Abstract
Nucleoporins mediate transport of macromolecules across the nuclear pore complex, yet the function of many individual nucleoporins is largely unresolved. To address this question, we depleted cells of the cytoplasmic nucleoporins Nup214/CAN and Nup358/RanBP2 by RNA interference. Depletion of Nup214 resulted in codepletion of its binding partner, Nup88. Nuclear pore complexes assembled in the absence of Nup214/Nup88 or Nup358 were fully functional in nuclear protein import, whereas nuclear mRNA export was slightly impaired. Depletion of Nup358 had only a minor effect on nuclear protein export. In contrast, depletion of Nup214/Nup88 led to strongly reduced CRM1-mediated export of the shuttling transcription factor NFAT as well as a human immunodeficiency virus-Rev derivative. A specific role of Nup214 in protein export is furthered by the biochemical properties of a high-affinity complex containing Nup214, CRM1, RanGTP, and an export cargo. Our results show that the Nup214/Nup88 complex is required for efficient CRM1-mediated transport, supporting a model involving a high-affinity binding site for CRM1 at Nup214 in the terminal steps of export.
Nucleocytoplasmic transport of most proteins and ribonucleoprotein particles is mediated by shuttling transport receptors of the importin β superfamily. These importins or exportins, also collectively referred to as karyopherins, bind to their transport cargoes via characteristic transport signals, termed NLS (nuclear localization signal) or NES (nuclear export signal) (19, 31). A common feature of all karyopherins is their ability to interact with RanGTP, a small GDP/GTP-binding protein. Nucleotide loading of Ran is controlled by the chromatin-bound nucleotide exchange factor RCC1 (10) and the cytoplasmic GTPase-activating protein RanGAP (9). Import of RanGDP into the nucleus is mediated by a dedicated transport factor, NTF2 (40). As a result of these activities, a gradient is established with a high concentration of RanGTP in the nucleus, providing the driving force for accumulation of cargo molecules against their own concentration gradient (23). Exportins interact with their substrate and RanGTP in the nucleus, forming a trimeric complex that translocates to the cytoplasm. Importins may bind either directly to their cargo molecules or via an adapter protein, like importin α, that interacts with a so-called classic NLS and with the actual transport receptor importin β. After transport into the nucleus, the import complex dissociates upon binding of RanGTP to the importin. The only exceptions to these rules appear to be NTF2-mediated import of Ran itself (40) and transport of the bulk of mRNA by the export factors NXF1 and p15 (38, 49).
Transport of all macromolecules across the nuclear envelope occurs through nuclear pore complexes (NPCs), channel-forming structures of ∼125 MDa in vertebrate cells that are embedded between the inner and the outer nuclear membranes (14). Nucleoporins, the constituents of the NPC, are mostly present at a copy number of eight or multiples of eight, reflecting the octagonal symmetry of the entire complex. In vertebrate cells, about 30 nucleoporins have been identified (12), most of which localize symmetrically on both sides of the NPC. Some nucleoporins, however, show an asymmetric distribution and may be found exclusively on one side of the pore. About a third of the identified nucleoporins contain FG (phenylalanine, glycine) repeats, which play an important role in various models that have been suggested to mechanistically explain the translocation of macromolecules across the NPC (19, 31, 39, 42). In these models, the gate-forming nucleoporins are mainly characterized by their propensity to generate a milieu that is dominated by FG repeats and that somehow facilitates transport. What, then, is the role of individual nucleoporins in different transport pathways? It has been speculated that nucleoporins with an asymmetric distribution serve as initial or terminal docking sites for transport complexes. In yeast, however, the FG-repeat asymmetry does not appear to be required for bulk nucleocytoplasmic transport (56). In fact, yeast cells that lack all of the five asymmetric FG domains are viable (48). Likewise, reconstituted Xenopus nuclei lacking the cytoplasmic nucleoporins Nup214/CAN and Nup358/RanBP2 do not exhibit any gross defects with respect to nuclear import (51).
We have previously identified Nup214 as a terminal binding site in nuclear protein export in vitro (27). Release of the export complex from Nup214 may be initiated by the soluble cytoplasmic protein RanBP1 or by the Ran-binding domains of Nup358 (27). Both Nup214 and Nup358 contain FG repeats (29, 50, 53, 54) and interact with various importins and exportins in vitro. Nup358 appears to be the major component of the cytoplasmic filaments (51). Nup214 also localizes to the cytoplasmic side of the NPC (29), where it forms a subcomplex with the nucleoporin Nup88 (3, 18). Nup214 interacts with CRM1 (18), a member of the importin β superfamily that binds to cargo molecules via so-called leucine-rich NESs and serves as an exportin for a large variety of nucleocytoplasmic shuttling proteins (17, 20, 36, 47). Our biochemical evidence suggested that Nup214 is the terminal binding site for CRM1-containing export complexes (25, 27). Others have advocated Nup358 as the major assembly/disassembly platform in CRM1-mediated export (13).
In this study, we investigated the effects of depleting Nup214 or Nup358 by RNA interference (RNAi) on various nucleocytoplasmic transport pathways in vivo. Our results point to a very prominent role of Nup214 in CRM1-mediated nuclear protein export in vivo.
MATERIALS AND METHODS
Plasmids.
The M9 region of human hnRNP A1 (amino acids 203 to 305) was amplified by PCR and inserted into the BglII and EcoRI sites of pEGFP-C1. Oligonucleotides coding for the simian virus 40 NLS (5′-GATCTCATGGGCCCAAAGAAAAAGAGGAAAGTTGGCA and 5′AGCTTGCCAACTTTCCTCTTTTTCTTTGGGCCCATGA) were annealed and inserted into the BglII and HindIII sites of pEGFP-C3. The coding sequence of green fluorescent protein (GFP) was PCR amplified and inserted into the NheI site of each of these constructs, leading to GFP-GFP fusion molecules in the plasmids pdGFP-M9 and pdGFP-NLS.
An expression clone for the C-terminal fragment of human Nup214 (amino acids 1859 to 2090; maltose-binding protein [MBP]-Nup214-C) was constructed by PCR amplification and insertion of the product into the EcoRI and XbaI sites of the vector pMal-C2 (New England Biolabs). The sequences encoding the N terminus (amino acids 1 to 507) and C terminus (amino acids 500 to 741) of human Nup88 were PCR amplified from cDNA generated from total HeLa RNA and inserted into the EcoRI and XbaI sites of pMal-C2.
Protein expression and nucleotide loading.
MBP-Nup214-C was expressed in Escherichia coli BL21(DE3) cells upon induction with 800 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. The protein was purified by affinity chromatography using amylose beads (New England Biolabs), followed by anion exchange chromatography. MBP-Nup88-N and MBP-Nup88-C were expressed in BL21(DE3) for 4 h at 25°C upon induction with 500 μM or 100 μM IPTG, respectively. Both proteins were purified by affinity chromatography using amylose beads in buffer A (50 mM Tris, pH 7.4, 300 mM NaCl, 1 mM MgCl2, 5% glycerol, 2 mM dithiothreitol [DTT], 1 μg/ml each of aprotinin, leupeptin, and pepstatin). For MBP-Nup88-C, buffer A contained 0.4% Triton X-100 for solubilization and 0.2% Triton X-100 during the binding and the washing steps. His-CRM1 (21), RanGAP (32), RanBP1 (25), and Ran (34) were expressed as described previously. MBP-Nup214-C, His-CRM1, RanGAP, RanBP1, and Ran were dialyzed against transport buffer [20 mM HEPES-KOH, pH 7.3, 110 mM potassium acetate [KOAc], 2 mM Mg(OAc)2, 1 mM EGTA, 2 mM DTT, 1 μg/ml each of aprotinin, leupeptin, and pepstatin]. MBP-Nup88-N and -C were dialyzed against buffer A, frozen in liquid nitrogen, and stored at −80°C. His-CRM1 was labeled with Cy2 (Amersham Pharmacia) according to the instructions of the manufacturer. Ran was loaded with GDP or GTPγS as described previously (27).
RNA interference.
Cells were transfected with small interfering RNA (siRNA) (Ambion) against Nup214 (Nup214-4, GUCACGGAAACAGUGAAAG, corresponding to nucleotides 646 to 664; and Nup214-2, GGTGAGAATCTTTGACTCC, corresponding to nucleotides 183 to 201; accession no. NM_005085), Nup358 (CACAGACAAAGCCGUUGAA, corresponding to nucleotides 351 to 369; accession no. NM_006267), or Nup88 (AUGCUUUGUUGAACACAUC, corresponding to nucleotides 1364 to 1382; accession no. NM_002532) at a final concentration of 100 nM (Nup214-4, Nup358, and Nup88) or 25 nM (Nup214-2) using Oligofectamine (Invitrogen), according to the instructions of the manufacturer, and retransfected after 2 days unless otherwise indicated. Where indicated, siRNA-treated cells were mixed with untransfected cells 1 day before analysis at a ratio of 1:3 to 1:2.
Antibodies.
Antibodies against Nup214 were raised in rabbits by injecting peptides corresponding either to the COOH terminus of Nup214 (CGSNNSSVQGFGGWRS) or to an internal sequence (CASSFGEQKPTGT), coupled to keyhole limpet hemocyanin (Calbiochem). Antibodies were purified using peptides coupled to CNBr-Sepharose. The mouse monoclonal RL1 antibody (46) and the goat anti-Nup358 antibody were provided by Larry Gerace (The Scripps Research Institute) and Frauke Melchior (Institut für Biochemie, Göttingen, Germany), respectively. The rabbit anti-GFP serum was provided by Barbara Müller (Hygiene Institut, Heidelberg, Germany). The mouse monoclonal anti-Nup88 antibody and mAB414 antibody were obtained from BD Biosciences/Pharmingen and Covance/BAbCO (Richmond, CA), respectively.
Cell culture and transfections.
HeLa or HeLa-P4 cells (11) were grown in Dulbecco's modified Eagle medium (DMEM) (GIBCO) containing 4,500 mg/liter glucose, 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Antibiotics were omitted in RNA interference experiments. To generate a stable cell line expressing GFP-NFAT, HeLa cells were transfected with pAd-4 and enriched by fluorescent-activated cell sorting (FACS) as described before (26). For the expression of Rev-GR-GFP, GFP-GFP-NLS, and GFP-GFP-M9, HeLa-P4 cells were transiently transfected with pXRGG (30), pdGFP-NLS, and pdGFP-M9, respectively, using Fugene6 (Roche).
Cell cycle and cell growth analysis.
Mock-treated or nucleoporin-depleted HeLa cells were trypsinized, fixed in cold ethanol, and stained with 50 μg/ml propidium iodide in phosphate-buffered saline (PBS) (Sigma) in the presence of 100 μg/ml RNase A for 45 min at 20°C. The DNA content was analyzed by flow cytometry (FACScalibur; Becton Dickinson) using ModFit LT (Verity Software House). For growth analysis, cells were seeded in triplicates in 24-well plates and transfected with siRNAs as described above. One to 4 days after transfection, cells were trypsinized and counted.
Immunofluorescence.
For immunofluorescence staining, cells were grown on poly-l-lysine-coated coverslips, fixed in 3.7% formaldehyde in PBS, and permeabilized with 0.5% Triton X-100 in PBS. After washing, cells were blocked for 10 min in blocking buffer (0.2% fish gelatin [Sigma] in PBS) and treated with primary antibodies in blocking buffer for 1 to 2 h. Anti-goat Alexa 568, anti-goat Alexa 594, anti-rabbit Alexa 488, anti-rabbit Alexa 568 (1:2,000; Molecular Probes), anti-rabbit Cy3, anti-mouse Cy3 (1:2,000; Dianova), and anti-mouse Alexa 647 (1:1,000; Molecular Probes) were used as secondary antibodies. After washing, cells were stained with 10 μg/ml Hoechst 33258 (Sigma) and mounted in Histogel (Linaris Histogel). Cells were analyzed by fluorescence microscopy using an Olympus IX70 or a Zeiss Axioskop2 microscope. Pictures were processed using Adobe Photoshop.
In situ hybridization.
For in situ hybridization, cells were grown on poly-l-lysine-coated coverslips, fixed for 15 min in 3.7% formaldehyde in PBS at 20°C, and permeabilized in 0.5% Triton X-100 in PBS at 4°C. After equilibration in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 25% formamide for 5 min on ice, cells were hybridized for 2 h at 37°C with 1 ng/ml digoxigenin-labeled oligo(dT) or, as a control, oligo(dA) (40-mers) in 2× SSC, 1 mg/ml tRNA, 0.02% bovine serum albumin (BSA), 2 mM ribonucleoside vanadyl complex (New England Biolabs), 25% formamide, 5% dextran sulfate, and 20 μg/ml of a random oligonucleotide (50-mer). Cells were washed twice with 2× SSC, 25% formamide at 37°C, once with 0.5× SSC, and once with 0.5% Triton X-100 in PBS. Cells were analyzed by indirect immunofluorescence using a fluorescein isothiocyanate-coupled sheep antidigoxigenin Fab fragment (1:300; Roche) for the detection of the labeled oligonucleotide. All buffers were treated with 0.1% diethyl pyrocarbonate (Sigma). For semiquantitative analysis of the subcellular localization of mRNA, 100 to 200 cells were scored into the following categories: N > C (more mRNA in the nucleus), N < C (more mRNA in the cytoplasm), and N = C (equal distribution of mRNA between nucleus and cytoplasm).
SDS-PAGE and immunoblotting.
To analyze RNAi efficiency, equal total protein amounts of cells were analyzed at day 4 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie staining or immunoblotting. Blots were blocked with 1% milk powder in TBST (50 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween). Horseradish peroxidase-coupled donkey anti-goat IgG, goat anti-mouse immunoglobulin G (IgG), goat anti-rabbit IgG, or donkey anti-rabbit IgG (1:10,000 in TBST containing 1% milk powder; Dianova) was used as secondary antibody. The ECL system (Pierce) was used for visualization of proteins.
Nuclear transport assays.
To induce the expression of GFP-NFAT, HeLa-NFAT cells were treated with 1 μM trichostatin A (Sigma) overnight. Import of GFP-NFAT was induced by the addition of 1 μM ionomycin (Sigma) for 20 min at 37°C. For export, cells were washed twice in DMEM and incubated in DMEM at 37°C for various periods of time, as indicated.
To induce the import of Rev-GR-GFP, cells were treated with 5 μM dexamethasone (Sigma) for 30 min at 37°C. For export, cells were washed three times with PBS and incubated in DMEM at 37°C for 1 h. After transport reactions, cells were subjected to indirect immunofluorescence staining for nucleoporins or directly lysed in SDS sample buffer for the analysis of the phosphorylation state of GFP-NFAT.
For the analysis of export efficiency, cells were scored into the following categories: N > C (more reporter protein in the nucleus), N < C (more reporter protein in the cytoplasm), and N = C (equal distribution of the reporter protein between nucleus and cytoplasm).
For import of recombinant CRM1, cells were grown on poly-l-lysine-coated coverslips, permeabilized with 0.015% digitonin in transport buffer, washed, and incubated in transport buffer for 30 min on ice or at 30°C with 12.5 μg/ml His-CRM1-Cy2 in the presence of an energy-regenerating system (1 mM ATP, 2.8 mM creatine phosphate, 0.4 U creatine phosphokinase; Sigma). In some reactions, cells were incubated with 320 μg/ml wheat germ agglutinin (WGA; Sigma) in transport buffer for 15 min on ice before adding the remaining reaction components, resulting in a final concentration of WGA of 200 μg/ml. After import reactions, cells were washed, subjected to immunofluorescence staining, and analyzed by fluorescence microscopy.
RanGAP assays.
RanGAP assays were performed as described previously (1, 25), using 100 nM RanGAP. For leptomycin B (LMB) modification, 1 μM CRM1 was preincubated for 2.5 h at 20°C in transport buffer with 10 μM LMB, added from a 1 mM stock solution in ethanol, or with ethanol alone.
Solid-phase binding assay.
Microtiter plate wells were coated with 80 ng MBP-Nup214 in 100 μl PBS and blocked in PBS-T (PBS with 0.1% Tween) containing 3% BSA. The wells were incubated with increasing concentrations of His-CRM1 in the absence or presence of 40 μM NES peptide (NS2 protein of minute virus of mice, CVDEMTKKFGTLTIHDTEK [1]) and 200 nM of Ran, loaded with either GDP or GTPγS. After 70 min at 4°C, the wells were washed four times with PBS-T. Bound CRM1 was detected using affinity-purified anti-CRM1 antibody (26) and horseradish peroxidase-coupled secondary antibody, both in PBS-T. Tetramethylbenzidine (100 μg/ml in 0.1 M NaOAc containing 0.02% H2O2) was used for the colorimetric reaction, which was stopped by the addition of 0.5 M H2SO4. The absorbance was measured at 450 nm, and the binding curve was fitted to the data using nonlinear regression (Prism 4; GraphPad Software).
RESULTS
We and others have previously identified Nup214 as a strong binding partner for CRM1 in vitro, implicating this cytoplasmic nucleoporin as a terminal binding site in nuclear protein export (18, 25, 27). Nup358, on the other hand, has been implicated as a release factor (27), promoting the dissociation of the export complex from Nup214. The functions of Nup214 and Nup358 in nuclear export in vivo, however, remain unclear. A depletion of Nup214 or Nup88 by RNA interference has recently been described to result in a concomitant reduction of Nup358 (7), precluding such cells for the analysis of specific Nup214/Nup88 functions. We did not observe such a codepletion (Fig. 1) and could therefore investigate individual functions of Nup214 and Nup358 in various transport pathways in vivo, using inducible nuclear import and export systems.
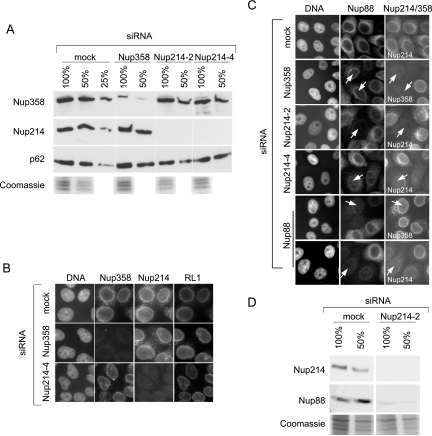
FIG. 1.
Efficient and specific depletion of Nup214 and Nup358 by RNA interference. HeLa cells were mock treated or transfected with specific siRNAs against Nup214 or Nup358. (A) Different amounts (100%, 50%, 25%) of cell lysates from mock-treated cells were compared to lysates from nucleoporin-depleted cells by immunoblotting, detecting Nup358, Nup214, and p62. A parallel Coomassie stain served as a loading control. Note the slightly lower protein concentration in the lysate obtained from Nup214-2-transfected cells. (B) Mock- or siRNA-treated cells were analyzed by triple immunofluorescence, using anti-Nup358, anti-Nup214, and the antinucleoporin antibody RL1, as indicated. (C) Mock- or siRNA-treated cells were analyzed by double immunofluorescence for Nup88 and Nup214 or Nup358. Nucleoporin-depleted cells are indicated by arrows. For a better comparison of protein levels, nucleoporin-depleted cells were mixed with untransfected cells. Antibodies used for detection of Nup214 or Nup358 are indicated in the individual pictures (right panel). (D) Different amounts (100%, 50%) of cell lysates from mock-treated cells and Nup214-depleted cells were analyzed by immunoblotting, detecting Nup214 and Nup88. A parallel Coomassie stain served as a loading control. Note that our commercial anti-Nup88 antibody did not allow a reliable quantitative analysis of the codepletion.
Efficient and specific depletion of Nup214 and Nup358 by RNA interference.
HeLa cells were either mock treated or transfected with siRNAs against Nup214 or Nup358. To determine the total or the NPC-associated levels of various nucleoporins, cells were analyzed by immunoblotting or by indirect immunofluorescence. As shown in Fig. 1A, Nup214 was hardly detectable after transfection with two different siRNAs (Nup214-2 and Nup214-4). The expression of Nup358 in the total population was reduced to an estimated level of 10% compared to mock-transfected cells. Importantly, the reduction of one of the two nucleoporins did not lead to a concomitant reduction of the other. The nucleoporins p62 (Fig. 1A) and Nup153 (data not shown) were not affected by the depletion of Nup358 or Nup214, in agreement with published results (7). When analyzed by indirect immunofluorescence, nucleoporins typically appear as a rim-like structure around the nucleus. In comparison to mock-transfected cells, about 95% of cells transfected with siRNAs against Nup358 or Nup214 showed a clear reduction in the nuclear rim after staining with specific antibodies (Fig. 1B). As predicted by the immunoblots, the depletion of Nup214 did not lead to a reduction of Nup358 at the NPC and vice versa. Moreover, cells depleted of Nup358 or Nup214 showed no reduced labeling after staining with the RL1 antibody, which recognizes a group of O-glycosylated nucleoporins (46), or with a specific antibody against p62 (data not shown). The only nucleoporin that we found to be affected by the depletion of Nup214 was its binding partner Nup88, as described previously (7). Cells with strongly reduced levels of Nup214 also exhibited a reduced staining for Nup88 at the NPC (Fig. 1C; in these experiments, we mixed nucleoporin-depleted cells with untransfected cells for a better comparison of protein levels). Likewise, depletion of Nup88 using a specific siRNA led to a reduction of Nup214 but, strikingly, not of Nup358 (Fig. 1C; compare to results in reference 7). These results could be confirmed for total protein levels by Western blotting (data not shown). In addition, cells depleted of Nup358 had normal levels of Nup88 at the NPC (Fig. 1C). We also analyzed total levels of Nup88 after depletion of Nup214 or Nup358 by Western blotting. As expected from the results of the immunofluorescence, depletion of Nup214 led to a concomitant reduction of Nup88 (Fig. 1D). Depletion of Nup358, in contrast, did not affect the total level of Nup88 (data not shown).
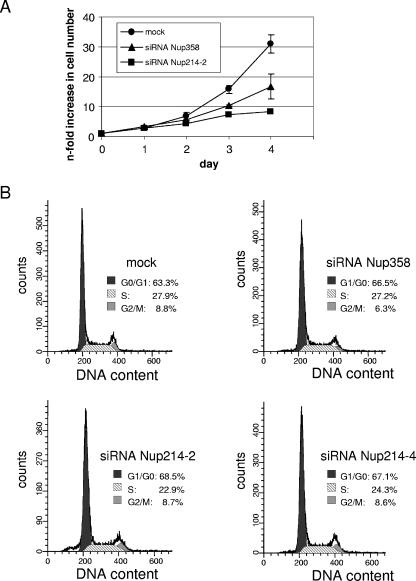
We next analyzed cell growth of mock- and siRNA-treated cells. Nup214/Nup88-depleted cells showed a strongly decreased proliferation rate compared to mock-treated cells. Nup358-depleted cells exhibited an intermediate phenotype (Fig. 2A). We therefore performed cell cycle analyses using propidium iodide staining followed by flow cytometry. No significant changes in the distribution of cells in different stages of the cell cycle could be observed in Nup214/Nup88- or Nup358-depleted cells compared to mock-transfected cells (Fig. 2B). Cells that were depleted of both nucleoporins grew very poorly and were not further analyzed. Taken together, we succeeded in specific depletion of Nup358 and the Nup214/Nup88 complex. We therefore set out to analyze different nucleocytoplasmic transport pathways in nucleoporin-depleted cells.
FIG. 2.
Reduced cell growth in Nup358- and Nup214-depleted cells. (A) Triplicates of mock-treated or nucleoporin-depleted cells were trypsinized and counted at various time points (days after transfection) during the siRNA treatment. Error bars indicate the standard deviation from the mean. (B) Mock- or siRNA-treated cells were analyzed by propidium iodide stain for cell cycle distribution and analyzed by flow cytometry. Percentages of cells in individual stages of the cell cycle are indicated.
Impaired nuclear mRNA export in cells depleted of Nup214/Nup88 or Nup358.
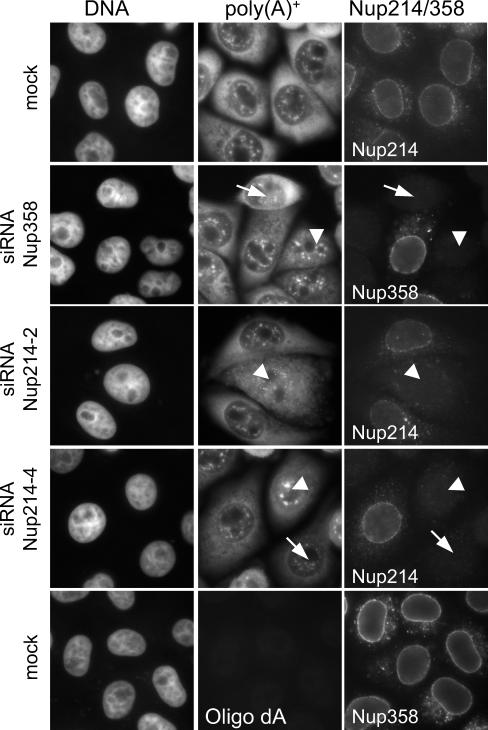
Recently, Forler et al. (16) reported a strong nuclear accumulation of poly(A)+ RNA upon partial depletion of either Nup358 or Nup214 in Drosophila melanogaster cells. We therefore analyzed the mRNA distribution in HeLa cells upon transfection of siRNAs against Nup214 or Nup358. Approximately 98% of mock-treated or untransfected cells showed a stronger fluorescent mRNA signal in the cytoplasm than the nucleus. In Nup358- and Nup214-depleted cells, this number decreased to ∼70% and ∼60%, respectively. Concomitantly, the number of cells with an equal distribution of mRNA between the nucleus and the cytoplasm increased from ∼1% in control cells to ∼25% or ∼37% in Nup358- or Nup214-depleted cells, respectively (Fig. 3). Very few nucleoporin-depleted cells showed a nuclear accumulation of mRNA. Taken together, the efficient depletion of Nup214 or Nup358 in human cells results in a mild phenotype with respect to mRNA export relative to that of insect cells, where a partial depletion of Nup214 or Nup358 resulted in strong nuclear accumulation of mRNA (16).
FIG. 3.
Poly(A)+ RNA export is slightly impaired in Nup358- or Nup214-depleted cells. Mock-treated or Nup214- or Nup358-depleted cells were analyzed by in situ hybridization for poly(A)+ RNA and in parallel by indirect immunofluorescence for the nucleoporins, as indicated. For a better comparison of nucleoporin levels, depleted cells were mixed with untransfected cells. Nucleoporin-depleted cells showed either the same pattern as mock-treated cells (arrows) or an equal distribution of poly(A)+ RNA between the nucleus and the cytoplasm (arrowheads). No signal was obtained with the control oligonucleotide oligo(dA) (lower panel).
Nuclear protein import is not affected in Nup214/Nup88- or Nup358-depleted cells.
We next analyzed the effects of Nup214 or Nup358 depletion on nuclear protein transport in transient transfection experiments. As examples for the “classic” import pathways, we used the simian virus 40 NLS, a substrate for the importin α/β receptor, and the M9 sequence of hnRNP A1, a substrate for the importin β-like receptor transportin (37), linked to a GFP-GFP fusion molecule. These proteins were imported into the nucleus, irrespective of the levels of Nup214 or Nup358 at the NPC (Fig. 4A and B). Likewise, no changes in the steady-state distribution of endogenous hnRNP A1 were observed in Nup214/Nup88- or Nup358-depleted cells compared to that of wild-type cells (data not shown). We also analyzed the subcellular localization of importin β by indirect immunofluorescence (data not shown). No changes of the steady-state distribution of this import factor could be observed in Nup214- or Nup358-depleted cells, suggesting that its nucleocytoplasmic shuttling is not affected by the depletion.
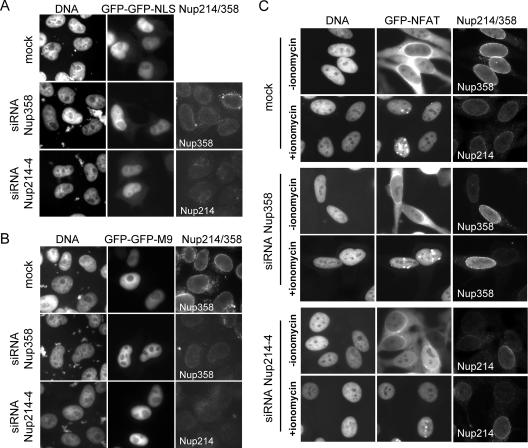
FIG. 4.
Nup214 and Nup358 are not required for nuclear protein import. Nup214- or Nup358-depleted HeLa cells were transiently transfected with constructs coding for GFP-GFP-NLS (A) or GFP-GFP-M9 (B), stained for Nup214 and Nup358, and analyzed by fluorescence microscopy. (C) Mock-treated or Nup214- or Nup358-depleted HeLa-GFP-NFAT cells were fixed before (−ionomycin) or after (+ionomycin) treatment with ionomycin, stained for residual nucleoporins at the NPC, and analyzed by fluorescence microscopy. For a better comparison of RNAi and transport efficiencies, nucleoporin-depleted HeLa-GFP-NFAT cells were mixed with untransfected cells. (A to C) Antibodies used for detection of Nup214 or Nup358 are indicated in the individual pictures (right panels).
In transfection experiments, even drastic changes in import rates may not become apparent, as long as the import substrate eventually accumulates in the nucleus. We therefore took advantage of an inducible nuclear transport system, using the nuclear factor of activated T cells (NFAT) (35) as a reporter protein. NFAT is a transcription factor that shuttles between the nucleus and the cytoplasm in a phosphorylation-dependent manner. It contains two “classic” NLS (4), mediating import via importin α/β, as well as a leucine-rich NES (28), mediating export via CRM1. Rapid nuclear import of NFAT can be induced by adding the calcium ionophore ionomycin to the medium, which leads to dephosphorylation of NFAT and unmasking of its NLSs (4). After washing the cells, NFAT gets rephosphorylated in the nucleus, promoting its export back into the cytoplasm (5, 44). Thus, the GFP-NFAT system is ideal for the analysis of rapid nuclear transport shortly after induction of either import or export. We have previously used GFP-NFAT-expressing HeLa cells to show that nuclear export in vitro requires the exportin CRM1 (26).
For the analysis of nuclear transport in vivo, HeLa-GFP-NFAT cells were first treated with siRNAs against Nup214 or Nup358. Depleted cells and mock-treated cells were then subjected to transport reactions in vivo and subsequently analyzed for the distribution of the reporter protein as well as for residual levels of nucleoporins at the NPC. In mock-treated as well as in Nup358- or Nup214-depleted cells, GFP-NFAT initially localized to the cytoplasm (Fig. 4C, −ionomycin). Upon addition of ionomycin, GFP-NFAT was efficiently imported into the nucleus within 20 min in control cells as well as in nucleoporin-depleted cells (Fig. 4C, +ionomycin). Very similar import efficiencies were also observed after shorter incubation times (e.g., 5 or 10 min at suboptimal temperature; data not shown).
We conclude from these experiments that Nup214/Nup88 and Nup358 are not required for efficient import of various nuclear proteins in HeLa cells, confirming previous results in Xenopus nuclei (51) and human cells (43).
Nup214/Nup88 is required for efficient CRM1-dependent nuclear protein export.
After nuclear accumulation of GFP-NFAT, its export can be induced by washing the cells to remove the ionomycin (26). As shown in Fig. 5A and B, GFP-NFAT was efficiently exported into the cytoplasm in untransfected cells (which were again mixed with nucleoporin-depleted cells for a better comparison of protein levels), in mock-treated cells, and in Nup358-depleted cells. Eighty-three percent of control cells exhibited a pronounced cytoplasmic GFP-NFAT signal after the export reaction (N < C), compared to 71% of Nup358-depleted cells. The level of cells with an equal distribution of the reporter protein between the nucleus and the cytoplasm (N = C) did not change significantly, whereas some cells with a reduced concentration of Nup358 at the NPC showed an accumulation of GFP-NFAT in the nucleus (N > C). This rather modest effect of the depletion of Nup358 on nuclear export is comparable to the effect described by Bernad et al. (7), who used a Rev-GFP construct as a reporter protein for the analysis of actinomycin D-induced nuclear export.
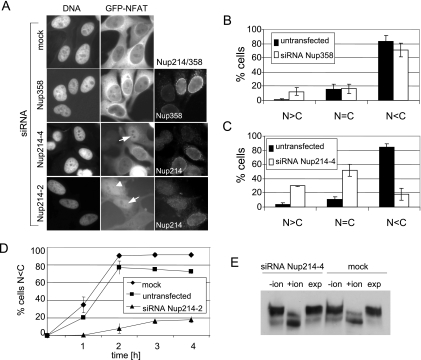
FIG. 5.
Depletion of Nup214 inhibits nuclear export of GFP-NFAT. HeLa-GFP-NFAT cells were either mock treated or transfected with siRNAs against Nup214 or Nup358 and subjected to one import/export cycle. Cells were stained for Nup358 or Nup214 and analyzed by fluorescence microscopy for GFP-NFAT distribution. For a better comparison of RNAi and transport efficiencies, nucleoporin-depleted cells were mixed with untransfected cells. (A) Nup214-depleted cells showed either a nuclear accumulation (arrows) or an equal distribution of GFP-NFAT between nucleus and cytoplasm (arrowhead). Export reactions were stopped after 90 min (mock; siRNA Nup358 and siRNA Nup214-4) or 120 min (siRNA Nup214-2). The mean distribution of cells in the three categories N > C, N = C, and N < C for GFP-NFAT fluorescence is shown for Nup358-depleted cells (B) and for Nup214-depleted cells (C). Bars indicate the standard deviation from the mean of three independent experiments. (D) Time course of cytoplasmic accumulation (N < C) of GFP-NFAT in mock-treated, untransfected, and Nup214-depleted cells (siRNA Nup214-2). Bars indicate the variation of the individual values from the mean of two independent experiments. (B to D) Only efficiently depleted cells (≥100 per experiment) were included in the analyses. Cells transfected with siRNA Nup214-2 (A and D) were analyzed at day 2 without retransfection. (E) Detection of GFP-NFAT in total cell lysates of mock-treated or Nup214-depleted cells before import (−ion), after import (+ion), and after export (exp) reactions by immunoblotting, using an anti-GFP antibody.
In contrast to Nup358, the depletion of Nup214/Nup88 from the NPC resulted in a dramatic reduction of nuclear export efficiencies (Fig. 5A, C, and D). The percentage of cells with a clear cytoplasmic signal for GFP-NFAT after the export reaction was reduced from 83% in the control cells to 18% in Nup214-depleted cells (Fig. 5C). The percentage of cells with an equal distribution or with a nuclear accumulation of GFP-NFAT increased accordingly (from 11% to 52% or from 4% to 30%, respectively). These results were confirmed using a second siRNA against Nup214 (siRNA Nup214-2; Fig. 5A). A quantitative analysis led to results very similar to those presented in Fig. 5C (data not shown). We next used this siRNA (Nup214-2) in a time course experiment of nuclear export of GFP-NFAT over a period of 4 h. In most of the control cells (mock treated or untransfected), the reporter protein accumulated in the cytoplasm after 2 h of incubation under export conditions (i.e., N < C; Fig. 5D). Nup358-depleted cells did not differ in export kinetics from control cells or mock-treated cells (data not shown). Only a few of the Nup214-depleted cells (18% compared to 92% in mock-treated cells), however, showed an efficient export of GFP-NFAT, even after 4 h of incubation. This result suggests that an (almost) complete depletion of Nup214 not only delays nuclear protein export but also leads to a strong transport block.
The shuttling of NFAT is controlled by its nuclear phosphorylation and cytoplasmic dephosphorylation. To exclude the possibility that the export defect of GFP-NFAT in Nup214/Nup88-depleted cells is a consequence of perturbed nuclear phosphorylation, we performed Western blot analysis of mock-treated and Nup214-depleted cells following transport reactions. After addition of ionomycin and subsequent nuclear import of GFP-NFAT, the protein migrated with a higher mobility in SDS-PAGE due to its dephosphorylation (Fig. 5E) (26). After removal of the ionomycin, GFP-NFAT in mock-treated and Nup214-depleted cells returned to the slower migrating form, indicating efficient rephosphorylation in the nucleus. This result shows that nuclear phosphorylation is not affected in Nup214-depleted cells, suggesting that the observed reduction in nuclear export of GFP-NFAT does not result from substrate-specific effects but rather from a general defect of the transport machinery.
To further confirm the export defect upon depletion of Nup214, we used a second reporter protein consisting of full-length Rev protein fused to the hormone-responsive element of the glucocorticoid receptor and GFP (30). Upon transient transfection in mock-treated or Nup214-depleted cells, Rev-GR-GFP localized exclusively in the cytoplasm (Fig. 6A). Following addition of the steroid dexamethasone, the majority of the protein was imported into the nucleus in both mock-treated and Nup214-depleted cells, showing a predominant nucleolar localization (Fig. 6B). Again, this result demonstrates that efficient nuclear import does not require Nup214/Nup88. The export of Rev-GR-GFP takes place in a CRM1-dependent manner and can be inhibited by LMB (30 and data not shown). Upon removal of the steroid, the reporter protein moved back into the cytoplasm in mock-treated cells, with the amount of residual protein in a nucleolar region depending on the expression level (Fig. 6C). A significant number of Nup214-depleted cells, however, showed a clear nuclear accumulation of the reporter protein after the export reaction (18% compared to 1% in mock-treated cells; mean of three independent experiments). Note that Rev-GR-GFP in Nup214-depleted cells does not concentrate in nucleoli anymore after the export reaction, suggesting that the release step from nucleoli is not inhibited in these cells. Taken together, our results show that Nup214/Nup88 is required for efficient export of proteins out of the nucleus. In contrast, an almost complete removal of the second cytoplasmic nucleoporin, Nup358, had only a very modest effect on nuclear protein export.
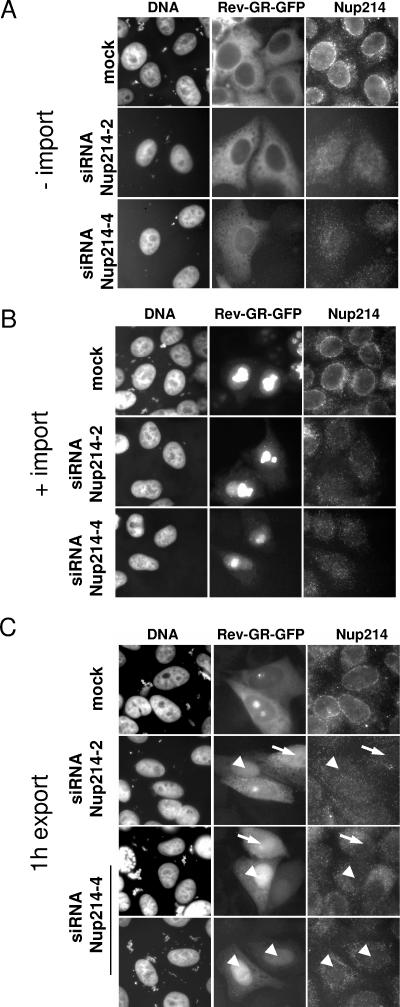
FIG. 6.
Depletion of Nup214 inhibits nuclear export of Rev-GR-GFP. Mock-treated or Nup214-depleted HeLa cells were transfected with a vector coding for Rev-GR-GFP and subjected to transport reactions. Cells were fixed before (A, −import), after the addition of dexamethasone (B, +import), or after the export reaction (C, 1 h export) and analyzed by fluorescence microscopy. Nup214-depleted cells showed either an equal distribution of Rev-GR-GFP between nucleus and cytoplasm (arrow) or a nuclear accumulation of the reporter (arrowhead).
Nup358-dependent binding of CRM1 to the NPC.
Nuclear import of CRM1 occurs in a Ran-, ATP-, and temperature-independent manner (57). Under certain conditions, binding of CRM1 to the NPC appears to be mediated by Nup358 (7, 13). We therefore compared nuclear import of CRM1 in control cells and in Nup214- or Nup358-depleted cells to rule out the possibility that the observed export defect simply results from perturbed import of CRM1 into the nucleus. Cells were treated with siRNAs against Nup214 or Nup358, permeabilized with digitonin, and subjected to nuclear import reactions using fluorescently labeled CRM1 as a substrate. As shown in Fig. 7A, CRM1 was efficiently imported into the nucleus at 30°C, irrespective of the presence of the two nucleoporins. When cells were incubated at 4°C, import still occurred, albeit to a lesser extent. Again, no difference was observed in mock-treated cells compared to nucleoporin-depleted cells (data not shown). However, we did observe a nuclear rim for CRM1 in mock-treated or Nup214-depleted cells which was strongly reduced or even absent in Nup358-depleted cells at either 30°C (Fig. 7A) or 4°C (data not shown). Although the excess of recombinant CRM1 makes it likely that the NPC staining reflects an initial binding site for CRM1 on its way into the nucleus, it could also represent reexported CRM1. To distinguish between these two possibilities, we preincubated cells with wheat germ agglutinin (WGA), a lectin that blocks nuclear import of proteins by binding to O-glycosylated nucleoporins (22, 55). Cells were then further incubated at 4°C in the presence of recombinant CRM1. This treatment resulted in a strong inhibition of CRM1 nuclear import in mock-treated and nucleoporin-depleted cells (Fig. 7B). Again, a prominent nuclear rim for CRM1 became apparent in mock-treated or Nup214-depleted cells but not in Nup358-depleted cells. We conclude that Nup358 represents an initial binding site for CRM1 on its way into the nucleus. This interaction, however, does not appear to be a rate-limiting step in nuclear import of CRM1 or in CRM1-dependent nuclear export.
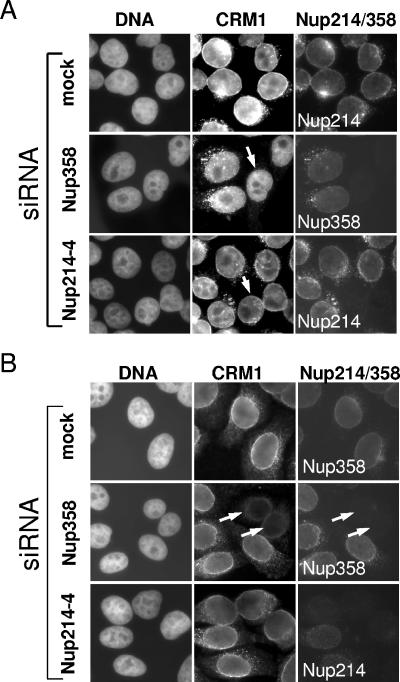
FIG. 7.
Cytoplasmic nucleoporins are not required for efficient CRM1 import. Mock- or siRNA-treated cells were permeabilized and incubated with recombinant His-CRM1-Cy2 for (A) 30 min at 30°C or (B) in the presence of WGA for 30 min at 4°C. Cells were stained for Nup358 or Nup214 and analyzed by fluorescence microscopy. (A) Arrows point to nucleoporin-depleted cells, showing CRM1 import similar to that of control cells. (B) Arrows point to Nup358-depleted cells with reduced nuclear rim for CRM1. For a better comparison of protein levels, nucleoporin-depleted cells were mixed with untransfected cells.
Characterization of Nup214-CRM1 complexes.
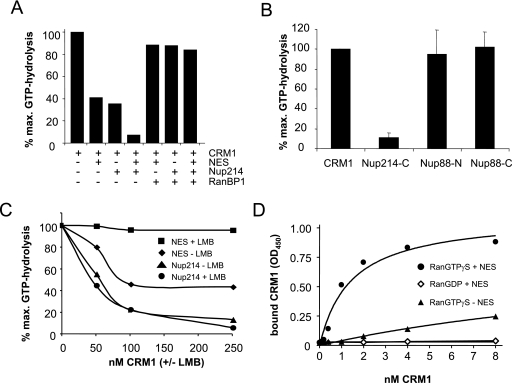
In light of the predominant effect of the depletion of Nup214 on the CRM1-mediated nuclear export pathway, we decided to analyze the interaction of the two proteins in detail. Upon binding to transport receptors, RanGTP becomes insensitive to the GTPase-activating protein RanGAP. RanGAP assays can therefore be used for the analysis of complexes involving transport receptors and RanGTP (8). In the case of CRM1, an NES-containing protein promotes the formation of a RanGAP-resistant complex (1). This is confirmed in Fig. 8A. An NES peptide, together with CRM1 (but not CRM1 alone), inhibited RanGAP-stimulated GTP hydrolysis on Ran. Surprisingly, a C-terminal fragment of Nup214, which is known to interact with CRM1, also reduced the GTP hydrolysis on Ran in the presence of CRM1, suggesting the formation of a GTPase-resistant Nup214-CRM1-RanGTP complex. A similar complex has previously been described for the nucleoporin Nup159, the yeast homologue of Nup214 (15). When added together to the reaction, the NES peptide and the Nup214 fragment further inhibited the GTPase reaction. This is likely to result from the formation of a tetrameric Nup214-CRM1-NES-RanGTP complex (see below). In the absence of CRM1, neither the NES peptide nor the Nup214 fragment inhibited GTP hydrolysis on Ran (data not shown). An NES-CRM1-RanGTP complex can be dissociated by the RanGTP-binding protein RanBP1 (27). We therefore tested if this is also the case for the Nup214-CRM1-RanGTP complex. As shown in Fig. 8A, RanBP1 abolished the inhibition of GTP hydrolysis by the NES peptide, by the Nup214 fragment, and by the two components when added together to the reaction. Hence, a complex containing CRM1, RanGTP, and Nup214 is likely to be dissociated by either soluble RanBP1 or the Ran-binding domains of NPC-associated Nup358.
FIG. 8.
CRM1 forms a high-affinity, RanGAP-resistant complex with RanGTP, Nup214, and export cargo. (A) RanGAP assays were performed with 220 nM CRM1 in the absence or presence of 40 μM NES peptide, 800 nM Nup214 fragment, and 1.1 μM RanBP1, as indicated. (B) RanGAP assays were performed with 240 nM CRM1 in the absence or presence of 450 nM Nup214-C, 2 μM Nup88-N, or 2.2 μM Nup88-C, as indicated. Error bars correspond to the standard deviations from the means of three (Nup214) or five (Nup88) independent experiments. (C) RanGAP assays were performed with NES peptide or Nup214 fragment as described for panel A and increasing concentrations of CRM1 that had been preincubated with (+LMB) or without (−LMB) leptomycin B, as indicated. (D) Solid-phase binding of CRM1 to Nup214 was analyzed in the absence or presence of RanGTPγS, RanGDP, or NES peptide, as indicated. OD450, optical density at 450 nm; max., maximum.
Since depletion of Nup214 led to a codepletion of Nup88, we also tested whether Nup88 is able to form a RanGAP-resistant complex with CRM1 and RanGTP. In contrast to the C-terminal fragment of Nup214, neither an N-terminal (amino acids 1 to 507) nor a C-terminal (amino acids 500 to 741) fragment of Nup88 inhibited the GTP hydrolysis by RanGAP in the presence of CRM1 (Fig. 8B).
What is the nature of the Nup214-CRM1-RanGTP complex? If a tetrameric complex containing CRM1, RanGTP, NES substrate, and Nup214 can be formed, one would predict distinct binding mechanisms of the latter two to CRM1. Interaction of CRM1 with an export substrate can be inhibited by leptomycin B (LMB), a fungal metabolite that efficiently inhibits CRM1-mediated export (17, 52). We therefore performed RanGAP assays with increasing concentrations of CRM1 that had been preincubated with or without LMB. LMB modification of CRM1 prevented the formation of a RanGAP-resistant NES-CRM1-RanGTP complex, leading to almost maximal GTP hydrolysis at all CRM1 concentrations (Fig. 8C, compare NES with and without LMB). In contrast, LMB-preincubated CRM1 was as efficient in the formation of RanGAP-resistant Nup214-CRM1-RanGTP complexes as unmodified CRM1 (Fig. 8C, compare Nup214 with and without LMB). This result shows that the interaction of CRM1 with the nucleoporin is fundamentally different from its binding to an export substrate. The region of CRM1 that is modified by LMB appears to interact with the NES but may not directly be involved in the binding of CRM1 to Nup214. Our result is consistent with the observation that LMB does not prevent the release of CRM1 from nuclei of digitonin-permeabilized cells (data not shown), as both modified and unmodified forms of the receptor protein are able to interact with Nup214.
What is the affinity of Nup214 for CRM1? Using RanGAP assays, we measured an apparent affinity of the Nup214 fragment for CRM1 of ∼100 to 200 nM (data not shown). This value may be significantly lower in the presence of an export substrate. RanGAP assays, however, are not suitable for such an analysis, as Nup214 and NES peptide independently lead to the formation of a RanGAP-resistant complex. We therefore performed solid-phase binding assays to determine the apparent affinity of the Nup214 fragment for CRM1 in the absence or presence of Ran that had been loaded with the nonhydrolyzable GTP analog GTPγS and/or an NES peptide. In the absence of the NES peptide and the presence of RanGTPγS, CRM1 binding above background to immobilized Nup214 could be detected, confirming the results from our RanGAP assays (Fig. 8D). Binding, however, was too low to determine an apparent Kd. In contrast, the addition of the NES peptide to the reaction led to a dramatic increase in apparent affinities of the interaction partners, with half-maximal binding between 1 and 2 nM of CRM1. No binding was detected when RanGDP instead of RanGTPγS was included in the reaction. These results suggest that the formation of a very high-affinity tetrameric complex containing CRM1, Nup214, RanGTP, and an export substrate is central to CRM1-mediated nuclear protein export.
DISCUSSION
In this study, we used in vivo and in vitro approaches to analyze the role of two cytoplasmic components of the NPC, Nup358 and the Nup214/Nup88 complex, on various nuclear transport pathways. The depletion of Nup214/Nup88 resulted in strongly reduced nuclear protein export efficiencies, whereas the depletion of Nup358 had only a modest effect on this transport pathway.
Depletion of Nup214 and Nup358 from the NPC.
Nup214 and Nup88 are known to form a subcomplex within the NPC (3, 18). Hence, it is not surprising that depletion of one of these nucleoporins led to a codepletion of the other, in agreement with earlier results (7). In contrast, Nup358 was not affected by the depletion of either Nup214 or Nup88, and the gross composition of the NPC was not compromised. Likewise, the depletion of Nup358 affected neither the Nup214/Nup88 complex nor other nucleoporins tested. These results are at variance with a recent study, where a depletion of Nup214 or Nup88 to ∼30% or ∼15%, respectively, of the control levels resulted in a concomitant reduction of Nup358 at the nuclear envelope (7). These discrepancies may result from the different methods that were used for the depletion of the nucleoporins (siRNAs versus a vector-based system) and/or the specific target sequences. Indeed, in a later study using a different target sequence, a codepletion of Nup358, was not apparent in Nup214 knockdown cells (13). We conclude from these results that the Nup214/Nup88 complex is not required to attach Nup358 to the NPC. Our findings are in agreement with results obtained in a study using Xenopus egg extracts (51). Here, NPCs assembled in vitro in the absence of Nup214 retained cytoplasmic filaments containing Nup358. Likewise, NPCs that lacked Nup358 were devoid of cytoplasmic filaments but still contained Nup214. Given the specificity of RNA interference, our nucleoporin-depleted cells are well suited for the analysis of the roles of Nup358 and Nup214/Nup88 in various nuclear transport pathways.
Impaired nuclear mRNA export upon depletion of Nup214/Nup88 or Nup358.
In Drosophila cells, a reduction of Nup358 to about 50% of the wild-type level as well as a reduction of Nup214 led to growth arrest and to a strong accumulation of poly(A)+ RNA in the nucleus (16). Our depleted HeLa cells, where Nup358 was almost undetectable, grew somewhat more slowly than control cells, but almost no nuclear mRNA accumulation could be observed. In some cells, however, the depletion of Nup358 led to an equal distribution of mRNA between the nucleus and the cytoplasm. These differences between human and insect cells may be explained by the lack of RanBP1 in Drosophila, which may have functions similar to those of the Ran-binding domains of Nup358. In both cell types, reduced mRNA export may result from effects on the RNA export factor NXF1, which has been shown to interact with Nup358 (16).
Nup214-depleted cells grew significantly more slowly than control cells. They also showed a modest mRNA export defect comparable to that observed in Nup358-depleted cells. This may be explained by disturbed binding of NXF1 to Nup214 (2, 24). Alternatively, the export defect as well as the growth defect may result from indirect effects after inhibition of nuclear protein export. Slowly growing nucleoporin-depleted cells did not accumulate at a specific stage of the cell cycle, suggesting an overall delay of cell growth. A detailed analysis of cell cycle progression will be required to further analyze this issue. In summary, we observed qualitative similarities between insect cells and human cells after depletion of two cytoplasmic nucleoporins with respect to mRNA transport. However, our results also point to differences between the analyzed species, as the observed mRNA export defect was by far less severe in human cells than in insect cells.
Depletion of Nup214/Nup88 or Nup358 does not affect nuclear protein import.
Two major nuclear transport pathways, importin β- and transportin-mediated import, were not affected in HeLa cells with a strongly reduced concentration of either Nup214 or Nup358, demonstrating that the depleted NPCs retain their principal transport capabilities. These results are in agreement with a number of other recent publications (16, 43). Even nuclei lacking both Nup214 and Nup358 showed only a minor reduction in nuclear protein import (51). We conclude that the cytoplasmic nucleoporins Nup214 and Nup358 are dispensable for efficient nuclear protein import, at least for the substrates and import receptors tested so far.
Depletion of Nup214/Nup88 inhibits CRM1-mediated export.
The most striking phenotype in Nup214/Nup88-depleted cells was observed for CRM1-dependent nuclear protein export. Two reporter proteins, GFP-NFAT and the human immunodeficiency virus-Rev derivative Rev-GR-GFP, were retained to a significant extent in nuclei of Nup214-depleted cells. Our analysis of the kinetics of GFP-NFAT export leads to the conclusion that Nup214 not only facilitates nuclear export (which would result in a delay of transport in its absence) but also is required for transport. Why do a few cells (about 20%; see Fig. 5C and D) with reduced levels of Nup214 at the NPC still export the reporter protein? This may result from a threshold effect with respect to the detection of the nucleoporin. Due to the inherently nonquantitative nature of indirect immunofluorescence, we can hardly distinguish between a 90% or 99% reduction in the absolute level of Nup214. The resulting export capacities of cells with either 10% or 1% of residual Nup214, which would both be scored as Nup214 depleted, may be very different. Also, different export cargoes may be affected by the depletion to different extents.
Depletion of Nup214 leads to a concomitant loss of Nup88 from the NPC, as described previously (7). Functionally, Nup88 was suggested to attenuate CRM1-dependent export, at least in Drosophila cells (41). Furthermore, we described a high-affinity, GTPase-resistant CRM1-Nup214 complex (see below). A GTPase-resistant CRM1-Nup88 complex, in contrast, was not observed. We conclude that Nup214 is the most relevant component for our observed effects upon depletion of the Nup214/Nup88 complex.
Our results support a model in which Nup214 serves as a terminal binding site for nuclear export complexes. Incubation of permeabilized cells with RanQ69L, a Ran mutant that is insensitive to RanGAP and is therefore predominantly in the GTP-bound form, or RanC4A, a mutant with low affinity for RanBP1/Nup358, leads to an efficient coprecipitation of Nup214 with an antibody against CRM1 (25, 27). We now further analyzed the binding characteristics of the export receptor and the nucleoporin using recombinant proteins. Under conditions that mimic those in nuclear export (i.e., in the presence of RanGTP and the NES peptide of the NS2 protein, which strongly binds to CRM1 [1]), Nup214 and CRM1 interact with a very high affinity in the low-nanomolar range, exceeding the highest affinities measured for importin β-nucleoporin interactions (6). In contrast to nuclear import receptors, the interaction of CRM1 with its preferred nucleoporin is strongly promoted by a transport cargo. In a situation where a transport complex within the aqueous channel of the NPC can move in either direction, this should contribute to the directionality of transport and, in combination with efficient release mechanisms promoted by cytoplasmic RanBP1 or the Ran-binding domains of Nup358, to efficient export. Nup214, CRM1, and RanGTP also interact in the absence of export cargo, albeit with much lower affinities. Given the high local concentration of Nup214 at the NPC, this trimeric complex may well be physiologically relevant. It is still resistant to GTPase activation by RanGAP, allowing it to persist until it encounters the releasing activity of soluble RanBP1 or the Ran-binding domains of Nup358. Certain export cargoes (e.g., with lower affinities for CRM1 than the NS2 protein) may dissociate from export complexes, even in the presence of RanQ69L, leaving CRM1 in association with Nup214. This interpretation can explain earlier observations of a low level of nuclear export of GFP-NFAT in the presence of RanQ69L in vitro without an accumulation of the reporter protein at the nuclear envelope (26, 27).
What is the role of Nup358 in nuclear export? The export defect in Nup358-depleted cells was only marginal compared to that observed in Nup214-depleted cells, yet it was comparable to that described before (7). Nup358 could have two functions. First, it may serve as a release factor, promoting the dissociation of RanGTP from export complexes containing RanGTP, CRM1, export cargo, and Nup214, or from “postexport complexes,” where the export cargo has already dissociated. Indeed, incubation of permeabilized cells with RanC4A, a mutant with a reduced affinity for RanBP1 (or for the Ran-binding domains of Nup358), resulted in accumulation of CRM1 at Nup214 (25). The GTPase-activating protein RanGAP is stably associated with Nup358 (32, 33) and may play a role in the final dissociation of the export complex. As Nup358 and RanGAP would act catalytically to release export complexes from Nup214, small amounts of the proteins that are left at the NPC after RNA interference may be sufficient for efficient release. In addition, soluble RanGAP and RanBP1 are expected to have functions in the dissociation of export complexes similar to those of Nup358 and Nup358-associated RanGAP. Second, Nup358 may interact with CRM1 after its release from Nup214, preventing uncontrolled diffusion of the export receptor into the cytoplasm. Nup358 has been shown to contain a domain that interacts with CRM1 in vitro (45), and Nup358-dependent association of CRM1 with the nuclear envelope has been described before (7, 13). Binding to Nup358 could allow CRM1 to translocate back into the nucleus without ever leaving the range of the NPC. A similar role for Nup358 has been suggested in the context of the import of the mRNA export factor NXF1 into the nucleus (16). Whatever the role of Nup358 in protein export may be, it is not rate limiting under our experimental conditions. In contrast, Nup214 is central to the late steps in nuclear protein export, as transport is significantly reduced in the absence of this nucleoporin. Being dispensable for nuclear protein import, Nup214 therefore plays a specific role in CRM1-mediated protein export in human cells, demonstrating that individual nucleoporins can have a major function in specific nucleocytoplasmic transport pathways. Of course, we cannot exclude the possibility that other, CRM1-independent export pathways are affected by the depletion of Nup214 as well.
Acknowledgments
We are grateful to Donna Love, John Hanover, Larry Gerace, Barbara Müller, and Minoru Yoshida for the gift of reagents. We thank Hans-Georg Kräusslich and Frauke Melchior for support of the project and Marc Arnold, Angelika Kehlenbach, Frauke Melchior, and Detlef Doenecke for critical reading of the manuscript.
S.H. was supported by a fellowship from the Marianne and Dr. Fritz Walter Fischer-Stiftung.
REFERENCES
- 1.Askjaer, P., A. Bachi, M. Wilm, F. R. Bischoff, D. L. Weeks, V. Ogniewski, M. Ohno, C. Niehrs, J. Kjems, I. W. Mattaj, and M. Fornerod. 1999. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Mol. Cell. Biol. 19:6276-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Görlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos, R., L. Ribas de Pouplana, M. Enarson, K. Bodoor, and B. Burke. 1997. Nup84, a novel nucleoporin that is associated with CAN/Nup214 on the cytoplasmic face of the nuclear pore complex. J. Cell Biol. 137:989-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beals, C. R., N. A. Clipstone, S. N. Ho, and G. R. Crabtree. 1997. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11:824-834. [DOI] [PubMed] [Google Scholar]
- 5.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Efraim, I., and L. Gerace. 2001. Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import. J. Cell Biol. 152:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernad, R., H. van der Velde, M. Fornerod, and H. Pickersgill. 2004. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol. Cell. Biol. 24:2373-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff, F. R., and D. Görlich. 1997. RanBP1 is crucial for the release of RanGTP from importin beta-related nuclear transport factors. FEBS Lett. 419:249-254. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff, F. R., C. Klebe, J. Kretschmer, A. Wittinghofer, and H. Ponstingl. 1994. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc. Natl. Acad. Sci. USA 91:2587-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff, F. R., and H. Ponstingl. 1991. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 354:80-82. [DOI] [PubMed] [Google Scholar]
- 11.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 12.Cronshaw, J. M., A. N. Krutchinsky, W. Zhang, B. T. Chait, and M. J. Matunis. 2002. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 158:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelsma, D., R. Bernad, J. Calafat, and M. Fornerod. 2004. Supraphysiological nuclear export signals bind CRM1 independently of RanGTP and arrest at Nup358. EMBO J. 23:3643-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahrenkrog, B., and U. Aebi. 2003. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat. Rev. Mol. Cell. Biol. 4:757-766. [DOI] [PubMed] [Google Scholar]
- 15.Floer, M., and G. Blobel. 1999. Putative reaction intermediates in Crm1-mediated nuclear protein export. J. Biol. Chem. 274:16279-16286. [DOI] [PubMed] [Google Scholar]
- 16.Forler, D., G. Rabut, F. D. Ciccarelli, A. Herold, T. Kocher, R. Niggeweg, P. Bork, J. Ellenberg, and E. Izaurralde. 2004. RanBP2/Nup358 provides a major binding site for NXF1-p15 dimers at the nuclear pore complex and functions in nuclear mRNA export. Mol. Cell. Biol. 24:1155-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 18.Fornerod, M., J. van Deursen, S. van Baal, A. Reynolds, D. Davis, K. G. Murti, J. Fransen, and G. Grosveld. 1997. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried, H., and U. Kutay. 2003. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 60:1659-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 21.Guan, T., R. H. Kehlenbach, E. C. Schirmer, A. Kehlenbach, F. Fan, B. E. Clurman, N. Arnheim, and L. Gerace. 2000. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol. Cell. Biol. 20:5619-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanover, J. A., C. K. Cohen, M. C. Willingham, and M. K. Park. 1987. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J. Biol. Chem. 262:9887-9894. [PubMed] [Google Scholar]
- 23.Izaurralde, E., U. Kutay, C. von Kobbe, I. W. Mattaj, and D. Görlich. 1997. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 16:6535-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katahira, J., K. Strasser, A. Podtelejnikov, M. Mann, J. U. Jung, and E. Hurt. 1999. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 18:2593-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehlenbach, R. H., R. Assheuer, A. Kehlenbach, J. Becker, and L. Gerace. 2001. Stimulation of nuclear export and inhibition of nuclear import by a Ran mutant deficient in binding to Ran-binding protein 1. J. Biol. Chem. 276:14524-14531. [DOI] [PubMed] [Google Scholar]
- 26.Kehlenbach, R. H., A. Dickmanns, and L. Gerace. 1998. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J. Cell Biol. 141:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kehlenbach, R. H., A. Dickmanns, A. Kehlenbach, T. Guan, and L. Gerace. 1999. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 145:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemm, J. D., C. R. Beals, and G. R. Crabtree. 1997. Rapid targeting of nuclear proteins to the cytoplasm. Curr. Biol. 7:638-644. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer, D., R. W. Wozniak, G. Blobel, and A. Radu. 1994. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc. Natl. Acad. Sci. USA 91:1519-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love, D. C., T. D. Sweitzer, and J. A. Hanover. 1998. Reconstitution of HIV-1 rev nuclear export: independent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA 95:10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan, R., C. Delphin, T. Guan, L. Gerace, and F. Melchior. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88:97-107. [DOI] [PubMed] [Google Scholar]
- 33.Matunis, M. J., E. Coutavas, and G. Blobel. 1996. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135:1457-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melchior, F., D. J. Sweet, and L. Gerace. 1995. Analysis of Ran/TC4 function in nuclear protein import. Methods Enzymol. 257:279-291. [DOI] [PubMed] [Google Scholar]
- 35.Northrop, J. P., S. N. Ho, L. Chen, D. J. Thomas, L. A. Timmerman, G. P. Nolan, A. Admon, and G. R. Crabtree. 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature 369:497-502. [DOI] [PubMed] [Google Scholar]
- 36.Ossareh-Nazari, B., F. Bachelerie, and C. Dargemont. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141-144. [DOI] [PubMed] [Google Scholar]
- 37.Pollard, V. W., W. M. Michael, S. Nakielny, M. C. Siomi, F. Wang, and G. Dreyfuss. 1996. A novel receptor-mediated nuclear protein import pathway. Cell 86:985-994. [DOI] [PubMed] [Google Scholar]
- 38.Reed, R., and H. Cheng. 2005. TREX, SR proteins and export of mRNA. Curr. Opin. Cell Biol. 17:269-273. [DOI] [PubMed] [Google Scholar]
- 39.Ribbeck, K., and D. Görlich. 2001. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribbeck, K., G. Lipowsky, H. M. Kent, M. Stewart, and D. Görlich. 1998. NTF2 mediates nuclear import of Ran. EMBO J. 17:6587-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth, P., N. Xylourgidis, N. Sabri, A. Uv, M. Fornerod, and C. Samakovlis. 2003. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J. Cell Biol. 163:701-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rout, M. P., J. D. Aitchison, M. O. Magnasco, and B. T. Chait. 2003. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 13:622-628. [DOI] [PubMed] [Google Scholar]
- 43.Salina, D., P. Enarson, J. B. Rattner, and B. Burke. 2003. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J. Cell Biol. 162:991-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibasaki, F., E. R. Price, D. Milan, and F. McKeon. 1996. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature 382:370-373. [DOI] [PubMed] [Google Scholar]
- 45.Singh, B. B., H. H. Patel, R. Roepman, D. Schick, and P. A. Ferreira. 1999. The zinc finger cluster domain of RanBP2 is a specific docking site for the nuclear export factor, exportin-1. J. Biol. Chem. 274:37370-37378. [DOI] [PubMed] [Google Scholar]
- 46.Snow, C. M., A. Senior, and L. Gerace. 1987. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J. Cell Biol. 104:1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 48.Strawn, L. A., T. Shen, N. Shulga, D. S. Goldfarb, and S. R. Wente. 2004. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 6:197-206. [DOI] [PubMed] [Google Scholar]
- 49.Stutz, F., and E. Izaurralde. 2003. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 13:319-327. [DOI] [PubMed] [Google Scholar]
- 50.von Lindern, M., M. Fornerod, S. van Baal, M. Jaegle, T. de Wit, A. Buijs, and G. Grosveld. 1992. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol. Cell. Biol. 12:1687-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walther, T. C., H. S. Pickersgill, V. C. Cordes, M. W. Goldberg, T. D. Allen, I. W. Mattaj, and M. Fornerod. 2002. The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import. J. Cell Biol. 158:63-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolff, B., J. J. Sanglier, and Y. Wang. 1997. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4:139-147. [DOI] [PubMed] [Google Scholar]
- 53.Wu, J., M. J. Matunis, D. Kraemer, G. Blobel, and E. Coutavas. 1995. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J. Biol. Chem. 270:14209-14213. [DOI] [PubMed] [Google Scholar]
- 54.Yokoyama, N., N. Hayashi, T. Seki, N. Pante, T. Ohba, K. Nishii, K. Kuma, T. Hayashida, T. Miyata, U. Aebi, M. Fukui, and T. Nishimoto. 1995. A giant nucleopore protein that binds Ran/TC4. Nature 376:184-188. [DOI] [PubMed] [Google Scholar]
- 55.Yoneda, Y., N. Imamoto-Sonobe, M. Yamaizumi, and T. Uchida. 1987. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp. Cell Res. 173:586-595. [DOI] [PubMed] [Google Scholar]
- 56.Zeitler, B., and K. Weis. 2004. The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo. J. Cell Biol. 167:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, X., M. Yamada, N. Mabuchi, and H. Shida. 2003. Cellular requirements for CRM1 import and export. J. Biochem. (Tokyo) 134:759-764. [DOI] [PubMed] [Google Scholar]