Abstract
Although it is clear that mitochondrial morphogenesis is a complex process involving multiple proteins in eukaryotic cells, little is known about regulatory molecules that modulate mitochondrial network formation. Here, we report the identification of a new yeast mitochondrial morphology gene called MFB1 (YDR219C). MFB1 encodes an F-box protein family member, many of which function in Skp1-Cdc53/Cullin-F-box protein (SCF) ubiquitin ligase complexes. F-box proteins also act in non-SCF complexes whose functions are not well understood. Although cells lacking Mfb1p contain abnormally short mitochondrial tubules, Mfb1p is not essential for known pathways that determine mitochondrial morphology and dynamics. Mfb1p is peripherally associated with the mitochondrial surface. Coimmunoprecipitation assays reveal that Mfb1p interacts with Skp1p in an F-box–dependent manner. However, Mfb1p does not coimmunoprecipitate with Cdc53p. The F-box motif is not essential for Mfb1p-mediated mitochondrial network formation. These observations suggest that Mfb1p acts in a complex lacking Cdc53p required for mitochondrial morphogenesis. During budding, Mfb1p asymmetrically localizes to mother cell mitochondria. By contrast, Skp1p accumulates in the daughter cell cytoplasm. Mfb1p mother cell-specific asymmetry depends on the F-box motif, suggesting that Skp1p down-regulates Mfb1p mitochondrial association in buds. We propose that Mfb1p operates in a novel pathway regulating mitochondrial tubular connectivity.
INTRODUCTION
Mitochondria are essential organelles required for diverse cellular functions, contributing to metabolism and calcium homeostasis and harboring molecules that activate or inhibit apoptosis (Saraste, 1999; Scheffler, 1999; Bianchi et al., 2004; Danial and Korsmeyer, 2004). In most eukaryotic cells, mitochondria form interconnected tubular networks that continuously change their shape and size (Yaffe, 1999; Jensen et al., 2000; Griparic and van der Bliek, 2001; Chen and Chan, 2004; Rube and van der Bliek, 2004; Okamoto and Shaw, 2005). The continuous remodeling of mitochondrial morphology and distribution maintains mitochondrial integrity and allows the organelle to respond to the changing physiological state of the cell (Westermann, 2002; Scott et al., 2003). Many molecules required for mitochondrial morphogenesis are conserved from yeast to humans. The importance of mitochondrial dynamics is highlighted by recent findings that defects in mitochondrial shape and transport are associated with neurodegenerative diseases, developmental lethality, obesity, and neuronal dysfunction (Chen and Chan, 2005; Niemann et al., 2005; Okamoto and Shaw, 2005). Additional studies reveal that proteins regulating mitochondrial morphology play key roles in programmed cell death (Bossy-Wetzel et al., 2003; Frank et al., 2003; Karbowski and Youle, 2003; Yoon, 2004; Okamoto and Shaw, 2005; Perfettini et al., 2005; Youle and Karbowski, 2005).
The budding yeast, Saccharomyces cerevisiae, has played a central role in the discovery and analysis of proteins and pathways that control mitochondrial dynamics. At least three of these pathways, termed tubulation, fusion, and fission, are directly involved in establishing and remodeling the interconnected mitochondrial tubular network (Okamoto and Shaw, 2005). In the tubulation pathway, Mmm1p (Burgess et al., 1994), Mmm2p (Youngman et al., 2004), Mdm10p (Sogo and Yaffe, 1994), Mdm12p (Berger et al., 1997), and Mdm31p and Mdm32p (Dimmer et al., 2005) are required to form elongated mitochondrial tubules. Mitochondrial fusion is mediated by Fzo1p (Hermann et al., 1998; Rapaport et al., 1998), Mgm1p (Jones and Fangman, 1992; Guan et al., 1993; Shepard and Yaffe, 1999; Wong et al., 2000), and Ugo1p (Sesaki and Jensen, 2001). Mitochondrial fission is mediated by Dnm1p (Otsuga et al., 1998; Bleazard et al., 1999; Sesaki and Jensen, 1999), Fis1p (Mozdy et al., 2000), Mdv1p (Fekkes et al., 2000; Tieu and Nunnari, 2000; Cerveny et al., 2001), and Caf4p (Griffin et al., 2005). Fusion and fission are two opposing pathways required to determine steady-state mitochondrial network morphology. Although it is thought that additional proteins regulate the connectivity of mitochondrial networks, much less is known about such molecules.
F-box protein family members play pivotal roles in a variety of cellular processes (Kipreos and Pagano, 2000). Many F-box proteins interact with Skp1 and Cdc53/Cullin to form Skp1-Cdc53/Cullin-F-box protein (SCF) ubiquitin ligase complexes. In these complexes, F-box proteins recruit specific substrates for ubiquitin/26S proteasome-mediated proteolysis (Cardozo and Pagano, 2004). Some F-box proteins interact with different components to form non-SCF complexes lacking ubiquitin ligase activity (Kipreos and Pagano, 2000). The functions of non-SCF complexes are currently not well understood. Recently, the yeast F-box protein Mdm30p was shown to play a role in maintenance of fusion-competent mitochondria by controlling the abundance of Fzo1p (Dimmer et al., 2002; Fritz et al., 2003). Whether additional F-box proteins regulate mitochondrial fission, tubulation, or other morphology pathways remains unclear.
Here, we show that the novel F-box protein Mfb1p peripherally associates with mitochondria where it functions to increase mitochondrial connectivity in yeast. Mfb1p interacts with Skp1p via the Mfb1p F-box motif. Mutational analyses reveal that the F-box motif is not essential for Mfb1p mitochondrial morphology function. During budding, Mfb1p preferentially localizes to mitochondria in the mother cell, whereas Skp1p is found predominantly in the daughter cell. The Mfb1p localization pattern depends on its F-box motif. These findings are consistent with the idea that Skp1p controls Mfb1p asymmetry by down-regulating Mfb1p mitochondrial association in buds.
MATERIALS AND METHODS
Strains and Growth Conditions
Yeast strains were generated in the W303 and S288C backgrounds. Yeast strains and plasmids used in this study are listed in Supplemental Tables S1 and S2, respectively. Standard genetic and molecular biology methods were used to grow, transform, and manipulate yeast (Burke et al., 2000) and bacterial (Sambrook and Russell, 2001) strains. Yeast cells were grown at 30°C in synthetic media containing 2% dextrose (SD), 2% galactose plus 2% raffinose (SGalRaf), or 0.1% dextrose + 2% galactose (SDGal). For serial dilutions, wild-type and mutant cells were grown to log phase (optical density [OD]600 = 1.0–1.5) at 30°C in rich media containing 2% dextrose (YPD), pelleted, and resuspended in 1 M sorbitol (OD600 = 0.02). Aliquots (3 μl) of 1:4 serial dilutions were spotted onto YPD and YPGly (containing 2% glycerol) plates and grown at 30°C for 2 and 2.5 d, respectively.
Quantification of Mitochondrial Morphology, Fusion, and Mfb1p-Skp1p Asymmetry
Mitochondrial morphology was scored in cells expressing a matrix-targeted green fluorescent protein (GFP) (mito-GFP), or a mitochondria-targeted DsRed (mito-red fluorescent protein [RFP]) grown to log phase (OD600 = 1.0–1.5) at 30°C in SD media. Phenotypes were quantified in 100 cells in three experiments. Data reported are the average of all experiments. Bars indicate standard deviations.
Mitochondrial fusion in zygotes was examined as described previously (Mozdy et al., 2000). Each haploid parent was labeled with mito-GFP or mito-RFP. The expression of mito-GFP was induced in SGalRaf at 30°C for 16 h. Large-budded zygotes were scored 3–4 h after mating. Phenotypes were quantified in 50 cells in three experiments. Data reported are the average of all experiments.
Mfb1p asymmetry was quantified in medium-budded cells expressing Mfb1p-GFP or Mfb1pL20A/P21A-GFP, and a mito-RFP grown to log phase (OD600 = 1.0–1.5) at 30°C in SD media. Cells containing stronger GFP signals in mother cells relative to buds but equivalent mito-RFP signals in mother and daughter cells were scored as having “asymmetric” Mfb1p localization. Phenotypes were quantified in 100 cells in three experiments. Data reported are the average of all experiments. Bars indicate standard deviations.
Using indirect immunofluorescence microscopy, Mfb1p and Skp1p localization patterns were quantified in the same budded cells expressing both Mfb1p-GFP and Skp1p-3HA grown to log phase (OD600 = 1.0–1.5) at 30°C in SD media. 4,6-Diamidino-2-phenylindole (DAPI) staining was performed to visualize mitochondrial DNA (mtDNA) as a mitochondrial marker. Phenotypes were quantified in 50 cells in four experiments. Data reported are the average of all experiments.
Protein Localization Assays
mfb1Δ cells (JSY7453) expressing Mfb1p-3HA or TMS-Mfb1p-3HA-GFP were grown in SDGal media to log phase (OD600 = 1.2–1.6) at 30°C, and the mitochondria were isolated as described previously (Kondo-Okamoto et al., 2003), except that Zymolyase 100T (Z1005; US Biological, Swampscott, MA) was used for spheroplast formation. Whole cell homogenates were separated into postmitochondrial supernatant (PMS) and mitochondria-enriched pellet by differential centrifugation. Isolated mitochondria (200 μg of protein) were subjected to protease protection and carbonate extraction assays as described previously (Kondo-Okamoto et al., 2003; Frederick et al., 2004). Samples (20 μg of protein per lane) were analyzed by Western blotting. Note that there are two distinct bands specific to Mfb1p. SDS-PAGE of recombinant Escherichia coli-expressed Mfb1p migrated with the lower band in yeast extracts, suggesting that the upper band is a posttranslationally modified Mfb1p species (our unpublished data). The biological significance of this putative modification is currently unknown.
In Vitro Binding of Mfb1p to Mitochondria
Mitochondria (90 μg of protein) from mfb1Δ cells (JSY7453) were pretreated with 50 μg/ml proteinase K (PK) in 0.5 ml of HS buffer (20 mM HEPES-KOH, pH 7.4, and 0.6 M sorbitol) on ice for 15 min. The reaction was stopped by the addition of 1 mM phenylmethylsulfonyl fluoride (PMSF) on ice for 5 min. Samples were subjected to centrifugation (18,000 × g) at 4°C for 10 min. The mitochondrial pellet was washed twice in 0.5 ml of HS buffer containing 1 mM PMSF and 0.4% protease inhibitor cocktail (PIC) (539134; Calbiochem, San Diego, CA), and collected by centrifugation (18,000 × g) at 4°C for 10 min. Mitochondria treated with or without PK were resuspended in 400 μl of a PMS (1.6 mg of protein) fraction isolated from JSY7453 (mfb1Δ) cells expressing Mfb1p-3HA and incubated at 4°C for 20 min. Then, 100 μl of the reaction mixture was layered onto a 500-μl sucrose cushion (25% sucrose, 20 mM HEPES-KOH, pH 7.4, 1 mM PMSF, and 0.4% PIC), and subjected to centrifugation (12,500 × g) at 4°C for 10 min. After collecting the supernatant (S), the sucrose cushion was discarded, and the mitochondrial pellet (P) was washed once in 0.2 ml of HS buffer containing 1 mM PMSF and collected by centrifugation (12,500 × g) at 4°C for 10 min. The P and S fractions (20 μg of protein per lane) were analyzed by Western blotting.
Immunoprecipitation Studies
Physical interactions of Mfb1p with Skp1p and Cdc53p were examined by immunoprecipitation as described previously (Galan et al., 2001). mfb1Δ cells (JSY7453) expressing Mfb1p-3HA or Mfb1pL20A/P21A-3HA were grown in SD media to log phase (OD600 = 1.0–1.6) at 30°C. The 16 and 8 OD600 units of cells expressing Mfb1p-3HA and Mfb1pL20A/P21A-3HA, respectively, were pelleted and resuspended in lysis buffer (30 μl/1.0 OD600 units of cells) (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1 mM EDTA, 0.2% Triton X-100, and 0.5% PIC). Cells were lysed with glass beads [20% (vol/vol) of total sample] by 10 cycles of vortexing (maximum speed) for 30 s followed by a 1 min rest on ice and subjected to centrifugation (18,000 × g) at 4°C for 10 min. Whole cell extracts were incubated with 20 μl of anti-hemagglutinin (HA) agarose conjugate (A2095; Sigma-Aldrich, St. Louis, MO) at 4°C for 2 h with gentle agitation. The beads were washed once with 500 μl of lysis buffer and twice with 500 μl of phosphate-buffered saline (PBS), and then they were collected by centrifugation (12,000 × g) at 4°C for 1 min. The proteins were eluted with SDS-sample buffer and analyzed by Western blotting. The whole cell extracts and eluted immunoprecipitates loaded per lane were 1 and 50% of the total extracts subjected to incubation with anti-HA–conjugated agarose, respectively.
Immunoprecipitation was also performed for cells (Y1459) expressing Cdc53p-13MYC and Mfb1p-3HA (or Cdc53p-13MYC and Mfb1p as a negative control), and cells (YPH499) expressing Mfb1p-3HA as described above using Anti-c-Myc agarose conjugate (A7470; Sigma-Aldrich). The whole cell extracts and eluted immunoprecipitates loaded per lane were 2.5 and 33% of the total extracts subjected to incubation with anti-HA or -c-Myc–conjugated agarose, respectively.
Immunoblot Analysis
Proteins were analyzed by immunoblotting with antibodies specific for Porin (1:20,000) and 3-phosphoglycerate kinase (3-PGK) (1:10,000) from Invitrogen (Carlsbad, CA); Cdc4p (yC-20; 1:500) and Cdc53p (yC-17; 1:500) from Santa Cruz Biotechnology (Santa Cruz, CA); HA (1:1000) and c-Myc (1:1000) from the University of Utah Core Facility (Salt Lake City, UT); Fzo1p (1:5000), Ugo1p (1:250), Dnm1p (1:8000), and Fis1p (1:1000) from the Shaw laboratory; Skp1p (1:3000) from the Kitagawa laboratory; Mgm1p (1:1000), a gift from A. Reichert (Ludwig-Maximilians-Universität München, Munich, Germany); Cyb2p (1:20,000), Mge1p (1:10,000), Tom35p (1:500), Tom37p (1:2000), Sam50p (1:300), Tom13p (1:250), and Tom40p (1:10,000), gifts from T. Endo (Nagoya University, Nagoya, Japan); mtHsp70 (1:10,000), a gift from W. Neupert (Ludwig-Maximilians-Universität München, Munich, Germany); Mdv1p (1:8000), a gift from J. Nunnari (University of California, Davis, Davis, CA); Tim44p (1:10,000), a gift from K. Hell (Ludwig-Maximilians-Universität München, Munich, Germany); and Tom22p (1:5000), a gift from N. Pfanner (Universität of Freiburg, Freiburg, Germany). The blots incubated with these primary antibodies were decorated with the goat anti-mouse or -rabbit, or rabbit anti-goat horseradish peroxidase conjugate (Sigma-Aldrich). Proteins were detected using ECL Plus (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) or SuperSignal West Femto (Pierce Chemical, Rockford, IL), followed by exposure to Hyperfilm ECL (GE Healthcare) or Blue Lite Autorad film (ISC BioExpress, Kaysville, UT).
Microscopy and Live Cell Imaging
Digital fluorescence and differential interference contrast (DIC) images of cells were acquired using a Zeiss Axioplan 2 Imaging microscope (Carl Zeiss, Thornwood, NY) equipped with DIC optics, epifluorescence capabilities, and a Zeiss Plan-Apochromat 100×/1.4D Oil DIC objective (numerical aperture 1.4). Zeiss filter sets (excitation/beamsplitter/emission) were BP 470 ± 20/FT 495/BP 525 ± 25 (no. 38) for GFP single imaging; and BP 470 ± 10/FT 493/BP 505–530 (no. 13) and BP 546 ± 6/FT 560/BP 575–640 (no. 20) for GFP and DsRed double imaging, respectively. Images were captured using a Zeiss AxioCam MRm monochrome digital camera and a Zeiss AxioVision 4.1 modular image processing and analysis system. For images of mitochondria in Figure 1A, Z-stacks of 0.25-μm slices were obtained and deconvolved using a Regularized Inverse Filter algorithm and a Clip mode for normalization. Three-dimensional projections of mitochondria were generated with a Maximum mode and converted into a single section image. Images were processed and assembled using Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA).
Figure 1.
The mfb1 null mutant contains short mitochondrial tubules but does not exhibit severe growth defects. (A) Mitochondrial morphology in wild-type (JSY7452), mfb1Δ (JSY7453), mdm30Δ (JSY7555), and mfb1Δ mdm30Δ (JSY7556) cells. Bar, 5 μm. (B) Strains used in A were spotted as serial dilutions on rich dextrose (YPD) and glycerol (YPGly) plates and grown at 30°C for 2 and 2.5 d, respectively.
Indirect Immunofluorescence for Skp1p Localization
Methods of indirect immunofluorescence staining were described previously (Palmer et al., 1992) but were modified. Wild-type (Y14) and skp1Δ cells expressing Skp1p-3HA (Y94) were grown to log phase and fixed with 3.7% formaldehyde for 15 min at 30°C. Cells were collected by centrifugation, washed twice with SK (1 M sorbitol and 50 mM KH2PO4, pH 7.5), and resuspended in 1 ml of SK plus 0.01% 2-mercaptoethanol and Zymolyase (final concentration 34 μg/ml). After digestion for 15 min at 37°C, cells were washed twice with SK and applied to polylysine-coated multiwell slides. Cells were washed twice with SK, blocked in 10% bovine serum albumin (BSA)-PBS for 20 min at room temperature and incubated with primary antibody for 60 min at 37°C. Primary antibody mouse anti-HA (Roche Diagnostics, Indianapolis, IN) was diluted to 1:200 in 10% BSA-PBS. Cells were washed seven times with PBS and incubated with the fluorescent secondary antibody for 90 min at 37°C. The secondary antibody, fluorescein isothiocyanate-conjugated AffiniPure goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), was diluted to 1:1000 in 10% BSA-PBS. Cells were washed seven times with PBS and incubated in 100 ng/ml DAPI. After washing several times with PBS, cells were observed through a Leica DM IRE2 motorized fluorescence microscope equipped with an HCX PL APO 100× oil immersion lens (Leica Microsystems, Deerfield, IL), an ARC LAMP power supply HBO100 DC IGN (Ludl Electronic Products, Hawthorne, NY), and an ORCA-ER high-resolution digital charge-coupled device camera (Hamamatsu Photonics, Bridgewater, NJ). Image acquisition and processing were performed using Openlab, version 4 scientific imaging software (Improvision, Lexington, MA).
For Mfb1p and Skp1p double staining, wild-type (Y1460) and skp1Δ cells expressing Skp1p-3HA plus Mfb1p-GFP (Y1461) were grown to log phase and fixed with 4% formaldehyde for 30 min at 30°C. Five to 10 OD600 units of cells were collected by centrifugation, washed once with H2O, and resuspended in 1 ml of TD (0.1 M Tris-HCl, pH 9.4, and 10 mM dithiothreitol). After incubation for 10 min, cells were collected by centrifugation and resuspended in 1 ml of buffer A (0.1 M Tris-HCl, pH 8.0, and 1 M sorbitol) containing 50 μg/ml Zymolyase 100T. After digestion for 20 min at 30°C, cells were washed twice with buffer A, and applied to polylysine-coated multiwell slides. Cells were blocked in buffer B (50 mM Tris-HCl, pH 8.0, and 150 mM NaCl) containing 2 mg/ml fatty acid-free BSA (A0281; Sigma-Aldrich) for 20 min, and incubated with primary antibody for 60 min. The primary antibody, mouse anti-HA (University of Utah Core Facility), was diluted to 1:500 in buffer B. Cells were washed four times with buffer B and incubated with the fluorescent secondary antibody for 60 min. The secondary antibody, Alexa Fluor 555-conjugated goat anti-mouse IgG (A21422; Invitrogen), was diluted to 1:10,000 in buffer B. Cells were washed four times with buffer B, incubated in 4 μg/ml DAPI for 3 min, and washed once again with buffer B. All steps were performed at room temperature unless otherwise indicated. Image capturing and data processing were performed using the live cell imaging system as described above.
RESULTS
Mitochondrial Tubules Are Abnormally Short in Cells Lacking Mfb1p
To identify additional pathways that regulate mitochondrial morphology and dynamics, we searched for genes encoding mitochondrial proteins predicted by the yeast GFP fusion localization database (Huh et al., 2003). Deletions of the selected genes that caused abnormal mitochondrial morphology in commercially available strains were introduced into our laboratory strain for further characterization. One of those genes, YDR219C, encodes a 465-amino acid protein (53.2 kDa). Because the encoded protein contains a consensus F-box motif, we named YDR219C mitochondria-associated F-box protein (MFB1). Proteins homologous to Mfb1p are found in some yeast and fungi.
We examined mitochondrial morphology in the mfb1 null mutant. Because Mdm30p is another F-box protein required for normal mitochondrial shape (Fritz et al., 2003), we compared mitochondrial morphologies in mfb1Δ cells with those in mdm30Δ and mfb1Δ mdm30Δ cells. Unlike wild-type cells, which display interconnected mitochondrial networks (94% of cells; Figure 1A and Table 1), cells lacking Mfb1p contained abnormal mitochondrial morphologies, including short tubules (76.7%), short tubules plus aggregates (15%), and aggregates plus fragments (3.3%) (Figure 1A and Table 1). The presence of short tubules in mfb1Δ cells suggests that Mfb1p promotes or maintains mitochondrial tubular connectivity. By contrast, cells lacking Mdm30p, a mitochondrial F-box protein that regulates fusion, exhibited a different distribution of aberrant mitochondrial morphologies (25.7% short tubules, 47.3% short tubules plus aggregates, and 21.3% aggregates plus fragments) (Figure 1A and Table 1). When both MFB1 and MDM30 were disrupted, the number of cells with short tubules decreased, whereas those with aggregated and fragmented mitochondria increased (3.7% short tubules, 18.3% short tubules plus aggregates, and 78% aggregates plus fragments; Figure 1A and Table 1). Thus, loss of both Mfb1p and Mdm30p causes synthetic mitochondrial morphology defects.
Table 1.
Mitochondrial morphology in wild-type and mutant cells
| Genotype | Tubular networks | Short tubules | Short tubules and aggregates | Aggregates and fragments |
|---|---|---|---|---|
| Wild-type (JSY7452) | 94.0 | 5.3 | 0.7 | 0.0 |
| mfb1Δ (JSY7453) | 5.0 | 76.7 | 15.0 | 3.3 |
| mdm30Δ (JSY7555) | 5.7 | 25.7 | 47.3 | 21.3 |
| mfb1Δ mdm30Δ (JSY7556) | 0.0 | 3.7 | 18.3 | 78.0 |
| fzo1Δ (JSY7562) | 0.0 | 0.0 | 3.0 | 97.0 |
| fzo1Δ mfb1Δ (JSY7561) | 0.0 | 0.0 | 1.7 | 98.3 |
| fzo1Δ mdm30Δ (JSY7614) | 0.0 | 0.0 | 4.0 | 96.0 |
| fzo1Δ mfb1Δ mdm30Δ (JSY7638) | 0.0 | 0.0 | 0.3 | 99.7 |
Mitochondria were visualized using mito-GFP. Numbers shown are percentages of cells (n = 300).
Despite the significant defects in mitochondrial connectivity, mfb1Δ cells grew as well as wild-type on rich dextrose- and glycerol-containing media (YPD and YPGly) at 25, 30, and 37°C (Figure 1B; our unpublished data). As reported previously (Fritz et al., 2003), mdm30Δ cells grew slower than wild type (Figure 1B). A severe synthetic growth defect was observed in cells lacking both Mfb1p and Mdm30p (Figure 1B). Additional studies indicated that the mfb1Δ mdm30Δ synthetic phenotype resulted from mtDNA instability and mitochondrial inheritance defects (our unpublished data).
Disruption of the endoplasmic reticulum or actin cytoskeleton organization can cause defects in mitochondrial morphology (Drubin et al., 1993; Hermann et al., 1997; Prinz et al., 2000; Singer et al., 2000). However, endoplasmic reticulum morphologies and actin cytoskeleton structures were normal in mfb1Δ cells (Supplemental Figures S1A and S1B).
Finally, using transmission electron microscopy, we found that mitochondrial membrane ultrastructure was not disrupted in cells lacking Mfb1p. Both wild-type and mfb1Δ sections contained mitochondrial profiles with normally organized outer and inner boundary membranes, and well-developed inner membrane cristae (Supplemental Figure S2). Together, these data suggest that deletion of MFB1 causes a primary defect in mitochondrial morphology.
Mitochondrial Fusion, Fission, and Tubulation Pathways Are Not Blocked in mfb1Δ Cells
Connectivity of the mitochondrial network is maintained, in part, by opposing fission and fusion events. Mutations in DNM1, a gene encoding a dynamin-related GTPase required for fission, cause formation of net-like or collapsed mitochondria due to ongoing fusion (Bleazard et al., 1999; Sesaki and Jensen, 1999). Mutations in FZO1, a gene encoding a transmembrane GTPase required for fusion, result in mitochondrial fragmentation due to ongoing fission (Bleazard et al., 1999; Sesaki and Jensen, 1999). Because mfb1Δ cells exhibit abnormal mitochondrial connectivity, we asked whether Mfb1p is important for mitochondrial fusion or fission.
For mitochondrial fusion assays in vivo, haploid cells of opposite mating type were labeled with mitochondria-targeted GFP or RFP and mated. Mitochondrial fusion and content mixing in the resulting diploid zygotes was indicated by complete overlap of green and red fluorescent signals. As shown in Table 2, mitochondrial fusion occurred at wild-type levels (98.7%) in mfb1Δ (96.7%) and dnm1Δ mfb1Δ (99.3%) zygotes. As shown previously (Bleazard et al., 1999; Sesaki and Jensen, 1999), mitochondria fused in dnm1Δ zygotes (99.3%; Table 2) but failed to fuse in dnm1Δ fzo1Δ zygotes (2.7%; Table 2). Our results suggest that mitochondria fuse efficiently in cells lacking Mfb1p.
Table 2.
Mitochondrial fusion in vivo
| Strains mated | % fusion observed |
|---|---|
| DNM1 FZO1 MFB1 (JSY7439) xDNM1 FZO1 MFB1 (JSY7452) | 98.7 |
| DNM1 FZO1 mfb1Δ (JSY7450) xDNM1 FZO1 mfb1Δ (JSY7453) | 96.7 |
| dnm1Δ FZO1 mfb1Δ (JSY7455) xdnm1Δ FZO1 mfb1Δ (JSY7449) | 99.3 |
| dnm1Δ FZO1 MFB1 (JSY7447) xdnm1Δ FZO1 MFB1 (JSY7438) | 99.3 |
| dnm1Δ fzo1Δ MFB1 (JSY7640) x dnm1Δ fzo1Δ MFB1 (JSY7639) | 2.7 |
One hundred and fifty large-budded zygotes containing both mito-GFP and mito-RFP were scored at 3–4 h after mating on a YPD plate.
Fission is required to generate mitochondrial fragments in fusion-defective fzo1Δ cells. To test whether Mfb1p is essential for fission, we evaluated mitochondrial morphology in an fzo1Δ mfb1Δ strain. Mitochondria in both fzo1Δ and fzo1Δ mfb1Δ cells fragmented with some aggregation (97 and 98.3%, respectively; Table 1). As reported previously (Fritz et al., 2003), fzo1Δ mdm30Δ cells also contained mitochondrial aggregates and fragments (96%; Table 1). Similar mitochondrial morphology defects were observed in fzo1Δ mfb1Δ mdm30Δ cells (99.7%; Table 1). Moreover, the localization and assembly patterns of the mitochondrial fission proteins Dnm1p, Fis1p, and Mdv1p were indistinguishable in wild-type and mfb1Δ cells (Supplemental Figure S3), indicating that these fission proteins behave properly in the absence of Mfb1p. Thus, Mfb1p is not essential for the mitochondrial fission machinery.
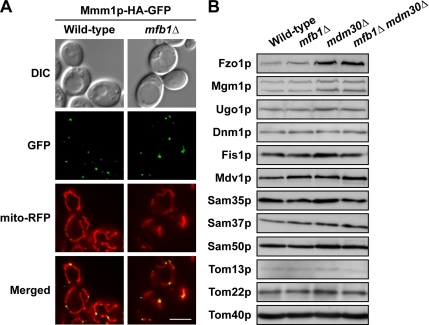
Tubulation of mitochondrial compartments in yeast requires four outer membrane proteins, Mmm1p, Mmm2p, Mdm10p, and Mdm12p, that form punctate foci. Mmm1p foci localize to the mitochondrial membrane contact sites, spanning the outer and inner membranes (Hobbs et al., 2001; Kondo-Okamoto et al., 2003; Meeusen and Nunnari, 2003). Mutations in MMM2, MDM10, and MDM12 disrupt formation of Mmm1p foci, converting mitochondrial tubular networks into large spheres (Boldogh et al., 2003; Youngman et al., 2004). As shown in Figure 2A, Mmm1p-HA-GFP formed mitochondrial foci in mfb1Δ cells similar to those observed in wild type, suggesting that Mmm1p, Mmm2p, Mdm10p, and Mdm12p function normally in the absence of Mfb1p.
Figure 2.
Localization and steady-state levels of previously characterized proteins involved in mitochondrial morphogenesis are not altered in cells lacking Mfb1p. (A) Mitochondrial foci formation of Mmm1p-HA-GFP in wild-type (JSY7452) and mfb1Δ (JSY7453) cells. Bar, 5 μm. (B) Steady-state levels of mitochondrial morphology and import/assembly proteins in strains used in Figure 1A. Proteins in whole cell extracts (0.2 OD600 units of cells/lane) (for Dnm1p) or isolated mitochondria (20 μg of protein/lane) were analyzed.
Despite the altered morphology, mitochondrial inheritance is not significantly disrupted in mfb1Δ cells. Mitochondria were found in both large and small buds at wild-type levels in cells lacking Mfb1p (Supplemental Figure S4). In addition, mitochondrial inheritance was not impaired in cells overexpressing Mfb1p (our unpublished data). These observations suggest that Mfb1p does not play an essential role in bud-directed mitochondrial movement.
Many F-box proteins function as components of SCF ubiquitin ligase complexes, which mediate degradation of specific substrates (Cardozo and Pagano, 2004). Loss of F-box protein function results in stabilization of substrates, leading to an increase in the steady-state protein levels. To determine whether the levels of mitochondrial morphology proteins increased in mfb1Δ cells, we analyzed whole cell extracts and enriched mitochondrial fractions by Western blotting. As shown in Figure 2B, deletion of MFB1 did not significantly alter the steady-state levels of the mitochondrial fusion proteins Fzo1p, Mgm1p, and Ugo1p or the fission proteins Dnm1p, Fis1p, and Mdv1p. In addition, steady-state levels of outer membrane import/assembly proteins required for normal mitochondrial morphology (Sam35p, Sam37p, Sam50p, Tom13p, Tom22p, and Tom40p) (Meisinger et al., 2004; Altmann and Westermann, 2005) were similar in wild-type and mfb1Δ cells.
A previous study showed that the steady-state level of Fzo1p increased in mdm30Δ cells (Fritz et al., 2003). Loss of Mfb1p did not change the steady-state level of Fzo1p in cells lacking Mdm30p (Figure 2B). Moreover, overexpression of Mfb1p did not rescue defects in mdm30Δ cells (our unpublished data). These combined results suggest that Mfb1p and Mdm30p have nonoverlapping functions and act in distinct pathways regulating mitochondrial morphology and dynamics.
Mfb1p Is a Cytoplasmic Protein That Peripherally Associates with Mitochondria
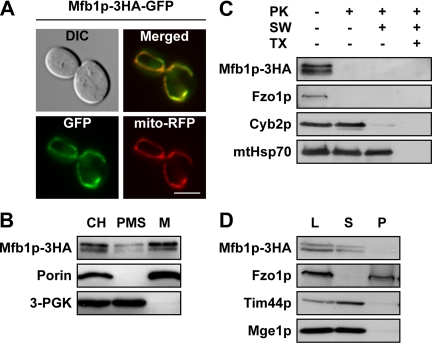
To determine the subcellular localization of Mfb1p in living cells, we fused a 3HA-GFP tag to the C terminus of Mfb1p. Mitochondrial tubular networks were restored in mfb1Δ cells expressing Mfb1p-3HA-GFP, indicating that the fusion protein was functional. When expressed in mfb1Δ cells, the Mfb1p-3HA-GFP signal overlapped completely with mito-RFP–labeled mitochondrial tubules (Figure 3A). In addition, we observed a hazy green fluorescence in the cytoplasm (Figure 3A). Western blot analyses indicated that this cytoplasmic staining was not due to clipping and release of GFP from the Mfb1p-3HA-GFP fusion protein (our unpublished data).
Figure 3.
Mfb1p is present in the cytoplasm and peripherally associated with mitochondria. (A) Localization of Mfb1p-3HA-GFP in mfb1Δ cells (JSY7453). Bar, 5 μm. (B) Subcellular fractionation of mfb1Δ cells (JSY7453) expressing Mfb1p-3HA. The cell homogenate (CH) was separated into PMS and M. 3-PGK (cytoplasmic protein) and Porin (mitochondrial protein) were monitored as controls. (C) Protease protection assays using mitochondria isolated from the strain used in B. Mitochondria were treated with PK under osmotic conditions, swelling (SW) to rupture the outer membrane, or Triton X-100 (TX) to solubilize all membranes. Fzo1p (outer membrane protein), Cyb2p (intermembrane space protein), and mtHsp70 (matrix protein) were monitored as controls. (D) Carbonate extraction assays of mitochondria isolated from the strain used in B. Mitochondria were treated with Na2CO3 and separated into S and P fractions. Fzo1p (integral membrane protein), Tim44p (peripheral membrane protein), and Mge1p (soluble protein) were monitored as controls. L, loaded mitochondria.
Subcellular fractionation assays supported our in vivo finding that Mfb1p is present in the cytoplasm and associated with mitochondria (Figure 3B). When whole cell homogenates from mfb1Δ cells expressing functional Mfb1p-3HA were separated into PMS and mitochondrial pellet (M) fractions, some Mfb1p-3HA was found in the PMS with the cytoplasmic protein marker 3-PGK, whereas the majority was present in M fractions containing the mitochondrial protein marker Porin.
To determine the submitochondrial localization of Mfb1p, protease protection assays were performed on mitochondria isolated from mfb1Δ cells expressing functional Mfb1p-3HA (Figure 3C). When mitochondria were treated with PK under osmotic conditions, Mfb1p-3HA and the outer membrane protein Fzo1p were readily degraded. By contrast, the intermembrane space protein Cyb2p was protected from degradation by externally added protease, indicating that the outer membrane remained intact during the experiment. Conditions that promoted swelling and rupture of the outer membrane allowed PK-mediated degradation of Cyb2p. Finally, the matrix protein mtHsp70 was degraded when mitochondria were solubilized using Triton X-100 in the presence of PK. These results indicate that Mfb1p localizes to the cytoplasmic face of the mitochondrial outer membrane.
Carbonate extraction assays demonstrated that Mfb1p is peripherally associated with the mitochondrial membrane (Figure 3D). When mitochondria treated with sodium carbonate under alkaline conditions were separated into supernatant and membrane pellet fractions, Mfb1p was released into the supernatant along with the peripheral membrane protein Tim44p and the soluble protein Mge1p. By contrast, the integral membrane protein Fzo1p remained with the membrane pellet. Mitochondrial association of Mfb1p did not seem to be mediated by ionic interactions, because Mfb1p-3HA remained membrane-associated upon high salt (1.2 M NaCl) treatment (our unpublished data).
Mfb1p Binds Mitochondria In Vitro via Interactions with Protease-sensitive Components
Our finding that Mfb1p localizes both to the cytoplasm and mitochondria raised the possibility that this F-box protein associates with mitochondria in a dynamic manner. To test whether soluble Mfb1p associates with mitochondria, we performed in vitro binding assays. mfb1Δ mitochondria were incubated with a PMS containing Mfb1p-3HA, separated into supernatant and mitochondrial pellet fractions by centrifugation through a sucrose cushion, and analyzed by Western blotting (Figure 4). Using these conditions, Mfb1p-3HA cofractionated with the mitochondrial pellet. In the same experiment, the cytoplasmic protein 3-PGK remained in the supernatant fraction, indicating that binding of Mfb1p to mitochondria was specific. By contrast, Mfb1p did not bind mitochondria that were pretreated with PK followed by the protease inhibitor PMSF to selectively degrade integral and peripheral outer membrane proteins. These conditions allowed degradation of the outer membrane protein Fzo1p. The protease inhibitor treatment successfully inactivated PK before Mfb1p-3HA addition, because Mfb1p was not degraded during incubation with PK/phenylmethylsulfonyl fluoride-pretreated mitochondria. These results suggest that Mfb1p associates with mitochondria via interactions with protease-sensitive components, most likely outer membrane proteins.
Figure 4.
Mfb1p binds mitochondria in vitro via interactions with protease-sensitive components. Mitochondria isolated from mfb1Δ cells (JSY7453) were pretreated with (+) or without (−) PK followed by PMSF and incubated with PMS prepared from mfb1Δ cells (JSY7453) expressing Mfb1p-3HA. The samples were separated into mitochondrial P and S fractions by sedimentation using a sucrose cushion. 3-PGK (binding and cytoplasmic loading controls), Fzo1p (PK treatment control), and Mge1p (mitochondrial loading control) were monitored. L, loaded mitochondria.
Mitochondria-associated Mfb1p Is Sufficient for Maintenance of Mitochondrial Connectivity
To determine whether mitochondria-associated Mfb1p can sufficiently maintain normal mitochondrial morphology, we fused a transmembrane segment (TMS) to the N terminus of Mfb1p-3HA-GFP (Figure 5A). This TMS is derived from 1 to 50 amino acid residues of Tom20p, a mitochondrial protein integrated into the outer membrane (Moczko et al., 1993; Ramage et al., 1993). We previously showed that the Tom20p TMS targets passenger proteins to the outer membrane (Kondo-Okamoto et al., 2003).
Figure 5.
Mitochondria-anchored Mfb1p rescues mitochondrial connectivity defects. (A) Schematic representation of Mfb1p-3HA-GFP (Mfb1p-HG), TMS-Mfb1p-3HA-GFP (TMS-Mfb1p-HG), and TMS-3HA-GFP (TMS-HG). Mfb1p is 465 amino acids in length. The Mfb1p F-box motif (F) is indicated. The TMS is derived from 1 to 50 amino acids of Tom20p. (B) Carbonate extraction assays performed on mitochondria isolated from mfb1Δ cells (JSY7453) expressing TMS-Mfb1p-3HA-GFP. Mitochondria were treated with Na2CO3 and separated into S and P fractions. Fzo1p (integral membrane protein), Tim44p (peripheral membrane protein), and Mge1p (soluble protein) were monitored as controls. L, loaded mitochondria. (C) Localization of TMS-Mfb1p-3HA-GFP expressed in mfb1Δ cells (JSY7453). Bar, 5 μm. (D) Mitochondrial morphology in mfb1Δ cells (JSY7453) containing an empty plasmid (p-empty), p-MFB1-HG, p-TMS-HG, or p-TMS-MFB1-HG. The percentage of cells with normal tubular mitochondria is shown (n = 300).
Cells lacking Mfb1p were transformed with a plasmid encoding TMS-Mfb1p-3HA-GFP. Subcellular fractionation and protease protection assays demonstrated that TMS-Mfb1p-3HA-GFP localized to the mitochondrial outer membrane without a detectable cytoplasmic fraction (our unpublished data). In addition, membrane-integration of TMS-Mfb1p-3HA-GFP was verified by carbonate extraction assays (Figure 5B). In mfb1Δ cells, mitochondria-anchored TMS-Mfb1p-3HA-GFP restored interconnected mitochondrial networks (Figure 5C). Seventy-one and 71.7% of mfb1Δ cells expressing Mfb1p-3HA-GFP and TMS-Mfb1p-3HA-GFP, respectively, contained wild-type tubular mitochondria (Figure 5D). By contrast, TMS-3HA-GFP failed to complement mitochondrial connectivity defects in mfb1Δ cells (Figure 5D). These observations suggest that cytoplasmic Mfb1p is not essential for mitochondrial morphology maintenance. Instead, mitochondria-associated Mfb1p is sufficient to regulate the connectivity of mitochondrial tubules.
The F-Box Motif Is Not Essential for Mfb1p Mitochondrial Morphology Function
In many proteins, the F-box motif serves as a site for direct interaction with Skp1p (Kipreos and Pagano, 2000). To determine whether the predicted F-box motif is required for Mfb1p function, we generated a double-point mutation in highly conserved leucine and proline residues of the F-box motif (L20A/P21A) (Figure 6A). Similar mutations in the F-box motifs of Cdc4p and Ctf13p have been shown to disrupt Skp1p interactions in vitro (Russell et al., 1999).
Figure 6.
The F-box motif is not essential for Mfb1p mitochondrial morphology function. (A) Amino acid sequence alignment of F-box motifs from yeast F-box proteins (left) and schematic representation of Mfb1p-3HA (p-WT) and Mfb1pL20A/P21A-3HA (p-LP) (right). Highly conserved leucine and alanine residues in the F-box motifs are highlighted. Amino acid substitutions generated in the Mfb1p F-box motif (L20A/P21A) are indicated. (B) Steady-state levels of Mfb1p-3HA and Mfb1pL20A/P21A-3HA expressed from the endogenous MFB1 promoter or overexpressed from the constitutive TPI promoter in mfb1Δ cells (JSY7453). Proteins in whole cell extracts (0.2 OD600 units of cells/lane) were analyzed. 3-PGK was monitored as a loading control. (C) Mitochondrial morphology in mfb1Δ cells (JSY7453) containing an empty plasmid (p-empty) and the strains used in (B). The expression was controlled by the MFB1 or TPI promoter. The percentage of cells containing normal tubular mitochondria is shown (n = 300). (D) Localization of Mfb1p(61-465)-3HA-GFP and Mfb1p(1-70)-3HA-GFP expressed in wild-type cells (JSY7452). Schematic representation of the truncated Mfb1p GFP-fusions is shown. Bar, 5 μm.
We investigated the effect of the L20A/P21A double-point mutation on cellular Mfb1p levels. Cells lacking Mfb1p were transformed with a low-copy plasmid expressing wild-type Mfb1p-3HA or mutant Mfb1pL20A/P21A-3HA from the endogenous MFB1 promoter. Analysis of whole cell extracts by Western blotting indicated that the steady-state level of the mutant protein was less than that of the wild-type protein (Figure 6B). Similar results were obtained when the constitutive TPI promoter was used to overexpress the wild-type and mutant proteins (Figure 6B). In addition, the steady-state level of Mfb1p(61-465)-3HA lacking the F-box motif was reduced (our unpublished data). These observations suggest that the F-box motif plays a role in controlling Mfb1p abundance.
When expressed from the endogenous MFB1 promoter, Mfb1p-3HA and Mfb1pL20A/P21A-3HA restored mitochondrial connectivity in 86.7 and 21.7% of mfb1Δ cells, respectively (Figure 6C). Overexpression of Mfb1pL20A/P21A-3HA using the TPI promoter rescued mitochondrial connectivity defects in 60% of mfb1Δ cells (Figure 6C). In addition, the F-box lacking Mfb1p(61-465)-3HA expressed from the TPI promoter completely complemented mitochondrial morphology defects in mfb1Δ cells (our unpublished data). Overexpression of Mfb1p-3HA neither increased nor decreased the percentage of mfb1Δ cells exhibiting wild-type mitochondrial networks (Figure 6C). Together, our results suggest that the F-box motif is not essential for Mfb1p-mediated mitochondrial network formation.
Our deletion analysis revealed that the F-box motif is not required to target Mfb1p to mitochondria (Figure 6D). Mfb1p(61-465)-3HA-GFP lacking the F-box motif colocalized with RFP-labeled mitochondria. By contrast, Mfb1p(1–70)-3HA-GFP, which contains the F-box motif but lacks C-terminal regions of the protein, localized diffusely in the cytoplasm. Thus, a segment within residues 61-465 of Mfb1p is required for mitochondrial targeting.
Mfb1p Interacts with Skp1p via the Mfb1p F-Box Motif
To determine whether the Mfb1p F-box motif is required for Skp1p interaction, we performed coimmunoprecipitation assays. Mfb1p-3HA and Mfb1pL20A/P21A-3HA were expressed in mfb1Δ cells from the MFB1 and TPI promoters, respectively, which established similar steady-state levels of each protein in strains used for these assays. As shown in Figure 7A, Skp1p coimmunoprecipitated with Mfb1p-3HA from whole cell extracts. The cytoplasmic protein 3-PGK did not coimmunoprecipitate with Mfb1p-3HA, indicating that the Mfb1p-Skp1p interaction was specific. We did not detect Cdc53p, a core component of the SCF complex, under these conditions, even when Mfb1p-3HA was overexpressed (our unpublished data). Importantly, Skp1p did not coimmunoprecipitate with Mfb1pL20A/P21A-3HA even when the F-box-mutated protein was overexpressed, indicating that Mfb1p-Skp1p interaction requires the F-box motif. Thus, Mfb1p is a bona fide F-box protein.
Figure 7.
Mfb1p interacts with Skp1p via the Mfb1p F-box motif. Whole cell extracts from mfb1Δ cells (JSY7453) expressing Mfb1p-3HA or Mfb1pL20A/P21A-3HA (A), cells (Y1459) expressing Cdc53p-13MYC plus Mfb1p or Mfb1p-3HA (B), and cells (YPH499 and Y1459) expressing Mfb1p-3HA plus Cdc53p or Cdc53p-13MYC (C) were subjected to immunoprecipitation using anti-HA and c-Myc antibody-conjugated agarose (IP: αHA and IP: αMYC, respectively). The whole cell extracts (W) and eluted immunoprecipitates (E) were analyzed by Western blotting with antibodies specific to HA, MYC, Skp1p, Cdc4p, Cdc53p, and 3-PGK. Arrowheads indicate the protein bands specific for Skp1p.
To further investigate the interaction between Mfb1p and Cdc53p, we used strains expressing functional Cdc53p-13MYC. This multiple MYC-tagged Cdc53p was detected with increased sensitivity in our assays. When whole cell extracts were subjected to immunoprecipitation using anti-HA antibodies, Cdc53p-13MYC was not detected as a coimmunoprecipitate, whereas Skp1p coimmunoprecipitated with Mfb1p-3HA (Figure 7B). In reciprocal assays using anti-c-Myc antibodies, Skp1p and the F-box protein Cdc4p coimmunoprecipitated with Cdc53p-13MYC (Figure 7C). However, we did not detect Mfb1p-3HA (Figure 7C). Together, these results suggest that Mfb1p does not form a stable complex with Cdc53p.
Mfb1p Asymmetrically Localizes to Mitochondria in Mother Cells
During the course of this study, we noticed that Mfb1p-GFP mitochondrial fluorescence was stronger in mother cells than in buds, whereas mito-RFP fluorescence was evenly distributed in both mother and daughter cells (Figure 8A). Quantification of these localization patterns confirmed that Mfb1p preferentially accumulated on mother cell mitochondria in 34% of budded cells (Figure 8B). The percentage of cells exhibiting Mfb1p mother cell asymmetry varies according to growth conditions and strain backgrounds (60–70% in strains derived from S288C; our unpublished data). Interestingly, the F-box mutant Mfb1pL20A/P21A-GFP, which is defective in Skp1p interaction, uniformly localized to mitochondria in budded cells (Figure 8B). These observations suggest that Skp1p regulates Mfb1p mitochondrial association in mother and daughter cells.
Figure 8.
Mfb1p and Skp1p asymmetrically accumulate on mother cell mitochondria and in the daughter cell cytoplasm, respectively. (A) Patterns of mitochondria-localized Mfb1p-GFP expressed in wild-type cells (JSY7452). Each pair of mother (m) and daughter (d) cells is indicated with the same number (1–3) in the DIC image. Bar, 5 μm. (B) Percentage of budded cells (JSY7452) with mother-asymmetry of Mfb1p-GFP or Mfb1pL20A/P21A-GFP is shown (n = 300). (C) A skp1Δ mutant expressing Mfb1p-GFP and Skp1p-3HA (Y1461) and a wild-type strain expressing Mfb1p and Skp1p (Y1460) were subjected to DAPI staining and indirect immunofluorescence using anti-HA primary and Alexa Fluor 555-conjugated secondary antibodies. Bar, 5 μm. (D) Budded cells with Mfb1p-GFP mother- (left) or Skp1p-3HA daughter-asymmetry (right) were scored for those exhibiting Skp1p-3HA or Mfb1p-GFP mother-asymmetry, symmetry, or daughter-asymmetry (n = 200).
One explanation for the observed asymmetric distribution of Mfb1p is that Skp1p accumulates in the mother cell where it promotes Mfb1p mitochondrial association. Alternatively, Skp1p might accumulate in the bud where it down-regulates Mfb1p mitochondrial association. To determine Skp1p localization in budded cells, we performed indirect immunofluorescence microscopy. Functional Skp1p-3HA exhibited several different localization patterns (Supplemental Figure S5, types I–IV). In the majority of dividing cells, Skp1p-3HA accumulated in buds (50.6%, type I), rather than in mother cells (8.2%, type II). In the remaining 38.2% of the cell population, Skp1p-3HA was equally distributed between mothers and buds (type III). In some cells (3%), Skp1p-3HA localized as foci in the cytoplasm or accumulated in the nucleus (type IV).
To determine Mfb1p and Skp1p localization patterns in the same cell, a strain expressing both Mfb1p-GFP and Skp1p-3HA was subjected to indirect immunofluorescence microscopy. For this assay, we also observed DAPI-stained mtDNA as a mitochondrial marker. As shown in Figure 8C, Mfb1p-GFP and Skp1p-3HA accumulated on mother cell mitochondria and in the daughter cell cytoplasm, respectively. In cells exhibiting Mfb1p-GFP mother-asymmetry, 82.5% displayed Skp1p-3HA daughter-asymmetry (16.0% symmetry and 1.5% mother-asymmetry) (Figure 8D, left). Conversely, in cells exhibiting Skp1p-3HA daughter-asymmetry, 82.5% displayed Mfb1p-GFP mother-asymmetry (16.5% symmetry and 1.0% daughter-asymmetry) (Figure 8D, right). These localization patterns are consistent with the idea that Skp1p negatively regulates Mfb1p mitochondrial association in the bud.
DISCUSSION
Mfb1p Defines a Novel Pathway That Regulates Mitochondrial Connectivity
In eukaryotes, F-box proteins act as adaptors for multiple proteins with different functions, forming heterologous complexes that play key roles in diverse cellular processes. Here, we report the identification and characterization of the new F-box protein Mfb1p that peripherally associates with the surface of mitochondria and regulates mitochondrial connectivity in yeast. Three lines of evidence suggest that Mfb1p is not a core component of known pathways that affect formation of mitochondrial tubular networks. First, the majority of cells lacking Mfb1p harbor mitochondria that are short tubules. This abnormal morphology phenotype is distinct from that found in mitochondrial fusion (fragments), fission (nets and collapsed tubules), and tubulation (spheres) mutants. Our additional results indicate that Mfb1p is not an essential component of these pathways. Moreover, mitochondrial morphology defects in mfb1Δ cells differ from those regulated by other proteins, including the inner membrane protein Mdm33p (rings, hollow spheres, and elongated sheets) (Messerschmitt et al., 2003) and the EF-hand GTPase Gem1p anchored on the outer membrane (large globules, collapsed thick tubules, and grape-like clusters) (Frederick et al., 2004). Second, defects in ER or actin organization, which are known to affect mitochondrial morphology and inheritance, are not observed in mfb1Δ cells. In addition, mitochondrial inheritance continues in the absence of Mfb1p. Third, the steady-state levels of mitochondrial import/assembly proteins of the outer membrane, which are important for biogenesis of morphology proteins, are not significantly altered in cells lacking Mfb1p. The morphology phenotype of mfb1Δ cells is also distinct from that of cells defective in import/assembly (aggregates and spheres) (Dimmer et al., 2002; Meisinger et al., 2004; Altmann and Westermann, 2005). Our data support the idea that Mfb1p mediates formation of mitochondrial networks via a novel pathway distinct from fusion, fission, tubulation, import/assembly, and inheritance. However, we do not exclude the possibility that Mfb1p acts as a regulatory factor in one or more of these pathways (for example, positive regulator for fusion and/or negative regulator for fission) or that Mfb1p has additional functions unrelated to mitochondrial morphology maintenance.
Yeast is predicted to encode three essential and 16 nonessential F-box proteins. Our observation (unpublished data) and a related study by Dürr et al. (2006) reveal that, with the exception of Mfb1p and Mdm30p, other nonessential F-box proteins are not required for normal mitochondrial morphology. A screen of strains containing Tet-regulated essential genes demonstrated that mitochondrial morphology was normal in cells depleted of the F-box protein Met30p, Ctf13p, or Cdc4p (Altmann and Westermann, 2005; Dürr et al., 2006). Thus, yeast has two F-box proteins, Mfb1p and Mdm30p, required for mitochondrial morphogenesis. Do Mfb1p and Mdm30p act in the same pathway? Studies reported here and elsewhere suggest that they do not. Mdm30p affects the steady-state level of Fzo1p, a large GTPase required for fusion. Deletion of MFB1, however, does not alter Fzo1p abundance (Figure 2B; Dürr et al., 2006). A large-scale study revealed that Mdm30p interacts with Skp1p and Cdc53p in a two-hybrid system (Uetz et al., 2000). In addition, systematic in vitro reconstitution assays demonstrate that Mdm30p forms a complex with Skp1p and Cdc53p (Kus et al., 2004). These results support the idea that Mdm30p acts in the mitochondrial fusion pathway, forming the SCFMdm30 complex for degradation of Fzo1p. Moreover, the overexpression of Mfb1p does not rescue mitochondrial morphology defects in cells lacking Mdm30p and vice versa (unpublished data; Dürr et al., 2006). Finally, a high-resolution study using electron microscopy and three-dimensional modeling reveals that mitochondrial aggregates in cells lacking Mfb1p consist of clumped, interconnected tubules, whereas mdm30Δ cells contain aggregated mitochondria comprised of distinct organelles (Dürr et al., 2006). We propose that Mfb1p and Mdm30p carry out nonoverlapping functions in formation of mitochondrial tubular networks.
Mitochondria-associated Mfb1p Is Sufficient for Formation of Tubular Networks
Our studies indicate that Mfb1p is present in the cytoplasm and peripherally associated with the surface of mitochondria. In vitro, cytoplasmic Mfb1p binds mitochondria via protease-sensitive components in the outer membrane. These observations raise the possibility that Mfb1p translocates from the cytoplasm to mitochondria via direct or indirect interactions with an outer membrane receptor protein. Mitochondria-associated Mfb1p may function primarily in formation of interconnected tubular networks. Supporting this idea, the expression of an outer membrane-anchored Mfb1p variant is sufficient to restore tubular networks in mfb1Δ cells. Although it is possible that Mfb1p cycles between the cytoplasm and the outer mitochondrial membrane, we have not identified physiological conditions that cause Mfb1p to dissociate from mitochondria and return to the cytoplasm. Whether cytoplasmic Mfb1p functions in additional cellular processes remains to be determined.
What protein(s) recruit Mfb1p to the mitochondrial surface? We found that Mfb1p is properly targeted to mitochondria in fzo1Δ and ugo1Δ cells defective in fusion, fis1Δ cells defective in fission, mmm1Δ, mmm2Δ, mdm10Δ and mdm12Δ cells defective in tubulation, and mas37Δ, tom7Δ, tom40-3 and sam50-1 cells defective in import/assembly (Dimmer et al., 2002; Meisinger et al., 2004; our unpublished data). Thus, these outer membrane proteins that affect mitochondrial morphology do not function in Mfb1p targeting to the organelle. Studies are currently underway to identify the mitochondrial receptor for Mfb1p and characterize its role in mitochondrial morphogenesis.
Mfb1p Acts in a Complex Lacking Cdc53p Required for Mitochondrial Network Formation
We demonstrate that mutations in highly conserved residues of the F-box motif, which disrupt Skp1p interaction, result in reduction of Mfb1p levels, suggesting that the F-box motif is necessary for normal Mfb1p abundance. When expressed from the endogenous promoter, the F-box–mutated Mfb1p does not support normal mitochondrial morphology, at least in part, due to the reduced expression level. However, the data presented here and in a related study by Dürr et al. (2006) suggest that the overexpression of the F-box-mutated Mfb1p significantly complements the mfb1Δ mutant phenotype without recovering Skp1p interaction. Thus, the F-box motif and Skp1p are not essential for Mfb1p-mediated mitochondrial network formation. In addition, our observation that Cdc53p does not coimmunoprecipitate with Mfb1p is consistent with the idea that Mfb1p forms a complex lacking Cdc53p and maintains mitochondrial networks independently of ubiquitin ligase activity.
Skp1p Regulates Mfb1p Mother Cell-specific Asymmetry
Surprisingly, during budding, Mfb1p preferentially localizes to mitochondria in the mother cell. To our knowledge, Mfb1p is the first mitochondria-associated protein that exhibits mother cell-specific asymmetry. How does Mfb1p asymmetrically associate with mitochondria in the mother cell? Our observation that the F-box–mutated Mfb1p localizes to mitochondria equally in the mother and daughter cells strongly suggests that Skp1p plays a critical role in establishing Mfb1p asymmetry. We also show that Mfb1p and Skp1p accumulate on mother cell mitochondria and in the daughter cell cytoplasm, respectively. These results support the idea that Skp1p negatively regulates Mfb1p mitochondrial association in the bud. Further analysis is required to determine whether Skp1p-dependent degradation in the bud is responsible for the Mfb1p mother cell-specific asymmetry, and how Skp1p asymmetrically localizes to the daughter cell.
The biological significance of Mfb1p asymmetric localization is not entirely clear, because mitochondria seem to form interconnected tubules in both the mother and daughter cells (Figure 8A). Although Mfb1p accumulates on mother cell mitochondria, it also localizes to daughter cell mitochondria to some extent (at a level detectable by microscopy; Figure 8A). Therefore, it is possible that there are enough mitochondria-associated Mfb1p molecules to maintain tubular organelles in the daughter cell. According to this scenario, it is conceivable that mitochondrial tubular connectivity may not be linked to Mfb1p mother cell-specific asymmetry. It should also be noted that the F-box–mutated Mfb1p, which localizes to mitochondria symmetrically, can function to maintain mitochondrial tubular networks. We speculate that accumulated Mfb1p on mother cell mitochondria may have an additional effect on mitochondrial behavior, which we have not yet resolved using our assay systems. For example, Mfb1p mother cell-specific asymmetry may contribute to mitochondrial distribution and transport. Numerous studies suggest that mitochondrial morphology, dynamics, and transport pathways are not mutually exclusive. For example, Mfb1p may contribute to retrograde transport of mitochondria toward the distal tip of the mother cell (Boldogh et al., 2005), and/or immobilization of “old” mitochondria during mother cell-specific aging (Jazwinski, 2005).
Is Mfb1p Function Evolutionarily Conserved?
Mfb1p is a new addition to the growing list of players that control mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Is the Mfb1p-mediated pathway conserved from yeast to humans? Although structural homologues of Mfb1p are only found in some yeast and fungi, there may be proteins that substitute for Mfb1p in multicellular organisms. Indeed, numerous F-box proteins are present in higher eukaryotes and many of them remain uncharacterized (Cenciarelli et al., 1999; Winston et al., 1999; Kipreos and Pagano, 2000; Cardozo and Pagano, 2004; Jin et al., 2004). Our ongoing studies of Mfb1p and its binding partners will advance our understanding of the mechanisms required to regulate mitochondrial tubular connectivity in eukaryotic cells.
Supplementary Material
ACKNOWLEDGMENTS
We greatly appreciate discussions with E. Amiott, R. Frederick, D. Bhar, M. A. Karren, E. Coonrod, and D. Otsuga and strains, constructs, protocols, and antibodies generated by all current and former members of the Shaw laboratory. We are grateful to B. Westermann (Universität Bayreuth, Bayreuth, Germany) for communicating results before publication. We thank W. Neupert, K. Hell, and A. Reichert (Ludwig-Maximilians-Universität München, Munich, Germany), N. Pfanner (Universität Freiburg, Freiburg, Germany), T. Endo (Nagoya University, Nagoya, Japan), and J. Nunnari (University of California, Davis, Davis, CA) for gifts of antibodies, B. Glick (University of Chicago, Chicago, IL) for a fast-folding DsRed variant (RFPff), M. Niederweis (Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany) for a brighter GFP variant (GFP+), R. Tsien (University of California, San Diego, San Diego, CA) for a monomeric DsRed variant (RFPm), and W. Prinz (National Institutes of Health, Bethesda, MD) for a plasmid encoding Sec63p-GFP. This work was supported by grants from the National Cancer Institute (CA-21765), the National Institutes of Health (GM-68418), and the American Lebanese Syrian Associated Charities awarded to K. K., the National Institutes of Health (GM-53466) and the University of Utah Research Foundation awarded to J.M.S., and the United Mitochondrial Disease Foundation and the American Heart Association awarded to K. O. The University of Utah Health Sciences DNA/Peptide and Sequencing Facilities are supported by National Cancer Institute Grant 5-P30CA42014.
Abbreviations used:
- 3-PGK
3-phosphoglycerate kinase
- DIC
differential interference contrast
- mito-GFP
mitochondria-targeted GFP
- mito-RFP
mitochondria-targeted DsRed
- PK
proteinase K
- PMS
postmitochondrial supernatant
- TMS
transmembrane segment.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0145) on June 21, 2006.
REFERENCES
- Altmann K., Westermann B. Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell. 2005;16:5410–5417. doi: 10.1091/mbc.E05-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. H., Sogo L. F., Yaffe M. P. Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J. Cell Biol. 1997;136:545–553. doi: 10.1083/jcb.136.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi K., Rimessi A., Prandini A., Szabadkai G., Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim. Biophys. Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Bleazard W., McCaffery J. M., King E. J., Bale S., Mozdy A., Tieu Q., Nunnari J., Shaw J. M. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat. Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I. R., Fehrenbacher K. L., Yang H. C., Pon L. A. Mitochondrial movement and inheritance in budding yeast. Gene. 2005;354:28–36. doi: 10.1016/j.gene.2005.03.049. [DOI] [PubMed] [Google Scholar]
- Boldogh I. R., Nowakowski D. W., Yang H. C., Chung H., Karmon S., Royes P., Pon L. A. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol. Biol. Cell. 2003;14:4618–4627. doi: 10.1091/mbc.E03-04-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Barsoum M. J., Godzik A., Schwarzenbacher R., Lipton S. A. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr. Opin. Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Burgess S. M., Delannoy M., Jensen R. E. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J. Cell Biol. 1994;126:1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Dawson D., Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Cardozo T., Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell. Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C., Chiaur D. S., Guardavaccaro D., Parks W., Vidal M., Pagano M. Identification of a family of human F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- Cerveny K. L., McCaffery J. M., Jensen R. E. Division of mitochondria requires a novel DMN1-interacting protein, Net2p. Mol. Biol. Cell. 2001;12:309–321. doi: 10.1091/mbc.12.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chan D. C. Mitochondrial dynamics in mammals. Curr. Top. Dev. Biol. 2004;59:119–144. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- Chen H., Chan D. C. Emerging functions of mammalian mitochondrial fusion and fission. Hum. Mol. Genet. 2005;14:R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Danial N. N., Korsmeyer S. J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dimmer K. S., Fritz S., Fuchs F., Messerschmitt M., Weinbach N., Neupert W., Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer K. S., Jakobs S., Vogel F., Altmann K., Westermann B. Mdm31 and Mdm32 are inner membrane proteins required for maintenance of mitochondrial shape and stability of mitochondrial DNA nucleoids in yeast. J. Cell Biol. 2005;168:103–115. doi: 10.1083/jcb.200410030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Jones H. D., Wertman K. F. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol. Biol. Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Escobar-Henriques M., Merz S., Geimer S., Langer T., Westermann B. Non-redundant roles of mitochondria-associated F-box proteins, Mfb1 and Mdm30, in maintenance of mitochondrial morphology in yeast. Mol. Biol. Cell. 2006;17:3745–3755. doi: 10.1091/mbc.E06-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes P., Shepard K. A., Yaffe M. P. Gag3p, an outer membrane protein required for fission of mitochondrial tubules. J. Cell Biol. 2000;151:333–340. doi: 10.1083/jcb.151.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Robert E. G., Youle R. J. Scission, spores, and apoptosis: a proposal for the evolutionary origin of mitochondria in cell death induction. Biochem. Biophys. Res. Commun. 2003;304:481–486. doi: 10.1016/s0006-291x(03)00620-x. [DOI] [PubMed] [Google Scholar]
- Frederick R. L., McCaffery J. M., Cunningham K. W., Okamoto K., Shaw J. M. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J. Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S., Weinbach N., Westermann B. Mdm30 is an F-box protein required for maintenance of fusion-competent mitochondria in yeast. Mol. Biol. Cell. 2003;14:2303–2313. doi: 10.1091/mbc.E02-12-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J. M., Wiederkehr A., Seol J. H., Haguenauer-Tsapis R., Deshaies R. J., Riezman H., Peter M. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin E. E., Graumann J., Chan D. C. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J. Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L., van der Bliek A. M. The many shapes of mitochondrial membranes. Traffic. 2001;2:235–244. doi: 10.1034/j.1600-0854.2001.1r008.x. [DOI] [PubMed] [Google Scholar]
- Guan K., Farh L., Marshall T. K., Deschenes R. J. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr. Genet. 1993;24:141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- Hermann G. J., King E. J., Shaw J. M. The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J. Cell Biol. 1997;137:141–153. doi: 10.1083/jcb.137.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann G. J., Thatcher J. W., Mills J. P., Hales K. G., Fuller M. T., Nunnari J., Shaw J. M. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. E., Srinivasan M., McCaffery J. M., Jensen R. E. Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol. 2001;152:401–410. doi: 10.1083/jcb.152.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O’Shea E. K. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M. Yeast longevity and aging—the mitochondrial connection. Mech. Ageing Dev. 2005;126:243–248. doi: 10.1016/j.mad.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Jensen R. E., Hobbs A. E., Cerveny K. L., Sesaki H. Yeast mitochondrial dynamics: fusion, division, segregation, and shape. Microsc. Res. Tech. 2000;51:573–583. doi: 10.1002/1097-0029(20001215)51:6<573::AID-JEMT7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Jin J., Cardozo T., Lovering R. C., Elledge S. J., Pagano M., Harper J. W. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. A., Fangman W. L. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992;6:380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- Karbowski M., Youle R. J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Pagano M. The F-box protein family. Genome Biol. 2000;1:3002.1–3002.7. doi: 10.1186/gb-2000-1-5-reviews3002. reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo-Okamoto N., Shaw J. M., Okamoto K. Mmm1p spans both the outer and inner mitochondrial membranes and contains distinct domains for targeting and foci formation. J. Biol. Chem. 2003;278:48997–49005. doi: 10.1074/jbc.M308436200. [DOI] [PubMed] [Google Scholar]
- Kus B. M., Caldon C. E., Andorn-Broza R., Edwards A. M. Functional interaction of 13 yeast SCF complexes with a set of yeast E2 enzymes in vitro. Proteins. 2004;54:455–467. doi: 10.1002/prot.10620. [DOI] [PubMed] [Google Scholar]
- Meeusen S., Nunnari J. Evidence for a two membrane-spanning autonomous mitochondrial DNA replisome. J. Cell Biol. 2003;163:503–510. doi: 10.1083/jcb.200304040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger C., et al. The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev. Cell. 2004;7:61–71. doi: 10.1016/j.devcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Messerschmitt M., Jakobs S., Vogel F., Fritz S., Dimmer K. S., Neupert W., Westermann B. The inner membrane protein Mdm33 controls mitochondrial morphology in yeast. J. Cell Biol. 2003;160:553–564. doi: 10.1083/jcb.200211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Gartner F., Pfanner N. The protein import receptor MOM19 of yeast mitochondria. FEBS Lett. 1993;326:251–254. doi: 10.1016/0014-5793(93)81801-6. [DOI] [PubMed] [Google Scholar]
- Mozdy A. D., McCaffery J. M., Shaw J. M. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Shaw J. M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- Otsuga D., Keegan B. R., Brisch E., Thatcher J. W., Hermann G. J., Bleazard W., Shaw J. M. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. E., Sullivan D. S., Huffaker T., Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 1992;119:583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini J. L., Roumier T., Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–183. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Prinz W. A., Grzyb L., Veenhuis M., Kahana J. A., Silver P. A., Rapoport T. A. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993;12:4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Brunner M., Neupert W., Westermann B. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:20150–20155. doi: 10.1074/jbc.273.32.20150. [DOI] [PubMed] [Google Scholar]
- Rube D. A., van der Bliek A. M. Mitochondrial morphology is dynamic and varied. Mol. Cell. Biochem. 2004:256–257. doi: 10.1023/b:mcbi.0000009879.01256.f6. 331–339. [DOI] [PubMed] [Google Scholar]
- Russell I. D., Grancell A. S., Sorger P. K. The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex. J. Cell Biol. 1999;145:933–950. doi: 10.1083/jcb.145.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. Molecular Cloning. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E. Mitochondria. New York: Wiley-Liss; 1999. [Google Scholar]
- Scott S. V., Cassidy-Stone A., Meeusen S. L., Nunnari J. Staying in aerobic shape: how the structural integrity of mitochondria and mitochondrial DNA is maintained. Curr. Opin. Cell Biol. 2003;15:482–488. doi: 10.1016/s0955-0674(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Sesaki H., Jensen R. E. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H., Jensen R. E. UGO1 encodes an outer membrane protein required for mitochondrial fusion. J. Cell Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard K. A., Yaffe M. P. The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial outer membrane to mediate mitochondrial inheritance. J. Cell Biol. 1999;144:711–720. doi: 10.1083/jcb.144.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. M., Hermann G. J., Shaw J. M. Suppressors of mdm20 in yeast identify new alleles of ACT1 and TPM1 predicted to enhance actin-tropomyosin interactions. Genetics. 2000;156:523–534. doi: 10.1093/genetics/156.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo L. F., Yaffe M. P. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J. Cell Biol. 1994;126:1361–1373. doi: 10.1083/jcb.126.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu Q., Nunnari J. Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J. Cell Biol. 2000;151:353–366. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P., et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–531. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J. T., Koepp D. M., Zhu C., Elledge S. J., Harper J. W. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- Wong E. D., Wagner J. A., Gorsich S. W., McCaffery J. M., Shaw J. M., Nunnari J. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P. The machinery of mitochondrial inheritance and behavior. Science. 1999;283:1493–1497. doi: 10.1126/science.283.5407.1493. [DOI] [PubMed] [Google Scholar]
- Yoon Y. Sharpening the scissors: mitochondrial fission with aid. Cell Biochem. Biophys. 2004;41:193–206. doi: 10.1385/CBB:41:2:193. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Karbowski M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell. Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Youngman M. J., Hobbs A. E., Burgess S. M., Srinivasan M., Jensen R. E. Mmm2p, a mitochondrial outer membrane protein required for yeast mitochondrial shape and maintenance of mtDNA nucleoids. J. Cell Biol. 2004;164:677–688. doi: 10.1083/jcb.200308012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.