terc−/− mice suggests that Myc induces papillomatosis by ways that are partially independent of telomerase activation.
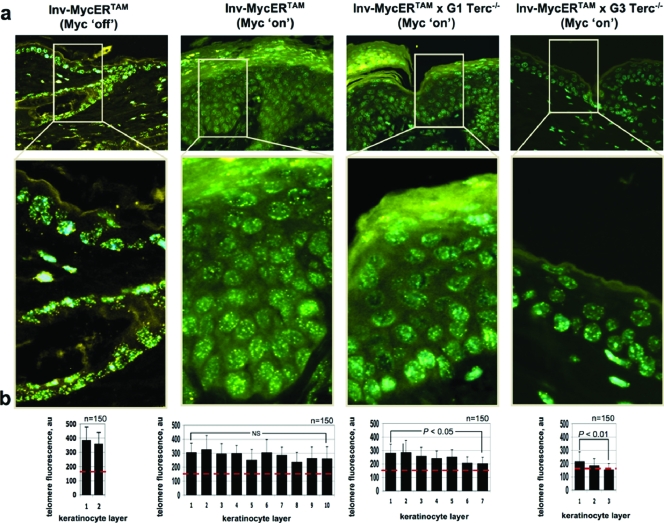
Next, we determined telomere length in Myc-induced skin from Inv-MycERTAM, Inv-MycERTAM × G1 Terc−/−, and Inv-MycERTAM × G3 Terc−/− mice by Q-FISH (see Materials and Methods). First, Myc activation for 14 days did not result in increased telomere length compared to the nontreated animals (see below), in agreement with previous reports showing that increased transgenic Tert expression in the skin does not lead to telomere elongation and supporting the notion that normal-length telomeres are not further elongated by telomerase (17). Next, we observed that the average telomere length per keratinocyte in Inv-MycERTAM 4-OHT-treated skin was maintained relatively constant (an average of 280 ± 29 arbitrary telomere fluorescence units) as keratinocytes migrated from the basal layer to the outermost viable layer of the epidermis (no significant differences between the innermost and outermost viable layers, P > 0.05 [Fig. 3a and b]), in agreement with the known telomerase expression in the skin mitotically active compartments (12). In contrast, both G1 and G3 Inv-MycERTAM × Terc−/− 4-OHT-treated mice showed progressive telomere attrition in skin keratinocytes as they migrate from the basal layer to the surface of the skin (Fig. 3a shows representative examples, and Fig. 3b shows quantification). This represented an ∼29% telomere reduction between the innermost and outermost keratinocyte layers (statistically significant for both G1 and G3 Terc × Inv-MycERTAM, P < 0.05 and P < 0.01, respectively [Fig. 3a and b]), in agreement with the fact that these mice lack telomerase activity because of Terc deficiency. The decline in average telomere length was similar for both Inv-MycERTAM × G1 Terc−/− and Inv-MycERTAM × G3 Terc−/− keratinocytes, but the initial telomere length (value of the innermost layer) was lower in Inv-MycERTAM keratinocytes in a G3 Terc−/− background (an average of 214 ± 63 arbitrary fluorescence units [Fig. 3b]), in agreement with three generations in the absence of telomerase activity. These data indicate the existence of a telomere length-dependent threshold that limits keratinocyte proliferation in the skin, similar to that previously shown for other mouse tissues (29). Furthermore, these results indicate that Myc-dependent mTert upregulation in the absence of Terc is not sufficient to prevent telomere shortening associated with increased hyperplasia.
FIG. 3.
Telomere fluorescence in Myc-induced hyperplastic skin in the presence or absence of Terc. (a) Illustrative images showing telomere fluorescence as determined by Q-FISH in EtOH-treated (Myc off) and 4-OHT-treated (Myc on) skin of Inv-MycERTAM, Inv-MycERTAM × G1 Terc−/−, and Inv-MycERTAM × G3 Terc−/− animals. (b) Quantification of average telomere fluorescence per keratinocyte of the indicated layers (black bars) plotted from the innermost/basal layer (left) to the outermost viable layer (right). Error bars indicate standard deviations. One hundred fifty nuclei per keratinocyte layer (n = 150) were captured from a total of three mice. The red line indicates an average fluorescence of 150 arbitrary units (au). Not significant (NS), P > 0.05; significant, P < 0.05; highly significant, P < 0.01. Note the progressive telomere shortening with increasing generations of Terc-deficient mice (G1 to G3).
Telomerase overexpression augments Myc-induced skin papillomatosis.
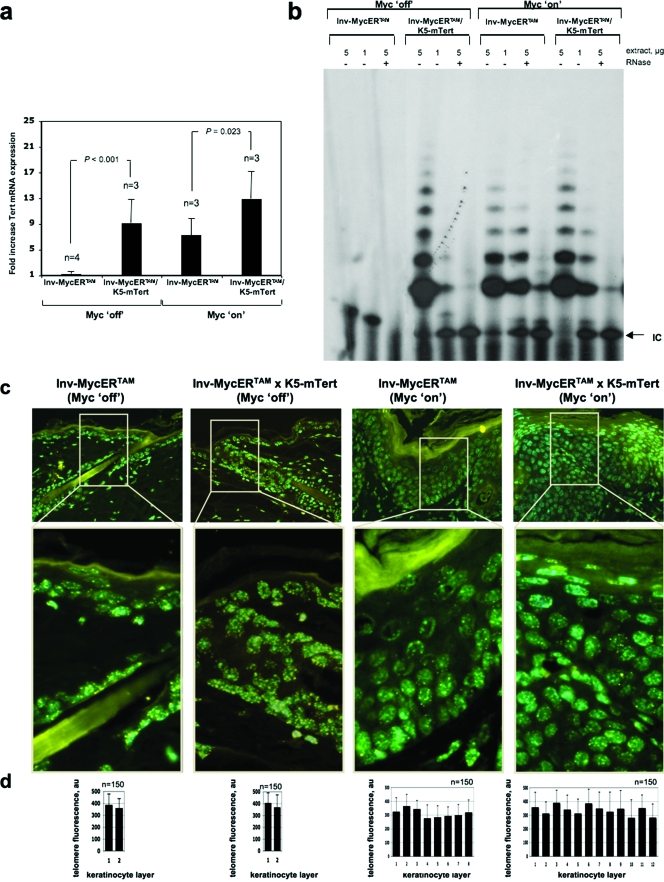
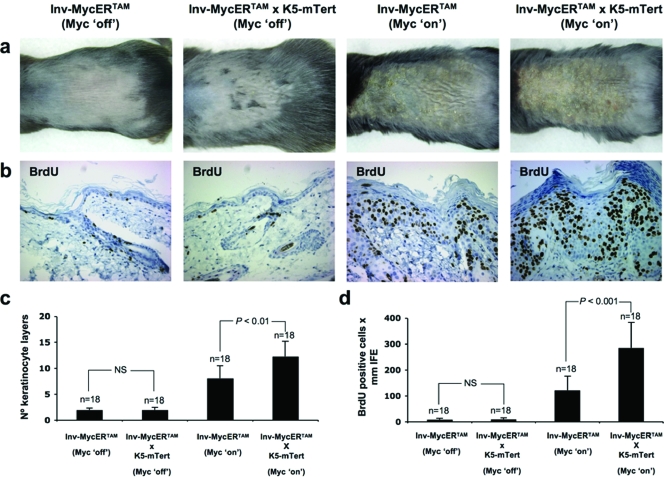
In the past, we have generated transgenic mice that overexpress mTert in the basal keratinocyte layer and the epidermal stem cell compartment of the skin epidermis (K5-mTert mice) (17) (see also Fig. 5a for mRNA levels). K5-mTert transgenic mice have high levels of telomerase activity in the skin and show an increased rate of wound healing and higher susceptibility to both spontaneous and induced skin tumorigenesis in the absence of significant changes in telomere length compared to WT mice (14, 17) (see also Fig. 5b for telomerase activity levels in the skin of K5-mTert mice). Furthermore, K5-mTert mice show an increased mobilization of epidermal stem cells and an increased hair anagen response following mitogenic treatments (12). Here, we have studied the effect of transgenic telomerase overexpression on Myc-induced papillomatosis. To this end, we generated Inv-MycERTAM × K5-mTert compound mice (see Materials and Methods). First, telomerase overexpression in untreated (Myc off) Inv-MycERTAM × K5-mTert mice did not show an increased hyperplastic response compared to untreated (Myc off) Inv-MycERTAM controls (Fig. 4a and b show representative examples; Fig. 4d shows quantification). In contrast, Myc activation (Myc on) in mTert-overexpressing skin led to an earlier onset of the full-blown skin hyperplastic lesions in compound Inv-MycERTAM × K5-mTert mice compared to similarly treated (Myc on) Inv-MycERTAM mice (Fig. 4a and b for representative examples). In particular, 10 days after treatment with 4-OHT, Inv-MycERTAM × K5-mTert mice presented full-blown skin lesions with up to 11 to 12 keratinocyte layers (highly significant difference, P < 0.01 [Fig. 4c]) and a roughly 2.3-fold increase in total BrdU-positive cells compared to similarly treated Inv-MycERTAM mice (extremely significant difference, P < 0.001 [Fig. 4d]). Continued activation of Myc for the subsequent 4 to 7 days progressively increased the number of keratinocyte layers only in Inv-MycERTAM skin, but not in Inv-MycERTAM × K5-mTert skin (data not shown), indicating that the lesion is self-limiting despite telomerase overexpression.
FIG. 5.
mTert overexpression increased mTert mRNA expression in Myc-induced skin, but not telomerase activity or telomere length. (a) mTert mRNA expression levels in the skin of mice of the indicated genotypes treated topically with either EtOH (Myc off) or 4-OHT (Myc on). Three or four skin samples (n) were collected in each treatment from three or four different mice. Averages (black bars) and standard deviations (error bars) are indicated. Statistical significance is indicated. (b) Telomerase TRAP activity in representative Inv-MycERTAM and Inv-MycERTAM × K5-mTert skin treated topically and daily for 10 days with either EtOH (Myc off) or 4-OHT (Myc on). +, treated with RNase; −, not treated with RNase; IC, internal PCR control. Skin samples were collected from three mice per genotype and treatment. (c) Illustrative images showing telomere fluorescence in skin from representative Inv-MycERTAM and Inv-MycERTAM × K5-mTert mice treated as indicated in panel a. (d) Quantification of average telomere fluorescence per keratinocyte of the indicated layers (black bars) plotted from the innermost/basal layer (left) to the outermost viable layer (right). Error bars represent standard deviations. One hundred fifty nuclei (n = 150) per keratinocyte layer were captured from a total of three mice. au, arbitrary units.
FIG. 4.
Tert overexpression augments Myc-induced hyperplasia. (a) Representative images of EtOH-treated (Myc off) and 4-OHT-treated (Myc on) skin from Inv-MycERTAM and Inv-MycERTAM × K5-mTert animals. EtOH or 4-OHT was applied topically and daily for 10 days to the upper back skin of three mice per genotype. (b) Representative images of BrdU incorporation in EtOH-treated (Myc off) and 4-OHT-treated (Myc on) skin from Inv-MycERTAM and Inv-MycERTAM × K5-mTert animals. (c and d) Quantification of average numbers of keratinocyte layers (black bars in panel c) and BrdU-positive cells within the IFE (black bars in panel d). Error bars represent standard deviations. Eighteen skin sections (n = 18) of 1 mm each from a total of three mice per genotype and treatment were used for quantification. Notice the exacerbation of Myc-induced papillomatosis in the K5-mTert background. Differences in the numbers of keratinocyte layers and BrdU-positive cells between genotypes were not significant (NS; P > 0.05) for Inv-MycERTAM (Myc off) versus Inv-MycERTAM × K5-mTert (Myc off) mice and highly significant (P < 0.01) and extremely significant (P < 0.001), respectively, for Inv-MycERTAM (Myc on) versus Inv-MycERTAM × K5-mTert (Myc on) mice.
Next, we studied whether the augmented hyperplastic response of 4-OHT-treated Inv-MycERTAM × K5-mTert mice compared to similarly treated Inv-MycERTAM controls could be explained by differences in mTert mRNA levels, telomerase activity, and/or telomere length. First, we determined mTert mRNA levels by quantitative reverse transcription-PCR (see Materials and Methods). Telomerase mRNA levels were similarly elevated in untreated (Myc off) Inv-MycERTAM × K5-mTert and 4-OHT-treated (Myc on) Inv-MycERTAM skin (Fig. 5a). Interestingly, as shown in Fig. 5a, there was a significant increase in mTert mRNA levels in 4-OHT-treated (Myc on) Inv-MycERTAM × K5-mTert skin compared to similarly treated Inv-MycERTAM controls (significant, P = 0.023 [Fig. 5a]), which is in agreement with mTert being simultaneously up-regulated in both basal (K5-mTert transgene) and suprabasal (Inv-MycERTAM transgene) skin layers. Next, we determined telomerase activity with the TRAP assay (see Materials and Methods). Untreated Inv-MycERTAM skin (Myc off) did not show detectable levels of telomerase activity as determined with the TRAP assay (Fig. 5b) (see Materials and Methods). In contrast, treated Inv-MycERTAM mouse skin (Myc on) and untreated Inv-MycERTAM × K5-mTert mouse skin (Myc off) showed similarly elevated levels of telomerase activity, in agreement with mTert up-regulation in both mouse models (Fig. 5b). Interestingly, telomerase activity levels in 4-OHT-treated compound Inv-MycERTAM × K5-mTert mice (Myc on) were similar to those of similarly treated Inv-MycERTAM mice (Fig. 5a), suggesting that mTert upregulation produced by either Myc activation or direct mTert overexpression is not further increased in the compound mice despite their elevated mTert mRNA levels. In particular, upon Myc activation the Inv-MycERTAM × K5-mTert/Inv-MycERTAM ratio of telomerase activity was 1.17 ± 0.23. Next, telomere Q-FISH indicated that there were no significant differences in the average telomere length of total keratinocytes within the IFE between 4-OHT-treated (Myc on) Inv-MycERTAM × K5-mTert skin and similarly treated Inv-MycERTAM skin (Fig. 5c and d). In particular, the Inv-MycERTAM × K5-mTert/Inv-MycERTAM ratio for telomere length in total keratinocytes within the IFE was 1.13 ± 0.48. These results suggest that combined Myc and mTert upregulation augments Myc-induced papillomatosis coincidentally with increased mTert mRNA levels but in the absence of significant changes in telomerase activity or telomere length (see Discussion).
DISCUSSION
Deregulation of Myc is a frequent event in human cancer, suggesting a possible role for Myc activation in tumor formation and/or maintenance. In support of this idea, several studies with animal models of carcinogenesis have shown that Myc is required for the maintenance of preneoplastic and neoplastic lesions (11, 26). Such Myc dependency or “addiction to Myc” shown by certain tumors types has made it plausible to consider the inhibition of this oncogene in the treatment of neoplasias (10).
By using mouse models, we show here that mTert is transcriptionally activated by Myc in the skin and that this results in Myc-induced telomerase activation. Furthermore, we show that increased telomerase activity partially contributes to Myc protumorigenic actions in vivo. In particular, Myc-mediated mTert upregulation in a Terc-deficient background, therefore in the absence of active telomerase complexes, leads to a modest but significant reduction in the Myc-induced hyperplastic response. Interestingly, this inhibitory effect of telomerase deficiency in Myc-induced papillomatosis is more pronounced in mice with short telomeres (a situation that resemble human tumors), supporting the idea that telomerase inhibitors may show some efficacy in Myc-selective therapeutics of human tumors.
These results highlight the fact that the abilities of skin keratinocytes to respond to mitogenic signals and to produce hyperplastic lesions require maintenance of telomere length above a certain threshold, which would allow keratinocyte proliferation for longer times and enlarge the proliferative compartment. It is important to note, however, that the expansion of the proliferative compartment in Myc-induced skin is self-limited since the execution of the final stages of the keratinocyte terminal differentiation program has been shown to prevail over the ability of Myc to maintain suprabasal keratinocyte proliferation (13).
Finally, we show that although mTert overexpression failed to substitute for Myc activation in inducing papillomatosis, in analogy with their different roles in transformation assays in vitro (18), it significantly augments hyperplastic lesions in Myc-transgenic mice. These results suggest additive effects of mTert overexpression in basal cell layers and Myc upregulation in the upper skin layers, leading to increased Myc-induced skin papillomatosis, which occurs in the absence of significant changes in telomere length. This effect of mTert overexpression augmenting Myc-induced papillomatosis could be explained in light of our recent findings demonstrating that mTert overexpression increases epidermal stem cell mobilization (12), which in turn could have additive effects with Myc expression in the basal-to-suprabasal skin layers.
Acknowledgments
I.F. is a Franco-Peral postdoctoral fellow of the Spanish Association Against Cancer (AECC). Research in the laboratory of M.A.B. is funded by the MCYT (SAF2001-1869, GEN2001-4856-C13-08), by the Regional Government of Madrid, CAM (08.1/0054/01), by the European Union (TELOSENS FIGH-CT.2002-00217, INTACT LSHC-CT-2003-506803, ZINCAGE FOOD-CT-2003-506850, RISC-RAD F16R-CT-2003-508842), and by the Josef Steiner Cancer Award 2003.
We do not have any conflicts of interest.
REFERENCES
- 1.Blasco, M. A. 2002. Telomerase beyond telomeres. Nat. Rev. Cancer 2:627-633. [DOI] [PubMed] [Google Scholar]
- 2.Blasco, M. A. 2005. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 6:611-622. [DOI] [PubMed] [Google Scholar]
- 3.Blasco, M. A., and W. C. Hahn. 2003. Evolving views of telomerase and cancer. Trends Cell Biol. 13:289-294. [DOI] [PubMed] [Google Scholar]
- 4.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 5.Blasco, M. A., M. Rizen, C. W. Greider, and D. Hanahan. 1996. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat. Genet. 12:200-204. [DOI] [PubMed] [Google Scholar]
- 6.Cayuela, M. L., J. M. Flores, and M. A. Blasco. 2005. The telomerase RNA component Terc is required for the tumour-promoting effects of Tert overexpression. EMBO Rep. 6:268-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerezo, A., H. Kalthoff, M. Schuermann, B. Schafer, and P. Boukamp. 2002. Dual regulation of telomerase activity through c-Myc-dependent inhibition and alternative splicing of hTERT. J. Cell Sci. 115:1305-1312. [DOI] [PubMed] [Google Scholar]
- 8.Drissi, R., F. Zindy, M. F. Roussel, and J. L. Cleveland. 2001. c-Myc-mediated regulation of telomerase activity is disabled in immortalized cells. J. Biol. Chem. 276:29994-30001. [DOI] [PubMed] [Google Scholar]
- 9.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 10.Felsher, D. W. 2003. Cancer revoked: oncogenes as therapeutic targets. Nat. Rev. Cancer 3:375-379. [DOI] [PubMed] [Google Scholar]
- 11.Felsher, D. W. Reversibility of oncogene-induced cancer. Curr. Opin. Genet. Dev. 14:37-42. [DOI] [PubMed]
- 12.Flores, I., M. L. Cayuela, and M. A. Blasco. 2005. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309:1253-1256. [DOI] [PubMed] [Google Scholar]
- 13.Flores, I., D. J. Murphy, L. B. Swigart, U. Knies, and G. I. Evan. 2004. Defining the temporal requirements for Myc in the progression and maintenance of skin neoplasia. Oncogene 23:5923-5930. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Suarez, E., J. M. Flores, and M. A. Blasco. 2002. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol. Cell. Biol. 22:7291-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Suarez, E., C. Geserick, J. M. Flores, and M. A. Blasco. 2005. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene 24:2256-2270. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Suarez, E., E. Samper, J. M. Flores, and M. A. Blasco. 2000. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat. Genet. 26:114-117. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Suarez, E., E. Samper, A. Ramirez, J. M. Flores, J. Martin-Caballero, J. L. Jorcano, and M. A. Blasco. 2001. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 20:2619-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg, R. A., R. C. O'Hagan, H. Deng, Q. Xiao, S. R. Hann, R. R. Adams, S. Lichtsteiner, L. Chin, G. B. Morin, and R. A. DePinho. 1999. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene 18:1219-1226. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 20.Herrera, E., E. Samper, J. Martin-Caballero, J. M. Flores, H. W. Lee, and M. A. Blasco. 1999. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18:2950-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Rivera, L., E. Herrera, J. P. Albar, and M. A. Blasco. 1998. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl. Acad. Sci. USA 95:10471-10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miliani de Marval, P. L., E. Macias, R. Rounbehler, P. Sicinski, H. Kiyokawa, D. G. Johnson, C. J. Conti, and M. L. Rodriguez-Puebla. 2004. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol. Cell. Biol. 24:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy, D. J., L. B. Swigart, M. A. Israel, and G. I. Evan. 2004. Id2 is dispensable for Myc-induced epidermal neoplasia. Mol. Cell. Biol. 24:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson, J. A., and J. L. Cleveland. 2003. Myc pathways provoking cell suicide and cancer. Oncogene 22:9007-9021. [DOI] [PubMed] [Google Scholar]
- 25.Patel, J. H., A. P. Loboda, M. K. Showe, L. C. Showe, and S. B. McMahon. 2004. Analysis of genomic targets reveals complex functions of MYC. Nat. Rev. Cancer 4:562-568. [DOI] [PubMed] [Google Scholar]
- 26.Pelengaris, S., M. Khan, and G. Evan. 2002. c-MYC: more than just a matter of life and death. Nat. Rev. Cancer 2:764-776. [DOI] [PubMed] [Google Scholar]
- 27.Pelengaris, S., T. Littlewood, M. Khan, G. Elia, and G. Evan. 1999. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3:565-577. [DOI] [PubMed] [Google Scholar]
- 28.Sarin, K. Y., P. Cheung, D. Gilison, E. Lee, R. I. Tennen, E. Wang, M. K. Artandi, A. E. Oro, and S. E. Artandi. 2005. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satyanarayana, A., S. U. Wiemann, J. Buer, J. Lauber, K. E. Dittmar, T. Wustefeld, M. A. Blasco, M. P. Manns, and K. L. Rudolph. 2003. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J. 22:4003-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shay, J. W., and W. E. Wright. 2002. Telomerase: a target for cancer therapeutics. Cancer Cell 2:257-265. [DOI] [PubMed] [Google Scholar]
- 31.Smith, L. L., H. A. Coller, and J. M. Roberts. 2003. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 5:474-479. [DOI] [PubMed] [Google Scholar]
- 32.Smogorzewska, A., and T. de Lange. 2004. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73:177-208. [DOI] [PubMed] [Google Scholar]
- 33.Wang, J., L. Y. Xie, S. Allan, D. Beach, and G. J. Hannon. 1998. Myc activates telomerase. Genes Dev. 12:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinrich, S. L., R. Pruzan, L. Ma, M. Ouellette, V. M. Tesmer, S. E. Holt, A. G. Bodnar, S. Lichtsteiner, N. W. Kim, J. B. Trager, R. D. Taylor, R. Carlos, W. H. Andrews, W. E. Wright, J. W. Shay, C. B. Harley, and G. B. Morin. 1997. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 17:498-502. [DOI] [PubMed] [Google Scholar]
- 35.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-MYC. Nat. Genet. 21:220-224. [DOI] [PubMed] [Google Scholar]