Abstract
Adhesion of T cells after stimulation of the T-cell receptor (TCR) is mediated via signaling processes that have collectively been termed inside-out signaling. The molecular basis for inside-out signaling is not yet completely understood. Here, we show that a signaling module comprising the cytosolic adapter proteins ADAP and SKAP55 is involved in TCR-mediated inside-out signaling and, moreover, that the interaction between ADAP and SKAP55 is mandatory for integrin activation. Disruption of the ADAP/SKAP55 module leads to displacement of the small GTPase Rap1 from the plasma membrane without influencing its GTPase activity. These findings suggest that the ADAP/SKAP55 complex serves to recruit activated Rap1 to the plasma membrane. In line with this hypothesis is the finding that membrane targeting of the ADAP/SKAP55 module induces T-cell adhesion in the absence of TCR-mediated stimuli. However, it appears as if the ADAP/SKAP55 module can exert its signaling function outside of the classical raft fraction of the cell membrane.
Within the immune system, integrins play important roles in regulating the interaction of T cells with other cells and with proteins of the extracellular matrix. By mediating T-cell adhesion, integrins control the homing and the trafficking of T cells as well as the interaction between T cells and antigen-presenting cells (34, 41). The major integrins expressed on T cells are the β2-integrin LFA-1 (αLβ2) as well as members of the β1-family of integrins (α4β1, α5β1, α6β1, and VLA) (25). The physiologic ligands of LFA-1 include the intercellular adhesion molecule 1 (ICAM-1), ICAM-2, and ICAM-3 (25), whereas ligands for β1-integrins are vascular cell adhesion molecule 1 (VCAM-1) or proteins of the extracellular matrix, such as fibronectin (13, 54).
On resting T cells, β1- and β2-integrins are expressed in an inactive state. However, ligation of the T-cell receptor (TCR) by antigen/major histocompatibility complexes results in a rapid increase in the activity of β1- and β2-integrins, thereby enhancing ligand binding (15, 46, 50). Two distinct mechanisms mediate the activation of integrins. First, the affinity of an integrin for its ligand is enhanced, and second, the lateral mobility becomes altered, which results in integrin clustering (avidity regulation) (14). The processes leading to the activation of integrins have collectively been termed inside-out signaling (14, 15, 28).
Several molecules have been suggested to play critical roles during TCR-mediated activation of β1- and β2-integrins (14, 28). Among these is the small GTPase Rap1, whose role for integrin activation has been a matter of intense research during the last few years (4, 29). The mechanisms for how Rap1 becomes activated are not yet completely understood (4). Rap1 activation has been shown to be mediated by particular guanine nucleotide exchange factors (GEFs), such as C3G, CalDAG-GEFI, and Epac (5, 8, 11). It has been proposed that Rap1 is associated with CalDAG-GEFI and that TCR-induced Rap1 activation is dependent upon the activation of phospholipase γ1 (27). Moreover, Medeiros et al. have most recently shown that plasma membrane targeting and activation of Rap1 is mediated via serine-threonine kinase PKD1, a downstream effector of protein kinase C (40). Importantly, the activation of Rap1 by PKD1 apparently does not depend on the enzymatic activity of PKD1 but seems to involve an interaction between the pleckstrin homology (PH) domain of PKD1 and Rap1. In addition, it appears as if the PKD1/Rap1 complex needs to bind to a C-terminal fragment within the cytoplasmic domain of the β1-intergin in order to facilitate adhesion.
Besides Rap1, several classical adapter proteins have been proposed to be involved in immunoreceptor-mediated integrin activation. These include the transmembrane adapter protein LAT (linker of activation of T cells) and the cytosolic adapter proteins SLP-76 (SH2 domain containing leukocyte phosphoprotein of 76 kDa), ADAP (adhesion and degranulation promoting adapter protein), and SKAP55 (Src kinase-associated phosphoprotein of 55 kDa) (18, 20, 23, 24, 32, 44, 45, 52, 53).
ADAP is expressed in T cells, thymocytes, myeloid cells, and dendritic cells. It possesses a central proline-rich region, two helical Src homology 3 (SH3)-like domains, and one Ena/VASP homology 1 (EVH1) binding domain (21, 31). ADAP also contains at least three potential tyrosine phosphorylation sites that likely become phosphorylated by the Src kinase Fyn after TCR engagement (2, 17).
Analysis of ADAP-deficient mice revealed a role of ADAP in regulating TCR-mediated inside-out signaling. Thus, ADAP−/− T cells show a defect in β1- and β2-integrin-mediated adhesion and a failure in integrin clustering after TCR stimulation (20, 45). More recently it was shown that mutation of two of the three tyrosine residues within ADAP (these sites are important for the inducible interaction with SLP-76) ablates the capability of ADAP to facilitate TCR-mediated activation of integrins (52). The present model therefore proposes that upon Fyn-mediated phosphorylation, ADAP interacts with the SH2 domain of SLP-76 (9, 42, 51). Subsequently, the ADAP/SLP-76 complex is recruited to the cell membrane via the small cytosolic adapter Gads, which binds to phosphorylated LAT and thus connects the TCR to integrin activation.
Another adapter protein that is believed to be involved in TCR-mediated activation of integrins is SKAP55. It contains a pleckstrin homology (PH) domain, a C-terminal Src homology 3 (SH3) domain, and three potential tyrosine phosphorylation sites. SKAP55 is structurally highly homologous to a widely expressed adapter protein called SKAP55-HOM (SKAP homologue, or SKAP55R) (36, 37, 39, 49). Most recently it was demonstrated that, similar to ADAP, SKAP55 is capable of enhancing TCR-mediated activation of β1- and β2-integrins and conjugating formation between T cells and antigen-presenting cells (53). Moreover, it was shown that after short interfering RNA (siRNA)-mediated knock down of SKAP55, conjugate formation and LFA-1 clustering are impaired in mouse T cells (24). Collectively, these data indicate that SKAP55 also regulates TCR-mediated activation of β2-integrins.
In T cells, SKAP55 constitutively associates with ADAP. This interaction involves the SH3 domain of SKAP55 and the proline-rich region in ADAP (36, 38). However, the molecular mechanism for how the ADAP/SKAP55 module regulates TCR-mediated adhesion is unknown, and a detailed structure-function analysis regarding this complex has not been performed so far.
In the present work, we have addressed these questions by using Jurkat T cells as a model system. We report that down modulation of SKAP55 expression by siRNA attenuates TCR-mediated adhesion to either ICAM-1 or fibronectin. Structure-function analysis further revealed that either the SH3 domain of SKAP55 or the central proline-rich region of ADAP is absolutely mandatory to facilitate activation of β1- or β2-integrins. We also show that Rap1-mediated activation of β1- and β2-integrins is impaired in cells in which the interaction between ADAP and SKAP55 is interrupted. However, this is not due to an alteration of Rap1 GTPase activity but rather due to displacement of Rap1 from the plasma membrane. Collectively, these data suggest that the ADAP/SKAP55 complex is required to target activated Rap1 to the plasma membrane. Surprisingly, our studies also revealed that the activation of integrins by the ADAP/SKAP55 module can take place outside of the “classical” lipid rafts.
MATERIALS AND METHODS
Knockout mice.
ADAP and SKAP-HOM knockout mice have been described previously (45, 49). Splenic T cells were prepared from wild-type, ADAP knockout, or SKAP-HOM knockout mice by standard separation techniques using AutoMACS (Miltenyl Biotech).
Cell culture, transfections, and flow cytometry.
HEK293 cells (American Type Culture Collection [ATCC]) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (FCS), 20 mM l-glutamine (Biochrom KG), and 1,000 U/ml penicillin-streptomycin (Biochrom KG) or 500 μg/ml hygromycin B (Roche) for HEK293 cells stably expressing secreted ICAM-1-Rg fusion protein. The Jurkat T-cell line (JE6.1; ATCC) was maintained in RPMI 1640 medium supplemented with 10% FCS and l-glutamine. Electroporation was performed as previously described (6). Briefly, Jurkat T cells (2 × 107) were transfected by electroporation using 30 μg for individual cDNA constructs or 60 μg in combinations of different cDNA constructs. An average of 60 to 70% of cells that overexpressed protein after our transfection protocol using 30- to 60-μg cDNA constructs was monitored on a regular basis by electroporation with green fluorescent protein (GFP). For siRNA of SKAP55, Jurkat T cells were transfected with 40 μg of the indicated vector and cultured for 48 to 72 h before use. For flow cytometric analysis, CD18 (BD Pharmingen), CD29 (BD Pharmingen), CD3 (OKT3; ATCC), or major histocompatibility complex class I (W6/32; ATCC) monoclonal antibodies (MAbs) were used, and samples were analyzed using a FACScalibur flow cytometer and CellquestPro software (Becton Dickinson).
Preparation of RNA and RT-PCR.
RNA was extracted from 1 × 107 purified splenic T cells using a NucleoSpin RNA II kit as specified by the manufacturer (Macherey-Nagel) and reverse transcribed into cDNA using the first-strand cDNA synthesis kit from Amersham Pharmacia according to the manufacturer's instructions. An aliquot (4 μg) of the reverse transcription (RT) reaction mixture was amplified with gene-specific primers by PCR (Hotstart Taq polymerase [QIAGEN]) and analyzed on a 2% Tris-acetate-EDTA agarose gel. The oligonucleotides used were SKAP-HOMfor (5′ CCT GTT GGC AGA TGT TGA AAC 3′), SKAP-HOMrev (5′ CAA ACC CCA GAA AGC TGT GAT C 3′), SKAP55for (5′ GAT CCG TTG GCT CCT GGA AG 3′), SKAP55rev (5′ AGG TCC CTT TGG GCT GCT TG 3′), β-Actinfor (5′ TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA 3′), and β-Actinrev (5′ CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG 3′). PCR products of SKAP55 and SKAP-HOM were cloned into the PCR2.1 TA vector according to the manufacturer's instructions (Invitrogen), and five independent clones were sequenced to ensure the specificity of the amplification.
cDNA constructs and siRNA of SKAP55.
For siRNA of SKAP55, the oligonucleotides SK4 (5′ GAAAGAATCCTGCTTTGAA 3′) and SK1 (5′ GCGATTAGAGATCATACTA 3′) were cloned into the pSuper (pS) vector (OligoEngine) according to the manufacturer's instructions. The same oligonucleotides, SK4 and SK1, were also cloned into the pCMS3-EGFP vector (kindly provided by Daniel D. Billadeau [Mayo Clinic, Minn.]). This vector contains a separate transcriptional cassette for GFP and is derived from the pSuper vector. The pEFBOS expression vectors encoding N-terminal FLAG-tagged mutants of SKAP55 and hemagglutinin (HA)-tagged SKAP55 have been described already (38). The N-terminal deletion cDNA construct of SKAP55 was generated by PCR and cloned in frame with the FLAG epitope into the pEFBOS vector, as previously described (38). GFP fusion proteins of the PH, the SH3, or the mutated SH3(W333K) domain of SKAP55 were generated by PCR and cloned into the pEGFP-N1 expression vector (Clontech). The SKAP55 cDNA construct was subcloned into the prokaryotic expression vector pGEX-4T1 (Amersham Pharmacia). pEFBOS containing N-terminal FLAG-tagged mutants of ADAP have been previously described (38), and the FADAP Pro cDNA construct was subcloned into the pEGFP-C1 expression vector (Clontech). The deletion of the SKAP55 binding site (amino acids 340 to 364) of the FADAP cDNA construct was created using the Quickchange II mutagenesis kit from Stratagene. The generation of the LAT-ADAP or the LAT-SKAP55 chimeras has been described already (3). The LAT partial cDNA was subcloned either into the FLAG-tagged SKAP55 or FLAG-tagged ADAP cDNA constructs, replacing the FLAG epitope. The construct of the secreted receptor-globulin fusion protein to the ICAM-1 extracellular domain (pCDM8-ICAM-Rg) was kindly provided by Waldemar Kolanus (University of Bonn, Germany). HARap1G12V or HARap1S17N cloned into pMT2 and pGEX RalGDS-RBD were kindly provided by Johannes L. Bos (Utrecht Medical Center, Utrecht, The Netherlands). The constructs for pDsRedC1-tagged Rap1wt were kindly provided by Ignacio Rubio (University of Jena, Germany).
Measurement of CD69 and calcium mobilization.
The measurement of CD69 upregulation and calcium mobilization have been described already (47). For TCR-induced CD69 expression, cells were cultured for 18 h in wells containing media alone, in wells with media containing 20 ng/ml phorbol myristate acetate (PMA), or in wells that had earlier been coated with the anti-CD3 MAb C305 (a kind gift of Arthur Weiss, University of California at San Francisco). After overnight culture, cells were stained with an anti-CD69 antibody (BD Pharmingen), and surface expression was analyzed by a fluorescent-activated cell sorter. For calcium mobilization, cells were loaded with Indo-1 AM (Molecular Probes) at 37°C for 45 min in RPMI 1640, without Phenol RED (Gibco BRL) and supplemented with 10% FCS. Cells were washed and reincubated for an additional 30 min at 37°C in the same RPMI medium. Baseline Ca2+ levels were measured for 30 s, and cells were stimulated with MAb C305 followed by ionomycin (2 μg/ml) treatment. Data for calcium mobilization were acquired on a FACSort flow cytometer (Becton Dickinson), and a ratiometric analysis was performed with Flow Jo software.
Adhesion assays.
Adhesion assays were performed as previously described (30, 43, 49). Briefly, Jurkat T cells (1 × 106 cells per dish) were washed with Hanks balanced salt solution (HBSS) and either left unstimulated or incubated with either MAb OKT3 (2 μg/ml), PMA (50 ng/ml; Calbiochem), or MnCl2 (1 mM) for 30 min at 37°C prior to the adhesion on fibronectin- or ICAM-1-coated dishes. After 30 min at 37°C, nonadherent cells were removed by washing three times with HBSS. The bound cell fraction was determined by counting four independent fields by microscopy using an ocular counting reticule. The fold induction of cell binding to either fibronectin or ICAM-1 was calculated as the ratio of the mean of stimulated (from four independent fields) to the mean of unstimulated empty vector (or GFP)-transfected cells (from four independent fields) for each experiment. Values are expressed as the means ± standard errors (SE) of three independent experiments. At a transfection efficiency of 60 to 70%, we generally observed a 2.0- to 3.0-fold increase in adhesion (compared to vector transfectants) in cells overexpressing wild-type SKAP55 or ADAP, as previously reported (53). These observations were noted in more than six experiments.
Immunoprecipitation and Western blotting.
Cell lysis and immunoprecipitations were performed as previously described (38, 49). Equivalent amounts of protein were used in precipitation experiments and Western blots. Cell lysates or immune complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Western blots were conducted with the indicated antibodies and developed with the appropriate horseradish peroxidase-conjugated secondary antibodies (Dianova) and the Luminol detection system (Roth). Anti-FLAG MAb M2 (Sigma), antitubulin MAb (Sigma), anti-GFP rabbit serum (Clontech), anti-SKAP55 rabbit serum (37), anti-SKAP55 MAb (BD Pharmingen), anti-SKAP-HOM rabbit serum (39), anti-β-actin MAb (Sigma), anti-EEA1 MAb (BD Pharmingen), anti-Na+/K+ ATPase α1-subunit rabbit serum (Sigma), anti-phospho-ERK1/2 rabbit serum (Cell Signaling), anti-ERK1/2 rabbit serum (Cell Signaling), anti-LAT rabbit serum (Upstate), anti-ADAP sheep serum (42), and anti-SLP-76 rabbit serum (19) were used for Western blotting and/or immunoprecipitation. Rat monoclonal antibodies were raised against the purified prokaryotic fusion protein encoded by the glutathione-S-transferase (GST)-SKAP55 expression plasmid as immunogen. Hybridoma supernatants were screened by Western blotting and immunofluorescence. Hybridomas secreting against SKAP55 were cloned twice, and among the various clones tested, the monoclonal antibody mABSK13B6F2 was selected.
Rap1 activity assay.
Jurkat T cells (5 × 106) were either left untreated or stimulated for 5 min with anti-CD3 MAb C305. Purified T cells (1 × 107 cells) from wild-type or ADAP knockout mice were incubated with biotinylated CD3 MAb (2C11; BD Pharmingen) and cross-linked with streptavidin (Sigma) for 3 min at 37°C. Subsequently, cells were lysed and active Rap1 was isolated using a GST-RalGDS-Rap1 binding domain (RBD) fusion protein (16), and bound Rap1 was quantified by Western blotting using an anti-Rap1 rabbit serum (Santa Cruz).
Isolation of lipid rafts and membrane fractions.
Preparation of lipid rafts by sucrose gradient centrifugation has been described previously (3, 47). Jurkat T cells (2 × 107 to 3 × 107 cells) were left unstimulated and lysed in morpholinepropanesulfonic acid (MES)-buffered saline (25 mM MES, pH 6.5, 150 mM NaCl) containing 1% Triton X-100 and supplemented with protease and phosphatase inhibitors. The lysate was mixed with 1 ml 80% sucrose in MES-buffered saline and overlaid with 2 ml of 30% and 1 ml of 5% sucrose in MES-buffered saline. Following centrifugation, fractions were removed sequentially starting from the top of the gradient, precipitated by trichloroacetic acid, and analyzed by Western blotting for LAT, SKAP55, or ADAP. Isolation of the cytosolic and plasma membrane fractions have been described elsewhere (43). Briefly, either resting or stimulated Jurkat T cells (1 × 107 to 2 × 107 cells) or purified splenic T cells from mice (4 × 107 to 6 × 107 cells) were washed in RPMI and resuspended on ice in a hypotonic buffer. Cells were sheared, and nuclei and unbroken cells were removed by low-speed centrifugation. The remaining supernatant was recentrifuged, and the cytosolic fraction (supernatant) was collected. The remaining pellet (membrane fraction) was washed twice with hypotonic buffer (washing fraction) and finally resuspended on ice in lysis buffer as described previously (38, 49). To ensure equal loading, the protein concentrations of the cytosolic and membrane fractions were determined using a Bradford assay (Roth). Western blot analysis revealed that the membrane fraction was strongly enriched for the plasma membrane markers Na+/K+ ATPase and LAT (an integral membrane protein) and contained only minor amounts of EEA1-positive membranes (EEA1 is an endosomal marker), whereas most of the EEA1-positive membranes were found in the washing fraction.
Confocal microscopy and quantification.
Jurkat T cells were transfected with either pCMS3E-C (scrambled) or pCMS3E-SK (for siRNA of SKAP55) in combination with pDsRed-tagged Rap1. After 48 h, either resting or stimulated (5 min with anti-CD3 MAb C305) Jurkat T cells were seeded onto poly-l-lysine-coated slides, fixed with 3.5% paraformaldehyde in phosphate-buffered saline, and mounted with Mowiol (Calbiochem). For each experiment, a minimum of at least 20 cells (coexpressing GFP and DsRed-tagged Rap1wt) were imaged with a LEICA TCS SP2 laser-scanning confocal system and LEICA software. COREL Photopaint histogram tools were used to obtain the number of red pixels per cell of Rap1 at the plasma membrane area or the total amount of Rap1.
RESULTS
Concomitant loss of SKAP55 and SKAP-HOM protein expression in ADAP-deficient T cells.
In T lymphocytes, SKAP55 and ADAP form a functional unit that is believed to be involved in the regulation of TCR-mediated adhesion via β1- and β2-integrins. Importantly, T lymphocytes not only express SKAP55 but also the homologue of SKAP55, SKAP-HOM (Fig. 1A). Previously, we had shown that in T cells, loss of SKAP-HOM does not alter the expression of ADAP or SKAP55, which suggested that SKAP-HOM does not influence the stability of ADAP or SKAP55 (49). In contrast, it was recently shown that loss of ADAP in Jurkat T cells destabilizes SKAP55 and induces its degradation (22). To assess the question of whether loss of ADAP also destabilizes SKAP55 and SKAP-HOM in primary T cells, we determined the expression levels of SKAP55 and SKAP-HOM protein in splenic T cells prepared from ADAP-deficient mice.
FIG. 1.
SKAP55 is critical for TCR-mediated adhesion of T cells to fibronectin and ICAM-1. (A) Postnuclear lysates prepared from purified splenic T cells of wild-type, ADAP−/−, or SKAP-HOM−/− mice were analyzed by anti-ADAP, anti-SKAP55, anti-SKAP-HOM, or anti-β-actin Western blotting. (B) RT-PCR analysis was performed using gene-specific primers for SKAP55, SKAP-HOM, and β-actin. (C) Jurkat T cells transfected with pS-SK1 (for siRNA of SKAP55 [SKAP55low]), pS-SK4 (scrambled), or pS (empty control vector) were either left unstimulated (non) or stimulated with phorbol ester (PMA), CD3 MAb OKT3 (TCR), or MnCl2 and subsequently analyzed for adhesion to fibronectin or ICAM-1. Adhesion data represent the means and SE of three independently performed experiments. (D) The efficiency of SKAP55 knockdown was assessed by anti-SKAP55 Western blotting. Antitubulin as well as anti-ADAP Western blotting served as a loading control. Note that the expression of ADAP is not affected by RNAi of SKAP55.
Figure 1A and B demonstrates that both SKAP55 and SKAP-HOM are undetectable in ADAP-deficient T cells on the protein level, whereas mRNA coding for both proteins is readily detectable. The specificity of the SKAP55 and the SKAP-HOM antisera was evident from analysis of SKAP-HOM-deficient T cells in which the SKAP55 antiserum clearly detected SKAP55, whereas the SKAP-HOM antiserum was nonreactive. Also, the SKAP55 antiserum did not detect a 55-kDa protein in B cells of wild-type, ADAP-deficient, or SKAP-HOM-deficient animals (data not shown), confirming previous data showing that B lymphocytes do not express ADAP or SKAP55. Thus, the data shown in Fig. 1A and B strongly suggest that in primary mouse T cells, loss of ADAP induces a concomitant destabilization of both SKAP55 and SKAP-HOM, thereby inducing their degradation. Further, they indicate that at least for the T-cell compartment the ADAP-deficient mouse is a triple knockout mouse that lacks the expression of ADAP, SKAP55, and SKAP-HOM.
Loss of SKAP55 alters TCR-mediated activation of β1- and β2-integrins.
The concomitant loss of SKAP55 and SKAP-HOM in ADAP-deficient T cells prompted us to ask whether the described loss of adhesiveness to β1- and β2-integrins in ADAP-deficient mice might be partially or even primarily due to the loss of SKAP55. To assess this question, we used siRNA interference of SKAP55 in Jurkat T cells which express ADAP and SKAP55 (but not SKAP-HOM) and therefore represent a suitable model to study the role of SKAP55 in TCR-mediated adhesion processes.
As shown in Fig. 1D, the SKAP55 siRNA treatment lowered the expression levels of endogenous SKAP55 to approximately 25%, whereas the expression levels of ADAP remained unaffected (Fig. 1D; see Fig. S1A in the supplemental material). The latter finding confirmed previously reported data showing that SKAP55 is not required for stable expression of ADAP (22). Flow cytometric analysis revealed comparable levels of CD18, CD29, and the TCR complex on the surface of SKAP55low Jurkat T cells (data not shown). Similarly, loss of SKAP55 did not influence TCR-mediated tyrosine phosphorylation, activation of ERK1/2, calcium mobilization, or upregulation of the activation marker CD69 following TCR engagement (see Fig. S1A to C in the supplemental material).
In marked contrast, SKAP55low Jurkat T cells displayed a clearly reduced capability to adhere to fibronectin or ICAM-1 after TCR triggering, while the basal adhesion to fibronectin or ICAM-1 or the activation of β1- or β2-integrins after PMA and/or Mn2+ stimulation was unaffected (Fig. 1C). In fact, the reduction of adhesion strongly correlated with the percent reduction in SKAP55 expression, which suggests that, similar to ADAP, SKAP55 also regulates TCR-mediated activation of integrins. Moreover, and in line with previously published work (24), these data show that loss of SKAP55 cannot be compensated for by ADAP.
TCR-mediated activation of β1- or β2-integrins requires the SH3 domain of SKAP55.
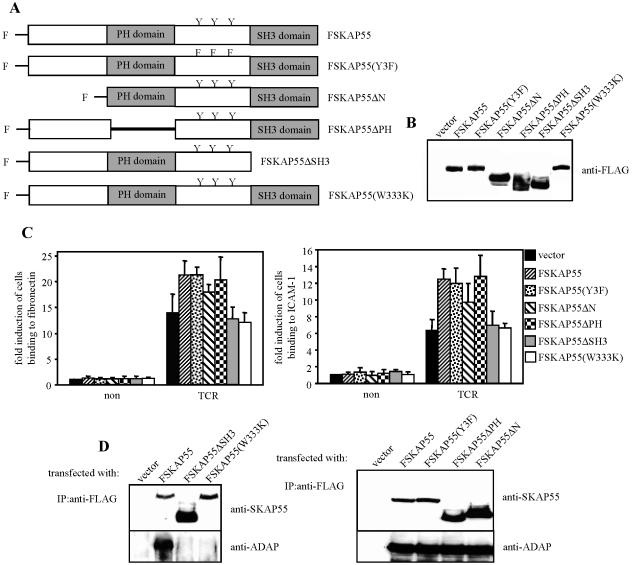
To assess which domain(s) of SKAP55 is important for SKAP55-mediated inside-out signaling, FLAG-tagged mutants of SKAP55 were transiently overexpressed in Jurkat T cells. The cDNAs coded for wild-type SKAP55 (FSKAP55) or SKAP55 mutants lacking either the PH domain (FSKAP55ΔPH), the SH3 domain (FSKAP55ΔSH3), the N-terminal segment (FSKAP55ΔN), or all three tyrosine residues (Fig. 2A). The transfection efficiency was analyzed 24 h posttransfection by anti-FLAG Western blotting (Fig. 2B), and the transfectants were subsequently assessed for their ability to adhere to ICAM-1 or fibronectin after antibody-mediated triggering of the TCR.
FIG. 2.
SH3 domain is critical for SKAP55-dependent β1- and β2-integrin activation. (A) Schematic representation of the individual FLAG (F)-tagged SKAP55 mutants used in this study. (B) Jurkat T cells were transiently transfected with either empty vector (vector), FSKAP55, FSKAP55(Y3F), FSKAP55ΔN, FSKAP55ΔPH, FSKAP55ΔSH3, or FSKAP55(W333K). The expression of the mutants was analyzed by anti-FLAG Western blotting. (C) The above transfectants were either left unstimulated (non) or were stimulated for 30 min with CD3 MAb OKT3 (TCR). Cells were analyzed for adhesion to fibronectin or to ICAM-1 as described in the legend to Fig. 1C. Data are the average means and SE from three independent experiments. (D) Jurkat T cells overexpressing the individual FLAG-tagged SKAP55 mutants were lysed, and FLAG-tagged proteins were immunoprecipitated using an anti-FLAG MAb. Precipitates were analyzed by Western blotting for the presence of SKAP55 and ADAP. IP, immunoprecipitation.
Figure 2C shows that overexpression of full-length SKAP55 enhanced the capability of Jurkat T cells to adhere to fibronectin or to ICAM-1, thus corroborating previously published data (53). Surprisingly, however, transfectants expressing either FSKAP55ΔPH, FSKAP55ΔN, or FSKAP55(Y3F) showed almost the same enhancement of adhesion to β1- and β2-ligands as cells overexpressing the wild-type protein (Fig. 2C). In fact, only FSKAP55ΔSH3, which lacks the binding site for ADAP, had an impaired ability to enhance TCR-mediated activation of β1- or β2-integrins. The same was true for an SKAP55 mutant carrying a W333K point mutation within the SH3 domain (Fig. 2C). These data suggest that the SH3 domain of SKAP55 and hence its interaction with ADAP (Fig. 2D) is primarily involved in regulating TCR-mediated adhesion, whereas the PH domain, the N-terminal region, and the three tyrosine residues within the interdomain appear to be dispensable.
TCR-mediated activation of β1- or β2-integrins requires the proline-rich fragment of ADAP.
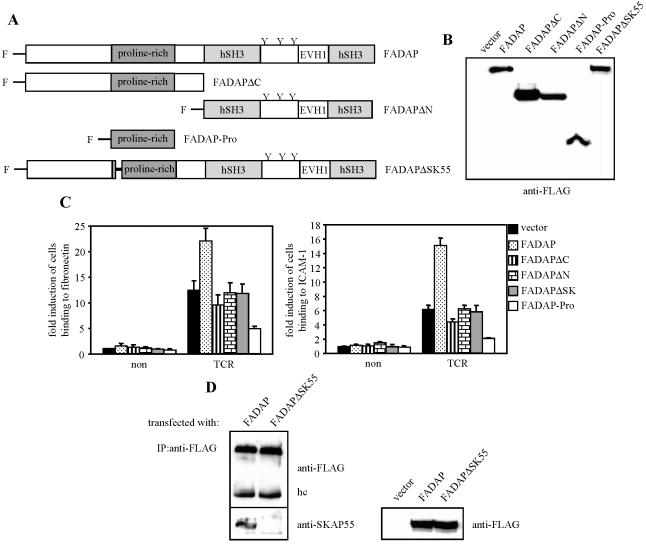
According to the currently proposed model, the interaction of ADAP with SKAP55 is mediated via a proline-rich segment within ADAP that binds to the C-terminal SH3 domain of SKAP55 (38). We thus assumed that any modification of ADAP affecting this interaction should also impede TCR-mediated adhesion of β1- and β2-integrins. To investigate this point, we overexpressed FLAG-tagged mutants of ADAP (Fig. 3A and B) in Jurkat T cells and subsequently analyzed TCR-mediated adhesion as described above.
FIG. 3.
Proline-rich region of ADAP is required for TCR-dependent β1- and β2-integrin activation. (A) Schematic representation of the FLAG (F)-tagged ADAP mutants used in this study. (B) Jurkat T cells were transiently transfected with either empty vector (vector), FADAP, FADAPΔN, FADAPΔC, FADAP-Pro, or FADAPΔSK. Expression of the ADAP mutants was analyzed by anti-FLAG Western blotting. (C) Jurkat T cells overexpressing the individual FLAG-tagged ADAP mutants were analyzed for adhesion to fibronectin or ICAM-1. Transfectants were either unstimulated (non) or stimulated with anti-CD3 MAb OKT3 (TCR) as described in the legend to Fig. 1C. Data represent the average means and SE of three independent experiments. (D) Jurkat T cells overexpressing FADAP or FADAPΔSK were lysed, and FLAG-tagged proteins were immunoprecipitated using an anti-FLAG MAb. Precipitates and total lysates were analyzed by Western blotting for the presence of SKAP55 and FLAG-tagged proteins, respectively. IP, immunoprecipitation; hc, heavy chain.
As expected, overexpression of wild-type FADAP augmented adhesion of Jurkat T cells to ICAM-1 or fibronectin (Fig. 3C), whereas overexpression of the C-terminal deletion mutant (FADAPΔC, which is incapable of binding to its putative upstream interaction partner, SLP-76) or the N-terminal deletion mutant (FADAPΔN, which cannot bind to SKAP55) did not enhance (or even slightly impaired) adhesion. Similarly, an ADAP mutant (FADAPΔSK55) in which 24 amino acids (amino acids 340 to 364) within the proline-rich segment that are involved in the binding of ADAP to SKAP55 (Fig. 3D) were eliminated also failed to facilitate TCR-mediated activation of β1- or β2-integrins (Fig. 3C).
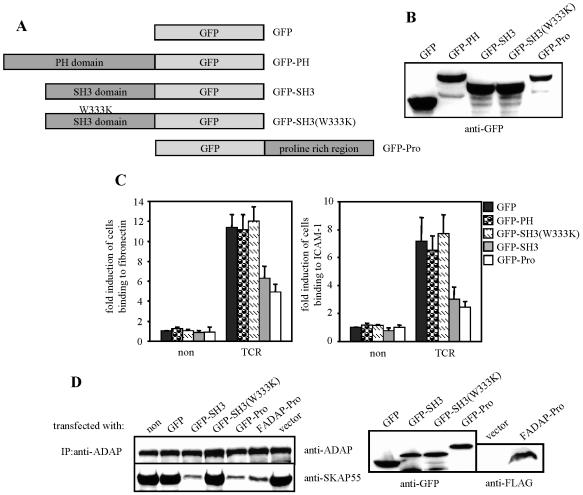
To further corroborate that the interaction between ADAP and SKAP55 is mandatory for mediating inside-out signaling, we next overexpressed the isolated Pro domain of ADAP (ADAP-Pro) as well as the isolated SH3 domain of SKAP55 (SKAP55-SH3) in Jurkat T cells (Fig. 4A and B) and subsequently investigated the ability of the transfectants to adhere to β1- and β2-integrins.
FIG. 4.
Isolated SH3 domain of SKAP55 or the proline-rich region of ADAP interferes with TCR-mediated activation of β1- and β2-integrins. (A) Scheme showing the GFP fusion proteins utilized in this study. (B) Jurkat T cells were transfected with either GFP, GFP-SKAP55-PH (GFP-PH), GFP-SKAP55-SH3 (GFP-SH3), GFP-SKAP55-SH3(W333K) [GFP-SH3(W333K)], or GFP-ADAP-Pro (GFP-Pro), and the expression of the GFP fusion proteins was assessed by anti-GFP Western blotting. (C) Transfectants were assessed for adhesion as described in the legend to Fig. 1C. non, nonstimulated; TCR, stimulated. Data represent the means and SE of at least three independent experiments. (D) Nontransfected (non) Jurkat cells or cells transfected with either GFP, GFP-SKAP55-SH3 (GFP-SH3), GFP-SKAP55-SH3(W333K) [GFP-SH3(W333K)], GFP-ADAP-Pro (GFP-Pro), FADAP-Pro, or empty vector (vector) were lysed, and endogenous ADAP was immunoprecipitated using a polyclonal sheep anti-ADAP serum. Precipitates (left) and total lysates (right) were analyzed by Western blotting for the presence of ADAP, SKAP55, GFP, or FLAG-tagged ADAP-Pro. IP, immunoprecipitation.
As shown in Fig. 4C and D, both ADAP-Pro and SKAP55-SH3 interrupted the interaction between endogenous SKAP55 and endogenous ADAP and consequently exerted a dominant-negative effect that strongly reduced TCR-mediated adhesion to ICAM-1 and fibronectin (see also Fig. 3C for the effect of the FLAG-tagged version of ADAP-Pro, FADAP-Pro). In contrast, neither the isolated PH domain of SKAP55 (SKAP55-PH) nor the isolated SKAP55-SH3(W333K) domain affected the activation of β1- and β2-integrins (Fig. 4C).
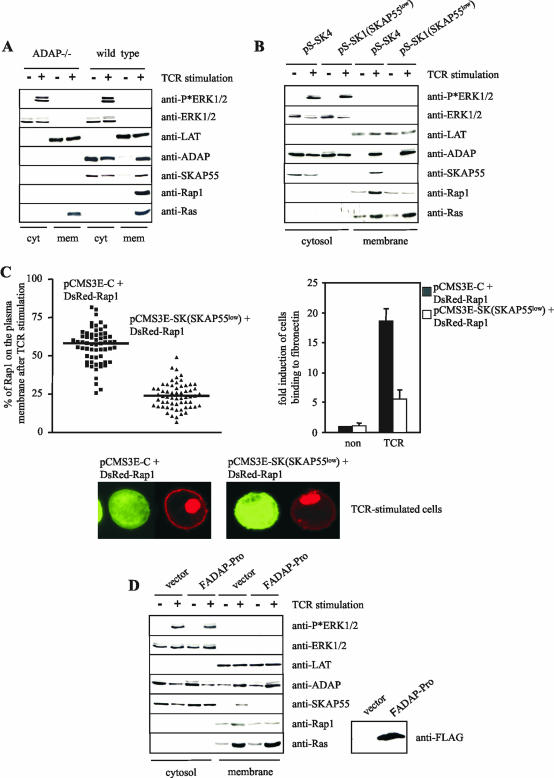
It is important to note that expression of either ADAP-Pro or SKAP55-SH3 did not alter the expression levels of CD18, CD29, or the TCR (data not shown). Also, TCR-mediated global tyrosine phosphorylation or ERK activation, TCR-mediated upregulation of CD69, and TCR-induced calcium influx were not affected (see Fig. S2A to C in the supplemental material). In summary, the data shown in Fig. 4 further corroborate the idea that the interaction between ADAP and SKAP55 is mandatory for TCR-mediated adhesion. Moreover, they demonstrate that overexpression of ADAP-Pro or SKAP55-SH3 is a suitable tool for specifically disrupting the ADAP/SKAP55 module without affecting other signaling events in these transfectants.
The SKAP55/ADAP module can signal outside of the “classical” lipid raft fraction of the plasma membrane.
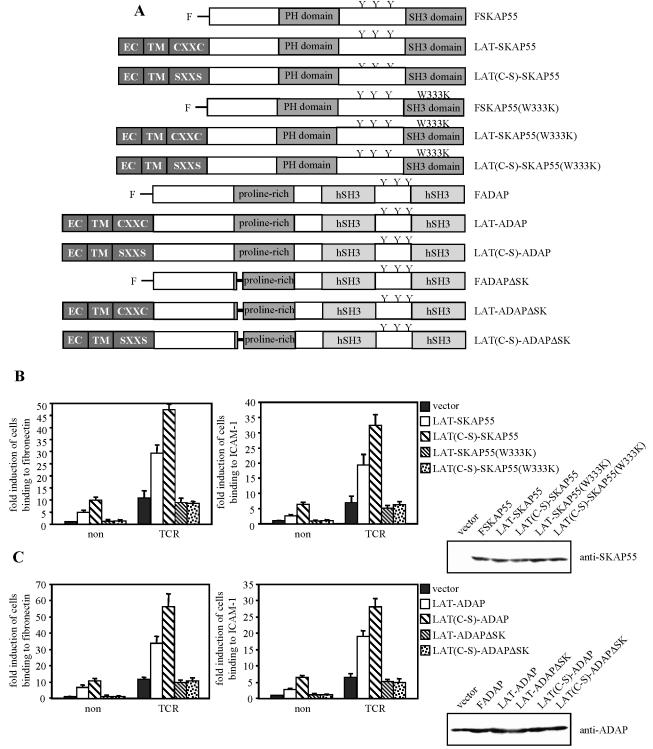
Previously, it has been suggested that SKAP55 translocates to the lipid rafts after TCR triggering (53). However, this study did not assess the percentage of endogenous SKAP55 that translocates from the nonrafts to the rafts after TCR engagement, and it also did not address the question of whether lipid-raft association of SKAP55 is required for TCR-mediated activation of β1- and β2-integrins. To investigate this latter point in more detail, we generated LAT chimeras of ADAP or SKAP55, which consisted of the extracellular and the transmembrane domains of the transmembrane adapter protein LAT fused to either full-length ADAP (LAT-ADAP) or SKAP55 (LAT-SKAP55), respectively (Fig. 5A). The LAT fraction of the chimeras included a small part of the cytoplasmic domain that contains two cysteine residues, C26 and C29, which have been reported to play a critical role in lipid-raft targeting of LAT (35, 55).
FIG. 5.
ADAP/SKAP55 complex can signal in the nonraft fraction of the plasma membrane. (A) Scheme showing the individual LAT-SKAP55 and LAT-ADAP chimeras used in these experiments. SXXS mutants carry cysteine-to-serine mutations at positions 26 and 29 of LAT. EC, extracellular domain; TM, transmembrane domain; y, tyrosine residue. (B) Jurkat T cells transiently overexpressing the LAT-SKAP55 chimeras were left unstimulated (non) or were stimulated with CD3 MAb OKT3 (TCR) for 30 min and then analyzed for adhesion to fibronectin or ICAM-1. The expression of the individual SKAP55 mutants was assessed by anti-SKAP55 Western blotting. Data represent the means and SE of at least three independent experiments. (C) Jurkat T cells transiently overexpressing the LAT-ADAP chimeras were either left unstimulated (non) or stimulated for 30 min with CD3 MAb OKT3 (TCR) and subsequently analyzed for adhesion to fibronectin or ICAM-1. The expression of the ADAP mutants was analyzed by anti-ADAP Western blotting. Data represent the means and SE of three independent experiments.
Figure 5B and C show that, in contrast to overexpression of wild-type FSKAP55 or FADAP, constitutive membrane targeting of either molecule induces T-cell adhesion to ICAM-1 or fibronectin, even in the absence of TCR stimulation (note the different scale of the y axis in Fig. 5B and C compared to those of previous figures). Moreover, overexpression of the LAT-SKAP55 or LAT-ADAP chimeras augmented TCR-mediated adhesion to a larger extent than the corresponding nonchimeric molecules. These data suggest that membrane recruitment of ADAP or SKAP55 alone is sufficient to activate integrins.
Surprisingly, however, both the basal as well as the TCR-induced adhesion were much stronger when LAT-SKAP55 or LAT-ADAP chimera was expressed that was not targeted to the “classical” (floating in sucrose gradients) lipid rafts because of mutation of the membrane-proximal palmitoyliation motif, CxxC of LAT [LAT(C-S)-SKAP55 or LAT(C-S)-ADAP chimera, Fig. 5B and C]. Indeed, the basal adhesion induced by the C-S mutants was of similar magnitude to the TCR-mediated adhesion of the vector transfectants. The functional differences between the LAT-X chimera versus the LAT(C-S)-X chimera could not be explained by different expression levels, as judged from Western blot analysis of the transfectants. Moreover, all four chimeras were detected by Western blotting in membrane fractions prepared from the individual transfectants, which rules out the possibility that some of the chimeras were not properly inserted into the cell membrane (see Fig. S3A and B in the supplemental material). Furthermore, analysis of the individual transfectants revealed that LAT(C-S)-SKAP55 and LAT(C-S)-ADAP chimeras were exclusively found in the nonfloating fractions (see Fig. S3C in the supplemental material). Thus, it seems as if the presence of the “classical” lipid rafts is not a prerequisite for the APAP/SKAP55 module to regulate integrin function.
Importantly, elimination of the ADAP binding site within the LAT-SKAP55 or the LAT(C-S)-SKAP55 chimera or mutation of the SKAP55 binding site within the LAT-ADAP or the LAT(C-S)-ADAP chimera extinguished the capability of the chimeras to induce the constitutive adhesion and to augment the TCR-mediated adhesion (Fig. 5B and C). Thus, also in this experimental setting an intact ADAP/SKAP55 complex is required for TCR-mediated activation of β1- and β2-integrins.
Functional interrelationship between the Rap1 and the ADAP/SKAP55 module-mediated signaling pathways.
The small GTPase Rap1 has previously been shown to be involved in regulating T-cell adhesion (4). To address the question of whether the ADAP/SKAP55 complex regulates the functional activity of Rap1, we overexpressed a constitutive active mutant of Rap1 (Rap1G12V) in Jurkat T cells in which expression of SKAP55 was downregulated by RNA interference. Subsequently, the transfectants were investigated for their capability to adhere to β1- or β2-integrins.
In line with previously published work (1, 10), overexpression of Rap1G12V induced a strong basal β1- and β2-mediated adhesion which could be further augmented by TCR stimulation (Fig. 6A). Importantly, however, down modulation of SKAP55 expression by RNAi strongly antagonized the functional effect of Rap1G12V, and the very same effect was produced when the interaction of ADAP and SKAP55 was inhibited (either by overexpression of ADAP-Pro or by overexpression of SKAP55-SH3 [see Fig. S4A and B in the supplemental material]). These data clearly suggest that an intact ADAP/SKAP55 complex is required for Rap1G12V to regulate adhesion.
FIG.6.
Loss of SKAP55 inhibits Rap1G12V-mediated adhesion but not TCR-induced Rap1 GTPase activity. (A) Jurkat T cells were transfected with pCMS3E-C (scrambled), HA-tagged Rap1G12V (HARap1G12V), and pCMS3E-SK (for siRNA of SKAP55 [SKAP55low]) alone or in combination. Prior to adhesion assay, the transfectants were left unstimulated (non) or were stimulated for 30 min with CD3 MAb OKT3 (TCR). Adhesion data represent the means and SE of three independent experiments. Expression of HARap1G12V was assessed by anti-HA Western blotting (lower panel). The reduction of SKAP55 expression was assessed by anti-SKAP55 Western blotting, and anti-ADAP Western blotting served as a control. (B) Jurkat T cells transiently overexpressing empty vector (vector) or FADAP-Pro were either left unstimulated (−) or were stimulated with CD3 MAb C305 (+) for 5 min. Endogenous Rap1 was precipitated using a GST-RalGDS-RBD fusion protein. Precipitates were probed with polyclonal rabbit anti-Rap1 serum to assess the amount of active Rap1 in the sample. Aliquots of total lysates were analyzed in parallel by anti-FLAG or anti-Rap1 Western blotting. (C) Jurkat T cells overexpressing only GFP or GFP-SKAP55-SH3 (GFP-SH3) were stimulated and assessed for Rap1 activity as described for panel B. Aliquots of total lysates were analyzed in parallel by anti-Rap1 or anti-GFP Western blotting. (D) Splenic T cells were prepared from ADAP-deficient (ADAP−/−) or wild-type mice and were either left unstimulated (−) or stimulated with CD3 MAb 2C11 (+) for 3 min at 37°C. Subsequently, Rap1 GTPase activity was analyzed as described above. (E) Nontransfected Jurkat T cells or cells transfected with pS-SK1 (for siRNA of SKAP55 [SKAP55low]), pS-SK4 (scrambled), or pS (empty control vector) were either left unstimulated (non) or were stimulated for 5 min with CD3 MAb C305 (+). The GTPase activity of endogenous Rap1 was subsequently assessed as described above. SKAP55 knockdown efficiency was investigated by anti-SKAP55 Western blotting, and antitubulin Western blotting served as a loading control.
Rap1 cycles between a GDP-bound inactive state and a GTP-bound active state. Switching between these two states is regulated by specific guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). To assess whether the ADAP/SKAP55 module is involved in regulating Rap1 activity, we determined the GTPase activity of endogenous Rap1 in Jurkat T cells in which either ADAP-Pro or SKA55-SH3 was overexpressed by using a previously described pull-down assay that employs the GST-coupled Rap binding domain (RBD) of RalGDS (RalGDS-RBD) (16).
As shown in Fig. 6B and C, neither ADAP-Pro nor SKAP55-SH3 influenced the TCR-induced activation of Rap1. Similarly, we did not find a significant alteration of TCR-mediated activation of Rap1 in ADAP-deficient T lymphocytes (Fig. 6D) or in Jurkat T cells in which the expression of SKAP55 was downregulated by siRNA (Fig. 6E). Note that this was also true for different time points after T-cell activation or different concentrations of CD3 MAb (data not shown).
Finally, overexpression of wild-type FSKAP55, LAT-SKAP55, and LAT(C-S)-SKAP55 or of FADAP, LAT-ADAP, and LAT(C-S)-ADAP did not influence the TCR-mediated GTPase activity of Rap1 (see Fig. S5A and B in the supplemental material). Still, the functional effects exerted by these molecules were clearly dependent on Rap1, as overexpression of a dominant-negative version of Rap1 (Rap1S17N) ablated the ability of LAT-SKAP55 and LAT(C-S)-SKAP55 to facilitate integrin activation (see Fig. S6A and B in the supplemental material).
Thus, although the APAP/SKAP55 complex appears to be located in the same TCR-mediated pathway that regulates the activation of integrins via Rap1, the ADAP/SKAP55 module does not interfere (directly or indirectly) with TCR-mediated activation of Rap1.
The ADAP/SKAP55 module facilitates TCR-mediated plasma membrane recruitment of Rap1.
A recent report had suggested that in activated T cells, GTP-bound (active) Rap1 is mainly detectable at the plasma membrane (1). Our finding showing that disruption of the ADAP/SKAP55 module impairs TCR-mediated adhesion without altering Rap1 activity prompted us to investigate whether the ADAP/SKAP55 complex might be required to recruit Rap1 to the plasma membrane.
To elucidate this point, we prepared cytosolic and plasma membrane fractions from resting and TCR-activated T cells and subsequently assessed the amount of Rap1 within the individual fractions by Western blotting.
Figure 7A shows that TCR stimulation of wild-type T cells induced a rapid recruitment of ADAP, SKAP55, and Rap1 to the plasma membrane fraction. In marked contrast, under the same experimental conditions, TCR-mediated membrane targeting of Rap1 was completely lost in ADAP-deficient T lymphocytes. This indicates that expression of the ADAP/SKAP55 module is mandatory for Rap1 to be recruited to the plasma membrane.
FIG.7.
Disruption of the ADAP/SKAP55 module interferes with the plasma membrane localization of Rap1. (A) Purified splenic T cells prepared from ADAP-deficient (ADAP−/−) or wild-type mice were either left unstimulated (−) or stimulated with CD3 MAb 2C11 (+) for 5 min at 37°C. Subsequently, membrane and cytosolic fractions were prepared from unstimulated (−) or stimulated (+) cells. Note that the membrane fraction was strongly enriched in plasma membranes, as indicated by the presence of Na+/K+ ATPase, the transmembrane adapter protein LAT, and almost complete absence of the endosomal marker EEA1. The individual samples were analyzed by Western blotting using anti-ADAP, anti-SKAP55, anti-Rap1, and anti-Ras antibodies. To control the fractionation efficiency and proper TCR stimulation, fractions were assessed for the presence of LAT (plasma membrane), ERK1/2 (cytosol), or phospho-ERK1/2 (stimulation). (B) Jurkat T cells were transiently transfected with either pS-SK4 (scrambled) or pS-SK1 (siRNA of SKAP55 [SKAP55low]) and were either left unstimulated (−) or were stimulated for 5 min with the CD3 MAb C305. Cytosolic and membrane fractions were analyzed as described above. (C) Jurkat T cells were transiently transfected with either pCMSE-C (scrambled) or pCMS3-SK (siRNA of SKAP55 [SKAP55low]) in combination with DsRed-tagged Rap1. Cells were stimulated for 5 min using CD3 MAb C305 and were imaged with a confocal laser-scanning microscope. Images are representative of three independent experiments. The graph indicates the percentage of Rap1 translocated on the plasma membrane of a total of 60 cells analyzed in three independent experiments. In parallel, transfectants were analyzed for their capability to adhere to fibronectin (unstimulated [non] or stimulated for 30 min with CD3 MAb OKT3 [TCR]). Adhesion data represent the means and SE of three independent experiments. (D) Jurkat T cells were transiently transfected with either empty vector or FADAP-Pro. Cells were either left unstimulated (−) or were stimulated using CD3 MAb C305 (+), and cytosolic and membrane fractions were subsequently prepared. The overexpression of FADAP-Pro was assessed by anti-FLAG Western blotting.
To next assess the question of whether loss of ADAP or loss of SKAP55 is primarily responsible for membrane recruitment of Rap1 in ADAP-deficient T cells (note that these T cells lack the expression of both ADAP and SKAP55 [Fig. 1A]), we determined TCR-mediated membrane recruitment of Rap1 in Jurkat T cells in which SKAP55 expression was downregulated by means of RNAi. The biochemical and confocal laser-scanning data shown in Fig. 7B and C clearly demonstrate that depletion of SKAP55 attenuated TCR-mediated membrane targeting of Rap1, while TCR-mediated membrane recruitment of ADAP and Ras were unaffected. Similarly, overexpression of ADAP-Pro (which disrupts the interaction between ADAP and SKAP55 [Fig. 7D]) displaced both SKAP55 and Rap1 from the plasma membrane, while TCR-mediated membrane association of ADAP remained normal (Fig. 7D). Collectively, these data indicate that ADAP can only regulate TCR-mediated adhesion processes if SKAP55 is coexpressed. Further, they suggest that the phenotype of ADAP-deficient T cells can be experimentally mimicked by depletion of SKAP55.
DISCUSSION
In the present study, we have performed a structure-function analysis of the ADAP/SKAP55 signaling complex and assessed its involvement in TCR-mediated activation of integrins. We show for the first time that primary peripheral blood T cells prepared from ADAP-deficient mice concomitantly lack expression of SKAP55 protein, while SKAP55 mRNA expression is readily detectable (Fig. 1A and B). These findings corroborate previous data obtained in ADAP-deficient Jurkat T cells which showed that ADAP is required to stabilize SKAP55 (22).
We also demonstrate that, in addition to SKAP55, expression of SKAP-HOM, the widely expressed homologue of SKAP55, is impaired in ADAP-deficient T cells (Fig. 1A). Thus, it appears as if in T cells ADAP stabilizes both SKAP55 and SKAP-HOM. Clearly, the opposite is not the case. Indeed, we have previously demonstrated that SKAP-HOM-deficient T cells express normal amounts of ADAP and SKAP55, indicating that expression of SKAP-HOM is dispensable for stable expression of the ADAP/SKAP55 complex (49). In addition, we corroborate the previous observation (22) that downregulation of SKAP55 expression by siRNA has no effect on the stability of ADAP (Fig. 1D; see Fig. S1A in the supplemental material). Thus, the data obtained in ADAP-deficient mice and in Jurkat T cells indicate that ADAP is required to stabilize the expression of both SKAP55 and SKAP-HOM, and perhaps more importantly, they suggest that at least in the T-cell compartment, the ADAP-deficient mouse is a triple knockout that lacks expression of ADAP, SKAP55, and SKAP-HOM. The molecular basis underlying the loss of SKAP55 and SKAP-HOM in the absence of ADAP is not yet clear.
Given the above findings, it was necessary to address the question of whether SKAP55 plays a role similar to that of ADAP with regard to TCR-mediated activation of integrins. Since SKAP55-deficient mice are not available yet, we utilized Jurkat T cells (which do not express SKAP-HOM) in which we downregulated the expression of SKAP55 by means of siRNA. While we did not observe obvious membrane-proximal signaling defects in the SKAP55low Jurkat T cells (see Fig. S1A to C in the supplemental material), these cells clearly showed a reduced capability to adhere to both β1- and β2-integrins after stimulation of the TCR (Fig. 1C). These findings indicate that SKAP55 is also an important component of the signaling machinery leading to the activation of integrins, and they corroborate previous findings showing augmented adhesion in murine T cells overexpressing SKAP55 (53).
Structure-function analysis of the ADAP/SKAP55 signaling module revealed that any mutation within ADAP or SKAP55 that impedes the binding between the two molecules also strongly attenuated activation of integrins (Fig. 2C and 3C). However, in contrast to ADAP, for which we could demonstrate the involvement of two different domains (N terminal and C terminal) in inside-out signaling, structure-function analysis of SKAP55 revealed the surprising result that only the C-terminal SH3 domain (that mediates the binding between SKAP55 and ADAP) appears to be required for functional activity. Indeed, neither deletion of the N-terminal part of SKAP55, deletion of the entire PH domain, nor mutation of the three tyrosine residues within the interdomain between the PH domain and the SH3 domain had a significant effect on the ability of SKAP55 to augment TCR-mediated adhesion (Fig. 2C). In contrast, deletion of the SH3 domain or introduction of a point mutation within the SH3 domain completely abrogated the ability of SKAP55 to augment TCR-mediated adhesion (Fig. 2C).
We are aware that our findings are in discordance with a recent report showing that the RKXXY294XXY297 motif of SKAP55 is involved in regulating TCR-mediated adhesion and TCR-mediated activation of an NFAT/AP1-luciferase reporter construct (12). However, in multiple transfection/overexpression experiments we did not observe an obvious effect of SKAP55 or any of its mutants upon TCR-mediated activation of various luciferase-based reporter genes (B. Schraven, unpublished data). Moreover, our present finding that TCR-mediated adhesion in Jurkat T cells overexpressing the SKAP55 triple tyrosine mutant is comparable to cells overexpressing wild-type SKAP55 is highly reproducible. Hence, at present we can only explain the discrepancy between the two studies by the usage of different Jurkat T-cell lines that might differ in their requirement to activate integrins after TCR stimulation or by different methods and treatments to activate the cells.
One possibility to explain the observation that only one domain of SKAP55 is involved in TCR-mediated integrin regulation would be that SKAP55 does not act as a typical adapter protein (meaning that it is not a component of a linear signaling pathway from the plasma membrane to the integrins). Rather, by binding to ADAP, SKAP55 could alter the conformation of ADAP, thereby modifying its signaling functions (see also below). Alternatively, it might be that the SH3 domain of SKAP55 is capable of binding two signaling molecules simultaneously, as described for the SH2 domain of the small adapter protein SAP (7) In this regard, it is important to note that we have previously shown that the SH3 domain of SKAP55 (besides regulating the interaction between SKAP55 and ADAP) is critically involved in mediating an association between SKAP55 and the negative regulatory receptor SHPS-1 (48). Importantly, however, the association between the SH3 domain of SKAP55 and SHPS-1 is not based on the classical SH3-proline motifs, because a SKAP55-SH3(W333K) mutant which has lost its ability to bind ADAP still can interact with SHPS-1 (48). Clearly, additional studies are required to solve the puzzle of how SKAP55 mediates protein-protein interactions that regulate TCR-mediated adhesion.
Another important observation that emerged from our studies is that membrane targeting of either ADAP or SKAP55 induced adhesion of T cells even in the absence of any stimuli. This indicated that TCR-mediated integrin activation depends on membrane recruitment of the ADAP/SKAP55 module. Indeed, cells expressing constitutively membrane-targeted SKAP55 or ADAP resembled Jurkat T cells overexpressing a constitutive active form of Rap1, Rap1G12V (compare Fig. 5B and C with Fig. 6A; see Fig. S4A and B in the supplemental material).
Surprisingly, however, we observed that LAT-ADAP and LAT-SKAP55 chimeras activated integrins much better when the CxxC-motif of LAT, which targets the chimeras (as well as endogenous LAT) to the “classical” lipid rafts (as defined by flotation in sucrose gradients [see Fig. S3C in the supplemental material]), was eliminated. These findings suggested that the ADAP/SKAP55 module can exert its signaling function outside of the raft fraction. In this regard, it is important to note that potent signaling via a nonraft compartment of the cell membrane has most recently also been described for the cytosolic adapter protein SLP-76 and the classical lipid raft-associated transmembrane adapter protein LAT (44, 56). However, whether the ADAP/SKAP55 module indeed signals nonclassical (i.e., nonfloating) types of rafts or a true “nonraft” compartment requires further investigation.
The membrane-targeting experiments also showed that integrin activation via ADAP/SKAP55 is dependent on Rap1, since coexpression of dominant-negative Rap1 antagonized the constitutive and TCR-mediated adhesion exerted by the LAT-SKAP55 (see Fig. S6 in the supplemental material) and LAT-ADAP chimeras (data not shown). However, the converse also holds true, because even constitutive active Rap1 was unable to mediate T-cell adhesion in the absence of a functional ADAP/SKAP55 complex (Fig. 6A; see Fig. S4A and B in the supplemental material). Further biochemical and confocal laser-scanning analysis revealed that the ADAP/SKAP55 module does not regulate the GTPase activity of Rap1 (Fig. 6B to E; see Fig. S5A and B in the supplemental material) but rather is required for plasma membrane recruitment of Rap1 (Fig. 7).
At present it is not clear whether, in T cells, the activation of Rap1 occurs exclusively at the plasma membrane or within an intracellular compartment that subsequently fuses with the plasma membrane (1, 26, 33). Hence, one possibility to explain our finding that disruption of the ADAP/SKAP55 module ablates membrane targeting of Rap1 without altering its GTPase activity would be that the ADAP/SKAP55 module is involved in membrane anchoring of Rap1 after the GTPase has been activated intracellulary. Another possibility would be to assume that the ADAP/SKAP55 module enhances the half life of active Rap1 at the plasma membrane. In such a model, the activation of Rap1 could take place at the plasma membrane (as recently suggested by Bivona et al. [1]), but in the absence of the ADAP/SKAP55 module, the half life of functional Rap1 at the plasma membrane would be too short to allow proper integrin activation. To assess both points, it will be necessary to study the trafficking of endogenous Rap1 in the presence and absence of the ADAP/SKAP55 module. Experiments are in progress in our laboratory to assess this question.
The molecular mechanism that underlies the interaction between the ADAP/SKAP55 module and Rap1 also remains to be determined. Again, one possibility would be to assume that the binding of SKAP55 to ADAP induces a conformational change in ADAP that allows Rap1 to bind to the ADAP/SKAP55 module. Whether this hypothesis is correct and, if so, whether Rap1 binds directly or indirectly (for example, via the recently described Rap1 effectors RIAM [33] and RAPL [26]) to the ADAP/SKAP55 module requires further analysis.
However, it is important to note that it has recently been shown that Rap1 is recruited to the cytoplasmic domain of the β1-integrins via the serine kinase PKD1, and that this event is critical for inside-out signaling and integrin activation (40). The molecular basis for the inducible association between PKD1 and Rap1 on the one hand and between PKD1 and the cytoplasmic domains of the β1-integrins on the other hand are as yet unclear. Thus, further studies are required to assess the relationship between the ADAP/SKAP55/Rap1- and the PKC/PKD1/Rap1-mediated signaling pathways leading to integrin activation.
Similarly, it will be necessary to investigate whether the ADAP/SKAP55 module not only regulates TCR-mediated adhesion but also other cellular processes, such as chemokine-mediated migration or adhesion mediated by cytokine receptors. In this regard, it is important to note that preliminary data obtained in our laboratory suggest that SDF-1-mediated migration is also affected in the absence of a functional ADAP/SKAP55 module (i.e., in ADAP-deficient primary mouse T cells or in SKAP55low Jurkat T cells), but additional studies are required to assess the molecular basis of these findings.
Nevertheless, our observation that TCR-mediated activation of integrins is regulated by the ADAP/SKAP55 module and that the function of this module is to recruit Rap1 to the plasma membrane adds an additional piece to the molecular puzzle as to how inside-out signaling and activation of integrins is regulated in T lymphocytes.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft grants KL1292/5-1 and GRK1167 as well as by grants from the U.S. National Institutes of Health.
We thank C. Merten, A. Nehring, J. Hoppe, and P. Glintschel for excellent technical assistance. We thank Vaclav Horejsi for providing monoclonal antibodies.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bivona, T. G., H. H. Wiener, I. M. Ahearn, J. Silletti, V. K. Chiu, and M. R. Philips. 2004. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J. Cell Biol. 164:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerth, N. J., B. A. Judd, and G. A. Koretzky. 2000. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J. Biol. Chem. 275:5143-5152. [DOI] [PubMed] [Google Scholar]
- 3.Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123-128. [DOI] [PubMed] [Google Scholar]
- 5.Bos, J. L., J. de Rooij, and K. A. Reedquist. 2001. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2:369-377. [DOI] [PubMed] [Google Scholar]
- 6.Bruyns, E., H. Kirchgessner, S. Meuer, and B. Schraven. 1998. Biochemical analysis of the CD45-p56(lck) complex in Jurkat T cells lacking expression of lymphocyte phosphatase-associated phosphoprotein. Int. Immunol. 10:185-194. [DOI] [PubMed] [Google Scholar]
- 7.Chan, B., A. Lanyi, H. K. Song, J. Griesbach, M. Simarro-Grande, F. Poy, D. Howie, J. Sumegi, C. Terhorst, and M. J. Eck. 2003. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5:155-160. [DOI] [PubMed] [Google Scholar]
- 8.Crittenden, J. R., W. Bergmeier, Y. Zhang, C. L. Piffath, Y. Liang, D. D. Wagner, D. E. Housman, and A. M. Graybiel. 2004. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 10:982-986. [DOI] [PubMed] [Google Scholar]
- 9.da Silva, A. J., Z. Li, C. de Vera, E. Canto, P. Findell, and C. E. Rudd. 1997. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA 94:7493-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruyn, K. M., S. Rangarajan, K. A. Reedquist, C. G. Figdor, and J. L. Bos. 2002. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J. Biol. Chem. 277:29468-29476. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij, J., F. J. Zwartkruis, M. H. Verheijen, R. H. Cool, S. M. Nijman, A. Wittinghofer, and J. L. Bos. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474-477. [DOI] [PubMed] [Google Scholar]
- 12.Duke-Cohan, J. S., H. Kang, H. Liu, and C. E. Rudd. 2006. Regulation and function of SKAP-55 non-canonical motif binding to the SH3c domain of ADAP. J. Biol. Chem. 281:13743-13750. [DOI] [PubMed] [Google Scholar]
- 13.Dustin, M. L. 2001. Role of adhesion molecules in activation signaling in T lymphocytes. J. Clin. Immunol. 21:258-263. [DOI] [PubMed] [Google Scholar]
- 14.Dustin, M. L., T. G. Bivona, and M. R. Philips. 2004. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 5:363-372. [DOI] [PubMed] [Google Scholar]
- 15.Dustin, M. L., J. Garcia-Aguilar, M. L. Hibbs, R. S. Larson, S. A. Stacker, D. E. Staunton, A. J. Wardlaw, and T. A. Springer. 1989. Structure and regulation of the leukocyte adhesion receptor LFA-1 and its counterreceptors, ICAM-1 and ICAM-2. Cold Spring Harb. Symp. Quant. Biol. 54:753-765. [DOI] [PubMed] [Google Scholar]
- 16.Franke, B., J. W. Akkerman, and J. L. Bos. 1997. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 16:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng, L., M. Raab, and C. E. Rudd. 1999. Cutting edge: SLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J. Immunol. 163:5753-5757. [PubMed] [Google Scholar]
- 18.Goda, S., A. C. Quale, M. L. Woods, A. Felthauser, and Y. Shimizu. 2004. Control of TCR-mediated activation of beta 1 integrins by the ZAP-70 tyrosine kinase interdomain B region and the linker for activation of T cells adapter protein. J. Immunol. 172:5379-5387. [DOI] [PubMed] [Google Scholar]
- 19.Gonen, R., D. Beach, C. Ainey, and D. Yablonski. 2005. T cell receptor-induced activation of phospholipase C-gamma1 depends on a sequence-independent function of the P-I region of SLP-76. J. Biol. Chem. 280:8364-8370. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, E. K., C. Krawczyk, Y. Y. Kong, M. Raab, S. J. Hyduk, D. Bouchard, V. S. Chan, I. Kozieradzki, A. J. Oliveira-Dos-Santos, A. Wakeham, P. S. Ohashi, M. I. Cybulsky, C. E. Rudd, and J. M. Penninger. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260-2263. [DOI] [PubMed] [Google Scholar]
- 21.Heuer, K., A. Arbuzova, H. Strauss, M. Kofler, and C. Freund. 2005. The helically extended SH3 domain of the T cell adaptor protein ADAP is a novel lipid interaction domain. J. Mol. Biol. 348:1025-1035. [DOI] [PubMed] [Google Scholar]
- 22.Huang, Y., D. D. Norton, P. Precht, J. L. Martindale, J. K. Burkhardt, and R. L. Wange. 2005. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280:23576-23583. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, A. J., N. Ottoson, N. Boerth, G. A. Koretzky, and Y. Shimizu. 2000. Cutting edge: a novel function for the SLAP-130/FYB adapter protein in beta 1 integrin signaling and T lymphocyte migration. J. Immunol. 164:1143-1147. [DOI] [PubMed] [Google Scholar]
- 24.Jo, E. K., H. Wang, and C. E. Rudd. 2005. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 201:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juliano, R. L. 2002. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 42:283-323. [DOI] [PubMed] [Google Scholar]
- 26.Katagiri, K., A. Maeda, M. Shimonka, and T. Kinashi. 2003. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4:741-748. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri, K., M. Shimonaka, and T. Kinashi. 2004. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. J. Biol. Chem. 279:11875-11881. [DOI] [PubMed] [Google Scholar]
- 28.Kellermann, S. A., C. L. Dell, S. W. Hunt III, and Y. Shimizu. 2002. Genetic analysis of integrin activation in T lymphocytes. Immunol. Rev. 186:172-188. [DOI] [PubMed] [Google Scholar]
- 29.Kinashi, T. 2005. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. 5:546-559. [DOI] [PubMed] [Google Scholar]
- 30.Kolanus, W., W. Nagel, B. Schiller, L. Zeitlmann, S. Godar, H. Stockinger, and B. Seed. 1996. Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell 86:233-242. [DOI] [PubMed] [Google Scholar]
- 31.Krause, M., A. S. Sechi, M. Konradt, D. Monner, F. B. Gertler, and J. Wehland. 2000. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/Vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex links T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon, J., C. K. Qu, J. S. Maeng, R. Falahati, C. Lee, and M. S. Williams. 2005. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 24:2331-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lafuente, E. M., A. A. F. L. van Pujienbroek, M. Krause, C. V. Carman, G. J. Freeman, A. Berezovskaya, E. Constantine, T. A. Springer, F. B. Gerber, and V. A. Boussiotis. 2004. RIAM, an Ena/VASP and profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell. 7:585-595. [DOI] [PubMed] [Google Scholar]
- 34.Laudanna, C., J. Y. Kim, G. Constantin, and E. Butcher. 2002. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186:37-46. [DOI] [PubMed] [Google Scholar]
- 35.Lin, J., A. Weiss, and T. S. Finco. 1999. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J. Biol. Chem. 274:28861-28864. [DOI] [PubMed] [Google Scholar]
- 36.Liu, J., H. Kang, M. Raab, A. J. da Silva, S. K. Kraeft, and C. E. Rudd. 1998. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. USA 95:8779-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marie-Cardine, A., E. Bruyns, C. Eckerskorn, H. Kirchgessner, S. C. Meuer, and B. Schraven. 1997. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 272:16077-16080. [DOI] [PubMed] [Google Scholar]
- 38.Marie-Cardine, A., L. R. Hendricks-Taylor, N. J. Boerth, H. Zhao, B. Schraven, and G. A. Koretzky. 1998a. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 273:25789-25795. [DOI] [PubMed] [Google Scholar]
- 39.Marie-Cardine, A., A. M. Verhagen, C. Eckerskorn, and B. Schraven. 1998b. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 435:55-60. [DOI] [PubMed] [Google Scholar]
- 40.Medeiros, R. B., D. M. Dickey, H. Chung, A. C. Quale, L. R. Nagarajan, D. D. Billadeau, and Y. Shimizu. 2005. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity 23:213-226. [DOI] [PubMed] [Google Scholar]
- 41.Montoya, M. C., D. Sancho, M. Vicente-Manzanares, and F. Sanchez-Madrid. 2002. Cell adhesion and polarity during immune interactions. Immunol. Rev. 186:68-82. [DOI] [PubMed] [Google Scholar]
- 42.Musci, M. A., L. R. Hendricks-Taylor, D. G. Motto, M. Paskind, J. Kamens, C. W. Turck, and G. A. Koretzky. 1997. Molecular cloning of SLAP-130, a SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 272:11674-11677. [DOI] [PubMed] [Google Scholar]
- 43.Nagel, W., L. Zeitlmann, P. Schilcher, C. Geiger, J. Kolanus, and W. Kolanus. 1998. Phosphoinositide 3-OH kinase activates the beta2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J. Biol. Chem. 273:14853-14861. [DOI] [PubMed] [Google Scholar]
- 44.Newbrough, S. A., A. Mocsai, R. A. Clemens, J. N. Wu, M. A. Silverman, A. L. Singer, C. A. Lowell, and G. A. Koretzky. 2003. SLP-76 regulates Fcγ receptor and integrin signaling in neutrophils. Immunity 19:761-769. [DOI] [PubMed] [Google Scholar]
- 45.Peterson, E. J., M. L. Woods, S. A. Dmowski, G. Derimanov, M. S. Jordan, J. N. Wu, P. S. Myung, Q. H. Liu, J. T. Pribila, B. D. Freedman, Y. Shimizu, and G. A. Koretzky. 2001. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293:2263-2265. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu, Y., G. A. Van Seventer, K. J. Horgan, and S. Shaw. 1990. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature 345:250-253. [DOI] [PubMed] [Google Scholar]
- 47.Singer, A. L., S. C. Bunnell, A. E. Obstfeld, M. S. Jordan, J. N. Wu, P. S. Myung, L. E. Samelson, and G. A. Koretzky. 2004. Role of the proline-rich domain in SLP-76 subcellular localization and T cell function. J. Biol. Chem. 279:15481-15490. [DOI] [PubMed] [Google Scholar]
- 48.Timms, J. F., K. D. Swanson, A. Marie-Cardine, M. Raab, C. E. Rudd, B. Schraven, and B. G. Neel. 1999. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9:927-930. [DOI] [PubMed] [Google Scholar]
- 49.Togni, M., K. D. Swanson, S. Reimann, S. Kliche, A. C. Pearce, L. Simeoni, D. Reinhold, J. Wienands, B. G. Neel, B. Schraven, and A. Gerber. 2005. Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 25:8052-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Kooyk, Y., P. van de Wiel-van Kemenade, P. Weder, T. W. Kuijpers, and C. G. Figdor. 1989. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature 342:811-813. [DOI] [PubMed] [Google Scholar]
- 51.Veale, M., M. Raab, Z. Li, A. J. da Silva, S. K. Kraeft, S. Weremowicz, C. C. Morton, and C. E. Rudd. 1999. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J. Biol. Chem. 274:28427-28435. [DOI] [PubMed] [Google Scholar]
- 52.Wang, H., F. E. McCann, J. D. Gordan, X. Wu, M. Raab, T. H. Malik, D. M. Davis, and C. E. Rudd. 2004. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 200:1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, H., E. Y. Moon, A. Azouz, X. Wu, A. Smith, H. Schneider, N. Hogg, and C. E. Rudd. 2003. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat. Immunol. 4:366-374. [DOI] [PubMed] [Google Scholar]
- 54.Woods, M. L., and Y. Shimizu. 2001. Signaling networks regulating beta1 integrin-mediated adhesion of T lymphocytes to extracellular matrix. J. Leukoc. Biol. 69:874-880. [PubMed] [Google Scholar]
- 55.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]
- 56.Zhu, M., S. Shen, Y. Liu, O. Granillo, and W. Zhang. 2005. Cutting edge: localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J. Immunol. 174:31-35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.