Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family that induces cancer cell death by apoptosis with some selectivity. TRAIL-induced apoptosis is mediated by the transmembrane receptors death receptor 4 (DR4) (also known as TRAIL-R1) and DR5 (TRAIL-R2). TRAIL can also bind decoy receptor 1 (DcR1) (TRAIL-R3) and DcR2 (TRAIL-R4) that fail to induce apoptosis since they lack and have a truncated cytoplasmic death domain, respectively. In addition, DcR1 and DcR2 inhibit DR4- and DR5-mediated, TRAIL-induced apoptosis and we demonstrate here that this occurs through distinct mechanisms. While DcR1 prevents the assembly of the death-inducing signaling complex (DISC) by titrating TRAIL within lipid rafts, DcR2 is corecruited with DR5 within the DISC, where it inhibits initiator caspase activation. In addition, DcR2 prevents DR4 recruitment within the DR5 DISC. The specificity of DcR1- and DcR2-mediated TRAIL inhibition reveals an additional level of complexity for the regulation of TRAIL signaling.
Apoptosis is one of the death phenotypes that can be triggered in tumor cells by anticancer agents. Resistance of tumor cells to apoptosis can account for treatment failure (18). One of the stimuli that can induce tumor cell death by apoptosis is the member of the tumor necrosis factor superfamily known as TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) (16). TRAIL has gained considerable interest in oncology since it displays specific antitumoral activity against a wide range of tumor cells (14, 41, 47) without significant side effects, at least in mice and monkeys (3, 21, 22).
TRAIL triggers apoptosis upon engagement of one of its two agonistic receptors, named DR4 (death receptor 4) (33) and DR5 (death receptor 5) (7, 46). In response to TRAIL, these receptors recruit the adaptor protein FADD (Fas-associated death domain), through death domain homophilic interactions (5), and the initiators procaspase-8 and -10, through death effector domain interactions with FADD, hence forming the macromolecular complex called DISC (death-inducing signaling complex). Within this complex, procaspase-8 and -10 are activated by autoproteolytic cleavage and initiate the caspase cascade leading to apoptosis (42).
In addition to the agonistic TRAIL receptors DR4 and DR5, TRAIL can bind to related but antagonistic receptors, including TRID or TRAIL-R3 (11, 27, 32) and TRUNDD or TRAIL-R4 (10), also coined DcR1 (decoy receptor 1) and DcR2 (decoy receptor 2), respectively. DcR1 is characterized by the presence of a glycosylphosphatidylinositol membrane anchor and therefore has no intracellular domain, whereas the death domain of DcR2 is truncated. Transient overexpression of DcR1 or DcR2 in TRAIL-sensitive tumor cells prevents cell death triggering by TRAIL (10, 11), and recent evidence indicates that tumor and normal cells can acquire resistance to TRAIL-induced killing by up-regulating TRAIL antagonistic receptors (6, 8, 9, 34).
Upon TRAIL binding, DcR1 and DcR2 fail to recruit FADD and, consequently, fail to induce downstream cell signaling events leading to apoptosis (10, 32). Initial binding experiments suggested that TRAIL binding affinities to TRAIL agonistic and antagonistic receptors were similar (11, 24), but subsequent studies demonstrated that DcR1 and DcR2 affinities to TRAIL were lower than that of DR4 or DR5 (8). To date, the molecular mechanisms by which DcR1 and DcR2 confer resistance to TRAIL-induced apoptosis remain unclear (1, 6, 9, 13).
In the current study, we demonstrate that DcR1 and DcR2 inhibit TRAIL-induced apoptosis by distinct mechanisms. DcR1 acts merely as a competitor for TRAIL binding, preventing DR5-associated DISC assembly, while DcR2 impairs TRAIL DISC processing and initiator caspase activation. DcR2 interacts with DR5 in the native DISC in a TRAIL-dependent manner and prevents DR4 corecruitment to DR5.
MATERIALS AND METHODS
Ligand production and antibodies.
Flag-tagged recombinant soluble human TRAIL and his-tagged TRAIL were produced and used as described previously (37). Flag-FasL was purchased from Axxora (San Diego, CA). Anti-Flag (M2) was purchased from Sigma-Aldrich (St. Quentin Fallavier, France). For Western blotting experiments, anti-DR4, anti-DR5, anti-DcR1, and anti-DcR2 antibodies were purchased from Chemicon (Temecula, CA), anti-FADD and anti-flotillin-1 were obtained from Transduction Laboratories (Lexington, KY), anti-caspase-8 and -10 were obtained from Medical and Biological Laboratories (Nagoya, Japan), and anti-FLIP was obtained from Alexis (Coger, Paris, France). Anti-caspase-3 was purchased from Cell Signaling (Ozyme, Saint Quentin Yvelines, France) and anti-poly(ADP-ribose) polymerase (anti-PARP) from Boehringer Mannheim (Germany). For flow cytometry experiments with Jurkat, HeLa, and HepG2, anti-DR4 (wB-K32), anti-DR5 (B-L27), anti-DcR1 (wB-B44), and anti-DcR2 (wB-P30) were kindly provided by Diaclone (Besançon, France). Fluorescein isothiocyanate-coupled goat anti-mouse secondary antibody was obtained from Molecular Probes (Invitrogen, Cergy Pontoise, France). For flow cytometry experiments with monocytes, phycoerythrin-conjugated anti-DR4 (B-N36), anti-DR5 (B-K29), anti-DcR1 (B-D44), and anti-DcR2 (B-R27) were provided by Diaclone. Anti-CD14 coupled to phycoerythrin was purchased from Pharmingen (BD Biosciences, California). For immunoprecipitation, the goat anti-caspase-8 (C20) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and the anti-DR5 (B-D37) and anti-DcR2 (wB-P30) antibodies were obtained from Diaclone. For cytotoxic assays, the agonistic anti-DR5 antibodies (B-T28 for Jurkat cells and B-D37 for HeLa and HepG2 cell lines) were provided by Diaclone.
Cell culture.
The HeLa (human cervix carcinoma) cell line was cultured with high-glucose Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (Gibco-BRL, Erigny, France) and penicillin-streptomycin (100 μg/ml of each). The Jurkat (human T-lymphoma) cells were cultured in RPMI 1640 medium (BioWhittaker Co., Fontenay-sous-bois, France) containing 10% of fetal calf serum and penicillin-streptomycin. The HepG2 (human hepatocarcinoma) cell line was cultured in 50% Ham's F-12 medium (BioWhittaker Co.) and 50% Dulbecco's modified Eagle's medium, supplemented with 5% fetal calf serum and penicillin-streptomycin. All cell lines were grown in 5% CO2 at 37°C. Monocytes were purified from peripheral blood mononuclear cells of healthy donors by use of a monocyte purification kit (Miltenyi Biotec, California).
Retrovirus production and cell transduction.
The retroviral vector pMSCV-puro and the generation of viruses have previously been described (30). DR4, DcR1, and DcR2 full-length constructs were subcloned from pCR-3 vector (Invitrogen) to pMSCV-puro. HeLa and Jurkat cells were transduced for 16 h with viral supernatants containing polybrene (8 μg/ml), washed in phosphate-buffered saline (PBS), and cultured in complete medium containing puromycin (2.5 μg/ml).
Measurement of cell viability and apoptosis.
In 96-well plates, 104 cells were incubated at 37°C for 24 h with increasing concentrations of his-TRAIL or Flag-FasL (from 0 to 10,000 ng/ml). For Jurkat cells, cell viability was determined by the PMS/MTS method, according to the manufacturer's specifications (Promega, Madison, Wis.), while cell viability was determined by methylene blue for HeLa cells (30). Apoptosis was assessed by Hoechst staining by determining the percentage of condensed nuclei from at least 300 cells per condition. When indicated, cells were additionally treated with 50 μM zVAD-fmk (Sigma-Aldrich).
Immunoprecipitations.
For TRAIL DISC analysis, 108 cells were stimulated with 5 μg of Flag-TRAIL cross-linked with 10 μg anti-Flag M2 in 1 ml medium for the indicated times at 37°C. Cells were then washed with cold PBS and lysed in 1 ml lysis buffer containing 1% of detergent (NP-40 or Brij 78), 20 mM of Tris-HCl (pH 7.5), 150 mM of NaCl, and 10% of glycerol. Lysates were precleared with Sepharose 6B (Sigma-Aldrich) and immunoprecipitated overnight at 4°C with protein G-Sepharose beads (Amersham Biosciences, Les Ullis, France). For caspase-8 or TRAIL receptor immunoprecipitations, cells were stimulated as described above with 5 μg/ml His-TRAIL and lysed in NP-40-containing lysis buffer, and lysates were precleared before 5 μg of immunoprecipitating antibody was added. In both cases, beads were then washed four times with the respective detergent, and immunoprecipitates were eluted in lysis buffer before being processed for immunobloting.
Lipid raft isolation.
Lipid rafts were isolated by sucrose density gradient centrifugation as previously described (50). Cells (108) were stimulated or not stimulated with 5 μg of His-TRAIL for 1 h at 37°C, washed in PBS, lysed on ice for 20 min in 1 ml of MNX buffer (1% of Triton X-100 in 25 mM of MES [morpholineethanesulfonic acid], 150 mM of NaCl, pH 6.5) supplemented with protease inhibitor cocktail (Roche Molecular Biochemicals, Meylan, France), and homogenized (10 strokes) with a loose-fitting glass Dounce homogenizer (Polylabo, Illkirch, France). The homogenates were mixed with 2 ml of 90% sucrose made with MNX buffer and placed on the bottom of a centrifuge tube. The samples were then overlaid with 4 ml of 35% sucrose and 4 ml of 5% sucrose and centrifuged at 175,000 × g for 20 h at 4°C. One-milliliter fractions were collected from the top of the gradient and analyzed by Western blotting or enzyme-linked immunosorbent assay (ELISA). The proteins of the different fractions obtained from monocytes were precipitated by 5% trichloroacetic acid before ELISAs. Cholesterol quantification was assessed by use of the Amplex Red cholesterol assay kit (Molecular Probes).
Western blotting.
Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked by incubation in PBS containing 0.05% Tween 20 and 5% powdered milk. Immunoblots were then incubated with specific primary antibody, followed by horseradish peroxidase-conjugated secondary antibody, and were developed by the enhanced chemiluminescence method according to the manufacturer's protocol (Pierce, Rockford, IL).
ELISA.
TRAIL or DcR1 protein content in the different fractions obtained after lipid raft isolation was quantified by ELISA according to the manufacturer's instructions (Diaclone).
RESULTS
DcR1 and DcR2 expression inhibits TRAIL-induced apoptosis.
TRAIL decoy receptors are expressed at low levels on the surface of most tumor cell lines, and when spontaneously present, their level of expression at the surface of a given cell line varies (not shown). In order to analyze the molecular mechanisms involved in the inhibition of TRAIL-induced cell death by these decoy receptors in a stable model system, we transduced the TRAIL-sensitive cell lines Jurkat and HeLa with retroviral expression vectors for DcR1 and DcR2. Compared to conventional transfections, the retroviral gene transfer enables a stable, reproducible, and limited expression of the gene.
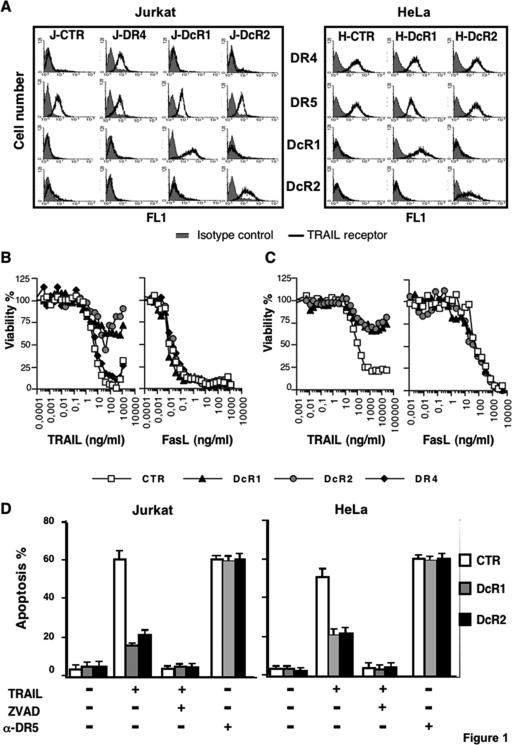
In line with previous reports (6, 10, 34, 36), overexpression of DcR1 or DcR2 at the cell surface (Fig. 1A) inhibited TRAIL-induced cell death (Fig. 1B and C). DcR1 and DcR2 were nearly as efficient as the pan caspase inhibitor zVAD-fmk at inhibiting TRAIL-induced apoptosis (Fig. 1D). All the cell populations remained sensitive to death induced by Flag-FasL (Fig. 1B and C) and to an agonistic monoclonal antibody targeting DR5 (Fig. 1D).
FIG. 1.
Inhibition of TRAIL-induced cell death by decoy receptors. (A) Jurkat (J) and HeLa (H) cells, stably transduced with retroviruses encoding DR4, DcR1, DcR2, or the empty vector pMSCV (CTR), were analyzed by fluorescence-activated cell sorter staining for TRAIL receptor expression as indicated. (B) Cellular viability in the different Jurkat cell populations was evaluated by PMS/MTS after a 24-h treatment with increasing concentrations of cross-linked recombinant Flag-tagged soluble TRAIL or FasL. (C) Cellular viability of HeLa cell populations was evaluated as described above by use of the methylene blue assay. (D) Jurkat and HeLa cell populations were stimulated for 6 h with Flag-TRAIL (100 ng/ml), alone or in combination with zVAD-fmk (100 μM) or with the agonistic anti-DR5 antibody (100 μg/ml), and apoptosis was determined by Hoechst staining. Data (means ± standard deviations) correspond to the percentage of apoptotic nuclei among the total nuclei counted (n = 3). Data are representative of at least three independent experiments.
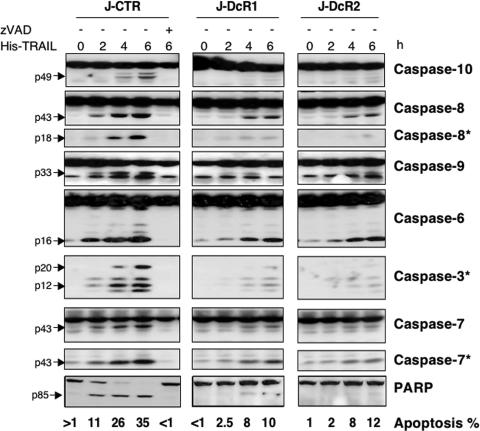
Analysis of caspase activation by use of caspase fluorogenic substrates (not shown) or by Western blotting (Fig. 2) revealed that both DcR1 and DcR2 inhibited early events of TRAIL signaling, as witnessed by the obvious reduction in caspase-8 and caspase-10 activation in cells expressing these receptors. As a consequence, activation of the effector caspases, caspase-3, -6, and -7, or cleavage of the downstream target PARP was severely impaired. Similar results were obtained with HeLa cell populations (not shown).
FIG. 2.
Inhibition of caspase activation in decoy receptor-expressing cells. Jurkat cell populations were treated with 100 ng/ml of His-TRAIL, alone or combined with 50 μM zVAD-fmk (+), for the indicated time periods. Cells were subsequently analyzed by Hoechst to determine the percentage of apoptosis (bottom), and the corresponding cellular lysates were analyzed by Western blotting using anti-caspase and anti-PARP antibodies, as described in Materials and Methods. Anti-caspase-7 and -8 antibodies recognizing caspase-7 (p43) and -8 (p18) active fragments are indicated by asterisks. Caspases cleavage products and PARP fragments are indicated by black arrows.
Expression of DR4 in the Jurkat cell line that spontaneously expresses only DR5 (Fig. 1A) did not increase cell sensitivity to TRAIL (Fig. 1B), probably because Jurkat cells are already highly sensitive to TRAIL-induced cell death.
DcR1 and DcR2 differentially inhibit TRAIL DISC formation.
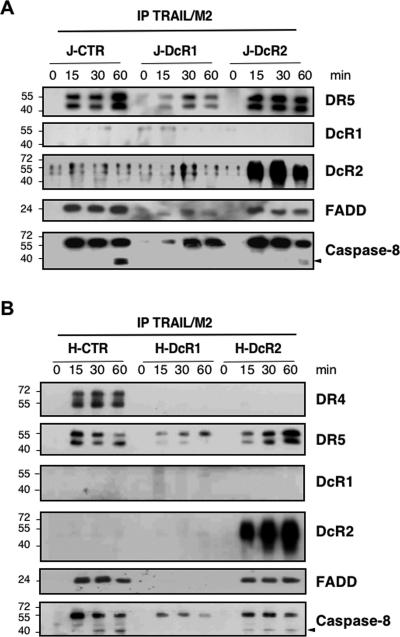
Since the protective effects of decoy receptors appear to be an upstream event during caspase activation, TRAIL DISC composition was analyzed by coimmunoprecipitation and immunoblotting using cross-linked recombinant Flag-tagged soluble TRAIL. In line with earlier reports (5), TRAIL DISC assembly in Jurkat cells involved the agonistic receptor DR5, the adaptor protein FADD, and caspase-8 (Fig. 3A). The same was true for HeLa cells, except that in addition, the endogenous DR4 coimmunoprecipitated with DR5 in a ligand-dependent manner (Fig. 3B).
FIG. 3.
DcR1 and DcR2 differentially affect TRAIL DISC formation. (A) Jurkat and (B) HeLa cell populations were stimulated with 5 μg/ml Flag-TRAIL in the presence of 10 μg/ml of M2 cross-linking antibody for the indicated time periods (see Materials and Methods). After cell lysis in NP-40-containing buffer, the DISC was immunoprecipitated (IP) and analyzed by Western blotting using antibodies to DR4, DR5, DcR1, DcR2, FADD, and caspase-8. Caspase-8 cleavage product, corresponding to the p43 fragment, is indicated by black arrowheads. Molecular masses are in kDa. Data are representative of at least three independent experiments.
In both Jurkat and HeLa cell populations, TRAIL DISC compositions differed significantly, depending on which decoy receptor was expressed. In DcR2-expressing cell populations, both DcR2 and DR5 coimmunoprecipitated with TRAIL (Fig. 3A and B). In contrast, DR4, whose expression was observed only in HeLa cells, was not identified in the DISC (Fig. 3B). The recruitment of procaspase-8 to the DISC was unaffected by DcR2, but its activation was impaired as there was little cleavage of procaspase-8 (Fig. 3). These results in addition to those shown in Fig. 1 suggested that DcR2 could inhibit caspase-8 processing within the DISC and thus impair TRAIL-induced cell death.
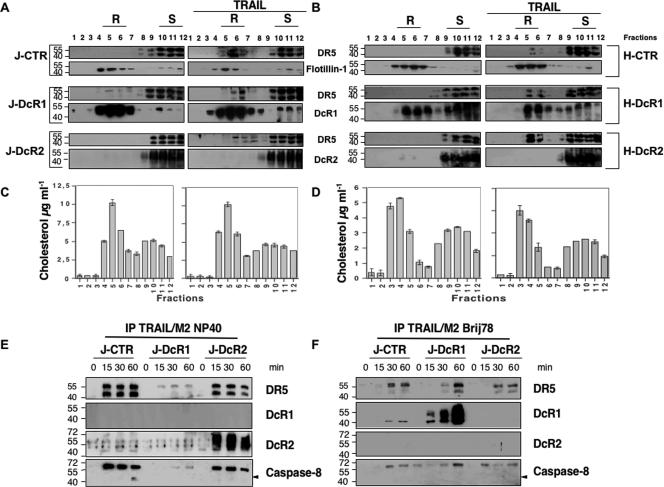
DISC analysis of cell populations expressing DcR1 provided strikingly different results. DISC assembly was severely inhibited by DcR1 expression, as only small amounts of DISC components were immunoprecipitated (Fig. 3A and B). In addition, under experimental conditions using NP-40 as a detergent, DcR1 was not immunoprecipitated by TRAIL. The glycosylphosphatidylinositol-anchored structure of DcR1 (39) suggested that the molecule could localize within lipid raft structures. We used Triton X-100 detergent and sucrose density gradient (25) to isolate these structures whose identification was checked by immunoblotting for the lipid raft-associated protein flotillin-1 (Fig. 4A and B) and by measuring the cholesterol content in each fraction (Fig. 4C and D). When expressed in HeLa and Jurkat cells, DcR1 localized mainly within lipid rafts, whereas DR4 (not shown), DR5, and DcR2 did not (Fig. 4A and B). TRAIL stimulation induced partial translocation of DR5 in lipid raft structures, but this redistribution occurred independently of DcR1 or DcR2 expression (Fig. 4A and B).
FIG.4.
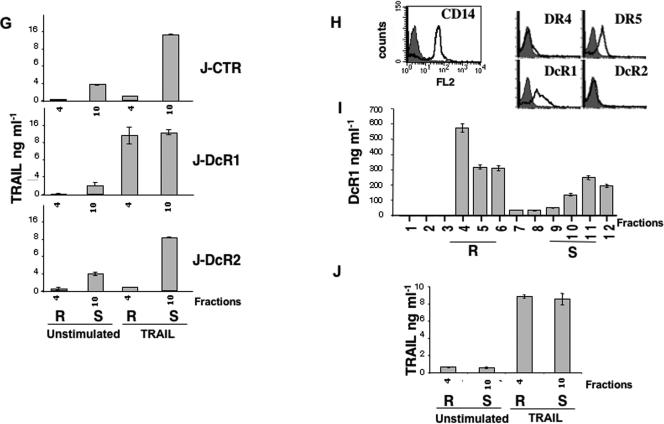
DcR1 inhibits TRAIL-DISC formation in lipid rafts. (A) Jurkat and (B) HeLa cell populations were left untreated or stimulated for 1 h with 100 ng/ml of His-TRAIL, lysed in Triton X-100, and subjected to density gradient fractionation. Fractions corresponding to lipid rafts (R) and soluble proteins (S) were immunoblotted for DR5, DcR1, DcR2, and flotillin-1 as indicated. Data are representative of at least three independent experiments. Fractions were analyzed for cholesterol content in Jurkat (C) and (D) HeLa cells by use of the Molecular Probes Amplex Red cholesterol assay kit. (E) TRAIL DISC formation in Jurkat cell populations was analyzed after Flag-TRAIL stimulation and immunoprecipitation (IP) in the presence of the M2 anti-Flag antibody as in Fig. 2, for the indicated time, from cell lysates in (E) NP-40- or (F) Brij78-containing lysis buffer. (G) Analysis of TRAIL protein content from density sucrose gradient fractions obtained from Jurkat cells treated or not treated with His-TRAIL, as described for panel A, was performed by use of an ELISA kit obtained from Diaclone (see Materials and Methods). (H) Freshly isolated monocytes were analyzed by fluorescence-activated cell sorter staining for CD14 and TRAIL receptor expression as indicated. (I) DcR1 protein content from freshly isolated monocytes was analyzed by use of a DcR1 ELISA kit from Diaclone after density sucrose gradient fractionation. Lipid rafts (R) and soluble protein fractions (S) are indicated. (J) Monocytes were stimulated with His-TRAIL as described for panel A, and TRAIL was measured by ELISA, as described above, in lipid rafts (R) and soluble protein fractions (S) as indicated.
The localization of DcR1 within raft microdomains was consistent with the insolubility of DcR1 in a lysis buffer containing NP-40 detergent (Fig. 4E). We therefore used Brij78, a detergent in which lipid rafts are solubilized (2), to reanalyze the DISC induced by TRAIL in DcR1-expressing Jurkat cells. Under these conditions, DcR1 coimmunoprecipitated with TRAIL but the other DISC components were hardly detectable in the cell population (Fig. 4F). Analysis of TRAIL distribution in unstimulated Jurkat cells or in Jurkat cells treated with recombinant TRAIL, in the fractions collected by sucrose gradient density, clearly indicated that a considerable amount of exogenous TRAIL was sequestered into lipid raft fractions in cells expressing DcR1, yet was absent from these fractions in control cells or cells expressing DcR2 (Fig. 4G). These results were confirmed in monocytes, which were recently shown to express DcR1 endogenously (44). In these cells, both DR5 and DcR1 are expressed (Fig. 4H), and DcR1 is localized mainly within rafts (Fig. 4I). According to the above results, upon stimulation, a significant fraction of recombinant TRAIL is sequestered in lipid rafts (Fig. 4J). Altogether, these results suggested that the expression of DcR1 could prevent DISC formation by titrating TRAIL within lipid rafts.
DcR2 is recruited to the DR5-containing complex, where it inhibits activation of apical caspases.
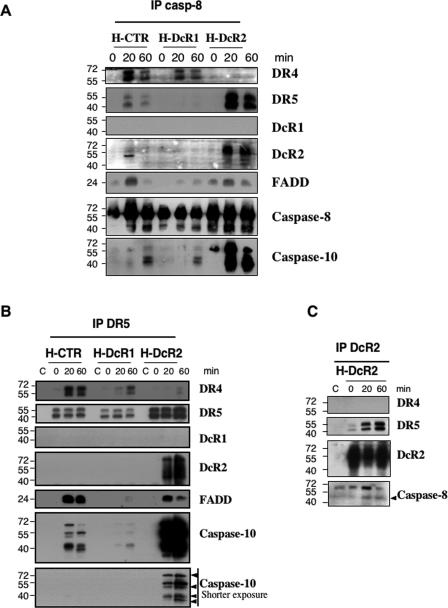
TRAIL DISC formation in HeLa cells was further analyzed by immunoprecipitation using antibodies targeting caspase-8, DR5, or DcR2. Immunoprecipitating caspase-8 after TRAIL stimulation enabled the pull-down of DISC components, including DR4, DR5, and FADD as well as the initiator caspase-10, in control HeLa cells (Fig. 5A). In line with experiments whose results are shown in Fig. 3, few DISC components were pulled down by immunoprecipitating caspase-8 from DcR1-overexpressing cells (Fig. 5A). In HeLa cells overexpressing DcR2, however, in addition to DcR2 and similar to TRAIL immunoprecipitation, caspase-8 immunoprecipitation also pulled down larger amounts of caspase-10 (Fig. 5A). This increase in caspase-8-associated caspase-10 could be the consequence of a reduced DISC processing activity. Accordingly, few caspase-10 cleavage products are found in DcR2-expressing cells compared to those found in control cells after TRAIL stimulation (Fig. 2). Caspase-10 activity as measured by fluorogenic substrates is also highly reduced in these cells (not shown). Similar findings have been documented for cells knocked down for the caspase-8 regulator c-FLIP (38). Immunoprecipitation of caspase-8 also confirmed that the presence of DcR2 within the caspase-8-containing complex partially excluded DR4 from the TRAIL-induced DISC (Fig. 3B and 5C).
FIG. 5.
DcR2 allows TRAIL-DISC formation but prevents its activation. (A) HeLa cell populations were stimulated with 5 μg/ml recombinant His-TRAIL for the indicated time periods. After cell lysis, DISCs were immunoprecipitated (IP) with an anti-caspase-8 (casp-8) antibody and analyzed by immunoblotting using antibodies to DR4, DR5, DcR1, DcR2, FADD, caspase-8, and caspase-10. (B) HeLa cell populations were stimulated as described above, and DISC analysis was performed using the anti-DR5 antibody or an isotype control. (C) The DcR2-expressing HeLa cell population was stimulated as described for panel A, and DISC analysis was performed using an anti-DcR2 antibody. Caspase-8 and caspase-10 cleavage products are indicated by black arrowheads.
The stoichiometry of TRAIL receptors within the DISC remains poorly understood, but indications exist that decoy receptors might interact with agonistic receptors (8, 24). To determine whether the receptors were present in unique or distinct complexes, we used anti-DR5 and anti-DcR2 antibodies to immunoprecipitate TRAIL DISC components. In TRAIL-treated control HeLa cells, immunoprecipitation with an anti-DR5 antibody indicated that DR5 and DR4 were within the same complex (Fig. 5B), together with FADD and caspase-10 and its cleavage products (Fig. 5B), while in DcR1-expressing HeLa cells, few DISC components associated to DR5 (Fig. 5B). When performed with HeLa cells expressing DcR2, the same experiments indicated for the first time that DcR2 could interact with DR5 in a ligand-dependent manner and confirmed the exclusion of DR4 from DR5 complexes containing DcR2 (Fig. 3B and 5C).
These experiments also confirmed that in the presence of DcR2, the amount of caspase-10 associated with the DISC components was increased (Fig. 5B). In addition, the use of an anti-DcR2 antibody strengthened the finding that DcR2 associates with DR5 in a ligand-dependent manner (Fig. 5C).
Endogenous DcR2 interacts with DR5 to inhibit DISC activation.
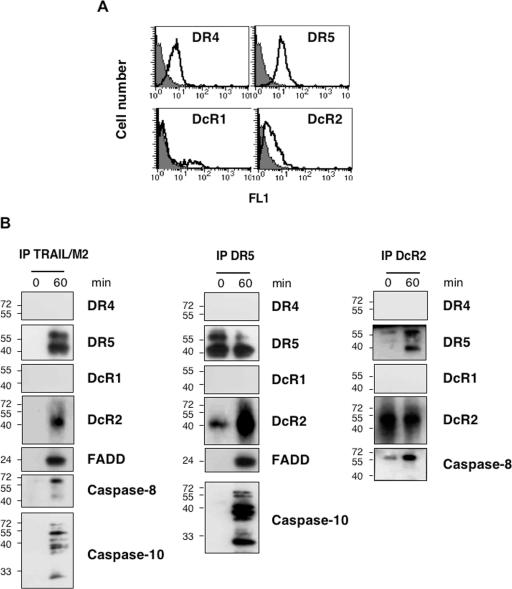
To confirm the finding that DcR2 interacts with DR5 via TRAIL in cells expressing endogenous levels of DcR2, we used the TRAIL-resistant HepG2 cell line that expresses DR4, DR5, DcR2, and, for a minor subpopulation only, DcR1 (Fig. 6A). In HepG2, stimulation with TRAIL triggered the formation of a DISC containing DR5, DcR2, FADD, and the initiators caspase-8 and -10 as seen by TRAIL immunoprecipitation. Similar to the results obtained using HeLa cells expressing DcR2, DR4 was not recruited into the DISC in HepG2 cells stimulated by TRAIL (Fig. 6B). In addition, DISC analysis using the anti-DR5 or anti-DcR2 antibodies provided similar information. Accordingly, quite substantial amounts of procaspase-10 were still detected within the DISC, and the ligand-dependent interaction of DcR2 with DR5 in these cells was confirmed (Fig. 6B). Altogether, these data demonstrate that DcR2 is able to interact with DR5 within the DISC, where it inhibits initiator caspase activation.
FIG. 6.
Endogenous DcR2 prevents TRAIL-DISC processing. (A) HepG2 cells were analyzed by fluorescence-activated cell sorter staining for TRAIL receptor expression as indicated. (B) HepG2 cells were stimulated as described for Fig. 2 for DISC analysis (IP TRAIL/M2). Alternatively, cells were stimulated with His-TRAIL as described for Fig. 5 and DISC analysis was performed using either the anti-DR5 or the anti-DcR2 antibody. Immunoprecipitations (IP) were then analyzed by immunoblotting using antibodies to DR4, DR5, DcR1, DcR2, FADD, caspase-8, and caspase-10.
DISCUSSION
The selective toxicity of TRAIL against tumor cells makes it an attractive candidate for treating cancers. However, all tumor cells are not equally sensitive to this cytokine. Identification of the molecular mechanisms that modulate the response to TRAIL may help to select those tumors that may optimally respond to this therapeutic approach. A first resistance mechanism is related to the lack of expression of DR4 and DR5 at the surface of some cancer cells (15). TRAIL-induced apoptosis has also been demonstrated to be inhibited by a number of intracellular regulators, such as proteins from the IAP and Bcl-2 families (48) and c-FLIP (19). The role of TRAIL decoy receptors in inducing tumor resistance towards TRAIL-induced cell death is now well established (12, 23, 39, 51). However, how these receptors prevent TRAIL-induced apoptosis is less clear. Our results provide, for the first time, strong evidence that the molecular mechanisms involved in TRAIL-induced cell death inhibition by DcR1 and DcR2 differ in important ways.
We provide strong evidence that DcR1, which localizes in sphingolipid- and cholesterol-enriched membranes, structures also known as raft microdomains, inhibits TRAIL-induced cell death merely by competition. In both Jurkat and HeLa cells engineered to express this decoy receptor, DcR1 bound TRAIL efficiently and impaired DISC assembly, as demonstrated by TRAIL immunoprecipitation using Brij78, a detergent that solubilizes lipid rafts (2). A significant fraction of recombinant TRAIL was sequestered into lipid rafts, as measured by ELISA, both in Jurkat cells engineered to express DcR1 and in HeLa cells (not shown) but also in monocytes that express endogenous levels of DcR1 at the cell surface. These findings are consistent with the current view that DcR1 is a decoy receptor that inhibits TRAIL-induced cell death by the competitive binding of TRAIL.
DcR2, on the other hand, allowed DR5-mediated DISC formation but prevented initiator caspase activation within the DISC. Using different antibodies targeting specifically DR5 or DcR2 to analyze TRAIL DISC composition, we show that the interaction of DcR2 with DR5 is mediated by TRAIL (Fig. 5C and 6B). Yet, recent findings indicate that the spontaneous ligand-independent interaction of DcR2 with DR5 which occurs through the PLAD (pre-ligand-associated domain) is required for the inhibition of TRAIL-induced cell death (8). These conclusions are in sharp contrast to our demonstration that the interaction of DcR2 with DR5 is indirect and is mediated by TRAIL. One possible explanation for this discrepancy could be that the PLAD-dependent interaction of DcR2 with DR5 prior to exogenous TRAIL stimulation is triggered either by endogenous soluble secreted TRAIL or by membrane-bound-expressed TRAIL in Jurkat cells. This hypothesis, however, remains to be tested.
How DcR2 inhibits caspase-8 and -10 processing remains to be determined. The prevailing view of TRAIL DISC assembly is based on homophilic homodimerization or homotrimerization of either the death domain or the death effector domain (49). Here, we show that DcR2 and DR5 form a heteromeric complex upon TRAIL binding. As initiator caspases were recently shown to be activated by dimerization (4, 31), DcR2, which is devoid of a functional death domain, might disrupt the DISC arrangement and prevent activation of caspase-8 and -10 by loosening the docking structure. It is very likely that the significant increase in procaspase-10 content in the DcR2-containing DISC is due to the impaired activation of caspase-8, as caspase-10 is unable to substitute for caspase-8 in cell death triggered by TRAIL (40).
The heteromeric complex that forms in response to TRAIL in DcR2-expressing cells could also change the composition of the DISC. In our hands, no obvious change in c-FLIP or FADD recruitment was observed in any of the tested cell lines (not shown). We did not identify any spontaneous association of c-FLIP to DR5, contrary to recent findings on Jurkat cells (17). The only change identified in the DISC composition of TRAIL-stimulated DcR2-expressing cells was the inhibition of DR4 recruitment to the DISC. As DcR2 inhibits TRAIL-induced cell death both in Jurkat cells, devoid of DR4, and in HeLa cells that express DR4, the lack of DR4 recruitment within the DISC may not account for the DcR2-mediated inhibition of TRAIL signaling. DR5 may prevail over DR4 to signal TRAIL-induced apoptosis in Jurkat cells, as ectopic expression of DR4 does not change cell sensitivity to TRAIL. However, this might not reflect the situation in all cell lines. The prevalence of either DR4 or DR5 for the triggering of the TRAIL cell death process has been demonstrated in different cell types (20, 28). Therefore, in addition to its ability to impair initiator caspase activation, DcR2-mediated DR4 exclusion from the DISC could also play an important role in regulating sensitivity to TRAIL-induced cell death depending on the cell type.
In addition, since DcR2 impairs caspase-8 and -10 processing within the TRAIL DISC, the question arises as to whether DcR2 could play a role in the triggering of TRAIL nonapoptotic functions. We have shown previously that active procaspases that are retained in the DISC may fail to activate a proapoptotic pathway, yet could cleave other substrates close to the DISC and participate in nonapoptotic signaling pathways due to changes in caspase-8, caspase-10, and c-FLIP ratios (31). Recent evidence indicates that TRAIL is involved in human intestinal differentiation, and interestingly, DcR1 and DcR2 have been shown to be up-regulated and expressed at the cell surface during the process (35). Whether DcR1 and DcR2 participate in this nonapoptotic event, e.g., by enhancing the formation of the recently described secondary complex that activates various kinases (43), remains to be determined.
Lastly, the understanding of the molecular mechanisms of TRAIL-mediated apoptosis inhibition by decoy receptors could be an important issue in oncology since chemotherapeutic drugs are known to sensitize tumor cells to TRAIL-induced apoptosis (29, 45). However, recent evidence indicates that cell surface expression of DcR2 could compromise not only TRAIL-induced cell death but also apoptosis induced by chemotherapeutic drugs (26). Therefore, further studies will be needed to determine whether TRAIL decoy receptors might affect antitumoral approaches aimed at combination therapy involving agents that target TRAIL or TRAIL derivatives, such as the newly developed anti-DR4 and anti-DR5 agonistic antibodies.
Acknowledgments
This work is supported by grants of the Ligue Nationale contre le Cancer (O.M. and E.S.), the Conseil Régional de Bourgogne (O.M.), the INCa (Institut National du Cancer) Cancéropôle Grand-Est (O.M.), and the INSERM (O.M. and E.S.). D.M. is a recipient of a fellowship from the Ministry of Research and Education. N.L. is a recipient of a joint fellowship from the INSERM and the Conseil Régional de Bourgogne. P.S. is supported by grants of the Swiss National Science Foundation, including a grant from the NCCR (National Center of Competence in Research) Molecular Oncology.
We would like to thank John Wijdenes and Diaclone for invaluable TRAIL-related reagents.
REFERENCES
- 1.Almasan, A., and A. Ashkenazi. 2003. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 14:337-348. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro, A., C. Gregoire, T. R. Bakker, L. Baldi, M. Jordan, L. Goffin, N. Boucheron, F. Wurm, P. A. van der Merwe, B. Malissen, and I. F. Luescher. 2001. CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J. Exp. Med. 194:1485-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., R. C. Pai, S. Fong, S. Leung, D. A. Lawrence, S. A. Marsters, C. Blackie, L. Chang, A. E. McMurtrey, A. Hebert, L. DeForge, I. L. Koumenis, D. Lewis, L. Harris, J. Bussiere, H. Koeppen, Z. Shahrokh, and R. H. Schwall. 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 104:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer, J. L., N. Holler, S. Reynard, P. Vinciguerra, P. Schneider, P. Juo, J. Blenis, and J. Tschopp. 2000. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat. Cell Biol. 2:241-243. [DOI] [PubMed] [Google Scholar]
- 6.Bouralexis, S., D. M. Findlay, G. J. Atkins, A. Labrinidis, S. Hay, and A. Evdokiou. 2003. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br. J. Cancer 89:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary, P. M., M. Eby, A. Jasmin, A. Bookwalter, J. Murray, and L. Hood. 1997. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7:821-830. [DOI] [PubMed] [Google Scholar]
- 8.Clancy, L., K. Mruk, K. Archer, M. Woelfel, J. Mongkolsapaya, G. Screaton, M. J. Lenardo, and F. K. Chan. 2005. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc. Natl. Acad. Sci. USA 102:18099-18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidovich, I. A., A. S. Levenson, and V. V. Levenson Chernokhvostov. 2004. Overexpression of DcR1 and survivin in genetically modified cells with pleiotropic drug resistance. Cancer Lett. 211:189-197. [DOI] [PubMed] [Google Scholar]
- 10.Degli-Esposti, M. A., W. C. Dougall, P. J. Smolak, J. Y. Waugh, C. A. Smith, and R. G. Goodwin. 1997. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813-820. [DOI] [PubMed] [Google Scholar]
- 11.Degli-Esposti, M. A., P. J. Smolak, H. Walczak, J. Waugh, C. P. Huang, R. F. DuBose, R. G. Goodwin, and C. A. Smith. 1997. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 186:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith, T. S., W. A. Chin, G. C. Jackson, D. H. Lynch, and M. Z. Kubin. 1998. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol. 161:2833-2840. [PubMed] [Google Scholar]
- 13.Griffith, T. S., C. T. Rauch, P. J. Smolak, J. Y. Waugh, N. Boiani, D. H. Lynch, C. A. Smith, R. G. Goodwin, and M. Z. Kubin. 1999. Functional analysis of TRAIL receptors using monoclonal antibodies. J. Immunol. 162:2597-2605. [PubMed] [Google Scholar]
- 14.Gura, T. 1997. How TRAIL kills cancer cells, but not normal cells. Science 277:768. [DOI] [PubMed] [Google Scholar]
- 15.Horak, P., D. Pils, G. Haller, I. Pribill, M. Roessler, S. Tomek, R. Horvat, R. Zeillinger, C. Zielinski, and M. Krainer. 2005. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol. Cancer Res. 3:335-343. [DOI] [PubMed] [Google Scholar]
- 16.Igney, F. H., and P. H. Krammer. 2002. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 17.Jin, T. G., A. Kurakin, N. Benhaga, K. Abe, M. Mohseni, F. Sandra, K. Song, B. K. Kay, and R. Khosravi-Far. 2004. Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5. J. Biol. Chem. 279:55594-55601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone, R. W., A. A. Ruefli, and S. W. Lowe. 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153-164. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka, T., M. Schroter, M. Hahne, P. Schneider, M. Irmler, M. Thome, C. J. Froelich, and J. Tschopp. 1998. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J. Immunol. 161:3936-3942. [PubMed] [Google Scholar]
- 20.Kelley, R. F., K. Totpal, S. H. Lindstrom, M. Mathieu, K. Billeci, L. Deforge, R. Pai, S. G. Hymowitz, and A. Ashkenazi. 2005. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J. Biol. Chem. 280:2205-2212. [DOI] [PubMed] [Google Scholar]
- 21.Kelley, S. K., L. A. Harris, D. Xie, L. Deforge, K. Totpal, J. Bussiere, and J. A. Fox. 2001. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299:31-38. [PubMed] [Google Scholar]
- 22.Lawrence, D., Z. Shahrokh, S. Marsters, K. Achilles, D. Shih, B. Mounho, K. Hillan, K. Totpal, L. DeForge, P. Schow, J. Hooley, S. Sherwood, R. Pai, S. Leung, L. Khan, B. Gliniak, J. Bussiere, C. A. Smith, S. S. Strom, S. Kelley, J. A. Fox, D. Thomas, and A. Ashkenazi. 2001. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 7:383-385. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc, H. N., and A. Ashkenazi. 2003. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 10:66-75. [DOI] [PubMed] [Google Scholar]
- 24.Lee, H. W., S. H. Lee, H. W. Lee, Y. W. Ryu, M. H. Kwon, and Y. S. Kim. 2005. Homomeric and heteromeric interactions of the extracellular domains of death receptors and death decoy receptors. Biochem. Biophys. Res. Commun. 330:1205-1212. [DOI] [PubMed] [Google Scholar]
- 25.Legler, D. F., O. Micheau, M. A. Doucey, J. Tschopp, and C. Bron. 2003. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 18:655-664. [DOI] [PubMed] [Google Scholar]
- 26.Liu, X., P. Yue, F. R. Khuri, and S. Y. Sun. 2005. Decoy receptor 2 (DcR2) is a p53 target gene and regulates chemosensitivity. Cancer Res. 65:9169-9175. [DOI] [PubMed] [Google Scholar]
- 27.MacFarlane, M., M. Ahmad, S. M. Srinivasula, T. Fernandes-Alnemri, G. M. Cohen, and E. S. Alnemri. 1997. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 272:25417-25420. [DOI] [PubMed] [Google Scholar]
- 28.MacFarlane, M., S. Inoue, S. L. Kohlhaas, A. Majid, N. Harper, D. B. Kennedy, M. J. Dyer, and G. M. Cohen. 2005. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 12:773-782. [DOI] [PubMed] [Google Scholar]
- 29.Micheau, O. 2003. Cellular FLICE-inhibitory protein: an attractive therapeutic target? Expert Opin. Ther. Targets 7:559-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Micheau, O., M. Thome, P. Schneider, N. Holler, J. Tschopp, D. W. Nicholson, C. Briand, and M. G. Grutter. 2002. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 277:45162-45171. [DOI] [PubMed] [Google Scholar]
- 32.Pan, G., J. Ni, Y. F. Wei, G. Yu, R. Gentz, and V. M. Dixit. 1997. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815-818. [DOI] [PubMed] [Google Scholar]
- 33.Pan, G., K. O'Rourke, A. M. Chinnaiyan, R. Gentz, R. Ebner, J. Ni, and V. M. Dixit. 1997. The receptor for the cytotoxic ligand TRAIL. Science 276:111-113. [DOI] [PubMed] [Google Scholar]
- 34.Riccioni, R., L. Pasquini, G. Mariani, E. Saulle, A. Rossini, D. Diverio, E. Pelosi, A. Vitale, A. Chierichini, M. Cedrone, R. Foa, F. Lo Coco, C. Peschle, and U. Testa. 2005. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica 90:612-624. [PubMed] [Google Scholar]
- 35.Rimondi, E., P. Secchiero, A. Quaroni, C. Zerbinati, S. Capitani, and G. Zauli. 2006. Involvement of TRAIL/TRAIL-receptors in human intestinal cell differentiation. J. Cell. Physiol. 206:647-654. [DOI] [PubMed] [Google Scholar]
- 36.Sanlioglu, A. D., E. Dirice, C. Aydin, N. Erin, S. Koksoy, and S. Sanlioglu. 2005. Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer 5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider, P. 2000. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 322:325-345. [DOI] [PubMed] [Google Scholar]
- 38.Sharp, D. A., D. A. Lawrence, and A. Ashkenazi. 2005. Selective knockdown of the long variant of cellular FLICE-inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 280:19401-19409. [DOI] [PubMed] [Google Scholar]
- 39.Sheridan, J. P., S. A. Marsters, R. M. Pitti, A. Gurney, M. Skubatch, D. Baldwin, L. Ramakrishnan, C. L. Gray, K. Baker, W. I. Wood, A. D. Goddard, P. Godowski, and A. Ashkenazi. 1997. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818-821. [DOI] [PubMed] [Google Scholar]
- 40.Sprick, M. R., E. Rieser, H. Stahl, A. Grosse-Wilde, M. A. Weigand, and H. Walczak. 2002. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but cannot functionally substitute caspase-8. EMBO J. 21:4520-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda, K., N. Yamaguchi, H. Akiba, Y. Kojima, Y. Hayakawa, J. E. Tanner, T. J. Sayers, N. Seki, K. Okumura, H. Yagita, and M. J. Smyth. 2004. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J. Exp. Med. 199:437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorburn, A. 2004. Death receptor-induced cell killing. Cell. Signal. 16:139-144. [DOI] [PubMed] [Google Scholar]
- 43.Varfolomeev, E., H. Maecker, D. Sharp, D. Lawrence, M. Renz, D. Vucic, and A. Ashkenazi. 2005. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 280:40599-40608. [DOI] [PubMed] [Google Scholar]
- 44.Vermot-Desroches, C., E. Sergent, B. Bonnin, and J. Wijdenes. 2005. Characterization of monoclonal antibodies directed against trail or trail receptors. Cell. Immunol. 236:86-91. [DOI] [PubMed] [Google Scholar]
- 45.Wajant, H., J. Gerspach, and K. Pfizenmaier. 2005. Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev. 16:55-76. [DOI] [PubMed] [Google Scholar]
- 46.Walczak, H., M. A. Degli-Esposti, R. S. Johnson, P. J. Smolak, J. Y. Waugh, N. Boiani, M. S. Timour, M. J. Gerhart, K. A. Schooley, C. A. Smith, R. G. Goodwin, and C. T. Rauch. 1997. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16:5386-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walczak, H., R. E. Miller, K. Ariail, B. Gliniak, T. S. Griffith, M. Kubin, W. Chin, J. Jones, A. Woodward, T. Le, C. Smith, P. Smolak, R. G. Goodwin, C. T. Rauch, J. C. Schuh, and D. H. Lynch. 1999. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5:157-163. [DOI] [PubMed] [Google Scholar]
- 48.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 49.Weber, C. H., and C. Vincenz. 2001. A docking model of key components of the DISC complex: death domain superfamily interactions redefined. FEBS Lett. 492:171-176. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, J., D. Cado, A. Chen, N. H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296-300. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, X. D., T. Nguyen, W. D. Thomas, J. E. Sanders, and P. Hersey. 2000. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 482:193-199. [DOI] [PubMed] [Google Scholar]