Abstract
Sphingosine kinase (Sphk) enzymes are important in intracellular sphingolipid metabolism as well as in the biosynthesis of sphingosine 1-phosphate (S1P), an extracellular lipid mediator. Here, we show that Sphk1 is expressed and is required for small intestinal tumor cell proliferation in ApcMin/+ mice. Adenoma size but not incidence was dramatically reduced in ApcMin/+ Sphk−/− mice. Concomitantly, epithelial cell proliferation in the polyps was significantly attenuated, suggesting that Sphk1 regulates adenoma progression. Although the S1P receptors (S1P1R, S1P2R, and S1P3R) are expressed, polyp incidence or size was unaltered in ApcMin/+ S1p2r−/−, ApcMin/+ S1p3r−/−, and ApcMin/+ S1p1r+/− bigenic mice. These data suggest that extracellular S1P signaling via its receptors is not involved in adenoma cell proliferation. Interestingly, tissue sphingosine content was elevated in the adenomas of ApcMin/+ Sphk1−/− mice, whereas S1P levels were not significantly altered. Concomitantly, epithelial cell proliferation and the expression of the G1/S cell cycle regulator CDK4 and c-myc were diminished in the polyps of ApcMin/+ Sphk1−/− mice. In rat intestinal epithelial (RIE) cells in vitro, Sphk1 overexpression enhanced cell cycle traverse at the G1/S boundary. In addition, RIE cells treated with sphingosine but not C6-ceramide exhibited reduced cell proliferation, reduced retinoblastoma protein phosphorylation, and cyclin-dependent kinase 4 (Cdk4) expression. Our findings suggest that Sphk1 plays a critical role in intestinal tumor cell proliferation and that inhibitors of Sphk1 may be useful in the control of intestinal cancer.
The concept that sphingolipid metabolism is an important source of signaling lipids has gained considerable acceptance. For example, sphingomyelin, an abundant membrane phospholipid, is metabolized into lipid mediators such as sphingosine, ceramide, ceramide 1-phosphate, and sphingosine 1-phosphate (S1P) (9). S1P, synthesized by the action of sphingosine kinase (Sphk), is secreted by cells and functions as an extracellular mediator by activating a family of G-protein-coupled receptors termed S1PR1-5 (13, 19, 30). The generality of S1P signaling in vertebrates is underscored by recent findings that S1PRs are needed for a multitude of physiological processes, including heart and vascular development, angiogenesis, and immune cell trafficking (12). However, the physiological and pathological significance of intracellular sphingolipid metabolism in vertebrates is virtually unknown. However, a recent study showed that the inhibition of S1P lyase resulted in alterations in tissue S1P levels, which influenced T-cell trafficking and immune responses (35). The importance of sphingolipid metabolism is underscored by its impact on cell death, stress responses, metabolism, and animal development in unicellular (Saccharomyces cerevisiae and Dictyostelium discoidium) and multicellular (Caenorhabditis elegans and Drosophila melanogaster) eukaryotes (4, 5, 10, 21, 26, 28).
Mammals express two functional Sphk isoenzymes, Sphk1 and Sphk2. Sphk isoenzymes are regulated by extracellular signals such as cytokines, growth factors, tumor promoters, and hormones and ultimately influence the activity of signaling molecules, transcriptional responses, cell proliferation, apoptosis, and oncogenesis (37). However, the role of Sphk in normal physiology and disease has not been addressed, even though mice deficient in Sphk1 appear normal with reduced tissue and plasma S1P levels (1). Deletion of Sphk1 and Sphk2 resulted in undetectable S1P levels and embryonic lethality (24), suggesting that Sphk isoenzymes are indispensable for the formation of S1P, an essential lipid mediator in embryonic development.
The role of S1P and Sphk isoenzymes in cancer is not well understood. Sphingosine and ceramide are generally growth inhibitory and induce cell death, whereas S1P acts in an opposite manner as an inducer of cell proliferation and survival. Nutritional studies have shown that dietary sphingomyelin inhibits intestinal cancer in murine models (33, 34). However, mechanisms by which this is achieved are not clear. Dietary sphingomyelin is degraded in the intestinal tract to ceramide and sphingosine by the action of sphingomyelinase and ceramidase, respectively (16, 25). Sphingosine is then taken up by the intestinal absorptive cells and is further metabolized, presumably by the Sphk and S1P lyase enzymes. Interestingly, neutral sphingomyelinase expression in the intestinal epithelial cells is downregulated during colorectal cancer (11). Sphk1 activity is induced by tumor promoters and oncogenes, and the overexpression of Sphk1 promotes NIH 3T3 cell transformation in vitro (28, 37). These studies suggest that sphingolipid metabolism influences tumorigenesis in general and intestinal cancer in particular, even though the mechanisms involved are poorly defined.
In this report, we addressed the role of Sphk1 in intestinal tumorigenesis. We show that Sphk as well as S1P receptors are expressed in intestinal tumor tissues. Deletion of the Sphk1 gene in ApcMin/+ mice resulted in the profound suppression of adenoma size but not incidence. Surprisingly, deletion of S1p2R and S1p3R and the loss of an allele of S1p1R did not phenocopy the Sphk1 deletion. Moreover, intracellular levels of S1P were not significantly altered, whereas the levels of the substrate sphingosine were elevated in Sphk1 null mice, suggesting that intracellular metabolism of sphingolipids is critical in adenoma progression.
MATERIALS AND METHODS
Mice.
Sphk1−/−, S1pr2−/−, S1pr3−/−, and S1pr1+/− mice in the 129sv-C57BL/6 background (1, 17) were derived as described in previous reports. ApcMin/+ mice (C57BL/6J background) were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were maintained on LM-485 Mouse/Rat Sterilizable Diet (Harlan Teklad, Indianapolis, IN) in the Center for Laboratory Animal Care at the University of Connecticut Health Center. These mice were crossbred to derive littermates. Analysis of intestinal polyps was performed on F2-generation mice, and littermates with similar genetic backgrounds were used for comparison.
Genotyping.
S1pr2, S1pr3, and S1pr1 and Sphk1 knockout mouse genotypes were determined by PCR analyses of genomic DNA isolated from tail biopsies (1, 17).
Analysis of intestinal polyps.
At 140 to 147 days of age, animals were sacrificed, and the intestinal tract was subdivided into five segments consisting of four parts of small intestine (duodenum, proximal jejunum, distal jejunum, and ileum) and the large intestine. Each segment was incised along its longitudinal axis and fixed, polyps were counted, and tumor diameter was measured with a dissecting microscope (StemiSV6; Carl Zeiss) at ×10 magnification. After fixation with 4% paraformaldehyde and/or 75% ethanol, the tissues were embedded in paraffin for histological analysis. Some individual tumors were selected for further molecular analysis. In these cases, after the intestinal segments were opened, polyps or mucosa were excised from the small intestine and flash frozen in liquid nitrogen for RNA extraction. Northern blot analysis of total RNA was conducted as described previously (15).
Production and purification of Sphk1 polyclonal antibody.
Rabbit polyclonal antibody against human Sphk1 was raised in rabbits with purified glutathione S-transferase-Sphk1 protein, and antiserum was obtained. Subsequently, the antibody against Sphk1 was affinity purified by protein A-Sepharose chromatography.
Western blot for Sphk1.
Cell extracts (10 μg) from mock and mouse Sphk1-, mouse Sphk-2-, human Sphk1-, and human Sphk-2-expressing HEK-293T cells were analyzed for expression of cognate proteins and reactivity to the purified Sphk1 antibody (1:2,000 dilution; 1.25 μg/ml) by an immunoblot analysis procedure described previously (31).
Immunohistochemistry (IHC) of human colon cancer tissues.
Formalin-fixed and paraffin-embedded human colon carcinoma (n = 27) specimens were immunostained with anti-Sphk1 antibody (1:200) as described previously (29).
For the analysis of polyps from ApcMin/+ mice, immunohistochemical analysis was conducted with anti-Sphk1 antibody (1:1,000), β-catenin (1:80) (Santa Cruz), cyclooxygenase 2 (COX-2) (1:300) (Cayman chemical), phospho-p44/42 extracellular signal-regulated kinase (ERK) (1:100) (Cell Signaling Technology, Beverly, MA), phospho-p38 mitogen-activated protein (MAP) kinase (1:100) (Cell Signaling Technology), Cdk4 (1:40) (Santa Cruz), c-myc (1:200) (Santa Cruz), PCNA (1:200) (Ki67; Calbiochem), villin (1:200) (Chemicon), and alkaline phosphatase (1:500) (Biotrend) with a Vectastain ABC kit (Vector) according to the manufacturer's protocol. The peroxidase staining was visualized with 3,3′-diaminobenzidine (Vector), and the sections were counterstained with methyl green.
BrdU incorporation assay.
Animals were injected intraperitoneally with 1 ml of 10 mM 5′-bromo-2′-deoxyuridine (BrdU) in phosphate-buffered saline 1 h before sacrifice as described previously (2). After sacrifice, intestinal tissues were fixed in 75% ethanol and embedded in paraffin. Slides were analyzed as described previously (2). The polyps were visualized by light microscopy (Axioskop2; Carl Zeiss) at ×100 magnification using Axiovision 4 software (Carl Zeiss), and the images were printed out. On the printed images, the total and BrdU-labeled nuclei of the epithelial cells were counted in the whole-polyp cross-section except for the basal region. The BrdU labeling index is defined as the percentage of epithelial cells that are BrdU positive.
Apoptosis assay.
After fixation with 4% paraformaldehyde and embedding in paraffin, sections of polyps from ApcMin/+ Sphk1+/+ or ApcMin/+ Sphk1−/− mice were evaluated for apoptosis with a commercial apoptosis detection kit (Chemicon International, Temecula, CA) according to the manufacturer's instructions. Positive signals from the luminal edge were not considered in the analysis.
Intratumoral MVD.
Polyps from ApcMin/+ Sphk1+/+ or ApcMin/+ Sphk1−/− mice were stained with rat monoclonal anti-PECAM-1 antibody (Chemicon) by the peroxidase method. Microvessel density (MVD) assessment was determined as previously reported (42). Briefly, the microscopic field that contained the highest number of capillaries was chosen for each sample by an initial scan at ×100 magnification. The vessels were counted at ×400 magnification from multiple random fields.
Measurement of S1P and sphingosine.
Lipids extracted from tissue homogenates or plasma were measured by high-performance liquid chromatography (HPLC) as described previously (23). Briefly, S1P was dephosphorylated by alkaline phosphatase, and the released sphingosine was derivatized with o-phthalaldehyde. The o-phthalaldehyde derivatives were then fluorometrically analyzed by HPLC at an excitation wavelength of 340 nm and an emission wavelength of 455 nm. For liquid chromatography/tandem mass spectrometry (LC/MS/MS) studies, tissue extracts were processed as described previously (8).
Real-time reverse transcriptase PCR.
Total RNA was isolated from the intestinal polyps using RNA-Stat 60 reagent (Tel Test, Friendswood, TX). Total RNA (1 μg) was analyzed for the transcripts of Cdk4 and c-myc and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as described previously (31).
Cell cycle analysis of RIE cells.
RIE cells were a kind gift of Raymond DuBois, Vanderbilt University Medical Center, Nashville, TN. They were grown as described previously (7) and serum starved in 0.5% fetal bovine serum (FBS). Cell cycle analysis was conducted using fluorescence-activated cell sorter (FACS) analysis as described previously (41). Cell growth was determined using the3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (15). Cell extracts were analyzed for immunoblot analysis using the above-mentioned antibodies.
Statistical analysis.
Statistical analysis was performed using Fisher's exact test to compare the expression level of Sphk1 in human tissues and Student's t test for all other experiments.
RESULTS
Expression of sphingolipid enzymes and receptors in intestinal adenomas.
We investigated the expression of sphingolipid metabolic enzymes and S1P receptors in the intestinal tissues of the ApcMin/+ mouse, a widely used model system in which intestinal adenomas develop spontaneously (6). S1P receptors (S1PR1, S1PR2, and S1PR3) were expressed in normal intestinal mucosa; however, S1PR1 and S1PR2 transcripts were induced in the adenomas. mRNAs for Sphk1 and Sphk2 were expressed in both the mucosa and the polyp tissue to an equivalent level. In contrast, S1P lyase was markedly downregulated in the polyp, whereas S1P phosphatase was induced (Fig. 1A). These data suggest that the S1P synthetic machinery and the receptors are present in both normal and adenomatous intestinal tissues.
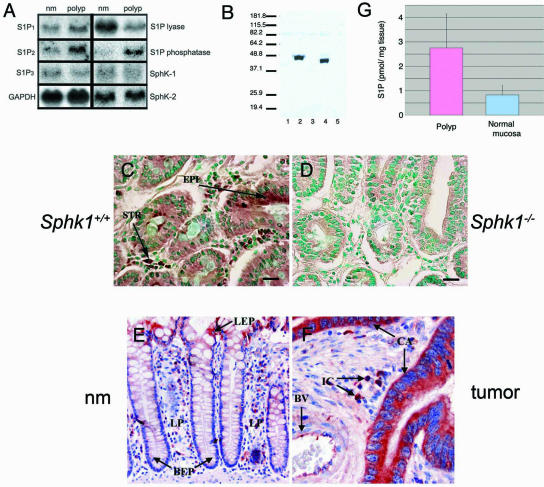
FIG. 1.
Expression of S1P receptors, Sphk1, and sphingolipid metabolic enzymes in intestinal tissues. (A) Northern blot analysis of S1P receptors and sphingolipid metabolic enzymes. nm, normal mucosa. Data show a representative blot from two to four animals that was repeated at least twice. (B) Immunoblot analysis of mouse and human Sphk1 in HEK-293T cells with anti-Sphk1 antiserum. Lanes: 1, mock; 2, mouse Sphk1; 3, mouse Sphk2; 4, human Sphk1; 5, human Sphk2. (C) Expression of Sphk1 in the polyp tissue of an ApcMin/+ Sphk1+/+ mouse (brown) is seen in epithelial and stromal cells. EPI, epithelial cells; STR, stromal cells. (D) Control staining of Sphk1 in the polyp tissue of an ApcMin/+ Sphk1−/− mouse. Scale bar, 10 μm in panels C and D. The counterstain in panels C and D is methyl green. (E) Expression of Sphk1 in normal colonic mucosal tissue adjacent to the colon cancer (red). (F) Expression of Sphk1 in human colonic mucosa and in colon cancer (red). BEP, basal epithelial cells; LP, lamina propria; LEP, luminal epithelial cells; CA, cancer cells; BV, blood vessel; IC, stromal mononuclear inflammatory cells; EP, epithelial cells. Original magnification is ×200 for panel E and ×400 for panel F. Sections in panels E and F are counterstained with hematoxylin and eosin (H&E). (G) S1P levels in the adenoma and the normal mucosa of an ApcMin/+ Sphk1+/+ mouse were quantified by HPLC methodology as described in the text. Data (mean ± standard deviation [SD]) were derived from three animals.
IHC of normal intestinal tissues from ApcMin/+ mice with anti-Sphk1 antiserum (Fig. 1B to D) indicated Sphk1 expression in the stromal as well as epithelial compartments. Immunoreactivity was substantially reduced in ApcMin/+ Sphk1−/− intestinal tissues, demonstrating that the antibody reacts specifically with Sphk1. Tumorigenic epithelial cells express moderate levels of Sphk1 in the adenoma. Stromal cells in the lamina propria also express moderate to high levels of Sphk1. Equivalent immunostaining was observed between normal mucosa and polyp tissues in ApcMin/+ mice. When intestinal polyps were extracted and the S1P content was analyzed, detectable levels (∼2.7 pmol/mg [wet weight]) were found (Fig. 1G). Polyp tissue contains higher levels of S1P than the surrounding small intestinal mucosa.
We also determined whether Sphk1 is expressed in the normal and pathological human intestinal tissues. In the human colon, differentiated luminal epithelial cells, stromal cells, and vascular cells expressed Sphk1 (Fig. 1E). Similar to mouse small intestinal tissues, moderate expression of Sphk1 was observed in epithelial cells, whereas moderate to high levels were seen in stromal cells in the lamina propria. Analysis of 27 colorectal cancer specimens indicates that 63% of the cases expressed moderate to high levels of Sphk1 in cancer cells (Fig. 1F and Table 1). In addition, blood vessels and inflammatory cells in the tumor stroma also expressed high levels of Sphk1. The presence of S1P synthetic machinery and receptors in human colorectal cancer suggests a possible role in tumor initiation and/or progression.
TABLE 1.
Sphk1 immunoreactivity in nonneoplastic epithelium adjacent to the tumor and in colorectal cancer cellsa
| Location of staining | Intensity of staining (arbitrary units)
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Nonneoplastic mucosa | ||||
| Basal epithelial cells | 15 (56) | 11 (41) | 1 (4) | 0 (0) |
| Luminal epithelial cells | 5 (19) | 7 (26) | 14 (52) | 1 (4) |
| Cancer cells | 2 (7) | 8 (30) | 11 (41) | 6 (22) |
n = 27.
Role of Sphk1 in intestinal polyposis.
To address whether Sphk1 expression is causally related to intestinal tumorigenesis, we analyzed intestinal tumors in mice that lack Sphk1 (1) in the ApcMin/+ background. Incidence of small intestinal adenomas was not significantly different between ApcMin/+ Sphk1+/+ and ApcMin/+ Sphk1−/− mice (Fig. 2A). In sharp contrast, tumor size was markedly reduced in ApcMin/+ Sphk1−/− mice (Fig. 2B to D). Histological features of the polyps from both genotypes were similar; except for the size difference, both stromal and epithelial cells were present (Fig. 2E and F). These data suggest that Sphk1 function is dispensable for initiation but is needed for the progression phase of polyposis in the ApcMin/+ model.
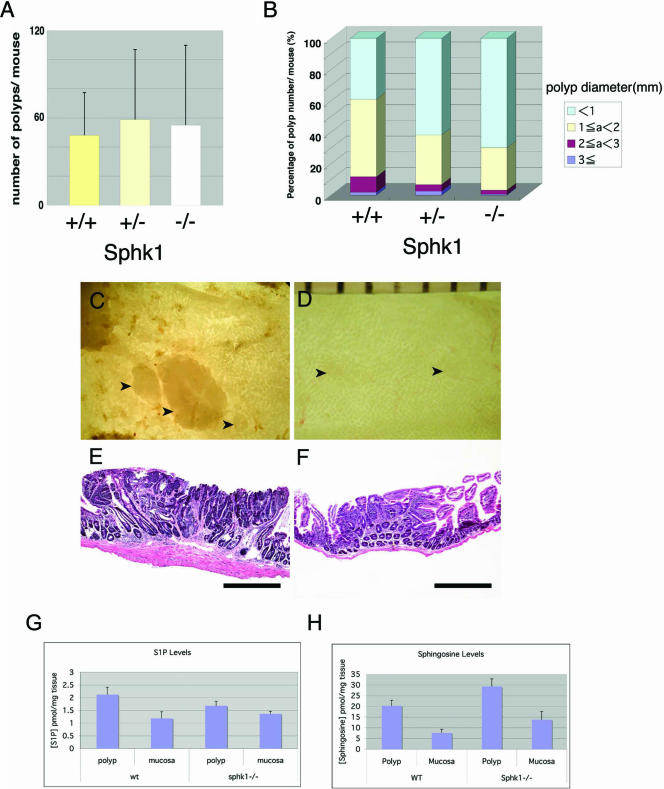
FIG. 2.
Analysis of intestinal adenomas in ApcMin/+ mice with a wild-type or Sphk1 deletion. (A) Numbers of polyps in the small intestine are shown (mean ± SD) (n = 19 [+/+], 18 [+/−], and 10 [−/−]). (B) Size distribution of the small intestinal polyps. Polyps were classified according to their diameters in millimeters, and an average percentage of each size class in each mouse is plotted. There is a significant change of polyp size distribution in <1 mm, 1 to 2 mm, and 2 to 3 mm (for <1 mm, 39.0 ± 12.3 [SD] for ApcMin/+ Sphk1+/+ and 69.8 ± 15.7 [SD] for ApcMin/+Sphk1−/− [P < 0.0001]; for 1 to 2 mm, 49.3 ± 11.6 [SD] for ApcMin/+ Sphk1+/+ and 27.1 ± 12.9 [SD] for ApcMin/+ Sphk1−/− [P < 0.0001]; for 2 to 3 mm, 9.8 ± 7.1 [SD] for ApcMin/+ Sphk1+/+ and 2.6 ± 3.9 [SD] for ApcMin/+ Sphk1−/− [P = 0.0065; Students' t test]). (C) An en face image of a cluster of distal jejunal polyps in an ApcMin/+ Sphk1+/+ mouse. (D) Similar image of a distal jejunal polyp in an ApcMin/+ Sphk1−/− mouse. (E) Histological section of an ileal polyp in an ApcMin/+ Sphk1+/+ mouse (H&E). (F) Histological section of an ileal polyp in an ApcMin/+Sphk1−/− mouse (H&E). Scale bars in D are in millimeters. Arrowheads show polyps in C and D. Scale bar, 500 μm in E and F. (G) S1P levels in polyps and mucosal scrapings from ApcMin/+ Sphk1+/+ or ApcMin/+ Sphk1−/− mice. Data represent means ± standard errors of the means (SEM) (n = 6 to 14). S1P levels in the polyps were not significantly different between wild-type (wt) and Sphk1−/− mice. (H) Sphingosine levels in polyps and mucosal scrapings from ApcMin/+ Sphk1+/+ or ApcMin+ Sphk1−/− mice. Data represent means ± SEM (n = 6 to 14) (P < 0.07; Student's t test).
Sphingolipid levels in polyps and mucosa were quantified by LC/MS/MS methodology (8). Surprisingly, S1P levels were not significantly different between ApcMin/+ Sphk1−/− and ApcMin/+ Sphk1+/+ polyps. Mucosa levels of S1P were not altered as well. As expected, mRNA for Sphk2 was detected to an equivalent level in both ApcMin/+ Sphk+/+ and ApcMin/+ Sphk1−/− adenoma tissues. In addition, mRNA for S1P phosphatase-1 and S1P phosphatase-2 were expressed similarly in both genotypes (see Fig. S1 in the supplemental material). Interestingly, 10-times-higher sphingosine levels were observed in the polyp tissues than in mucosal tissues, and ApcMin/+ Sphk1−/− mice contained higher levels of sphingosine than their ApcMin/+ Sphk1+/+ counterparts (Fig. 2G and H). These data suggest that Sphk1 gene deletion results in alterations in the levels of the substrate sphingosine. The levels of S1P were not altered significantly, presumably due to the action of Sphk2, which is expressed in the intestinal tissues.
Role of S1PRs in intestinal adenoma development.
The requirement for Sphkl in polyp progression could be due to the action of its product, S1P, an extracellular ligand for the S1PRs. Therefore, we tested whether S1PR1, S1PR2, and S1PR3 are involved in intestinal polyposis. Since S1pr2−/− and S1pr3−/− mice are viable (13, 17), we crossed these mice with the ApcMin/+ mice and analyzed intestinal tumor development in the bigenic mice. As shown in Fig. 3A to D, the lack of either S1PR2 or S1PR3 failed to influence polyp number or size.
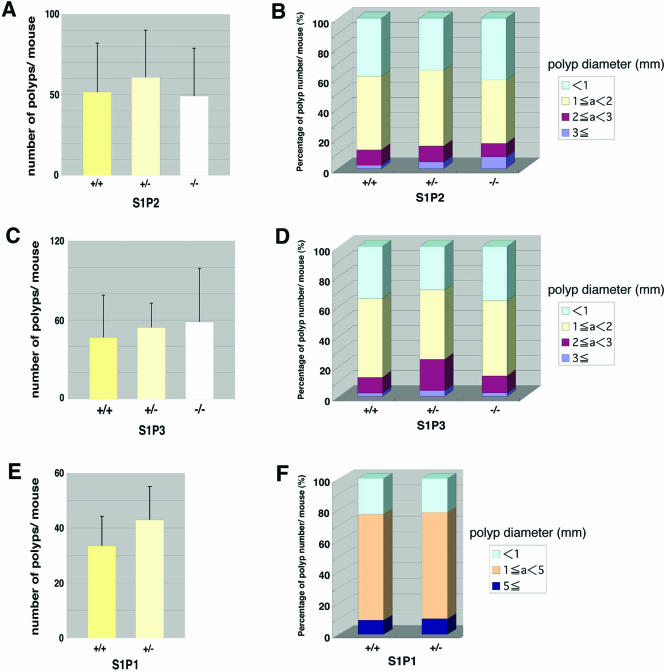
FIG. 3.
Intestinal polyposis in ApcMin/+ mice with the wild type or S1pr1-3 deletion. (A, C, and E) Numbers of polyps in small intestines of S1PR2 (A), S1PR3(C), and S1PR1 (E) mutant mice are shown (means ± SD). There is no significant change between each genotype. In the small intestine, 33.6 ± 10.7 polyps were detected in ApcMin/+ S1pr1+/+ mice, whereas in ApcMin/+ S1pr1+/− littermates, 43.0 ± 12.1 (SD) polyps were detected (P = 0.0073; Students' t test). (B, D, and F) Size distribution of the small intestinal polyps in S1PR2 (B), S1PR3 (D), and S1PR1 (F) mutant mice. Polyps were classified according to their diameters in millimeters, and an average percentage of each size class in each mouse is plotted (for S1P2, n = 12 [+/+], 15 [+/−], and 11 [−/−]; for S1P3, n = 5 [+/+], 6 [+/−], and 5 [−/−]; and for S1P2, n = 31 [+/+] and 19 [+/−]. There were no significant changes among the genotypes (Student's t test).
Since the S1pr1−/− mice are embryonically lethal due to a vascular maturation defect (20), we could not test the role of the null mutation of this receptor. Instead, we used the heterozygote S1pr1+/− mice in the ApcMin/+ background. Compared with their wild-type counterparts, S1pr1+/− ApcMin/+ mice had a 27% higher incidence of polyp numbers (P < 0.01) without a difference in size distribution (Fig. 3E and F), suggesting that S1PR1 signal transduction may inhibit tumor initiation events without affecting progression. Indeed, expression studies show that S1PR1 is expressed in differentiated luminal epithelial cells, and overexpression of this receptor inhibited the growth of intestinal cancer cells, supporting the notion that extracellular S1P signaling through this receptor is not capable of promoting tumorigenesis (data not shown). These data indicate that the three S1P receptors that are expressed in the intestinal polyps are unlikely to mediate the positive effects of Sphk1 on tumor progression.
Role of Sphk1 in cell proliferation, apoptosis, differentiation, and angiogenesis of the small intestinal polyps.
Sphk1 could influence polyp size by regulating cell proliferation and/or death. A BrdU incorporation assay was conducted to quantify cell proliferation. As shown in Fig. 4A to C, BrdU-positive nuclei were abundant in the crypts of normal intestinal mucosa and throughout the polyps of the ApcMin/+ Sphk1+/+ mice. In contrast, BrdU-positive nuclei were dramatically reduced in the polyps but not in the crypts of the ApcMin/+ Sphk1−/− mice. Quantitative analysis clearly showed that epithelial cell proliferation was reduced ∼45% (P < 0.05) in the polyps of ApcMin/+ Sphk1−/− mice compared with their ApcMin/+ Sphk1+/+ counterparts. Similarly, immunostaining of polyp tissues with Ki67 antibody, which detects the expression of the cell proliferation marker PCNA, showed that cell proliferation is markedly reduced in ApcMin/+ Sphk1−/− adenoma tissues (see Fig. S2 in the supplemental material). Together, these data suggest that intestinal development and turnover were normal in the ApcMin/+ Sphk1−/− mice, whereas polyp progression was dramatically reduced.
FIG. 4.
Regulation of adenoma cell proliferation by Sphk1. (A to C) Cell proliferation in intestinal polyps. Polyps from an ApcMin/+ Sphk1+/+ mouse (A) and an ApcMin/+ Sphk1−/− mouse (B) were sectioned and assessed for BrdU incorporation. Scale bar, 100 μm. (C) BrdU labeling index. The graph shows the percentage of BrdU-positive cells in polyps of ApcMin+ Sphk1+/+ and ApcMin/+ Sphk1−/− mice (24.3 ± 4.67 for ApcMin/+ Sphk1+/+ and 13.4 ± 6.33 for ApcMin/+ Sphk1−/−). *P = 0.0051 (Students' t test). (D to F) Apoptosis in intestinal polyps. Polyps from an ApcMin/+ Sphk1+/+ mouse (D) and an ApcMin/+ Sphk1−/− mouse (E) were sectioned and assessed for cell death by TUNEL staining as described in the text. Arrowheads show the positive cells, and cells at the luminal edge were not considered (no arrowheads shown). Scale bar, 100 μm. (F) Apoptotic index. The graph shows the percentage of TUNEL-positive cells in polyps of ApcMin/+ Sphk1+/+ and ApcMin/+ Sphk1−/− mice (0.428% ± 0.146% for ApcMin/+ Sphk1+/+ and 0.622% ± 0.423% for ApcMin/+ Sphk1−/−). *P = 0.385 (Students' t test). (G to I) IHC of PECAM-1 to detect intratumoral MVD. (G and H) Intestinal polyps from ApcMin/+ Sphk1+/+ (G) and ApcMin+ Sphk1−/− (H) mice were analyzed for the expression of the PECAM-1 protein by immunohistochemistry as described in the text. Scale bar, 10 μm. (I) MVD is shown (51.0 ± 13.5 [SD] for ApcMin/+ Sphk1+/+ and 38.6 ± 13.7 [SD] for ApcMin/+ Sphk1−/− [P = 0.137; Student's t test]).
We also determined whether the lack of Sphk1 influenced cell death (apoptosis) in the intestine. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis showed that apoptotic cells were present at a small frequency (∼0.63%) throughout the polyp structure, excluding the luminal epithelial cells, which show high extent of TUNEL positivity due to normal cell turnover (Fig. 4D to F). Although the ApcMin/+ Sphk1−/− mice had a somewhat higher incidence of apoptotic cells in the polyp body, the difference between the knockout and the wild type did not reach statistical significance, suggesting that the inhibition of apoptosis of the intestinal epithelial cells by the Sphk1 is not the primary mechanism in the control of tumor progression.
Extracellular S1P is well established to regulate angiogenesis (also known as new blood vessel growth), which is needed for optimal tumorigenesis (12). Microvessel counts in the intestinal polyps of ApcMin/+ Sphk+/+ and ApcMin/+ Sphk1−/− mice did not exhibit significant differences (Fig. 4G to I), suggesting that alteration of polyp angiogenesis is not the primary mechanism regulated by Sphk1.
We also examined the expression patterns of villin and alkaline phosphatase, which are markers of epithelial cell differentiation in the intestine (32). Expression patterns of villin and alkaline phosphatase are indistinguishable in the differentiated epithelial cells of the intestinal mucosa in both ApcMin/+ Sphk+/+ and ApcMin/+ Sphk1−/− mice (see Fig. S3 and S4 in the supplemental material). In general, these markers are not expressed significantly in the adenoma tissues of both genotypes. These data suggest that Sphk1 does not influence the crypt-to-villus transition and differentiation of intestinal cells.
Molecular mechanisms involved in Sphk1 regulation of cell proliferation.
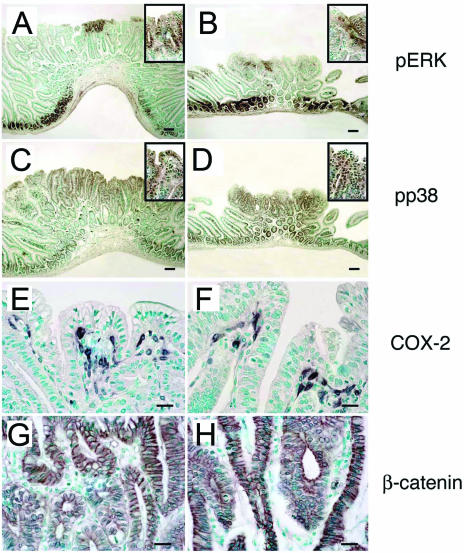
To gain insight into the molecular mechanisms involved in Sphk1 regulation of tumorigenesis, intestinal adenomas were analyzed for various signaling proteins implicated previously in S1P-mediated biology. Regions of the polyp and particularly the epithelial cells were positive for immunostaining of phospho-ERK and phospho-p38 MAP kinase. However, significant differences in the intensities or patterns of staining or subcellular localization were not observed between mice that express Sphk1 and those that lack Sphk1, even when polyps of similar sizes were analyzed (Fig. 5). This is consistent with the fact that extracellular S1P signaling via its G-protein-coupled receptors regulates MAP kinase activation (12) and further supports the notion that S1PRs do not regulate polyp progression. Similarly, expression of the COX-2 gene was observed in the stromal cells of the polyps without major differences in intensities and patterns between mice that express or lack Sphk1 (Fig. 5). Thus, the COX-2/prostaglandin E2 pathway, which is a critical regulator of adenoma size and angiogenesis in the ApcMin/+ model (27), is unlikely to account for the effect of Sphk1 on polyp progression. Similarly, the expression level, pattern, or subcellular localization of β-catenin was unaltered by Sphk1 gene dosage. These data suggest that intracellular Sphk1 action is distinct from that the canonical Wnt signaling pathway, which is known to regulate nuclear localization of β-catenin and intestinal progenitor cell proliferation during polyposis (32).
FIG. 5.
Gene expression in intestinal polyps of Sphk1+/+ and Sphk1−/− mice. Intestinal polyps from ApcMin/+ Sphk1+/+ (A, C, E, and G) and ApcMin/+ Sphk1−/− (B, D, F, and H) mice were analyzed for the expression of phospho-ERK (A and B), phospho-p38 (C and D), COX-2 (E and F), and β-catenin (G and H) by IHC as described in the text. Scale bar, 100 μm in panels E, F, G, and H and 10 μm in panels A, B, C, and D.
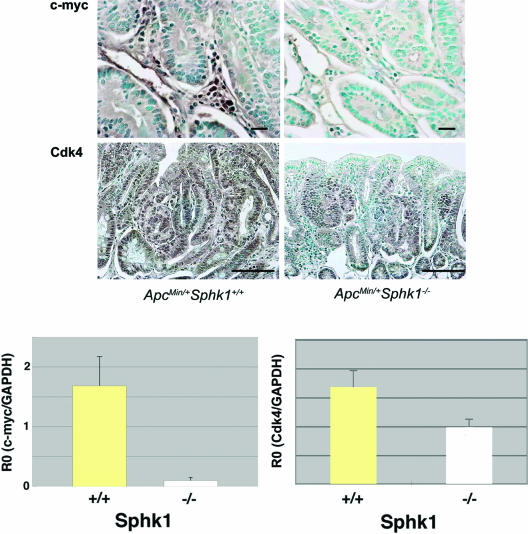
Markers of cell proliferation and cell cycle control were analyzed by immunohistochemistry of intestinal adenomas from ApcMin/+ Sphk+/+ and ApcMin/+ Sphk−/− mice. We found that cell cycle kinase Cdk4 mRNA and protein were dramatically downregulated in the epithelial cells of small intestinal polyps in ApcMin/+ Sphk−/− mice (Fig. 6). In addition, the proto-oncogene c-myc, which is implicated in cell proliferation, is also downregulated at both the mRNA and protein levels in the mice that lack the Sphk1 gene (Fig. 6). Interestingly, c-myc expression was observed predominantly in the stromal cells, whereas epithelial expression was equivocal. Other cell cycle regulators, such as cyclin D1, cyclin A1, cyclin E, p27, and p21, were not altered in the ApcMin/+ Sphk1−/− mice compared to their wild-type counterparts (data not shown). These data suggest that the regulation of expression of Cdk4 and c-myc in epithelial cells and in the tumor stromal microenvironment, respectively, by the Sphk1 pathway may be involved in the regulation of cell proliferation in the polyp.
FIG. 6.
Downregulation of cell cycle regulatory factors in ApcMin/+ Sphk1−/− adenoma tissues. (A) Tissue sections of adenomas from ApcMin/+ Sphk1+/+ and ApcMin/+ Sphk1−/− mice were analyzed for the expression of c-myc and Cdk4 by immunohistochemistry as described in the text. Note that c-myc expression is high in the stromal cells, whereas Cdk4 is expressed primarily in the epithelial compartment. Scale bar, 10 μm for c-myc panels and 100 μm for Cdk4. (B) Expression of c-Myc and Cdk4 mRNA in the intestinal polyp tissues of ApcMin/+ Sphk1+/+ (n = 5) and ApcMin/+ Sphk1−/− (n = 5) mice. Relative expression of mRNAs (R0) was determined as described in the text. (P < 0.0001 for c-myc and P < 0.05 for Cdk4; Student's t test). Data represent means ± SD of an experiment that was repeated at least two times.
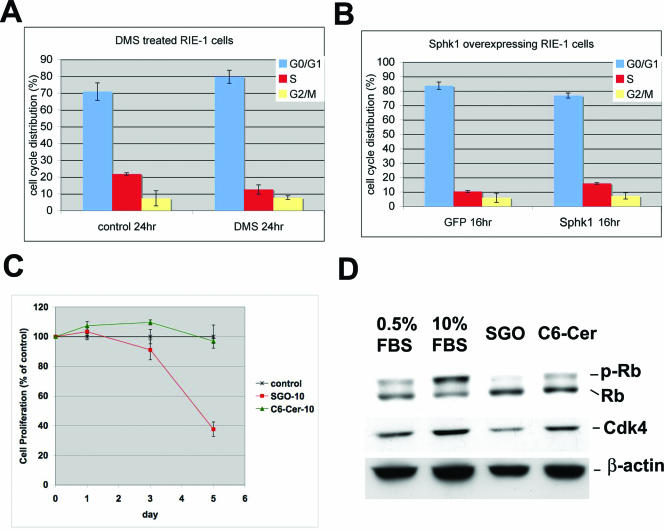
To determine whether the Sphk1 enzyme is directly involved in the regulation of the cell cycle, we explored the effects of Sphk1 in RIE cells, which have been used as an in vitro model system (7). When Sphk1 was overexpressed using an adenoviral construct, a decrease in the G0/G1 population and a concomitant increase in the S-phase population was observed. In addition, when RIE cells were treated with N,N′-dimethylsphingosine (DMS), an inhibitor of the Sphk enzyme, accumulation of G0/G1 population and a reduction in the S-phase population were observed (Fig. 7A and B). These data suggest that Sphk1 can directly modulate cell cycle traverse at the G1/S boundary in intestinal epithelial cells.
FIG. 7.
Regulation of G1/S cell cycle traverse in RIE cells by Sphk1. (A) RIE cells were treated with vehicle or DMS (10 μM) for 24 h, and cell cycle analysis was conducted by FACS as described in the text. Results represent means ± SEM (n = 2). (B) RIE cells were transduced with adenoviral vectors expressing Sphk1 as described in the text. Cell cycle analysis at 16 h after serum stimulation was conducted by FACS. Results represent means ± SEM (n = 2) of a representative experiment. (C) RIE cells were starved in 0.5% FBS for 2 days and treated with sphingosine (SGO) (10 μM), C6-ceramide (C6-Cer) (10 μM), or vehicle for various time points. Cell proliferation was quantified by an MTT assay as described in the text. S1P treatment under similar conditions did not inhibit cell proliferation (data not shown). Results represent means ± SD of a representative experiment that was repeated twice (n = 3). (D) RIE cells were treated as in C for 24 h in 0.5% FBS containing sphingosine (SGO) or C6-ceramide (C6-cer) for 24 h as described above. Some cells were treated with 10% FBS to stimulate optimal cell proliferation. Cell extracts were analyzed for Rb phosphorylation (p-Rb) and Cdk4 expression by immunoblot analysis. A representative blot from two independent experiments is shown.
Inhibition of the Sphk1 enzyme or deletion of the Sphk1 gene would result in the accumulation of the substrate sphingosine and a reduction in the levels of S1P. Indeed, sphingosine levels were elevated in the polyps of Sphk1−/− ApcMin/+ mice (Fig. 2H), whereas S1P levels were not significantly changed. Thus, we tested whether sphingosine treatment of RIE cells would influence cell proliferation. Treatment of RIE cells with sphingosine (10 μM) resulted in a profound suppression of cell proliferation. The effect was specific because treatment of RIE cells with C6-ceramide (10 μM) did not result in growth inhibition (Fig. 7C). In addition, sphingosine suppressed retinoblastoma (Rb) phosphorylation and Cdk4 protein expression levels (Fig. 7D). Expression of cyclin A, cyclin D1, and cyclin E was not altered by sphingosine treatment in RIE cells (see Fig S5 in the supplemental material). These data suggest that the intracellular action of sphingosine modulates the G1/S cell cycle regulators, such as the phosphorylation of Rb and expression of the Cdk4 protein, and thereby modulates intestinal epithelial cell proliferation.
DISCUSSION
The role of sphingolipids in cancer development and progression is poorly understood at the mechanistic level.Intestinal tumors are particularly relevant in this respect because dietary sphingolipids have been implicated as inhibitors of intestinal carcinogenesis (18, 22). In addition, S1P is a potent angiogenic factor that regulates tumor angiogenesis by signaling through the S1P1R (3). It is known that dietary sphingomyelin is metabolized in the intestinal tract into ceramide and sphingosine by the sequential actions of sphingomyelinase and ceramidase, respectively. Sphingosine is thought to be rapidly converted into S1P and then ultimately into palmitate by intracellular metabolism (25). Thus, the metabolic fate of S1P as well as receptor-dependent S1P signaling may be important in the intestinal cells. In this report, we show that transcripts for S1P1, S1P2, and S1P phosphatase are upregulated and that S1P lyase is downregulated in intestinal adenomas compared to the normal mucosa in ApcMin/+ mice. Transcripts for other S1P metabolic enzymes and receptors are expressed at equivalent levels. These data suggest that S1P is metabolized in the normal and pathological intestinal tissues and is likely to be involved in signaling events via its receptors.
In contrast to μM levels of S1P found in blood, intestinal tissues contain low levels (nM) of S1P. However, the polyp tissues from the ApcMin/+ mice contain S1P levels that are about twofold higher than those of the normal mucosa, suggesting that either activation of sphingosine kinases or decreased activity of degradative enzymes (such as S1P lyase and phosphatase) influences the steady-state levels of S1P. Alternatively, increased vascular permeability in the adenoma tissue would lead to enhanced deposition of plasma-borne S1P.
In this study, we focused on the role of the Sphk1 isoenzyme. Expression studies indicate that several cell types in the intestinal adenomas of ApcMin/+ mice express this enzyme. Although inflammatory cells in the stroma express the highest levels of Sphk1, the epithelial cells also express significant levels. These data suggest that Sphk1 may be involved in the generation of S1P in both tumor cells and the stromal microenvironment. However, Sphk1 protein levels are present in both normal mucosa and adenoma tissues, suggesting that this enzyme may be involved in multiple functions in both normal and pathological intestinal tissues.
We also examined the expression of Sphk1 in human colorectal cancer tissues and normal mucosa in the colon. In normal colonic mucosa, stromal inflammatory cells express high levels of Sphk1, which is similar to the pattern in mouse small intestinal tissues. Differentiated colonic epithelial cells near the lumen express high levels of Sphk1, suggesting that this gene is expressed during the differentiation pathway of epithelial cells. In colon carcinoma cells, a high level of expression was seen in the cancer cells themselves. However, endothelial cells in the blood vessels as well as inflammatory cells also express significant levels. Analysis of multiple tumor samples indicated that ∼63% of tumors express high levels of Sphk1, suggesting that Sphk1 may have a regulatory function in intestinal tumorigenesis. Recently, Kawamori and colleagues reported that Sphk1 is overexpressed in human colorectal cancer and in azoxymethane-induced aberrant crypt foci and tumors in the rodent colon (14).
To definitively address the role of Sphk1 in intestinal tumors, we analyzed the small intestinal polyps in bigenic ApcMin/+ Sphk1−/− mice. The incidence of adenomas was unchanged, but the size distribution was markedly diminished in ApcMin/+ Sphk1−/− mice, suggesting that Sphk1 expression is critical for the progression phase of tumor development. It also suggests that Sphk1 does not regulate the initiation of intestinal tumors. In this respect, this phenotype resembles the cPLA2 knockout mice described previously by Takaku and colleagues (39).
It is known that an ApcMin/+ mutation drives the initiation of tumors by the Wnt/β-catenin signaling pathway (6). Our findings suggest that the function of Sphk1 is distinct from that of the β-catenin signaling pathway. In agreement with this, expression analysis of β-catenin in the intestinal adenoma tissues indicated that the level as well as the subcellular distribution of this critical oncogenic protein are unaltered by an Sphk1 gene deletion. The fact that Sphk1 function is independent of the β-catenin pathway is further underscored by the finding that expression of COX-2, which is required for the β-catenin-dependent development of polyp progression (40), is unaltered by the Sphk1 gene deletion. These results are somewhat discrepant from those reported in the recent work by Kawamori et al., who showed that COX-2 expression is correlated with Sphk1 in colorectal cancer tissues and in azoxymethane-induced tumors in rodents (14). The reason for this incongruity is unclear but may be related to the specifics of the model under study. In the azoxymethane model, tumors are found primary in the colon, whereas small intestinal adenomas develop in the ApcMin/+ model. In the ApcMin/+ model, COX-2 is induced primarily in the stromal cells, which is distinctly different from the epithelial expression observed in the azoxymethane model. Thus, Sphk1 induction of COX-2 may be cell type specific.
Bigenic crosses between ApcMin/+ mice and S1p1r+/−, S1p2r−/−, and S1p3r−/− mice indicated that the null mutation in each of these receptors does not influence intestinal polyposis. Indeed, ApcMin/+ S1p1r+/− mice contained significantly higher numbers of intestinal adenomas even though the size distribution was not altered. The data suggest that the ability of Sphk1 to influence adenoma progression is independent of S1P1-3 receptors. In support of this, levels ofphosphorylated MAP kinases (phospho-ERK and phospho-p38), which are downstream targets of S1PRs, were unaltered by the Sphk1 gene deletion. These findings strongly suggest that the intracellular function of Sphk1, as opposed to the extracellular signaling of the product S1P, is involved in the regulation of intestinal adenoma progression.
Sphingolipids in the polyp tissues of ApcMin/+ Sphk+/+ mice and ApcMin/+ Sphk1−/− mice were quantified using a highly sensitive LC/MS/MS assay. Although there was a trend towards reduced S1P levels in the polyps of ApcMin/+ Sphk1−/− mice, the alterations did not reach statistical significance. The residual S1P is likely synthesized by the Sphk2 isoenzyme. In contrast, tissue sphingosine levels were higher in the polyps (∼10-fold above S1P levels), and ApcMin/+ Sphk1−/− mice contained significantly higher levels than the ApcMin/+ Sphk1+/+ mice. These data suggest that a lack of the Sphk1 enzyme caused the accumulation of the substrate sphingosine. Enhanced sphingosine levels may be one reason why adenoma progression is attenuated in the ApcMin/+ Sphk1−/− mice. This is consistent with the proposal by Schmelz et al. that sphingosine may be a tumor suppressor lipid (34).
S1P and sphingosine are potent regulators of cell proliferation, apoptosis, and angiogenesis (12). We therefore quantified these parameters in the intestinal adenomas of ApcMin/+ Sphk1−/− mice and their ApcMin/+ Sphk1+/+ counterparts. A significant reduction in tumor cell proliferation was observed in the ApcMin/+ Sphk1−/− mice, suggesting that the ability of Sphk1 to suppress adenoma progression is likely to be due to the suppression of cell proliferation. Although a slight suppression of angiogenesis and induction of apoptosis were observed, such alterations were not as dramatic, suggesting that these processes are unlikely to be primarily responsible for the growth-inhibited phenotype. Furthermore, expression patterns of villin and alkaline phosphatase were not altered by the Sphk1 gene deletion, suggesting that progenitor cell differentiation along the crypt-villus border and maturation along the villus are not involved. Thus, the primary reason for the suppression of tumor progression appears to be due to the requirement for Sphk1 in tumor cell proliferation. Since Sphk1 is expressed in both epithelial and stromal cell compartments, we cannot at present ascertain if it functions in a cell-autonomous manner or whether cell-cell interactions between epithelial and stromal compartments are required. Indeed, c-myc expression in the stromal cells and Cdk4 expression in the epithelial cells are markedly downregulated in Sphk1 null intestinal tissues, suggesting the function of Sphk1 on both epithelial cell and stromal compartments.
Sphingosine levels in intestinal adenomas are increased by the Sphk1 gene deletion, suggesting that the intracellular action of sphingosine may mediate the growth-suppressive action. It is known that sphingosine is a potent inhibitor of cell growth and an inducer of apoptosis (36). How sphingosine induces these effects is not entirely clear; however, the modulation of intracellular phosphorylation mechanisms by sphingosine-dependent kinase as well as the modulation of mitochondrial pathway of apoptosis may be involved (38).
Given that Sphk1 function is likely to be critical for intestinal epithelial cell proliferation, we used RIE cells, an immortalized nontransformed in vitro model (7). Inhibition of Sphk1 by DMS inhibited cell cycle progression, and the overexpression of Sphk1 resulted in an enhancement in the G1/S transition of the cell cycle in RIE cells. To directly test whether sphingosine is capable of regulating cell cycle traverse, we treated RIE cells with sphingosine or C6-ceramide. Sphingosine treatment inhibited cell proliferation and downregulated Cdk4 expression and phosphorylation of phospho-Rb. These data strongly suggest that the intracellular action of sphingosine is involved in the regulation of the G1/S traverse of the cell cycle in intestinal epithelial cells. It is likely that the downregulation of Cdk4 is a major mechanism involved. Since Cdk4 phosphorylates the Rb protein during G1/S cell cycle traverse, downregulation of this critical protein by sphingolipid metabolism may be an important regulatory mechanism.
A major finding of this study is that the intracellular function of Sphk1, independent of the extracellular signaling of its product, S1P, is critical for the growth of intestinal adenomas. We speculate that within the sphingolipid-rich environment of the intestinal mucosa, sphingosine, a by-product of sphingomyelin metabolism, may play a regulatory role as a suppressor of intestinal tumorigenesis. Indeed, sphingolipid feeding studies have suggested that sphingosine is capable of inhibiting aberrant crypt focus formation in a carcinogen (azoxymethane)-induced model in rodents (18, 22, 34). In the context of a tumor suppressor gene mutation (i.e., ApcMin/+), a reduction of sphingosine levels by the action of Sphk1 is needed for optimal cell proliferation and polyp progression. The main finding of this study, that Sphk1 regulates cell proliferation and adenoma size, is potentially significant because these data suggest Sphk1 as a novel target in the control of intestinal cancer. We speculate that alternative ways of enhancing sphingosine levels in the intestinal tract by either metabolic alterations or dietary manipulation may be tumor suppressive.
Supplementary Material
Acknowledgments
This work is supported by NIH grants HL67330, CA77839, and HL70694 to T.H. and CA77528 to J.D.S.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allende, M. L., T. Sasaki, H. Kawai, A. Olivera, Y. Mi, G. van Echten-Deckert, R. Hajdu, M. Rosenbach, C. A. Keohane, S. Mandala, S. Spiegel, and R. L. Proia. 2004. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279:52487-52492. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, K., Y. Tamai, S. Horiike, M. Oshima, and M. M. Taketo. 2003. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/− compound mutant mice. Nat. Genet. 35:323-330. [DOI] [PubMed] [Google Scholar]
- 3.Chae, S. S., J. H. Paik, H. Furneaux, and T. Hla. 2004. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J. Clin. Investig. 114:1082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson, R. C., and R. L. Lester. 1999. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1438:305-321. [DOI] [PubMed] [Google Scholar]
- 5.Dobrosotskaya, I. Y., A. C. Seegmiller, M. S. Brown, J. L. Goldstein, and R. B. Rawson. 2002. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 296:879-883. [DOI] [PubMed] [Google Scholar]
- 6.Dove, W. F., R. T. Cormier, K. A. Gould, R. B. Halberg, A. J. Merritt, M. A. Newton, and A. R. Shoemaker. 1998. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBois, R. N., J. Awad, J. Morrow, L. J. Roberts II, and P. R. Bishop. 1994. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J. Clin. Investig. 93:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fyrst, H., D. R. Herr, G. L. Harris, and J. D. Saba. 2004. Characterization of free endogenous C14 and C16 sphingoid bases from Drosophila melanogaster. J. Lipid Res. 45:54-62. [DOI] [PubMed] [Google Scholar]
- 9.Hannun, Y. A., C. Luberto, and K. M. Argraves. 2001. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40:4893-4903. [DOI] [PubMed] [Google Scholar]
- 10.Herr, D. R., H. Fyrst, V. Phan, K. Heinecke, R. Georges, G. L. Harris, and J. D. Saba. 2003. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development 130:2443-2453. [DOI] [PubMed] [Google Scholar]
- 11.Hertervig, E., A. Nilsson, M. Nilbert, and R. D. Duan. 2003. Reduction in alkaline sphingomyelinase in colorectal tumorigenesis is not related to the APC gene mutation. Int. J. Colorect. Dis. 18:309-313. [DOI] [PubMed] [Google Scholar]
- 12.Hla, T. 2004. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15:513-520. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, I., N. Fukushima, X. Ye, and J. Chun. 2004. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73:321-354. [DOI] [PubMed] [Google Scholar]
- 14.Kawamori, T., W. Osta, K. R. Johnson, B. J. Pettus, J. Bielawski, T. Tanaka, M. J. Wargovich, B. S. Reddy, Y. A. Hannun, L. M. Obeid, and D. Zhou. 2006. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 20:386-388. [DOI] [PubMed] [Google Scholar]
- 15.Kluk, M. J., and T. Hla. 2001. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ. Res. 89:496-502. [DOI] [PubMed] [Google Scholar]
- 16.Kono, M., J. L. Dreier, J. M. Ellis, M. L. Allende, D. N. Kalkofen, K. M. Sanders, J. Bielawski, A. Bielawska, Y. A. Hannun, and R. L. Proia. 2006. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J. Biol. Chem. 281:324-7331 [DOI] [PubMed] [Google Scholar]
- 17.Kono, M., Y. Mi, Y. Liu, T. Sasaki, M. L. Allende, Y. P. Wu, T. Yamashita, and R. L. Proia. 2004. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279:29367-29373. [DOI] [PubMed] [Google Scholar]
- 18.Lemonnier, L. A., D. L. Dillehay, M. J. Vespremi, J. Abrams, E. Brody, and E. M. Schmelz. 2003. Sphingomyelin in the suppression of colon tumors: prevention versus intervention. Arch. Biochem. Biophys. 419:129-138. [DOI] [PubMed] [Google Scholar]
- 19.Liu, H., M. Sugiura, V. E. Nava, L. C. Edsall, K. Kono, S. Poulton, S. Milstien, T. Kohama, and S. Spiegel. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275:19513-19520. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Y., R. Wada, T. Yamashita, Y. Mi, C. X. Deng, J. P. Hobson, H. M. Rosenfeldt, V. E. Nava, S. S. Chae, M. J. Lee, C. H. Liu, T. Hla, S. Spiegel, and R. L. Proia. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 106:951-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendel, J., K. Heinecke, H. Fyrst, and J. D. Saba. 2003. Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. J. Biol. Chem. 278:22341-22349. [DOI] [PubMed] [Google Scholar]
- 22.Merrill, A. H., Jr., E. M. Schmelz, E. Wang, D. L. Dillehay, L. G. Rice, F. Meredith, and R. T. Riley. 1997. Importance of sphingolipids and inhibitors of sphingolipid metabolism as components of animal diets. J. Nutr. 127:830S-833S. [DOI] [PubMed] [Google Scholar]
- 23.Min, J. K., H. S. Yoo, E. Y. Lee, W. J. Lee, and Y. M. Lee. 2002. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal. Biochem. 303:167-175. [DOI] [PubMed] [Google Scholar]
- 24.Mizugishi, K., T. Yamashita, A. Olivera, G. F. Miller, S. Spiegel, and R. L. Proia. 2005. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25:11113-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson, A., and R. D. Duan. 2006. Absorption and lipoprotein transport of sphingomyelin. J. Lipid Res. 47:154-171. [DOI] [PubMed] [Google Scholar]
- 26.Olivera, A., H. M. Rosenfeldt, M. Bektas, F. Wang, I. Ishii, J. Chun, S. Milstien, and S. Spiegel. 2003. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. J. Biol. Chem. 278:46452-46460. [DOI] [PubMed] [Google Scholar]
- 27.Oshima, M., J. E. Dinchuk, S. L. Kargman, H. Oshima, B. Hancock, E. Kwong, J. M. Trzaskos, J. F. Evans, and M. M. Taketo. 1996. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803-809. [DOI] [PubMed] [Google Scholar]
- 28.Pitson, S. M., P. Xia, T. M. Leclercq, P. A. Moretti, J. R. Zebol, H. E. Lynn, B. W. Wattenberg, and M. A. Vadas. 2005. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J. Exp. Med. 201:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ristimaki, A., A. Sivula, J. Lundin, M. Lundin, T. Salminen, C. Haglund, H. Joensuu, and J. Isola. 2002. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 62:632-635. [PubMed] [Google Scholar]
- 30.Saba, J. D., and T. Hla. 2004. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 94:724-734. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez, T., T. Estrada-Hernandez, J. H. Paik, M. T. Wu, K. Venkataraman, V. Brinkmann, K. Claffey, and T. Hla. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278:47281-47290. [DOI] [PubMed] [Google Scholar]
- 32.Sancho, E., E. Batlle, and H. Clevers. 2004. Signaling pathways in intestinal development and cancer. Annu. Rev. Cell Dev. Biol. 20:695-723. [DOI] [PubMed] [Google Scholar]
- 33.Schmelz, E. M., A. S. Bushnev, D. L. Dillehay, D. C. Liotta, and A. H. Merrill, Jr. 1997. Suppression of aberrant colonic crypt foci by synthetic sphingomyelins with saturated or unsaturated sphingoid base backbones. Nutr. Cancer 28:81-85. [DOI] [PubMed] [Google Scholar]
- 34.Schmelz, E. M., P. C. Roberts, E. M. Kustin, L. A. Lemonnier, M. C. Sullards, D. L. Dillehay, and A. H. Merrill, Jr. 2001. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 61:6723-6729. [PubMed] [Google Scholar]
- 35.Schwab, S. R., J. P. Pereira, M. Matloubian, Y. Xu, Y. Huang, and J. G. Cyster. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309:1735-1739. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel, S., and A. H. Merrill, Jr. 1996. Sphingolipid metabolism and cell growth regulation. FASEB J. 10:1388-1397. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel, S., and S. Milstien. 2002. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277:25851-25854. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, E., K. Handa, M. S. Toledo, and S. Hakomori. 2004. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc. Natl. Acad. Sci. USA 101:14788-14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaku, K., M. Sonoshita, N. Sasaki, N. Uozumi, Y. Doi, T. Shimizu, and M. M. Taketo. 2000. Suppression of intestinal polyposis in Apc(delta 716) knockout mice by an additional mutation in the cytosolic phospholipase A(2) gene. J. Biol. Chem. 275:34013-34016. [DOI] [PubMed] [Google Scholar]
- 40.Takeda, H., M. Sonoshita, H. Oshima, K. Sugihara, P. C. Chulada, R. Langenbach, M. Oshima, and M. M. Taketo. 2003. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Res. 63:4872-4877. [PubMed] [Google Scholar]
- 41.Trifan, O. C., R. M. Smith, B. D. Thompson, and T. Hla. 1999. Overexpression of cyclooxygenase-2 induces cell cycle arrest. Evidence for a prostaglandin-independent mechanism. J. Biol. Chem. 274:34141-34147. [DOI] [PubMed] [Google Scholar]
- 42.Weidner, N., J. P. Semple, W. R. Welch, and J. Folkman. 1991. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N. Engl. J. Med. 324:1-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.