Abstract
Human DNA polymerase ι (Pol ι) differs from other DNA polymerases in that it exhibits a marked template specificity, being more efficient and accurate opposite template purines than opposite pyrimidines. The crystal structures of Pol ι with template A and incoming dTTP and with template G and incoming dCTP have revealed that in the Pol ι active site, the templating purine adopts a syn conformation and forms a Hoogsteen base pair with the incoming pyrimidine which remains in the anti conformation. By using 2-aminopurine and purine as the templating residues, which retain the normal N7 position but lack the N6 of an A or the O6 of a G, here we provide evidence that whereas hydrogen bonding at N6 is dispensable for the proficient incorporation of a T opposite template A, hydrogen bonding at O6 is a prerequisite for C incorporation opposite template G. To further analyze the contributions of O6 and N7 hydrogen bonding to DNA synthesis by Pol ι, we have examined its proficiency for replicating through the 6O-methyl guanine and 8-oxoguanine lesions, which affect the O6 and N7 positions of template G, respectively. We conclude from these studies that for proficient T incorporation opposite template A, only the N7 hydrogen bonding is required, but for proficient C incorporation opposite template G, hydrogen bonding at both the N7 and O6 is an imperative. The dispensability of N6 hydrogen bonding for proficient T incorporation opposite template A has important biological implications, as that would endow Pol ι with the ability to replicate through lesions which impair the Watson-Crick hydrogen bonding potential at both the N1 and N6 positions of templating A.
Human DNA polymerase ι (Pol ι), a member of the Y family of polymerases, differs from other DNA polymerases in that it incorporates nucleotides opposite template purines with a much higher efficiency and fidelity than opposite template pyrimidines (4, 6, 19, 21, 26). Pol ι exhibits the highest efficiency and fidelity opposite template A, where it misincorporates nucleotides with frequencies of ∼10−4 to 10−5; opposite template G, Pol ι incorporates a C with an efficiency that is 5- to 10-fold reduced compared to a T opposite template A, and a T is misincorporated opposite template G with an efficiency that is only a fewfold-lowered than that for a C. Pol ι's efficiency and fidelity are much reduced opposite pyrimidine templates, particularly opposite template T (4, 6, 19, 21, 26).
The ternary crystal structures of Pol ι with template A and incoming dTTP and with template G and incoming dCTP have shown that the purine template adopts a syn conformation in the Pol ι active site and forms a Hoogsteen base pair with the incoming nucleotide which remains in the anti conformation (14, 16). The binding of the pyrimidine nucleotide induces a conformational change in the template purine from anti to syn in the Pol ι active site, and that results because the active site can accommodate only the ∼8.6-Å C1′-C1′ distance of sugars in a Hoogsteen base pair but not the C1′-C1′ distance of ∼10.5 Å in a Watson-Crick base pair (14-16).
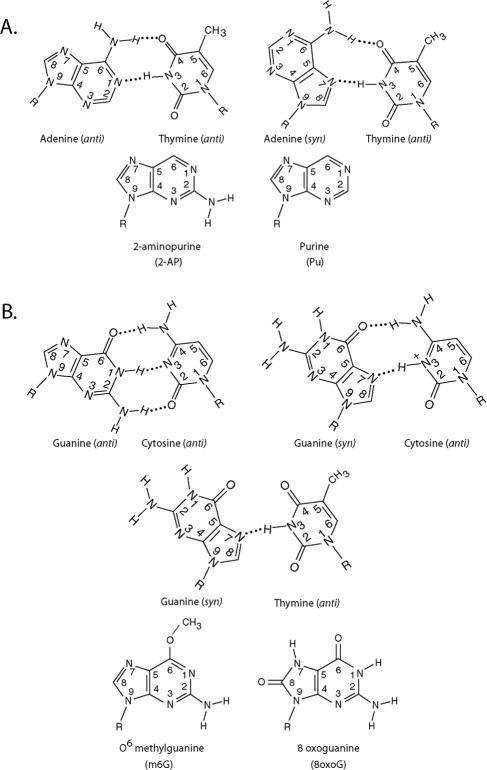
In Pol ι, in the A · T Hoogsteen base pair, two hydrogen bonds are formed between the “Hoogsteen edge” of template A (N7 and N6) and the Watson-Crick (W-C) edge of incoming T (N3 and O4), and in the G · C Hoogsteen base pair, the Hoogsteen edge of G (N7 and O6) hydrogen bonds with the W-C edge of incoming C (N3 and N4) (Fig. 1). To evaluate the contribution that hydrogen bonding at the “Hoogsteen edge” of template purines makes to the efficiency and fidelity of nucleotide incorporation by Pol ι, here we have examined the effects of substitutions of templates A and G by their analogs which disrupt the hydrogen bonding potential of the Hoogsteen edge. In addition, we have analyzed the impact of DNA lesions 6O-methyl guanine (m6G) and 8-oxoguanine (8-oxoG), which affect the Hoogsteen edge of template G, on DNA synthesis by Pol ι. We conclude from these studies that whereas hydrogen bonding at the N7 position is indispensable for nucleotide incorporation opposite both the purine nucleotides, hydrogen bonding at the N6 of A versus the O6 of G has very different effects on the efficiency and fidelity of nucleotide incorporation by Pol ι. We discuss the implications of these observations for Pol ι's role in promoting synthesis through the DNA lesions which eliminate the Watson-Crick hydrogen bonding potential of the templating purine.
FIG. 1.
Watson-Crick versus Hoogsteen base pairing. (A) Hydrogen bonding in an A-T base pair. The structures of 2-aminopurine and purine are also shown. (B) Hydrogen bonding in a G-C base pair. The hydrogen bonding for a G · T Hoogsteen base pair is also shown, and the structures of 6O-methyl guanine and 8-oxoguanine are shown.
MATERIALS AND METHODS
Protein purification and DNA substrates.
Full-length human Pol ι was expressed and purified from Saccharomyces cerevisiae harboring plasmid pBJ956 as described previously (4). The glutathione S-transferase tag was removed by treatment with Prescission protease (Amersham Pharmacia), which resulted in an N-terminal 7-amino-acid leader peptide attached to Pol ι. Oligonucleotides containing a site-specific A, 2-aminopurine (2AP), purine (Pu), m6G, or 8-oxoG residue were purchased from Midland Scientific (Midland, Texas). Oligonucleotides were polyacrylamide gel electrophoresis purified before use. To assay insertion opposite A, 2-AP, or Pu, the oligodeoxynucleotide primer (32-mer), 5′ GTTTTCCCAG TCACGACGAT GCTCCGGTAC TC 3′, was annealed to a 52-mer template, 5′ TTCGTATAAT GCCTACACTX GAGTACCGGA GCATCGTCGT GACTGGGAAAAC 3′, where the X denotes an A, 2-AP, or Pu residue.
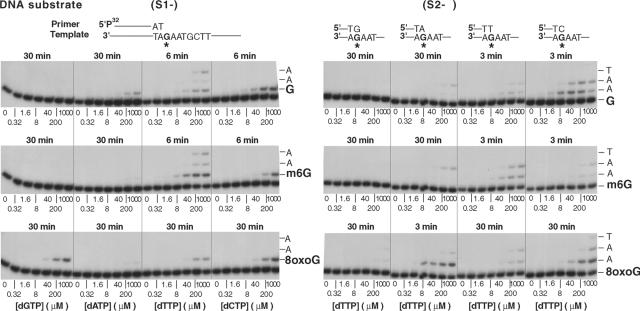
S1 DNA substrates, shown in Fig. 3, were generated by annealing a 75-nucleotide oligomer template (5′ AGC TAC CAT GCC TGC CTC AAG AAT TCG TAA XAT GCC TAC ACT GGA GTA CCG GAG CAT CGT CGT GAC TGG GAA AAC 3′), containing a G, a m6G, or an 8-oxoG at position 31 (X), to the 5′ 32P-labeled oligonucleotide primer, N4309 (5′ GTT TTC CCA GTC ACG ACG ATG CTC CGG TAC TCC AGT GTA GGCAT 3′). For S2 DNA substrates, also shown in Fig. 3, the same 75-nucleotide template oligonucleotides were annealed to four different 5′ 32P-labeled oligonucleotide primers, 5′-GTT TTC CCA GTC ACG ACG ATG CTC CGG TAC TCC AGT GTA GGCATN-3′, where N is G, A, T, or C.
FIG. 3.
Steady-state kinetic analysis of insertion and extension reactions catalyzed by Pol ι on m6G- and 8-oxoG-containing DNA templates. Gel assay of nucleotide incorporation (left) and extension (right) reactions across from m6G and 8-oxoG residues by Pol ι are shown. DNA substrate (30 nM) was incubated with Pol ι (1 nM) and different concentrations of a single nucleotide at 37°C for various times (3 to 30 min), as indicated.
Oligonucleotide primers were 5′-32P end-labeled using polynucleotide kinase (Roche Molecular Biochemicals) and [γ-32P]ATP (Amersham Pharmacia Biotech) and subsequently annealed to the template by heating a mixture of primer-template at a 1:1.5 molar ratio to 95°C and allowing it to cool to room temperature over several hours.
DNA polymerase assays.
The standard DNA polymerase reaction (5 μl) contained 25 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM dithiothreitol, 100 μg/ml bovine serum albumin, 10% glycerol, one of the four deoxynucleotides (dGTP, dATP, dTTP, or dCTP) at the indicated concentrations, and DNA substrate at the indicated concentration. Reactions, containing 1 nM Pol ι, were carried out at 37°C for the indicated time and then were terminated by the addition of 5 volumes of loading buffer (95% formamide, 0.06% cyanol blue, 0.06% bromophenol blue) before resolving the products on 12% polyacrylamide gels containing 8 M urea. Gels were dried before analysis on a phosphorimager.
Steady-state kinetic analysis.
For steady-state kinetic analyses shown in Table 1, the standard DNA polymerase assay was used and the deoxynucleotide concentrations were varied from 0.5 to 2,500 μM. Reactions were carried out for 5 to 15 min. The concentration of Pol ι varied from 0.5 nM to 5 nM, depending on the DNA substrate and incoming deoxynucleotide combination. For steady-state kinetic analyses for deoxynucleotide incorporation opposite 8-oxoG and m6G residues (Table 2), or primer extension opposite from these lesions (Table 3), Pol ι (1 nM) was incubated with DNA substrate (30 nM) in the presence of different concentrations of a single deoxynucleotide for various times (3 to 30 min) as indicated in Fig. 3. The reaction products were resolved on 15% polyacrylamide gels containing 8 M urea. Gel band intensities of the substrates and products were quantitated with a PhosphorImager, and the observed rate of deoxynucleotide incorporation was plotted as a function of deoxynucleoside triphosphate (dNTP) concentration. The data were fit by nonlinear regression using SigmaPlot to the Michaelis-Menten equation describing a hyperbola, v = (Vmax × [dNTP])/(Km+[dNTP]). The kcat and Km steady-state parameters were obtained from the fit and were used to calculate the efficiency of nucleotide incorporation (kcat/Km), the frequency of deoxynucleotide incorporation (finc), and the intrinsic efficiency of mispair extension (f oext) for each primer/template pair by using the following equation: finc or foext = (kcat/Km)mispaired/(kcat/Km)paired. To facilitate the comparison of the insertion reactions opposite undamaged G, m6G, and 8-oxoG, the relative insertion efficiency (frel) was calculated by using the following equation: frel = (kcat/Km)damaged/(kcat/Km)undamaged C:G pair.
TABLE 1.
Efficiency and fidelity of human Pol ι opposite templates A, 2AP, and Pu
| Template base | Incoming nucleotide | kcat (min−1) | Km (μM) | kcat/Km | finc | Efficiency relative to template A |
|---|---|---|---|---|---|---|
| A | G | NDa | >1,250 | 1.4 × 10−5 | 2.3 × 10−5 | |
| A | 0.12 ± 0.007 | 600 ± 100 | 2.0 × 10−4 | 3.3 × 10−4 | ||
| T | 2.8 ± 0.12 | 4.6 ± 0.7 | 0.6 | 1 | ||
| C | ND | >1,250 | 5.2 × 10−6 | 8.7 × 10−6 | ||
| 2AP | G | 0.03 ± 0.002 | 918 ± 117 | 3.3 × 10−5 | 1.0 × 10−4 | ↑2.4 |
| A | 0.09 ± 0.002 | 570 ± 34 | 1.6 × 10−4 | 5.0 × 10−4 | ↓1.3 | |
| T | 1.2 ± 0.03 | 3.8 ± 0.3 | 0.32 | 1 | ↓0.5 | |
| C | 0.12 ± 0.01 | 530 ± 107 | 2.3 × 10−4 | 7.2 × 10−4 | ↑44 | |
| Pu | G | 0.09 ± 0.01 | 1,400 ± 300 | 6.4 × 10−5 | 1.1 × 10−4 | ↑4.6 |
| A | 0.3 ± 0.01 | 310 ± 46 | 9.7 × 10−4 | 1.6 × 10−3 | ↑4.9 | |
| T | 2.1 ± 0.08 | 3.5 ± 0.5 | 0.6 | 1 | 1 | |
| C | 0.15 ± 0.01 | 290 ± 65 | 5.2 × 10−4 | 8.7 × 10−4 | ↑100 |
ND, not determined.
TABLE 2.
Steady-state kinetic parameters of insertion reactions opposite m6G and 8-oxoG template residues by human Pol ι
| Template base | Incoming nucleotide | kcat (min−1) | Km (μM) | kcat/Km | finc | Efficiency relative to template G |
|---|---|---|---|---|---|---|
| G | G | NDa | ≥500 | 2.4 × 10−5 | 1.5 × 10−3b | |
| A | 0.06 ± 0.01 | 340 ± 80 | 1.8 × 10−4 | 1.1 × 10−2 | ||
| T | 0.75 ± 0.09 | 120 ± 20 | 6.3 × 10−3 | 3.9 × 10−1 | ||
| C | 0.8 ± 0.05 | 49 ± 6 | 1.6 × 10−2 | 1 | ||
| m6G | G | ND | ≥500 | 1.2 × 10−5 | 7.5 × 10−4b | ↓2.0 |
| A | 0.022 ± 0.004 | 680 ± 300 | 3.2 × 10−5 | 2 × 10−3 | ↓5.6 | |
| T | 1 ± 0.05 | 42 ± 5 | 2.4 × 10−2 | 1.5 | ↑3.8 | |
| C | 0.65 ± 0.03 | 420 ± 120 | 1.5 × 10−3 | 9.4 × 10−2 | ↓10 | |
| 8oxo-G | G | 0.16 ± 0.03 | 270 ± 50 | 5.9 × 10−4 | 3.6 × 10−2 | ↑25 |
| A | 0.021 ± 0.003 | 260 ± 30 | 8 × 10−5 | 5 × 10−3 | ↓2.3 | |
| T | 0.07 ± 0.008 | 190 ± 20 | 3.7 × 10−4 | 2.3 × 10−2 | ↓17 | |
| C | 0.14 ± 0.03 | 310 ± 90 | 4.5 × 10−4 | 2.8 × 10−2 | ↓36 |
ND, not determined.
Because the nucleotide incorporation rate remained linear throughout the nucleotide concentration range used, the kcat/Km value was obtained from the slope of the line.
TABLE 3.
Steady-state kinetic parameters of extension from primers paired opposite m6G and 8-oxoG catalyzed by human Pol ι
| Template:primer base pair | Incoming nucleotide | kcat (min−1) | Km (μM) | kcat/Km | foextc |
|---|---|---|---|---|---|
| G:G | T | NDa | ≥500 | 2.0 × 10−5 | 7.1 × 10−5b |
| G:A | T | 0.052 ± 0.007 | 180 ± 30 | 2.9 × 10−4 | 1 × 10−3 |
| G:T | T | 0.63 ± 0.07 | 46 ± 6 | 1.4 × 10−2 | 5 × 10−2 |
| G:C | T | 2.1 ± 0.2 | 7.4 ± 0.5 | 0.28 | 1 |
| m6G:G | T | ND | ≥500 | 1.0 × 10−5 | 3.6 × 10−5b |
| m6G:A | T | 0.065 ± 0.009 | 140 ± 30 | 4.6 × 10−4 | 1.6 × 10−3 |
| m6G:T | T | 2.4 ± 0.14 | 38 ± 3 | 6.3 × 10−2 | 0.23 |
| m6G:C | T | 0.75 ± 0.04 | 130 ± 15 | 5.8 × 10−3 | 2 × 10−2 |
| 8-oxoG:G | T | 0.028 ± 0.004 | 190 ± 40 | 1.5 × 10−4 | 5.4 × 10−4 |
| 8-oxoG:A | T | 2.3 ± 0.1 | 13 ± 2 | 0.17 | 0.6 |
| 8-oxoG:T | T | 0.083 ± 0.009 | 180 ± 20 | 4.6 × 10−4 | 1.6 × 10−3 |
| 8-oxoG:C | T | 0.33 ± 0.05 | 110 ± 13 | 3.0 × 10−3 | 1.1 × 10−2 |
ND, not determined.
Because the nucleotide incorporation rate remained linear throughout the nucleotide concentration range used, the kcat/Km value was obtained from the slope of the line.
foext depicts the efficiency of extension of different primer termini relative to the extension from a G:C base pair.
RESULTS
Base analogs and DNA lesions.
To examine the contribution that hydrogen bonding at the N6 position of adenine makes to nucleotide incorporation by Pol ι, we used 2AP, which is similar to adenine except that it lacks the N6 amino group and has an additional amino group at the second position. To rule out the possibility of any effects arising from the presence of the additional amino group at the second position in 2AP, we used Pu, which lacks this amino group as well as the N6 amino group (Fig. 1A). The results with 2AP and Pu are also informative for the contribution that hydrogen bonding at the O6 position makes to C versus T incorporation opposite guanine.
Additionally, we have examined the effects of m6G and 8-oxoG lesions on DNA synthesis by Pol ι. The presence of a methyl group at the O6 position in m6G could sterically hinder nucleotide incorporation, and 8-oxoG could affect nucleotide incorporation because of the protonation at N7 (Fig. 1B). Since, at physiological pH, 8-oxoG exists predominantly in a 6,8-diketo form and is protonated at N7 (2, 20), the presence of a hydrogen atom at N7 would disrupt hydrogen bonding at this position because of a steric clash with the N3 hydrogen in thymine, and that could impair the incorporation of T that is normally observed opposite an undamaged G. The incorporation of a C opposite 8-oxoG would also be hindered if the dCTP is protonated in the Pol ι active site as we have suggested before (14).
Hydrogen bonding at the N6 of adenine is dispensable for thymine incorporation.
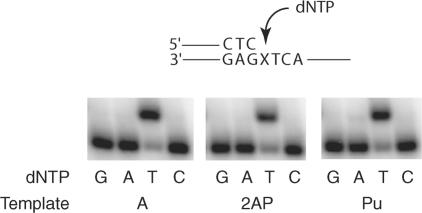
To assess the role of hydrogen bonding at the N6 position of adenine, we determined the efficiencies of correct and incorrect nucleotide incorporations opposite templates A, 2AP, and Pu by steady-state kinetic analyses. As for template A, Pol ι predominantly incorporates a T opposite 2AP and Pu (Fig. 2). As is shown in Table 1, Pol ι incorporates a T opposite template A with an efficiency (kcat/Km) that is ∼10−4- to 10−5-fold greater than the efficiency for the incorporation of incorrect nucleotides. Interestingly, the efficiency of T incorporation opposite the 2AP and Pu templates remains almost the same as that opposite template A, which suggests that hydrogen bonding at the N6 position is not a prerequisite for proficient T incorporation opposite template A by Pol ι. The N6 amino group, however, does contribute to the fidelity of nucleotide incorporation opposite template A, as the misincorporation of C is enhanced opposite the 2AP and Pu templates ∼40- and 100-fold, respectively. The lowered efficiency of C incorporation opposite template A compared to that opposite templates 2AP and Pu could arise from the steric clash of amino groups at the N6 of A and N4 of C.
FIG. 2.
Nucleotide insertion opposite an A, 2AP, or Pu by DNA polymerase ι. Pol ι (1 nM) was incubated with DNA substrate (10 nM) and one of the four dNTPs (50 μM G, A, T, or C) for 10 min at 37°C. The substrate is shown on the top, and the X indicates the presence of either an A, a 2-AP, or a Pu residue.
Hydrogen bonding at the O6 of guanine contributes to C incorporation.
Pol ι is less efficient at incorporating the correct nucleotide opposite template G than opposite an A; moreover, it incorporates a T opposite template G with only a fewfold reduction in efficiency compared to that for C incorporation. As shown in Fig. 1B, in a G · C Hoogsteen base pair, the N7 and O6 of template G hydrogen bond with the N3 and N4 of C, respectively, and hydrogen bonding between the N7 of G and N3 of C requires that the N3 of C be protonated. In the G · C Hoogsteen base pair seen in the ternary structure of Pol ι, the geometry of the nascent base pair is consistent with the formation of two such hydrogen bonds and with the protonation of N3 of dCTP (14). The template G could also form a Hoogsteen base pair with a T held by a single hydrogen bond between the N7 of G and N3 of T (Fig. 1B).
The kinetic analyses we have performed with the 2AP and Pu templates are also informative in regard to the contribution of hydrogen bonding at the O6 position of guanine to C versus T incorporation, since as far as the Hoogsteen edge is concerned, 2AP and Pu retain the N7 hydrogen bonding but lack the O6 hydrogen bonding of a G. Even though we find that C incorporation is enhanced opposite the 2AP and Pu templates compared to that opposite an A, still, it is incorporated quite inefficiently, with an finc of less than 10−3 (Table 1). Also, a C is incorporated opposite template G better than opposite an 2AP or Pu template (compare Tables 1 and 2). Our observation that T is efficiently incorporated opposite the 2AP and Pu templates, but C is incorporated quite poorly (Table 1), would suggest a role for hydrogen bonding at the O6 position in the incorporation of a C opposite template G.
Preferential incorporation of a T opposite m6G by Pol ι.
The proficiency of Pol ι for C incorporation opposite m6G is reduced ∼10-fold compared with the incorporation of a C opposite undamaged G; moreover, the incorporation of a T opposite m6G is over 15-fold more efficient than the incorporation of a C opposite this lesion (Table 2; Fig. 3). Thus, by contrast to an undamaged G, where Pol ι incorporates a C somewhat better than a T, the situation is reversed for the m6G lesion opposite which a T is preferentially incorporated over a C (Fig. 3; Table 2).
The inhibitory effect of the presence of a methyl group at the O6 position in template G on the incorporation of C is in line with the inference drawn from the studies with the 2AP and Pu templates that hydrogen bonding at O6 is an important determinant for C incorporation opposite template G.
An 8-oxoG lesion is inhibitory to the incorporation of a C or a T by Pol ι.
The protonation of N7 in the 8-oxoG lesion would be expected to lower the efficiency of T incorporation by Pol ι because of the adverse effect that would have on the hydrogen bonding of N7 of 8-oxoG with the N3 of T (Fig. 1B). Accordingly, we observe an ∼20-fold reduction in the efficiency with which a T is incorporated opposite the 8-oxoG lesion compared to that opposite the undamaged G template (Table 2; Fig. 3).
In the G · C Hoogsteen base pair formed in the Pol ι active site, the O6(G)-HN4(C) and N7(G)-HN3+(C) distances of 2.87 and 2.98 Å, respectively, are typical for hydrogen bonds. This geometry, indicative of a hydrogen bond between the N7 of guanine and N3 of cytosine, however, requires that the N3 of dCTP be protonated, and we have suggested before that the pKa of dCTP is elevated when it is bound in the Pol ι active site (14). If such a protonation of dCTP indeed occurs in the Pol ι active site, in that case, an 8-oxoG should be inhibitory for the incorporation of a C also, since the protonated N7 of 8-oxoG would sterically clash with the protonated dCTP in the Pol ι active site. In fact, we find an ∼40-fold reduction in the efficiency of C incorporation opposite 8-oxoG compared to that opposite undamaged G. The reduced efficiency of C incorporation opposite 8-oxoG supports the inference of dCTP protonation in the Pol ι active site arrived at from structural studies (14).
In most DNA polymerases, including the replicative Pols, 8-oxoG adopts a syn conformation and hydrogen bonds via its Hoogsteen edge with dATP in the anti conformation. Nuclear magnetic resonance and X-ray crystallographic studies of DNA containing an 8-oxoG(syn) · dA(anti) base pair have shown that this base pair fits well in B-form DNA without causing any significant distortion (1, 7, 10, 12, 17). The incorporation of an A(anti) opposite 8-oxoG(syn), however, will be disfavored in the Pol ι active site because of the ∼10.5-Å distance between the C1′-C1′ atoms of an 8-oxoG(syn) · dA(anti) base pair. Accordingly, we find that Pol ι is highly inefficient at incorporating an A opposite the 8-oxoG lesion (Table 2).
Extension of primers opposite from the m6G and 8-oxoG lesions by Pol ι.
Although upon the binding of the incoming dNTP, the templates A and G adopt a syn conformation in the Pol ι active site and engage in Hoogsteen base pairing with the incoming nucleotide, upon translocation of Pol ι to the next templating base, the base pair at the primer terminus adopts a normal W-C geometry (14-16). This implies that when extending the primer termini opposite from DNA lesions, Pol ι would preferentially extend the primer termini that conform to a normal W-C base pairing geometry. To test whether the W-C base pairing indicated at the primer terminus from structural studies also prevails in solution conditions, we examined the ability of Pol ι to extend from primer termini bearing a G, an A, a T, or a C opposite the m6G and 8-oxoG lesions. As shown in Table 3 and Fig. 3, Pol ι extends most efficiently from a T opposite m6G, and this was only ∼4-fold less efficient than the extension from a C opposite the undamaged G. Opposite the 8-oxoG lesion, Pol ι extended from the A primer terminus with nearly the same efficiency as the extension from a C opposite undamaged G. In the Discussion, we elaborate upon the implications of these observations for the adoption of a W-C base pairing geometry at the primer terminus in Pol ι active site.
DISCUSSION
The Hoogsteen base pairing of templates A and G with incoming T and C, respectively, involves the formation of two hydrogen bonds between the atoms at the seventh and sixth positions of the purine and at the third and fourth positions of the pyrimidine, respectively (Fig. 1). Here, by using 2AP and Pu as the templating residues, which lack the N6 amino group of an A or the O6 group of a G, we have examined the contribution that hydrogen bonding at the N6 or the O6 position of template purines makes to the efficiency and fidelity of dNTP incorporation. From our observation that the efficiency of T incorporation by Pol ι is not affected by these modified bases, we conclude that hydrogen bonding at N6 is not obligatory for the efficient incorporation of T opposite template A. Hydrogen bonding at the N7 position, however, is imperative for proficient T incorporation opposite A, as the efficiency of T incorporation opposite 7-deazaA is reduced by over 200-fold relative to that opposite an A (5).
The substitution of 2AP or Pu for an A in the template leads to an ∼40- to 100-fold increase in the efficiency of C incorporation by Pol ι, which suggests that the N6 amino group is inhibitory to C incorporation opposite template A. Presumably, this results from the steric clash of the N6 amino group in A with the N4 amino group of C (Fig. 1). Although the efficiency of C incorporation increases opposite 2AP and Pu, still, it is incorporated over 1,000-fold less well than a T. In the Hoogsteen base pairing of 2AP or Pu with a C, only the N7 of the purine can hydrogen bond with the N3 of C and would require the protonation of C. Since the residues in the vicinity of the N3 atom of C in Pol ι are hydrophobic (Val64 and Leu78) (14), the much-reduced efficiency of C incorporation opposite 2AP or Pu could reflect the less-efficient binding of a positively charged dCTP versus neutral dTTP in the Pol ι active site.
By contrast to the incorporation of a T opposite an A, which requires only the hydrogen bonding of N7 of A with N3 of T, the proficient incorporation of a C opposite template G requires, in addition to the hydrogen bonding at N7 of G with N3 of C, the hydrogen bonding at O6 of G with N4 of C. Our previous studies with 7-deaza guanine have indicated a requirement of hydrogen bonding at N7 for C incorporation opposite template G (5). Our results with 2AP and Pu support the requirement of O6 hydrogen bonding for C incorporation opposite template G, and our observation that a T is incorporated about 15-fold more efficiently than a C opposite m6G is also in accord with such an inference.
From our studies with 7-deaza adenine and 7-deaza guanine that we reported previously (5), and from the studies presented in this paper, we draw the following conclusions for the contribution that hydrogen bonding at the Hoogsteen edge of template purines makes to dNTP incorporation by Pol ι: (i) T incorporation opposite template A requires only the hydrogen bonding at the N7 position of A, and hydrogen bonding at the N6 position is dispensable; and (ii) C incorporation opposite template G requires hydrogen bonding at both the N7 and O6 positions, and if the O6 hydrogen bonding is impaired, then a T is preferentially incorporated opposite template G.
The dispensability of hydrogen bonding at the N6 amino group of adenine for proficient T incorporation could have important implications for lesion bypass by Pol ι. Because of its reactivity, a number of adducts would bind at the N6 position of an A and adversely affect the potential of this position to participate in hydrogen bonding. Pol ι, however, could still remain effective at proficiently incorporating a T opposite such N6 adenine adducts. Moreover, since N6 also contributes to W-C base pairing, exocyclic adducts that would form between the N1 and N6 of an A, and which thereby completely disrupt the Watson-Crick edge, may have little or no inhibitory affect on the proficiency of T incorporation by Pol ι, as the hydrogen bonding potential of the N7 position would not be affected by such lesions.
1,N6-Ethenodeoxyadenosine (ɛdA), a promutagenic and potentially carcinogenic lesion, is formed in DNA from the interaction of bases with aldehydes derived from lipid peroxidation. A variety of highly reactive aldehydes, such as acrolein, malonaldehyde, and trans-4-hydroxy-2-nonenal (HNE), are generated in vivo from lipid peroxidation. Enals such as HNE are converted to epoxyaldehyde upon further oxidation reactions, and the reaction of epoxyaldehyde with adenine in DNA generates the ɛdA adduct (3, 11). Since the exocyclic ring present between the N1 and N6 positions of ɛdA completely disrupts the Watson-Crick edge of adenine, this adduct is highly inhibitory to synthesis by replicative DNA polymerases and also to synthesis by translesion synthesis polymerases η and κ (9). However, Pol ι efficiently incorporates a T opposite the ɛdA adduct with only an about 10-fold reduction in efficiency compared to that opposite a normal A (13). In the crystal structure of Pol ι · ɛdA · dTTP ternary complex, the ɛdA adduct adopts a syn conformation in the Pol ι active site and forms a Hoogsteen base pair with dTTP which remains in the anti conformation, and as expected, a single hydrogen bond is established between the N7 of ɛdA and N3 of dTTP (13). Following the incorporation of a T opposite the ɛdA adduct by Pol ι, the subsequent extension by Pol ζ would provide for efficient and accurate synthesis through the lesion (13).
The role of Pol ι in translesion synthesis thus would involve not only the proficient incorporation of a C opposite the large variety of N2 adducted guanines that we have proposed before (22, 24, 25) but could include, also, the ability for proficient T incorporation opposite the N6 adducted adenines as well as opposite the lesions that impair the W-C hydrogen bonding potential of both the N1 and N6 positions of adenine. In this regard, it is noteworthy that since Pol ι exhibits the highest efficiency and fidelity of nucleotide incorporation opposite template A, its dependence upon only the N7 hydrogen bonding for efficient T incorporation would endow it with the ability for promoting proficient replication through a large variety of adducts that bind the templating A and disrupt its W-C edge.
As inferred from ternary crystal structures, following the nucleotide incorporation step, the resulting primer terminal base pair adopts a normal W-C geometry in the Pol ι active site (14-16). Our observation that Pol ι is most proficient at extending from a T opposite the m6G lesion and from an A opposite the 8-oxoG lesion suggests that in solution also, Pol ι extends most proficiently from the primer termini that more closely approximate the W-C base pairing geometry. The high-fidelity replicative DNA polymerases which depend strictly upon a proper W-C base pairing geometry synthesize DNA across the m6G and 8-oxoG lesions by incorporating a T or an A, respectively, and by extending therefrom. This is because an m6G · T mispair closely resembles a W-C base pair (8, 18, 23) and an 8-oxoG(syn) · A(anti) Hoogsteen base pair also has the geometric features that resemble a W-C base pair (1, 7, 12).
Acknowledgments
This work was supported by NIH grants ES012411 and CA115856.
REFERENCES
- 1.Brieba, L. G., B. F. Eichman, R. J. Kokoska, S. Doublie, T. A. Kunkel, and T. Ellenberger. 2004. Structural basis for the dual coding potential of 8-oxoguanosine by a high-fidelity DNA polymerase. EMBO J. 23:3452-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho, B. P., F. F. Kadlubar, S. J. Culp, and F. E. Evans. 1990. 15N nuclear magnetic resonance studies in the tautomerism of 8-hydroxy-2′-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem. Res. Toxicol. 3:445-452. [DOI] [PubMed] [Google Scholar]
- 3.Chung, F.-L., L. Zhang, J. E. Ocando, and R. G. Nath. 1999. Role of 1,N2-propanodeoxygunosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci. Publ. 150:45-53. [PubMed] [Google Scholar]
- 4.Haracska, L., R. E. Johnson, I. Unk, B. B. Phillips, J. Hurwitz, L. Prakash, and S. Prakash. 2001. Targeting of human DNA polymerase ι to the replication machinery via interaction with PCNA. Proc. Natl. Acad. Sci. USA 98:14256-14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, R. E., L. Prakash, and S. Prakash. 2005. Biochemical evidence for the requirement of Hoogsteen base pairing for replication by human DNA polymerase ι. Proc. Natl. Acad. Sci. USA 102:10466-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, R. E., M. T. Washington, L. Haracska, S. Prakash, and L. Prakash. 2000. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 406:1015-1019. [DOI] [PubMed] [Google Scholar]
- 7.Kouchakdjian, M., V. Bodepudi, S. Shibutani, M. Eisenberg, F. Johnson, A. P. Grollman, and D. J. Patel. 1991. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry 30:1403-1412. [DOI] [PubMed] [Google Scholar]
- 8.Leonard, G. A., J. Thomson, W. P. Watson, and T. Brown. 1990. High-resolution structure of a mutagenic lesion in DNA. Proc. Natl. Acad. Sci. USA 87:9573-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine, R. L., H. Miller, A. Grollman, E. Ohashi, H. Ohmori, C. Masutani, F. Hanaoka, and M. Moriya. 2001. Translesion DNA synthesis catalyzed by human Pol η and Pol κ across 1,N6-ethenodeoxyadenosine. J. Biol. Chem. 276:18717-18721. [DOI] [PubMed] [Google Scholar]
- 10.Lipscomb, L. A., M. E. Peek, M. L. Morningstar, S. M. Verghis, E. M. Miller, A. Rich, J. M. Essignman, and L. D. Williams. 1995. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc. Natl. Acad. Sci. USA 92:719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luczaj, W., and E. Skrzydlewska. 2003. DNA damage caused by lipid peroxidation products. Cell. Mol. Biol. Lett. 8:391-413. [PubMed] [Google Scholar]
- 12.McAuley-Hecht, K. E., G. A. Leonard, N. J. Gibson, J. B. Thomson, W. P. Watson, W. N. Hunter, and T. Brown. 1994. Crystal structure of a DNA duplex containing 8-hydroxydeoxyguanine-adenine base pairs. Biochemistry 33:10266-10270. [DOI] [PubMed] [Google Scholar]
- 13.Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash, and A. K. Aggarwal. 2006. Hoogsteen base pair formation promotes synthesis opposite the 1,N6-ehthenodeoxyadenosine lesion by human DNA polymerase ι. Nat. Struct. Mol. Biol. 13:619-625. [DOI] [PubMed] [Google Scholar]
- 14.Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash, and A. K. Aggarwal. 2005. Human DNA polymerase ι incorporates dCTP opposite template G via a G.C+ Hoogsteen base pair. Structure 13:1569-1577. [DOI] [PubMed] [Google Scholar]
- 15.Nair, D. T., R. E. Johnson, S. Prakash, L. Prakash, and A. K. Aggarwal. 2006. An incoming nucleotide imposes an anti to syn conformational change on the templating purine in the human DNA polymerase-ι active site. Structure 14:749-755. [DOI] [PubMed] [Google Scholar]
- 16.Nair, D. T., R. E. Johnson, S. Prakash, L. Prakash, and A. K. Aggarwal. 2004. Replication by human DNA polymerase ι occurs via Hoogsteen base-pairing. Nature 430:377-380. [DOI] [PubMed] [Google Scholar]
- 17.Oda, Y., S. Uesugi, M. Ikehara, S. Nishimura, Y. Kawase, H. Ishikawa, H. Inoue, and E. Ohtsuka. 1991. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 19:1407-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan, H.-B., P. F. Swann, and E. M. Chance. 1994. Kinetic analysis of the coding properties of O6-methylguanine in DNA: the crucial role of the conformation of the phosphodiester bond. Biochemistry 33:5335-5346. [DOI] [PubMed] [Google Scholar]
- 19.Tissier, A., J. P. McDonald, E. G. Frank, and R. Woodgate. 2000. Pol ι, a remarkably error-prone human DNA polymerase. Genes Dev. 14:1642-1650. [PMC free article] [PubMed] [Google Scholar]
- 20.Uesugi, S., and M. Ikehara. 1977. Carbon-13 magnetic resonance spectra of 8-substituted purine nucleosides. Characteristic shifts for the syn conformation. J. Am. Chem. Soc. 99:3250-3253. [DOI] [PubMed] [Google Scholar]
- 21.Washington, M. T., R. E. Johnson, L. Prakash, and S. Prakash. 2004. Human DNA polymerase ι utilizes different nucleotide incorporation mechanisms dependent upon the template base. Mol. Cell. Biol. 24:936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washington, M. T., I. G. Minko, R. E. Johnson, W. T. Wolfle, T. M. Harris, R. S. Lloyd, S. Prakash, and L. Prakash. 2004. Efficient and error-free replication past a minor groove DNA adduct by the sequential action of human DNA polymerases ι and κ. Mol. Cell. Biol. 24:5687-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams, L. D., and B. R. Shaw. 1987. Protonated base pairs explain the ambiguous pairing properties of O6-methylguanine. Proc. Natl. Acad. Sci. USA 84:1779-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfle, W. T., R. E. Johnson, I. G. Minko, R. S. Lloyd, S. Prakash, and L. Prakash. 2005. Human DNA polymerase ι promotes replication through a ring-closed minor-groove adduct that adopts a syn conformation in DNA. Mol. Cell. Biol. 25:8748-8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfle, W. T., R. E. Johnson, I. G. Minko, R. S. Lloyd, S. Prakash, and L. Prakash. 2006. Replication past a trans-4-hydroxynonenal minor-groove adduct by the sequential action of human DNA polymerase ι and κ. Mol. Cell. Biol. 26:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Y., F. Yuan, X. Wu, and Z. Wang. 2000. Preferential incorporation of G opposite template T by the low-fidelity human DNA polymerase ι. Mol. Cell. Biol. 20:7099-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]