Abstract
Xist is the trigger for X inactivation in female mammals. The long noncoding Xist RNA localizes along one of the two female X chromosomes and initiates chromosome-wide silencing in the early embryo. In differentiated cells, Xist becomes dispensable for the maintenance of the inactive X, and its function for initiation of silencing is lost. How Xist mediates gene repression remains an open question. Here, we use an inducible Xist allele in adult mice to identify cells in which Xist can cause chromosome-wide silencing. We show that Xist has the ability to initiate silencing in immature hematopoietic precursor cells. In contrast, hematopoietic stem cells and mature blood cells are unable to initiate ectopic X inactivation. This indicates that pathways critical for silencing are transiently activated in hematopoietic differentiation. Xist-responsive cell types in normal female mice show a change of chromatin marks on the inactive X. However, dosage compensation is maintained throughout hematopoiesis. Therefore, Xist can initiate silencing in precursors with concomitant maintenance of dosage compensation. This suggests that Xist function is restricted in development by the limited activity of epigenetic pathways rather than by a change in the responsiveness of chromatin between embryonic and differentiated cell types.
Mammals achieve dosage compensation by inactivation of one of the two X chromosomes in female cells during early embryogenesis. In the mouse, the paternally inherited X chromosome is inactivated in the cells of preimplantation embryos, giving rise to an imprinted pattern of X inactivation in extraembryonic tissues (41). Concomitant with the establishment of pluripotency in the inner cell mass of the blastocyst, the inactive X chromosome (Xi) is reactivated in cells contributing to the embryo proper (33, 36). Subsequently, the cells of the epiblast possess two active X chromosomes and are not dosage compensated between days 3.5 and 5.5 postcoitum (dpc). During gastrulation, one of the first epigenetic reprogramming events is the establishment of X inactivation to achieve dosage compensation by 6.5 dpc in all cells of the embryo (30, 43). The choice of the X chromosome for inactivation is random, resulting in a mosaic pattern of cells transcribing genes of either X. Female embryonic stem (ES) cells resemble epiblast cells in having two transcriptionally active X chromosomes (37, 42). During the differentiation of ES cells, the process of random X inactivation is recapitulated (21). X inactivation is a multistep process involving an ordered series of chromosomal modifications, which include the specific methylation and ubiquitination of histones, DNA methylation, and the recruitment of Polycomb group proteins (5, 14, 15).
Xist is the trigger for the initiation of chromosome-wide silencing and is required for X inactivation in early embryos (4, 6-8, 34, 37). The mechanism of establishing transcriptional silencing is presently not well understood. The Xist gene encodes a long nontranslated RNA that physically associates with the Xi (12). Silencing requires a conserved repeat sequence located at the 5′ end of Xist. Deletion of this element results in Xist RNA that associates with chromatin and spreads over the chromosome but does not affect transcriptional repression (47). This suggests a function of the 5′ end of Xist in binding putative silencing factors. However, it is clear that Xist alone is not sufficient for initiation of silencing. Formation of an Xi is dependent on the stage of the cell in development or differentiation. It has been shown that XIST cannot establish silent chromatin in differentiated human cells either by employing chromosomal translocations involving the inactive X chromosome (40) or by inducing XIST expression from the active X chromosome by DNA demethylation (11, 44). In differentiated cells, the X-inactivation center is not required for maintenance of X chromosome inactivation, and Xist RNA, DNA methylation, and histone hypoacetylation cooperate in maintaining the Xi (9, 13). We have previously used an inducible Xist expression system in male ES cells to analyze the function of Xist in initiating gene silencing during differentiation (46). Induction of Xist expression from transgenes integrated on the X chromosome or autosomes led to chromosome-wide silencing and histone H3 lysine 27 trimethylation (H3K27me3) in undifferentiated ES cells (28, 47). Xist induction in cells that had differentiated for 2 days or more did not trigger gene repression showing that silencing of the X chromosome depends on a particular cellular context, which subsists in ES cells only for two cell divisions following the onset of differentiation (46). It is not known what restricts Xist function in differentiation. One explanation might be differences in chromatin composition and function between embryonic cells and differentiated cells. Chromatin in differentiated cells might have undergone irreversible changes and modifications which impede the establishment of silent chromatin in response to Xist expression. This view is supported by the observation that the initiation of X inactivation in ES cell differentiation has been linked to a time window when chromosomal silencing is reversible. Alternatively, Xist might require factors whose activity is only present in early embryonic cells.
X chromosome inactivation has been assumed to be complete in all cells of female mouse embryos at about 6.5 dpc, and no transcriptional activity from the Xi was found in embryos at 9.5 dpc (30). However, reactivation of the Xi has been observed in primordial germ cells in the female germ line (43). The human Xi displays changes in chromatin marks and function in certain types of cancer. A relationship between XIST expression and responses of ovarian cancer to chemotherapy has been reported (24). XIST is also expressed in testicular germ cell tumors with multiple inactive X chromosomes, and evidence for initiation of X inactivation in tumor cells has been reported (26, 27, 32). The loss of a normal Xi including the absence of proper XIST RNA localization has been observed in breast cancer cells lacking wild-type BRCA1 (16). Finally, an ectopic human XIST transgene has been found to induce chromosome inactivation in human HT-1080 fibrosarcoma cells (19). These observations suggest that cells providing an appropriate context for initiation of silencing by Xist could be present in adult mammals. Here, we used an inducible system for ectopic Xist expression to identify Xist-responsive cells during embryogenesis and in the adult mouse. We observe that silencing of the X chromosome by Xist can be initiated in adult mice specifically in immature hematopoietic cells. Deregulated Xist expression ultimately leads to lethality due to hematopoietic failure.
MATERIALS AND METHODS
Xist induction in mice.
Mice carrying a tetracycline-inducible Xist allele (TX) were generated by blastocyst injection of correctly targeted ES cells (47) and were subsequently crossed with R26rtTA/rtTA mice (46). The TX and R26rtTA alleles (46, 47) were genotyped, and the sex of the embryos was determined as previously described (29). The Xist gene was induced in TX/Y R26rtTA/rtTA embryos and adult mice by adding doxycycline to the drinking water (1 g of doxycycline [Sigma] and 100 g of sucrose [Fluka] per liter). Water bottles were protected from light and changed every second day. The blood of anesthetized mice was collected by cardiac puncture into heparin-containing vials, and the blood parameters including hematocrit and thrombocytes were determined by a veterinary laboratory (Firma In Vitro, Vienna, Austria).
Fluorescence-activated cell sorter (FACS) analysis and cell sorting.
The following fluorescein isothiocyanate-, phycoerythrin (PE)-, CyChrome-, or allophycocyanin-coupled antibodies were used for flow cytometry: anti-B220 (RA3-6B2), CD3 (145-2C11), CD4 (L3T4), CD8a (53-6.7), CD11b/Mac1 (M1/70), CD19 (1D3), CD25/interleukin-2Rα (PC61), CD117/c-Kit (2B8), Gr1 (RB6-8C5), immunoglobulin D (IgD; 1.19), IgM (M41.42), Ly5.1 (A20), Ly5.2 (104), macrophage colony-stimulating factor receptor (AFS98), NK1.1 (PK136), Sca1/Ly6A (D7), T-cell receptor β (TCRβ; H57-597), Ter119 (TER119), and Thy1.2/CD90 (53-2.1) antibodies. Nonspecific antibody binding was suppressed by preincubation with CD16/CD32 Fc-block solution (PharMingen). Stained single-cell suspensions were analyzed on a FACSCalibur flow cytometer (Becton-Dickinson) by using a wide forward scatter/side scatter gate, which included all hematopoietic cells except for small erythrocytes. For isolating Lin− Sca1high c-Kithigh (LSK) cells, the bone marrow was stained with PE-coupled lineage marker antibodies (B220, CD3ɛ, CD4, CD8, NK1.1, Gr1, Mac1, and Ter119), and Lin+ cells were eliminated by magnetic cell sorting (MACS) with anti-PE beads (Miltenyi Biotec). Following staining with fluorescein isothiocyanate-anti-Sca1 and allophycocyanin-anti-cKit antibodies, LSK progenitors were sorted with a FACS Aria cell sorter (Becton-Dickinson). Lymphoid progenitors (LPs) were isolated as C19− B220+ c-Kit+ cells from the bone marrow.
Competitive bone marrow transplantation.
The TX and R26rtTA alleles were crossed for four generations into the C57BL/6 background, before bone marrow transfer experiments were performed as previously described (39). The bone marrow of TX/Y R26rtTA/rtTA Ly5.2+ mice, which were treated with doxycycline for 6 weeks, was mixed at a ratio of 1:10, 1:1, or 10:1 with the bone marrow of wild-type Ly5.1+ C57BL/6 mice prior to injection of 2 × 106 cells into the tail veins of Ly5.1+ C57BL/6 mice 24 h after lethal γ-irradiation (12 Gy). Chimeric mice were analyzed 6 months after transplantation.
Cell culture and RNA analysis.
ES cells were cultured as previously described (28). ES cells were established from TX/Y R26rtTA/rtTA blastocysts in Dulbecco's modified Eagle's medium (Biochrome) supplemented with 15% fetal calf serum (Euroclone), 250 U of leukemia inhibitory factor/ml and 50 μM PD98059 (Cell Signaling Technology). B220+ bone marrow cells were isolated by MACS, and pro-B cells were cultured on γ-irradiated ST2 cells in interleukin-7 containing Iscove's modified Dulbecco's medium as previously described (35). c-Kit+ pro-B cells were isolated by MACS sorting prior to RNA isolation. Allele-specific reverse transcription-PCR (RT-PCR) was performed as previously described (25) using a Superscript One-Step RT-PCR Platinum Taq kit (Invitrogen). Northern blot analysis was performed as previously described (46). RNA fluorescence in situ hybridization (FISH) using a directly Cy3-labeled Xist cDNA as a probe and immunofluorescence staining was performed as described previously (28). DAPI (4′,6′-diamidino-2-phenylindole) was used to stain the DNA. Magnification of cells varied in order to allow better visibility of Xist and H3K27me3 foci.
Methylation analysis.
Methylation-specific Southern blotting of the Xist promoter was performed by a HpaII/EcoRI digest of genomic DNA and detection of the fragments was performed with an XB1K probe (46).Southern blotting for promoter methylation at the Mecp2 locus was performed as described previously (50).
RESULTS
Induction of ectopic X inactivation in embryos.
We have generated a tetracycline-inducible Xist allele, named TX, by inserting a tet operator upstream of the Xist transcription initiation site in ES cells (Fig. 1A and B) (47). TX/Y mice were obtained and crossed with R26rtTA/rtTA mice expressing the tetracycline-responsive transactivator nls-rtTA (18) from the ROSA26 locus (46, 49). Hemizygous TX/Y R26rtTA/rtTA males and homozygous TX/TX R26rtTA/rtTA females were born at the expected Mendelian ratio (see Fig. S1A in the supplemental material) and appeared healthy and fertile, demonstrating that the inducible promoter in the absence of doxycycline did not interfere with Xist regulation. The inducible TX allele was functional, as a male ES cell line established from TX/Y R26rtTA/rtTA blastocysts showed silencing of X-linked genes and decreased cell viability after 4 days of doxycycline treatment (see Fig. S1B and C in the supplemental material). Xist expression was detected by RNA-FISH in TX/Y R26rtTA/rtTA embryos that were exposed, via the drinking water of their mother, to doxycycline for 2 days starting at 9.5 or 13.5 dpc in 90% and 80% of the cells, respectively, and resulted in focal H3K27me3 staining (see Fig. S1 in the supplemental material) (28).
FIG. 1.
Ectopic X inactivation upon Xist induction in the embryo. (A) Generation of the inducible TX allele. The tet operator sequence (green) was introduced into the SacII site upstream of the P1 promoter of Xist. The probe XB1K is indicated. (B) Doxycycline (dox)-induced DNA binding of the transactivator protein nls-rtTA results in Xist expression. (C and D) Northern blot analysis of X-linked (C) and erythroid cell-specific (D) gene transcripts in male TX/Y R26rtTA/rtTA embryos, in which Xist was induced with doxycycline (+) for 4 days starting at the indicated time point. (E) Lateral view of TX/Y R26rtTA/rtTA embryos (+) after Xist induction for 4 days after the time point shown. Untreated (−) embryos were used as control.
To investigate the ability of Xist to initiate gene silencing, we studied the expression of X-linked genes by Northern analysis of TX/Y R26rtTA/rtTA embryos that were treated with doxycycline for 4 days (Fig. 1C). Induction of Xist before 9.5 dpc led to the destruction of the embryo within 3 days, precluding a molecular analysis. Induction at 9.5 dpc resulted in clear repression of the X-linked Hprt and Pgk1 genes in 13.5 dpc embryos. This repression became progressively less pronounced in embryos induced at later time points between 9.5 and 12.5 dpc, indicating that the cells of midgestation embryos gradually lose the potential to initiate X inactivation. The conclusion that Xist-responsive cells persist in the embryo until 12.5 dpc is furthermore supported by the observation that Xist induction at 9.5 dpc resulted in severely malformed embryos at 13.5 dpc (Fig. 1E). However, ectopic Xist expression starting at 12.5 dpc was compatible with the birth of mice that had no major anatomic defects (Fig. 1E) and could feed normally, as indicated by the presence of milk in their stomachs. These mice died within 1 day after birth, possibly due to hematopoietic failure, as the blood hematocrit was severely reduced compared to untreated newborn mice (see Fig. S1D in the supplemental material). Furthermore, the erythroid-specific X-linked Gata1 and autosomal Epor genes were reduced in doxycycline-treated embryos at 16.5 dpc, indicating the loss of red blood cells (Fig. 1D). We therefore conclude that ectopic Xist expression during late embryogenesis selectively affects the hematopoietic system.
Xist induction causes lethal anemia in adult mice.
We next investigated the function of Xist in adult hematopoiesis, as the ROSA26 locus is expressed in blood cells, allowing for efficient Xist activation in all lineages (46, 49). For this, we induced Xist expression in 4-week-old male TX/Y R26rtTA/rtTA or female TX/TX R26rtTA/rtTA mice by continuous addition of doxycycline to the drinking water. As early as 2 weeks after Xist induction, these mice became weak, and most of them died between 5 and 6 weeks, with no mouse surviving 10 weeks (Fig. 2A). Mice at the first appearance of disease symptoms had a hematocrit of one-tenth of controls (Fig. 2B), suggesting a defect in hematopoiesis. Upon histological examination, the bone marrow was hypocellular, and the thymus was severely reduced or absent at 6 weeks of doxycycline treatment (Fig. 2C), showing that ectopic Xist expression affects multiple hematopoieticlineages in adult mice. Importantly, doxycycline had no effect on control X/Y R26rtTA/rtTA males, heterozygous TX/X R26rtTA/rtTA females, or TX-carrying mice lacking the R26rtTA allele. This shows that deregulated Xist expression in adult mice results in lethality due to hematopoietic failure and anemia.
FIG. 2.
Xist induction leads to anemia in adult mice. (A) Survival of TX/Y R26rtTA/rtTA and control X/Y R26rtTA/rtTA males (n = 15 each) after doxycycline treatment for 10 weeks. (B) Hematocrit levels of male TX/Y R26rtTA/rtTA and control X/Y R26rtTA/rtTA mice (n = 15 each) after doxycycline treatment for 3 to 6 weeks. (C) Hypocellularity of the bone marrow and absence of the thymus (thy) in a TX/Y R26rtTA/rtTA male after 6 weeks of doxycycline treatment (+). The thymus of an untreated (−) mouse is indicated by a dashed line.
Immature hematopoietic progenitors support Xist-mediated silencing.
We next performed FACS analysis to study the cellular composition of the bone marrow (20, 38) of TX/Y R26rtTA/rtTA males after 5 and 7 days of Xist induction. B lymphocyte development is initiated in pro-B cells and proceeds via pre-B and immature B cells to the mature B-cell stage (10). Pro-B cells (CD19+c-Kit+) were reduced twofold by Xist induction, consistent with a similar reduction of the bone marrow cellularity (Fig. 3A and G) and with the fact that the proliferation of in vitro cultured TX/Y R26rtTA/rtTA pro-B cells was minimally affected by the presence of doxycycline (see Fig. S2D in the supplemental material). In contrast, the pre-B cells (CD19+ CD25+ IgM−) were largely eradicated (Fig. 3B and G), and the immature B cells (B220+ IgM+ IgD−) were severely reduced after 5 days of Xist induction (Fig. 3C and G). The mature B cells (B220+ IgD+ IgM+) in the bone marrow (Fig. 3C and G) and spleen (see Fig. S2B and C in the supplemental material) were, however, mainly resistant to Xist-mediated killing. Within the erythro-myeloid lineages, the macrophages (Gr1int Mac1+) were most rapidly eliminated, and the cell counts of granulocytes (Gr1hi Mac1+) declined significantly but more moderately (Fig. 3D and G; see also Fig. S2A in the supplemental material), whereas Ter119+ erythroid cells even increased after 7 days of ectopic Xist expression (Fig. 3E and G). This expansion of erythroid cells was temporary and specific to more mature cell types as early erythroid progenitors were significantly reduced by 7 days of Xist induction (see Fig. S5C and D in the supplemental material). Consistent with this, we found that the erythroid system collapsed after 4 weeks of Xist induction, when animals became anemic.
FIG. 3.
Depletion of immature hematopoietic cell types by ectopic X inactivation. FACS analysis of the bone marrow (A to E) and thymus (F) of male TX/Y R26rtTA/rtTA mice without and with doxycycline treatment for 5 and 7 days (d). Pro-B cells (A), pre-B cells (B), immature and mature recirculating B cells (C), granulocytes and macrophages (D), erythroblasts (E), and DN, DP, and SP thymocytes (F) are shown with their percentage in the respective quadrant. Five mice per time point were analyzed to determine the absolute cell number (106) of the indicated cell types in the bone marrow (G) and thymus (H). (I) Northern blot analysis of in vitro cultured pro-B cells and ex vivo sorted pre-B cells and splenic IgD+ B cells before (−) or after (+) 4 days of doxycycline treatment. (K) Xist RNA FISH and H3K27me3 staining in 4-day-induced pre-B cells of a male TX/Y R26rtTA/rtTA mouse. The percentage of cells (n > 100) containing a signal is indicated. DNA is stained by DAPI (blue). BM, bone marrow; I, immature; R, recirculating; G, granulocytes; M, macrophages.
In the thymus, all T-cell subsets rapidly declined upon Xist induction (Fig. 3F and H), consistent with a strong reduction of the total cellularity (see Fig. S2C in the supplemental material). The double-positive (DP) pre-T cells (CD4+ CD8+) were the most rapidly eliminated compared to the double-negative (DN) pro-T cells (Thy1.2+ CD4− CD8−) and the more mature CD4+ and CD8+ single-positive (SP) thymocytes. As an important control, heterozygous TX/X R26rtTA/rtTA females did not reveal any hematopoietic abnormalities after 4 weeks of doxycycline treatment (data not shown). These data therefore indicate that ectopic Xist expression severely affects the development of multiple hematopoietic lineages in TX/Y males.
To demonstrate that Xist-induced gene silencing and ectopic X inactivation were, indeed, initiated in hematopoietic cells, we analyzed the expression of X-linked genes in cultured TX/Y R26rtTA/rtTA pro-B cells as well as in ex vivo sorted pre-B and mature B cells of TX/Y R26rtTA/rtTA males before and after 4 days of doxycycline treatment. Upon doxycycline treatment, the Pgk1 and Hprt genes were repressed in pre-B cells in contrast to pro-B and mature B cells (Fig. 3I). Moreover, focal H3K27me3 staining was observed in a significant number (>10%) of the doxycycline-treated pre-B cells but was undetectable in pro-B and mature B cells, although Xist induction was equally efficient (>80%) in all three cell types (Fig. 3K; also data not shown). Ectopic Xist expression also initiated silencing of the Hprt and Pgk1 genes in DP pre-T cells and CD4+ SP thymocytes of TX/Y R26rtTA/rtTA males (see Fig. S2E in the supplemental material). Hence, we conclude that Xist induction leads to gene silencing and subsequent cell loss in lineage-committed immature cells of the hematopoietic system.
The context for initiation of Xist-mediated silencing is established after stem cell differentiation.
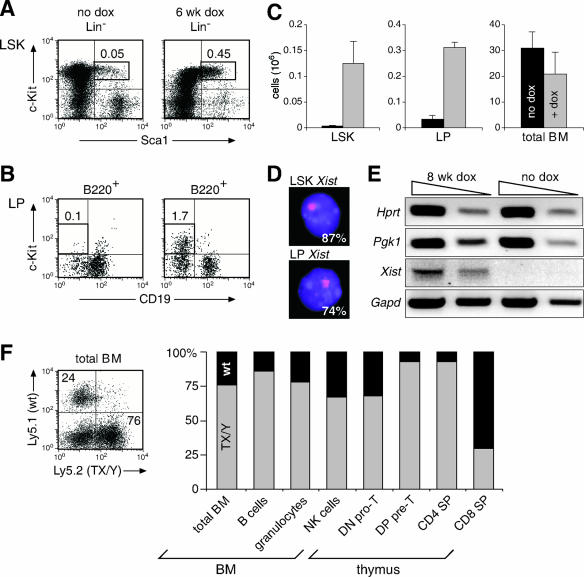
To our surprise, the population of LSK (Lin− Sca1high c-Kithigh) progenitors, which includes hematopoietic stem cells (HSCs), was not reduced in the bone marrow of TX/Y R26rtTA/rtTA males but, instead, started to increase in absolute cell numbers after 2 weeks of doxycycline treatment to reach a 20-fold expansion after 4 to 6 weeks in anemic mice (Fig. 4A and C). Similarly, uncommitted LPs (CD19− B220+ c-Kit+; pre-pro-B cells) (2, 20, 38) were also 10-fold expanded during the same period, while the cellularity of the bone marrow was moderately reduced in doxycycline-treated versus unstimulated TX/Y R26rtTA/rtTA mice (Fig. 4B and C). Moreover, c-Kit+ progenitors constituted a majority of the accumulating bone marrow cells after 6 weeks of doxycycline treatment (see Fig. S3A to D in the supplemental material). At this time point, strong Xist RNA signals were detected by RNA FISH in 87% of sorted LSK and 74% of LP cells, although no H3K27me3 foci were discernible (Fig. 4D; also data not shown). Finally, the X-linked Pgk1 and Hprt genes were equally expressed in LSK cells of anemic or untreated TX/Y R26rtTA/rtTA males (Fig. 4E). Hence, ectopic Xist expression did not initiate X inactivation in uncommitted hematopoietic progenitors, which accumulate possibly by homeostatic expansion due to the loss of the differentiated blood cells. Cells apparently could initiate ectopic X inactivation only once expression of the stem cell marker c-Kit was down-regulated.
FIG. 4.
Absence of ectopic X inactivation in HSCs. (A to E) Male TX/Y R26rtTA/rtTA mice were induced with doxycycline for 6 weeks or left untreated. FACS analysis of multipotent LSK (A) and LPs (B). The percentage of total bone marrow cells in the respective gate is indicated. (C) Absolute cell numbers (106) of LSK, LPs, and total bone marrow cells (n = 5 each). (D) Xist RNA FISH signals with their detection frequency are shown for FACS-sorted LSK and LPs. DAPI staining is shown in blue. (E) RT-PCR analysis of X-linked gene expression in sorted LSK cells. Tenfold cDNA dilutions were analyzed. (F) FACS analysis of Ly5.1+ recipient mice 6 months after transplantation of a 1:1 mixture of bone marrow from a wild-type (wt) Ly5.1+ mouse and a TX/Y R26rtTA/rtTA Ly5.2+ mouse that was treated for 6 weeks with doxycycline. The relative contributions of wild-type (wt) Ly5.1+ HSCs (black bars) and TX/Y Ly5.2+ HSCs (gray bars) to the different lineages are shown. BM, bone marrow; dox, doxycycline.
The potency of the expanded HSCs was next assessed by competitive bone marrow transfer experiments after crossing the TX and R26rtTA alleles into the C57BL/6 background. Six weeks after doxycycline treatment, the bone marrow of anemic TX/Y R26rtTA/rtTA males expressing the pan-hematopoietic marker Ly5.2 was mixed at a ratio of 1:10, 1:1, or 10:1 with the bone marrow of wild-type Ly5.1+ C57BL/6 mice prior to injection into lethally irradiated Ly5.1+ C57BL/6 recipients. Six months after the transplantation and absence of doxycycline, 76% of the bone marrow cells of a chimeric mouse receiving a 1:1 graft expressed the Ly5.2 marker of the TX/Y R26rtTA/rtTA donor cells (Fig. 4F). Moreover, these Ly5.2+ cells constituted the majority of all hematopoietic cell types in the bone marrow and thymus except for CD8+ SP T cells, which were predominantly derived from the Ly5.1+ HSCs (Fig. 4F; see also Fig. S4 in the supplemental material). The analysis of bone marrow chimeras generated with different graft ratios confirmed that the HSCs were more abundant in the bone marrow of anemic mice compared to wild-type mice (see Fig. S4A in the supplemental material). Importantly, these data indicate that the HSCs of anemic mice are functional, as the Xist-induced effects on hematopoiesis were fully reversible. In support of this conclusion, anemic TX/Y R26rtTA/rtTA mice, which were doxycycline-treated for 5 weeks, recovered and showed normalized hematopoiesis 2 weeks after doxycycline withdrawal (data not shown).
Loss of markers of the Xi in immature hematopoietic cells.
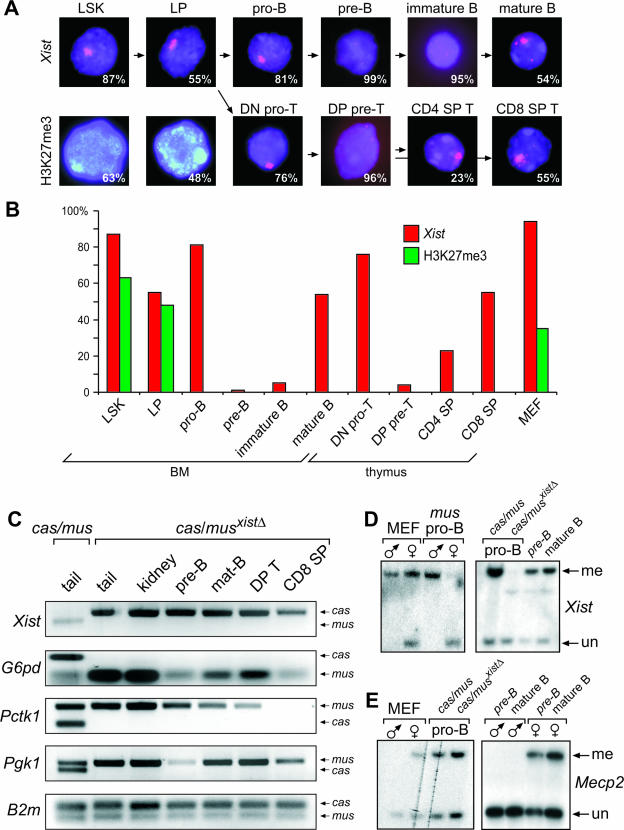
The observation that Xist can initiate gene silencing in immature hematopoietic cells predicts that an epigenetic context enabling initiation of X inactivation is established in these cells. To study if establishment of this context was characterized by changes in chromatin composition and function, we characterized the Xi in sorted hematopoietic cells of wild-type female mice by Xist RNA FISH and H3K27me3 staining (Fig. 5A and B; see also Fig. S5A and B in the supplemental material). A clear focal Xist RNA cluster and one intense H3K27me3 focus were observed in LSKs and LPs. However, the Xi changed in appearance during lymphopoiesis with the loss of focal H3K27me3 staining in pro-B and DN pro-T cells. Moreover, none of the committed cell types downstream of the LPs showed any focal H3K27me3 staining (Fig. 5B; see also Fig. S5B in the supplemental material). Although a focal Xist RNA cluster was detected in most pro-B and DN pro-T cells, Xist became undetectable in all pre-B cells and was diffusely localized in the majority of DP pre-T cells. Focal Xist clusters were again present in a significant fraction of mature B cells as well as CD4+ and CD8+ SP T cells. The absence of Xist staining in wild-type female pre-B cells was not attributable to a technical failure, since focal Xist staining was detected reproducibly in pre-B cells of TX/Y R26rtTA/rtTA mice after Xist induction (Fig. 3K). We conclude that changes in the chromatin of the Xi occur in cell types that become endowed with the potential to initiate X inactivation.
FIG. 5.
Absence of markers of the Xi in immature lymphocytes of female mice. (A) Xist RNA FISH (red) and H3K27me3 staining (green) in hematopoietic cell types (n = 200 each) of a wild-type female mouse. The percentage of cells is indicated. DNA is stained by DAPI (blue). (B) Statistical evaluation of Xist and H3K27me3 signals in hematopoietic cells and mouse embryo fibroblasts. (C) X-linked G6pd, Pgk1, Pctk1, Xist, and autosomal control B2m expression was analyzed by allele-specific RT-PCR in the tissues indicated from an F1 cas/mus(xistΔ) female mouse, containing a maternal M. musculus X bearing a deletion of the Xist gene and a paternal M. castaneus X, and from a control wild-type cas/mus female. (D and E) Methylation analysis of the Xist (D) and Mecp2 promoter (E) in the indicated cell types. Mouse embryo fibroblasts and pro-B cells of all indicated sexes and genotypes were cultured, while pre-B and mature B cells were sorted ex vivo. MEF, mouse embryo fibroblast.
To address the possibility that genes on the Xi might be reactivated in immature lymphocytes, we made use of sequence polymorphisms that distinguish the Mus castaneus and Mus musculus genes in a genetic setting, where X inactivation is completely nonrandom. We used M. musculus × M. castaneus (mus/cas) hybrid mice carrying a deletion in the Xist gene on the maternal inherited M. musculus X chromosome to force inactivation of the paternally inherited M. castaneus X in all cells. Reactivation of the M. castaneus Xi could then be detected by RT-PCR. In this genetic setting, M. castaneus X-linked genes were not expressed, suggesting that reactivation of genes on the Xi does not normally occur in vivo (Fig. 5C). This finding was corroborated by the observation that DNA methylation was maintained on the promoter of the Xist gene on the active X and X-linked genes, such as Mecp2, on the inactive X chromosome in cells of the hematopoietic system (Fig. 5D and E; also data not shown). These data are consistent with earlier reports describing stable X inactivation in hematopoietic cells of the mouse and clonal patterns of X inactivation in leukemia (23). We conclude that ectopic X inactivation can be initiated in pre-B cells while dosage compensation is simultaneously maintained.
DISCUSSION
Initiation window for X inactivation in embryogenesis.
Using an inducible Xist expression system, we have analyzed when silencing of the X chromosome can be initiated during embryogenesis and in the adult mouse. We find that the ability of Xist to initiate silencing is progressively lost in cells of the embryo and that Xist induction at 12.5 dpc does not interfere with development to term. The initiation phase of X inactivation therefore extends over a considerable time span in embryogenesis from its initiation in gastrula embryos at 6.5 dpc until 12.5 dpc. This finding is compatible with our previous report that Xist does not induce silencing in cultured fibroblasts established from embryos at 13.5 dpc (46). Xist induction in embryos before 12.5 dpc leads to developmental malformations and cell loss. Histological analysis of the phenotype suggests that differentiated cells are maintained as observed on sections (data not shown). However, the formation of facial structures, limbs, heart, and brain are severely retarded or completely arrested. We believe that this is caused by a loss of multipotent progenitors in the embryo, which results in a depletion of organ-forming cells. Previous studies in ES cell differentiation have indicated that the function of Xist for initiation of silencing is lost shortly after its expression is initiated. In this study, we demonstrate that Xist-responsive cells are present in the embryo and critically contribute to development between 3.5 dpc and 12.5 dpc.
Transient potential for initiation of X inactivation in adult hematopoiesis.
We observe that the hematopoietic system contains cells that are capable of initiating silencing of the X chromosome in the adult mouse. Xist induction in mice led to a loss of blood cells, causing lethality due to anemia after 4 to 6 weeks. We show that cells, which can support Xist-mediated gene silencing, are present and critical for survival in adult mice. This demonstrates that deregulated Xist expression can be pathological leading to a fatal condition. All hematopoietic lineages were severely affected by Xist induction, with the pre-B cells, the DP pre-T cells, and the bone marrow macrophages being lost most rapidly. Notably, we find that lymphoid lineage cells before and after the pre-B- and pre-T-cell stages were either less or not at all responsive to Xist induction. Hence, immature precursor cells of the B- and T-cell lineages temporarily reestablish epigenetic pathways, as seen in ES cells, and thus become Xist responsive. The epigenetic context for initiation of X inactivation is therefore established throughout the lifetime of the mouse during normal hematopoiesis after commitment of the HSCs to differentiation and lineage choice.
In ES cells, initiation of Xist-mediated silencing is restricted to an early time point in differentiation, when silencing is reversible. In contrast to embryonic cells, pre-B and pre-T cells can initiate X inactivation although these cells maintain proper dosage compensation. In support of this notion, no cells with two active X chromosomes were detected in female mice beyond 9.5 dpc (30, 43). Reactivation of the X-linked Pgk1 gene was also not observed in the blood, bone marrow, or thymus of female mice with a translocation-induced nonrandom pattern of X inactivation (42). Our results, using mice with a nonrandom pattern of X inactivation due to a deletion in the Xist gene, support the view that non-dosage-compensated cells are extremely rare or absent in the adult mouse. Yet we demonstrate the loss of markers of the Xi in pre-B and pre-T cells, which both have the potential to initiate X inactivation. In somatic cells, the Xi is maintained by redundant mechanisms including DNA methylation (13). Maintenance of the Xi together with the ability to initiate silencing might parallel the situation in ES cells, when X inactivation is initiated and imprinting marks are maintained. It has been shown that maintenance of genomic imprinting is dependent on DNA methylation (31, 45). We observe maintenance of DNA methylation on the promoters on the Xi, when other marks of the Xi are lost at the pre-B- and pre-T-cell stages.
Xist becomes competent to induce gene silencing at a stage in lymphoid development, which is characterized by the establishment of allelic exclusion at the immunoglobulin heavy chain (Igh) or TCRβ loci, respectively. Functional V(D)J (indicating variable, diversity, and joining regions, respectively) rearrangement of one of the two Igh alleles during pro-B development leads to the expression of the Ig(μ) protein as part of the pre-B-cell receptor (3). Likewise, the generation of a functional TCRβ gene in DN pro-T cells leads to cell surface expression of the pre-TCR. Transient signaling of the pre-B-cell receptor or pre-TCR promotes differentiation to pre-B or DP pre-T cells and establishes allelic exclusion, which prevents further V-to-DJ rearrangement at the second incompletely rearranged Igh or TCRβ locus, respectively (3, 17). The responsiveness of pre-B and pre-T cells to Xist could thus result from a change in cellular signaling, which likely activates pathways controlling long-range gene activity and Xist function. V(D)J recombination of the antigen receptor loci is under epigenetic control and regulated by the accessibility of the DNA segments subject to allelic exclusion (3, 17). It is tempting to speculate about a mechanistic link between allelic exclusion of the antigen receptor genes in lymphocytes and X inactivation in the embryo. Counting and choice in X inactivation could be regarded as an allelic exclusion mechanism before Xist activation and initiation of chromosomal silencing in early embryonic development. Recently, understanding of counting and choice in random X inactivation has been advanced by the observation of X inactivation center pairing in ES cells at the onset of X inactivation (1, 48). It will be interesting to see if similarities in the mechanisms between the choice in X inactivation and allelic exclusion at the antigen receptor loci will be uncovered in the future. In conclusion, Xist can initiate chromosome wide-silencing in hematopoietic cells that are still able to maintain the Xi.
Difference in responsiveness to Xist between embryonic and HSCs.
Blood cell production is maintained throughout the lifetime of the mouse by HSCs, which differentiate into all blood cell types. Stem cell maintenance is of critical importance, and the cellular identity of the HSC must be stably maintained. At the same time, the cell identity has to be changed once the stem cell has been activated and cellular differentiation progresses to produce mature blood cells. Our data show that HSCs and uncommitted progenitors, like most somatic cells, do not have the ability to initiate X-linked gene silencing upon ectopic Xist expression and are thus resistant to Xist-mediated killing. The Xi in female HSCs and multipotent progenitors appears to be stable, as revealed by the presence of an Xist RNA cluster and a corresponding H3K27me3 staining similar to other somatic cells. This highlights a difference between adult and embryonic stem cells. Only following differentiation of HSCs do the lineage-restricted hematopoietic precursors establish an epigenetic environment resembling that of embryonic stem cells. It needs to be shown if the establishment of a context for initiation of X inactivation is paralleled by a general ability for epigenetic reprogramming of these hematopoietic precursors. Our work identifies a change in epigenetic activity at a time in hematopoietic development when epigenetic patterns may be fixed after reprogramming of gene expression at lineage commitment. Previously, reprogramming of the X chromosome after gastrulation has been observed in cells that enter the germ line. The absence of markers of the Xi was also observed in certain human tumors (16). A question that needs to be addressed in the future is whether an epigenetic context for reprogramming the X chromosome is also established during tumorigenesis. Recently, it has been shown that ectopic expression of the transcription factor Oct4, which is associated with pluripotency of ES cells and the early embryo, induces reversible hyperproliferation of progenitor cells of the intestinal epithelium (22). Finally, the identification of developmental transitions during which cells gain and lose their ability to initiate Xist-mediated gene silencing will facilitate efforts to find yet elusive factors involved in the earliest steps of X inactivation and gene silencing.
Supplementary Material
Acknowledgments
We thank M. Dezic, R. Flannery, and C. Beard for mouse husbandry; G. Stengl for cell sorting; C. Cobaleda for advice with pro-B cell cultures; N. Brockdorff for the Mecp2 probe; T. Jenuwein and H. Beug for helpful discussions; and R. Grosschedl for critical reading of the manuscript.
This research was supported by Boehringer Ingelheim, the Austrian GEN-AU initiative (financed by the Ministry of Science) and EU FP6 funding for the Epigenome Network of Excellence.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bacher, C. P., M. Guggiari, B. Brors, S. Augui, P. Clerc, P. Avner, R. Eils, and E. Heard. 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell. Biol. [DOI] [PubMed]
- 2.Balciunaite, G., R. Ceredig, S. Massa, and A. G. Rolink. 2005. A B220+ CD117+ CD19− hematopoietic progenitor with potent lymphoid andmyeloid developmental potential. Eur. J. Immunol. 2019-2030. [DOI] [PubMed]
- 3.Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109(Suppl.):S45-S55. [DOI] [PubMed] [Google Scholar]
- 4.Borsani, G., R. Tonlorenzi, M. C. Simmler, L. Dandolo, D. Arnaud, V. Capra, M. Grompe, A. Pizzuti, D. Muzny, C. Lawrence, et al. 1991. Characterization of a murine gene expressed from the inactive X chromosome. Nature 351:325-329. [DOI] [PubMed] [Google Scholar]
- 5.Brockdorff, N. 2002. X-chromosome inactivation: closing in on proteins that bind Xist RNA. Trends Genet. 18:352-358. [DOI] [PubMed] [Google Scholar]
- 6.Brockdorff, N., A. Ashworth, G. F. Kay, P. Cooper, S. Smith, V. M. McCabe, D. P. Norris, G. D. Penny, D. Patel, and S. Rastan. 1991. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351:329-331. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C. J., A. Ballabio, J. L. Rupert, R. G. Lafreniere, M. Grompe, R. Tonlorenzi, and H. F. Willard. 1991. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349:38-44. [DOI] [PubMed] [Google Scholar]
- 8.Brown, C. J., R. G. Lafreniere, V. E. Powers, G. Sebastio, A. Ballabio, A. L. Pettigrew, D. H. Ledbetter, E. Levy, I. W. Craig, and H. F. Willard. 1991. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 349:82-84. [DOI] [PubMed] [Google Scholar]
- 9.Brown, C. J., and H. F. Willard. 1994. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature 368:154-156. [DOI] [PubMed] [Google Scholar]
- 10.Busslinger, M. 2004. Transcriptional control of early B cell development. Annu. Rev. Immunol. 22:55-79. [DOI] [PubMed] [Google Scholar]
- 11.Clemson, C. M., J. C. Chow, C. J. Brown, and J. B. Lawrence. 1998. Stabilization and localization of Xist RNA are controlled by separate mechanisms and are not sufficient for X inactivation. J. Cell Biol. 142:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemson, C. M., J. A. McNeil, H. F. Willard, and J. B. Lawrence. 1996. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132:259-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csankovszki, G., A. Nagy, and R. Jaenisch. 2001. Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J. Cell Biol. 153:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Napoles, M., J. E. Mermoud, R. Wakao, Y. A. Tang, M. Endoh, R. Appanah, T. B. Nesterova, J. Silva, A. P. Otte, M. Vidal, H. Koseki, and N. Brockdorff. 2004. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7:663-676. [DOI] [PubMed] [Google Scholar]
- 15.Fang, J., T. Chen, B. Chadwick, E. Li, and Y. Zhang. 2004. Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation. J. Biol. Chem. 279:52812-52815. [DOI] [PubMed] [Google Scholar]
- 16.Ganesan, S., D. P. Silver, R. A. Greenberg, D. Avni, R. Drapkin, A. Miron, S. C. Mok, V. Randrianarison, S. Brodie, J. Salstrom, T. P. Rasmussen, A. Klimke, C. Marrese, Y. Marahrens, C. X. Deng, J. Feunteun, and D. M. Livingston. 2002. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell 111:393-405. [DOI] [PubMed] [Google Scholar]
- 17.Goldmit, M., and Y. Bergman. 2004. Monoallelic gene expression: a repertoire of recurrent themes. Immunol. Rev. 200:197-214. [DOI] [PubMed] [Google Scholar]
- 18.Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 19.Hall, L. L., M. Byron, K. Sakai, L. Carrel, H. F. Willard, and J. B. Lawrence. 2002. An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells. Proc. Natl. Acad. Sci. USA 99:8677-8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, R. R., and S. A. Shinton. 2004. Characterization of B lymphopoiesis in mouse bone marrow and spleen. Methods Mol. Biol. 271:1-24. [DOI] [PubMed] [Google Scholar]
- 21.Heard, E. 2004. Recent advances in X-chromosome inactivation. Curr. Opin. Cell Biol. 16:247-255. [DOI] [PubMed] [Google Scholar]
- 22.Hochedlinger, K., Y. Yamada, C. Beard, and R. Jaenisch. 2005. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121:465-477. [DOI] [PubMed] [Google Scholar]
- 23.Hotta, T. 1997. Clonality in hematopoietic disorders. Int. J. Hematol. 66:403-412. [DOI] [PubMed] [Google Scholar]
- 24.Huang, K. C., P. H. Rao, C. C. Lau, E. Heard, S. K. Ng, C. Brown, S. C. Mok, R. S. Berkowitz, and S. W. Ng. 2002. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol. Cancer Ther. 1:769-776. [PubMed] [Google Scholar]
- 25.Huynh, K. D., and J. T. Lee. 2003. Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426:857-862. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami, T., K. Okamoto, O. Ogawa, and Y. Okada. 2004. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet 363:40-42. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami, T., K. Okamoto, H. Sugihara, T. Hattori, A. E. Reeve, O. Ogawa, and Y. Okada. 2003. The roles of supernumerical X chromosomes and XIST expression in testicular germ cell tumors. J. Urol. 169:1546-1552. [DOI] [PubMed] [Google Scholar]
- 28.Kohlmaier, A., F. Savarese, M. Lachner, J. Martens, T. Jenuwein, and A. Wutz. 2004. A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol. 2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert, J. F., B. O. Benoit, G. A. Colvin, J. Carlson, Y. Delville, and P. J. Quesenberry. 2000. Quick sex determination of mouse fetuses. J. Neurosci. Methods 95:127-132. [DOI] [PubMed] [Google Scholar]
- 30.Lebon, J. M., P. P. Tam, J. Singer-Sam, A. D. Riggs, and S. S. Tan. 1995. Mouse endogenous X-linked genes do not show lineage-specific delayed inactivation during development. Genet. Res. 65:223-227. [DOI] [PubMed] [Google Scholar]
- 31.Li, E., C. Beard, and R. Jaenisch. 1993. Role for DNA methylation in genomic imprinting. Nature 366:362-365. [DOI] [PubMed] [Google Scholar]
- 32.Looijenga, L. H., A. J. Gillis, R. J. van Gurp, A. J. Verkerk, and J. W. Oosterhuis. 1997. X inactivation in human testicular tumors. XIST expression and androgen receptor methylation status. Am. J. Pathol. 151:581-590. [PMC free article] [PubMed] [Google Scholar]
- 33.Mak, W., T. B. Nesterova, M. de Napoles, R. Appanah, S. Yamanaka, A. P. Otte, and N. Brockdorff. 2004. Reactivation of the paternal X chromosome in early mouse embryos. Science 303:666-669. [DOI] [PubMed] [Google Scholar]
- 34.Marahrens, Y., B. Panning, J. Dausman, W. Strauss, and R. Jaenisch. 1997. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11:156-166. [DOI] [PubMed] [Google Scholar]
- 35.Nutt, S. L., P. Urbanek, A. Rolink, and M. Busslinger. 1997. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 11:476-491. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto, I., A. P. Otte, C. D. Allis, D. Reinberg, and E. Heard. 2004. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303:644-649. [DOI] [PubMed] [Google Scholar]
- 37.Penny, G. D., G. F. Kay, S. A. Sheardown, S. Rastan, and N. Brockdorff. 1996. Requirement for Xist in X chromosome inactivation. Nature 379:131-137. [DOI] [PubMed] [Google Scholar]
- 38.Rolink, A. G. 2004. B-cell development and pre-B-1 cell plasticity in vitro. Methods Mol. Biol. 271:271-281. [DOI] [PubMed] [Google Scholar]
- 39.Souabni, A., C. Cobaleda, M. Schebesta, and M. Busslinger. 2002. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity 17:781-793. [DOI] [PubMed] [Google Scholar]
- 40.Surralles, J., and A. T. Natarajan. 1998. Position effect of translocations involving the inactive X chromosome: physical linkage to XIC/XIST does not lead to long-range de novo inactivation in human differentiated cells. Cytogenet. Cell Genet. 82:58-66. [DOI] [PubMed] [Google Scholar]
- 41.Takagi, N., and M. Sasaki. 1975. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256:640-642. [DOI] [PubMed] [Google Scholar]
- 42.Takagi, N., M. A. Yoshida, O. Sugawara, and M. Sasaki. 1983. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell 34:1053-1062. [DOI] [PubMed] [Google Scholar]
- 43.Tam, P. P., S. X. Zhou, and S. S. Tan. 1994. X-chromosome activity of the mouse primordial germ cells revealed by the expression of an X-linked lacZ transgene. Development 120:2925-2932. [DOI] [PubMed] [Google Scholar]
- 44.Tinker, A. V., and C. J. Brown. 1998. Induction of XIST expression from the human active X chromosome in mouse/human somatic cell hybrids by DNA demethylation. Nucleic Acids Res. 26:2935-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucker, K. L., C. Beard, J. Dausmann, L. Jackson-Grusby, P. W. Laird, H. Lei, E. Li, and R. Jaenisch. 1996. Germ-line passage is required for establishment of methylation and expression patterns of imprinted but not of nonimprinted genes. Genes Dev. 10:1008-1020. [DOI] [PubMed] [Google Scholar]
- 46.Wutz, A., and R. Jaenisch. 2000. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol. Cell 5:695-705. [DOI] [PubMed] [Google Scholar]
- 47.Wutz, A., T. P. Rasmussen, and R. Jaenisch. 2002. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat. Genet. 30:167-174. [DOI] [PubMed] [Google Scholar]
- 48.Xu, N., C. L. Tsai, and J. T. Lee. 2006. Transient homologous chromosome pairing marks the onset of X inactivation. Science. [DOI] [PubMed]
- 49.Zambrowicz, B. P., A. Imamoto, S. Fiering, L. A. Herzenberg, W. G. Kerr, and P. Soriano. 1997. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94:3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zvetkova, I., A. Apedaile, B. Ramsahoye, J. E. Mermoud, L. A. Crompton, R. John, R. Feil, and N. Brockdorff. 2005. Global hypomethylation of the genome in XX embryonic stem cells. Nat. Genet. 37:1274-1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.