Abstract
R-Ras3/M-Ras is a member of the RAS superfamily of small-molecular-weight GTP-binding proteins. Previous studies have demonstrated high levels of expression in several regions of the central nervous system, and a constitutively active form of M-Ras promotes cytoskeletal reorganization, cellular transformation, survival, and differentiation. However, the physiological functions of M-Ras during embryogenesis and postnatal development have not been elucidated. By using a specific M-Ras antibody, we demonstrated a high level of M-Ras expression in astrocytes, in addition to neurons. Endogenous M-Ras was activated by several trophic factors in astrocytes, including epidermal growth factor (EGF), basic fibroblast growth factor, and hepatocyte growth factor. Interestingly, M-Ras activation by EGF was more sustained compared to prototypic Ras. A mouse strain deficient in M-Ras was generated to investigate its role in development. M-Ras null mice appeared phenotypically normal, and there was a lack of detectable morphological and neurological defects. In addition, primary astrocytes derived from Mras−/− mice did not appear to display substantial alterations in the activation of both the mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in response to trophic factors.
R-Ras3/M-Ras was initially isolated based on its sequence similarity to the prototypic Ras oncogene through PCR-based methodology (22), expressed sequence tag (EST) database search (14, 7, 32), and subtractive hybridization (20). It is closely related (∼60%) to TC21/R-RAS2, which was identified through low-stringency hybridization method (6) and as a mutated oncogene in a human ovarian tumor cell line (5). M-Ras belongs to the Ras subgroup of small G proteins which includes Ha-, N-, and Ki-Ras; R-Ras; Rap1A/Krev1; Rap1B; Rap2A; Rap2B; TC21/R-Ras2; RalA; RalB; Rheb; Rin; and Rit (reviewed in references 3 and 34).
M-Ras possesses functional motifs shared by other Ras-related GTPases. For example, residues essential for guanine nucleotide-binding and catalysis are highly conserved. In addition, the canonical effector-binding switch I and switch II domains are identical to that of the prototypic Ras oncogenes. Thus, sequences that can confer M-Ras-specific biological properties are likely to be located in other regions of this unique GTPase. Indeed, M-Ras has a unique amino-terminal sequence, MATSAVPSDNLPT, that is not found in other GTPases. In the carboxyl-terminal region, M-Ras harbors a polybasic stretch (residue 187 to 191: KKKTK) that is reminiscent of that of K-Ras4B (4). Also, the membrane-targeting consensus sequence in the C terminus, CVIL, would predict M-Ras to be modified by the geranylgeranyl transferase.
The biochemical properties of M-Ras resemble that of H-Ras more than other related members, such as R-Ras and TC21 (28). For example, both H-Ras and M-Ras share the same exchange factors, Sos, RasGRF, and CalDAG-GEFII (RasGRP) and GTPase activating protein (GAP) proteins, such as p120 GAP and NF-1 (28). Apart from the conventional Ras effectors, several downstream signaling molecules have been implicated in mediating M-Ras-dependent signaling (28, 32). Interestingly, guanine nucleotide exchange factors (GEFs) for Rap (MR-GEF/RA-GEF-2) (32) and Ral (RGL3) (8) have been shown to interact with M-Ras in a GTP-dependent manner. These findings suggest that M-Ras may coordinate biological events through cross talk with other GTPases. In fibroblasts, M-Ras has been shown to activate the phosphatidylinositol 3-kinase (PI3-K)-dependent signaling cascade while only weakly stimulating the mitogen-activated protein kinase (MAPK) pathway (15). On the contrary, M-Ras strongly stimulates MAPK in PC12 cells through binding and activation of B-Raf (16).
By far, the highest level of M-Ras expression is found in the central nervous system (CNS). Indeed, by in situ hybridization, high levels of M-Ras are found in the mouse hippocampus and the Purkinje layer of the cerebellum (16). H-Ras also displays a similar pattern of expression in the CNS except that it is present in both the granular and Purkinje layers of the cerebellum (40). On the contrary, both K- and N-Ras show a significantly lower level of expression in the juvenile rat brain (40). The regional expression of R-Ras and TC21 in the CNS has not been reported. However, recent data suggest that R-Ras expression is more restricted to both endothelial and vascular smooth muscle cells (18). Apart from the CNS, M-Ras expression has also been reported in muscle cells (22) and in interleukin-9 (IL-9)-induced T-cell clones (20). Interestingly, M-Ras expression was also shown to be elevated after food search memory tests (35), as well as during thermal adaptation in newborn chicks (19). These findings implicate a role for M-Ras in synaptic plasticity in both the hippocampal and the hypothalamic regions of the CNS.
The bona fide physiological functions of M-Ras during development and in somatic tissues are still largely unknown. M-Ras was initially referred to as Microspike-Ras based on its ability to induce protrusions in the cell periphery of Swiss 3T3 fibroblasts (22). Ectopic expression of a constitutively active M-Ras in PC12 cells leads to neurite outgrowth (16). In mammary epithelial cell system, M-Ras expression has been implicated in the process of transformation and epithelial-to-mesenchymal transition (39). M-Ras null mice have not yet been reported. Prior studies involving the generation of knockout (KO) mice targeting different members of the Ras family revealed potential functional overlap among GTPases. For example, H-Ras or N-Ras null mice are viable with no apparent phenotypes. Surprisingly, mice lacking both H- and N-Ras also display no prominent defects (10). However, K-Ras null mice are embryonic lethal due to defects in the fetal liver (13, 17). In addition, mice heterozygous for K-Ras in a N-Ras null background display hematopoietic defects and die at embryonic day 10 (E10) to E12 (13). The high level of M-Ras expression in the CNS raises the possibility of a unique role for this G protein in this system. In the present study, we report the generation of an M-Ras KO mouse and provide an initial characterization of these animals at the cellular and biochemical levels.
MATERIALS AND METHODS
Generation of M-Ras KO mouse strain.
M-Ras KO mice were obtained from Lexicon Genetics, Inc., and the detailed derivation of this mouse strain was previously described (44). Briefly, M-Ras was inactivated by insertional mutagenesis with the VICTR20 retroviral vector in Lex-1 embryonic stem (ES) cells (129/SvEvBrd). A selected ES cell clone, OST108223, was implanted into C57BL/6 albino blastocysts. A 129Sv/C57BL/6 mixed background of both wild-type (WT) and KO progenies from the same littermates were used in all experiments. Genotyping was carried by PCR using 100 ng of tail DNA. A 288-bp product from the WT allele was amplified by using two primers—OSTg3 (5′-ACCACCTAAATACCTGCTTCTCAG-3′; 15 ng) and OSTg5 (5′-GATGATGTCATGTCCCCTCTAAC-3′; 15 ng)—in a 25-μl reaction mixture containing 3 mM MgCl2, 0.25 μl of Taq polymerase (Invitrogen), and 0.2 mM deoxynucleoside triphosphate in a 1× reaction buffer. The cycling condition was 95°C for 30 s and 67°C for 2 min for 33 cycles. A 139-bp PCR product specific for the KO allele was generated by a nested amplification strategy using first the primer pair LTR2 (5′-AAATGGCGTTACTTAAGCTAGCTTGC-3′; 15 ng) and OSTg5 (5′-GATGATGTCATGTCCCCTCTAAC-3′; 15 ng). PCRs were carried out under the same conditions for 10 cycles. Next, 7 μl of this reaction was subjected to a second round of amplification using the primer pair LTR2 and OSTg2 (5′-TTACCCATAACACCATCTTACATG-3′; 15 ng) for an additional 28 cycles.
Characterization of M-Ras null mice.
All experiments involving in the use of mice were approved by the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine under contract number 98-407CA. Morphological and histological characterization of M-Ras null mice were conducted by Frimorpho, Inc. (Fribourg, Switzerland). Three 3-month-old WT and KO mice were subjected to morphological, histological, and biochemical analysis. The full report is available upon request.
Antibodies.
Two anti-M-Ras rabbit polyclonal antibodies, R3N16 and R3C18, were raised against N-terminal (amino acids 1 to 16; MATSAVPSDNLPTYKLC) and C-terminal (amino acids 187 to 204; CKKKTKWRGDRATGTHKLQ) peptides (Covance) (20), respectively. Antibodies against actin (sc-8432; 1:10,000), c-Erk2 (sc-154; 1:5,000), GFAP (sc-6171; 1:50), and TC21 (sc-883; 1:100) were purchased from Santa Cruz Biotech. p-Akt (Thr308) (catalog no. 9275; 1:300), c-Akt (catalog no. 9272; 1:1,000), p-Erk2 (catalog no. 5120; 1:2,000), c-ERM (catalog no. 3142; 1:500), and p-ERM (catalog no. 3141; 1:500) were from Cell Signaling. H-Ras monoclonal antibody (catalog no. 05-516; 1:1,500) was purchased from Upstate Biotechnology. Cy3-GFAP (C-9205; 1:500) was obtained from Sigma. The R-Ras polyclonal antibody, RN23, was raised against the N-terminal 23 amino acids of human R-Ras.
Western blot analysis.
Oligodendrocyte lysates were kindly provided by the Pfeiffer's Laboratory (University of Connecticut). Cells were solubilized in a Tris lysis buffer composed of 50 mM Tris-HCl (pH 8.0), 1% Triton X-100, 10 mM MgCl2, 5 mM EGTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg of leupeptin/ml, and 1 μg of aprotinin/ml. Unless otherwise stated, 50 μg of total cell lysates were resolved on a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto nitrocellulose membrane (Whatman), and Western blot analysis was performed for individual antibodies under conditions recommended by the manufacturers. Membranes were incubated with either horseradish peroxidase-conjugated anti-mouse or -rabbit immunoglobulin G (1:5,000; Amersham), followed by the standard enhanced chemiluminescence detection method (Amersham). Filters were then exposed to X-ray films, and the band intensity was quantified by using a GS-800 densitometer (Bio-Rad Laboratories).
Primary neural cell cultures.
All primary cell cultures were established based on published procedures (2). For primary neuronal cultures, E16 to E18 embryos were removed from timed pregnant female mice, and the hippocampi or the cerebral cortexes were microdissected and acutely dissociated by repeated pipetting. Approximately 5 × 105 cells were plated onto each well of a six-well plate previously coated with poly-d-lysine (10 μg/ml). Neurons were maintained in Neurobasal medium supplemented with B-27 and 0.5 mM glutamate (Invitrogen). For primary astrocyte cultures, cerebral cortexes were microdissected from postnatal day 1 pups. Astrocytes were plated on poly-l-lysine-coated dishes and allowed to proliferate for 10 days in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. When the cultures reached confluence, microglia were eliminated by an overnight treatment with cytosine β-d-arabinofuranoside hydrochloride (20 μM). The purity of the astrocyte cultures was monitored by immunofluorescence analysis by a Cy3-conjugated anti-GFAP antibody (Sigma) and was normally about 80 to 90%. All astrocytes used in the present study were passaged for no more than two times.
Fear conditioning.
Two groups (WT and KO mice, n = 8 per group) were used in this experiment as previously described (38). All animals were exposed to handling, transportation, and environment training for 7 days prior to the test. On training day 1, mice were transported to the conditioning room and placed in the experimental chamber, and the light was turned on. The baseline freezing level (as a percentage of total test time) was measured during the first 3 min. Freezing was defined as the lack of movement except that necessitated by respiration. After that, the mice received two footshocks (0.7 mA, 2 s, 60-s intertrial interval). At 1 min after the second footshock, the light was turned off, and the mice were immediately returned to their home cages. On day 2, fear in relation to the context of the conditioning chamber was assessed by returning the mice to the chamber and measuring freezing behavior expressed as a percentage of the total measured time.
Morris water maze.
The spatial memory task was conducted with Mras+/+ and Mras−/− mice (n = 4 for each group) (24). A Plexiglas platform (15 by 15 cm) was submerged 1 cm below the water's surface located at a fixed position. A training session consisted of a series of trials. For each trial, a mouse was launched from one of eight random positions along the side of the tank. The mouse was given 60 s to find the submerged platform. If the mouse did not mount the platform within 60 s, it was guided to the platform. The time to reach the platform was recorded as the training latency for each trial. During the first 2 days of the 5 days of trials, the platform was black, and there was an extra black cue hanging over the platform to guide the mouse to find the platform. In the last 3 days, the extra cue was removed, and the platform was white. One hour after the last trial of day 5, the platform was removed, and the animals were tested again. Mice were allowed to swim for 60 s. The time spent in each quadrant was then analyzed.
GTP-loading assay.
The levels of GTP-bound M-Ras in primary mouse astrocytes were assayed by an affinity pull-down assay using the Ras-binding domain (RBD) of the p110 subunit of PI3-K (16). Glutathione S-transferase-fused p110α RBD was purified from 1 liter of bacterial culture and affinity absorbed onto 300 μl of glutathione-Sepharose beads (Pharmacia). For a typical assay, a near-confluent astrocyte culture was solubilized in 400 μl of a lysis buffer composed of 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 1 mM PMSF, 1 μg of leupeptin/ml, and 1 μg of aprotinin/ml. Binding reactions were carried out using 1 mg of a total cell extract and 30 μl (∼30 μg) of the p110-RBD-coupled glutathione beads in a final volume of 400 μl. After incubation at 4°C for 1 h, binding reactions were washed three times with the lysis buffer, and bound proteins were eluted with 60 μl of a 2× Laemmli buffer. To detect the amount of M-Ras bound to p110-RBD, 30 μl of the eluted materials were resolved by 12.5% SDS-PAGE, and Western blot analysis was carried out using an anti-M-Ras antibody, R3C18. To detect the levels of GTP-bound Ras, similar assays were performed based on a previously published method (41). A glutathione S-transferase-Raf-RBD probe was used in affinity pull-down assays with reactions composed of 10% glycerol, 1% NP-40, 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 2.0 mM MgCl2, 10 mM NaF, 1 mM Na3VO4, 250 μM PMSF, 2 μg of aprotinin/ml, and 1 μg of leupeptin/ml.
Activation of kinase cascades by trophic factors.
Mouse epidermal growth factor (EGF) was purchased from Invitrogen. Both human basic fibroblast growth factor (bFGF) and human hepatocyte growth factor (HGF) were obtained from Preprotech. These factors were added to cultures that had been deprived of serum for 48 h at final concentrations of 50 (HGF) and 100 (mEGF and bFGF) ng/ml. Interleukin-1β (IL-1β) and transforming growth factor β1 (TGF-β1) were used at 10 ng/ml. At the indicated time points, total cell extracts were prepared from treated cultures by solubilization with a Tris-based lysis buffer (see above). Cell lysates were subjected to Western blot analysis with selected antibodies.
Immunofluorescence analysis.
Primary cells were plated on poly-l-lysine-coated glass coverslips (Carolina Biotech). Cells were routinely fixed in 4% paraformaldehyde in phosphate-buffered saline for 10 min and then permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 15 min. Both blocking and incubation of primary antibodies were carried out in 3% bovine serum albumin. To stain for p-ERM, cells were fixed on ice in 0.5% dodecyltrimethylammonium chloride (DOTMAC)-1% paraformaldehyde for 5 min instead. Anti-rabbit immunoglobulin G secondary antibodies, Alexa 594 (A-21207; 1:1,000) and Alexa 488 (A-21206; 1:1,000) were purchased from Invitrogen. Glass coverslips were then mounted on glass slides and viewed under a Nikon fluorescence microscope.
RESULTS
Expression of M-Ras in the mammalian brain cells.
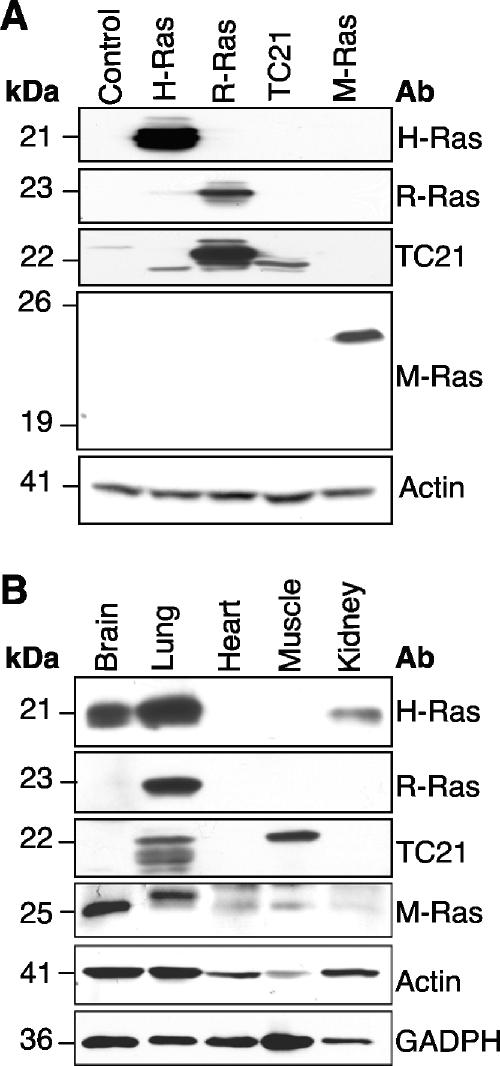
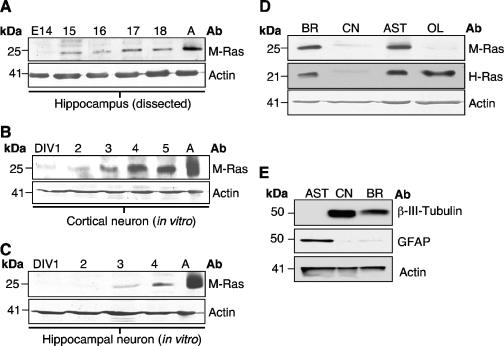
M-Ras transcripts were detected in both human and mouse brains (7, 14, 16, 20, 22, 32). By in situ hybridization, it was demonstrated that M-Ras showed a broad pattern of expression throughout the entire brain but was particularly abundant in the hippocampus and the Purkinje layer of the cerebellum (16). To investigate the expression of M-Ras in the CNS at the protein level, we generated an antibody directed against the C-terminal portion of this GTPase. As shown in Fig. 1A, R3C18 was able to detect the ∼25-kDa M-Ras protein exogenously expressed in 293T cells. As expected, it failed to cross-react with three related members of the Ras subfamily: H-Ras, R-Ras, and TC21/R-Ras2. The presence of Ras-related proteins was confirmed by using their respective antibodies. To validate the expression of M-Ras in the brain, cell extracts were prepared from several mouse organs. As expected, the highest level of M-Ras was detected in the brain, with moderate levels in both heart and muscle (Fig. 1B). The higher-molecular-weight species present in the lung sample was unlikely to be other Ras-related GTPases such as H-Ras, R-Ras, and TC21 since M-Ras has the slowest migratory property on an SDS-PAGE gel. Furthermore, H-Ras expression was found in the brain, lung, and kidney. Interestingly, the two closely related members, R-Ras and TC21, displayed high levels of expression in the lung and muscle, respectively (Fig. 1B). More importantly, their expression was substantially lower than M-Ras in the brain. The R3C18 antibody was, however, not amenable for either immunofluorescence or immunohistochemical analysis. For this reason, we utilized microdissected CNS regions or primary neural cell cultures to examine M-Ras expression in the CNS. When dissected hippocampi from different embryonic stages were analyzed, M-Ras expression was first detected at E15 and gradually elevated to a higher level in the adult (>8 weeks) (Fig. 2A). We have further established primary neuronal cultures from both cerebral cortical and hippocampal regions. M-Ras expression was barely detectable at day in vitro 1 (DIV1) but increased from DIV3 to DIV5, which coincided with the onset of neurite outgrowth (Fig. 2B and C). To further explore the expression of M-Ras in other cell types, primary cultures were also established for astrocytes and oligodendrocytes. Culture purity was confirmed by immunofluorescence staining with GFAP and Oligo-1, respectively, as well as by Western blot analysis (Fig. 2E). As shown in Fig. 2D, M-Ras was highly expressed in astrocytes but was undetectable in oligodendrocytes. Interestingly, the expression of H-Ras showed a similar pattern of expression, with the distinct exception that it was expressed in oligodendrocytes. In addition, M-Ras was not expressed in microglia while H-Ras protein was present (data not shown). From these data, it appears that M-Ras expression correlates with the onset of neurogenesis, and its expression may be required for certain neurological functions in adult life.
FIG. 1.
Expression of M-Ras in mouse tissues. (A) The specificity of M-Ras antibody, R3C18, was tested with lysates from 293T cells transfected with H-Ras, R-Ras, TC21, and M-Ras expression plasmids. Western blot analysis was performed with antibodies raised against individual G proteins. The anti-TC21 antibody cross-reacted with H-Ras and R-Ras. (B) The expression of Ras-related proteins in various mouse organs was analyzed by Western blot analysis with the corresponding antibodies. Note the high level of M-Ras expression in the brain.
FIG. 2.
Expression of M-Ras in the CNS. (A) The expression of M-Ras in microdissected hippocampi from different embryonic stages was analyzed. Cell extracts from DIV cultures of cortical (B) and hippocampal (C) neurons were analyzed for M-Ras expression. Total cell extracts derived from adult brain (A) were used as a positive control. (D) A total brain lysate and primary cultures of cortical neurons (CN), astrocytes (AST), and oligodendrocytes (OL) were analyzed for the expression of both M-Ras and H-Ras. Note the lack of M-Ras expression in OL. (E) Lysates from DIV5 astrocytes, cortical neurons, and total brain extracts were subjected to Western blotting analysis with antibodies against GFAP and β-III-tubulin. All lysates were probed with an anti-actin antibody to ensure similar protein loading.
Generation and validation of an Mras null mouse strain.
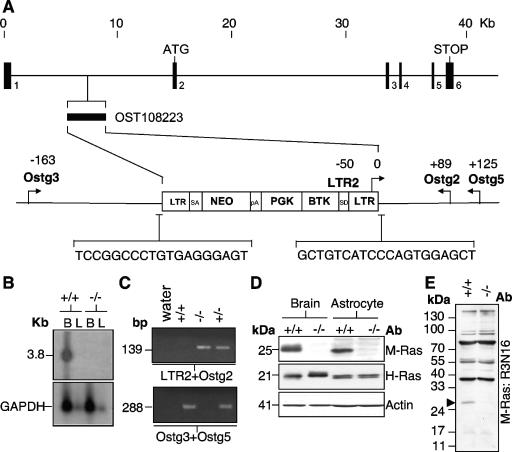
In an attempt to unravel the role M-Ras in the CNS, a mouse strain deficient in M-Ras expression was generated based on a retrovirus-mediated gene trap strategy (44). The ES cell clone, OST108223, was selected based on the presence of a single insertion site mapped to the first intron of MRas gene ∼7.6 kb from the start of exon 2 as verified by a reverse PCR-based methodology (Lexicon Genetics, Inc.) (Fig. 3A). Thus, the gene trap most likely inactivated M-Ras transcription by the splicing of exon 1 to the splice acceptor site within the retroviral sequence. Indeed, Northern blot analysis of poly(A)+ RNA from both the brains and livers of Mras−/− mice lacked the 3.8-kb M-Ras transcripts (Fig. 3B and C).
FIG. 3.
Generation of the M-Ras null mouse. (A) The retroviral integration site within the first intron of the mouse MRas gene in the ES cell clone, OST108223, is indicated. The nucleotide sequences flanking the insertion site are shown. The locations of the PCR primers (LTR2, Ostg2, Ostg3, and Ostg5) used in genotyping are indicated. Their positions (in base pairs) relative to the sites of integration are shown (not drawn to scale). (B) Poly(A)+ RNA extracted from the brain and liver of Mras+/+ and Mras−/− mice were analyzed with an M-Ras cDNA probe (upper panel). Loading control was provided by a GAPDH probe (lower panel). (C) A typical genotyping analysis showing the 139- and 288-bp PCR fragments specific for the Mras−/− and Mras+/+ alleles, respectively. (D) The expression of M-Ras was completely absent in both brain and astrocyte lysates derived from Mras−/− mice (upper panel). The levels of H-Ras are, however, not significantly altered (lower panel). (E) The lack of expression of M-Ras was further confirmed with an anti-M-Ras polyclonal antibody, R3N16, raised against the N terminus of M-Ras (solid arrowhead). The multiple bands of >30 kDa in size are likely due to nonspecific cross-reactivity.
To ascertain that Mras−/− mice lacked the M-Ras encoded product, Western blot analysis was performed using the C-terminal anti-M-Ras antibody, R3C18. As expected, M-Ras was absent in total brain and astrocyte lysates derived from Mras−/− mice (Fig. 3D). A similar observation was also made using an antibody raised against the N-terminal 16 amino acids of M-Ras (Fig. 3E). These data provide strong evidence that M-Ras gene was effectively inactivated by the gene trap technique in mice derived from the ES clone, OST108223.
Gross morphological analysis of M-Ras-deficient mice.
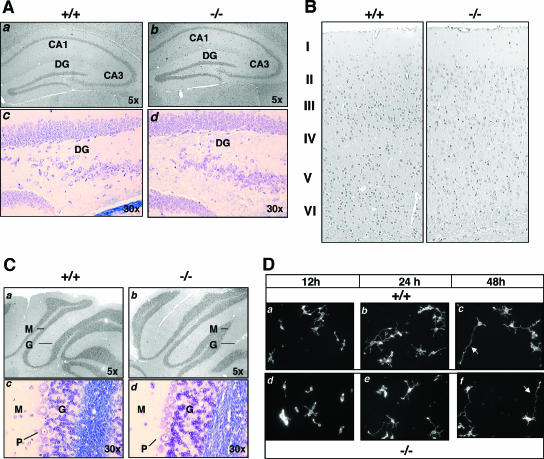
Mras−/− mice were produced by crossing heterozygous animals. The frequency of offspring genotypes maintained a close to expected Mendelian ratio of inheritance of 22.0% (+/+):50.8% (+/−):27.2% (−/−) (n = 126). Also, there was not a significant bias toward a certain gender (male:female = 67:59). There was a lack of gross morphological differences between the Mras+/+ and Mras−/− mice at both the anatomical and the histological levels. A detailed histological analysis was performed on brain sections derived from Mras−/− mice. The hippocampus appeared to be normal, with the neuronal density similar to the WT counterpart (Fig. 4A). The cortex showed a laminated structure throughout its extent. The normal six-layer structure was present in the neocortex, whereas the piriform region had three layers. The cell density of the cortex appeared to be correct, and the proportion of astrocytes was not increased (Fig. 4B). Finally, the cerebral cortex possessed the normal three-laminar structure of a molecular layer, a Purkinje cell layer, and an internal granular layer (Fig. 4C).
FIG. 4.
Brain morphology of Mras−/− mice. (A) Hippocampal regions of wild-type (+/+, panels a and c) and M-Ras KO mice (−/−, panels b and d) are compared. Hematoxylin-and-eosin (H&E) (panels a and b)- and Nissl (panel c and d)-stained paraffin sections show the CA1, CA3, and dentate gyrus (DG) regions, with the original magnifications indicated. (B) Coronal sections of cerebral cortex were stained with H&E, and the six-layered structure is indicated. (C) H&E (panels a and b)- and Nissl (panels c and d)-stained coronal sections of cerebellum from WT (+/+, panels a and c) and M-Ras KO (−/−, panels b and d) mice show the molecular (M), granular (G), and Purkinje (P) cell layers. (D) Primary hippocampal neurons were prepared from WT (+/+, panels a to c) and M-Ras KO (−/−, panels d to f) mice. At the indicated time points, cells fixed with 4% paraformaldehyde and actin were stained with Texas Red-conjugated phalloidin (converted to grayscale). Note the polarized extension of a single axon from the cell body (red arrows, panels c and f). Magnification, ×10.
Morphological and physiological characterization of Mras−/− mice.
To explore whether Mras−/− mice displayed any morphological defects in neuronal cells, we performed analysis utilizing both in vitro and in vivo systems. Primary hippocampal neurons (PHNs) were established by acute dissociation from E18 embryos. Both Mras+/+ and Mras−/− PHNs displayed equivalent number of sprout sites on DIV1, and this was followed by the polarized protrusion of a single axon on DIV2 (Fig. 4D). Exposure of Mras+/+ and Mras−/− PHNs to excitotoxin (glutamate) did not result in any significant changes in neuronal cell death (data not shown). Also, the depolarization of neurons by high levels of potassium led to a similar extent of phospho-Creb in cell lysates from Mras+/+ and Mras−/− PHNs (data not shown). The expression of M-Ras in the hippocampus prompted us to investigate whether Mras−/− mice could have deficits in memory-related functions. Both Mras+/+ and Mras−/− mice were subjected to fear conditioning and the Morris water maze assays, but these tests failed to reveal any significant differences (see Fig. S1 and S2 in the supplemental material).
M-Ras in astrocyte signaling.
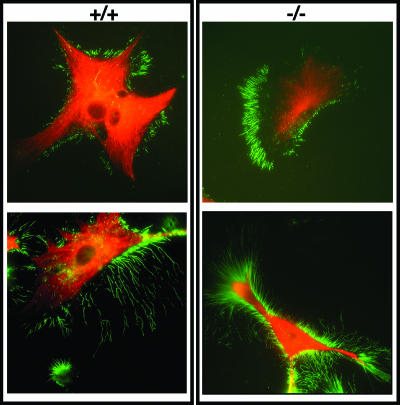
M-Ras was highly expressed in mouse astrocytes. It is widely believed that astrocytes play critical roles in providing trophic support for neurons during neurotransmission and injuries (26, 33). Primary astrocytes prepared from Mras+/+ and Mras−/− mice were first analyzed for morphological differences. Two cytoskeletal markers, GFAP and p-ERM, were used in immunocytochemical analysis. The glia-specific intermediate filament, GFAP, displayed the characteristic mesh-like cytoplasmic distribution in both Mras+/+ and Mras−/− astrocytes (Fig. 5). However, the activated form of the membrane-cytoskeleton linker, p-ERM, which marked the cell periphery, revealed subtle differences between Mras+/+ and Mras−/− astrocytes. Although these astrocytes all displayed long and arborized-filopodia-like structures, the density of these structures appeared to be greater in Mras−/− astrocytes. However, Western blot analysis of Mras+/+ and Mras−/− astrocytes failed to reveal any significant difference in the levels of p-ERM (data not shown), indicating that this difference likely reflects a change in the subcellular distribution.
FIG. 5.
Morphology of M-Ras KO astrocytes. (A) Immunofluorescence analysis of WT (+/+) and M-Ras KO (−/−) astrocytes. Astrocytes are stained with a Cy3-conjugated anti-GFAP antibody (red), and the actin cytoskeleton at the cell periphery was detected with an anti-phospho-ERM antibody (green). Two representative astrocytes are shown for each genotype. Magnification, ×60.
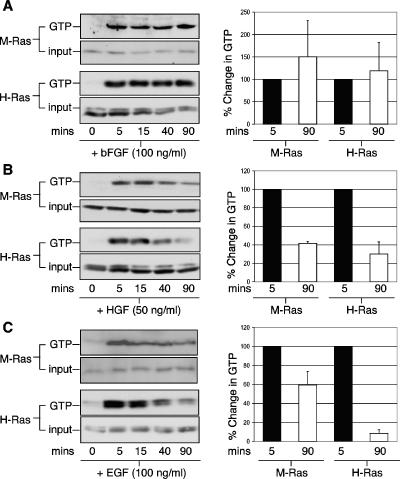
To further delineate the role of M-Ras in astrocyte functions, the ability of several trophic factors in activating M-Ras was investigated. For this, affinity pull-down assays were conducted utilizing the RBDs of p110α-PI3-K and c-Raf to monitor the GTP-bound states of endogenous M-Ras and H-Ras, respectively. Trophic factors such as bFGF, HGF, and EGF induced robust activation (>10-fold) of both M-Ras and H-Ras within 5 min of stimulation (Fig. 6, left panels). Kinetically, bFGF induced a sustained activation of both M-Ras and H-Ras for up to 1.5 h (Fig. 6A, right panel). In contrast, the levels of both M-Ras and H-Ras in their GTP states were reduced by 60 and 70%, respectively, after 1.5 h of stimulation by HGF (Fig. 6B, right panel). More importantly, M-Ras and H-Ras differed in their relative responsiveness to EGF stimulation. Although the amount of H-Ras in the GTP-bound state returned almost to the basal level (<10%) after 1.5 h, M-Ras retained more than 60% of its active state when experiments were carried out under similar conditions (Fig. 6C, right panel). Furthermore, cytokines such as TGF-β1 and IL-1β both failed to significantly activate either M-Ras or H-Ras (see Fig. S3 in the supplemental material). Finally, while ATP modestly stimulated M-Ras by ∼5-fold, it robustly activated H-Ras by >20-fold (see Fig. S3 in the supplemental material). These data suggest that M-Ras could potentially propagate signals initiated by trophic factors implicated in the maintenance of cell growth and survival.
FIG. 6.
Activation of M-Ras by trophic factors in astrocytes. Primary astrocytes were deprived of serum for 48 h and stimulated with bFGF (A), HGF (B), and EGF (C) for the indicated durations (minutes). Cells were solubilized, and about 1.0 to 1.5 mg of total cell lysates was used in affinity pull-down assays to assess the GTP-bound levels (GTP) of M-Ras (upper panel) and H-Ras (lower panel). The levels of total M-Ras and H-Ras are shown (input). The relative levels of GTP-bound M-Ras at peak (5-min, black bars) verses late (90-min, white bars) phase are quantified (right panels). The data are the means ± standard deviations derived from two to four independent mating experiments.
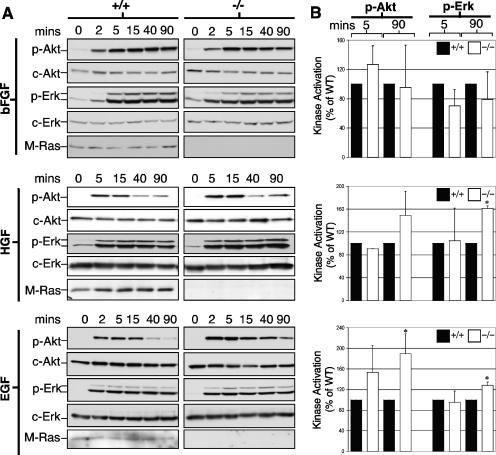
Next, we investigated whether M-Ras was required for the downstream signaling events in response to trophic factor stimulations. For this, we tested the ability of bFGF, HGF, and EGF to activate the MAPK and PI3-K pathways in Mras+/+ and Mras−/− astrocytes. As shown in Fig. 7, both c-Akt and c-Erk were activated by >20-fold in both Mras+/+ and Mras−/− astrocytes within 2 min of bFGF addition (Fig. 7A and B, top panels). Activation peaked around 5 to 15 min and sustained for over 1.5 h. There were, however, no significant differences between Mras+/+ and Mras−/− astrocytes prepared from littermates of up to four separate mating experiments. Next, HGF induced a transient activation of c-Akt that peaked at around 5 to 15 min, whereas c-Erk activation was more sustained (Fig. 7A and B, middle panels). Once again, both Mras+/+ and Mras−/− astrocytes responded similarly to this trophic factor except that the activation state of c-Erk in the sustained phase (90 min) was marginally (1.6-fold) elevated in Mras−/− astrocytes when compared to the Mras+/+ cells. Finally, EGF stimulated transient activations of both c-Akt and c-Erk (Fig. 7A and B, bottom panels). The magnitudes of activation in the late phase (90 min) for both were significantly elevated by 1.8- and 1.2-fold, respectively, in Mras−/− astrocytes.
FIG. 7.
Activation of kinase cascades in M-Ras KO astrocytes. (A) WT (+/+) and M-Ras KO (−/−) astrocytes were serum starved for 48 h and stimulated with bFGF, HGF, and EGF for the indicated durations. Cells were lysed, and Western blot analysis was performed to examine the activation states of c-Akt and c-Erk using phospho-specific antibodies directed against each protein. (B) The relative activation of c-Akt and c-Erk at peak (5-min) and late (90-min) time points was compared between Mras+/+ (black bars) and Mras−/− (white bars) astrocytes. The data are mean values derived from three independent experiments with the standard deviations shown (*, P < 0.05).
DISCUSSION
In this report, we characterized the expression and signaling properties of M-Ras in the mouse CNS. Expression of M-Ras is relatively low in the early embryonic stage (<E14) but steadily increases to significant levels in the adult CNS. These observations may partially explain the lack of detectable morphological and histological abnormalities during development in Mras−/− mice. The fact that adult mice failed to show any signs of neurological disorders may suggest compensatory actions from functionally redundant genes.
One interesting observation is the significant level of expression of M-Ras in primary astrocyte cultures. It is important to point out that the astrocyte cultures were derived from the cerebral cortexes of newborn pups. Whether M-Ras still maintains a high level of expression in adult astrocytes in vivo remains to be determined. It is believed that astrocytes confer neuroprotective effects on neurons by secreting trophic factors, such as glial cell-derived growth factor (45). Astrocytes also control neuronal electrical activity at the synaptic junctions by modulating the glutamate concentration in a calcium-dependent manner (26, 30, 33).
In response to injuries, astrocytes undergo a process of astrogliosis that is characterized by a drastic remodeling of the actin cytoskeleton (9, 12, 27). It has been reported that M-Ras is capable of inducing microspikes when microinjected into murine fibroblasts (22). The ability of M-Ras to regulate actin-based cytoskeleton raises the possibility of a role in astrogliosis. However, scratch wounds introduced in primary astrocytes fail to activate M-Ras. Furthermore, both Mras+/+ and Mras−/− astrocytes displayed very similar rates of wound closure (data not shown). We are currently investigating whether Mras−/− mice may display different degrees of neuronal survival in experimental models where astrogliosis is induced after lesions are introduced in the CNS.
The in vivo function of M-Ras in neurons is at present unclear. The prospect of detecting neuronal deficits may be very much dependent on the experimental conditions. For instance, K-Ras heterozygote mice are neurologically normal. However, treatment with a subthreshold dose of mitogen-activated/extracellular signal-regulated kinase inhibitor unmasks defects in long-term potentiation and contextual learning (29). Another explanation for a lack of phenotype is that M-Ras function may be required only under certain physiological states. Indeed, Robles et al. have uncovered M-Ras as one of the genes that is upregulated in the hippocampus of rats that have been subjected to food search memory tasks (35). More interestingly, Labunskay and Meiri have identified M-Ras as a gene being induced in the preoptic anterior hypothalamus of 3-day-old chicks that were undergoing thermal adaptation (19). In both cases, adaptative neuroplasticity mechanisms (1) are involved and probably reinforced by an increase in M-Ras levels in either astrocytes or neurons. Finally, we cannot rule out the possibility of revealing neuronal defects in Mras−/− mice under certain stressed conditions. Indeed, a recent study has demonstrated that a KO mouse model for a Ras-related gene, R-Ras, while phenotypically normal, displayed enhanced angiogenic response to arteriole injuries (18). In addition, it is also necessary to perform a detailed characterization of the behavioral and histological deficits associated with aging in Mras−/− mice.
Both H-Ras and M-Ras display similar responses to different trophic factors. It is not surprising since these two related G proteins have very similar biochemical properties (28). We have examined the activation states of H-Ras and another closely related Ras-related protein, Rap1A, but failed to detect any compensatory increase in either their expression levels or GTP-bound states (data not shown). The sustained activation of both M-Ras and H-Ras by bFGF closely parallels the prolonged activation of c-Akt and c-Erk. bFGF levels are upregulated in injured brain (36) and, together with extracellular ATP, have been implicated in promoting astrogliosis through the PI3-K and MAPK cascades (25). It is possible that M-Ras and H-Ras are components of these signaling events in response to neuronal injuries. HGF induces transient activations of both M-Ras and H-Ras with kinetics that mirror those of p-Akt but not of p-Erk. The role of HGF in astrocyte function is not entirely clear. Increased expression of HGF is observed in astroglial cells surrounding senile plaques in Alzheimer's disease brains (11, 37). EGF has been implicated in the metabotropic glutamate receptor signaling to the MAPK pathway (31). In combination with bFGF, these trophic factors promote calcium oscillation in astrocytes upon exposure to neurotransmitters such as glutamate, ATP, or thimerosal (23). The observation that M-Ras shows a relatively sustained activation compared to H-Ras in response to EGF is interesting. A similar observation was reported for Rap1A upon NGF stimulation in PC12 cells (21). In this case, the sustained activation of Rap1A occurs in the endosomes, and this causes the prolonged activation of MAPK pathway, leading presumably to neurite outgrowth (41, 43).
Unlike bFGF, the activation state of c-Akt in EGF-treated astrocytes starts to attenuate after 15 min, kinetics resembling those of H-Ras. In contrast, M-Ras retains >60% of the peak activation level even after 90 min. We speculate that M-Ras may be involved in the regulation of the PI3-K pathway in the late phase. For instance, M-Ras may be part of a negative feedback loop of the PI3-K pathway since p-Akt is more elevated in the late phase of EGF-treated Mras−/− astrocytes (Fig. 7). Along this line, the recently resolved crystal structure of M-Ras in its GTP-bound state reveals a distinct switch I conformation rendering it in an “off” state. This would predict a weak binding capacity of M-Ras to certain Ras substrates (42). Thus, the possibility of M-Ras acting as a negative regulator of trophic factor signaling cannot be excluded.
In summary, M-Ras appears to be dispensable for mouse development, and adult Mras−/− mice do not display any detectable aberrations. Since any potential defects in Mras−/− can be compensated for during embryogenesis, the generation of a conditional KO mouse would be necessary to clearly define the role of M-Ras in somatic cells. In addition, Mras−/− astrocytes show responsiveness to different trophic factors similar to that of the WT counterpart. Given the similarity of M-Ras and H-Ras at the biochemical level, it is very likely that H-Ras may substitute for the loss of M-Ras. We are currently generating a double-KO mouse strain of H-Ras and M-Ras in an attempt to further unravel the interplay between these two related G proteins in neurogenesis.
Supplementary Material
Acknowledgments
We thank Cristina Alberini, Deanna Benson, Maria Diverse, Mitchell Goldfarb, Garrett John, Kevin Kelley, and Matthew Shapiro (Mt. Sinai) for invaluable advice and Paula Bos for technical assistance.
This study was funded by NIH grants MH59771 and CA78509 to A.M.-L.C. and an NCI Shared Resources grant (CA88302). A.C.K. is a recipient of the Leonard B. Holman Research Pathway fellowship. T.H. is supported by DOD IDEA award DAMD17-03-1-0682.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allen, N. J., and B. A. Barres. 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr. Opin. Neurobiol. 15:542-548. [DOI] [PubMed] [Google Scholar]
- 2.Bankers, G., and K. Goslin. 1998. Culturing nerve cells, 2nd ed. MIT Press, Cambridge, Mass.
- 3.Bos, J. L. 1997. Ras-like GTPases. Biochim. Biophys. Acta 1333:M19-M31. [DOI] [PubMed] [Google Scholar]
- 4.Cadwallader, K. A., H. Paterson, S. G. Macdonald, and J. F. Hancock. 1994. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol. Cell. Biol. 14:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, A. M., T. Miki, K. A. Meyers, and S. A. Aaronson. 1994. A human oncogene of the RAS superfamily unmasked by expression cDNA cloning. Proc. Natl. Acad. Sci. USA 91:7558-7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drivas, G. T., A. Shih, E. Coutavas, M. G. Rush, and P. D'Eustachio. 1990. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol. Cell. Biol. 10:1793-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt, G. R., K. B. Leslie, F. Lee, J. S. Wieler, and J. W. Schrader. 1999. M-Ras, a widely expressed 29-kD homologue of p21 Ras: expression of a constitutively active mutant results in factor-independent growth of an interleukin-3-dependent cell line. Blood 94:2433-2444. [PubMed] [Google Scholar]
- 8.Ehrhardt, G. R., C. Korherr, J. S. Wieler, M. Knaus, and J. W. Schrader. 2001. A novel potential effector of M-Ras and p21 Ras negatively regulates p21 Ras-mediated gene induction and cell growth. Oncogene 20:188-197. [DOI] [PubMed] [Google Scholar]
- 9.Eng, L. F., and R. S. Ghirnikar. 1994. GFAP and astrogliosis. Brain Pathol. 4:229-237. [DOI] [PubMed] [Google Scholar]
- 10.Esteban, L. M., C. Vicario-Abejon, P. Fernandez-Salguero, A. Fernandez-Medarde, N. Swaminathan, K. Yienger, E. Lopez, M. Malumbres, R. McKay, J. M. Ward, A. Pellicer, and E. Santos. 2001. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol. Cell. Biol. 21:1444-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton, H., P. W. Finch, J. S. Rubin, J. M. Rosenberg, W. G. Taylor, V. Kuo-Leblanc, M. Rodriguez-Wolf, A. Baird, H. M. Schipper, and E. G. Stopa. 1998. Hepatocyte growth factor (HGF/SF) in Alzheimer's disease. Brain Res. 779:262-270. [DOI] [PubMed] [Google Scholar]
- 12.Hatten, M. E., R. K. Liem, M. L. Shelanski, and C. A. Mason. 1991. Astroglia in CNS injury. Glia 4:233-243. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, L., D. Greenbaum, K. Cichowski, K. Mercer, E. Murphy, E. Schmitt, R. T. Bronson, H. Umanoff, W. Edelmann, R. Kucherlapati, and T. Jacks. 1997. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 11:2468-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimmelman, A., T. Tolkacheva, M. V. Lorenzi, M. Osada, and A. M. Chan. 1997. Identification and characterization of R-ras3: a novel member of the RAS gene family with a non-ubiquitous pattern of tissue distribution. Oncogene 15:2675-2685. [DOI] [PubMed] [Google Scholar]
- 15.Kimmelman, A. C., M. Osada, and A. M. Chan. 2000. R-Ras3, a brain-specific Ras-related protein, activates Akt and promotes cell survival in PC12 cells. Oncogene 19:2014-2022. [DOI] [PubMed] [Google Scholar]
- 16.Kimmelman, A. C., N. Nunez Rodriguez, and A. M. Chan. 2002. R-Ras3/M-Ras induces neuronal differentiation of PC12 cells through cell-type-specific activation of the mitogen-activated protein kinase cascade. Mol. Cell. Biol. 22:5946-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koera, K., K. Nakamura, K. Nakao, J. Miyoshi, K. Toyoshima, T. Hatta, H. Otani, A. Aiba, and M. Katsuki. 1997. K-ras is essential for the development of the mouse embryo. Oncogene 15:1151-1159. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu, M., and E. Ruoslahti. 2005. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat. Med. 11:1346-1350. [DOI] [PubMed] [Google Scholar]
- 19.Labunskay, G., and N. Meiri. 2006. R-Ras3/(M-Ras) is involved in thermal adaptation in the critical period of thermal control establishment. J. Neurobiol. 66:56-70. [DOI] [PubMed] [Google Scholar]
- 20.Louahed, J., L. Grasso, C. De Smet, E. Van Roost, C. Wildmann, N. C. Nicolaides, R. C. Levitt, and J. C. Renauld. 1999. Interleukin-9-induced expression of M-Ras/R-Ras3 oncogene in T-helper clones. Blood 94:1701-1710. [PubMed] [Google Scholar]
- 21.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, K., T. Asano, and T. Endo. 1997. Novel small GTPase M-Ras participates in reorganization of actin cytoskeleton. Oncogene 15:2409-2417. [DOI] [PubMed] [Google Scholar]
- 23.Morita, M., C. Higuchi, T. Moto, N. Kozuka, J. Susuki, R. Itofusa, J. Yamashita, and Y. Kudo. 2003. Dual regulation of calcium oscillation in astrocytes by growth factors and pro-inflammatory cytokines via the mitogen-activated protein kinase cascade. J. Neurosci. 23:10944-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalbantoglu, J., G. Tirado-Santiago, A. Lahsaini, J. Poirier, O. Goncalves, G. Verge, F. Momoli, S. A. Welner, G. Massicotte, J. P. Julien, and M. L. Shapiro. 1997. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387:500-505. [DOI] [PubMed] [Google Scholar]
- 25.Neary, J. T., Y. Kang, and Y. F. Shi. 2004. Signaling from nucleotide receptors to protein kinase cascades in astrocytes. Neurochem. Res. 29:2037-2042. [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard, M., B. Ransom, and S. A. Goldman. 2003. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26:523-530. [DOI] [PubMed] [Google Scholar]
- 27.Norton, W. T., D. A. Aquino, I. Hozumi, F. C. Chiu, and C. F. Brosnan. 1992. Quantitative aspects of reactive gliosis: a review. Neurochem. Res. 17:877-885. [DOI] [PubMed] [Google Scholar]
- 28.Ohba, Y., N. Mochizuki, S. Yamashita, A. M. Chan, J. W. Schrader, S. Hattori, K. Nagashima, and M. Matsuda. 2000. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J. Biol. Chem. 275:20020-20026. [DOI] [PubMed] [Google Scholar]
- 29.Ohno, M., P. W. Frankland, A. P. Chen, R. M. Costa, and A. J. Silva. 2001. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat. Neurosci. 4:1238-1243. [DOI] [PubMed] [Google Scholar]
- 30.Parri, H. R., T. M. Gould, and V. Crunelli. 2001. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4:803-812. [DOI] [PubMed] [Google Scholar]
- 31.Peavy, R. D., M. S. Chang, E. Sanders-Bush, and P. J. Conn. 2001. Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J. Neurosci. 21:9619-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quilliam, L. A., A. F. Castro, K. S. Rogers-Graham, C. B. Martin, C. J. Der, and C. Bi. 1999. M-Ras/R-Ras3, a transforming Ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 274:23850-23857. [DOI] [PubMed] [Google Scholar]
- 33.Ransom, B., T. Behar, and M. Nedergaard. 2003. New roles for astrocytes (stars at last). Trends Neurosci. 26:520-522. [DOI] [PubMed] [Google Scholar]
- 34.Reuther, G. W., and C. J. Der. 2000. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr. Opin. Cell Biol. 12:157-165. [DOI] [PubMed] [Google Scholar]
- 35.Robles, Y., P. E. Vivas-Mejia, H. G. Ortiz-Zuazaga, J. Felix, X. Ramos, and S. Pena de Ortiz. 2003. Hippocampal gene expression profiling in spatial discrimination learning. Neurobiol. Learn. Mem. 80:80-95. [DOI] [PubMed] [Google Scholar]
- 36.Smith, C., M. Berry, W. E. Clarke, and A. Logan. 2001. Differential expression of fibroblast growth factor-2 and fibroblast growth factor receptor 1 in a scarring and nonscarring model of CNS injury in the rat. Eur. J. Neurosci. 13:443-456. [DOI] [PubMed] [Google Scholar]
- 37.Sun, W., H. Funakoshi, and T. Nakamura. 2002. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 22:6537-6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taubenfeld, S. M., M. H. Milekic, B. Monti, and C. M. Alberini. 2001. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat. Neurosci. 48:813-818. [DOI] [PubMed] [Google Scholar]
- 39.Ward, K. R., K. X. Zhang, A. M. Somasiri, C. D. Roskelley, and J. W. Schrader. 2004. Expression of activated M-Ras in a murine mammary epithelial cell line induces epithelial-mesenchymal transition and tumorigenesis. Oncogene 23:1187-1196. [DOI] [PubMed] [Google Scholar]
- 40.Wei, W., S. S. Schreiber, M. Bandry, G. Tocco, and D. Broek. 1993. Localization of the cellular expression pattern of cdc25NEF and ras in the juvenile rat brain. Brain Res. Mol. Brain Res. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 41.Wu, C., C. F. Lai, and W. C. Mobley. 2001. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21:5406-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye, M., F. Shima, S. Muraoka, J. Liao, H. Okamoto, M. Yamamoto, A. Tamura, N. Yagi, T. Ueki, and T. Kataoka. 2005. Crystal structure of M-Ras reveals a GTP-bound “off” state conformation of Ras family small GTPases. J. Biol. Chem. 280:31267-33175. [DOI] [PubMed] [Google Scholar]
- 43.York, R. D., H. Yao, T. Dillon, C. L. Ellig, S. P. Eckert, E. W. McCleskey, and P. J. Stork. 1998. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392:622-626. [DOI] [PubMed] [Google Scholar]
- 44.Zambrowicz, B. P., G. A. Friedrich, E. C. Buxton, S. L. Lilleberg, C. Person, and A. T. Sands. 1998. Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature 392:608-611. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, Z., S. Alam, R. W. Oppenheim, D. M. Prevette, A. Evenson, and A. Parsadanian. 2004. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp. Neurol. 190:356-372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.