Abstract
Genetic analyses in Caenorhabditis elegans demonstrate that sel-12 and hop-1, homologues of the Alzheimer’s disease-associated presenilin genes, modify signaling through LIN-12 and GLP-1, homologues of the Notch cell surface receptor. To gain insight into the biochemical basis of this genetic interaction, we tested the possibility that presenilin-1 (PS1) physically associates with the Notch1 receptor in mammalian cells. Notch1 and PS1 coimmunoprecipitated from transiently transfected human embryonic kidney 293 cell lysates in a detergent-sensitive manner, consistent with a noncovalent physical association between the two proteins. The interaction predominantly occurred early in the secretory pathway prior to Notch cleavage in the Golgi, because PS1 immunoprecipitation preferentially recovered the full-length Notch1 precursor. When PS1 was immunoprecipitated from 293 cells that had been metabolically labeled with [35S]methionine and [35S]cysteine, Notch1 was the primary protein detected in PS1 immunoprecipitates, suggesting that this interaction is specific. Furthermore, endogenous Notch and presenilin coimmunoprecipitated from cultured Drosophila cells, indicating that physical interaction can occur at physiological expression levels. These results suggest that the genetic relationship between presenilins and the Notch signaling pathway derives from a direct physical association between these proteins in the secretory pathway.
The most common known cause of autosomal dominantly inherited early onset Alzheimer’s disease (AD) is mutation in the presenilin genes (collectively PS) (reviewed in refs. 1 and 2). The PS genes encode widely expressed integral membrane proteins localized primarily in the endoplasmic reticulum (ER) and Golgi (3–5). Mutations in presenilin-1 (PS1) and its homologue presenilin-2 (PS2) are scattered throughout the coding region, and most lead to single amino acid substitutions in the encoded protein (1, 2). PS mutations result in elevated levels of Aβ42, a highly amyloidogenic subspecies of Aβ that is the primary protein deposited in senile plaques, a hallmark of AD pathology (1, 2, 6, 7). Aβ42 is generated most likely in the ER (8) from the β-amyloid precursor protein (APP) by two unidentified enzymatic activities, β-secretase, which cleaves in the extracellular domain, and γ-secretase, which cleaves within the transmembrane domain (1, 2). In contrast to cells expressing PS1 mutations, neurons lacking PS1 produce less Aβ because of decreased cleavage of APP at the γ-secretase site, indicating that PS1 functions either directly or indirectly in APP processing, and that PS1 mutations do not result in a loss of function (9). Because a biochemical activity for PS1 has not been identified, it is not known how this regulation of APP cleavage occurs. However, coimmunoprecipitation experiments suggest that PS1 and APP physically interact early in the secretory pathway, making it possible that PS1 regulates APP processing directly (10).
In addition to the role of PS1 in APP processing, genetic evidence indicates that the PS function in the Notch pathway. A screen for suppressors/enhancers of lin-12 (a nematode homologue of Notch) hypermorphic mutations in Caenorhabditis elegans led to the identification of sel-12, a PS homologue (11). sel-12 mutants are rescued by human PS1, demonstrating their functional homology (12, 13). When a second PS homologue, hop-1, was disrupted by antisense RNA in a sel-12 mutant background, a severe developmental phenotype resulted which resembles the phenotype caused by mutations in both lin-12 and glp-1, a lin-12 homologue (also known as the LAG phenotype for lin-12 and glp-1) (14). Mice lacking PS1 die shortly after birth and exhibit developmental abnormalities, including skeletal defects and impaired neurogenesis (15, 16). Furthermore, Notch1 and Dll1, a mammalian homologue of the Notch ligand delta, were expressed at lower than normal levels in the presomitic mesoderm of these animals (15). Both Notch1 expression levels and the developmental defects were rescued by wild-type PS1 and by PS1 containing the E246A AD-linked mutation (17, 18). This result suggests that the AD-causing mutations do not result in a complete loss of PS1 function.
Like APP, Notch is a large, single transmembrane cell surface protein that undergoes proteolytic processing within the secretory pathway. Notch has thus far been best described in terms of its role as a receptor that mediates several cell fate decisions during development (19–22). In vertebrate cells, Notch is synthesized as a large precursor, but is cleaved in the Golgi lumen by furin (23) to generate two fragments that remain associated and form the functional receptor (24, 25). Notch is activated by binding a member of the delta/serrate/lag-1 (DSL) family of cell-surface proteins (19–22). Following activation, Notch is cleaved within the transmembrane domain by an unidentified protease, releasing the Notch intracellular domain (NICD) from the membrane (26–28). The NICD then translocates to the nucleus where it can modulate gene expression via association with CSL [CBF-1, Su(H), Lag-1] proteins, and thereby affect cell fate choice (26–28).
PSs could modulate any of the above steps in the Notch signaling pathway. Genetic data from C. elegans indicates that sel-12 mutations act within the cell expressing LIN-12 (20). Furthermore, sel-12 mutations do not modify the phenotype observed when the LIN-12 intracellular domain is expressed alone, suggesting that SEL-12 acts before or concurrent with ligand-activated cleavage (29). Combined, these results suggest a role for PSs in the biosynthesis, trafficking, and/or proteolysis of Notch. We now report that PSs form a complex with the Notch receptor in mammalian and Drosophila cells, and thus are likely to mediate Notch signaling via a direct interaction with the receptor in the secretory pathway.
MATERIALS AND METHODS
Cell Culture and Transient Transfection.
Human embryonic kidney 293 cells (American Type Culture Collection) were grown in 10% fetal bovine serum in DMEM (GIBCO) plus penicillin/streptomycin. For transfection, cells were seeded at 2 × 105 cells/35-mm dish, grown for 24 h, then transiently transfected with 5 μg of plasmid DNA as described (26). Total plasmid concentration was held constant by the addition of pGreenLantern (GIBCO). Lysates were prepared 16–18 h posttransfection. Cultures of Drosophila clone 8 cells (30) and S2 cells stably transfected with an inducible Notch cDNA (31) were provided by Ross Cagan and used directly.
Plasmids.
All cDNAs were cloned into pcDNA3 (Invitrogen), except FLN6mt and HA-ΔE, which were cloned into pCS2+ and have been described (32). All PS1 constructs are human sequence -VRSQ (33). APP is the human 695 splice form (John Hardy). IκBα and expresses full-length mouse protein (Alain Israel).
Antibodies.
The following antibodies were used. In several cases the name of the antibody was replaced with an abbreviation for clarity. The abbreviation used in this manuscript is given, followed by the name of the antibody if applicable, and a reference or commercial source. PS1: α-PS1CTF (PS1 loop, ref. 34); α-PS1NTF (Ab14, ref. 5); α-PS1CTF199 (199A, ref. 5); mAb NT-1 (Paul Matthews). Drosophila PS affinity-purified polyclonal antiserum was Drosophila PS–C-terminal fragment (DPS-CTF) (raised against residues 360–378). PS-2: α-PS2, raised against the loop region (34). Myc epitope: α-myc [9E10 hybridoma supernatant or ascites fluid (Sigma)]. HA epitope: monoclonal α-HA (Babco, Richmond, CA). Notch1 C-terminal: mN1A (Laurie Milner), Drosophila Notch: Notch intracellular domain (31). APP: α-APP (6E10, Senetek, St. Louis). IκBα [α-IκBα, Alain Israel (35)]. All antibodies were diluted 1:100 for immunoprecipitation and 1:1000 for immunoblot, except 9E10 (1:5), mN1A (neat), NT1 (5 μg IgG/ml), DPS-CTF (1:400), and Notch intracellular domain (1:500).
Western Blots and Immunoprecipitations.
For Western blot analyses, cells were lysed in 200 μl of SDS lysis buffer [2% SDS/62.5 mM Tris, pH 6.8/10% glycerol/1% 2-mercaptoethanol/1× complete protease inhibitors (CPI; Boehringer Mannheim)] and passed through a 20-gauge needle. For coimmunoprecipitation, cells were lysed in 500 μl coimmunoprecipitation lysis buffer [1% Nonidet P-40/0.5% Triton X-100/50 mM Tris, pH 7.6/500 mM NaCl/2 mM EDTA/1× CPI], resulting in a final protein concentration of ≈0.4 mg/ml. Lysates were cleared overnight at 4°C with 20 μl protein A-agarose beads (Repligen), incubated at 4°C with antibody for 1 h, recovered with 40 μl protein A beads, then washed twice each with wash A (coimmunoprecipitation lysis buffer minus Nonidet P-40 and Triton X-100), and wash B (wash A with 150 mM NaCl). Proteins were eluted into SDS buffer at 55°C (for PS1 immunoblots) or 100°C (all other immunoblots), resolved by SDS/PAGE, transferred to nitrocellulose (Amersham), immunoblotted, and visualized by enhanced chemiluminescence (Amersham). Stringent myc immunoprecipitations were performed in 1% SDS as described (26). For stringent APP and PS1 immunoprecipitation, cells were lysed in 1 ml of immunoprecipitation lysis buffer (1% Triton X-100/0.5% SDS/0.25% deoxycholate/0.25% BSA/1 mM PMSF in PBS) then immunoprecipitated as above.
Metabolic labeling.
Cells were washed into DMEM minus methionine and cysteine, incubated for 30 min at 37°C, then given [35S]methionine and [35S]cysteine (Easy Tag Express; NEN) to 0.2 mCi/ml (1 Ci = 37 GBq) for 4.5 h. Fetal bovine serum was added to 10% for an additional 60 min, then cells were washed with PBS before lysis.
RESULTS
Expression in 293 Cells.
Based on the observations that PSs act upstream of Notch activation in C. elegans (11, 29) and that PS1 coimmunoprecipitates with APP (10), we hypothesized that PS1 modifies Notch signaling by directly binding the Notch receptor. To test this hypothesis, coimmunoprecipitation experiments were performed by using human embryonic kidney 293 cells. Because 293 cells express very low levels of endogenous Notch1 (see Fig. 6), cultures were transiently transfected with FLN6mt, which encodes amino acids 1–2192 of mouse Notch1 followed by a hexameric myc epitope tag. Cells transfected with FLN6mt express the ≈300-kDa Notch1 precursor (FL-N, full-length Notch) as well as the 100-kDa C-terminal fragment (transmembrane-intracellular domain of Notch, TMIC-N) (Fig. 1). The N-terminal extracellular fragment of Notch was not detected because it is not epitope tagged. Also present were a series of bands between FL-N and TMIC-N. These species result artifactually from boiling Notch1 protein in SDS lysis buffer and are not products of cellular metabolism (compare Fig. 1, lane 1 to Fig. 3, lane 1). In contrast to the low levels of Notch1 expression, 293 cells endogenously express moderate levels of both PS1 and PS2 (4, 34). As reported elsewhere, endogenous PS1 in 293 cells exists predominantly as endoproteolytic cleavage fragments with little or no full-length PS1 (FL-PS1) detected (4, 5). Consistent with other reports, transfection with PS1 cDNA led to an increase in levels of FL-PS1 with no apparent increase in levels of the cleavage products (4, 5). Thus, in PS1-transfected cells, the only species that is overexpressed is FL-PS1.
Figure 6.
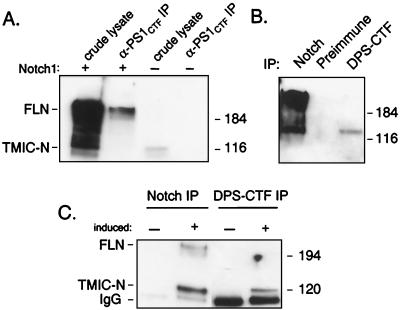
Interaction between PS1 and Notch at endogenous expression levels. (A) 293 cells were transfected with completely full-length, wild-type mouse Notch1 bearing no epitope tag (+) or were untransfected (−) and either lysed in SDS lysis buffer and analyzed directly (crude lysate) or immunoprecipitated with α-PS1CTF. Following SDS/PAGE, Notch1 was detected with antibody mN1A. Crude lysates represent 20% of the protein analyzed in the immunoprecipitations (IP). (B) Drosophila clone 8 cells were lysed in coimmunoprecipitation lysis buffer and immunoprecipitated with the indicated antibody or with preimmune serum. Precipitating proteins were resolved by SDS/PAGE followed by Notch intracellular domain immunoblot. Each lane represents an equal amount of lysate. (C) A Drosophila S2 cells stable cell line expressing a metal-inducible promoter driving expression of Notch were either untreated (−) or induced with 700 μM copper sulfate overnight (+). Cells were then lysed in coimmunoprecipitation lysis buffer and immunoprecipitated with Notch or DPS-CTF as indicated. Precipitating proteins were analyzed by SDS/PAGE followed by Notch immunoblot. In this experiment, IgG heavy plus light chain was detected and is indicated. Sizes of molecular weight markers is given on the right.
Figure 1.
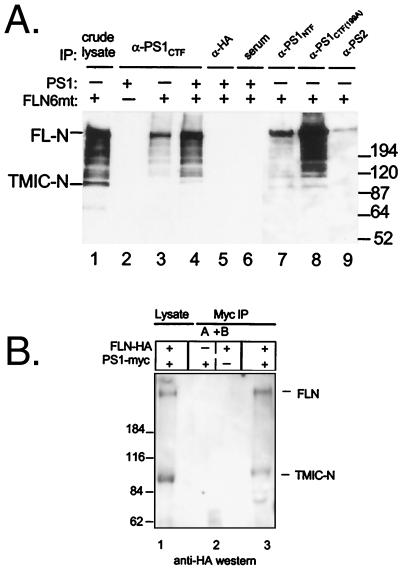
(A) Notch1 is coimmunoprecipitated with PS1. 293 cells were transiently transfected with (+) or without (−) FLN6mt and PS1 as indicated. Cells were lysed in SDS buffer and analyzed directly (crude lysate), or in coimmunoprecipitation (IP) lysis buffer and immunoprecipitated with the indicated antibody or with normal rabbit serum. Precipitated proteins were resolved by SDS/PAGE and immunoblotted with α-myc, which detects the myc epitope tag at the C terminus of FLN6mt. Crude lysates equal 20% of the protein used in coimmunoprecipitations. Bands migrating at ≈64 kDa (lane 1) and ≈95 kDa (lanes 7 and 8) below TMIC-N are apparently degradation products that occur postlysis in some experiments and not specific products of cellular metabolism. Migration of molecular weight markers (in kDa) is shown on right. (B) Notch and PS1 do not associate postlysis. HEK 293 cells were transfected with FL-N with an HA epitope tag at the C terminus and full-length human PS bearing a C-terminal myc epitope as indicated (+ or −). In lane 1, cells were lysed in SDS and analyzed directly. In lane 2, cultures, one expressing Notch-HA (A) and one expressing PS1-myc (B), were mixed immediately following lysis with coimmunoprecipitation lysis buffer and incubated overnight. Lysates were immunoprecipitated with anti-myc as under coimmunoprecipitation conditions. Lane 3 is the positive control in which both proteins were coexpressed in the same culture before lysis. Immunoblot was with polyclonal anti-HA; migration of molecular weight markers (in kDa) is shown on the left.
Figure 3.
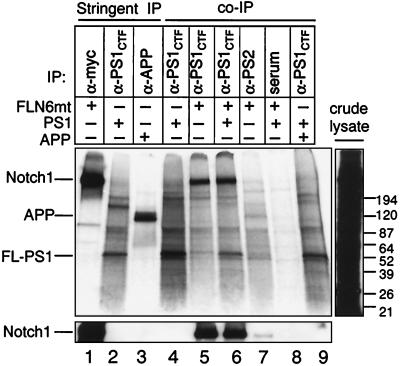
Cellular proteins which coimmunoprecipitate with PS1. 293 cells were transfected with (+) or without (−) APP, FLN6mt, or PS1 as indicated. Cells were metabolically labeled for 5.5 h with [35S]methionine and [35S]cysteine and either lysed in SDS buffer and analyzed directly (crude lysate) or lysed in stringent immunoprecipitation buffers (stringent IP, lanes 1–3, see Materials and Methods), or coimmunoprecipitation lysis buffer (coimmunoprecipitation, lanes 4–9) and immunoprecipitated with the indicated antibodies or normal rabbit serum. The precipitating proteins were split into two samples, resolved by SDS/PAGE, and either used to establish a fluorograph (Upper) or immunoblotted with α-myc (Lower). The immunoprecipitations were exposed 10-fold longer than the crude lysate. Each lane represents an equal amount of lysate. Sizes of molecular weight markers in kDa are given on the right.
Notch1 Coimmunoprecipitates with PS1.
293 cells transfected with FLN6mt were lysed in coimmunoprecipitation lysis buffer containing 1% Nonidet-P40 and 0.5% Triton X-100 and preabsorbed with protein A-agarose beads to clear proteins that bind nonspecifically. Endogenous (Fig. 1A, lane 3) or overexpressed (Fig. 1A, lane 4) PS1 was then immunoprecipitated with α-PS1CTF, which recognizes the C-terminal fragment (CTF-PS1). When the PS1 immunoprecipitates were analyzed by Western blot with anti-myc, the 300-kDa Notch1 precursor was readily detectable, as were the SDS-dependent artifactual bands from 120 to 300 kDa. Approximately 10–20% of cellular Notch precursor was recovered by PS1 antibodies. Trace amounts of the 100-kDa TMIC-N cleavage fragment were also recovered.
Specificity of the Interaction.
The presence of Notch1 in PS1 immunoprecipitates suggests that it is physically associated with PS1. Several lines of evidence support this conclusion. First, three antibodies raised against distinct regions of PS1 and one antiserum to PS2 all coimmunoprecipitated Notch1, whereas immunoprecipitation with an irrelevant antiserum (α-HA) or with normal rabbit serum failed to immunoprecipitate Notch1 (Fig. 1A). To confirm that Notch and PS1 interact within the cells before lysis and not during the coimmunoprecipitation procedure, epitope-tagged Notch and PS1 were mixed and coimmunoprecipitated. Cells expressing full-length Notch with a C-terminal HA epitope were lysed and mixed with a separate culture of cells expressing PS1 with a C-terminal myc epitope. The lysates were mixed overnight, and then PS1 was recovered by myc immunoprecipitation by using the same conditions as in Fig. 1A. Immunoblot with anti-HA for Notch showed that Notch did not coimmunoprecipitate with PS1 when lysates were mixed (Fig. 1B). When both proteins were expressed in the same cell, Notch was abundantly recovered. Thus post-lysis interactions between PS1 and Notch did not account for the coimmunoprecipitation results.
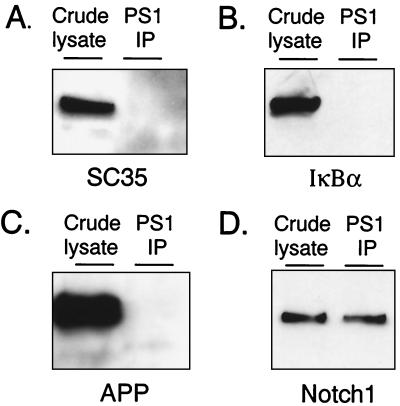
Second, because the myc epitope tag is derived from a leucine zipper protein, and the CDC/ankyrin region of Notch codes for a protein–protein interaction domain (36), we controlled for the possibility that these domains nonspecifically interact with PS1. Neither SC35, which like FLN6mt bears a myc epitope tag, nor IκBα, which contains CDC/ankyrin repeats homologous to those in Notch (36), were coimmunoprecipitated with PS1 from doubly transfected cells (Fig. 2).
Figure 2.
Specificity of the coimmunoprecipitation assay. 293 cells were transfected with plasmids encoding PS1 plus (A) SC35 bearing a myc epitope tag, (B) IκBα, or (C) APP. Cells were lysed in SDS buffer and analyzed directly (crude lysate), or in coimmunoprecipitation lysis buffer and immunoprecipitated with the indicated antibody. Precipitating proteins were immunoblotted with α-myc (A and D), α-IκBα (B), or α-APP (C). (D) A brief exposure of Notch1 coimmunoprecipitated with endogenous PS1 is given for comparison. In all samples, crude lysate equals 20% of the total protein assayed by immunoprecipitation.
Third, we tested for an interaction between PS1 and APP, a cell surface protein that is also trafficked through the secretory pathway where PS1 is localized. An APP–PS1 interaction has been reported by using a similar procedure (10). However, this association appears to involve a very small percentage of cellular APP, because relatively large numbers of stably transfected cells were required to detect the interaction. By using immunoprecipitation conditions that readily detect the PS1–Notch1 association, no interaction with APP was detected (Fig. 2). Together, these data indicate that PS1 is not interacting promiscuously with other overexpressed proteins, including one with the myc epitope tag (SC35), one which shares a protein binding motif with Notch (IκBα), and one that interacts with PS1 at quantitatively low levels (APP).
Finally, we tested the possibility that PS1 interacts specifically with a large number of cellular proteins in addition to Notch1. To estimate the number of proteins in 293 cells that coimmunoprecipitate with PS1, cells were transiently transfected as indicated in Fig. 3 and metabolically labeled with [35S]methionine and [35S]cysteine for 5.5 h, followed by immunoprecipitation with α-PS1CTF, α-PS2, or normal rabbit serum. Precipitates were split into two samples, one for fluorography and one for immunoblot. For comparison, Notch1, PS1, and APP were immunoprecipitated in high SDS concentrations expected to disrupt most protein–protein interactions. Because PS1 forms insoluble aggregates at 100°C, samples were heated to 50°C before electrophoresis. Under these conditions the Notch1 cleavage products were not separated and migrated at the size of FL-N, consistent with the finding that they remain tightly associated after cleavage (24, 25). FL-PS1 migrated at 50 kDa [Fig. 3, lane 2; PS1 cleavage products were not sufficiently labeled for detection as observed in other cell lines (37)]. Also present in the high SDS PS1 immunoprecipitation were bands migrating at ≈150 kDa and ≈200 kDa, which are likely to be high molecular weight PS1 aggregates often detected following SDS/PAGE (5, 37, 38), or may be other proteins that strongly associate with PS1. In the absence of FL-N, several bands were immunoprecipitated by α-PS1CTF (Fig. 3, lane 4). However, most of these bands comigrate with FL-PS1 or PS1 aggregates (Fig. 3, lane 2) or were also precipitated nonspecifically by normal rabbit serum (Fig. 3, lane 8). When FLN6mt was overexpressed, a ≈300-kDa species abundantly coimmunoprecipitated with PS1. Lower amounts of this band were also coimmunoprecipitated with PS2. Immunoblotting with α-myc verified that these bands contained Notch1 (Fig. 3, Lower). Thus, Notch1 was the primary protein coimmunoprecipitated with PS1 in our assay. Consistent with the previous experiment (Fig. 2), when cells were transfected with both APP and PS1, no band comigrating with APP was coimmunoprecipitated with PS1. These results support the conclusion that PS1 is interacting robustly and specifically with Notch1 and that the PS1–Notch1 interaction is more vigorous than the PS1–APP interaction.
Notch1 Binding to PS1 Is Detergent Sensitive.
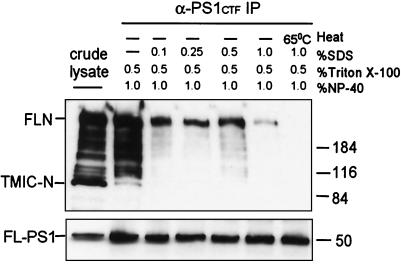
To determine whether PS1 and Notch coimmunoprecipitate because of noncovalent interactions, the above coimmunoprecipitation procedure was repeated in the presence of SDS, a strongly denaturing ionic detergent that disrupts most noncovalent protein interactions. As shown in Fig. 4, the PS1–Notch1 interaction was partially resistant to 1% SDS. Increasing detergent concentration diminished the recovery of Notch1, but not PS1. In addition, heating the lysate in 1% SDS before immunoprecipitation completely prevented the recovery of Notch1 but not PS1. Thus, the PS1–Notch1 association is mediated by noncovalent interactions that are partially resistant to SDS.
Figure 4.
Sensitivity of the PS1–Notch1 interaction to SDS. 293 cells were transiently transfected with both FLN6mt and PS1 and either lysed in SDS buffer and analyzed directly (crude lysate) or lysed in coimmunoprecipitation (IP) lysis buffer with the indicated concentration of SDS. The far right sample was also heated for 5 min at 65°C before immunoprecipitation. All samples were then immunoprecipitated with α-PS1CTF and either boiled (Upper) or heated to 50°C (Lower) before immunoblot. (Upper) The sample was blotted with α-myc. (Lower) The sample was blotted with the anti-PS1 antibody NT1. Sizes of molecular weight markers in kDa are given on the right.
PS1 Is Coimmunoprecipitated with Notch1.
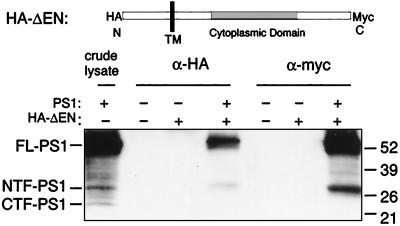
If Notch1 and PS1 form a complex, then PS1 should be recovered during Notch1 immunoprecipitation. To test this, 293 cells were transfected with a Notch1 construct encoding a small portion of the ectodomain, the transmembrane domain, and most of the cytoplasmic domain (Fig. 5). This construct, HA-ΔEN (26), was chosen because it has an HA epitope tag at the N terminus and a myc epitope tag at the C terminus, allowing for immunoprecipitation to be performed independently with two antibodies. Lysates were immunoprecipitated with α-HA or α-myc, and the immunoprecipitates assayed for the presence of PS1 by immunoblot with both α-PS1NTF and α-PS1CTF simultaneously. When HA-ΔEN and PS1 were cotransfected, FL-PS1 and NTF-PS1 were recovered with HA-ΔEN by both antibodies (Fig. 5). Thus PS1 was coimmunoprecipitated with Notch1 in doubly transfected cells, confirming that the two proteins physically interact. However, coimmunoprecipitation of Notch1 protein with PS1 antibodies (Fig. 1A) is apparently more efficient than the reverse experiment (Fig. 5), because endogenous PS1 was not recovered when HA-ΔEN was transfected alone. Similar results were observed with full-length Notch1 (data not shown), indicating that the absence of the ectodomain of Notch1 in this construct does not account for this result. This difference in efficiency could be caused by selective recovery of the population of Notch1 that is not bound to PS1 in transfected cells, where the abundance of overexpressed Notch1 is much greater than that of endogenous PS1.
Figure 5.
PS1 is coimmunoprecipitated with Notch1. (Upper) Diagram of the Notch1 construct HA-ΔEN. The N terminus (N), C terminus (C), TM domain, and the positions of the HA and myc epitopes are shown. The location of the CDC/ankyrin repeats is shaded. 293 cells were transfected with (+) or without (−) HA-ΔEN and PS1 as indicated. Cells were lysed in SDS buffer (crude lysate) or were lysed in coimmunoprecipitation (IP) lysis buffer and immunoprecipitated with α-HA or α-myc. Precipitating proteins were resolved by SDS/PAGE and immunoblotted with both α-PS1CTF and α-PS1NTF. Crude lysates equal 10% of the protein used in the coimmunoprecipitations. FL-PS1, NTF-PS1, and CTF-PS1 are indicated. Sizes of molecular weight markers in kDa are given on the right.
Interaction Between Notch1 and PS1 at Endogenous Expression Levels.
As shown in Fig. 1, physiological levels of PS1 are sufficient to abundantly coimmunoprecipitate overexpressed Notch1. However when PS1 was immunoprecipitated from untransfected 293 cells, endogenous Notch1 was not detected in the precipitate (Fig. 6A). This result could stem from the very low levels of FL-N present in 293 cells, or might indicate that Notch1 containing the complete C terminus and no epitope tag does not interact with PS1. To address this issue, we first determined whether Notch1 containing all amino acids and no epitope tag also interacts with PS1 in transfected cells. As shown in Fig. 6, untagged, full-length Notch1 coimmunoprecipitated with PS1 in transfected 293 cells.
To determine whether Notch and PS1 interact at physiological expression levels, we assayed Drosophila clone 8 cells (30), which were derived from the imaginal disk and endogenously express readily detectable levels of Notch (Fig. 6) as well as DPS (P.N. and A.G., unpublished data). As in transfected mammalian cells, Notch was immunoprecipitated with antiserum to DPS. However, the relative abundance of TMIC-N recovered with DPS differed significantly from that recovered with PS1 in 293 cells: DPS immunoprecipitation preferentially recovered TMIC-N. The difference in the PS–Notch interaction between Drosophila cells and mammalian 293 cells cannot simply be explained by overexpression, because a similar preference for TMIC-N was observed in Drosophila S2 cells stably overexpressing Notch (Fig. 6). Because Notch processing in Drosophila cells has not been characterized to the same extent as mammalian cells (23, 25), it is not possible to deduce the site of subcellular interaction between Drosophila Notch and PS from this result. Nonetheless, these experiments demonstrate that Notch can interact with PS at physiological expression levels, and that the Notch-binding activity of PSs is conserved in Drosophila.
DISCUSSION
Genetic analyses in C. elegans and mice have implicated the PSs in Notch signaling (11–14, 29). This functional interaction probably occurs upstream of signal transduction by the NICD, because sel-12 mutations act within the cell expressing LIN-12 (11), and sel-12 mutants do not suppress the activity of LIN-12 mutants in which the extracellular and transmembrane domains have been deleted (29). Potential sites of regulation upstream of NICD release include biosynthesis, receptor maturation, trafficking, ligand binding, and ligand-activated cleavage within the transmembrane domain. To address the molecular basis of this genetic interaction, we tested whether PS1 physically interacts with the Notch receptor. The data presented here demonstrate that PS1 and the Notch1 precursor protein noncovalently interact in transfected mammalian cells. The presence of a PS1–Notch1 complex in the early secretory pathway supports the conclusions derived from the genetic analyses, namely that PS1 participates in the Notch signaling pathway by regulating some aspect of receptor maturation and/or processing before signal transduction (29).
Evidence for a PS1–Notch1 Complex.
The recovery of Notch1 when PS1 was immunoprecipitated from the lysates of transfected 293 cells suggests that a physical association between these two proteins occurs (Fig. 1). The specificity and robustness of this interaction is demonstrated by a number of control experiments. First, to ensure that Notch1 is recovered because of its interaction with PS1 and not because of cross-immunoreactivity in any particular antibody preparation, multiple antibodies to PS1 were tested. All antibodies tested [three antibodies to distinct regions of PS1, one to PS2 (Fig. 1A), and one to Drosophila PS (Fig. 6)] coimmunoprecipitated Notch. Control experiments indicate that the interaction occurs within the cell before lysis (Fig. 1B). Furthermore, PS1 was recovered by two antibodies to Notch1, confirming the coimmunoprecipitation result (Fig. 5). Finally, to confirm this result by using an independent method, the Notch1 intracellular domain and the PS1 N-terminal hydrophilic domain interacted in a yeast two-hybrid assay (data not shown).
To control against that possibility that PS1 binds nonspecifically with transfected proteins, three different proteins were overexpressed in 293 cells and assayed for coimmunoprecipitation with PS1. Two of the three proteins were chosen for their potential to interact nonspecifically: SC35, which bears a myc epitope tag, and IκBα, which shares the ankyrin repeat structural motif with Notch which mediates protein–protein interactions (36). The third, APP, interacts with PS1 at quantitatively low levels when overexpressed (10). None of these overexpressed proteins coimmunoprecipitated with PS1, demonstrating the stringency of the coimmunoprecipitation procedure (Fig. 2). By using sensitivity to SDS and heat as a rough measure of interaction strength, the PS1–Notch1 complex appears relatively stable as it is partially resistant to 1% SDS, 1% Nonidet-P-40, and 0.5% Triton X-100 (Fig. 3). Thus, Notch1 and PS1 appear to specifically and strongly interact via noncovalent bonds.
It is possible that PS1 binds many proteins as part of its normal function, which if true would suggest a general role for PS1 in protein maturation. To estimate the number of proteins that interact with PS1, 293 cellular proteins were metabolically labeled before PS1 immunoprecipitation. Only a few species were distinctly present in the PS1 immunoprecipitated material, and when Notch1 was overexpressed, it was the dominant protein recovered (Fig. 3). Thus, PS1 does not appear to interact strongly with a large number of proteins under these conditions. As we did not detect the interaction between PS1 and APP, which apparently involves a small percentage of cellular APP at any one time, it remains possible that PS1 interacts specifically with other proteins at similarly low levels.
Interaction at Physiological Expression Levels.
In HEK 293 cells, endogenous PS1 was expressed at sufficient levels that Notch1 could be abundantly coimmunoprecipitated when it was overexpressed (Figs. 1A and 6A). However, when both proteins were present at physiological expression levels in 293 cells, Notch1 protein was not present in the PS1 immunoprecipitate (Fig. 6A). These cells express very low levels of Notch1 precursor, making it difficult to assay for the PS1–Notch1 interaction. The experiment with Drosophila cells demonstrates that the two proteins can interact at physiological expression levels (Fig. 6). Detection here is facilitated by substantially higher levels of Notch expression.
Subcellular Site of Interaction.
In 293 cells, PS1 preferentially associates with the Notch1 precursor (Figs. 1 and 3). Notch is proteolytically processed in the medial Golgi, and very little Notch precursor reaches the plasma membrane (15, 16, 38). Thus, PS1 most likely interacts with Notch before or concurrent with transit through the medial Golgi. The most likely subcellular site of interaction is the ER, where PS1 is predominantly localized (3–5). Because small amounts of the C-terminal fragment of Notch1 were recovered (Fig. 1), it is possible that either a subset of Notch remains complexed with PS1 after furin cleavage or PS1 interacts with the mature protein following reinternalization from the cell surface. In Drosophila cells, where Notch processing has not been extensively characterized, cleaved Notch was preferentially recovered (Fig. 6). This difference could be because of cell type- or species-specific differences in Notch processing or PS1 localization.
Functional Significance of the Interaction.
The purpose of the PS1–Notch1 physical interaction is not known, but this complex is likely to be the site at which PS1 regulates Notch signaling. Interestingly, both the Notch1 precursor and the immature form of APP (which has not been O-glycosylated in the Golgi) bind PS1 in the secretory pathway (10), and both proteins share the rare characteristic of being cleaved within the transmembrane domain (1, 2, 26, 27). Identifying the role of PSs in Notch signaling may provide clues as to how PSs regulate the cleavage of APP, and how that regulation is altered in PS-linked FAD.
Acknowledgments
We thank Drs. Ross Cagan, Jonathan Gitlin, David Harris, and Jeanne Nerbonne (Washington University); Dr. Alain Israel (Pasteur Institute); Drs. Sam Gandy and Paul Matthews (Nathan Kline Institute); Dr. Laurie Milner (Fred Hutchinson Cancer Institute); Dr. Peter Seubert (Athena Neurosciences); and Dr. John Hardy (Mayo Clinic, Jacksonville, FL) for generously sharing reagents. We thank Julie Lotharius, Eric Schroeter, and Patricia Powell for their assistance. This work was supported by Career Development Award AG000634 (National Institutes of Health) and the Metropolitan Life Foundation (A.G.), the Alzheimer’s Disease Research Center (Grant AG5681 to A.G., J.W., and R.K.), the Missouri Alzheimer’s Disease and Related Disorder Foundation (J.W.), the American Health Assistance Foundation (J.W.) and National Institutes of Health Grant GM55479-01 (R.K.). W.J.R. is supported by a W. M. Keck Postdoctoral Fellowship, and J.M. is supported by a Lucille P. Markey Predoctoral Fellowship.
ABBREVIATIONS
- AD
Alzheimer’s disease
- PS
presenilin
- APP
β-amyloid precursor protein
- FL-N
full-length Notch
- TMIC-N
transmembrane-intracellular domain of Notch
- FL-PS1
full-length PS1
- NTF-PS1
N-terminal fragment of PS1
- CTF-PS1
C-terminal fragment of PS1
- TM
transmembrane
- ER
endoplasmic reticulum
- NICD
Notch intracellular domain
- DPS
Drosophila PS
- CTF
C-terminal fragment
References
- 1.Lendon C L, Ashall F, Goate A M. J Am Med Assoc. 1997;277:825–831. [PubMed] [Google Scholar]
- 2.Hutton M, Hardy J. Hum Mol Gen. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 3.Doan A, Thinakaran G, Borchelt D, Slunt H H, Ratovitsky T, Podlisny M, Selkoe D J, Seeger M, Gandy S E, Price D L, Sisodia S S. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 4.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovitsky T, Davenport F, Norstedt C, Seeger M, et al. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 5.Podlisny M B, Citron M, Amarante P, Sherrington R, Xia W, Zhang J M, Diehl T, Levesque G, Fraser P, Haass C, et al. Neurobiol Dis. 1997;3:325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- 6.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 8.Wild-Bode C, Yamazaki T, Capel A, Leimer U, Steiner H, Ihara Y, Haass C. J Biol Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 10.Xia W, Zhang J, Perez A, Koo E H, Selkoe D J. Proc Natl Acad Sci USA. 1997;94:8208–8213. doi: 10.1073/pnas.94.15.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitan D, Greenwald I. Nature (London) 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C. Genes Funct. 1997;1:149–159. doi: 10.1046/j.1365-4624.1997.00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Levitan D, Doyle T G, Brousseau D, Lee M K, Slunt H H, Sisodia S S, Greenwald I. Proc Natl Acad Sci USA. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Greenwald I. Proc Natl Acad Sci USA. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong P C, Zheng H, Chen H, Becher M W, Sirinathsinghji D J S, Trumbauer M E, Chen H Y, Price D L, Van der Ploeg L H T, Sisodia S S. Nature (London) 1997;387:288–291. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Bronson R T, Chen D F, Xia W, Selkoe D, Tonegawa S. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 17.Davis J A, Naruse S, Chen H, Eckman C, Younkin S, Price D L, Borchelt D R, Sisodia S S, Wong P C. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 18.Qian S, Jiang P, Guan X-M, Singh G, Trumbauer M E, Yu H, Chen H Y, Van der Ploeg L H T, Zheng H. Neuron. 1998;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 19.Artavanis-Tsakonas S, Matsuno K, Fortini M E. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 20.Kopan R, Turner D. Curr Opin Neurobiol. 1996;6:594–601. doi: 10.1016/s0959-4388(96)80090-0. [DOI] [PubMed] [Google Scholar]
- 21.Weinmaster G. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- 22.Nye J S, Kopan R. Curr Opin Biol. 1995;5:966–969. doi: 10.1016/s0960-9822(95)00189-8. [DOI] [PubMed] [Google Scholar]
- 23.Logeat F, Bessia C, Brou C, Lebail O, Jarriault S, Seidah N G, Israel A. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crittendon S L, Tromel E R, Evans T C, Kimble J. Development (Cambridge, UK) 1994;120:2901–2911. doi: 10.1242/dev.120.10.2901. [DOI] [PubMed] [Google Scholar]
- 25.Blaumueller C M, Qi H L, Zagouras P, Artavanis-Tsakonas S. Cell. 1997;90:281–291. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 26.Kopan R, Schroeter E H, Weintraub H, Nye J S. Proc Natl Acad Sci USA. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeter E H, Kisslinger J A, Kopan R. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 28.Struhl G, Adachi A. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 29.Levitan D, Greenwald I. Development (Cambridge, UK) 1998;125:3599–3606. doi: 10.1242/dev.125.18.3599. [DOI] [PubMed] [Google Scholar]
- 30.Peel D J, Milner M J. W Roux Arch Dev Biol. 1992;201:120–123. doi: 10.1007/BF00420423. [DOI] [PubMed] [Google Scholar]
- 31.Fehon R G, Kooh P J, Rebay I, Regan C L, Xu T, Muskavitch M, Artavanis-Tsakonas S. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 32.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 33.Alzheimer’s Disease Collaborative Group. Nat Genet. 1995;11:219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- 34.Malin S A, Guo W-X A, Jafari G, Goate A M, Nerbonne J M. Neurobiol Dis. 1998;4:398–409. doi: 10.1006/nbdi.1998.0167. [DOI] [PubMed] [Google Scholar]
- 35.Weil R, Whiteside S T, Israel A. Immunobiology. 1997;198:14–23. doi: 10.1016/s0171-2985(97)80023-x. [DOI] [PubMed] [Google Scholar]
- 36.Blank V, Kourilsky P, Israel A. Trends Biochem Sci. 1992;17:135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- 37.Ratovitski T, Slunt H H, Thinakaran G, Price D L, Sisodia S S, Borchelt D. J Biol Chem. 1997;272:24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- 38.Capell A, Grunberg J, Pesold B, Diehlman A, Citron M, Nixon R, Beyreuther K, Selkoe D J, Haass C. J Biol Chem. 1998;273:3205–3211. doi: 10.1074/jbc.273.6.3205. [DOI] [PubMed] [Google Scholar]