Abstract
Histone H3 lysine 9 (H3K9) methylation has broad roles in transcriptional repression, gene silencing, maintenance of heterochromatin, and epigenetic inheritance of heterochromatin. Using Xenopus laevis oocytes, we have previously shown that targeting G9a, an H3K9 histone methyltransferase, to chromatin increases H3K9 methylation and consequently represses transcription. Here we report that treatment with trichostatin A induces histone acetylation and is sufficient to activate transcription repressed by G9a, and this activation is accompanied by a reduction in dimethyl H3K9 (H3K9me2). We tested the possibility that the reduction in H3K9me2 was due to the replacement of methylated H3 with unmethylated H3.3. Surprisingly, we found that both free H3 and H3.3 are continually exchanged with chromatin-associated histones. This dynamic exchange of chromatin-associated H3 with free H3/H3.3 was not affected by alterations in transcriptional activity, elongation, acetylation, H3K9 methylation, or DNA replication. In support of this continual histone exchange model, we show that maintenance of H3K9 methylation at a specific site requires the continual presence of an H3K9 histone methyltransferase. Upon dissociation of the methyltransferase, H3K9 methylation decreases. Taken together, our data suggest that chromatin-associated and non-chromatin-associated histones are continually exchanged in the Xenopus oocyte, creating a highly dynamic chromatin environment.
Posttranslational modifications of histones play crucial roles in the regulation of chromatin structure, chromatin assembly, DNA repair, and transcription. The list of posttranslational modifications includes acetylation, methylation, phosphorylation, ubiquitylation, sumoylation, and ADP-ribosylation (41). All of these modifications in some way affect the local structure of chromatin, influence the binding of chromatin-associated factors, and influence histone-DNA interactions and thereby regulate related cellular activities such as chromatin remodeling and transcription (9, 23).
Histone methylation has broad functions in the regulation of chromatin biology as it influences a broad range of chromatin-related processes from transcription to DNA repair (16, 25, 35, 59). Histone methylation can take place on either lysine (K) or arginine (R). Many sites on histones can be methylated; notable sites for H3 include K4, K9, R17, K27, K36, and K79. Lysine residues can be mono-, di-, or trimethylated, whereas arginine residue can be mono- or dimethylated (symmetrically or asymmetrically) (5, 35). Unlike acetylation, the effect of histone methylation on transcription seems to be site specific and is also dependent upon the level of methylation. For example, histone H3 lysine 4 (H3K4) methylation is generally enriched in actively transcribed genes (47, 48), whereas H3K9 methylation is associated with repressed chromatin and is sufficient to induce transcriptional repression (39, 40, 55).
Earlier studies indicate that histone methylation is metabolically stable (13, 21). The finding that H3K9 methylation is important for establishing and maintaining heterochromatin also leads to the notion that H3K9 methylation serves as a epigenetic mark for the stable heredity of heterochromatin (31). However, several studies have reported a rapid reduction in H3K9 methylation coinciding with transcriptional activation (33, 46). This indicates that either H3K9 must be demethylated or the entire H3 molecule must be replaced with an unmethylated H3 during gene activation. Both of these mechanisms have been previously proposed (6, 7). Consistent with this idea, several enzymes have been identified that can decrease histone methylation: PADI4/PAD4 (peptidyl arginine deiminase 4), LSD1/KIAA0601 (lysine-specific demethylase 1), JHDM1 (JmjC domain-containing histone demethylase 1), JHDM2A/JMJD1A, and JMJD2 family proteins (14, 36, 51, 58, 61, 65). Thus, demethylation is clearly a viable mechanism for the observed dynamics of H3K9 methylation.
Replacement of H3 with the variant H3.3 during transcriptional activation represents another mechanism for dynamic regulation of H3K9 methylation (1). The genes encoding canonical H3 (H3.1 and H3.2) are intronless and transcribed exclusively during S phase to produce short-lived nonpolyadenylated mRNA (52). The H3.1 protein is utilized for replication-dependent chromatin assembly by the histone chaperone CAF1 (chromatin assembly factor 1) (56). In contrast, the human gene encoding H3.3 contains introns and is transcribed continuously throughout the cell cycle to produce polyadenylated mRNA (60, 64). H3.3 is preferentially utilized for replication-independent chromatin assembly by the histone chaperone HIRA (histone regulator A) (3, 56). H3.3 differs from H3.1 by only 4 amino acids, but this slight difference allows for the specific interaction with HIRA. In vivo, H3.3 is enriched at sites of active transcription, such as Drosophila rRNA genes or the HSP70 locus following heat shock (3, 37, 50).
Using Xenopus laevis oocytes as a model system, we have shown that targeting G9a, an H3K9-specific histone methyltransferase (HMT), to chromatin induces H3K9 methylation and consequently transcriptional repression (55). In an effort to study the reversibility of H3K9 methylation, we found that increasing histone acetylation with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) was sufficient to cause a reduction in the amount of dimethyl H3K9 (H3K9me2) associated with a chromatinized reporter. The underlying mechanism for this TSA-induced reduction of H3K9 methylation appears to involve the continual exchange of free histones with chromatin-associated histones.
MATERIALS AND METHODS
Plasmid constructs and antibodies.
The 4xUAS-TRβA-CAT reporter plasmid was previously described (63). The construct for in vitro synthesis of mRNA encoding the Gal4 DNA-binding domain (DBD) fused to the SET domain of G9a [Gal-G9a(SET)] was previously reported (55). The construct for in vitro synthesis of mRNA encoding TetR-G9a(SET) was created by N-terminally fusing amino acids 1 to 207 of TetR with the G9a SET domain (amino acids 831 to 1172) in the modified vector pSP64(polyA). The constructs for in vitro synthesis of mRNAs encoding Flag-tagged histones H3 and H4 or hemagglutinin (HA)-tagged H3 were created by cloning Xenopus histones H3 and H4 into the MS2-Flag or MS2-HA vector. The construct for in vitro synthesis of mRNA encoding the Xenopus H3.3 variant was created by PCR-directed mutagenesis of MS-Flag-xH3. The three amino acids substituted were S87A, V89I, and M90G. These three amino acids confer the replication-independent chromatin assembly property of H3.3 (2).
Anti-pan acetyl H3, pan acetyl H4, H3K9ac, H3K14ac, H4K8ac, H4K5ac, H3K9me2, H3K4me2, H3K27me2, H3S10ph, and H3S10ph/H3K14ac antibodies were purchased from Upstate Biotechnology (Lake Placid, NY) or Abcam. The antibody against the C terminus of histone H3 was purchased from Novus Biologicals (Littleton, CO). Gal4 (DBD) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Flag tag (M2) antibodies were purchased from Sigma (St. Louis, MO). HA tag antibodies were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal G9a antibodies were generated from purified recombinant glutathione S-transferase (GST)-G9a(SET) protein.
Microinjection of Xenopus oocytes.
Preparation and microinjection of mRNA and reporter DNA into stage VI Xenopus oocytes were performed as previously described (63). All capped poly(A) mRNAs used for injection were synthesized using an SP6 mESSAGE mACHINE kit (Ambion, Austin, TX). The preparation of single-stranded DNA (ssDNA) from the 4xUAS-TRβA-CAT reporter was previously described (63). mRNA was injected at a concentration of 100 ng/μl (18.4 nl/oocyte), and reporter DNA was injected at a concentration of 65 ng/μl (18.4 nl/oocyte) according to the experimental scheme described in each figure.
Western blotting.
For Xenopus oocyte whole-cell extracts, 5 to 10 oocytes were homogenized in 100 mM Tris-10 mM EDTA (pH 8.0) (10 μl/oocyte) and centrifuged to remove insoluble material. An equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added to each sample and either used immediately for Western analysis or stored at −20C. Xenopus oocyte nuclear extracts were prepared by manually dissecting the germinal vesicles from 10 oocytes and dissolving them in 40 μl of 2× SDS-PAGE loading buffer. Ten microliters was used for Western blotting. HeLa core histones were prepared from confluent cultures in 10-cm dishes as described (34). Primary antibodies were diluted 1:1,000, except for anti-Gal4(DBD), anti-HA, and anti-Flag, which were diluted 1:5,000.
ChIP.
Chromatin immunoprecipitation (ChIP) assays were performed exactly as previously described (55). Immunoprecipitated DNA was resuspended in 20 μl of 10 mM Tris (pH 8.0) (80 μl for input samples). Four microliters was used for each PCR. Standard PCR was performed in 20-μl volumes with the inclusion of 1 μCi of [32P]dCTP. The products were visualized by autoradiography. PCR primers amplified a 100-bp region in the promoter of the 4xUAS-TRβA-CAT reporter and have been previously described (33). The PCR consisted of 20 of cycles of the following temperatures: 94°C for 45 s, 63°C for 45 s, and 72°C for 45 s.
Primer extension.
Primer extension was used to analyze the quantity of RNA transcripts produced from reporter genes in Xenopus oocytes. The procedure used for primer extension has been previously described (63). The Xenopus oocyte storage histone H4 mRNA was used as an internal control in all primer extension assays (62).
Immunofluorescence using Xenopus laevis lampbrush chromosome spreads.
Capped poly(A) mRNA encoding Flag-tagged histone H3 was injected into the cytoplasm of stage V oocytes (2 ng RNA/oocyte). Nuclei were isolated and chromosomes were spread within 15 min at 1, 2, 4, and 6 h after injection. Dispersal and spreading of nuclear contents were performed as described previously (53). After fixation in 70% ethanol, spread preparations were immunostained using anti-Flag (1:5,000) and fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulin G (1:20,000). Preparations were counterstained with DAPI (4′,6′-diamidino-2-phenylindole), and microscopic images were acquired as described previously (54).
RESULTS
TSA treatment relieves H3K9 methylation-induced repression and reduces preexisting H3K9 methylation in chromatin.
Previously we reported that tethering the SET domain of human G9a to chromatin as a Gal-G9a(SET) fusion protein is sufficient to induce H3K9 methylation and consequently transcriptional repression (55). We also showed that TSA treatment prior to Gal-G9a(SET) expression blocks H3K9 methylation and transcriptional repression induced by Gal-G9a(SET). In this study, we tested whether TSA is able to activate transcription if we allowed H3K9 methylation to be established first. Thus, we first injected Xenopus oocytes with or without mRNA encoding Gal-G9a(SET) and the ss4xUAS-TRβA-CAT reporter plasmid and then incubated the oocytes overnight to establish H3K9 methylation and transcriptional repression (Fig. 1A). Previous studies have shown that ssDNA is rapidly converted into double-stranded DNA (dsDNA) and assembled into chromatin through a replication-coupled pathway within a few hours after injection (4). Then, the injected oocytes were treated with TSA (1.65 μM) and collected at the indicated time points for analysis of transcriptional activity by primer extension and effect on H3K9 methylation by ChIP. Consistent with our previous result (55), Gal-G9a(SET) repressed transcription of the TRβA-CAT reporter gene (Fig. 1B, compare lane 2 with lane 1) after overnight incubation. Addition of TSA reversed this repression and further enhanced transcription of the reporter gene over the 6-h treatment period (Fig. 1B, lanes 2 to 6). Similarly, we found that TSA relieved H3K9 methylation-induced repression of a thymidine kinase-chloramphenicol acetyltransferase (TK-CAT) reporter (Fig. 1C). Thus, TSA treatment is able to activate transcription from chromatin templates repressed by H3K9 methylation.
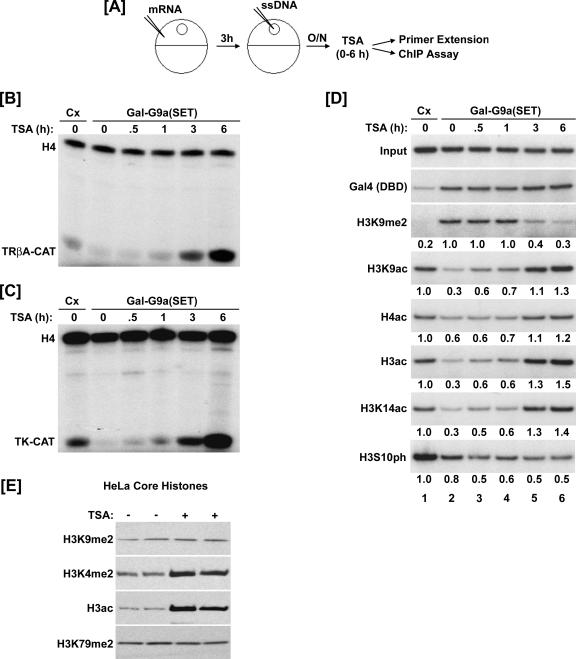
FIG. 1.
TSA treatment relieves H3K9 methylation-induced repression and reduces preexisting H3K9 methylation in chromatin. (A) Experimental design for panels B to D. Xenopus oocytes were microinjected with nothing (control [Cx]) or mRNA encoding the Gal-G9a(SET). After 3 h, oocytes were microinjected with single-stranded reporter plasmid. The following day, TSA was added to the media (1.65 nM) for 0, 0.5, 1, 3, or 6 h. O/N, overnight. (B and C) Results of primer extension analyses. Methylation of H3K9 by Gal-G9a(SET) repressed transcription of the chromatin-assembled 4xUAS-TRβA-CAT (B) and 4xUAS-TK-CAT (C) reporter genes. TSA relieved this repression and stimulated transcriptional activity during the 6-h treatment period. A primer to the Xenopus oocyte storage histone H4 mRNA was used as an internal control. (D) Results of ChIP assays. Gal-G9a(SET) increased the level of H3K9me2 and decreased the level of histone acetylation and H3S10ph. TSA decreased H3K9me2 and increased histone acetylation. H3S10ph did not change during TSA treatment. The ChIP data were quantitated by densitometry and are presented as difference (fold) from the control. (E) HeLa cells were treated with or without TSA for 16 h. Core histones were isolated and used for Western blotting. The steady-state levels of H3K9me2 and H3K79me2 in the cells were not affected by TSA treatment; however, TSA treatment did increase the amount of H3K4me2 and acetyl H3.
As previously reported (55), the ChIP results indicate that after overnight incubation Gal-G9a(SET) induced H3K9 dimethylation and reduced acetylation of both histones H3 and H4 (Fig. 1D, compare lane 2 with lane 1). As expected and consistent with TSA-induced transcriptional activation, TSA treatment led to a gradual increase of acetylated H3 and H4. Importantly, ChIP analysis revealed that TSA treatment also resulted in a gradual reduction of H3K9me2. The reduction in the level of H3K9 methylation became obvious at 3 h and further decreased after 6 h. However, TSA treatment did not affect the binding of Gal-G9a(SET) as determined by ChIP using an anti-Gal4(DBD) antibody. In additional experiments, we confirmed that TSA treatment induced a significant reduction of H3K9 methylation around 3 h and reached a plateau around 6 h (data not shown). Furthermore, the reduction of H3K9 methylation upon TSA treatment was consistently observed in multiple independent experiments using three different antibodies from two sources (Upstate #07-521 and #07-441 and Abcam ab7312).
One concern we had was that the observed reduction of H3K9 methylation was an artifact due to the inability of the H3K9me2 antibodies to recognize H3K9me2 in the context of other histone modifications induced by TSA. As a site adjacent to K9, phosphorylation of H3 at serine 10 (H3S10ph) may affect recognition of H3K9me2 by an anti-H3K9me2 antibody. We therefore analyzed how TSA affected the levels of H3S10ph by ChIP. The results in Fig. 1D show that TSA did not increase H3S10 phosphorylation in our experiment, thus excluding interference by H3S10ph as an explanation for the observed H3K9me2 reduction. In addition, we found that TSA treatment did not affect the ability for the H3K9me2 antibody to detect H3K9me2 in bulk histones prepared from TSA-treated HeLa cells (Fig. 1E). As expected, TSA treatment led to increase in histone H3 acetylation and H3K4me2, but did not affect the steady-state levels of H3K9me2 or H3K79me2 (Fig. 1E). These results complement the conclusions of Maison and colleagues that inhibition of HDAC activity with TSA does not affect the global level of cellular H3K9 methylation (34). Taken together, we conclude that TSA treatment leads to an authentic reduction of the H3K9 methylation preestablished by targeting G9a to chromatin.
Both H3 and H3.3 can be exchanged into preassembled chromatin.
The above results demonstrate that TSA treatment not only reverses the transcriptional repression induced by H3K9 methylation but also leads to a reduction of preexisting H3K9 methylation. Given the information in the literature, we envision two underlying mechanisms. One is that TSA treatment somehow induces H3K9 demethylation. Although it cannot be completely excluded, the fact that TSA treatment in HeLa cells did not reduce the level of H3K9 methylation argues against this mechanism. In support, we found that TSA treatment also did not affect the overall level of H3K9 methylation in bulk histones from Xenopus oocytes (unpublished data). The second mechanism is that TSA-induced transcriptional activation leads to replacement of the K9 methylated H3 with an unmethylated H3.3 variant. This is plausible because TSA treatment activates transcription from the repressed reporter (Fig. 1B and C) and the H3.3 variant is utilized for the replication-independent chromatin assembly that follows transcription elongation (50).
To determine if replacement by H3.3 variant is involved in TSA-induced reduction of H3K9 methylation, we tested if TSA treatment induces preferential incorporation of Flag-H3.3 into chromatin. The histone H3.3 variant differs from H3 at four amino acids, namely S31, A87, I89, and G90 in H3.3 versus A31, S87, V89, and M90 in H3. It was shown that the sequence differences at positions 87, 89, and 90 between H3.3 and H3 are responsible for replication-independent chromatin assembly activity of H3.3 (3). We thus generated by site-directed mutagenesis an H3 mutant by converting S87, V89, and M90 in Flag-H3 to A87, I89, and G90A. For simplicity, we termed this mutant H3.3. In a pilot test, we first demonstrated that the Flag-H3, -H4, and -H3.3 proteins were all incorporated into chromatin during replication-coupled chromatin assembly and TSA treatment had no effect on this process (Fig. 2A). Western analysis showed that the Flag-histone proteins were appropriately expressed and that TSA did not affect the steady-state level of protein in the oocyte extract (Fig. 2B).
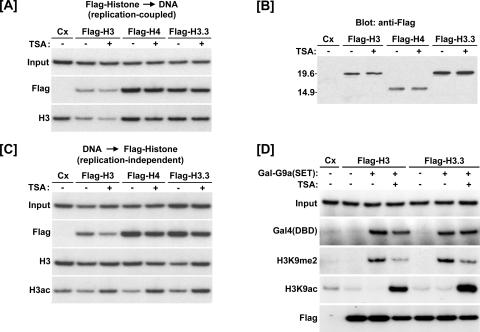
FIG. 2.
Both H3 and H3.3 can be exchanged into preassembled chromatin. (A) ChIP assays showing Flag-H3, -H4, and -H3.3 were efficiently utilized for replication-coupled chromatin assembly. TSA did not affect the replication-independent incorporation. Cx, control (no coinjection). (B) TSA treatment did not affect expression of the Flag-histones as determined by Western blotting. (C) Flag-H3, -H4, and -H3.3 can be incorporated into preassembled chromatin. Oocytes were incubated overnight following injection of the single-stranded reporter DNA to allow more than sufficient time for complete chromatin assembly. Then, mRNA encoding Flag-H3, -H4, or -H3.3 was introduced and the indicated groups were treated with TSA for 6 h. As determined by ChIP, each Flag-histone fusion protein was incorporated into the preassembled minichromosome. TSA did not affect this activity. (D) ChIP assays showing histone exchange is unaffected by H3K9 methylation or acetylation. Gal-G9a(SET) was expressed overnight in the presence of the 4xUAS-TRβA reporter. The following day, oocytes were injected with Flag-H3 or -H3.3 mRNA and treated with or without TSA for 6 h. H3K9 methylation did not affect the replication-independent incorporation of Flag-H3 or -H3.3.
Having confirmed that both Flag-H3 and Flag-H3.3 could be utilized for replication-coupled chromatin assembly, we next tested if H3.3 is preferentially incorporated into preassembled chromatin during TSA treatment. For this purpose, Xenopus oocytes were first injected with single-stranded reporter DNA and incubated overnight to ensure complete chromatin assembly (data not shown) (see Fig. 3). The oocytes were then injected with mRNA encoding Flag-H3.3, -H3, or -H4, treated with or without TSA for 6 h, and assayed for Flag-histone incorporation by ChIP. The ChIP results show that both Flag-H3 and Flag-H3.3 proteins were incorporated into preassembled chromatin and that TSA did not enhance incorporation of either histone (Fig. 2C). In fact, TSA treatment appears to confer a slight reduction in the amount of Flag-histone and total H3 on the construct, likely due to a slight reduction in the number of nucleosomes associated with the chromatin template due to increased transcription (J. Jun and J. Wong, unpublished data). To determine if incorporation of Flag-H3 leads to an increase in the overall level of chromatin-associated histone H3, we performed ChIP using an antibody against the H3 C terminus. The H3 C terminus is not known to be subject to posttranslational modification, and this antibody has been widely used for determining the total amount of H3 (10, 44). The results in Fig. 2C showed that the overall level of histone H3 did not increase, in agreement with the idea that the reporter DNA is fully assembled into chromatin prior to mRNA injection. In addition, ChIP assays using anti-acetyl H3 verified the expected effect of TSA on induction of histone acetylation. Since the overall level of chromatin-associated H3 did not increase, these data imply that both Flag-H3 and Flag-H3.3 were incorporated into chromatin by replacing chromatin-associated H3.
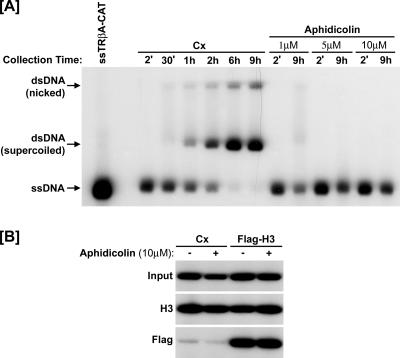
FIG. 3.
Histone exchange occurs independently of DNA replication. (A) Oocytes were injected with single-stranded 4xUAS-TRβA reporter plasmid and harvested at the indicated time points for Southern blotting. Additional groups were pretreated with aphidicolin 2 h prior to injection of the ssDNA. Complete conversion of the ssDNA to dsDNA was observed within 6 to 9 h. Addition of aphidicolin completely blocked DNA replication at 10 μM. Cx, control (no coinjection). (B) Oocytes were injected with the single-stranded reporter plasmid and incubated overnight to allow complete chromatin assembly. Then, the indicated groups were treated with aphidicolin (10 μM) 2 h prior to injection of Flag-H3 mRNA. The ChIP was performed 6 h later using anti-Flag or H3 antibodies, and the results showed that the replication-independent incorporation of Flag-H3 was not affected by aphidicolin.
Neither H3K9 methylation nor acetylation affects histone exchange.
Although the above result indicates that both H3 and H3.3 are exchanged with equal efficiency into preassembled chromatin regardless of TSA treatment, it is possible that H3.3 is preferentially utilized to replace histone H3 when K9 is methylated. To test this possibility, we compared incorporation of Flag-H3 and Flag-H3.3 into a preassembled chromatin template in the presence or absence of Gal-G9a(SET). As before, the Gal-G9a(SET) mRNA and reporter plasmid were injected 1 day prior to the injection of Flag-H3 or -H3.3 mRNA to ensure complete chromatin assembly and efficient H3K9 methylation prior to the introduction of the Flag-tagged histones. Oocytes were treated with or without TSA immediately following injection of Flag-H3/H3.3 mRNA and collected for ChIP analysis 6 h later (Fig. 2D). The ChIP analysis confirmed that H3K9 dimethylation was significantly increased and H3 acetylation was significantly decreased in the presence of Gal-G9a(SET). As shown previously, TSA treatment reduced the level of H3K9me2 and increased the level of H3K9ac. Importantly, we did not observe any preferential incorporation of Flag-H3.3 over the Flag-H3 in the sample with Gal-G9a(SET) and TSA treatment (Fig. 2D). These results indicate that there is no preferential incorporation of H3.3 over H3 into chromatin in which H3K9 methylation is preestablished.
Taken together, our data indicate that TSA-induced reduction of preexisting H3K9 methylation cannot be simply explained by the H3.3 replacement model. Instead, we find that both H3 and H3.3 can be efficiently incorporated into preassembled chromatin. Since there was no increase in the total amount of histone H3 on the reporter, we hypothesized that the incorporation of Flag-H3 or -H3.3 is at the expense of the chromatin-associated histone H3 through an exchange or replacement mechanism. We next provide several lines of evidence to demonstrate that in Xenopus oocytes histone exchange indeed occurs and chromatin-associated histones are dynamic.
Histone exchange occurs independently of DNA replication.
First, we wished to exclude the possibility that the observed incorporation of Flag-H3 and Flag-H3.3 into preassembled chromatin was actually due to residual DNA replication. For this purpose, we first analyzed whether the single-stranded plasmid DNA was indeed completely converted to double-stranded DNA prior to introduction of the Flag-histone constructs. Xenopus oocytes were injected with ssTRβA-CAT reporter DNA. DNA was recovered from injected oocytes over a 9-h time course and analyzed by Southern blotting using a fragment of the TRβA promoter as a probe. The results of the Southern indicate that ssDNA is gradually but completely converted to double-stranded DNA within 6 h (Fig. 3A). This result confirms that the injected ssDNA was indeed converted to dsDNA prior to injection of Flag-H3 (which was done 14 to 16 h after injection of ssDNA).
We also tested if aphidicolin, a DNA polymerase inhibitor, inhibits incorporation of Flag-H3 into preassembled chromatin. As shown in Fig. 3A, addition of aphidicolin inhibited the conversion of injected ssDNA to dsDNA in a dose-dependent manner and 10 μM aphidicolin completely blocked formation of dsDNA. If the incorporation of Flag-H3 into chromatin was due to residual conversion of ssDNA to dsDNA, we would expect that blocking DNA synthesis with aphidicolin would inhibit the incorporation of Flag-H3. However, the results of the ChIP assays indicate that aphidicolin did not affect incorporation of Flag-H3 (Fig. 3B). Taken together, we conclude that the observed histone exchange is not an artifact of residual DNA replication.
Histone exchange is not dependent on transcriptional elongation.
Previous reports suggest that replication-independent replacement of H3-H4 dimers with H3.3-H4 dimers occurs at sites of active transcription and is dependent on elongation of RNA polymerase (2, 22, 50). To test if the observed histone exchange is dependent on transcriptional elongation, we first examined if α-amanitin affects TSA-induced reduction of preexisting H3K9 methylation. The oocytes were first injected with the ss4xUAS-TRβA reporter and with or without Gal-G9a(SET) mRNA. After overnight incubation, the oocytes were treated with or without α-amanitin (1 μg/ml) 2 h prior to the 6-h TSA treatment and processed for transcriptional analysis and ChIP assays. Primer extension analysis (Fig. 4A) showed that Gal-G9a(SET) suppressed transcription of the reporter and this repression was reversed upon addition of TSA. As expected, addition of α-amanitin completely blocked the transcriptional activation induced by TSA. However, ChIP analysis showed that TSA treatment increased the level of acetyl H3/H4 and decreased the level of H3K9me2, and this effect was not affected by α-amanitin (Fig. 4B). Thus, transcriptional elongation is not required for the reduction in H3K9 methylation following TSA treatment.
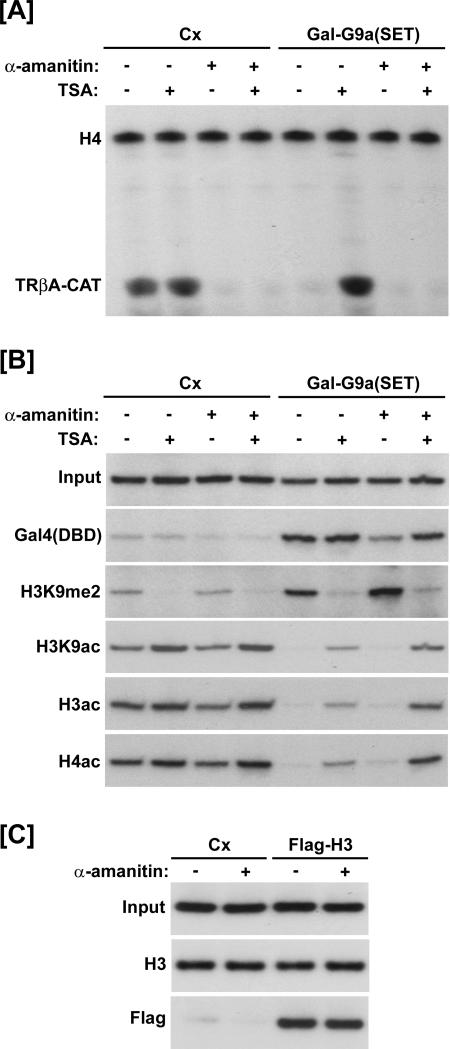
FIG. 4.
Histone exchange and the TSA-induced reduction in H3K9 methylation is not depended on transcriptional elongation. (A) Results of primer extension analysis. Gal-G9a(SET) repressed transcription of the reporter gene, and TSA reversed this repression; however, transcription was completely inhibited in all groups that received α-amanitin. Cx, control (no coinjection). (B and C) Results of ChIP assays. (B) The H3K9 methylation induced by Gal-G9a(SET) was reduced upon treatment with TSA. Likewise, acetylation of H3 and H4 decreased in the presence of Gal-G9a(SET) and increased in the presence of TSA. The same effects were observed in the presence of α-amanitin. (C) The replication-independent incorporation of Flag-H3 was not affected by α-amanitin. Oocytes were incubated overnight following injection of the single-stranded reporter DNA. Then they were treated with or without α-amanitin for 2 h prior to injection of Flag-H3 mRNA. ChIP was performed 6 h after the Flag-H3 mRNA injection. Incorporation of Flag-H3 was unaffected by α-amanitin treatment. As a control for the total amount of histone H3 associated with the reporter, a ChIP was performed using an antibody raised against the H3 C terminus.
We also tested if α-amanitin affects the replication-independent incorporation of Flag-H3 into chromatin. Similar to the experimental design for Fig. 3B, oocytes were injected with the ss4xUAS-TRβA reporter and incubated overnight to ensure complete chromatin assembly. Then the oocytes were treated with or without α-amanitin for 2 h prior to injection of Flag-H3 mRNA and processed for ChIP 6 h after the Flag-H3 mRNA injection. The results in Fig. 4C show that α-amanitin did not affect the replication-independent incorporation of Flag-H3 into chromatin. In addition, ChIP analysis revealed that α-amanitin did not affect the overall level of chromatin-associated H3. Thus, the observed histone exchange is not dependent on transcriptional elongation.
Histone exchange occurs in the Xenopus oocyte endogenous lampbrush chromosomes.
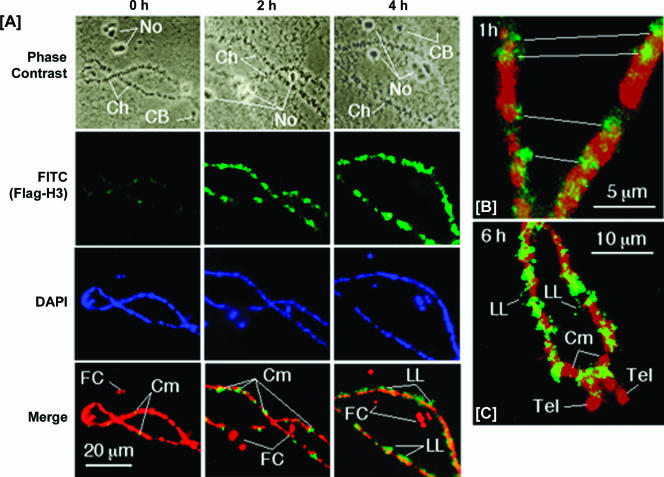
To be certain that the histone exchange we observed is not specific to the chromatin-assembled plasmid DNA, we also determined if Flag-H3 could be deposited into the chromatin of the endogenous lampbrush chromosomes of the Xenopus oocyte. Since the Xenopus oocyte is arrested in metaphase II of meiosis, the endogenous lampbrush chromosomes are nonreplicating. Nuclear spreads made within 15 min of injection of Flag-H3 mRNA showed only a few isolated spots of immunofluorescence over the chromosomes, whereas after 2 h, Flag-H3 incorporation was seen to be extensive along the chromomeric axes, and by 4 h, immunofluorescence was seen to extend to the fringes of chromomeres (Fig. 5A). In none of the preparations made up to 6 h was Flag-H3 detected in nucleoli or Cajal bodies (Fig. 5A). Details of differences in localization with time can be discerned at higher magnification: whereas at 1 h, Flag-H3 incorporation was restricted to small foci located in or close to the chromomeres (Fig. 5B), after 6 h incorporation had extended out into the lateral loops (Fig. 5C). Although most of the label was seen at loop inserts, close to the condensed chromatin of the chromomeres, occasional small foci were located at points around the extended loop axes (Fig. 5C). Interestingly, incorporation of Flag-H3 was excluded from heterochromatin in many of the chromomeres and in the telomeres of the lampbrush chromosomes (Fig. 5C). These results confirm that exchange of free and chromatin-associated histones in the Xenopus oocyte occurs independently of DNA replication. Furthermore, these data indicate that histone exchange occurs in stable endogenous chromatin and is more dynamic in euchromatin than heterochromatin.
FIG. 5.
Histone exchange occurs in the Xenopus oocyte endogenous lampbrush chromosomes. Oocytes were injected with mRNA encoding Flag-H3 and processed for immunofluorescence immediately (0 h) and after 1, 2, 4, and 6 h. Representative images of chromosomes were recorded by phase contrast and immunofluorescence for Flag-H3 (FITC) and DNA (DAPI). Merged images are colored green for Flag-H3 and red for DNA: the overlap of signal is orange/yellow. (A) Only a few labeled foci were seen over the chromomeres (Cm) immediately after injection (0 h), whereas incorporation of Flag-H3 became more extensive over chromomeres by 2 h and extended into lateral chromatin loops (LL) by 4 h following injection. Incorporation was restricted to the chromosomes (Ch) and was not seen in nucleoli (No) or Cajal bodies (CB). (B) Higher magnification showing distinct foci of incorporation of Flag-H3 at 1 h over and adjacent to chromomeres. Immunofluorescence is located at sites in register in homologous chromosomes (white lines). (C) Higher magnification of chromosome arms in a preparation made at 6 h after injection showing punctate incorporation of Flag-H3 around the axes of lateral loops and no incorporation in many chromomeres or in telomeres (Tel).
Time course of histone incorporation.
We next attempted to roughly estimate the rate at which Flag-H3 protein is incorporated into preassembled chromatin. The ss4xUAS-TRβA reporter plasmid was microinjected into the oocyte nuclei and then allowed to assemble into chromatin overnight. We then injected purified recombinant Flag-H3 protein into the oocyte nuclei and collected samples for ChIP at the indicated time points (Fig. 6A). Flag-H3 incorporation was detected within 15 min of injection and steadily increased over the short 1-h time course (Fig. 6B). In a longer 6-h time course, incorporation of Flag-H3 steadily increased and reached maximal levels 3 to 6 h after injection (Fig. 6C).
FIG. 6.
Time course of histone incorporation. (A) Experimental design. Oocytes were microinjected with the ss4xUAS-TRβA reporter and incubated overnight to allow chromatin assembly. Then, recombinant Flag-H3 protein was microinjected into the oocyte nuclei. Extracts were prepared for ChIP analysis at the indicated time points following injection of the Flag-H3 protein. (B and C) Results of ChIP assays. (B) Flag-H3 incorporation was detectable 15 min following injection and increased over the 60-min time course. (C) Flag-H3 incorporation reached maximal levels 3 to 6 h following injection. The ChIP data were quantitated by densitometry and are presented as difference (fold) from the control.
Preassembled histone H3 can be replaced by newly synthesized histone H3.
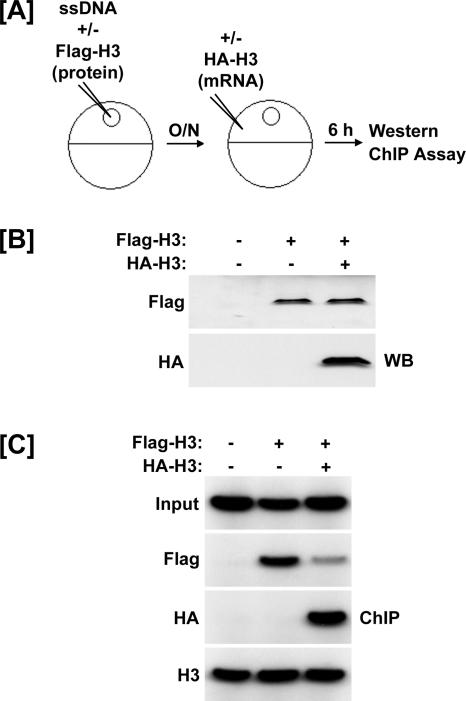
In the previous experiments, we illustrated that Flag-tagged histones can be incorporated into preassembled chromatin. To further test the histone exchange model, we sought to determine if preassembled histone H3 could be replaced by newly synthesized histone H3. For this purpose, we first injected the oocytes with the ss4xUAS-TRβA reporter and recombinant Flag-H3 protein to allow the incorporation of the Flag-H3 protein. After overnight incubation, the oocytes were injected with mRNA encoding HA-tagged H3 to express HA-H3 (Fig. 7A). Oocytes were harvested for Western and ChIP analyses 6 h following injection of the HA-H3 mRNA.
FIG. 7.
Histone H3 preassembled into chromatin can be competed away by excess histone H3. (A) Experimental design. The ss4xUAS-TRβA reporter was coinjected with nothing or recombinant Flag-H3 protein. After overnight incubation, the indicated group was injected with mRNA encoding HA-H3. Extracts were prepared 6 h after the HA-H3 injection. (B) Western blots showing appropriate expression of Flag- and HA-H3. (C) Results of ChIP assays. Flag-H3 was utilized for chromatin assembly. Overexpression of HA-H3 resulted in replication-independent incorporation of HA-H3 and a decrease in the amount of Flag-H3 associated with the reporter. There was no change in the total amount of H3 associated with the reporter as determined by ChIP using an antibody raised against the H3 C terminus.
Western blots using anti-Flag antibodies showed that the level of Flag-H3 protein was not affected by the expression of HA-H3 (Fig. 7B). Although we do not know the precise ratio of Flag-H3 to HA-H3, we are confident that the HA-H3 is present in excess to that of the Flag-H3 because HA-H3 was readily detected by Western blotting and detection of Flag-H3 required a supersensitive chemiluminescent reagent (SuperSignal West Femto; Pierce Biotechnology). The ChIP results in Fig. 7C show that Flag-H3 was incorporated into chromatin prior to injection of HA-H3 mRNA. Importantly, ChIP analysis showed that 6 h after injection of HA-H3 mRNA, the level of chromatin-associated Flag-H3 was significantly reduced and the incorporation of HA-H3 was clearly detected (Fig. 7C). These results provide compelling evidence that chromatin-associated histone H3 can be replaced by newly synthesized histone H3 and further support our hypothesis that chromatin-associated and non-chromatin-associated histones are continually exchanged in the Xenopus oocyte.
Methylated H3K9 is lost upon dissociation of the methyltransferase from chromatin.
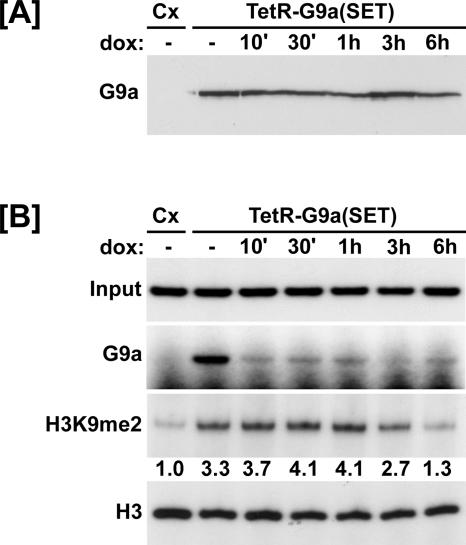
The data presented thus far indicate that free and chromatin-associated histones are continually exchanged in the Xenopus oocyte. If this conclusion is correct, then histone modifications at specific sites within chromatin would have to be maintained by the close association of the modifying enzyme. According to this model, methylated H3K9 would diminish upon dissociation of the H3K9 methyltransferase from chromatin. To test this model, we created a Tet repressor (TetR)-G9a(SET) fusion construct (Tet-off system) and modified the 4xUAS-TRβA reporter to contain seven binding sites for the TetR (2xtetO-4xUAS-TRβA). Xenopus oocytes were injected with the new reporter and mRNA encoding TetR-G9a(SET) and incubated overnight to allow establishment of H3K9 methylation. Then, the oocytes were treated with doxycycline (10 μg/ml) to dissociate the methyltransferase from the chromatin-assembled reporter and extracts were prepared at the indicated time points for analysis of TetR-G9a(SET) expression by Western blotting and H3K9 methylation by ChIP. A Western blot using an anti-G9a antibody showed that the level of TetR-G9a(SET) protein remained constant in each treatment group (Fig. 8A). The results of the ChIPs indicated that after overnight incubation, TetR-G9a(SET) efficiently increased the level of H3K9me2 on the reporter (Fig. 8B). The TetR-G9a(SET) quickly (within 10 min) dissociated from the chromatin template upon treatment with doxycycline. Importantly, the results of the ChIP assay revealed a reduction in H3K9me2 3 to 6 h after addition of doxycycline. As a control, we found that doxycycline treatment did not affect the total amount of histone H3 associated with the reporter (Fig. 8B).
FIG. 8.
Methylated H3K9 is lost upon dissociation of the methyltransferase from chromatin. (A) Western blot using anti-G9a antibodies indicating the level of TetR-G9a(SET) protein in each group. Cx, control (no coinjection). (B) Oocytes were injected with mRNA encoding TetR-G9a(SET) and the 2xtetO-4xUAS-TRβA reporter. After overnight incubation, oocytes were treated with doxycycline (10 μg/ml) for the indicated times and processed for ChIP analysis. TetR-G9a(SET) was targeted to the reporter and induced dimethylation of H3K9 as determined by ChIP using anti-G9a or anti-H3K9me2 antibodies, respectively. TetR-G9a(SET) quickly dissociated from the reporter upon doxycycline treatment. The amount of H3K9me2 associated with the reporter began to detectably decrease 3 to 6 h following dissociation of the methyltransferase. There was no change in the amount of total histone H3 associated with the reporter. The ChIP data were quantitated by densitometry and are presented as difference (fold) from the control.
DISCUSSION
In this study, we have investigated the potential mechanisms underlying TSA-induced reduction of H3K9 methylation in Xenopus oocytes. Until recently, lysine methylation was thought to be stable; however, the identification of two families of lysine demethylases clearly demonstrates that, like other histone modifications, lysine methylation is reversible (51, 58, 61, 65). Thus, the reduction in H3K9 methylation observed during TSA treatment could occur through a mechanism involving demethylation. However, we believe this is unlikely because TSA does not affect the overall level of H3K9 methylation in either Xenopus oocytes or HeLa cells as shown here and reported by others (34). If TSA somehow promoted H3K9 demethylation, we would expect to see a reduction of the overall level of H3K9 methylation, much like its effect on histone acetylation. Regardless of whether TSA treatment promotes H3K9 demethylation, we present strong evidence that chromatin-associated histones in Xenopus oocytes are dynamic and that this dynamic histone exchange with free pool histones is likely to account for TSA-induced reduction of H3K9 methylation in chromatin.
The key finding of this study is that, in Xenopus oocytes, chromatin-associated histone H3 undergoes constant exchange with non-chromatin-associated histone H3. Several lines of evidence support this novel finding. First, we showed that both Flag-H3 and Flag-H3.3 can be incorporated into preassembled chromatin in a DNA replication-independent manner. Since there was no increase in the overall level of chromatin-associated histone H3, the Flag-histones must be incorporated by replacing the chromatin-associated H3. Second, we showed that Flag-H3 is incorporated into the endogenous lampbrush chromosomes. Xenopus oocytes are arrested at meiosis II, and DNA replication does not occur during this stage; thus, the incorporation of histone H3 is clearly DNA replication independent. Finally, overexpression of HA-H3 can replace Flag-H3 preassembled into chromatin. Together these data demonstrate that in Xenopus oocytes chromatin-associated histones are constantly exchanged with non-chromatin-associated histones.
From the results in Fig. 7, we estimate that ∼70 to 80% of chromatin-associated Flag-H3 can be replaced by HA-H3 within 6 h. In addition, we found that injected Flag-H3 proteins are gradually incorporated into preassembled chromatin and reach a plateau 3 to 6 h after injection. This estimation is in good correlation with the data in Fig. 1D and Fig. 8B showing that it takes ∼3 h to see a 50% reduction in H3K9 methylation upon TSA or doxycycline treatment. Thus, it is possible that dynamic histone exchange, regardless of the underlying mechanism, can account for the TSA-induced reduction of H3K9 methylation. To explain how TSA treatment induces reduction of H3K9 methylation in chromatin through histone exchange, we propose the following working model. First Gal-G9a binds and establishes local H3K9 methylation in chromatin. Although chromatin-associated histones are constantly exchanged with histones in the free pool, the chromatin-bound Gal-G9a(SET) methylates newly incorporated, unmethylated H3 and maintains local levels of H3K9me2. When oocytes are treated with TSA, acetylation of H3K9 (as well as most other lysines) increases and consequently protects H3K9 from methylation. Thus, in the presence of TSA, newly incorporated H3 is preferentially acetylated on H3K9 and consequently cannot be methylated by Gal-G9a(SET). The continual exchange of unmethylated (acetylated) H3 with chromatin-associated H3 eventually leads to the replacement of K9 methylated H3 with acetylated histone H3. This model explains how TSA is able to reduce preexisting H3K9me2 from chromatin, despite its lack of effect on HMT activity or the rate of histone exchange.
The dynamic exchange between non-chromatin-associated and chromatin-associated histones reported here is not the same phenomenon as the replacement of H3 with variant H3.3 during transcription. Previous reports have shown that deposition of H3.3 only occurs at sites of active transcription and is dependent upon transcriptional elongation (2, 3, 22, 50). In contrast, the process of histone exchange in Xenopus oocytes was neither specific for H3.3 nor affected by inhibition of elongation with α-amanitin. Furthermore, histone exchange was unaffected by alterations in transcriptional activity [i.e., repression by Gal-G9a(SET) and activation by TSA], methylation of H3K9, or histone acetylation. However, the precise mechanism through which histone exchange occurs is currently unknown. For instance, we do not know if histones are exchanged individually or as dimers or tetramers (H3-H4). Additionally, it is not known whether continual histone exchange requires the assistance of chaperones and/or ATP-dependent chromatin remodeling complexes as in the case of H2A/H2A.Z exchange, which is catalyzed by the SWR1 chromatin remodeling complex (24, 38).
Previous reports using mammalian or insect cells indicate that H3 and H4 are much less mobile than H2A and H2B (27, 28, 57). Additionally, expression of GFP-H3.3 in nonreplicating cells results in its specific incorporation at transcriptionally active loci; whereas GFP-H3 is restricted from nonreplicating chromatin (2). Several factors may explain why chromatin-associated H3 in Xenopus oocytes is more dynamic than that in many other cells. Histone exchange is likely catalyzed or facilitated by histone chaperones and/or distinct protein complexes with ATPase activities (24, 38). In this regard, Xenopus oocytes are known to contain large stores of free histones, histone chaperones, and ATP-dependent chromatin remodeling factors. For instance, Xenopus oocytes contain the abundant histone chaperones nucleoplasmin and N1/N2 that have the capacity to remove and deposit histones onto DNA (15, 29, 42, 43). The Xenopus oocyte is also enriched in ISWI and SWI/SNF ATPases (18, 20, 26), which have been shown to increase chromatin fluidity through their ability to assemble and disassemble chromatin. The presence of these types of chromatin metabolic activities in the Xenopus oocyte helps to rationalize our continual histone exchange model. It is likely that some combination of these or similar chaperones contributes to the histone exchange activity we observed on the exogenous minichromosome and in the endogenous lampbrush chromosomes.
Like the Xenopus oocyte, the early Drosophila embryo contains a large maternal pool of histones and chromatin assembly factors for use in the many rapid cell divisions that occur during the initial stages of development. Extracts from preblastoderm embryos can assemble chromatin in a similar fashion to that of Xenopus oocyte extracts (8). Investigation into the mechanism of chromatin assembly by the Drosophila embryo extract has revealed the importance of several histone chaperones (dCAF-1, dCAF-4, and dNAP-1), as well as ATP, for efficient assembly (12). Thus, like Xenopus oocytes, histone exchange may also occur in the early Drosophila embryo. This matter warrants further investigation.
Although chromatin-associated histones, especially H3 and H4, are less mobile in mammalian and insect cells, to a certain extent the dynamic histone exchange observed here may also operate in these cells. First, histone chaperones and chromatin remodeling activities that likely make chromatin more dynamic in oocytes and early embryos also exist in somatic cells. For example, a somatic form of the histone chaperone NASP (nuclear autoantigenic sperm protein), a homolog of Xenopus N1/N2, is expressed by many different cell types (45). Second, there is a free pool of histones in mammalian cells. It has been shown that in mammalian cells hAsf1 complexes maintain a “free pool” of excess histones that can be immediately utilized for chromatin assembly (19). In addition, in yeast histones are constantly displaced from gene promoters and actively transcribed coding regions (11, 17, 30, 32, 49). While the histone H3.3 variant is clearly preferentially incorporated into active transcription sites (22, 37, 50), the displaced H3 is unlikely simply designated for degradation and more likely reincorporated into chromatin via replication-independent pathways. Third, a recent study demonstrated, although at a low rate, free histones H3 and H4 are exchanged into chromatin via a replication-independent mechanism (57). In the same study, the transcribed regions of the rRNA gene loci were found to exhibit rapid exchange of H3/H4 tetramers. Interestingly, we found that while exchange of H3/H4 into the minichromosomes derived from injected ssDNA is independent of transcription (insensitive to α-amanitin), histone exchange into Xenopus oocyte lampbrush chromosomes is more prevalent in euchromatin than heterochromatin (Fig. 6). While this difference could be simply explained by the more compact nature of heterochromatin that reduces histone exchange, transcription may also facilitate the exchange of H3/H4 into the euchromatin regions. Taken together, it is likely that both transcription-dependent (including H3.3 variant) and -independent histone exchanges operate in all cells. The extent to which the transcription-dependent or -independent histone exchange contributes to chromatin dynamics is likely cell-type dependent.
In summary, the results of this study have uncovered a previously unidentified mechanism of chromatin metabolism. We have shown that chromatin-associated and non-chromatin-associated histones are continually exchanged in the Xenopus oocyte. Although only H3 was analyzed here, this dynamic histone exchange is likely true for all other histones. Continual histone exchange creates an environment in which posttranslational histone modifications are quite dynamic as the maintenance of any specific modification requires the continual presence of the modifying enzyme. Future experiments should be designed to address the molecular mechanism of histone exchange and identify the chaperones and/or ATP-dependent chromatin remodeling factors involved in the process. Furthermore, it will be interesting to determine if histone exchange also occurs to some extent in other cell types.
Acknowledgments
We thank Bert O'Malley, Ming-Jer Tsai, David Moore, Michelle Barton, and Sharon Dent for valuable discussion.
This work was supported by National Institutes of Health grant DK58679 to J.W. and by a grant to J.S. from The Wellcome Trust.
REFERENCES
- 1.Ahmad, K., and S. Henikoff. 2002. Epigenetic consequences of nucleosome dynamics. Cell 111:281-284. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, K., and S. Henikoff. 2002. Histone H3 variants specify modes of chromatin assembly. Proc. Natl. Acad. Sci. USA 99(Suppl. 4):16477-16484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad, K., and S. Henikoff. 2002. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9:1191-1200. [DOI] [PubMed] [Google Scholar]
- 4.Almouzni, G., and M. Mechali. 1988. Assembly of d chromatin promoted by DNA synthesis in extracts from Xenopus eggs. EMBO J. 7:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannister, A. J., and T. Kouzarides. 2004. Histone methylation: recognizing the methyl mark. Methods Enzymol. 376:269-288. [DOI] [PubMed] [Google Scholar]
- 6.Bannister, A. J., and T. Kouzarides. 2005. Reversing histone methylation. Nature 436:1103-1106. [DOI] [PubMed] [Google Scholar]
- 7.Bannister, A. J., R. Schneider, and T. Kouzarides. 2002. Histone methylation: dynamic or static? Cell 109:801-806. [DOI] [PubMed] [Google Scholar]
- 8.Becker, P. B., and C. Wu. 1992. Cell-free system for assembly of transcriptionally repressed chromatin from Drosophila embryos. Mol. Cell. Biol. 12:2241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 10.Boeger, H., J. Griesenbeck, J. S. Strattan, and R. D. Kornberg. 2003. Nucleosomes unfold completely at a transcriptionally active promoter. Mol. Cell 11:1587-1598. [DOI] [PubMed] [Google Scholar]
- 11.Boeger, H., J. Griesenbeck, J. S. Strattan, and R. D. Kornberg. 2004. Removal of promoter nucleosomes by disassembly rather than sliding in vivo. Mol. Cell 14:667-673. [DOI] [PubMed] [Google Scholar]
- 12.Bulger, M., T. Ito, R. T. Kamakaka, and J. T. Kadonaga. 1995. Assembly of regularly d nucleosome arrays by Drosophila chromatin assembly factor 1 and a 56-kDa histone-binding protein. Proc. Natl. Acad. Sci. USA 92:11726-11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byvoet, P., G. R. Shepherd, J. M. Hardin, and B. J. Noland. 1972. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch. Biochem. Biophys. 148:558-567. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbert, G. L., S. Daujat, A. W. Snowden, H. Erdjument-Bromage, T. Hagiwara, M. Yamada, R. Schneider, P. D. Gregory, P. Tempst, A. J. Bannister, and T. Kouzarides. 2004. Histone deimination antagonizes arginine methylation. Cell 118:545-553. [DOI] [PubMed] [Google Scholar]
- 15.Earnshaw, W. C., B. M. Honda, R. A. Laskey, and J. O. Thomas. 1980. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell 21:373-383. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenhofer-Murray, A. E. 2004. Chromatin dynamics at DNA replication, transcription and repair. Eur. J. Biochem. 271:2335-2349. [DOI] [PubMed] [Google Scholar]
- 17.Ercan, S., M. J. Carrozza, and J. L. Workman. 2004. Global nucleosome distribution and the regulation of transcription in yeast. Genome Biol. 5:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelius, B., P. Wade, A. Wolffe, O. Wrange, and A. K. Ostlund Farrants. 1999. Characterization of a chromatin remodelling activity in Xenopus oocytes. Eur. J. Biochem. 262:426-434. [DOI] [PubMed] [Google Scholar]
- 19.Groth, A., D. Ray-Gallet, J. P. Quivy, J. Lukas, J. Bartek, and G. Almouzni. 2005. Human Asf1 regulates the flow of S phase histones during replicational stress. Mol. Cell 17:301-311. [DOI] [PubMed] [Google Scholar]
- 20.Guschin, D., T. M. Geiman, N. Kikyo, D. J. Tremethick, A. P. Wolffe, and P. A. Wade. 2000. Multiple ISWI ATPase complexes from Xenopus laevis. Functional conservation of an ACF/CHRAC homolog. J. Biol. Chem. 275:35248-35255. [DOI] [PubMed] [Google Scholar]
- 21.Honda, B. M., P. M. Candido, and G. H. Dixon. 1975. Histone methylation. Its occurrence in different cell types and relation to histone H4 metabolism in developing trout testis. J. Biol. Chem. 250:8686-8689. [PubMed] [Google Scholar]
- 22.Janicki, S. M., T. Tsukamoto, S. E. Salghetti, W. P. Tansey, R. Sachidanandam, K. V. Prasanth, T. Ried, Y. Shav-Tal, E. Bertrand, R. H. Singer, and D. L. Spector. 2004. From silencing to gene expression: real-time analysis in single cells. Cell 116:683-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 24.Jin, J., Y. Cai, B. Li, R. C. Conaway, J. L. Workman, J. W. Conaway, and T. Kusch. 2005. In and out: histone variant exchange in chromatin. Trends Biochem. Sci. 30:680-687. [DOI] [PubMed] [Google Scholar]
- 25.Khorasanizadeh, S. 2004. The nucleosome: from genomic organization to genomic regulation. Cell 116:259-272. [DOI] [PubMed] [Google Scholar]
- 26.Kikyo, N., P. A. Wade, D. Guschin, H. Ge, and A. P. Wolffe. 2000. Active remodeling of somatic nuclei in egg cytoplasm by the nucleosomal ATPase ISWI. Science 289:2360-2362. [DOI] [PubMed] [Google Scholar]
- 27.Kimura, H. 2005. Histone dynamics in living cells revealed by photobleaching. DNA Repair (Amsterdam) 4:939-950. [DOI] [PubMed] [Google Scholar]
- 28.Kimura, H., and P. R. Cook. 2001. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 153:1341-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinschmidt, J. A., and W. W. Franke. 1982. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell 29:799-809. [DOI] [PubMed] [Google Scholar]
- 30.Korber, P., T. Luckenbach, D. Blaschke, and W. Hörz. 2004. Evidence for histone eviction in trans upon induction of the yeast PHO5 promoter. Mol. Cell. Biol. 24:10965-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachner, M., R. J. O'Sullivan, and T. Jenuwein. 2003. An epigenetic road map for histone lysine methylation. J. Cell Sci. 116:2117-2124. [DOI] [PubMed] [Google Scholar]
- 32.Lee, C. K., Y. Shibata, B. Rao, B. D. Strahl, and J. D. Lieb. 2004. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 36:900-905. [DOI] [PubMed] [Google Scholar]
- 33.Li, J., Q. Lin, H.-G. Yoon, Z.-Q. Huang, B. D. Strahl, C. D. Allis, and J. Wong. 2002. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol. Cell. Biol. 22:5688-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maison, C., D. Bailly, A. H. Peters, J. P. Quivy, D. Roche, A. Taddei, M. Lachner, T. Jenuwein, and G. Almouzni. 2002. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30:329-334. [DOI] [PubMed] [Google Scholar]
- 35.Martin, C., and Y. Zhang. 2005. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell. Biol. 6:838-849. [DOI] [PubMed] [Google Scholar]
- 36.Metzger, E., M. Wissmann, N. Yin, J. M. Muller, R. Schneider, A. H. Peters, T. Gunther, R. Buettner, and R. Schule. 2005. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437:436-439. [DOI] [PubMed] [Google Scholar]
- 37.Mito, Y., J. G. Henikoff, and S. Henikoff. 2005. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 37:1090-1097. [DOI] [PubMed] [Google Scholar]
- 38.Mizuguchi, G., X. Shen, J. Landry, W. H. Wu, S. Sen, and C. Wu. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343-348. [DOI] [PubMed] [Google Scholar]
- 39.Peters, A. H., S. Kubicek, K. Mechtler, R. J. O'Sullivan, A. A. Derijck, L. Perez-Burgos, A. Kohlmaier, S. Opravil, M. Tachibana, Y. Shinkai, J. H. Martens, and T. Jenuwein. 2003. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 12:1577-1589. [DOI] [PubMed] [Google Scholar]
- 40.Peters, A. H., J. E. Mermoud, D. O'Carroll, M. Pagani, D. Schweizer, N. Brockdorff, and T. Jenuwein. 2002. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat. Genet. 30:77-80. [DOI] [PubMed] [Google Scholar]
- 41.Peterson, C. L., and M. A. Laniel. 2004. Histones and histone modifications. Curr. Biol. 14:R546-R551. [DOI] [PubMed] [Google Scholar]
- 42.Philpott, A., and G. H. Leno. 1992. Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell 69:759-767. [DOI] [PubMed] [Google Scholar]
- 43.Philpott, A., G. H. Leno, and R. A. Laskey. 1991. Sperm decondensation in Xenopus egg cytoplasm is mediated by nucleoplasmin. Cell 65:569-578. [DOI] [PubMed] [Google Scholar]
- 44.Reinke, H., and W. Horz. 2003. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11:1599-1607. [DOI] [PubMed] [Google Scholar]
- 45.Richardson, R. T., I. N. Batova, E. E. Widgren, L. X. Zheng, M. Whitfield, W. F. Marzluff, and M. G. O'Rand. 2000. Characterization of the histone H1-binding protein, NASP, as a cell cycle-regulated somatic protein. J. Biol. Chem. 275:30378-30386. [DOI] [PubMed] [Google Scholar]
- 46.Saccani, S., and G. Natoli. 2002. Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev. 16:2219-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein, N. C. Emre, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419:407-411. [DOI] [PubMed] [Google Scholar]
- 48.Schneider, R., A. J. Bannister, F. A. Myers, A. W. Thorne, C. Crane-Robinson, and T. Kouzarides. 2004. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 6:73-77. [DOI] [PubMed] [Google Scholar]
- 49.Schwabish, M. A., and K. Struhl. 2004. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 24:10111-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz, B. E., and K. Ahmad. 2005. Transcriptional activation triggers deposition and removal of the histone variant H3.3. Genes Dev. 19:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi, Y., F. Lan, C. Matson, P. Mulligan, J. R. Whetstine, P. A. Cole, and R. A. Casero. 2004. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941-953. [DOI] [PubMed] [Google Scholar]
- 52.Sittman, D. B., R. A. Graves, and W. F. Marzluff. 1983. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc. Natl. Acad. Sci. USA 80:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smillie, D. A., A. J. Llinas, J. T. Ryan, G. D. Kemp, and J. Sommerville. 2004. Nuclear import and activity of histone deacetylase in Xenopus oocytes is regulated by phosphorylation. J. Cell Sci. 117:1857-1866. [DOI] [PubMed] [Google Scholar]
- 54.Sommerville, J., C. L. Brumwell, J. C. Politz, and T. Pederson. 2005. Signal recognition particle assembly in relation to the function of amplified nucleoli of Xenopus oocytes. J. Cell Sci. 118:1299-1307. [DOI] [PubMed] [Google Scholar]
- 55.Stewart, M. D., J. Li, and J. Wong. 2005. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol. Cell. Biol. 25:2525-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tagami, H., D. Ray-Gallet, G. Almouzni, and Y. Nakatani. 2004. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116:51-61. [DOI] [PubMed] [Google Scholar]
- 57.Thiriet, C., and J. J. Hayes. 2005. Replication-independent core histone dynamics at transcriptionally active loci in vivo. Genes Dev. 19:677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsukada, Y., J. Fang, H. Erdjument-Bromage, M. E. Warren, C. H. Borchers, P. Tempst, and Y. Zhang. 2006. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439:811-816. [DOI] [PubMed] [Google Scholar]
- 59.Vidanes, G. M., C. Y. Bonilla, and D. P. Toczyski. 2005. Complicated tails: histone modifications and the DNA damage response. Cell 121:973-976. [DOI] [PubMed] [Google Scholar]
- 60.Wells, D., and L. Kedes. 1985. Structure of a human histone cDNA: evidence that basally expressed histone genes have intervening sequences and encode polyadenylylated mRNAs. Proc. Natl. Acad. Sci. USA 82:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whetstine, J. R., A. Nottke, F. Lan, M. Huarte, S. Smolikov, Z. Chen, E. Spooner, E. Li, G. Zhang, M. Colaiacovo, and Y. Shi. 2006. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125:467-481. [DOI] [PubMed] [Google Scholar]
- 62.Wong, J., D. Patterton, A. Imhof, D. Guschin, Y. B. Shi, and A. P. Wolffe. 1998. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J. 17:520-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong, J., Y. B. Shi, and A. P. Wolffe. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 9:2696-2711. [DOI] [PubMed] [Google Scholar]
- 64.Wu, R. S., S. Tsai, and W. M. Bonner. 1982. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell 31:367-374. [DOI] [PubMed] [Google Scholar]
- 65.Yamane, K., C. Toumazou, Y. I. Tsukada, H. Erdjument-Bromage, P. Tempst, J. Wong, and Y. Zhang. 2006. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125:485-495. [DOI] [PubMed] [Google Scholar]