Abstract
Gene amplification and protein overexpression of MDM2, which is often found in certain types of cancers, indicate that MDM2 plays an important role in tumorigenesis. Interestingly, several clinical reports have demonstrated that amplification of the MDM2 gene correlates with the metastatic stage. Using an antibody array assay, we identified E-cadherin as an MDM2-binding protein and confirmed that E-cadherin is a substrate for the MDM2 E3 ubiquitin ligase. We demonstrate that MDM2 interacts in vivo with E-cadherin, resulting in its ubiquitination and degradation. This regulation appears to be clinically relevant, as we found a significant correlation between high MDM2 and low E-cadherin protein levels in resected tumor specimens recovered from breast cancer patients with lymph node metastases. Ectopic expression of MDM2 in breast cancer cells was found to disrupt cell-cell contacts and enhance cell motility and invasive potential. We found that E-cadherin and MDM2 colocalized on the plasma membrane and in the early endosome, where ubiquitin moieties were attached to E-cadherin. Blocking endocytosis with dominant-negative mutants of dynamin abolished the association of MDM2 with E-cadherin, prevented E-cadherin degradation, and attenuated cell motility as observed by fluorescence microscopy. Thus, we provide evidence to support a novel role for MDM2 in regulating cell adhesions by a mechanism that involves degrading and down-regulating the expression of E-cadherin via an endosome pathway. This novel MDM2-regulated pathway is likely to play a biologically relevant role in cancer metastasis.
MDM2 has been shown to play an important role in a variety of physiological and pathological processes. Overexpression of the human homologue of MDM2, referred to as HDM2, occurs in diverse human malignancies including soft tissue sarcomas and cancers of the brain, breast, ovary, cervix, lung, colon, and prostate (40, 54). Overexpression in tumors correlates with a poor prognosis for those patients (4, 5, 24, 25, 39, 43, 44, 50, 53, 56, 58, 61). Furthermore, several studies have shown that amplification of the MDM2 gene occurs more frequently in metastatic and recurrent cancers than in primary tumors (23, 46). One study examined 100 tumor samples from patients with esophageal squamous cell carcinoma and found that MDM2 expression was the most significant risk factor for distant metastases (52), implicating its potential role in metastasis. Hypoxia, a common condition within solid tumors, has been shown to lead to the up-regulation of MDM2 expression and to increase the metastatic efficiency of tumor cells (77). Additionally, MDM2 expression was shown to correlate with increased levels of vascular endothelial growth factor, which may facilitate the intravasation and metastatic seeding of tumor cells (42, 68, 80). Thus, MDM2 expression appears to correlate with an increased risk of distant metastases, which may contribute to an overall poorer prognosis for patients with tumors that overexpress MDM2. However, the precise role and underlying mechanism of MDM2 in cancer metastasis are not completely understood at present.
MDM2 is a RING finger-containing E3 enzyme involved in eukaryotic protein degradation via the ubiquitin proteasome system. A well-established function of MDM2 involves its negative regulation of the p53 pathway through the inhibition of p53 transactivation and the promotion of p53 degradation via a ubiquitin-mediated proteolytic process (32, 45). Disrupting the interaction between MDM2 and p53 has been used as an approach in anticancer drug development (74). Although it is believed that one major function of MDM2 is to regulate the stability of the p53 protein, recent studies suggest that MDM2 may also play a critical role in tumorigenesis via p53-independent mechanisms (27, 55, 65, 67, 73). Therefore, identifying additional molecules that interact with MDM2 would be important for the further understanding of its oncogenic function and may reveal additional targets for anticancer therapy.
We have previously shown that HER2/neu-mediated resistance to DNA-damaging agents requires the activation of Akt, which leads to the phosphorylation and nuclear localization of MDM2 and enhancement of MDM2-mediated ubiquitination and degradation of p53 (79). Although MDM2 was originally discovered as a nuclear protein, it has since been reported to interact with molecules outside the nucleus, such as the insulin-like growth factor I receptor (30), the β2-adrenergic receptor (β2-AR), and its adaptor protein β-arrestin (62). Recent studies have shown that membrane protein trafficking and degradation involve E3 ligase-mediated ubiquitination of these proteins. Indeed, multiple endocytic pathways are now known to be involved in the regulation of membrane-bound proteins. The cytoplasmic domain of plasma membrane “cargo” proteins is recognized by adaptor proteins, and attachment of ubiquitin (Ub) to either “cargo” protein or adaptor protein by an E3 ligase is believed to serve as a sorting signal for the endocytic machinery (2, 35). As an E3 ligase, MDM2 has been shown to interact with and ubiquitinate β-arrestin, a requisite step for the internalization of the β2-AR (62, 66). In contrast, direct ubiquitination of the β2-AR has been shown to lead to its immediate degradation (62, 66). Aside from G-protein-coupled receptors, two recent reports have shown that the epithelial growth factor receptor may also be a target for Ub-mediated endocytosis (1, 16, 63). One study has demonstrated ubiquitination of E-cadherin, and this process was shown to be mediated by a c-Cbl-like E3 ligase called Hakai (28). Thus, there is mounting evidence to support a role for E3 ligase in the ubiquitination of membrane-bound proteins, which leads to either their intracellular trafficking or degradation. Although the details of this mechanism are incomplete, one current model proposes that when membrane proteins are ubiquitinated, interaction with adaptor proteins is triggered, which leads to the internalization and sorting of the proteins and, ultimately, to the late-endosome/lysosome compartment.
The loss of epithelial differentiation in carcinomas is accompanied by increased motility and invasiveness of the tumor cells, often as a consequence of reduced intercellular adhesion (9, 15, 19, 41, 69, 72). The down-regulation of cell-cell adherent junctions is a hallmark of the epithelial-to-mesenchymal transition, which involves the loss of functional E-cadherin protein by either transcriptional repression or silencing mutations of its gene (8, 10, 18, 47, 57). Indeed, we have previously shown that E-cadherin transcription is repressed by SNAIL through a pathway mediated by glycogen synthase kinase 3β (78). In several experimental systems and in a significant percentage of invasive tumors, others have previously demonstrated that the E-cadherin gene is normal, raising the possibility that the down-regulation of E-cadherin and the reduction of cell-cell junction stability that leads to the increased migratory potential of tumor cells could be dependent on posttranscriptional or posttranslational modification of E-cadherin as well (71). Interestingly, endocytosis of E-cadherin was recently shown to be an important step in the regulation of cadherin function in remodeling adhesive contacts, although the precise mechanism has not been fully elucidated (60).
In the present study, we identified E-cadherin as a new substrate of MDM2, demonstrating that MDM2 ubiquitinates E-cadherin and decreases its protein level. Furthermore, when we examined primary tumor cells from breast cancer patients who had lymph node metastases, we found an inverse correlation between MDM2 and E-cadherin protein levels. Ectopic expression of MDM2 in breast cancer cells was found to increase cell-cell dissociation, cell motility, and invasion. Interestingly, MDM2 and E-cadherin were demonstrated to colocalize on the plasma membrane and in the early endosome. E-cadherin was ubiquitinated in both fractions of the plasma membrane and the early endosome; furthermore, ubiquitination of E-cadherin in the early endosome was enhanced by MDM2. Dominant-negative mutants of dynamin (dn-dynamin), which interfere with endocytosis, blocked both the interaction of MDM2 with E-cadherin and MDM2-induced cell motility, indicating that endocytosis is necessary. Therefore, this study provides new evidence that MDM2 plays a role in modulating cell-cell adhesions by a mechanism that involves the down-regulation of E-cadherin via an early endosomal pathway. This novel MDM2-mediated pathway is likely to play a biologically significant role in cancer metastasis.
MATERIALS AND METHODS
Reagents, antibodies, and plasmids.
MG132 was purchased from Sigma. The anti-E-cadherin antibody was purchased from Transduction Laboratories (Lexington, KY). The anti-MDM2 (N-20) antibody was purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA), and antibodies against hemagglutinin (HA) and Myc were purchased from Roche (Nutly, NJ). The anti-ubiquitin antibody was purchased from Neomark (Fremont, CA). E-cadherin cDNA was inserted into pcDNA6A (Invitrogen) to generate a Myc-His-tagged fusion E-cadherin protein. Expression plasmids for MDM2 and deletion mutants of MDM2 were kind gifts from Jiandong Chen. pAdTrack-CMV/MDM2 was generated by inserting MDM2 into the pAdTrack-CMV vector (encodes enhanced green fluorescent protein [GFP] [EGFP] under the control of a separate cytomegalovirus [CMV] promoter) (33) using the NotI/XhoI restriction enzyme sites. pGEX6p-1/E-cadherin was generated by in-frame cloning of the E-cadherin coding sequence into the pGEX6p-1 plasmid (Pharmacia) using the SmalI/XhoI restriction enzyme sites. The expression vector of MDM2-2B was generated by ligating two separated PCR products into pcDNA6A. The expression plasmids for GFP-tagged dynamin 2 (13), dominant-negative mutant K44A (14), and GFP-tagged EPS15 (7) were kindly provided by Mark A. McNiven and Alexandre Benmerah.
Antibody array.
A standard antibody array was performed according to the manufacturer's procedure (Hypromatrix, Worcester, MA).
Cell culture.
All the cell cultures were maintained in Dulbecco's modified Eagle's medium-F12 (1:1) supplemented with 10% fetal bovine serum at 5% CO2 in a humidified incubator at 37°C. Stable clones were selected and maintained in the above-described medium supplemented with 400 μg/ml G418 or G418 plus 200 μg/ml hygromycin depending on the selection markers.
Patients and tumor specimens.
One hundred thirty archived, formalin-fixed, and paraffin-embedded blocks of infiltrating breast carcinomas were obtained from the Department of Pathology, Shanghai East Breast Disease Hospital, People's Republic of China. All blocks were from female patients who had undergone mastectomy and axillary lymph node dissection between 1988 and 1994 (75).
Immunofluorescence staining and immunohistochemical staining.
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 90 min and then permeabilized for 5 min in PBS containing 0.1% Triton X-100. Cells were sequentially incubated with primary antibodies followed by secondary antibodies diluted in bovine serum albumin-containing PBS as a blocking buffer. Cells were mounted in mounting solution. Immunohistochemical staining was performed as described previously (75). For negative controls, all incubation steps were identical except that PBS instead of primary antibody was used. Tumor samples were examined by light microscopy and scored by an H-score method that combines the values of immunoreaction intensity and the percentages of tumor cell staining as described previously (75).
Preparation of cell lysates, immunoprecipitation, SDS-PAGE, and immunoblotting.
All experiments were performed according to standard protocols. Briefly, for direct Western analysis, cell lysates were prepared with ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 25 mM NaF). After adjusting to equal protein concentrations, samples were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled for 5 min. For coimmunoprecipitation experiments, cell lysates were extracted with NP-40 lysis buffer (components were same as those for RIPA, except that 1% NP-40 was used instead of Triton X-100 and deoxycholate) as described above. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with the indicated antibodies. Proteins were detected by immunoblotting (IB) and visualized by the chemiluminescence method.
In vivo ubiquitination and in vivo [35S]methionine labeling.
Expression plasmids of either wild-type human MDM2 or its RING finger domain deletion mutant were individually cotransfected into HeLa cells with His-tagged E-cadherin and HA-tagged ubiquitin plasmids. His-tagged E-cadherin was pulled down using Ni-nitrilotriacetic acid (NTA) agarose beads and then washed with 8 M urea containing 3 mM imidazole to eliminate nonspecific binding. After separation by SDS-PAGE, membranes were immunoblotted with anti-HA or anti-Ub to detect E-cadherin ubiquitination. E-cadherin stability was determined by a pulse-chase labeling assay. Briefly, cells were preincubated with methionine- and cysteine-free medium containing dialyzed fetal calf serum and labeled for 20 min (pulse) with 35S-mix (NEN) bound to methionine and cysteine followed by an additional incubation with excess unlabeled methionine and cysteine (chase) for the indicated times. Cells were extracted, and lysates were subjected to immunoprecipitation with an anti-E-cadherin antibody. After SDS-PAGE, gels were fixed, dried, and subjected to autoradiography (with an intensifying screen).
Cell surface biotinylation.
Cells were incubated on ice for 1 h with 1.5 mg/ml sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (sulfo-HNS-SS- biotin; Pierce, Rockford, IL). This was followed by washing with sulfo-NHS-SS-biotin blocking reagent (50 mM NH4Cl in PBS containing 1 mM MgCl2 and 0.1 mM CaCl2) to quench free sulfo-NHS-SS-biotin and several additional washes with PBS. Cells were extracted with RIPA buffer, and after centrifugation at 14,000 × g for 10 min at 4°C, the collected supernatant was subjected to a Bradford protein assay to measure protein concentrations. Equal amounts of protein lysates were subjected to pull-down with streptavidin-agarose beads (Pierce), and biotinylated E-cadherin was detected by immunoblotting with an anti-E-cadherin antibody.
Transmission electron microscopy.
The procedures for transmission electron microscopy were performed according to previously published methods (49). Briefly, MCF-7 cells were fixed in 0.4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), following osmication and dehydration. Ultrathin sections of cells were prepared using an ultramicrotome (Reichert E). After blocking with 5% normal goat serum, sections were hybridized with specific primary antibodies. Sections were then incubated with gold particle-labeled goat anti-mouse or an anti-rabbit secondary antibody (Amersham Biosciences). After washing, the sections were stained with uranyl acetate and Reynolds's lead citrate prior to examination using a Jeol 1200EX microscope. The pictures were obtained at the high-resolution electron microscopy facility at the M.D. Anderson Cancer Center.
Subcellular fractionation.
All experiments for subcellular fractionation were carried out as previously described (11). Cell fractionation was carried out at 4°C unless otherwise noted. The postnuclear supernatants (PNS) were obtained by homogenizing cells through a 22-gauge needle with homogenization buffer (HB) containing 250 mM sucrose, 3 mM imidazole (pH 7.4), 1 mM EDTA, protease inhibitors, and cycloheximide. Homogenization was assessed by phase-contrast microscopy. After centrifugation at 3,000 rpm for 10 min, the supernatant was collected as PNS for additional centrifugation using a two-step sucrose gradient. Briefly, the sucrose concentration of the collected PNS was first adjusted to 40.6 to 41% by the addition of 62% sucrose. After thorough mixing, the sucrose-containing PNS was loaded into the bottom of an SW40 centrifuge tube (Beckman). Sucrose cushion overlays at concentrations of 35% and 25% were then added sequentially and topped with HB buffer. The samples were then sedimented in a Beckman Coulter SW40 rotor at 35,000 rpm (14,000 × g) for 1.5 h at 4°C. Fractions were collected, subjected to Bradford protein assay, and analyzed by immunoprecipitation/immunoblotting after diluting with 2× RIPA buffer. Typically, the fraction of the late-endosome sediments accumulate to the interphase between the 8.6% and 25% sucrose concentrations, and the fraction of the early endosome accumulates between the 25% and 35% sucrose concentrations.
Cell motility assay, cell dissociation assay, and invasion assay.
Cell motility assay using time lapse microscopy was conducted using Zeiss Axiovert 200 with a cell observer, AxioCam, and incubator/heating stage. Cells expressing GFP constructs were kept at a constant temperature of 37°C in 5% CO2. Images were taken with intervals ranging 10 to 30 min. The average migrating distances (arbitrary) were measured from a population of GFP-positive cells and control cells using Carl Zeiss AxioVision software analysis. On average, 10 cells from each field and three fields were counted for each experiment. The cell dissociation assay was performed as described elsewhere previously (64), with some modifications. Cells were grown in regular growth medium, fixed, and stained with an anti-E-cadherin antibody for immunofluorescence microscopy. The number of cells based on their attachment to adjacent cells was scored by fluorescence microscopy. The extent of dissociation of the cells was represented by the index Nd/Nt, where Nd is the number of separated cells and Nt is the total number of cells. Cell invasion was examined as described previously (48, 70).
RESULTS
Identification of a protein that binds to MDM2.
In order to identify novel proteins that may interact with MDM2, we performed an antibody array assay using cell lysates from human breast cancer MDA-MB-453 cells and an anti-MDM2 antibody as the probe. Among the antibody candidates, we identified a positive signal in the position on the filter that was immobilized with an anti-E-cadherin antibody.
MDM2 interacts with and facilitates degradation of E-cadherin.
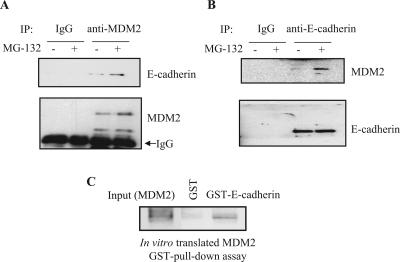
We next confirmed and characterized the interaction between E-cadherin and MDM2 in vitro and in vivo and examined the functional significance of this interaction for protein stability, given the known role of MDM2 as an E3 ligase. The interaction between endogenous E-cadherin and MDM2 was examined by a sequential immunoprecipitation and immunoblotting assay. Endogenous E-cadherin was detected in anti-MDM2 immunoprecipitates (Fig. 1A), and this association was confirmed in the reciprocal immunoprecipitation/immunoblotting experiment (Fig. 1B). These results indicate that endogenous E-cadherin binds to endogenous MDM2. This interaction was enhanced when cells were treated with the proteasome inhibitors MG132 (Fig. 1A and B) and lactacystin (data not shown). Furthermore, in a glutathione S-transferase (GST) pull-down assay, an in vitro-translated MDM2 protein interacted with purified GST-E-cadherin protein (Fig. 1C), which suggests that the interaction between MDM2 and E-cadherin could be direct and independent of a third binding partner.
FIG. 1.
(A and B) Endogenous MDM2 associates with endogenous E-cadherin. Exponentially growing MCF-7 cells were treated with 20 μM MG132, a proteasome inhibitor, for 4 h as indicated. The cell lysates were subjected to immunoprecipitation (IP) with antibodies against MDM2 (N-20) (A), E-cadherin (B), or control immunoglobulin G (IgG). The immunoprecipitates were separated by SDS-PAGE and subjected to IB with an anti-E-cadherin or an anti-MDM2 antibody for the detection of endogenous E-cadherin and MDM2, respectively. (C) MDM2 interacts with E-cadherin directly. In vitro-translated MDM2 was mixed with either purified GST or a GST-E-cadherin fusion protein in RIPA buffer. The reactions were pulled down with glutathione-agarose beads that were then boiled and separated by SDS-PAGE followed by IB with an anti-MDM2 antibody. As a control, 5% of the in vitro-translated MDM2 was loaded into the input lane.
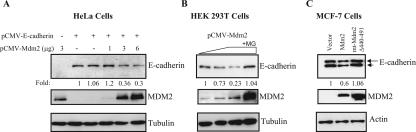
We next examined the effect of MDM2 on the protein level of E-cadherin. Transient transfection assays demonstrated that both exogenous and endogenous E-cadherin levels were decreased as the MDM2 level was increased in a dose-dependent manner (Fig. 2A and B). The E-cadherin protein level was dependent on the ligase activity of MDM2 since an E3 ligase-inactive MDM2 mutant failed to decrease E-cadherin levels (Fig. 2C). The E-cadherin from MCF-7 cells appeared as a double band, the upper part of which represents the unprocessed form of E-cadherin, as also reported previously by others (28). Moreover, the loss of E-cadherin protein was abrogated by using the proteasome inhibitor MG132 (Fig. 2B), suggesting that proteasome degradation is necessary for MDM2-mediated suppression of the E-cadherin protein. Taken together, these results demonstrate that E-cadherin is a novel MDM2-interacting protein that is down-regulated by MDM2.
FIG. 2.
(A) MDM2 facilities the degradation of E-cadherin protein. pCMV-Mdm2, a full-length human MDM2 expression plasmid, was cotransfected at increasing concentrations together with pCMV-E-cadherin into HeLa cells. Cell lysates were analyzed by SDS-PAGE and IB with the indicated antibodies. (B) E-cadherin protein accumulates after treatment with MG132 (+MG), a proteasome inhibitor. HEK 293T cells were transiently transfected with pCMV-Mdm2, and at 48 h after transfection, the cells were treated for 4 h with 20 μM MG132 prior to the detection of endogenous E-cadherin by SDS-PAGE and IB. (C) A deletion mutant of MDM2 fails to down-regulate E-cadherin. MCF-7 cells were transiently transfected with expression plasmids for Mdm2 and deletion mutant Mdm2 (mt-Mdm2 Δ440-491) and cell lysates were subjected to IB with the indicated antibodies. The arrow and arrowhead indicate the positions of the unprocessed and processed forms of E-cadherin, respectively. Quantifications using densitometry are indicated below the respective panels.
MDM2 and E-cadherin protein expression are inversely correlated in primary tumors of breast cancer patients with lymph node metastasis.
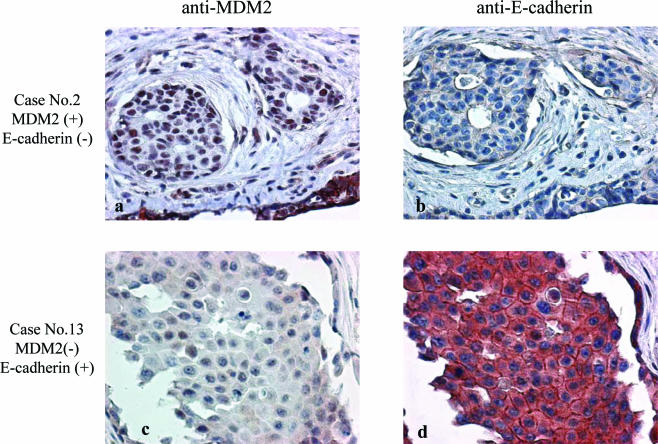
Based on our observation of E-cadherin protein down-regulation by MDM2 in a cell culture system, we further explored the relationship of MDM2 and E-cadherin in primary tumors cells by performing immunohistochemical staining analysis of MDM2 and E-cadherin expression in primary breast cancer tissue from 120 patients. Two subgroups were analyzed: 57 patients were pathologically axillary lymph node (ALN) positive, and 63 patients were ALN negative. Representative immunostaining patterns with anti-MDM2 and anti-E-cadherin are shown in Fig. 3. Case 2 demonstrates negative E-cadherin staining with positive MDM2 staining. The inverse relationship was also observed in tumors such as case 13, which demonstrated positive E-cadherin staining with little or no MDM2 staining. All tissue samples were scored for membrane E-cadherin and cytoplasmic MDM2 staining. Of 57 tissue samples from patients with positive ALN metastases, high MDM2 and low E-cadherin levels were found in 21 tumors, while the inverse staining pattern, with low MDM2 and high E-cadherin levels, was observed in 11 samples. Of 63 samples from patients with a negative ALN status, high MDM2 and low E-cadherin levels were observed in 11 samples, while low MDM2 and high E-cadherin levels were found in 11 samples. Statistical analysis with the Pearson chi-square test using SPSS software suggested that the expression patterns of cytoplasmic MDM2 and membrane E-cadherin are significantly correlated with axillary lymph node metastasis (P = 0.034) (Table 1). Furthermore, we used a logistic regression model to test the hypothesis that having both high MDM2 and low E-cadherin levels is associated with an increased likelihood of ALN metastasis. The results of the logistic regression model shown in Table 2 indicate that patients with low E-cadherin levels were 2.83 times more likely to have ALN metastasis than patients with high E-cadherin levels (P = 0.01). Patients with high MDM2 levels were only 1.26 times more likely to have ALN metastasis than patients with low MDM2 levels (P = 0.66). However, patients with low E-cadherin but high MDM2 levels were 6.83 times more likely to have ALN metastasis than patients with high E-cadherin but low MDM2 levels or neither. Therefore, patients with low E-cadherin but high MDM2 levels are significantly associated with an increased risk of ALN metastasis (P = 0.02). These results indicate that MDM2 expression is significantly correlated with E-cadherin suppression in primary tumors, particularly in those of breast cancer patients with ALN metastases.
FIG. 3.
Overexpression of MDM2 correlates with the down-regulation of E-cadherin. The 120 surgical specimens from breast cancer patients were analyzed by immunohistochemical staining with an anti-MDM2 antibody (a and c), an anti-E-cadherin antibody (b and d), and control serum (data not shown). Consecutive sections were stained using antibodies against MDM2 (Santa Cruz) and E-cadherin (Transduction Laboratories).
TABLE 1.
Expression profiles of cytoplasmic MDM2 and membrane-associated E-cadherin in the surgical specimens of 120 breast cancer patients with or without lymph node metastasesa
| Expression pattern | No. of axillary lymph node metastases (%)
|
||
|---|---|---|---|
| Negative (n = 63) | Positive (n = 57) | Total | |
| High MDM2, low E-cadherin | 11 (9) | 21 (18) | 32 (27) |
| High MDM2, high E-cadherin | 24 (20) | 19 (16) | 43 (36) |
| Low MDM2, high E-cadherin | 11 (9) | 11 (9) | 22 (18) |
| Low MDM2, low E-cadherin | 17 (14) | 6 (5) | 23 (19) |
| Total | 63 (53) | 57 (47) | 120 (100) |
Staining patterns were scored as described in Materials and Methods. Analysis using Pearson chi-square test showed a statistically significant correlation between MDM2 and E-cadherin expression profiles and axillary lymph node status (P = 0.034).
TABLE 2.
High levels of cytoplasmic MDM2 and low levels of membrane-associated E-cadherin are associated with an increased risk for axillary lymph node metastasis in breast cancer patientsa
| Term | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| E-cadherin (low vs high) | 2.83 | 0.81, 9.9 | 0.010 |
| MDM2 (high vs low) | 1.26 | 0.45, 3.54 | 0.66 |
| E-cadherin and MDM2 | 6.83 | 1.42, 32.78 | 0.02 |
A logistic regression analysis was performed to determine if levels of E-cadherin, MDM2, or both conferred an increased risk for axillary lymph node metastasis. The odds ratios and 95% confidence intervals are shown. The reverse correlation between E-cadherin and MDM2 is significantly associated with an increased risk of ALN metastasis (P = 0.02).
Expression of ectopic MDM2 enhances cell dissociation, motility, and invasive activity of breast cancer cells.
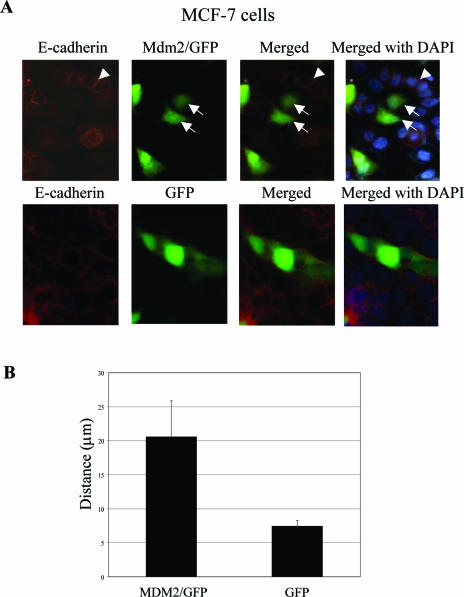
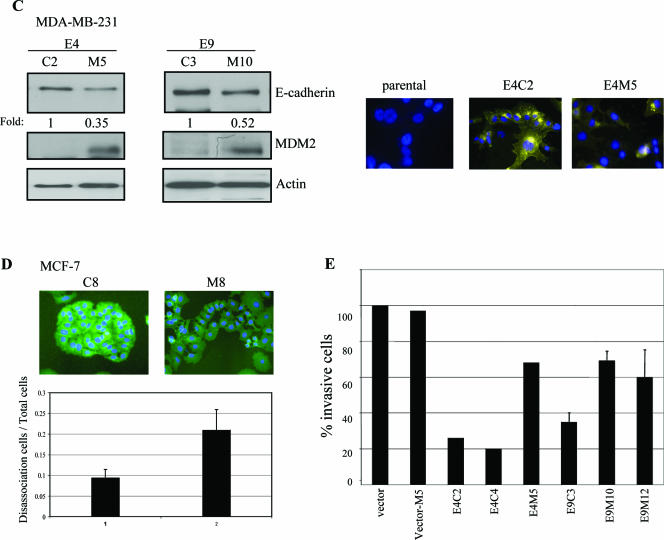
To further characterize the biologic effect of MDM2 expression in human cancer cells, MDM2/GFP expression plasmids or GFP alone (pAdTrack/MDM2) (see Materials and Methods) was transiently transfected into MCF-7 human breast cancer cells and examined by fluorescence microscopy. E-cadherin was visualized by anti-E-cadherin immunostaining. In pAdTrack/MDM2-transfected cultures of MCF-7 cells, cells that did not express GFP were considered mock-transfected control cells, and in these control cells, E-cadherin was found to localize at cell-cell contact sites, with the cells exhibiting the typical clustered epithelial morphology (Fig. 4A, upper panel). In contrast, GFP-expressing cells (which express MDM2) assumed an irregular shape lacking cell-cell contacts, and the E-cadherin staining was very subtle and nearly nondiscernible (Fig. 4A, upper panel). As a control, MCF-7 cells transfected with GFP alone did not exhibit changes in cell morphology compared to parental cells (Fig. 4A, lower panel). We also monitored cell movement by time lapse microscopy. Individual cells that expressed MDM2/GFP demonstrated much greater motility than the GFP control. On average, MDM2/GFP-expressing cells displayed two- to threefold-increased motility compared to those expressing GFP alone (Fig. 4B). To further investigate the biological role of MDM2-mediated regulation of E-cadherin and to determine if p53 is involved in the observed MDM2-induced cell motility, we generated two stable human breast cancer cell lines that expressed MDM2: one was in MCF-7 cells, which are known to express wild-type p53 protein, and the other was in MDA-MB-231 cells, which express codon 280 mutant p53 protein (51). Since MDA-MB-231 cells lack endogenous E-cadherin expression, we transfected these cells with E-cadherin prior to MDM2 to generate stably transfected E-cadherin-expressing cell lines. From several stable clones that expressed exogenous MDM2 (data not shown), two independent MDA-MB-231 clones (E4 and E9) were used for further analyses. Ectopic expression of MDM2 led to reduced levels of E-cadherin (Fig. 4C, left panel), and an alteration of cell morphology was consistent with the loss of cell-cell contacts as visualized by anti-E-cadherin immunostaining (Fig. 4C, right panel, E4C2 versus E4M5). Not surprisingly, there was no detectable E-cadherin by anti-E-cadherin immunostaining in parental MDA-MB-231 cells due to their lack of endogenous E-cadherin expression (Fig. 4C, right panel). Since we observed changes in the morphology of MDM2-expressing cells, we next performed cell dissociation assays as described in Materials and Methods. Control cells were found to display compact cell-cell architecture compared to those that expressed MDM2 (MDA-MB-231 cells in Fig. 4C, right panel, and MCF-7 cells in Fig. 4D). A cell dissociation index (see Materials and Methods) was calculated (dissociated cells/total cells) to quantify this phenomenon. The MDM2-expressing cells had a greater dissociation index, reflecting their increased propensity to scatter away from each other rather than to remain clustered together (Fig. 4D). Furthermore, reexpression of E-cadherin in MDA-MB-231 cells decreased their in vitro invasive ability as expected. Notably, the concomitant expression of MDM2 in these E-cadherin-expressing cells resulted in the partial restoration of their invasive potential (Fig. 4E). Since cell invasion was not further enhanced by expression of MDM2 alone compared to the vector control cells (Fig. 4E, vector versus vector-M5), and MDA-MB-231 cells are known to not harbor wild-type p53, the results suggest that the increased invasive potential imparted by MDM2 is dependent on the down-regulation of E-cadherin and independent of p53. Thus, these observations point to a role for MDM2 in regulating breast cancer cell dissociation, cell motility, and cell invasiveness by a mechanism involving the regulation of E-cadherin function.
FIG.4.
Biological effect of MDM2 overexpression in MCF-7 and MDA-MB-231 cells. (A) MDM2 enhances MCF-7 cell scattering. MCF-7 cells were transiently transfected with pAd-Track-CMV/MDM2 (MDM2/GFP) (upper panel) or pAdTrack (lower panel). After 48 h, cells were fixed and immunostained with an anti-E-cadherin antibody (red). Arrows indicate GFP-positive cells. Arrowheads indicate the cell-cell junctions. (B) The motilities of control (pAd-Track-CMV) and pAd-Track-CMV/MDM2-transfected MCF-7 cells were monitored by time lapse microscopy. The average migrating distances (arbitrary) measured from a population of MDM2-positive cells and control cells using Carl Zeiss AxioVision software are shown. Ten different cells from each field and three fields were calculated. (C) Expression levels of MDM2 and E-cadherin were determined in two representative stably transfected E-cadherin-expressing MDA-MB-231 cell lines (E4 and E9) that were each stably transfected with pAd-Track-CMV/MDM2 (M clones) or pAd-Track-CMV control plasmid (C clones) as indicated. Cell lysates were immunoblotted with the antibodies indicated in each panel. Parental MDA-MB-231 cells and two independent clones were chosen for immunofluorescent staining using an anti-E-cadherin antibody (yellow) and DAPI (4′,6′-diamidino-2-phenylindole) (blue) in the right panel. Photographs were acquired using identical exposure conditions and times. (D) Control and stably transfected MDM2-expressing MCF-7 cells were analyzed by fluorescence microscopy to determine a cell dissociation index as described in Material and Methods. The ratio of the number of separate cells, Nd, to the total number of cells, Nt, was calculated. Cells were scored in 6 to 12 high-power fields for each experiment. Photographs show a representative field. (E) Effect of MDM2 on the invasive ability of MDA-MB-231 cells. The indicated stable transfectants were used for in vitro invasion assays with Matrigel. vector, mock transfectant; Vector-M5, MDM2 transfectant on control cells; E4C2 and E4C4, two independent control vector transfectants on E-cadherin stable clone 4; E4M5, MDM2 transfectant on E-cadherin stable clone 4; E9C3, control vector transfectant on E-cadherin stable clone 9; E9M10 and E9M12, two independent MDM2 transfectants on E-cadherin stable clone 9. Results are the averages of at least two independent experiments; standard deviations are shown for three independent experiments.
MDM2 facilitates ubiquitination of E-cadherin in vivo.
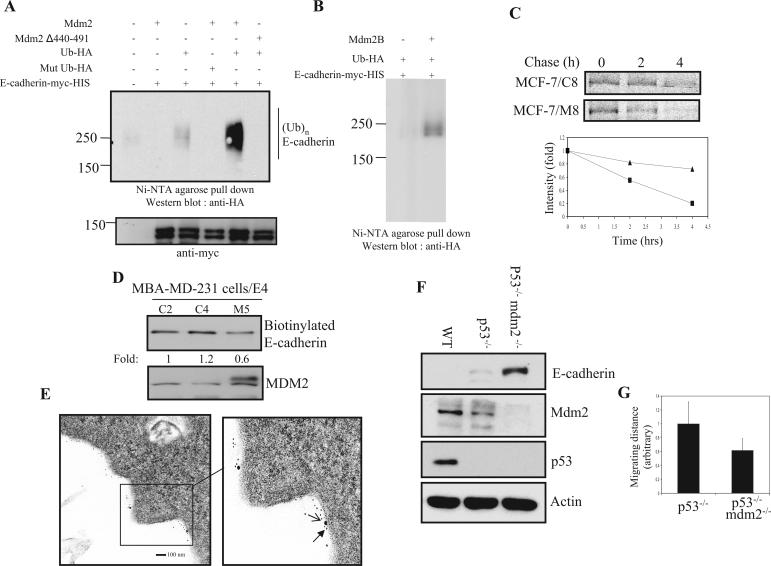
To further elucidate the mechanism by which MDM2 alters E-cadherin protein levels, we next investigated whether MDM2 can function as a ubiquitin ligase for E-cadherin. It has been previously reported that MDM2 possesses a ubiquitin ligase activity for p53 via its RING finger domain, and indeed, an MDM2 mutant that lacks the RING finger domain was shown to have dramatically reduced ubiquitin ligase activity (36-38). As such, expression plasmids of either wild-type human MDM2 or its RING finger domain deletion mutant were separately cotransfected into HEK 293T cells together with Myc-His-tagged E-cadherin and HA-tagged ubiquitin expression plasmids in the presence of MG132 to prevent proteasome degradation. Following Myc-His-E-cadherin pull-down with Ni-NTA agarose beads (which binds His-tagged proteins) and immunoblotting with an anti-HA antibody (which detects HA-ubiquitin moieties), Myc-His-E-cadherin was found to be clearly ubiquitinated when cotransfected with wild-type MDM2 compared to E-cadherin that was cotransfected with the deletion mutant in which no ubiquitination could be detected (Fig. 5A). Consistent with this, E-cadherin ubiquitination was also detected by immunoprecipitating and immunoblotting with an anti-E-cadherin antibody (data not shown). No ubiquitination of E-cadherin could be detected by using mutant Ub, K48R, which prevents the formation of polyubiquitin chains via Lys48 linkages with monoubiquitin molecules (3). The broad E-cadherin band on immunoblots suggests that it is polyubiquitinated. Moreover, an aberrantly spliced MDM2 variant, MDM2-B, which lacks p53-binding activity, is still capable of promoting E-cadherin ubiquitination (Fig. 5B). It further suggested that the effect of E-cadherin on MDM2 is independent of p53. Since the level of E-cadherin was reduced with increasing MDM2 expression, we carried out pulse-chase experiments to determine if MDM2 alters the half-life of the E-cadherin protein (Fig. 5C). Control and stably transfected MDM2-expressing MCF-7 cells were labeled with [35S]methionine/cysteine and subjected to immunoprecipitation with anti-E-cadherin and autoradiography. As shown in Fig. 5C, total E-cadherin turnover was increased in MDM2-expressing cells. Consistent with these results, biotin-labeled cell surface E-cadherin was also reduced in MDM2-expressing cells compared to control cells (Fig. 5D), indicating that the MDM2-induced effect of increasing turnover of the total cellular pool of E-cadherin also affected the stability of the membrane-bound fraction of E-cadherin. Since E-cadherin is known to be a membrane protein, and recent data have shown that MDM2 can ubiquitinate β-arrestin, an adapter for a membrane receptor, we next asked whether E-cadherin and MDM2 could be colocalized at the plasma membrane. Colocalization of MDM2 and E-cadherin at the cell surface could be observed by using immunoelectron microscopy with gold particles of two different sizes that were attached to either an anti-MDM2 or an anti-E-cadherin antibody (Fig. 5E). The staining pattern of E-cadherin closely resembled that previously observed for epithelial growth factor receptor, which was localized at the plasma membrane (63). Therefore, these results indicate that MDM2 functions as a ubiquitin ligase for E-cadherin, affecting the stability of total and membrane-bound E-cadherin. Moreover, these results demonstrate that E-cadherin colocalizes with MDM2 at the plasma membrane. To further elucidate that the effect of E-cadherin by MDM2 is not due to ectopic overexpression, three different mouse embryo fibroblasts (MEF), the wild type, p53−/−, and p53−/−/Mdm2−/−, were used. As shown in Fig. 5F, the protein level of E-cadherin was dramatically increased in p53−/−/Mdm2−/− double knockout MEF compared to the other controls. A slightly reduced MDM2 level in p53−/− MEF was likely due to negative feedback loop regulation as reported previously (76). More importantly, cell motility of double knockout MEF was slower than that of p53−/− alone (Fig. 5G). These data were consistent with our previous observations using human breast cancer cells as a model.
FIG. 5.
MDM2 facilitates the ubiquitination of E-cadherin in vivo. (A) MDM2 induces E-cadherin ubiquitination. HEK 293T cells were cotransfected with an expression plasmid for Myc-His-tagged E-cadherin and the indicated expression plasmids. Cells were treated with MG132. Immunoprecipitation was performed with Ni-NTA agarose beads washed with 8 M urea and subjected to IB with an anti-HA antibody. The filter was stripped and reprobed with an anti-Myc antibody. Mut, mutant; (Ub)n, polyubiquitination. (B) MDM2-B is capable of promoting E-cadherin ubiquitination. The in vivo ubiquitination assay was performed as described above (A). (C) Control and stably transfected MDM2-expressing MCF-7 cells were treated with methionine/cysteine-free medium overnight, pulsed with [35S]Met/Cys treatment for 20 min, and chased for the indicated time intervals. Cell lysates were immunoprecipitated with an anti-E-cadherin antibody, separated by SDS-PAGE, dried, and subjected to autoradiography. Densitometry results were plotted (triangles, control cells; squares, MDM2-overexpressing cells). (D) Cells were labeled with sulfo-NHS-SS-biotin for 30 min on ice, and after quenching with NH4Cl, cells were lysed with RIPA buffer, and biotin-labeled complexes were recovered with streptavidin beads. The samples were subjected to SDS-PAGE and analyzed by immunoblotting (upper panel). Total cell lysates were immunoblotted with an anti-MDM2 antibody (lower panel). (E) Electron microscopy sections were prepared as described in Materials and Methods. MDM2 is represented by the larger particle, while E-cadherin is represented by the smaller particle. Scale bar represents 100 nm. (F) MEF lysates from three different cell lines were analyzed. Immunoblots were hybridized with anti-E-cadherin and anti-MDM2 antibodies. The anti-actin blot served as a protein loading control. WT, wild type. (G) Motility of p53−/− and p53−/−/Mdm2−/− MEF were monitored using time lapse microscopy for 24 h. The average migrating distances (arbitrary) were measured from 10 different cells for each field, and three different fields were subjected to Carl Zeiss AxioVision software analysis.
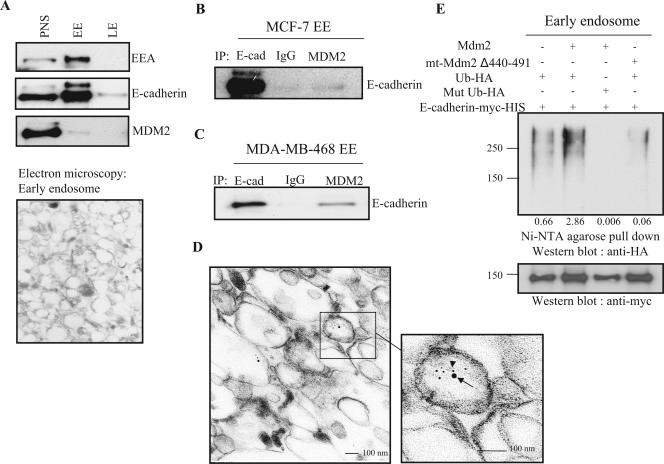
MDM2 and E-cadherin colocalize in the early endosome, where ubiquitin moieties remain attached to E-cadherin.
To further investigate the downstream fate of E-cadherin during endocytosis, we used flotation gradient fractionation to isolate early and late endosomes. The early-endosome marker EEA and electron microscopy were used to confirm the isolation of the endosomes as shown in Fig. 6A (upper and lower panels). MDM2 and E-cadherin appear to be cotransported at least during the early stages of endocytosis, as we found that the two proteins were colocalized after immunoprecipitation of the early-endosome fraction. This observation was confirmed in two different cell lines as shown in Fig. 6B and C. The association of both MDM2 and E-cadherin with the early endosome was further demonstrated by electron microscopy using gold particles of two different sizes labeled with either an anti-MDM2 or an anti-E-cadherin antibody as described above (Fig. 6D). Furthermore, ubiquitin moieties were found to be attached to E-cadherin in early endosomes (Fig. 6E).
FIG. 6.
MDM2 associates with and ubiquitinates E-cadherin in the early endosome. (A) MCF-7 cells were treated with MG132 for 4 h, and cell fractionation was performed as described in Materials and Methods. EE, early endosome; LE, late endosome. (B and C) MDM2 associates with E-cadherin (E-cad) in the early endosome. The early endosomes from MCF-7 cells and MDA-MB-468 cells were isolated as described above and then coimmunoprecipitated and immunoblotted with the indicated antibodies. Immunoglobulin G (IgG) was used as a control. (D) Early endosomes from MCF-7 cells were subjected to electron microscopy staining with an anti-MDM2 antibody (larger particle, arrow) and an anti-E-cadherin antibody (smaller particle, arrowhead). (E) HEK 293T cells were transiently transfected with the indicated expression plasmids. The early endosomes were recovered as described above and then subjected to pull-down with Ni-NTA agarose beads followed by SDS-PAGE and immunoblotting with an anti-HA antibody. Filters were stripped and reprobed with an anti-Myc antibody. The relative intensity was calculated by the entire signal from an anti-HA blot divided by the signal from an anti-Myc blot.
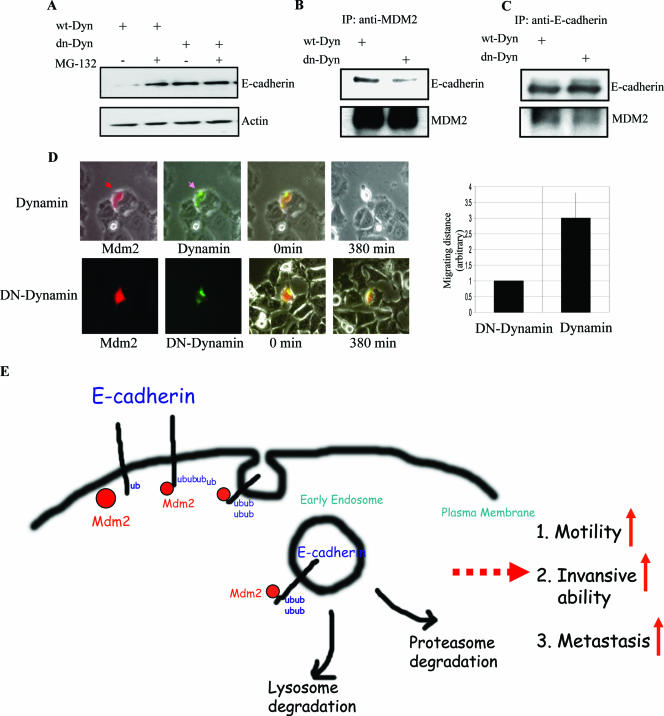
Endocytosis is necessary for MDM2-mediated regulation of E-cadherin function.
Endocytosis of E-cadherin would also appear to play a critical role in its regulation, as its fate would be determined by either terminal degradation or recycling back to the plasma membrane. To address whether endocytosis may be required for the regulation of E-cadherin by MDM2, we first examined the potential role of dynamin, a GTPase whose involvement has been implicated in various types of endocytosis (12, 22, 29). MCF-7 cells were transiently transfected with a well-characterized dominant-negative mutant of dynamin (K44A), which functions as a general endocytosis inhibitor (21, 34). Transfection with dn-dynamin led to a significant increase in E-cadherin protein and interfered with the interaction between E-cadherin and MDM2 (Fig. 7A, B, and C), suggesting that endocytosis is necessary for the degradation of E-cadherin by MDM2. Increased levels of E-cadherin have been shown to correlate with decreased cell motility. As such, we used time lapse microscopy to monitor cell movement for over 6 h. Cells that expressed both MDM2 and wild-type dynamin were observed to detach from neighboring cells after 380 min, while cells that expressed both MDM2 and dn-dynamin retained a greater degree of attachment to other cells as shown in Fig. 7D. The motility of dn-dynamin-expressing cells was significantly suppressed (Fig. 7D, right panel). Together, these results indicate that MDM2 facilitates cell motility by degrading E-cadherin through a mechanism that involves endocytosis. We confirmed that the interaction of MDM2 and E-cadherin is facilitated by endocytosis by using a dominant-negative mutant of Eps15, a Ub-interacting motif-containing protein that has also been implicated in the endocytosis machinery (data not shown; 17, 20, 26). Taken together, these data support a role for dynamin and Eps15 in the regulation of E-cadherin function by MDM2. Overall, our results suggest that MDM2 is capable of ubiquitinating E-cadherin, a process that may serve as a sorting signal for endocytosis, leading to E-cadherin cycling to the early endosome. Furthermore, MDM2 appears to be physically associated with E-cadherin during the endocytic process, and this implicates a possible role for MDM2 in the endocytic machinery.
FIG. 7.
An intact endocytic machinery is required for MDM2 association with E-cadherin and regulation of its function. (A) HEK 293T cells were transiently transfected with wild-type dynamin (wt-Dyn) or dn-dynamin (dn-Dyn) and lysed following treatment with or without 20 μM MG132 for 4 h. Protein extracts were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. (B and C) Dominant-negative mutants of dynamin disrupt the interaction between MDM2 and E-cadherin. HEK 293T cells were transiently transfected with either the wild type or a dominant-negative mutant of dynamin, and after 48 h, cells were treated with MG132 prior to protein extraction, reciprocal coimmunoprecipitation, and immunoblotting with the indicated antibodies. (D) MCF-7 cells were cotransfected with MDM2 expression plasmid and red florescence protein expression plasmid (DsRed) (at a 10:1 ratio) plus pEGFP-dynamin or pEGFP-dn-dynamin. Cells were monitored by time lapse fluorescence microscopy, and photographs were acquired at the indicated times beginning 24 h after transfection. The relative cell migrating distances (arbitrary units) are shown in the right panel. (E) Model proposed to illustrate that E-cadherin degradation by MDM2 is through endocytosis.
DISCUSSION
In this study, we showed that E-cadherin is an MDM2-associated protein that serves as a substrate for MDM2 ligase activity. We demonstrated that MDM2 triggers the ubiquitination of E-cadherin in vivo, leading to the down-regulation of E-cadherin protein levels. Furthermore, our study revealed a previously undescribed function for MDM2 in down-regulating E-cadherin-mediated cell-cell contacts. This is likely to be clinically relevant, as we found a statistically significant correlation between MDM2 overexpression and E-cadherin down-regulation in tumor specimens recovered from human breast cancer patients with lymph node metastases. Our study also points to a novel role for MDM2 in an E-cadherin endocytosis pathway, as MDM2 and E-cadherin were both colocalized at the plasma membrane and in the early endosome. Lending further support to these results, we found that E-cadherin both at the plasma membrane and in early endosomes was ubiquitinated and that an intact endocytic machinery was required for the observed interaction between MDM2 and E-cadherin in the early endosome (Fig. 7E).
MDM2 is an oncogene that is amplified and/or overexpressed in many human cancers including approximately 30% of soft tissue sarcomas and 7% of all solid tumors (54). MDM2 has been shown to function as a negative regulator of p53 by binding to the protein and shuttling it out of the nucleus and into the cytoplasm, where it is degraded by the proteasome. There is an increasing body of evidence suggesting that MDM2 binds to a number of other cellular proteins, and those targeted molecules are further subjected to the proteasome degradation pathway. Furthermore, expression of alternatively or aberrantly spliced MDM2 variants lacking the p53-binding domain have been observed in both human and murine tumors (6, 31). Some of these MDM2 variants appear to retain their capacity to induce tumor formation in Eμ-Myc transgenic mice, indicating that MDM2 may mediate tumorigenesis through p53-independent mechanisms (27). In this study, we have shown that one of variants, MDM2-B, lacks p53-binding activity but is still capable of promoting E-cadherin ubiquitination. Clinical investigators have shown that the amplification of the MDM2 gene occurs more frequently in cancer patients with metastases (23, 46). Thus, in addition to the well-known p53-dependent mechanism, MDM2 down-regulation of E-cadherin may also promote tumor development through a p53-independent mechanism; in addition, the loss of E-cadherin function seems to be directly correlated with the invasive and metastatic potential of cancer cells. Thus, it is evident from both in vitro and clinical studies that reduced or a loss of E-cadherin expression is a common feature shared by both primary tumors and cancer cell lines (41). Aside from silencing gene mutations, several mechanisms could account for the down-regulation of E-cadherin function observed in cancer cells, including promoter hypermethylation, transcriptional repression, proteolytic degradation of the extracellular domain of cadherin, as well as targeting of E-cadherin to a protein degradation pathway. This latter process appears to be mediated by at least two distinct pathways, i.e., the 26S proteasome pathway and the endosome/lysosome recycling pathway. Posttranslational modification by ubiquitination has been established as an important step in both pathways. One recent study has shown that Hakai, a c-Cbl-like E3 ubiquitin ligase, can induce the ubiquitination and the endocytosis of E-cadherin in epithelial cells in response to Src activation and has thus implicated Hakai as a potential physiologic regulator of cell adhesion (28). Another study reported that during an Src-induced epithelial-to-mesenchymal transition, the ubiquitin tagging of E-cadherin is a necessary step for proper sorting to the lysosome and for subsequent degradation (59). The results of our current study lead us to conclude that under pathological conditions such as cancer, the overexpression of MDM2 with a concomitant down-regulation of E-cadherin may be a potential p53-independent mechanism underlying tumorigenesis and metastasis, as we also observed that E-cadherin was ubiquitinated by MDM2-B, a spliced variant of MDM2 lacking p53-binding ability (Fig. 5B).
Although it is believed that the monoubiquitination of integral plasma membrane proteins and of endocytic machinery proteins may serve as a sorting signal for their internalization to the endocytic pathway, polyubiquitination of cytosolic proteins appears to be essential for degradation via the 26S proteasome. Important questions that remain to be elucidated include how and what type of ubiquitin modification that occurs on the substrate is determined and how these modifications ultimately lead to distinct cellular outcomes. First, the type of modification may depend upon whether E3 is stably or transiently associated with the substrate, as a stable interaction could allow for possible polymerization of an ubiquitin chain. Second, the interaction of a specific adaptor protein with its ubiquitinated partner may specify their intracellular trafficking destination and ultimately their mode of degradation. While others have previously shown that monoubiquitination is sufficient for ligand-mediated receptor endocytosis (35), we detected the polyubiquitinated form of E-cadherin at the plasma membrane (data not shown) and in the early endosomal compartment, which suggests that polyubiquitination of proteins may also serve as a signal for internalization via the endocytic pathway. In addition, we found that the proteasome inhibitor MG132 blocked E-cadherin degradation by MDM2, which indicates that the polyubiquitinated form of E-cadherin undergoes degradation by a proteasome pathway.
Other than by direct degradation of E-cadherin, the proteasome may be involved in regulating one or more steps of the endocytic pathway. As such, an indirect role for proteasome-mediated E-cadherin degradation by acting as a sorting signal to lysosomal degradation cannot be excluded. However, the questions remain open as to which endosomal compartment is actually responsible for directing E-cadherin toward lysosomal degradation and how MDM2 might be involved in signaling this process. When MDM2, as an E3 ligase, catalyzes the ubiquitination of β-arrestin, the β2-adrenerigic receptor is rapidly internalized. Because MDM2 can potentially ubiquitinate either β2-AR or β-arrestin, it appears that the ubiquitination of β-arrestin plays a more important role in the process of rapid endocytosis. While β-arrestin is not known to be a component of the transport machinery, it may instead function as a ubiquitinated transport modifier for certain proteins such as the β2-AR. Presently, it is unknown whether such a modifier exists for the MDM2-mediated E-cadherin endocytosis observed here.
Because of the relatively high frequency of MDM2 overexpression in human breast cancers as well as its known role in regulating the cell cycle, it is reasonable to suggest that MDM2 may be a key player in controlling cancer initiation, growth, progression, and metastasis. In line with this, there have been many recent studies that have examined MDM2 as a potential target for human cancer therapy. Although these strategies are driven by the most current and advanced molecular approaches, including the use of small-molecule inhibitors that disrupt the interaction between MDM2 and p53, we may ultimately discover that these therapeutics have limited anticancer efficacy, as it is already becoming clear that MDM2 serves multiple functions, and targeting the interaction of MDM2 with p53 as well as with other critical molecules, such as E-cadherin, will likely be necessary. In conclusion, our findings here not only contribute to the basic understanding of MDM2 functions but also reveal novel strategic approaches to cancer treatment.
Acknowledgments
We thank Jiandong Chen (H. Lee Moffitt Comprehensive Cancer Center and Research Institute, Tampa, FL), Mark A. McNiven, and Alexandre Benmerah for their generosity in providing the expression plasmids. We thank Guillermina Lozano for the knockout MEF cells. We also thank Dipak K Giri, Peter Zhou, and Lei Shen for their early contributions to this work and Ulrich Hermanto and Jeng C. Cheng for editing the manuscript.
This work was supported by grants from NIH P01 CA 099031 and M. D. Anderson Cancer Center Core Grant CA16672 and was also partially supported by the National Breast Cancer Foundation and the Patel Memorial Breast Cancer Research Foundation.
REFERENCES
- 1.Aguilar, R. C., and B. Wendland. 2005. Endocytosis of membrane receptors: two pathways are better than one. Proc. Natl. Acad. Sci. USA 102:2679-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, R. C., and B. Wendland. 2003. Ubiquitin: not just for proteasomes anymore. Curr. Opin. Cell Biol. 15:184-190. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli, A., R. Crinelli, M. Bianchi, A. Cerasi, L. Gentilini, G. Serafini, and M. Magnani. 1999. Efficient inhibition of macrophage TNF-alpha production upon targeted delivery of K48R ubiquitin. Br. J. Haematol. 104:475-481. [DOI] [PubMed] [Google Scholar]
- 4.Arora, S., R. Mathew, M. Mathur, T. K. Chattopadhayay, and R. Ralhan. 2001. Alterations in MDM2 expression in esophageal squamous cell carcinoma: relationship with p53 status. Pathol. Oncol. Res. 7:203-208. [DOI] [PubMed] [Google Scholar]
- 5.Bardeesy, N., D. Falkoff, M. J. Petruzzi, N. Nowak, B. Zabel, M. Adam, M. C. Aguiar, P. Grundy, T. Shows, and J. Pelletier. 1994. Anaplastic Wilms' tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat. Genet. 7:91-97. [DOI] [PubMed] [Google Scholar]
- 6.Bartel, F., H. Taubert, and L. C. Harris. 2002. Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell 2:9-15. [DOI] [PubMed] [Google Scholar]
- 7.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berx, G., A. M. Cleton-Jansen, F. Nollet, W. J. de Leeuw, M. van de Vijver, C. Cornelisse, and F. van Roy. 1995. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 14:6107-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birchmeier, W., and J. Behrens. 1994. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 1198:11-26. [DOI] [PubMed] [Google Scholar]
- 10.Bolos, V., H. Peinado, M. A. Perez-Moreno, M. F. Fraga, M. Esteller, and A. Cano. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116:499-511. [DOI] [PubMed] [Google Scholar]
- 11.Bomsel, M., R. Parton, S. A. Kuznetsov, T. A. Schroer, and J. Gruenberg. 1990. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62:719-731. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. [DOI] [PubMed] [Google Scholar]
- 13.Cao, H., F. Garcia, and M. A. McNiven. 1998. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9:2595-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, H., H. M. Thompson, E. W. Krueger, and M. A. McNiven. 2000. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J. Cell Sci. 113:1993-2002. [DOI] [PubMed] [Google Scholar]
- 15.Chan, J. K., and C. S. Wong. 2001. Loss of E-cadherin is the fundamental defect in diffuse-type gastric carcinoma and infiltrating lobular carcinoma of the breast. Adv. Anat. Pathol. 8:165-172. [DOI] [PubMed] [Google Scholar]
- 16.Chen, H., and P. De Camilli. 2005. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc. Natl. Acad. Sci. USA 102:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, H., S. Fre, V. I. Slepnev, M. R. Capua, K. Takei, M. H. Butler, P. P. Di Fiore, and P. De Camilli. 1998. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394:793-797. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, C. W., P. E. Wu, J. C. Yu, C. S. Huang, C. T. Yue, C. W. Wu, and C. Y. Shen. 2001. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 20:3814-3823. [DOI] [PubMed] [Google Scholar]
- 19.Christofori, G., and H. Semb. 1999. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 24:73-76. [DOI] [PubMed] [Google Scholar]
- 20.Confalonieri, S., A. E. Salcini, C. Puri, C. Tacchetti, and P. P. Di Fiore. 2000. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 150:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damke, H., D. D. Binns, H. Ueda, S. L. Schmid, and T. Baba. 2001. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol. Biol. Cell 12:2578-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta, M. W., E. Macri, S. Signoretti, A. A. Renshaw, and M. Loda. 2001. Transition from in situ to invasive testicular germ cell neoplasia is associated with the loss of p21 and gain of mdm-2 expression. Mod. Pathol. 14:437-442. [DOI] [PubMed] [Google Scholar]
- 24.Dei Tos, A. P., C. Doglioni, S. Piccinin, R. Sciot, A. Furlanetto, M. Boiocchi, P. Dal Cin, R. Maestro, C. D. Fletcher, and G. Tallini. 2000. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. J. Pathol. 190:531-536. [DOI] [PubMed] [Google Scholar]
- 25.Fontana, X., P. Ferrari, M. Abbes, J. Monticelli, M. Namer, and F. Bussiere. 1994. Study of mdm2 gene amplification in primary breast tumors. Bull. Cancer 81:587-592. (In French.) [PubMed] [Google Scholar]
- 26.Ford, M. G., I. G. Mills, B. J. Peter, Y. Vallis, G. J. Praefcke, P. R. Evans, and H. T. McMahon. 2002. Curvature of clathrin-coated pits driven by epsin. Nature 419:361-366. [DOI] [PubMed] [Google Scholar]
- 27.Fridman, J. S., E. Hernando, M. T. Hemann, E. de Stanchina, C. Cordon-Cardo, and S. W. Lowe. 2003. Tumor promotion by Mdm2 splice variants unable to bind p53. Cancer Res. 63:5703-5706. [PubMed] [Google Scholar]
- 28.Fujita, Y., G. Krause, M. Scheffner, D. Zechner, H. E. Leddy, J. Behrens, T. Sommer, and W. Birchmeier. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4:222-231. [DOI] [PubMed] [Google Scholar]
- 29.Giri, D. K., M. Ali-Seyed, L. Y. Li, D. F. Lee, P. Ling, G. Bartholomeusz, S. C. Wang, and M. C. Hung. 2005. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol. Cell. Biol. 25:11005-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girnita, L., A. Girnita, and O. Larsson. 2003. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl. Acad. Sci. USA 100:8247-8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris, L. C. 2005. MDM2 splice variants and their therapeutic implications. Curr. Cancer Drug Targets 5:21-26. [DOI] [PubMed] [Google Scholar]
- 32.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 33.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 36.Honda, R., H. Tanaka, and H. Yasuda. 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420:25-27. [DOI] [PubMed] [Google Scholar]
- 37.Honda, R., and H. Yasuda. 2000. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene 19:1473-1476. [DOI] [PubMed] [Google Scholar]
- 38.Honda, R., and H. Yasuda. 1999. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 18:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeguchi, M., T. Ueda, K. Fukuda, K. Yamaguchi, S. Tsujitani, and N. Kaibara. 2002. Expression of the murine double minute gene 2 oncoprotein in esophageal squamous cell carcinoma as a novel marker for lack of response to chemoradiotreatment. Am. J. Clin. Oncol. 25:454-459. [DOI] [PubMed] [Google Scholar]
- 40.Iwakuma, T., and G. Lozano. 2003. MDM2, an introduction. Mol. Cancer Res. 1:993-1000. [PubMed] [Google Scholar]
- 41.Jiang, W. G., and R. E. Mansel. 2000. E-cadherin complex and its abnormalities in human breast cancer. Surg. Oncol. 9:151-171. [DOI] [PubMed] [Google Scholar]
- 42.Kim, K. J., B. Li, J. Winer, M. Armanini, N. Gillett, H. S. Phillips, and N. Ferrara. 1993. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841-844. [DOI] [PubMed] [Google Scholar]
- 43.Koga, T., S. Hashimoto, K. Sugio, I. Yoshino, K. Nakagawa, Y. Yonemitsu, K. Sugimachi, and K. Sueishi. 2001. Heterogeneous distribution of P53 immunoreactivity in human lung adenocarcinoma correlates with MDM2 protein expression, rather than with P53 gene mutation. Int. J. Cancer 95:232-239. [DOI] [PubMed] [Google Scholar]
- 44.Korkolopoulou, P., P. Christodoulou, P. Kapralos, M. Exarchakos, A. Bisbiroula, M. Hadjiyannakis, C. Georgountzos, and E. Thomas-Tsagli. 1997. The role of p53, MDM2 and c-erb B-2 oncoproteins, epidermal growth factor receptor and proliferation markers in the prognosis of urinary bladder cancer. Pathol. Res. Pract. 193:767-775. [DOI] [PubMed] [Google Scholar]
- 45.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 46.Ladanyi, M., C. Cha, R. Lewis, S. C. Jhanwar, A. G. Huvos, and J. H. Healey. 1993. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 53:16-18. [PubMed] [Google Scholar]
- 47.Leptin, M. 1991. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5:1568-1576. [DOI] [PubMed] [Google Scholar]
- 48.Li, Y. M., Y. Pan, Y. Wei, X. Cheng, B. P. Zhou, M. Tan, X. Zhou, W. Xia, G. N. Hortobagyi, D. Yu, and M. C. Hung. 2004. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 6:459-469. [DOI] [PubMed] [Google Scholar]
- 49.Lo, H. W., S. C. Hsu, M. Ali-Seyed, M. Gunduz, W. Xia, Y. Wei, G. Bartholomeusz, J. Y. Shih, and M. C. Hung. 2005. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 7:575-589. [DOI] [PubMed] [Google Scholar]
- 50.Lukas, J., D. Q. Gao, M. Keshmeshian, W. H. Wen, D. Tsao-Wei, S. Rosenberg, and M. F. Press. 2001. Alternative and aberrant messenger RNA splicing of the mdm2 oncogene in invasive breast cancer. Cancer Res. 61:3212-3219. [PubMed] [Google Scholar]
- 51.Lyakhovich, A., and M. P. Shekhar. 2003. Supramolecular complex formation between Rad6 and proteins of the p53 pathway during DNA damage-induced response. Mol. Cell. Biol. 23:2463-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew, R., S. Arora, R. Khanna, M. Mathur, N. K. Shukla, and R. Ralhan. 2002. Alterations in p53 and pRb pathways and their prognostic significance in oesophageal cancer. Eur. J. Cancer 38:832-841. [DOI] [PubMed] [Google Scholar]
- 53.McCann, A. H., A. Kirley, D. N. Carney, N. Corbally, H. M. Magee, G. Keating, and P. A. Dervan. 1995. Amplification of the MDM2 gene in human breast cancer and its association with MDM2 and p53 protein status. Br. J. Cancer 71:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Momand, J., D. Jung, S. Wilczynski, and J. Niland. 1998. The MDM2 gene amplification database. Nucleic Acids Res. 26:3453-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore, L., S. Venkatachalam, H. Vogel, J. C. Watt, C. L. Wu, H. Steinman, S. N. Jones, and L. A. Donehower. 2003. Cooperativity of p19ARF, Mdm2, and p53 in murine tumorigenesis. Oncogene 22:7831-7837. [DOI] [PubMed] [Google Scholar]
- 56.Morgan, R. J., P. V. Newcomb, R. H. Hardwick, and D. Alderson. 1999. Amplification of cyclin D1 and MDM-2 in oesophageal carcinoma. Eur. J. Surg. Oncol. 25:364-367. [DOI] [PubMed] [Google Scholar]
- 57.Nieto, M. A. 2002. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3:155-166. [DOI] [PubMed] [Google Scholar]
- 58.Osman, I., M. Drobnjak, M. Fazzari, J. Ferrara, H. I. Scher, and C. Cordon-Cardo. 1999. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin. Cancer Res. 5:2082-2088. [PubMed] [Google Scholar]
- 59.Palacios, F., J. S. Tushir, Y. Fujita, and C. D'Souza-Schorey. 2005. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 25:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paterson, A. D., R. G. Parton, C. Ferguson, J. L. Stow, and A. S. Yap. 2003. Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 278:21050-21057. [DOI] [PubMed] [Google Scholar]
- 61.Saito, H., S. Tsujitani, S. Oka, M. Ikeguchi, M. Maeta, and N. Kaibara. 2002. The expression of murine double minute 2 is a favorable prognostic marker in esophageal squamous cell carcinoma without p53 protein accumulation. Ann. Surg. Oncol. 9:450-456. [DOI] [PubMed] [Google Scholar]
- 62.Shenoy, S. K., P. H. McDonald, T. A. Kohout, and R. J. Lefkowitz. 2001. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307-1313. [DOI] [PubMed] [Google Scholar]
- 63.Sigismund, S., T. Woelk, C. Puri, E. Maspero, C. Tacchetti, P. Transidico, P. P. Di Fiore, and S. Polo. 2005. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skoudy, A., M. D. Llosas, and A. Garcia de Herreros. 1996. Intestinal HT-29 cells with dysfunction of E-cadherin show increased pp60src activity and tyrosine phosphorylation of p120-catenin. Biochem. J. 317:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinman, H. A., E. Burstein, C. Lengner, J. Gosselin, G. Pihan, C. S. Duckett, and S. N. Jones. 2004. An alternative splice form of Mdm2 induces p53-independent cell growth and tumorigenesis. J. Biol. Chem. 279:4877-4886. [DOI] [PubMed] [Google Scholar]
- 66.Strous, G. J., and J. A. Schantl. 2001. Beta-arrestin and Mdm2, unsuspected partners in signaling from the cell surface. Sci. STKE 2001:PE41. [DOI] [PubMed] [Google Scholar]
- 67.Swaroop, M., and Y. Sun. 2003. Mdm2 ligase dead mutants did not act in a dominant negative manner to re-activate p53, but promoted tumor cell growth. Anticancer Res. 23:3167-3174. [PubMed] [Google Scholar]
- 68.Takahashi, Y., Y. Kitadai, C. D. Bucana, K. R. Cleary, and L. M. Ellis. 1995. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 55:3964-3968. [PubMed] [Google Scholar]
- 69.Takeichi, M. 1993. Cadherins in cancer: implications for invasion and metastasis. Curr. Opin. Cell Biol. 5:806-811. [DOI] [PubMed] [Google Scholar]
- 70.Tan, M., J. Yao, and D. Yu. 1997. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 57:1199-1205. [PubMed] [Google Scholar]
- 71.Thiery, J. P. 2003. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15:740-746. [DOI] [PubMed] [Google Scholar]
- 72.Thiery, J. P., and D. Chopin. 1999. Epithelial cell plasticity in development and tumor progression. Cancer Metast. Rev. 18:31-42. [DOI] [PubMed] [Google Scholar]
- 73.Vargas, D. A., S. Takahashi, and Z. Ronai. 2003. Mdm2: a regulator of cell growth and death. Adv. Cancer Res. 89:1-34. [DOI] [PubMed] [Google Scholar]
- 74.Vassilev, L. T., B. T. Vu, B. Graves, D. Carvajal, F. Podlaski, Z. Filipovic, N. Kong, U. Kammlott, C. Lukacs, C. Klein, N. Fotouhi, and E. A. Liu. 2004. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844-848. [DOI] [PubMed] [Google Scholar]
- 75.Xia, W., J. S. Chen, X. Zhou, P. R. Sun, D. F. Lee, Y. Liao, B. P. Zhou, and M. C. Hung. 2004. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin. Cancer Res. 10:3815-3824. [DOI] [PubMed] [Google Scholar]
- 76.Zauberman, A., D. Flusberg, Y. Haupt, Y. Barak, and M. Oren. 1995. A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic Acids Res. 23:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang, L., and R. P. Hill. 2004. Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res. 64:4180-4189. [DOI] [PubMed] [Google Scholar]
- 78.Zhou, B. P., J. Deng, W. Xia, J. Xu, Y. M. Li, M. Gunduz, and M. C. Hung. 2004. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6:931-940. [DOI] [PubMed] [Google Scholar]
- 79.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]
- 80.Zietz, C., M. Rossle, C. Haas, A. Sendelhofert, A. Hirschmann, M. Sturzl, and U. Lohrs. 1998. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am. J. Pathol. 153:1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]