Abstract
Wnt ligands bind receptors of the Frizzled (Fz) family to control cell fate, proliferation, and polarity. Canonical Wnt/Fz signaling stabilizes β-catenin by inactivating GSK3β, leading to the translocation of β-catenin to the nucleus and the activation of Wnt target genes. Noncanonical Wnt/Fz signaling activates RhoA and Rac, and the latter triggers the activation of c-Jun N-terminal kinase (JNK). Here, we show that exposure of B-lymphocytes to Wnt3a-conditioned media activates JNK and raises cytosolic β-catenin levels. Both the Rac guanine nucleotide exchange factor Asef and the mitogen-activated protein kinase kinase kinase kinase germinal center kinase-related enzyme (GCKR) are required for Wnt-mediated JNK activation in B cells. In addition, we show that GCKR positively affects the β-catenin pathway in B cells. Reduction of GCKR expression inhibits Wnt3a-induced phosphorylation of GSK3β at serine 9 and decreases the accumulation of cytosolic β-catenin. Furthermore, Wnt signaling induces an interaction between GCKR and GSK3β. Our findings demonstrate that GCKR facilitates both canonical and noncanonical Wnt signaling in B lymphocytes.
Wnt genes encode secreted cysteine-rich glycoproteins that regulate many cellular functions, including proliferation, differentiation, survival, cell polarity, and migration (15, 28, 49). Wnt proteins activate the well-characterized canonical signaling pathway as well as others, referred to as noncanonical pathways. A noncanonical pathway, the Drosophila planar cell polarity (PCP) pathway, and a likely vertebrate equivalent, the convergent extension (CE) pathway, involve the activation of small GTPases and c-Jun N-terminal kinase (JNK) (3, 29, 54). Another noncanonical pathway, the Wnt/Ca2+ pathway, triggers the release of intracellular calcium perhaps via the activation of heterotrimeric G proteins (20, 41). This pathway can suppress canonical Wnt signaling (16). Interestingly, Wnt5a−/− mice possess B-cell progenitors that are hyperproliferative and that show evidence of a defective activation of the Wnt/Ca2+ pathway (24).
The canonical β-catenin pathway is the best studied of the Wnt-signaling cascades. In the absence of Wnt ligand, cells regulate β-catenin levels by a protein complex containing Axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3β (GSK3β), which phosphorylates β-catenin (15). The phosphorylated β-catenin is ubiquitinated, transported to the proteasome, and degraded. Wnt ligands bind to a receptor complex containing the seven-transmembrane protein Frizzled (Fz) and the low-density lipoprotein receptor-related protein 5 or 6 (48). Wnt binding leads to the activation of the downstream element Dishevelled (Dvl), which recruits FRAT (frequently rearranged in T-cell lymphoma) (22). Dvl dissociates the GSK3β/APC/Axin complex, reducing β-catenin phosphorylation, ubiquitination, and degradation. A portion of the accumulated cytoplasmic β-catenin translocates to the nucleus, where it associates with members of the T-cell factor (TCF)/Lef-1 transcription family, to activate the transcription of Wnt target genes (2, 30).
Lef-1 is a member of the lymphocyte enhancer factor (LEF)/TCF family of canonical Wnt-signaling transcription factors, which was originally identified in pre-B and T cells. Lef-1-deficient mice exhibit defects in pro-B-cell proliferation and survival (39). Further arguing for the importance of Wnt signaling in B-cell development, Wnt-conditioned media enhanced the proliferation of B-cell progenitors. Tcf-1-deficient mice have major defects in T-cell differentiation in the thymus, while Lef-1−/− Tcf-1−/− mice die at embryonic day 10, with developmental defects reminiscent of Wnt3a−/− mice (12, 45). These and more recent studies demonstrating that Wnt proteins and β-catenins affect lymphocyte progenitor fate and stem cell renewal indicate that early B- and T-cell progenitors utilize Wnt signals for their survival and proliferation (38, 52). Suggesting that signaling through the canonical Wnt-signaling pathway also regulates more mature B cells, Wnt proteins stimulate the accumulation of β-catenin in malignant plasma cells and promote their proliferation (10) and Wnt proteins may have a role in the pathogenesis of B-cell chronic lymphocytic leukemia (26).
The PCP pathway is manifested in Drosophila wing, eye, and sensory-bristle development, and the CE pathway is required in Xenopus for cell movements during gastrulation (19, 29, 49, 50). The PCP/CE pathway shares two key components of the canonical pathway, Fz and Dvl, but its downstream components are distinct and likely to include RhoA, RhoA-associated kinase (ROCK), Rac, and JNK but not β-catenin (3, 11, 23). In 293 cells, Wnt/Fz signaling coactivates RhoA and Rac. Wnt binding to Fz leads to Dvl activation and assembly of a complex including Dvl, Daam1, and RhoA, which leads to activation of ROCK and cytoskeletal changes (14). A parallel signaling pathway also initiated by Dvl recruits and activates Rac, leading to JNK activation (13). The mechanism by which Dvl activates Rac is unknown. Interestingly, APC, which binds to β-catenin and induces its degradation, also interacts with the Rac-specific guanine nucleotide exchange factor (GEF) Asef and stimulates its activity. Experiments based on RNA interference and on the expression of dominant negative mutants implicate the APC-Asef complex in cell migration and in E-cadherin-mediated cell-cell adhesion (17, 18).
No signaling intermediates linking Wnt-mediated Rac-activation to the JNK pathway have been identified. One possibility is one of the germinal center kinases (GCKs). The GCK family includes 28 mammalian kinases, subdivided into 8 subfamilies, which are homologous to Ste20p, a direct upstream activator of the Saccharomyces cerevisiae mitogen-activated protein kinase kinase kinase (MAP3K) Ste11p (9, 21). Members of subfamily 1 include GCK (MAP4K2), hematopoietic progenitor (MAP4K1), germinal center kinase-like kinase (MAP4K3), and GCK-related enzyme (GCKR) (MAP4K5). Making them attractive candidates as an intermediary in Wnt-mediated JNK activation is that they are selective upstream activators of the JNK pathway. All have an N-terminal catalytic domain, a highly variable intermediate region with several proline-rich motifs, a citron homology domain that likely mediates interactions with small GTPases (27), and a conserved C-terminal extension. GCK and GCKR link tumor necrosis factor alpha (TNF-α) signaling to JNK activation (4, 42, 44). TNF-α signaling to GCKR depends upon the ubiquitination of TNF receptor-associated factor 2 and its interaction with GCKR (43). CD40, which belongs to the TNF receptor superfamily, is a critical receptor for the regulation of B-cell function. Treating B cells with CD40 ligand activates GCKR and the JNK pathway (5).
Here, we provide evidence that Wnt signaling triggers an intracellular signaling complex, likely organized by Dvl, which includes GCKR, Asef, and Rac. We show that APC, Asef, and Rac are required for Wnt signaling to activate GCKR and the JNK pathway in B lymphocytes. Surprisingly, we also found that GCKR interacts with GSK3β and affects the β-catenin pathway. These data indicate that GCKR is a positive regulator of canonical and noncanonical Wnt signaling in B lymphocytes and perhaps in other cell types.
MATERIALS AND METHODS
Cell lines, conditioned media, and antibodies.
WEHI-231, BAJI, and Raji B-cell lines were obtained from the American Tissue Culture Collection (Manassas, VA). Those cells were maintained in RPMI 1640 medium with 10% fetal bovine serum. The L-M (TK) cell clone (American Tissue Culture Collection) is transfected with a Wnt3a expression vector. The conditioned media were made according to the accompanying protocol and used in all the B-cell experiments. The negative-control media were prepared with the same cell line that lacks the Wnt3a expression construct. In some experiments, purified Wnt3a purchased from R&D Systems (Minneapolis, MN) was used. The mouse Wnt3a cDNA was a gift from Roel Nusse. This cDNA was cloned into PCR3.1 vector and used to transfect 293 cells for 48 h (10-cm dishes with 5 μg DNA). The media were harvested and used in the Wnt-signaling assays performed with 293 cells. The control media were generated by transfection of the empty vector. The conditioned media were assayed for activity by measurement of TCF luciferase reporter activation. The antibodies against the following proteins were purchased: GCKR (sc-6429), Asef (sc-13278), Dvl (sc-8025), GSK3β (sc-9166), JNK (sc-571), and actin (sc-1616) from Santa Cruz Biotechnology; phospho-JNK (no. 9255), phospho-GSK3β-Ser9 (no. 9336), and GCK (no. 3782) from Cell Signaling; and β-catenin (c-7207; Sigma).
Generation of Gckr−/− mice.
The Gckr targeting vector was constructed by using a 1,260-bp DNA fragment that spanned a portion of intron 7, exon 8, and intron 8. The long arm of the targeting construct started 4 bp inside the beginning of exon 6 and extended 10 kb upstream. In this strategy, a Neo gene cassette replaced Gckr exons 6 and 7, which encoded a portion of the kinase domain. Ten micrograms of linearized targeting vector was electroporated into 129/Sv embryonic stem cells. After selection in G418, surviving colonies were expanded, and clones that had undergone homologous recombination were identified. Correctly targeted embryonic stem cell lines were microinjected into C57BL/6J blastocysts. Chimeric mice that demonstrated germ line transmission of the disrupted Gckr gene were generated. Screening for homozygous Gckr−/− mice was performed by PCR analysis of genomic DNA with Gckr-specific primers. The Gckr mutation were backcrossed onto a C57BL/6 background six times. On both a mixed genetic background and a C57BL/6 background, Gckr−/− mice were born at a normal Mendelian frequency. Those Gckr−/− mice present in litters were viable, showed no obvious phenotypic abnormalities, and lacked GCKR mRNA and protein expression. The mice were housed in specific pathogen-free conditions and used in accordance with the guidelines of the Institutional Animal Care Committee at the NIH.
The production of wild-type and Gckr−/− progenitor B cells.
B-cell progenitor cells were prepared as previously detailed (6). Total bone marrow cells were flushed from femurs and tibias. After red blood cell lysis, 107 bone marrow cells were cultured in tissue culture dishes (100 mm2) in 10 ml/dish of RPMI medium containing 10 ng/ml of interleukin-7 (R&D Systems), 10% fetal calf serum, β-mercaptoethanol (50 μm), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). On day 3 of the culture, another 10 ml of fresh medium containing 10 ng/ml of interleukin-7 was added. On day 7 of the culture, nonadherent B cells were washed two times with medium and harvested. The resulting cells were >95% B220+ CD21− CD23− immunoglobulin G- and D-negative B cells.
Generation of shRNA and stable cell lines.
The sequences used for the GCKR short-hairpin RNA (shRNA) constructs were 5′-CATCGTTGCCTACTTTGGG-3 ′ and 5′-ACAGGAATGCCAGAGCTGA-3′, which were cloned into pSIREN-RetroQ vector (Clontech) and named GCKR1 shRNA and GCKR2 shRNA. A control shRNA, consisting of a scrambled luciferase shRNA in pSIREN-RetroQ vector and referred to as Luc shRNA, was purchased from Clontech. The sequence for the other control shRNA was 5′-GCAGAAGAACGGCATCAAG-3′, which targets green fluorescent protein (GFP) and was cloned into the same vector and called GFP shRNA. The APC and Asef shRNA constructs were kind gifts from Tetsu Akiyama. The GCKR1 shRNA, APC shRNA, and Asef shRNA and the control shRNA from Clontech were transfected into BJAB and Raji cells with a Nucleofector V kit (Amaxa) following the company's protocol. One day after transfection, the cells were placed under selection with either G418 or puromycin. The cell lines were selected for 4 weeks prior to their use in signaling assays.
Immunoprecipitation and immunoblotting.
Cells (5 × 106) were lysed with 0.5 ml lysis buffer (20 mM HEPES [pH 7.4], 2 mM EGTA, 50 mM β-glycerophosphate, 1% Triton-100, 10% glycerol, 2 mM Na3VO4, and a protease inhibitor cocktail). The lysates were incubated with 2 μg of antibody for 2 h at 4°C and the immunocomplexes absorbed to protein G-conjugated Sepharose 6B beads for 1 h at 4°C. The beads were washed eight times with 0.5 ml of the above-described buffer prior to the addition of sodium dodecyl sulfate (SDS) sample buffer. The eluted proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane for immunoblotting with specific antibodies. For detection of cytosolic β-catenin levels, the cells were lysed with lysis buffer (10 mM Tris [pH 7.4], 10 mM NaCl, 3 mM MgCl2, 0.25% NP-40) on ice for 10 min. The broken cells with the buffer were spun for 5 min at 1,500 rpm. The supernatant was spun at 100,000 × g for 150 min at 4°C to generate a supernatant or cytosolic fraction, which was immunoblotted for β-catenin levels.
GCKR, RhoA, and Rac activation assays.
For the GCKR in vitro kinase assay, 5 × 106 cells were lysed and GCKR was immunoprecipitated as described above. The beads were washed with lysis buffer and twice with wash buffer (500 mM LiCl, 100 mM Tris [pH 7.6], 0.1% Triton-100) and resuspended in reaction buffer (20 mM MOPS [morpholinepropanesulfonic acid; pH 7.4], 2 mM EGTA, 10 mM MgCl2, 5 mM MnCl, 0.1% Triton-100) plus [γ-32P]ATP and myelin basic protein. The reactions were terminated by the addition of SDS-PAGE sample buffer and the samples fractionated by SDS-PAGE to examine substrate phosphorylation and to determine the amount of GCKR in the immunoprecipitations. For assessment of RhoA and Rac activation, 5 × 106 cells were lysed with lysis buffer (50 mM Tris [pH 7.4], 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 2 mM Na3VO4, and a protease inhibitor cocktail). The lysates were spun at 14,000 rpm at 4°C for 3 min. The cleared lysates were incubated on ice for 60 to 90 min, with beads conjugated with glutathione S-transferase (GST) fusion to residues 7 to 89 of the mouse Rhotekin Rho binding domain (Upstate), which binds only Rho-GTP, or beads conjugated with a GST fusion protein corresponding to the P21-binding domain (residues 67 to 150) of human PAK-1 (Upstate), which binds Rac-GTP. The beads were washed six times (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, and a protease inhibitor cocktail). The proteins bound to beads were fractionated by 12% SDS-PAGE gel, transferred to membrane, and blotted with antibody against Rho or Rac.
Luciferase assay.
Fifteen nanograms of Super8XTOPFlash or Super8XFOPFlash luciferase reporter (mutated TCF/LCF binding site) as a control luciferase reporter (gifts from Randall Moon) and 3 ng of the Renilla luciferase vector phRL-TK (Promega) to control for transfection efficiency were transfected into 293 cells by use of SuperFect transfection reagent (QIAGEN) following the manufacturer's directions. Twenty-four hours after transfection or treatment with Wnt3a for 6 h, the cells were collected and luciferase activity was detected. Each sample was done in duplicate, and the averages and standard deviations for data from three experiments are shown (see Fig. 5).
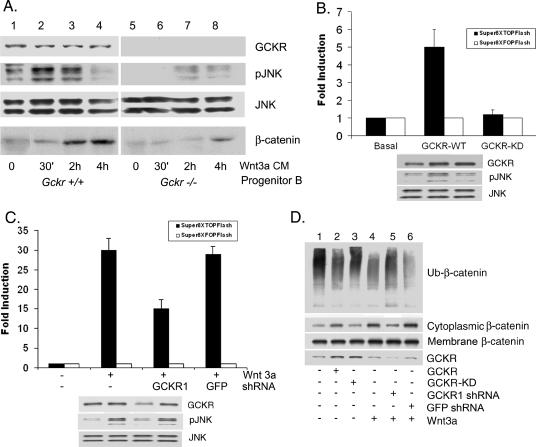
FIG. 5.
Role for GCKR in noncanonical and canonical Wnt signaling. (A) Gckr−/−progenitor B cells are defective in Wnt3a-induced signaling pathways. Bone marrow-derived progenitor B cells from wild-type and Gckr−/− mice were treated with Wnt3a-conditioned media (Wnt3a CM) for various durations and cell lysates prepared. Immunoblots show GCKR, phospho-JNK (pJNK), JNK1/JNK2, and β-catenin levels in the cell lysates. Each blot was exposed for a similar duration. Two experiments were performed, with similar results. (B) Expression of GCKR in 293 cells activates a TCF reporter. Super8XTOPFlash (15 ng) or Super8XFOPFlash (control) were transfected along with a construct expressing either GCKR (100 ng) or a kinase-dead form of GCKR (GCKR-KD; 100 ng). Twenty-four hours later, cell lysates were prepared and luciferase activities measured. The averages and standard deviations for the inductions obtained from triplicate determinations are shown. Experiments were performed a minimum of four times, with similar results. Immunoblots show GCKR, phospho-JNK, and JNK in the cell lysates from the SuperXTOPFlash-transfected cells (lanes 1 to 3). (C) Reducing GCKR expression in 293 cells impairs Wnt3a-induced TCF reporter gene activation. Super8XTOPFlash or Super8XFOPFlash luciferase reporter genes were transfected along with constructs expressing an shRNA targeted at GCKR or GFP. Following the transfection, Wnt3a (30 ng) was added or not added. Twenty-four hours later, cell lysates were prepared and luciferase activities measured. The averages and standard deviations for the inductions of luciferase activities obtained from triplicate determinations are shown. The experiment was performed twice, with similar results. Immunoblots show GCKR, phospho-JNK, and JNK in the cell lysates from the Super8XTOPflash transfections (lanes 1 to 4). (D) GCKR is important for the stabilization of cytoplasmic β-catenin. Constructs expressing GCKR, kinase-dead GCKR, GCKR shRNA, and GFP shRNA were transfected into 293 cells, and Wnt3a (30 ng) was added or not added. The cytoplasmic fraction and cellular membranes were separated. Immunoblots show β-catenin levels (cytoplasmic, membrane associated, and presumably ubiquitinated) and GCKR levels (total cell lysate). The Ub-β-catenin immunoblot shown is a long exposure of the upper portion (above 100 kDa) of the cytoplasmic β-catenin immunoblot. The experiment was performed twice, with similar results.
Cell imaging, FRET, and acceptor photobleaching.
For the imaging studies, 293 cells were plated in glass coverslip bottom microwell dishes (MatTek) in 2 ml complete medium, incubated at 37°C until they were 60 to 70% confluent, and immunofluorescently stained with GCKR- and GSK3β-specific antibodies. A Perkin-Elmer Ultraview confocal system was mounted on a Zeiss Axiovert 200 microscope and equipped with an argon/krypton laser and suitable filters. For live studies, the CO2 was maintained at 5% and the temperature of the sample was maintained at 37°C by use of an environmental controller system (Carl Zeiss). For fluorescence resonance energy transfer (FRET), 293 cells were transfected with the plasmid combinations, each containing appropriate cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) tags on the C termini of GCKR and GSK3β. Cells were incubated with 20 ng/ml of Wnt3a for various times as indicated during imaging. FRET was performed using a Dual-View system (Optical Insights, LLC) attached to an Axiovert 135 inverted epifluorescence microscope (Zeiss) equipped with Sensicam EM high-performance camera (Cooke) and CFP/YFP FRET filter sets. For image acquisition, processing, and analysis, IPlab3.6.5 software was used (Scanalytics, Inc.). FRET images were quantified by calculation of the ratio of the fluorescence intensity emission signal at 480 nm to that at 535 nm when excited at the CFP excitation wavelength at 436 nm. Spillover of CFP into the 535-nm channel was subtracted with the IPlab3.6.5 FRET Ratio Acquire program to give a corrected ratio. Spillover of YFP into the 480-nm channel was negligible. FRET between CFP-GCKR and YFP-GSK3β was further studied by donor dequenching after acceptor photobleaching. FCFP-GCKR was recorded before and after YFP-GSK3β photobleaching with a 1-min illumination at 525/40× (Chroma YFP bleaching set). The increase in FCFP-GCKR after acceptor photobleaching was determined. Photobleaching of cells expressing YFP-GSK3β alone did not show an enhancement of the CFP signal, ruling out the possibility that FRET could result from photoconversion of YFP into a CFP-like species under the condition used in this study. Direct bleaching of CFP by the photobleaching procedure was determined for cells expressing CFP-GCKR alone (5.8%), and all data were corrected for this effect. In all cases, a 63× Plan-Aprochromax 1.4 oil immersion objective was employed.
RESULTS
In B cells, Wnt3a predominantly induces Rac activation.
In 293 cells, both Rac and RhoA can be activated by Wnt signaling and by the expression of Dvl1 or Dvl2 (13). To determine whether Wnt signaling leads to the activation of either Rac or RhoA, we treated three B-cell lines, WEHI-231, Raji, and BJAB, with Wnt3a-conditioned media for 30 min, 2 h, and 4 h. In cell lysates prepared from the treated cells, we checked the amount of Rac-GTP and Rho GTP by using pulldown assays with the appropriate recombinant binding protein. Wnt3a treatment at 30 min and 2 h increased the amounts of Rac-GTP in each of the cell lines, and by 4 h, they had returned to basal levels. The amounts of RhoA-GTP in the various B cell lines were larger than those in Rac-GTP; however, Wnt3a treatment did not induce any significant elevation (Fig. 1A). To confirm that Wnt3a caused the Rac activation, we used recombinant mouse Wnt3a instead of conditioned media to treat BJAB cells for 15 min, 30 min, and 1 h. We found elevated levels of Rac-GTP at each time point, with a peak at 30 min (Fig. 1B). This result was similar to what we had observed with the Wnt3a-conditioned media.
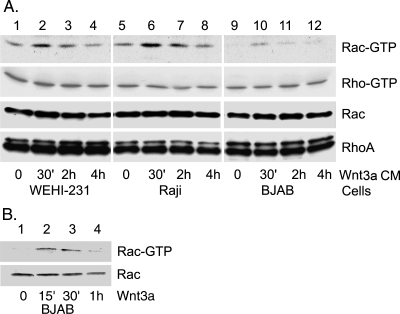
FIG. 1.
Wnt3a-conditioned media (Wnt3a CM) or a recombinant mouse Wnt3a ligand induces Rac but not RhoA activation in B-cell lines. (A) Wnt3a CM induces Rac but not RhoA activation in three B cell lines. WEHI-231 cells, Raji, and BJAB B-cell lines were exposed to Wnt3a CM, and Rac and RhoA activation was measured in cell lysates prepared at various time points following exposure. The levels of GTP-bound Rac and RhoA were determined by pulldown assays and subsequent immunoblotting for Rac (top row) and RhoA (second row). The third and fourth rows show the levels of Rac and RhoA proteins in cell lysates prepared from the WEHI-231, Raji, and BJAB B-cell lines. The anti-Rac antibodies recognize Rac1 and Rac2, while the RhoA antibody recognizes RhoA, RhoB, and RhoC. Experiments were performed three times, with similar results. (B) A recombinant mouse Wnt3a ligand induces Rac activation in the BJAB B-cell line. The BJAB cells were treated with Wnt3a ligand (20 ng/ml) for the indicated times. The GTP-bound Rac was detected by a pulldown assay. The first panel shows the GTP-Rac levels, and the second shows total Rac protein levels.
Exposure of B cells to Wnt3a causes GCKR and JNK activation and the cytosolic accumulation of β-catenin.
After showing that Wnt signaling activates Rac in B cells, we tested whether Wnt3a also caused JNK activation. Because of previous findings implicating GCKR in TNF-α-mediated JNK activation and preliminary experiments in which we had found that activated forms of Rac stimulate GCKR kinase activity, we also examined whether Wnt3a treatment activated GCKR kinase activity. We treated WEHI-231, Raji, and BJAB cells with Wnt3a-conditioned media and checked the endogenous GCKR kinase activity by an in vitro kinase assay and the level of phospho-JNK. The results show that Wnt3a induced GCKR kinase activity and raised the level of phospho-JNK at 30 min and 2 h after exposure, although by 4 h, the level had returned to basal (Fig. 2A). To confirm that the Wnt3a-conditioned media contained active Wnt3a, we competitively inhibited its activity by adding to the conditioned media secreted Frizzled-related protein 1 (sFRP-1), a protein that contains a domain homologous to the Wnt-binding domain of Frizzled receptors. The results show that the added sFRP-1 significantly impaired the previously observed Wnt3a-conditioned-media-induced activation of GCKR and JNK (Fig. 2B). Because Wnt3a signaling also prevents β-catenin from ubiquitin-mediated degradation, causing the cytosolic accumulation of β-catenin, we checked whether Wnt3a-conditioned media led to accumulation of β-catenin in B cells. We found that Wnt3a caused β-catenin accumulation detectable at 30 min, which persisted even at 4 h posttreatment (Fig. 2C), demonstrating that Wnt proteins can trigger the canonical pathway in immature and mature B-cell lines.
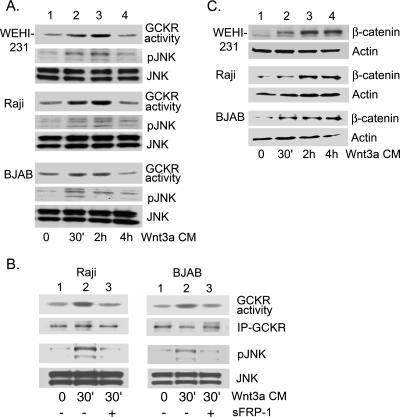
FIG. 2.
Wnt3a-conditioned medium (Wnt3a CM) stimulates GCKR kinase activity and JNK phosphorylation and protects β-catenin from degradation. (A) WEHI-231, Raji, and BJAB B-cell lines were stimulated with Wnt3a CM for 30 min, 2 h, and 4 h. An in vitro kinase assay was used to determine the level of GCKR kinase activity, and JNK activation was assessed by immunoblotting with phospho-specific JNK antibodies. The amounts of JNK1/JNK2 expression in the cell lysates were assessed by immunoblotting with JNK-specific antibodies and are shown. Two experiments were performed, with similar results. (B) sFRP-1 specifically impairs Wnt3a ligand-induced GCKR kinase activity and JNK phosphorylation. Wnt3a CM was incubated with sFRP-1 (100 ng/ml) for 1/2 hour to neutralize Wnt3a ligand in the conditioned media; subsequently, the media with sFRP-1 were used to treat Raji and BJAB cells. Wnt3a CM without sFRP-1 was used to treat the cells for 0 minutes as a basal control and for 30 min as a positive control. Myelin basic protein was used as a substrate in a GCKR in vitro kinase assay. An immunoblot of GCKR from immunoprecipitated GCKR used in the in vitro kinase assay is shown. Cell lysates were immunoblotted for phospho-JNK (pJNK) and total JNK. (C) Wnt3a CM also stimulate the accumulation of β-catenin. The levels of β-catenin and β-actin, which served as a loading control, were determined by immunoblotting of the cell lysates prepared as described above. Three experiments were performed, with similar results.
GCKR, APC, Asef, and Rac involvement in Wnt-induced JNK activation in B cells.
The observation that Wnt3a treatment led to GCKR and JNK activation encouraged us to test the relative importance of GCKR in a Wnt/Fz/Dvl/Rac/JNK pathway. To do so, we created shRNAs targeting two different sites of GCKR mRNA. We tested those shRNAs by blotting the endogenous GCKR following their expression in 293 cells and compared the results to those obtained with two different control shRNAs. The two GCKR shRNAs silenced GCKR expression but did not significantly impair the expression of GCK, a highly related kinase (data not shown). Next, we stably expressed GCKR1 shRNA and a control shRNA in the BJAB and Raji cell lines. We verified that we had reduced GCKR expression and then tested the responses of these and the control cell lines to Wnt signaling. The reduction of GCKR expression in Raji and BJAB cells significantly reduced Wnt3a-induced JNK activation (Fig. 3). To confirm this result, the GCKR1 and GCKR2 shRNAs were both used to reduce GCKR expression in 293 cells. A GFP shRNA was used as a negative control. Both GCKR shRNAs reduced recombinant-Wnt3a-induced JNK activation (data not shown).
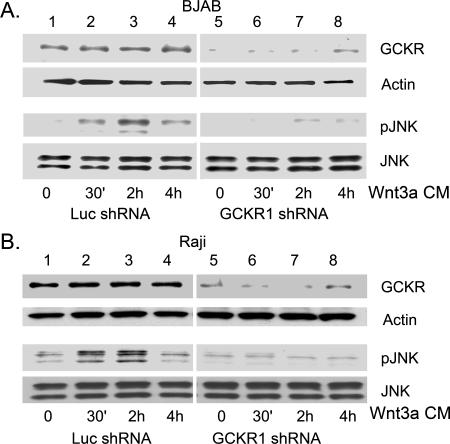
FIG. 3.
Silencing GCKR expression in BJAB and Raji cells impairs JNK activation. BJAB (A) and Raji (B) cells were stably transfected with an shRNA construct designed to interfere with GCKR expression or a control construct. The indicated cell lines were treated with Wnt3a-conditioned media (Wnt3a CM) for 30 min, 2 h, and 4 h. Cell lysates were immunoblotted for GCKR, β-actin, phospho-JNK (pJNK), and JNK1/JNK2. Two experiments were performed, with similar results.
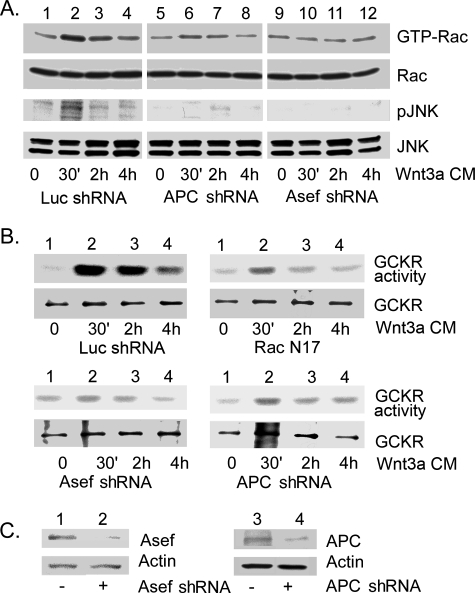
In the Wnt pathway, APC binds to Axin, which is a scaffold protein that facilitates the phosphorylation of β-catenin by GSK3β. APC also binds to the Rac-specific GEF Asef via the Armadillo repeat domain of APC. APC stimulates the GEF activity of Asef and subsequent Rac activation (17, 18). To test whether Asef functions in Wnt signaling to Rac activation, we expressed shRNAs previously shown to target either APC or Asef. We found that the reduction of either APC or Asef significantly impaired Wnt3a-induced Rac activation and the appearance of phospho-JNK (Fig. 4A). Next, we examined Wnt3a-induced GCKR activation in the various cell lines and in cells expressing a dominant negative form of Rac, Rac-N17. In each instance, we monitored GCKR activity by in vitro kinase assays using immunoprecipitated GCKR. In the control cells, Wnt3a-conditioned media induced GCKR activation to a maximal level at 30 min, which nearly returned to basal at 4 h. The cell line expressing the Rac dominant negative form had an increased GCKR basal activity but impaired GCKR activation. The Asef and APC shRNA-expressing cell lines had defective GCKR activation (Fig. 4B). We verified that the amounts of immunoprecipitated GCKR used in the in vitro kinase assay were similar by immunoblotting for GCKR using the upper portion of the gel (Fig. 4B). To demonstrate that we had silenced APC and Asef expression, we blotted cell lysates from the control and the corresponding cell line (Fig. 4C). These results link Wnt signaling in B cells to Rac activation via APC/Asef and to JNK activation via APC/Asef/Rac/GCKR.
FIG. 4.
Wnt3a activates Rac via Asef and APC, and Rac activation is required for Wnt3a-induced GCKR and JNK activation. (A) Effect of silencing APC or Asef expression on Wnt3a-induced Rac and JNK activation. Raji B cells were stably transfected with shRNA constructs targeted at APC or Asef or with a control shRNA construct. The various cell lines were stimulated with Wnt3a-conditioned media (Wnt3a CM) and the cell lysates subjected to a GTP-Rac pulldown assay and immunoblotted for the amount of Rac, phospho-JNK (pJNK), or JNK1/JNK2. Two experiments were performed, with similar results. (B) Wnt3a-induced GCKR activation depends upon Rac activation. Raji cells were stably transfected with constructs expressing either Rac-N17, Asef shRNA, APC shRNA, or a control shRNA and were stimulated with Wnt3a CM for various durations. GCKR immunoprecipitates were subjected to an in vitro kinase assay, using myelin basic protein as a substrate. Each blot was exposed for a similar duration. To verify that equivalent amounts of GCKR were immunoprecipitated, the upper portion of the gel for the kinase assay was removed and immunoblotted for GCKR expression. Each experiment was performed at least twice. (C) Verification that APC and Asef silencing reduce protein expression. Cell lysates from the Raji cells transfected with constructs expressing either APC or Asef shRNAs were subjected to immunoblotting with either APC- or Asef-specific antiserum.
GCKR positively regulates Wnt-induced JNK and β-catenin pathways.
To further document that GCKR is required for Wnt-induced JNK activation, we made use of progenitor B cells prepared from Gckr−/− mice. We checked the levels of phospho-JNK and β-catenin following the treatment of wild-type or Gckr−/− progenitor B cells with Wnt3a-conditioned media. We found that the Gckr−/− cells had a significant defect in Wnt-mediated JNK activation compared to wild-type controls. Gckr−/− progenitor B cells had both a delayed and a suboptimal response. Interestingly, the Gckr−/− progenitor cells also had a defect in their induction of β-catenin following exposure to Wnt3a-conditioned media, suggesting that GCKR may also have a role in the canonical pathway (Fig. 5A).
This result prompted us to further study the potential role of GCKR in the canonical pathway. We used a TCF reporter construct to test whether GCKR positively regulates this pathway. The results show that the expression of GCKR in 293 cells enhanced TCF reporter gene activation, while a kinase-dead version of GCKR did not. GCKR did not affect the activity of the mutant TCF reporter. As expected, GCKR expression caused JNK activation (Fig. 5B). Reduction of GCKR expression in 293 cells by use of GCRK shRNA1 impaired Wnt3a-induced TCF reporter and JNK activation, while the control shRNA did not (Fig. 5C). When we examined levels of cytoplasmic β-catenin and of ubiquitinated β-catenin following Wnt3a stimulation, we found that expression of GCKR, but not its kinase-dead form, reduced β-catenin ubiquitination and increased cytosolic β-catenin levels (Fig. 5D). To determine whether the other GCKR shRNA had a similar effect on the Wnt3a β-catenin pathway, the TCF luciferase reporter assay was carried out. The result indicates that the second GCKR shRNA, like the first one, can impair Wnt3a-induced TCF reporter activation in 293 cells (data not shown).
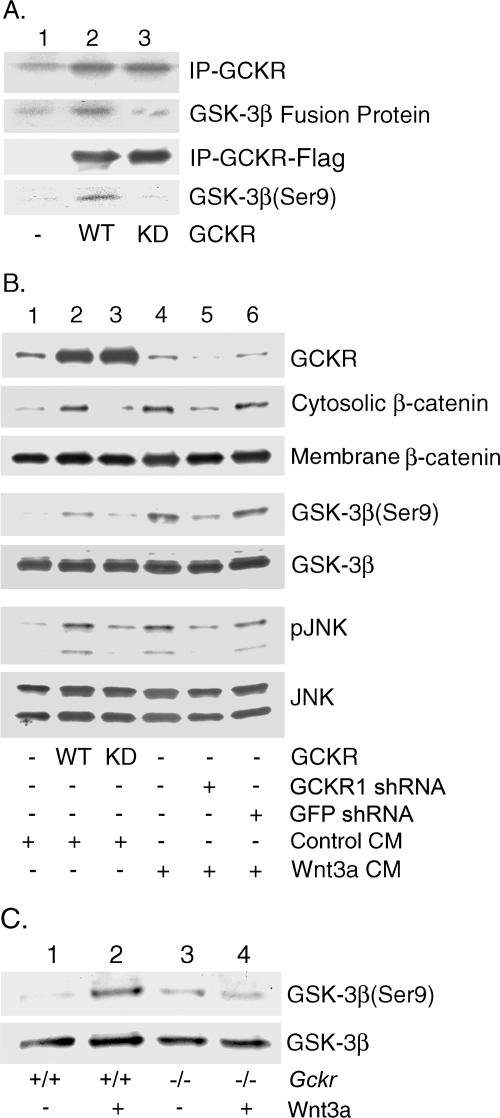
GCKR regulates serine 9 phosphorylation of GSK3β.
GCKR could alter β-catenin degradation by altering the activity of GSK3β. Phosphorylation of GSK3β on serine 9 creates a pseudosubstrate that binds to the active site of the enzyme, thereby competitively inhibiting the binding of the true substrates (8). To test whether GCKR can phosphorylate GSK3β, we used an N-terminal peptide of GSK3 fused to GST (Cell Signaling, Beverly, MA) as a substrate in a GCKR kinase assay. We found that immunoprecipitated GCKR, but not kinase-dead GCKR, phosphorylated the fusion protein in vitro. To determine whether this phosphorylation included serine 9 of GSK3β, the N-terminal peptides of GSK3 phosphorylated by the immunoprecipitated Flag-tagged GCKR or its kinase-dead form were detected with an antibody specific for the phosphorylated residue. Based on this analysis, GCKR can phosphorylate the GSK3β peptide on serine 9 (Fig. 6A). Next, we checked whether GCKR participates in the endogenous phosphorylation of GSK3β in 293 cells. The expression of GCKR, but not its kinase-dead form, led to serine 9 phosphorylation of GSK3β as assessed by immunoblotting with the phospho-specific antibody. Furthermore, treating 293 cells with Wnt3a-conditioned media caused serine 9 GSK3β phosphorylation and reducing GCKR expression impaired this phosphorylation (Fig. 6B). Expression of GCKR, but not its kinase-dead form, led to JNK activation, while silencing GCKR impaired Wnt3a-induced JNK activation (Fig. 6B). Finally, we showed that Wnt3a induced serine 9 phosphorylation of GSK3β in progenitor B cells prepared from the wild type but did so poorly with cells from GCKR−/− mice (Fig. 6C). These results indicated that Wnt signaling activates GCKR, which either directly or indirectly facilitates the inactivation of GSK3β.
FIG. 6.
Involvement of GCKR in the phosphorylation of GSK3β. (A) GCKR phosphorylates an N-terminal GSK3 peptide, and a portion is on serine 9 (Ser9). Constructs expressing GCKR or a kinase-dead form were transfected into 293 cells, and GCKR immunoprecipitates were used in an in vitro kinase assay using as a substrate a 20-amino-acid peptide that spans serines 9 and 21 in the N-terminal portion of GSK3β fused to GST. Shown are the levels of GCKR in the immunoprecipitates (top row) and the phosphorylated fusion protein (second row). Endogenous GCKR in 293 cells accounts for the band in lane 1. Also, Flag empty vector, Flag-GCKR, and Flag-GCKR KD (kinase dead) were transfected into 293 cells. Bead-linked anti-flag antibodies were used to immunoprecipitate the expressed proteins. After being washed six times, the beads were incubated with GST-GSK3 peptide in reaction buffer for 30 min. The third row shows the immunoprecipitated (IP) Flag GCKR. The fourth row shows the serine 9 phosphorylation of GSK3 with a phospho-specific antibody. (B) GCKR is important for serine 9 phosphorylation of GSK3β in 293 cells. Constructs expressing GCKR, the GCKR kinase-dead form, GCKR shRNA, and GFP shRNA were transfected into 293 cells. Media obtained from 293 cells transfected with a control construct or a Wnt3a expression construct were used to treat the cells shown in lanes 1 to 3 and 4 to 6, respectively, for 30 min prior to cell lysis. Immunoblots of cell lysates for GCKR, phosphorylated (serine 9) and total GSK3β, and phosphorylated (pJNK) and total JNK are shown. In addition, a portion of each cell lysate was fractionated into cytosolic and membrane fractions and immunoblotted for β-catenin. WT, wild type. (C) GCKR is important for serine 9 phosphorylation of GSK3β in progenitor B cells. Bone marrow-derived progenitor B cells from wild-type and Gckr−/− mice were prepared and stimulated with Wnt3a for 15 min. Immunoblots of cell lysates for phosphorylated (serine 9) and total GSK3β are shown. All experiments were performed at least twice, with similar results.
Wnt signaling triggers the association of GCKR with Dvl, Asef, and Rac and with GSK3β.
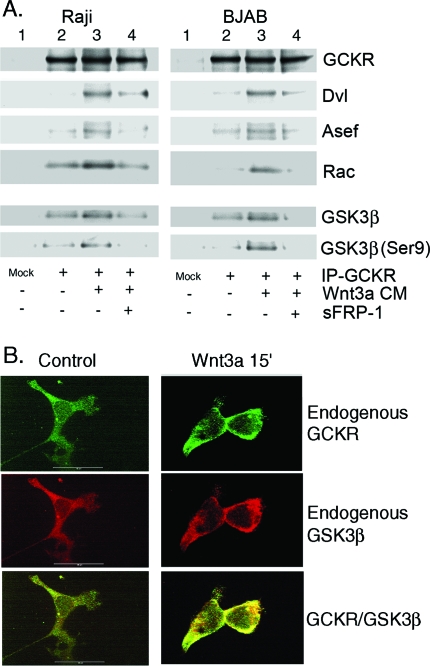
Wnt protein binds to the Fz receptor, which recruits Dvl proteins (51). Dvl organizes a protein complex that includes Rac, which is needed for activation of the JNK pathway (13). Based on our signaling data, Dvl, Asef, and Rac might recruit GCKR into such a protein complex. To provide evidence for this possibility, we treated Raji and BJAB cell lines with Wnt3a-conditioned media, added or did not add s-FRP-1, and examined GCKR immunoprecipitates for the presence of Dvl, Asef, and Rac. In the absence of Wnt signaling or Wnt ligand with s-FRP-1, the GCKR immunoprecipitates contained modest amounts of Rac and smaller amounts of Dvl and Asef. Wnt3a treatment increased the presence of all the proteins in the GCKR immunoprecipitates but more so for Dvl and Asef (Fig. 7A, top panels).
FIG. 7.
Wnt3a enhances the association of GCKR with Dvl, Asef, Rac, and GSK3β. (A) Wnt3a-conditioned media augment the immunoprecipitation of GCKR with Dvl, Asef, Rac, and GSK3β. Raji and BJAB B-cell lines were treated with Wnt3a-conditioned media for 30 min, or sFRP-1 (100 ng/ml) was incubated with Wnt3a-conditioned media for 1/2 hour to neutralize the activity of the Wnt3a ligand before treatment of the cells. The GCKR immunoprecipitates prepared from the cell lysates were immunoblotted for the presence of Dvl, Asef, and Rac proteins. The same GCKR immunoprecipitates were also examined for the presence of GSK3β and serine 9 (Ser9)-phosphorylated GSK3β. Two experiments were performed, with similar results. (B) Wnt3a enhances colocalization of endogenous GCKR with GSK3β. 293 cells were treated with Wnt3a ligand (15 ng/ml) for 15 min. The cells were fixed and stained with antibodies reactive with GCKR (green) and GSK3β (red). Colocalization of GCKR and GSK3β is indicated by yellow.
The data implicating GCKR in the regulation of the activity of GSK3β prompted us to examine the GCKR immunoprecipitates for the presence of GSK3β and to assess its phosphorylation status. Immunoblotting the GCKR immunoprecipitates with a GSK3β-specific antibody and stripping and reblotting them with the GSK3β phosphoserine 9-specific antibody revealed that in the absence of Wnt ligand, the GCKR immunoprecipitates contained some GSK3β, while the addition of Wnt3a-conditioned media enhanced the interaction and led to a larger amount of GSK3β phosphorylated on serine 9 (Fig. 7A, bottom panels). Next, we attempted to colocalize the endogenous proteins by confocal microscopy. We immunofluorescently stained 293 cells with GCKR- and GSK3β-specific antibodies and examined their intracellular locations in the presence or absence of Wnt ligand. The confocal images show that GCKR and GSK3β similarly localized in the cytoplasm and to a lesser degree at the plasma membrane. Wnt3a ligand enhanced the colocalization of GCKR and GSK3β as assessed by a pixel-by-pixel overlay of the confocal images (Fig. 7B).
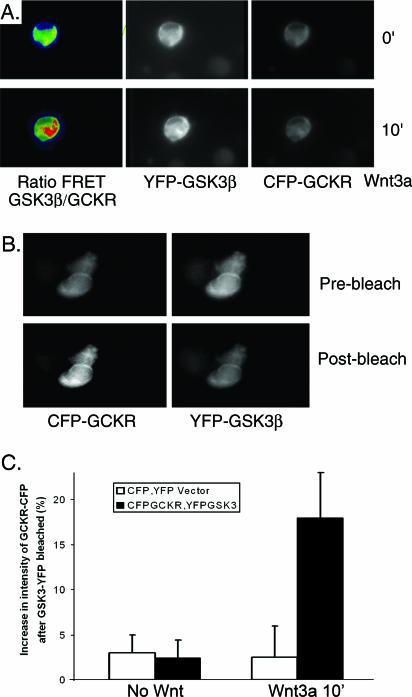
Finally, we used FRET to verify that a direct interaction between GCKR and GSK3β can occur in both live and fixed cells. For the live cells, the interaction between CFP-GCKR and YFP-GSK3β was monitored by the emission ratio between YFP-GSK3β and CFP-GCKR. CFP-GCKR and YFP-GSK3β recapitulated the localization of the endogenous proteins (Fig. 8A). A 10-min exposure to Wnt3a (20 ng/ml) increased the emission ratio between YFP-GSK3β and CFP-GCKR, consistent with a FRET response within the cytoplasm (Fig. 8B). To confirm the ratio-imaging result, we used YFP-GSK acceptor photobleaching with fixed cells previously cotransfected with CFP-GCKR and YFP-GSK3β and exposed to Wnt3a ligand or left unexposed. If FRET occurs between CFP-GCKR and YFP-GSK3β, acceptor photobleaching (YFP-GSK3β) leads to an increase in donor (CFP-GCKR) fluorescence intensity. One minute of photobleaching reduced the fluorescence intensity of YFP-GSK 72 ± 12% and the CFP-GCKR signal 5.8%. Following photobleaching of the Wnt3a-exposed cells, the intensity of CFP-GCKR fluorescence increased 19 ± 5% (n = 8), while no such increase in CFP-GCKR fluorescence occurred in the nonexposed cells (Fig. 8C). As a control, cells expressing CFP and GFP were used. As expected, no FRET could be observed with these proteins, as they do not interact despite their homogenous distribution in the cells (Fig. 8C). These results strongly suggest that Wnt signaling triggers an interaction between GCKR and GSK3β.
FIG. 8.
Further evidence that Wnt signaling triggers an interaction between GCKR and GSK3β. (A) FRET reveals a Wnt-induced interaction between GKCR and GSK3β in live cells. Constructs expressing CFP-GCKR, YFP-GSK3β, or both were transfected into 293 cells. The following day, the cells were incubated in media without serum for 4 h and transferred to the microscope stage, where they were maintained at 37°C with 5% CO2, and Wnt3a (20 ng/ml) was added, during which time the cells were imaged. We briefly illuminated the cells with a 436-nm laser line and simultaneously measured the emission of CFP and YPF and the spillover-corrected FYFP/FCFP ratio. Shown are images simultaneously acquired in the CFP and YFP channels at time zero and 10 min after addition of Wnt3a ligand and the corrected ratio images. The YFP images and the YFP-GSK/CFP-GCKR emission ratio images in response to Wnt3a (20 ng/ml) are shown. The color scale goes from green (no FRET) to yellow to red (high FRET). Similar results were obtained in three experiments. (B) Donor dequenching after acceptor photobleaching confirms an interaction between GCKR and GSK3β. 293 cells were transfected with CFP-GCKR and YFP-GSK3β for 24 h, after which the cells were incubated for 4 h without serum media. Before being fixed, the cells were treated with Wnt3a (20 ng/ml) for 10 min. The cells were illuminated with a 436-nm laser line, and images in the CFP and YFP emission ranges were acquired. Thereafter, the YFP was photobleached and a second CFP image was acquired. Note the increase in the intensity of the CFP image following photobleaching. Experiments were performed four times, with similar results. (C) Quantification of the increase in the intensity of CFP-GCKR after acceptor photobleaching. 293 cells were transfected with CFP-GCKR and YFP-GSK3β for 24 h, after which the cells were incubated for 4 h without serum media. Before being fixed, the cells were treated with Wnt3a (20 ng/ml) for 10 min or left untreated. A minimum of five cells was analyzed in each of three different experiments. As a control, similar experiments were performed on cells transfected with constructs that express CFP and YFP.
DISCUSSION
Normal mouse B-cell progenitors, a mouse pre-B-cell line, and two human B-cell lines of germinal center origin all respond to Wnt3a-conditioned media by activating the canonical and a PCP/CE-like signaling pathway. Additional evidence indicates that the Wnt/Ca2+ pathway exists in lymphocytes since Wnt5a exposure increases nuclear factor in activated T cells (32) and Wnt5a−/− mice possess B cells that show defects consistent with an impaired Wnt/Ca2+-signaling pathway (24). We also showed that the exposure of B cells to Wnt3a-conditioned media activates Rac, and we identified Asef/APC as a critical exchange factor in the pathway. Although we did not detect Wnt-induced RhoA activation in the cell lines we examined, a previous study has demonstrated RhoA activation in a myeloma cell line (37). We provide a link between Wnt-mediated Rac activation and JNK activation, implicating the MAP4K GCKR in the pathway. Previous data had demonstrated a Wnt-induced signaling complex that contains Dvl and Rac (13), and our data suggest that this complex in B lymphocytes also contains Asef and GCKR. We also provide evidence that GCKR positively regulates the canonical pathway. We show that Wnt signaling induces an interaction between GCKR and GSK3β, that Wnt signaling results in decreased levels of β-catenin in Gckr−/− progenitor B cells, and that silencing GCKR expression in 293 cells reduces the accumulation of cytosolic β-catenin and the activity of a TCF reporter gene following Wnt3a stimulation.
Based on genetic and biochemical studies, the PCP-signaling pathway in vertebrates diverges at the level of Dvl, which independently activates RhoA and Rac (13, 31). Interference with either Rac or RhoA function impairs Xenopus laevis gastrulation (13, 14). Our study provides evidence that the elements of the PCP pathway are found in B and presumably T lymphocytes. It is known that expression of Dvl in a variety of cell types leads to both Rac and RhoA activation. Daam1, a formin homology protein, is required for RhoA activation by Dvl, the formation of a Dvl-RhoA complex, and CE movements during Xenopus gastrulation (14). Although germinal center B cells express Daam1 (based on extended-sequence-tag expression), we did not detect a significant increase in the amount of activated RhoA in the different B-cell lines that we tested. This may be secondary to the large amount of GTP- bound RhoA already present. The cell lines contained lower basal levels of Rac-GTP, and Wnt signaling caused a significant increase in them. While lymphocytes express a multitude of Rac exchange factors, including the Vav proteins, Elmo/Dock2, ARHGEF18, ARHGEF6, and ARHGEF2 (36), we show that Asef, ARHGEF4, mediates Wnt-induced Rac activation and signaling to JNK activation in B cells. While APC negatively regulates the Wnt/β-catenin pathway via its interaction with Axin, our data support another role for APC in Wnt signaling, that is, to act along with Asef to enhance Rac activation. Previous work had highlighted the activation of Asef by truncated forms of APC; however, the signal that activated Asef in the presence of wild-type APC remained obscure. Perhaps Wnt proteins are one such signal.
A mechanism that leads from Rac activation to JNK activation in the Wnt/Dvl/Rac/JNK-signaling pathway was also previously unknown (13). We have shown that in B cells, GCKR acts as a link to the JNK pathway. Further studies are needed to determine whether GCKR or other GCK family members perform a similar function in other cell types. The mechanism by which Rac activation causes GCKR activation also needs elucidation. Rac may directly interact with GCKR via its citron homology domain. Such an interaction would be predicted to recruit GCKR into a Dvl organized signaling complex, leading to its activation by one of the components of the complex.
Recently, KLHL12 has been identified to control Dvl protein stability, which servers as a substrate-specific adapter for the cullin-3-based ubiquitin ligase complex. This complex is recruited to Dvl in a Wnt-dependent manner that promotes its polyubiquitination and degradation. Functionally, this complex antagonizes the Wnt β-catenin pathway (1). In TNF-induced JNK activation, the E2/E3 complex of Ubc13-Uev1A and TNF receptor-associated factor 2 are required for GCKR-mediated JNK activation (43). Is polyubiquitination involved in Wnt-induced JNK activation mediated by GCKR? Preliminary data show that the expression of ubiquitin K48R or ubiquitin K63R in 293 cells minimally increases Wnt3a-induced JNK activation (data not shown). These data indicate that a K63-dependent polyubiquitination, which is required for TNF-induced JNK activation, is likely not involved in Wnt-mediated JNK activation. Although the effect of expressing the K48R ubiquitin might be predicted to enhance Wnt3a-induced JNK activation by stabilizing Dvl proteins by interrupting their proteasome degradation, we observed only a minimal effect.
There is a single report of a GCK family member subjected to gene targeting. Mice that lack Nik protein, a class IV GCK family member, die postgastrulation between embryonic days 9.5 and 10.5, with the striking failure of certain mesodermal and endodermal cells to migrate to their correct locations (53). The improper migration of these cells results in embryos that do not develop somites or a hindgut. Since mesodermal and somite development are not perturbed in Jnk1−/− Jnk2−/− mice, a lack of JNK activation in Nik−/− mice probably does not to account for the observed phenotype. Interestingly, Nik protein was originally isolated as a mammalian homolog of a Drosophila Ste20 kinase encoded by misshapen (msn). Genetic experiments on flies indicate that Msn functions downstream of the Frizzled receptor (34, 46). msn mutants show a defect in planar polarity in both wing and eyes due to the failure of Dvl to activate JNK, arguing that another GCK family member may serve a similar function in mammalian development.
An interesting observation during the course of our studies was that the Gckr−/− progenitor B cells had low basal levels of β-catenin compared to wild-type cells and a delayed increased in β-catenin levels following exposure to Wnt-conditioned media. This observation suggests that GCKR may regulate the β-catenin pathway. Providing further evidence for such a role are the following observations. First, expression of GCKR stabilizes β-catenin in the cytosol and induces TCF-dependent reporter gene activation, while silencing GCKR expression reduces Wnt3a-induced increases in cytosolic β-catenin level and TCF-dependent reporter gene activation. Second, GCKR can phosphorylate an N-terminal recombinant fusion protein of GSK3β and enhance the in vivo phosphorylation of GSK3β on serine 9, while silencing GCKR reduces such phosphorylation. Third, the intracellular expression patterns of the two proteins overlap and GCKR immunoprecipitates contain GSK3β, whose presence increases following Wnt signaling. Fourth, ratio imaging of CFP- and YFP-tagged proteins and FRET studies using acceptor photobleaching indicates a dynamic interaction between GCKR and GSK3β following Wnt signaling.
Other kinases involved in Wnt signaling function only in one pathway or to enhance one pathway at the expense of the other. For example, casein kinase Iα is an Axin-associated negative regulator of Wnt signaling that functions as a priming kinase, whose phosphorylation of β-catenin is required for subsequent phosphorylation by GSK3β (25). Casein kinase Iɛ functions as a molecular switch to direct Dvl from the JNK pathway to the β-catenin pathway by altering the conformation of the C terminus of Dvl (7, 35, 40). Par-1 kinase associates with Dvl. Treating cells with Wnt increases Par-1 kinase activity, coincident with Dvl phosphorylation. Par-1 potentiates Wnt activation of the β-catenin pathway but blocks the JNK pathway (47). In contrast, GCKR functions as a positive regulator of Wnt-mediated JNK activation and Wnt-mediated stabilization of β-catenin. It does so presumably by acting downstream of Rac and upstream of the JNK module and via its interaction with and phosphorylation of GSK3β, respectively.
A recent study links JNK activation and the β-catenin-signaling pathway (33). Phosphorylated c-Jun was found to interact with TCF4 to form a ternary complex that contained β-catenin. This complex bound the c-Jun promoter and regulated intestinal cancer development in mice (33). Our study uniquely positions GCKR as a modulator of Wnt-mediated c-Jun phosphorylation and Wnt-mediated translocation of β-catenin to the nucleus and thereby should facilitate the integration of these two signaling pathways.
Acknowledgments
We thank Mary Rust for excellent editorial assistance, Anthony Fauci and the National Institutes of Allergy and Infectious Diseases for their continued support, Tetsu Akiyama for providing us with the APC and Asef expression vectors and the APC and Asef RNA interface constructs, and Randall Moon for giving us the Super8XTOPFlash and Super8XFOPFlash luciferase reporter constructs.
REFERENCES
- 1.Angers, S., C. J. Thorpe, T. L. Biechele, S. J. Goldenberg, N. Zheng, M. J. Maccoss, and R. T. Moon. 2006. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8:348-357. [DOI] [PubMed] [Google Scholar]
- 2.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. [DOI] [PubMed] [Google Scholar]
- 3.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 4.Chadee, D. N., T. Yuasa, and J. M. Kyriakis. 2002. Direct activation of mitogen-activated protein kinase kinase kinase MEKK71 by the Ste20p homologue GCK and the adapter protein TRAF2. Mol. Cell. Biol. 22:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin, A. I., J. Shu, C. Shan Shi, Z. Yao, J. H. Kehrl, and G. Cheng. 1999. TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol. Cell. Biol. 19:6665-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 7.Cong, F., L. Schweizer, and H. Varmus. 2004. Casein kinase Iɛ modulates the signaling specificities of Dishevelled. Mol. Cell. Biol. 24:2000-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 9.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 10.Derksen, P. W., E. Tjin, H. P. Meijer, M. D. Klok, H. D. MacGillavry, M. H. van Oers, H. M. Lokhorst, A. C. Bloem, H. Clevers, R. Nusse, R. van der Neut, M. Spaargaren, and S. T. Pals. 2004. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc. Natl. Acad. Sci. USA 101:6122-6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanto, M., U. Weber, D. I. Strutt, and M. Mlodzik. 2000. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 10:979-988. [DOI] [PubMed] [Google Scholar]
- 12.Galceran, J., I. Farinas, M. J. Depew, H. Clevers, and R. Grosschedl. 1999. Wnt3a(−/−)-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 13:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habas, R., I. B. Dawid, and X. He. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17:295-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habas, R., Y. Kato, and X. He. 2001. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107:843-854. [DOI] [PubMed] [Google Scholar]
- 15.Huelsken, J., and J. Behrens. 2002. The Wnt signalling pathway. J. Cell Sci. 115:3977-3978. [DOI] [PubMed] [Google Scholar]
- 16.Ishitani, T., S. Kishida, J. Hyodo-Miura, N. Ueno, J. Yasuda, M. Waterman, H. Shibuya, R. T. Moon, J. Ninomiya-Tsuji, and K. Matsumoto. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 23:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki, Y., R. Sato, and T. Akiyama. 2003. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat. Cell Biol. 5:211-215. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki, Y., T. Senda, T. Ishidate, R. Koyama, T. Morishita, Y. Iwayama, O. Higuchi, and T. Akiyama. 2000. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 289:1194-1197. [DOI] [PubMed] [Google Scholar]
- 19.Keller, R. 2002. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298:1950-1954. [DOI] [PubMed] [Google Scholar]
- 20.Kuhl, M., L. C. Sheldahl, M. Park, J. R. Miller, and R. T. Moon. 2000. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16:279-283. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 22.Li, L., H. Yuan, C. D. Weaver, J. Mao, G. H. Farr III, D. J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, L., H. Yuan, W. Xie, J. Mao, A. M. Caruso, A. McMahon, D. J. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 24.Liang, H., Q. Chen, A. H. Coles, S. J. Anderson, G. Pihan, A. Bradley, R. Gerstein, R. Jurecic, and S. N. Jones. 2003. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4:349-360. [DOI] [PubMed] [Google Scholar]
- 25.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 26.Lu, D., Y. Zhao, R. Tawatao, H. B. Cottam, M. Sen, L. M. Leoni, T. J. Kipps, M. Corr, and D. A. Carson. 2004. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 101:3118-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madaule, P., T. Furuyashiki, T. Reid, T. Ishizaki, G. Watanabe, N. Morii, and S. Narumiya. 1995. A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 377:243-248. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. R., A. M. Hocking, J. D. Brown, and R. T. Moon. 1999. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene 18:7860-7872. [DOI] [PubMed] [Google Scholar]
- 29.Mlodzik, M. 2002. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 18:564-571. [DOI] [PubMed] [Google Scholar]
- 30.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 31.Moriguchi, T., K. Kawachi, S. Kamakura, N. Masuyama, H. Yamanaka, K. Matsumoto, A. Kikuchi, and E. Nishida. 1999. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274:30957-30962. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, L. L., and C. C. Hughes. 2002. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J. Immunol. 169:3717-3725. [DOI] [PubMed] [Google Scholar]
- 33.Nateri, A. S., B. Spencer-Dene, and A. Behrens. 2005. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437:281-285. [DOI] [PubMed] [Google Scholar]
- 34.Paricio, N., F. Feiguin, M. Boutros, S. Eaton, and M. Mlodzik. 1999. The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 18:4669-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters, J. M., R. M. McKay, J. P. McKay, and J. M. Graff. 1999. Casein kinase I transduces Wnt signals. Nature 401:345-550. [DOI] [PubMed] [Google Scholar]
- 36.Pruitt, K. D., K. S. Katz, H. Sicotte, and D. R. Maglott. 2000. Introducing RefSeq and LocusLink: curated human genome resources at the NCBI. Trends Genet. 16:44-47. [DOI] [PubMed] [Google Scholar]
- 37.Qiang, Y. W., Y. Endo, J. S. Rubin, and S. Rudikoff. 2003. Wnt signaling in B-cell neoplasia. Oncogene 22:1536-1545. [DOI] [PubMed] [Google Scholar]
- 38.Reya, T., A. W. Duncan, L. Ailles, J. Domen, D. C. Scherer, K. Willert, L. Hintz, R. Nusse, and I. L. Weissman. 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409-414. [DOI] [PubMed] [Google Scholar]
- 39.Reya, T., M. O'Riordan, R. Okamura, E. Devaney, K. Willert, R. Nusse, and R. Grosschedl. 2000. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13:15-24. [DOI] [PubMed] [Google Scholar]
- 40.Sakanaka, C., P. Leong, L. Xu, S. D. Harrison, and L. T. Williams. 1999. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc. Natl. Acad. Sci. USA 96:12548-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saneyoshi, T., S. Kume, Y. Amasaki, and K. Mikoshiba. 2002. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295-299. [DOI] [PubMed] [Google Scholar]
- 42.Shi, C. S., and J. H. Kehrl. 1997. Activation of stress-activated protein kinase/c-Jun N-terminal kinase, but not NF-kappaB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem. 272:32102-32107. [DOI] [PubMed] [Google Scholar]
- 43.Shi, C. S., and J. H. Kehrl. 2003. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278:15429-15434. [DOI] [PubMed] [Google Scholar]
- 44.Shi, C. S., A. Leonardi, J. Kyriakis, U. Siebenlist, and J. H. Kehrl. 1999. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J. Immunol. 163:3279-3285. [PubMed] [Google Scholar]
- 45.Staal, F. J., and H. C. Clevers. 2003. Wnt signaling in the thymus. Curr. Opin. Immunol. 15:204-208. [DOI] [PubMed] [Google Scholar]
- 46.Su, Y. C., J. E. Treisman, and E. Y. Skolnik. 1998. The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev. 12:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun, T. Q., B. Lu, J. J. Feng, C. Reinhard, Y. N. Jan, W. J. Fantl, and L. T. Williams. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3:628-636. [DOI] [PubMed] [Google Scholar]
- 48.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 49.Veeman, M. T., J. D. Axelrod, and R. T. Moon. 2003. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 5:367-377. [DOI] [PubMed] [Google Scholar]
- 50.Wallingford, J. B., S. E. Fraser, and R. M. Harland. 2002. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2:695-706. [DOI] [PubMed] [Google Scholar]
- 51.Wharton, K. A., Jr. 2003. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253:1-17. [DOI] [PubMed] [Google Scholar]
- 52.Willert, K., J. D. Brown, E. Danenberg, A. W. Duncan, I. L. Weissman, T. Reya, J. R. Yates III, and R. Nusse. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448-452. [DOI] [PubMed] [Google Scholar]
- 53.Xue, Y., X. Wang, Z. Li, N. Gotoh, D. Chapman, and E. Y. Skolnik. 2001. Mesodermal patterning defect in mice lacking the Ste20 NCK interacting kinase (NIK). Development 128:1559-1572. [DOI] [PubMed] [Google Scholar]
- 54.Yamanaka, H., T. Moriguchi, N. Masuyama, M. Kusakabe, H. Hanafusa, R. Takada, S. Takada, and E. Nishida. 2002. JNK functions in the noncanonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]