Abstract
P-TEFb phosphorylates RNA polymerase II and negative elongation factors to stimulate general transcriptional elongation. It is kept in a functional equilibrium through alternately interacting with its positive (the Brd4 protein) and negative (the HEXIM1 protein and 7SK snRNA) regulators. To investigate the physiological significance of this phenomenon, we analyzed the responses of HeLa cells and murine erythroleukemia cells (MELC) to hexamethylene bisacetamide (HMBA), which inhibits growth and induces differentiation of many cell types. For both cell types, an efficient, albeit temporary disruption of the 7SK-HEXIM1-P-TEFb snRNP and enhanced formation of the Brd4-P-TEFb complex occurred soon after the treatment started. When the P-TEFb-dependent HEXIM1 expression markedly increased as the treatment continued, the abundant HEXIM1 pushed the P-TEFb equilibrium back toward the 7SK/HEXIM1-bound state. For HeLa cells, as HMBA produced only a minor, temporary effect on their growth, the equilibrium gradually returned to its pretreatment level. In contrast, long-term treatment of MELC induced terminal division and differentiation. Concurrently, the P-TEFb equilibrium was shifted overwhelmingly toward the 7SK snRNP side. Together, these data link the P-TEFb equilibrium to the intracellular transcriptional demand and proliferative/differentiated states of cells.
The elongation stage of eukaryotic transcription is a highly regulated process crucial for not only generating the full-length mRNA transcripts but also coupling transcription with pre-mRNA processing (24). Central to the elongation control is the positive transcription elongation factor b (P-TEFb), which stimulates the processivity of RNA polymerase II elongation and antagonizes the effects of negative elongation factors (1, 10, 20). The predominant form of P-TEFb in many human cell types consists of cyclin-dependent kinase 9 (CDK9) and its regulatory subunit cyclin T1 (CycT1) (20). It phosphorylates the carboxy-terminal domain of the largest subunit of polymerase II as well as the negative elongation factors DSIF and NELF (1, 10, 20). These phosphorylation events are crucial for the transition from the abortive to the productive phase of transcriptional elongation. For this reason, P-TEFb is considered a general transcription factor essential for the expression of a vast array of protein-coding genes (4, 22).
Recent evidence indicates that nuclear P-TEFb is kept in a functional equilibrium through alternately interacting with its positive or negative regulators (13, 29). For negative regulation, the associations with the HEXIM1 protein and 7SK snRNA sequester P-TEFb into a kinase-inactive 7SK-HEXIM1-P-TEFb snRNP (17-19, 30, 31). Within this complex (termed the 7SK snRNP), HEXIM1 inhibits the CDK9 kinase activity, whereas 7SK stabilizes the HEXIM1-P-TEFb interaction (17, 18, 31, 32). Besides HEXIM1 and 7SK, P-TEFb also binds to the bromodomain protein Brd4 to form a separate complex (13, 29). The association with Brd4 forms the transcriptionally active P-TEFb and recruits P-TEFb to cellular promoters. It is believed that the abilities of Brd4 to bind to acetylated histones and the transcriptional Mediator complex may facilitate the recruitment of P-TEFb to chromatin templates (13, 29).
In HeLa cells under normal growth conditions, about half of nuclear P-TEFb is sequestered into the 7SK snRNP, whereas the other half probably binds to Brd4 (13, 17, 29-31). However, treatment of cells with certain stress-inducing agents, particularly those that can globally interrupt transcription, such as actinomycin D, DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole), and UV irradiation, causes a rapid disruption of the 7SK snRNP and enhanced formation of the Brd4-P-TEFb complex (19, 30, 31). Furthermore, treatment of cardiac myocytes with conditions that cause cardiac hypertrophy has also been shown to induce the disruption of the 7SK snRNP and activation of P-TEFb (21). Because P-TEFb activity is limiting in normal cardiac myocytes, the activation of P-TEFb leads to a global increase in cellular RNA and protein contents and consequently the enlargement of heart cells, which is the cause of hypertrophy (21).
Despite demonstrations that P-TEFb can interact alternately with its positive and negative regulators and that the 7SK snRNP can be converted quantitatively into the Brd4-P-TEFb complex under certain conditions, the physiological significance of the P-TEFb equilibrium remains largely unknown. Moreover, only the conditions that can cause the disruption of the 7SK snRNP and enhanced formation of the Brd4-P-TEFb complex have been described so far (5, 19, 29-31). It is not clear whether there may exist reagents that can shift the P-TEFb balance in the opposite direction to increase the sequestration of P-TEFb into the 7SK snRNP.
Here, we addressed these two issues by analyzing the effects of hexamethylene bisacetamide (HMBA), a hybrid bipolar compound, on both P-TEFb activity and growth of human HeLa cells and murine erythroleukemia cells (MELC). Whereas HMBA retarded HeLa cell growth only weakly and transiently, it is by far the best-characterized suppressor of growth and inducer of terminal differentiation of MELC (15). Our studies have revealed intricate regulations of P-TEFb activity throughout the course of HMBA treatment. For both cell types, an efficient, albeit temporary disruption of the 7SK snRNP and activation of P-TEFb-dependent transcription were observed during the initial phase of the treatment. When the P-TEFb-dependent HEXIM1 expression was markedly elevated as the treatment progressed, the abundance of HEXIM1 caused the P-TEFb equilibrium to shift back toward the 7SK snRNP direction. For HeLa cells, the equilibrium quickly returned to the pretreatment level. In contrast, significantly more P-TEFb was sequestered into the 7SK snRNP in HMBA-treated MELC than in untreated cells. Together, our data indicate that the functional P-TEFb equilibrium is tightly regulated to accommodate the overall transcriptional demand as well as the growth or differentiated states of cells.
MATERIALS AND METHODS
Materials.
HeLa and 293T cells were purchased from the American Type Culture Collection (Manassas, VA). MELC were a generous gift from the laboratory of Alan C. Sartorelli. HIV LTR-luciferase cells are a HeLa-based cell line stably transfected with a luciferase reporter construct driven by the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) (30). F1C2 cells are a HeLa-based cell line stably expressing Flag-tagged CDK9 (CDK9-f) (30). Rabbit anti-CDK9 and -CycT1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit immunoglobulin G, FLAG peptide, mouse anti-FLAG antibody, and anti-FLAG antibody conjugated to agarose beads were obtained from Sigma. Rabbit anti-HEXIM1 and anti-Brd4 antibodies were described previously (29, 31). Buffer D contained 20 mM HEPES-KOH, pH 7.9, 15% glycerol, 0.2 mM EDTA, 0.2% NP-40, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and various concentrations of KCl as indicated below. All other chemicals were from Sigma unless otherwise noted.
HMBA treatment.
Fresh stocks of HMBA prepared in tissue culture medium were used for all experiments. HeLa cells or MELC were treated for the indicated time periods with either 5 or 10 mM HMBA as specified in Results. For growth rate assays, HeLa cells and MELC were treated with HMBA for the indicated periods, diluted in fresh media without HMBA at 1 × 105 per well in six-well plates, and allowed to grow for 2 or 4 days. Cell counts were determined using a hemacytometer.
Luciferase assay.
HIV LTR-luciferase cells were seeded at 1.5 × 105 cells per well in six-well plates 1 day before the HMBA treatment and treated with HMBA. Luciferase activity was measured 48 h later with an assay kit from Promega.
Affinity purification of CDK9, CDK9-f, and their associated factors.
CDK9-f and CDK9 and their associated factors were isolated by anti-Flag or anti-CDK9 immunoprecipitation from nuclear extracts (NEs) of the indicated cell lines. For most experiments, NEs prepared in buffer D containing 0.35 M KCl were used. For Brd4 detection, however, the extracts were dialyzed against buffer D containing 0.15 M KCl. After incubation at 4°C for 2 h, the immunoprecipitates were washed extensively. The Flag peptide-eluted (for anti-Flag immunoprecipitates) or sodium dodecyl sulfate-eluted (for anti-CDK9 immunoprecipitates) materials were analyzed by Western blotting with the indicated antibodies and Northern blotting using the full-length 7SK antisense RNA as a probe. The signals of HEXIM1 and CDK9 in the NEs or immunoprecipitates were quantified using an Innotech AlphaImager 2200.
Transcription assay.
In vitro transcription reactions with mixtures containing NE from untreated or HMBA-treated HeLa cells and an HIV-1 template were carried out as described previously (34). G-less RNA fragments derived from in vitro-transcribed HIV-1 transcripts were isolated after RNase T1 digestion and analyzed on 6% polyacrylamide sequencing gels.
RT-PCR and Northern blot analysis.
Total RNA from HMBA-treated or untreated cells was isolated using TRIzol (Invitrogen). Twenty micrograms of total RNA was resolved on a 1% formaldehyde gel, transferred to a nylon membrane, and analyzed by Northern blotting. 32P-labeled ([α-32P]dATP or [α-32P]CTP) cDNA probes for HEXIM1 and luciferase were generated using a Stratagene Prime-It random primer labeling kit. The 7SK antisense RNA probe was generated as previously described (30). For reverse transcriptase PCR (RT-PCR), DNA contamination was eliminated using DNA-free DNase treatment and removal reagents (Ambion). First-strand cDNA synthesis was carried out using Moloney murine leukemia virus reverse transcriptase as instructed by the supplier (Promega).
Chromatin immunoprecipitation.
HIV LTR-luciferase cells (30) (2 × 106) were seeded into a 15-cm dish 1 day prior to HMBA treatment. Cells were treated with HMBA for 0 or 3 h, harvested, and subjected to a chromatin immunoprecipitation (ChIP) assay as described recently (29). After DNA purification, PCRs containing α-[32P]dCTP (800 Ci/mmol) were carried out for 20 cycles, and the products were analyzed on a 6% polyacrylamide-urea gel. Input and immunoprecipitated chromatin were analyzed first in pilot experiments to ensure that PCRs occurred in the linear range of amplification.
siRNAs.
HeLa cells were cotransfected in a 1:10 ratio with pBabe-puro empty vector and pSuper-based constructs expressing small interfering RNAs (siRNAs) specific for the indicated proteins. To deplete CycT1, a CycT1-specific siRNA called siCycT1 was expressed from the pSuper vector containing a short hairpin sequence, 5′-CTCGTGTCCCTCATTCGAAACGCTTCCTGTCACGTTTCGAATGAGGGACACGAG-3′ (with the central hairpin region underlined). For Brd4 depletion, a Brd4-specific siRNA called siBrd4 was derived from a short hairpin sequence, 5′-GAACCTCCCTGATTACTATAAGCTTCCTGTCACTTATAGTAATCAGGGAGGTTC-3′. At 24 h posttransfection, untransfected cells were killed by the addition of 1.5 μg/ml puromycin into the culturing media. NEs were prepared at 48 h posttransfection. For HEXIM1 depletion, siRNA530 and siRNA562 (31), with the numbers referring to the positions of the beginning nucleotides of two separate 20-nucleotide regions within the HEXIM1 open reading frame, were employed. Two independent stable transfectants for each siRNA were selected with and maintained in 1.5 μg/ml puromycin and used in the experiment.
RESULTS
HMBA induces transcription from the HIV-1 promoter.
We previously reported that HEXIM1 expression could be induced by HMBA in a variety of cell lines, including HeLa cells (33). Given HEXIM1's important role as an inhibitor of P-TEFb, we asked whether treatment of HeLa cells with HMBA and the consequent induction of HEXIM1 would have a negative effect on transcription. A stable HeLa-based cell line containing an integrated luciferase reporter gene driven by the HIV-1 LTR (30), which is known to be highly sensitive to P-TEFb (4, 9, 30), was used. Surprisingly, rather than causing an inhibition of transcription, HMBA induced expression from the reporter construct by several hundred-fold over an 8-h time period (Fig. 1A). Northern blot and semiquantitative RT-PCR analyses revealed that this increase occurred at the luciferase mRNA level (Fig. 1B). However, no increase was detected for the expression of the cellular gene encoding either the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) protein or the 7SK snRNA. Finally, NE prepared from HMBA-treated HeLa cells caused a significant increase in transcription from the HIV-1 promoter compared to NE from untreated cells (Fig. 1C), indicating that the HMBA-mediated increase in HIV-1 gene expression could be attributed directly to an activation of transcription.
FIG. 1.
HMBA stimulates transcription from the HIV-1 promoter. (A) HMBA induces expression of the luciferase reporter gene driven by the HIV-1 LTR. A stable HeLa-based cell line containing an integrated HIV-1 LTR-luciferase reporter construct was treated with 10 mM HMBA for the indicated time periods. The level of induction (n-fold) in luciferase activity compared to that in untreated cells is shown. (B) The HMBA-induced increase in luciferase activity occurs at the mRNA level. Semiquantitative RT-PCR and Northern blot analyses were performed to detect luciferase mRNA from among total RNA isolated at various time points of a continuous HMBA treatment. The levels of the GAPDH mRNA and 7SK snRNA were also analyzed as internal controls. (C) NE from HMBA-treated HeLa cells significantly increases HIV-1 transcription in vitro. Reaction mixtures contained the transcription template HIV+TAR-G400 and NE prepared from cells treated with HMBA for 0 or 3 h. RNA fragments transcribed from a G-less cassette inserted into the template at a position ∼1 kb downstream of the HIV-1 promoter are indicated.
HMBA disrupts the 7SK snRNP.
To seek an explanation for this dramatic increase in HIV-1 transcription, we examined whether, like certain stress-inducing agents and hypertrophic signals, HMBA could induce the disruption of the 7SK snRNP, thus releasing P-TEFb for transcriptional activation. Indeed, treatment of F1C2 cells, a HeLa-based cell line stably expressing FLAG-tagged CDK9, with HMBA caused a significant reduction in the amounts of HEXIM1 and 7SK associated with the immunoprecipitated CDK9-f (Fig. 2A). In contrast, the CDK9-CycT1 heterodimer formation was completely unaffected. The peak 7SK snRNP disruption occurred approximately 2 h after the treatment commenced (Fig. 2A, lane 8). Interestingly, a prolonged incubation with the drug (with fresh changes every 4 h) actually led to a gradual recovery of the 7SK snRNP (Fig. 2A, compare lanes 9 and 10 with lane 8), a point that will become clear later.
FIG. 2.
HMBA treatment of HeLa cells disrupts the 7SK snRNP and enhances the binding of P-TEFb to the HIV-1 chromatin template. (A) Treatment of HeLa cells with HMBA induces the dissociation of HEXIM1 and 7SK from P-TEFb. F1C2, a HeLa-based cell line stably expressing CDK9-f, was treated with HMBA for the indicated numbers of hours. CDK9-f, CycT1, HEXIM1, and 7SK present in NEs (lanes 1 to 5) and the anti-FLAG immunoprecipitates (αFLAG IP) (lanes 6 to 10) were detected by Western and Northern blotting. The presence of endogenous CDK9 (endo. CDK9) in NEs was revealed by anti-CDK9 Western blotting. (B) HMBA enhances the binding of P-TEFb to the HIV-1 chromatin template. The HeLa-based cell line with the integrated luciferase reporter gene driven by the HIV-1 LTR was treated with HMBA for 0 or 3 h. ChIP with anti-CDK9 antibody was performed. Three regions corresponding to the promoter region, interior region, and 3′ untranslated region (3′ UTR) of the integrated HIV-1 LTR-luciferase gene, as well as an interior region of the endogenous GAPDH gene, were PCR amplified from the precipitated and purified DNA. Numbers in parentheses indicate nucleotides. Amplified signals from 10% of the input chromatin are also shown.
HMBA-induced disruption of the 7SK snRNP causes more P-TEFb to bind to the HIV-1 chromatin template.
We next performed a ChIP assay to determine whether the HMBA-induced release of P-TEFb from the 7SK snRNP would result in the association of more P-TEFb with the chromatin template containing an integrated HIV-1 LTR-luciferase reporter gene, thus causing activation of transcription. Quantification of the data shown in Fig. 2B revealed that treatment of the HeLa-based cell line with HMBA for 3 h indeed increased the binding of P-TEFb to the HIV-1 promoter region by 6.5-fold. In addition, the treatment also caused 2.5- and 1.5-fold more P-TEFb to bind to the interior of the luciferase gene as well as the 3′ untranslated region, respectively. In contrast, HMBA did not result in the association of more P-TEFb with the coding sequence of the GAPDH gene, in agreement with the observation that the expression of this gene was unaffected by HMBA (Fig. 1B, upper panel).
Prolonged treatment of HeLa cells with HMBA leads to reformation of the 7SK snRNP.
The above-described partial restoration of the 7SK snRNP detected at 8 h of the HMBA treatment (Fig. 2A) prompted us to investigate the long-term effect of this drug on the formation of this complex. Quantification of the amounts of HEXIM1 associated with the immunoprecipitated CDK9-f at different time points since the commencement of HMBA treatment (with fresh changes of the drug every 4 h) confirmed that the HMBA-induced disruption of the 7SK snRNP was transient (Fig. 3A). By 15 to 18 h into the treatment, the amount of the 7SK snRNP present in the treated HeLa cells was restored almost to the pretreatment level and remained this way until the end of the 72-h incubation period.
FIG. 3.
Transient disruption of the 7SK snRNP, formation of the Brd4-P-TEFb complex, and activation of HIV-1 transcription in HMBA-treated HeLa cells. (A) Prolonged treatment with HMBA leads to reformation of the 7SK snRNP. HeLa cells were incubated with HMBA for the indicated time periods. Levels of HEXIM1 associated with the immunoprecipitated CDK9 derived from NEs were detected by Western blotting, quantified, and normalized to CDK9 levels, and they are shown as percentages relative to the pretreatment level, which was set to 100%. (B) HMBA transiently induces luciferase mRNA synthesis. Results are from Northern blot analysis of luciferase mRNA transcribed from the HIV-1 LTR in HeLa cells treated with HMBA for the indicated time periods. As a loading control, 7SK snRNA in total RNA samples was also examined. (C) Transient induction of luciferase activity in HMBA-treated cells. The level of induction (n-fold) in luciferase activity, expressed from the integrated HIV-1 LTR-luciferase reporter gene, was measured with a HeLa-based cell line treated with HMBA for the indicated time periods. pHIV, HIV-1 promoter. (D) P-TEFb alternately interacts with HEXIM1 and Brd4 throughout the course of HMBA treatment. NEs were prepared from HeLa cells treated with HMBA for the indicated durations and subjected to immunoprecipitation with anti-CDK9 antibody (αCDK9) (lanes 5 to 7) or, as a negative control (cntl.), anti-CDK4 antibody (lane 4). Brd4, HEXIM1, CycT1, and CDK9 present in the immunoprecipitates (IP) and NEs were examined by Western blotting.
Consistent with this observation, the luciferase mRNA transcribed from the HIV-1 promoter also showed a transient increase, peaking around 6 h after the treatment began, but was hardly detectable 72 h after the treatment began (Fig. 3B). Similarly, the HMBA-mediated induction of luciferase activity peaked around 12 h and then started to decline (Fig. 3C). A combination of factors such as the rates of mRNA processing, export, and degradation coupled with the time required for protein synthesis and maturation may contribute to the apparent 6-h delay in the accumulation of peak luciferase activity relative to the peak mRNA level. Furthermore, the relatively high stability of the luciferase protein may result in the detection of a considerable amount of its enzymatic activity in HMBA-treated cells even after the nuclear 7SK snRNP returns to the pretreatment level.
P-TEFb alternately interacts with its positive and negative regulators throughout the course of HMBA treatment.
We have shown previously that the treatment of HeLa cells with certain stress-inducing agents, such as actinomycin D, DRB, and UV irradiation, causes a quantitative conversion of the HEXIM1-7SK-P-TEFb snRNP into the Brd4-P-TEFb complex (29). To determine whether HMBA could also shift the balance between these two P-TEFb subpopulations, the amounts of Brd4 and HEXIM1 bound to the immunoprecipitated CDK9 at 0, 6, and 24 h into a continuous HMBA treatment were analyzed by Western blotting. In contrast to its effect on the HEXIM1-P-TEFb binding, which decreased dramatically at the 6-h time point and returned to the pretreatment level at 24 h as described above (Fig. 3A), HMBA produced the opposite effect on the binding of Brd4 to P-TEFb by significantly enhancing it at 6 h and restoring it to the pretreatment level at 24 h (Fig. 3D). It has been proposed that P-TEFb is normally maintained in a functional equilibrium through alternately interacting with its positive (Brd4) and negative (HEXIM1 and 7SK) regulators (13, 29). The observation that P-TEFb switched back and forth between these two functional states during the course of HMBA treatment suggests that HMBA caused an initial, transient shift of the P-TEFb equilibrium toward the active, Brd4-bound state. However, over a more extended period of time, the treatment did not produce any long-lasting effect on the relative concentrations of the two P-TEFb subpopulations, as the equilibrium soon returned to the pretreatment level.
HMBA induces HEXIM1 expression in a P-TEFb-dependent manner.
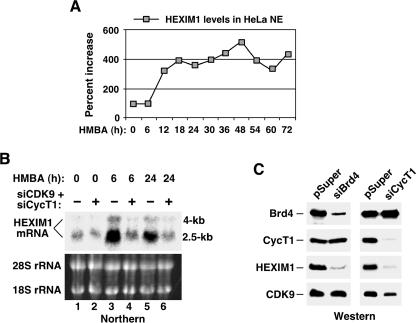
What might have caused a rapid resequestration of P-TEFb into the 7SK snRNP after the initial response to HMBA, a possible stress-inducing agent to HeLa cells, had subsided? One potential clue came from the observation that sometime between 6 and 12 h after the HMBA treatment started, the HEXIM1 expression began to increase significantly and its nuclear concentration remained four to five times higher than the pretreatment level for at least 72 h (Fig. 4A). It is possible that this major increase in HEXIM1 concentration coupled with a constitutively high level of 7SK snRNA (Fig. 1B and 3B) (27) might have pushed the P-TEFb equilibrium back toward the HEXIM1/7SK-bound state.
FIG. 4.
P-TEFb-dependent induction of HEXIM1 expression by HMBA. (A) HMBA treatment of HeLa cells increases HEXIM1 expression. HEXIM1 levels in NE of HMBA-treated cells were detected by Western blotting at various time points of the treatment. The HEXIM1 signals were quantified and normalized to those of CDK9 for each time point, and they are shown as percentages relative to the pretreatment level, which was set to 100%. (B) P-TEFb is required for HMBA-induced HEXIM1 mRNA synthesis. HeLa cells transfected with either the empty pSuper vector (−) or vectors expressing the CDK9- and CycT1-specific siRNAs (siCDK9 and siCycT1, respectively) were treated with HMBA for 0, 6, or 24 h. Total RNA was isolated and subjected to Northern blot analysis to detect the major 2.5-kb and minor 4.0-kb HEXIM1 mRNA. The 18S and 28S rRNA present in total RNA were stained with ethidium bromide and used as loading controls. (C) CycT1- and Brd4-dependent production of the HEXIM1 protein. Western blotting was performed to examine the levels of Brd4, CycT1, HEXIM1, and CDK9 in NEs prepared from HeLa cells transfected with either the empty pSuper vector or the indicated siRNA-expressing pSuper constructs at 48 h posttransfection.
Next, we asked how the HMBA-induced HEXIM1 expression was accomplished. We noticed that the onset of this induction occurred soon after the HMBA-mediated disruption of the 7SK snRNP and activation of P-TEFb. To determine whether P-TEFb was required for the HMBA-induced HEXIM1 expression, we performed Northern analysis to measure HEXIM1 mRNA levels in HeLa cells that expressed the siRNAs specific for CDK9 and CycT1. Indeed, compared to an empty vector, the siRNA-mediated depletion of P-TEFb caused a drastic reduction in levels of the HMBA-induced HEXIM1 mRNA at both 6 and 24 h of the treatment (Fig. 4B). Notably, the HEXIM1 mRNA was quite stable in the absence of the siRNAs, as its level remained relatively high at 24 h (Fig. 4B, lane 5), when the freshly liberated P-TEFb had been recaptured by 7SK/HEXIM1 (Fig. 3A).
The P-TEFb-dependent HEXIM1 gene expression was further confirmed by Western analysis (Fig. 4C), which showed that the CycT1-specific siRNA reduced the expressions of not only its intended target, CycT1, but also HEXIM1. Moreover, introduction of a siRNA specific for Brd4, the positive regulator of P-TEFb, also caused the codepletion of both Brd4 and HEXIM1 (Fig. 4C). Because these two proteins act in opposite directions to control the activity of P-TEFb, their coregulation implicated the existence of a cellular mechanism to maintain a balance between the two P-TEFb subpopulations for proper cell growth (see below). In summary, the observed strong dependence on P-TEFb for HMBA-induced HEXIM1 expression may explain why the P-TEFb equilibrium was shifted transiently toward the Brd4-bound active state during the initial phase of HMBA treatment, which in turn produced abundant HEXIM1 to eventually drive the P-TEFb equilibrium back to the pretreatment level.
HMBA exerts only a minor, short-term effect on HeLa cell growth.
To understand why HeLa cells would want to resequester P-TEFb into the inactive 7SK snRNP once HEXIM1 gene expression was induced, we examined the effect on HeLa cell growth by either a 6- or a 24-h treatment with HMBA. Compared to results with untreated cells, whose numbers were artificially set to 100%, incubation with HMBA for 6 h had no detectable effect on cell numbers, which were counted at 2 and 4 days after the removal of the drug and are presented as percentages relative to those of untreated cells (Fig. 5A). Similarly, the 24-h HMBA treatment slowed cell growth only temporarily by about 30% measured at 2 days posttreatment, and the cell numbers returned largely to normal after another 2 days (Fig. 5A). Thus, despite the demonstrations that HMBA induced a transient disruption of the 7SK snRNP and induction of HEXIM1 expression, it apparently had only a relatively minor, short-term effect on HeLa cell growth. It is likely that this lack of a major, long-lasting effect by HMBA led to the resequestration of P-TEFb into the 7SK snRNP and restoration of the complex to its original level. Notably, the human embryonic kidney cell line 293T displayed exactly the same responses as HeLa cells to the HMBA treatment in terms of the kinetics and degrees of 7SK snRNP disruption/reformation as well as the growth rates (data not shown).
FIG. 5.
HeLa cells strive to maintain a constant level of nuclear 7SK snRNP for optimal growth. (A) HMBA suppresses HeLa cell growth only mildly and temporarily. Cells were either untreated or treated with HMBA for 6 or 24 h. After removal of the drug, cells were placed in fresh media at low but equal concentrations and allowed to grow for 2 or 4 days. Cell counts were determined and are shown as percentages relative to those of untreated cells, which were set to 100%. (B) Diversion of free HEXIM1 into the 7SK snRNP in HeLa cells expressing the HEXIM1-specific siRNAs. NEs were prepared from HeLa-based cell lines either containing the empty pSuper vector or expressing the indicated HEXIM1-specific siRNAs and examined for their HEXIM1 and CDK9 levels by Western blotting (lanes 1 to 5). Two independent cell clones (denoted by -1 and -2 after the siRNA designations) for each siRNA were analyzed. The NEs were also subjected to immunoprecipitation with either anti-CDK4 (αCDK4) (lane 6) or anti-CDK9 (lanes 7 to 11) antibody, and the indicated factors present in the immunoprecipitates (IP) were examined by Northern and Western blotting.
HeLa cells strive to maintain a constant amount of 7SK snRNP for optimal growth.
The observations discussed above raised an intriguing possibility that the intracellular concentrations of 7SK snRNP may define and even help establish the growth states of cells. According to this hypothesis, when cells are not ready to undergo a major alteration of their growth state, they strive hard to maintain their nuclear 7SK snRNP at levels appropriate for the overall transcriptional demand of that particular state. Besides the P-TEFb homeostasis observed with HMBA-treated HeLa and 293T cells, additional evidence in support of this notion came from studies involving several HeLa-based cell lines that stably expressed the HEXIM1-specific siRNAs. It is known that in normal log-phase HeLa cells, a major portion of nuclear HEXIM1 exists outside of the 7SK snRNP and probably in free forms (3, 18, 31). Interestingly, when the total HEXIM1 levels in NEs were markedly reduced as a result of the expression of the HEXIM1 siRNAs (Fig. 5B, left panel), the amounts of HEXIM1 associated with 7SK/P-TEFb were barely affected (Fig. 5B, right panel). It is important to point out that the growth rates of these siRNA-producing cells were reduced only slightly and that we were able to establish and propagate them as stable cell lines without much difficulty. Thus, there was apparently an effort by these cells to compensate for the loss of HEXIM1 by mobilizing virtually all of the remaining free HEXIM1 into the 7SK snRNP in order to avoid a major perturbation of the P-TEFb equilibrium.
Enhanced sequestration of P-TEFb into the 7SK snRNP during HMBA-induced MELC differentiation.
Despite the demonstration that HMBA displayed no long-term effect on HeLa cell growth, the above-described behavioral changes of P-TEFb during the course of HMBA treatment have nevertheless revealed an interesting connection between the functional P-TEFb equilibrium and the overall growth state of cells. To further test the hypothesis that nuclear 7SK snRNP levels are intimately associated with the critical cellular decision between growth and differentiation, we turned our attention to the effect of HMBA on MELC. It has long been recognized that HMBA is a highly effective and the best-characterized inducer of terminal differentiation (including terminal division) of MELC, which serves as a model for examining the control of erythroid differentiation (15, 16). In contrast to HeLa cells, whose growth was affected only mildly by treatment with either 5 (Fig. 6A) or 10 (Fig. 5A) mM HMBA, MELC were severely growth arrested when treated with 5 mM HMBA for 72 h (Fig. 6A) and, moreover, underwent efficient terminal differentiation under these conditions (15, 16; data not shown). At 10 mM HMBA, a significant portion of MELC was led to apoptosis after a prolonged incubation (23; data not shown).
FIG. 6.
Enhanced sequestration of P-TEFb into the 7SK snRNP and elevated expression of HEXIM1 in HMBA-treated MELC. (A) HMBA treatment of MELC severely inhibits cell growth. HeLa cells and MELC were either untreated or treated with HMBA for 72 h. Upon removal of the drug, cells were placed in fresh media at low but equal concentrations and allowed to grow for 2 days. Cell counts were determined and are shown as percentages relative to those of untreated cells, which were set to 100%. (B) NEs were prepared from MELC treated with HMBA for the indicated time periods and subjected to anti-CDK9 (αCDK9) immunoprecipitation. HEXIM1 associated with the immunoprecipitates (IP) or present in NEs were detected by Western blotting. The HEXIM1 signals were normalized to those of CDK9 for each time point and are shown as percentages relative to the pretreatment levels, which were set to 100%. (C) Diagram depicting the regulation of P-TEFb activity during the course of HMBA treatment. The 7SK snRNP is proposed to contain two copies of P-TEFb and HEXIM1 and one copy of 7SK (14). During the first several hours of HMBA treatment, the 7SK snRNP was converted into the Brd4-P-TEFb complex, which stimulated P-TEFb-dependent HEXIM1 gene expression. The elevated HEXIM1 levels then pushed the P-TEFb equilibrium back toward the 7SK snRNP direction during a prolonged HMBA treatment. For details, see Discussion.
Quantification of the amounts of HEXIM1 associated with the immunoprecipitated CDK9 indicated that the initial response of MELC to either 5 or 10 mM HMBA was a swift disruption of the 7SK snRNP (Fig. 6B), reminiscent of the situations seen with HeLa and 293T cells. However, unlike the last two cell lines, in which 7SK snRNP returned almost to the pretreatment level after a prolonged incubation (Fig. 3A; data not shown), MELC produced up to 2.9-fold more 7SK snRNP than was produced in the untreated cells as the treatment proceeded further (Fig. 6B, left panel). Moreover, the HMBA-mediated induction of HEXIM1 expression in MELC (11- to 14-fold) (Fig. 6B, right panel) was significantly higher than that in HeLa and 293T cells (4- to 5-fold) (Fig. 4A; data not shown), which could be responsible for the enhanced sequestration of P-TEFb into the 7SK snRNP in these HMBA-treated MELC, which were undergoing terminal division/differentiation. Taken together, our analyses of HeLa and 293T cells and MELC yielded consistent data which revealed a strong correlation between nuclear 7SK snRNP concentrations and the growth/differentiated states of cells.
DISCUSSION
Despite the demonstrations that P-TEFb is maintained in a functional equilibrium through alternately interacting with its positive and negative regulators (13, 29), the physiological significance of this phenomenon has not been demonstrated clearly. In this study, we analyzed the responses of HeLa cells and MELC to HMBA treatment in aspects such as growth properties, associations of P-TEFb with its regulators, and nuclear P-TEFb activities. Our data strongly support the notion that the functional P-TEFb equilibrium is tightly and dynamically regulated to reflect and accommodate the intracellular transcriptional demand as well as the proliferative or differentiated states of cells. Furthermore, among all of the agents that are known to affect the P-TEFb equilibrium (5, 18, 19, 30, 31), HMBA has been identified as the only one that can shift the P-TEFb balance toward the HEXIM1/7SK-bound state to increase the formation of the inactive P-TEFb complex in MELC.
As indicated in the diagram shown in Fig. 6C, the initial responses of both HeLa cells and MELC to HMBA are an efficient, albeit temporary disruption of the 7SK snRNP and liberation of P-TEFb. Since transcription from the HIV-1 LTR is known to be exquisitely sensitive to P-TEFb (4, 9, 30), the HMBA-induced activation of P-TEFb provides a molecular explanation for the previously described stimulatory effect of this drug on expression from the HIV-1 LTR, which has been characterized as independent of the NF-κB binding sites and other enhancer elements within the LTR (26). The HMBA-induced dissociation of HEXIM1/7SK from P-TEFb is also reminiscent of the situations seen with HeLa cells treated with certain stress-inducing agents that globally disrupt transcription and suppress cell growth (5, 18, 19, 30, 31). Since HMBA is able to inhibit the growth of both MELC and HeLa cells, albeit only transiently in the latter case, the induced shift of the P-TEFb equilibrium toward the active, Brd4-bound state could simply be an instinctive cellular response to this growth-arresting and stressful event. One conceivable consequence could be the activation of various stress-responsive genes. In addition, the activation of P-TEFb in MELC could lead to elevated expression of genes that play key roles during the commitment and establishment of the differentiated state (15, 16).
It remains to be determined how many genes can be activated directly by P-TEFb during the initial phase of HMBA treatment, what they are, and what roles they may play in the subsequent differentiation process of MELC. Nevertheless, we now know that there exists at least one gene, namely, the HEXIM1 gene, whose elevated expression is indeed caused by the HMBA-induced P-TEFb activation (Fig. 4). It is conceivable that accumulation of abundant HEXIM1 during a prolonged HMBA treatment is able to push the P-TEFb equilibrium back toward the 7SK/HEXIM1-bound state (Fig. 6C). After that, the intrinsic differences between HeLa cells and MELC in their responses to HMBA determine the final states of their nuclear P-TEFb equilibriums. For HeLa cells, since the treatment with HMBA produces no long-lasting effect on their growth, the P-TEFb equilibrium quickly returns to the pretreatment level. In contrast, MELC undergo terminal division and differentiation once they have passed through the commitment stage, which occurs as early as 10 to 12 h after commencement of the treatment (6). As a result, the P-TEFb equilibrium is shifted overwhelmingly toward the inactive 7SK snRNP side to accommodate an altered transcriptional demand in these cells that can no longer divide. Despite the implication of P-TEFb in this process, it is important to stress that we actually cannot tell at this stage whether the increased formation of the inactive P-TEFb complex in HMBA-treated MELC is a consequence of or a contributor to growth arrest and terminal differentiation of these cells.
Nevertheless, the tight coupling of the P-TEFb equilibrium with the global control of cell growth and differentiation agrees well with the demonstrated growth-regulatory functions of the P-TEFb-associated factors. For instance, as a negative regulator of P-TEFb activity, HEXIM1 has been shown to display an antigrowth function in cardiac myocytes, where the deletion of the mouse HEXIM1 (CLP-1) gene causes the enlargement of heart cells, reminiscent of a pathological condition known as hypertrophy (12). Interestingly enough, hypertrophic agents that induce the disruption of the 7SK snRNP also cause the same disease (21). In both cases, the deletion of the HEXM1 gene or dissociation of HEXIM1 from P-TEFb achieves the same goal of activating P-TEFb, which provides the necessary means to deal with an increased transcriptional demand under these highly proliferative conditions. As with its role in cardiac myocytes, HEXIM1 has been recognized as an inhibitor of breast epithelial cell proliferation, as its expression is down-regulated by estrogens and decreased in breast tumors (28). Finally, a recent report indicates that ectopic expression of HEXIM1 causes growth inhibition and promotes neuronal differentiation (25).
In contrast to HEXIM1, Brd4, the positive regulator and recruitment factor for P-TEFb, has been implicated to play a growth-stimulatory role. While mice lacking both alleles of the Brd4 gene are embryonic lethal, Brd4-heterozygotic mice display pre- and postnatal growth defects associated with a reduced proliferation rate (11). In addition, Brd4 is required for the proper progression of the cell cycle, as heterozygotes display a significantly reduced number of mitotic cells compared to wild-type tissues (11).
The opposing effects on cell growth exerted by Brd4 and HEXIM1, both of which target P-TEFb but produce antagonizing results, further support the idea that controlling the activity of the general transcription factor P-TEFb, which affects expression of a vast array of genes, is critical to the cellular decision between growth and differentiation. Besides its role in MELC, P-TEFb has been implicated to play a role in regulating the differentiation programs of several other cell types, including skeletal muscle cells, monocytes, lymphocytes, and neurons (2, 7, 8). However, only the expressions of CDK9 and CycT1 have been monitored in these studies. Future investigation of the P-TEFb equilibriums and their control by Brd4 and HEXIM1 in these cells will be very informative toward the understanding of the general roles of P-TEFb and transcriptional elongation in the global control of cell growth and differentiation.
Acknowledgments
We thank Rieko Nishimura for experiments involving the HEXIM1 siRNAs and Vivien Lee for technical assistance. We also thank Dalibor Blazek and Matija Peterlin for providing us with good-quality HMBA.
This work was supported by grants from the National Institutes of Health (AI41757) and the American Cancer Society (RSG-01-171-01-MBC) to Q.Z.
REFERENCES
- 1.Barboric, M., and B. M. Peterlin. 2005. A new paradigm in eukaryotic biology: HIV Tat and the control of transcriptional elongation. PLoS Biol. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellan, C., G. De Falco, S. Lazzi, P. Micheli, S. Vicidomini, K. Schurfeld, T. Amato, A. Palumbo, L. Bagella, E. Sabattini, S. Bartolommei, M. Hummel, S. Pileri, P. Tosi, L. Leoncini, and A. Giordano. 2004. CDK9/CYCLIN T1 expression during normal lymphoid differentiation and malignant transformation. J. Pathol. 203:946-952. [DOI] [PubMed] [Google Scholar]
- 3.Byers, S. A., J. P. Price, J. J. Cooper, Q. Li, and D. H. Price. 2005. HEXIM2, a HEXIM1-related protein, regulates positive transcription elongation factor b through association with 7SK. J. Biol. Chem. 280:16360-16367. [DOI] [PubMed] [Google Scholar]
- 4.Chao, S.-H., and D. H. Price. 2001. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J. Biol. Chem. 276:31793-31799. [DOI] [PubMed] [Google Scholar]
- 5.Chen, R., Z. Yang, and Q. Zhou. 2004. Phosphorylated positive transcription elongation factor b (P-TEFb) is tagged for inhibition through association with 7SK snRNA. J. Biol. Chem. 279:4153-4160. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., J. Banks, R. A. Rifkind, and P. A. Marks. 1982. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc. Natl. Acad. Sci. USA 79:471-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Falco, G., C. Bellan, A. D'Amuri, G. Angeloni, E. Leucci, A. Giordano, and L. Leoncini. 2005. Cdk9 regulates neural differentiation and its expression correlates with the differentiation grade of neuroblastoma and PNET tumors. Cancer Biol. Ther. 4:277-281. [DOI] [PubMed] [Google Scholar]
- 8.De Falco, G., and A. Giordano. 2002. CDK9: from basal transcription to cancer and AIDS. Cancer Biol. Ther. 1:342-347. [PubMed] [Google Scholar]
- 9.Flores, O., G. Lee, J. Kessler, M. Miller, W. Schlief, J. Tomassini, and D. Hazuda. 1999. Host-cell positive transcription elongation factor b kinase activity is essential and limiting for HIV type 1 replication. Proc. Natl. Acad. Sci. USA 96:7208-7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garriga, J., and X. Grana. 2004. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 337:15-23. [DOI] [PubMed] [Google Scholar]
- 11.Houzelstein, D., S. L. Bullock, D. E. Lynch, E. F. Grigorieva, V. A. Wilson, and R. S. Beddington. 2002. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22:3794-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, F., M. Wagner, and M. A. Siddiqui. 2004. Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech. Dev. 121:559-572. [DOI] [PubMed] [Google Scholar]
- 13.Jang, M. K., K. Mochizuki, M. Zhou, H. S. Jeong, J. N. Brady, and K. Ozato. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19:523-534. [DOI] [PubMed] [Google Scholar]
- 14.Li, Q., J. P. Price, S. A. Byers, D. Cheng, J. Peng, and D. H. Price. 2005. Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing Cdk9 phosphorylated at threonine 186. J. Biol. Chem. 280:28819-28826. [DOI] [PubMed] [Google Scholar]
- 15.Marks, P. A., V. M. Richon, H. Kiyokawa, and R. A. Rifkind. 1994. Inducing differentiation of transformed cells with hybrid polar compounds: a cell cycle-dependent process. Proc. Natl. Acad. Sci. USA 91:10251-10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks, P. A., V. M. Richon, and R. A. Rifkind. 1996. Cell cycle regulatory proteins are targets for induced differentiation of transformed cells: molecular and clinical studies employing hybrid polar compounds. Int. J. Hematol. 63:1-17. [DOI] [PubMed] [Google Scholar]
- 17.Michels, A. A., A. Fraldi, Q. Li, T. E. Adamson, F. Bonnet, V. T. Nguyen, S. C. Sedore, J. P. Price, D. H. Price, L. Lania, and O. Bensaude. 2004. Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor. EMBO J. 23:2608-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michels, A. A., V. T. Nguyen, A. Fraldi, V. Labas, M. Edwards, F. Bonnet, L. Lania, and O. Bensaude. 2003. MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol. Cell. Biol. 23:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen, V. T., T. Kiss, A. A. Michels, and O. Bensaude. 2001. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322-325. [DOI] [PubMed] [Google Scholar]
- 20.Price, D. H. 2000. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20:2629-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano, M., M. Abdellatif, H. Oh, M. Xie, L. Bagella, A. Giordano, L. H. Michael, F. J. DeMayo, and M. D. Schneider. 2002. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat. Med. 8:1310-1317. [DOI] [PubMed] [Google Scholar]
- 22.Shim, E. Y., A. K. Walker, Y. Shi, and T. K. Blackwell. 2002. CDK-9/cyclin T (P-TEFb) is required in two postinitiation pathways for transcription in the C. elegans embryo. Genes Dev. 16:2135-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel, D. S., X. Zhang, R. Feinman, T. Teitz, A. Zelenetz, V. M. Richon, R. A. Rifkind, P. A. Marks, and J. Michaeli. 1998. Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells. Proc. Natl. Acad. Sci. USA 95:162-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims, R. J., III, R. Belotserkovskaya, and D. Reinberg. 2004. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18:2437-2468. [DOI] [PubMed] [Google Scholar]
- 25.Turano, M., G. Napolitano, C. Dulac, B. Majello, O. Bensaude, and L. Lania. 2006. Increased HEXIM1 expression during erythroleukemia and neuroblastoma cell differentiation. J. Cell. Physiol. 206:603-610. [DOI] [PubMed] [Google Scholar]
- 26.Vlach, J., and P. M. Pitha. 1993. Hexamethylene bisacetamide activates the human immunodeficiency virus type 1 provirus by an NF-kappa B-independent mechanism. J. Gen. Virol. 74:2401-2408. [DOI] [PubMed] [Google Scholar]
- 27.Wassarman, D. A., and J. A. Steitz. 1991. Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol. Cell. Biol. 11:3432-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittmann, B. M., N. Wang, and M. M. Montano. 2003. Identification of a novel inhibitor of breast cell growth that is down-regulated by estrogens and decreased in breast tumors. Cancer Res. 63:5151-5158. [PubMed] [Google Scholar]
- 29.Yang, Z., J. H. Yik, R. Chen, N. He, M. K. Jang, K. Ozato, and Q. Zhou. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19:535-545. [DOI] [PubMed] [Google Scholar]
- 30.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 31.Yik, J. H., R. Chen, R. Nishimura, J. L. Jennings, A. J. Link, and Q. Zhou. 2003. Inhibition of P-TEFb (CDK9/cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol. Cell 12:971-982. [DOI] [PubMed] [Google Scholar]
- 32.Yik, J. H., R. Chen, A. C. Pezda, C. S. Samford, and Q. Zhou. 2004. A human immunodeficiency virus type 1 Tat-like arginine-rich RNA-binding domain is essential for HEXIM1 to inhibit RNA polymerase II transcription through 7SK snRNA-mediated inactivation of P-TEFb. Mol. Cell. Biol. 24:5094-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yik, J. H., R. Chen, A. C. Pezda, and Q. Zhou. 2005. Compensatory contributions of HEXIM1 and HEXIM2 in maintaining the balance of active and inactive positive transcription elongation factor b complexes for control of transcription. J. Biol. Chem. 280:16368-16376. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, Q., and P. A. Sharp. 1995. Novel mechanism and factor for regulation by HIV-1 Tat. EMBO J. 14:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]