Abstract
Recently, we characterized a novel endothelial nitric-oxide synthase (eNOS)-interacting protein, NOSTRIN (for eNOS-trafficking inducer), which decreases eNOS activity upon overexpression and induces translocation of eNOS away from the plasma membrane. Here, we show that NOSTRIN directly binds to caveolin-1, a well-established inhibitor of eNOS. Because this interaction occurs between the N terminus of caveolin (positions 1–61) and the central domain of NOSTRIN (positions 323–434), it allows for independent binding of each of the two proteins to eNOS. Consistently, we were able to demonstrate the existence of a ternary complex of NOSTRIN, eNOS, and caveolin-1 in Chinese hamster ovary (CHO)-eNOS cells. In human umbilical vein endothelial cells (HUVECs), the ternary complex assembles at the plasma membrane upon confluence or thrombin stimulation. In CHO-eNOS cells, NOSTRIN-mediated translocation of eNOS involves caveolin in a process most likely representing caveolar trafficking. Accordingly, trafficking of NOSTRIN/eNOS/caveolin is affected by altering the state of actin filaments or cholesterol levels in the plasma membrane. During caveolar trafficking, NOSTRIN functions as an adaptor to recruit mediators such as dynamin-2 essential for membrane fission. We propose that a ternary complex between NOSTRIN, caveolin-1, and eNOS mediates translocation of eNOS, with important implications for the activity and availability of eNOS in the cell.
INTRODUCTION
Endothelial nitric-oxide synthase (eNOS) is the major enzyme generating nitric oxide (NO) in endothelial and epithelial cells (Ortiz and Garvin, 2003; Sessa, 2004). Because NO is an extremely reactive signaling molecule its production needs to be tightly regulated. Regulation seems to take place at three levels: direct interaction of eNOS with accessory proteins such as caveolin and Ca2+/calmodulin, reversible phosphorylation, and differential localization of the enzyme within cells. Subcellular distribution of eNOS is in part governed by lipid modification, i.e., myristoylation and dual palmitoylation, which bring about the association of the enzyme with the Golgi and plasma membrane (PM), respectively (Govers and Rabelink, 2001). Importantly, eNOS seems to be regulated by different modes in different subcellular locations, e.g., Ca2+/calmodulin stimulation is mainly effective at the PM, whereas Akt-driven activation is most pronounced at the Golgi (Fulton et al., 2004).
Differential subcellular localization of eNOS is subject to dynamic regulation, e.g., after certain stimuli, the enzyme also occurs at vesicular structures throughout the cytoplasm (Nuszkowski et al., 2001; Thuringer et al., 2002). At present, however, it remains largely unknown how the differential distribution of eNOS to various subcellular locales is achieved. Emerging determinants of eNOS trafficking are eNOS-interacting proteins, which may guide eNOS to a distinct destination within the cell. In support of this model, we described two novel eNOS-interacting proteins termed NOSIP (for eNOS-interacting protein) and NOSTRIN (for eNOS-trafficking inducer), which both influence localization of eNOS. For NOSIP, targeting of eNOS to the cytoskeleton seems to be one of the protein’s major functions (Schleicher et al., 2005). Overexpression of NOSTRIN, in contrast, leads to translocation of eNOS from the PM to intracellular vesicular structures (Zimmermann et al., 2002), possibly involving an endocytic process (Icking et al., 2005).
Within membranes, eNOS associates with raft-like membrane microdomains, specifically with caveolae at the PM (Shaul et al., 1996). The major constituent of caveolae is caveolin-1 (Fra et al., 1995), which is expressed in many cell types, including endothelial and epithelial cells. Caveolae function in multiple contexts such as signal transduction as well as endocytosis and transcytosis. Interestingly, a major phenotype of caveolin-1 knockout mice relates to NO signaling. NO release from caveolin-1–deficient cells is higher compared with wild-type cells, and secondary effects of NO are increased, suggesting activation of eNOS through deinhibition in the absence of caveolin-1 (Drab et al., 2001). Conversely, direct binding of eNOS to the scaffolding domain (positions 82–101) of caveolin-1 had previously been shown to decrease NO production in vitro and in cell culture (Feron et al., 1996; Ju et al., 1997; Ghosh et al., 1998). On in vivo delivery of the caveolin scaffolding domain, the peptide is taken up into endothelial cells where it attenuates NO-mediated effects (Bucci et al., 2000). In spite of this strong effect of caveolin-1 on eNOS, association of the two proteins is only partial in microscopic as well as biochemical analyses (Sowa et al., 2001; Bernatchez et al., 2005) and varies between different types of blood vessels (Andries et al., 1998), suggesting that the eNOS–caveolin interaction might be subject to further regulation.
In general, caveolae are viewed as rather rigid and immobile structures (Mundy et al., 2002; Thomsen et al., 2002). However, there is good evidence for caveolar endocytosis of certain cargoes (Pelkmans and Helenius, 2002; Parton and Richards, 2003). Mechanistically, caveolar endocytosis has begun to be understood in the past few years, mainly by studying internalization of simian virus 40 and cholera toxin (Le and Nabi, 2003; Smith and Helenius, 2004). Fission of caveolar vesicles from the PM requires the large GTPase dynamin-2, which is transiently recruited to the neck of caveolae (Henley et al., 1998; Oh et al., 1998). Additionally, rearrangement of the actin cytoskeleton is required during the process of pinching off (Pelkmans et al., 2002). Once released from the PM, caveolar vesicles target their cargo to tubular membrane organelles termed caveosomes due to their lack of “conventional” endosomal markers. During the transport and fusion process, caveolin remains associated with these vesicles (Pelkmans et al., 2004). Similarly, caveolar domains assemble at the Golgi and traffic to the PM as stable transport platforms (Tagawa et al., 2005).
Recently, we showed that overexpression of NOSTRIN triggers redistribution of eNOS from the PM to intracellular vesicular structures (Zimmermann et al., 2002). Further analysis indicated that this observation, at least partially, reflects NOSTRIN-facilitated vesicular transport of eNOS from the PM to intracellular compartments (Icking et al., 2005). In this process, NOSTRIN functions as an adaptor protein through homotrimerization and recruitment of eNOS, dynamin-2 and N-WASP to its Src homology 3 (SH3) domain. Concomitant with the NOSTRIN-induced translocation of eNOS, we also observed a pronounced attenuation of the NO-producing capacity (Zimmermann et al., 2002). At present, the mechanisms underlying the NOSTRIN-mediated effects are not fully understood, and it remains unclear whether translocation and inhibition are causally linked. Here, we have set out to study the role of NOSTRIN in the basic mechanisms underlying eNOS trafficking in the cell.
MATERIALS AND METHODS
Antibodies and Reagents
Antibodies against eNOS (monoclonal and polyclonal) and caveolin-1 (polyclonal) were from BD Transduction Laboratories (Heidelberg, Germany); antibodies against hemagglutinin (HA)-tag and transferrin receptor (TfR) were from Covance (Berkeley, CA) and Invitrogen (Karlsruhe, Germany), respectively; monoclonal anti-NOSTRIN directed against glutathione S-transferase (GST)-NOSTRIN242-506 were from nanoTools (Teningen, Germany); A23187 was from Calbiochem (Schwalbach, Germany); filipin, methyl-β-cyclodextrin (MβCD), N-octylglycopyranoside, thrombin, and mouse anti-GST were from Sigma (Taufkirchen, Germany); [14C]l-arginine (100 μCi/ml) was from Hartmann Analytics (Braunschweig, Germany); latrunculin A was from Invitrogen (Carlsbad, CA); Pefabloc was from Roche (Penzberg, Germany); and OptiPrep was from Axis-Shield (Oslo, Norway).
Yeast Mating Assay
Yeast mating assays were performed using the Matchmaker yeast two-hybrid (Y2H) system, according to the manufacturer’s instructions (Clontech, Palo Alto, CA). To narrow down the binding sites between NOSTRIN and caveolin-1, the following constructs were produced in pEG or pJG vectors: NOSTRIN242-506, NOSTRIN1-288, NOSTRIN250-434, NOSTRIN323-470, NOSTRIN433-506, or caveolin1-101 and caveolin128-178. Yeast strains EGY48/pSH18-34 and RFY206 were transformed with pEG and pJG plasmids, respectively. Empty vectors served as negative controls. Interaction was tested using an X-galactosidase assay on 4× deficient plates (−His, −Ura, −Trp, −Leu).
Cell Culture and Transfection
Chinese hamster ovary (CHO) cells stably expressing eNOS (CHO-eNOS) were cultured in DMEM supplemented with 10% fetal calf serum (FCS) and 200 nM methothrexate (Dedio et al., 2001). CHO-eNOS cells were transiently transfected using PolyFect (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. Production of Semliki forest virus (SFV) encoding enhanced green fluorescent protein (GFP)-tagged NOSTRIN, and infection of CHO-eNOS cells was done as described previously (Zimmermann et al., 2002). Human umbilical vein endothelial cells (HUVECs) were cultured in gelatin-coated dishes using Endothelial Cell Medium (PAA Laboratories, Pasching, Austria) plus 10% FCS. Stimulation with thrombin was performed by adding 10 U/μl into the culture medium for 30 min.
Pull-Down Assays
For GST pull-down assays, full-length NOSTRIN and deletion constructs of NOSTRIN (as in yeast two-hybrid assay) cloned into pGEX2T vectors (GE Healthcare, Freiburg, Germany) were expressed in Escherichia coli BL21. The resultant GST fusion proteins were purified on gluthathione (GSH)-Sepharose (GE Healthcare). Untransfected NIH-3T3 or CHO-eNOS cells were lysed in OG buffer (60 mM N-octylglycopyranoside, 50 mM Tris-HCl, pH 7.4, 125 mM NaCl, 2 mM dithiothreitol, 50 μM EGTA, and 100 μM Pefabloc) and incubated with GST fusion proteins immobilized on GSH-Sepharose for 2 h at 4°C. Stimulation with 5 μM A23187 was performed for 15 min before lysis, as indicated. Precipitated proteins were washed two times with OG buffer and one time with phosphate-buffered saline (PBS). Subsequently, bound proteins were eluted with sample buffer (63 mM Tris-HCl, pH 6.8, 2.5% SDS, 5% glycerol, 5% β-mercaptoethanol, and 0.005% bromphenol blue) followed by immunoblotting analysis using anti-caveolin-1 and anti-eNOS. Full-length (His)6-tagged NOSTRIN (pET22b vector; GE Healthcare) was purified from E. coli BL21 using a Ni-NTA matrix according to manufacturer’s instructions (QIAGEN). GST-caveolin constructs 1–61, 61–100, and 137–178 cloned into vector pGEX2T were a kind gift of Dr. W. C. Sessa (Yale University, New Haven, CT). GST and GST-caveolin constructs were expressed in E. coli BL21 and purified on GSH-Sepharose. (His)6-NOSTRIN immobilized on Ni-NTA matrix was incubated with purified GST and GST-caveolin constructs in OG buffer for 2 h at 4°C and washed two times with OG buffer and one time with PBS. Bound proteins were eluted with sample buffer and analyzed by SDS-PAGE and amido black staining or immunoblotting using anti-GST.
Immunoprecipitation
CHO-eNOS cells on 5-cm plates were infected with SFV-NOSTRIN242-506 for 7 h. Cells were lysed in 0.5 ml of OG buffer on ice for 1 h and precleared using 25 μl of Pansorbin. The lysates were incubated for 30 min at 4°C in presence of 50 μM caveolin peptides cav61-81 or cav82-101 or unrelated peptide RLC24, followed by overnight incubation with anti-eNOS (polyclonal) or anti-HA. To precipitate protein complexes, protein A/G-Sepharose (GE Healthcare) was added for 2 h at 4°C. Sepharose beads were washed three times with OG buffer. Bound proteins were eluted with sample buffer and analyzed by SDS-PAGE and immunoblotting using anti-eNOS (monoclonal) or anti-HA.
HUVECs from 3 × 10-cm dishes were lysed on ice for 1 h in 2 ml of OG buffer including 1 mM EGTA, 50 mM NaF, 1 mM Na3VO4, and 10 mM Na4P2O7 and precleared with 100 μl of Pansorbin (Calbiochem, San Diego, CA). Rabbit anti-NOSTRIN (AS532) bound to protein A-coated Dynal magnetic beads (Invitrogen, Carlsbad, CA) was used for immunoprecipitation from the lysates (4°C, overnight). Beads were washed four times with OG buffer without N-octylglycopyranoside. Bound proteins were eluted with sample buffer and analyzed by SDS-PAGE, followed by immunoblotting with monoclonal anti-eNOS, anti-NOSTRIN, or anti-caveolin-1.
Gradient Centrifugation
Raft fractions were isolated using the original method (Harder et al., 1998) with modifications (Manes et al., 1999). Transfected cells were washed on ice with PBS and extracted with cold 1% Triton X-100 in TNE buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 5 mM EDTA) for 30 min on ice. The extract was adjusted to 35% OptiPrep and loaded to the bottom of an OptiPrep step gradient (35, 30, and 5%). After ultracentrifugation (175,000 × g; 4°C; 4 h; Beckman SW50.1 rotor), fractions of 600 μl were collected from the top. Sample buffer was added to the fractions which were then analyzed by SDS-PAGE and immunoblotting using anti-NOSTRIN, anti-caveolin-1, and anti-TfR.
Confocal Immunofluorescence Microscopy
CHO-eNOS cells on coverslips were infected with SFV-NOSTRIN for 8 h or transfected with pcDNA3.1-NOSTRIN and pEGFP-dynamin-2 for 24 h. pEGFP-dynamin-2 (aa isoform) was kindly provided by Mark A. McNiven (Mayo Clinic College of Medicine, Rochester, NY). Treatment with 1 μm LatA was done for the last 2 h of infection, with 5 μg/ml filipin or 10 mM MβCD for 30 min. CHO-eNOS cells or HUVECs (P1) were fixed with ice-cold methanol for 7 min, blocked with BPT (1% BSA and 0.1% Tween 20 in PBS), incubated with primary antibodies (dilution 1:100 in BPT if not stated otherwise), and probed with Cy3-, Cy5- and/or fluorescein isothiocyanate-coupled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). The coverslips were embedded in Gelmount mounting medium (Biomeda, Foster City, CA) and analyzed with an LSM 510 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany).
NOS Activity Assay
eNOS activity was quantified in living cells by monitoring the conversion of [14C]l-arginine into [14C]l-citrulline as described previously (Nuszkowski et al., 2001). Briefly, CHO-eNOS cells cultured in 24-well plates were transfected with NOSTRIN or control protein and treated with inhibitors, as indicated. Cells were preincubated at 37°C for 30 min with NOS activation buffer (10 mM HEPES, pH 7.4, 145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 1 mM glucose, and 10 μM l-arginine) in the presence or absence of 100 μM Nω-nitro-l-arginine (l-NNA). Reaction was started by the addition of NOS activation buffer including 16 mM l-valine, 1 mM NADPH, 3 μM tetrahydrobiopterine, 1 μM FAD, 1 μM FMN, and 0.2 mCi of [14C]l-arginine in the presence or absence of 1 mM A23187 and/or 100 μM l-NNA. After 15 min, the reaction was stopped with ice-cold PBS containing 5 mM l-arginine and 4 mM EDTA, and cells were fixed with 96% ethanol. After evaporation, the soluble cellular components were dissolved in 20 mM HEPES buffer, pH 5.5, and applied to 1 ml of preequilibrated Dowex AG 50WX-8 resin. The radioactivity corresponding to the [14C]l-citrulline content of the eluate was quantified by liquid scintillation counting.
RESULTS
NOSTRIN Directly Interacts with Caveolin-1
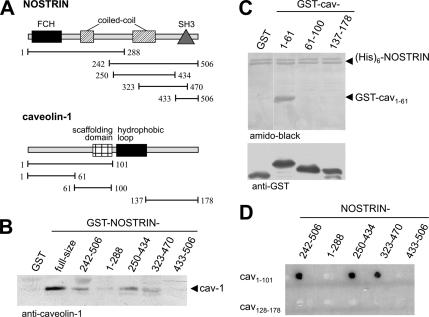
Assuming that NOSTRIN may exert its effect on eNOS in cooperation with other eNOS-interacting proteins, we analyzed whether NOSTRIN is capable of binding to caveolin-1. To this end, we studied the potential of various GST fusion constructs of NOSTRIN (Figure 1A) to precipitate caveolin-1 from cell lysates of NIH-3T3 cells, which do not express eNOS. NOSTRIN did indeed bring down caveolin-1 (Figure 1B). Strongest interaction was observed with full-size NOSTRIN, but the N-terminally truncated fragment NOSTRIN242-506 and the central fragments NOSTRIN250-434 and NOSTRIN323-470 also precipitated caveolin-1. This indicated that the region covering positions 323–434 of NOSTRIN is sufficient for the interaction with caveolin-1. Of note, the SH3 domain of NOSTRIN (positions 433–506), which mediates binding to eNOS (Zimmermann et al., 2002), is not required for association with caveolin-1. To demonstrate that the interaction between NOSTRIN and caveolin-1 is direct and to map the NOSTRIN binding site within caveolin-1, we used purified proteins in a (His)6 pull-down assay. GST-caveolin-11-61 displayed a strong interaction with (His)6-NOSTRIN, whereas constructs containing the scaffolding domain (61–100) or the C-terminal region of caveolin (137–178) failed to bind to NOSTRIN (Figure 1C).
Figure 1.
NOSTRIN directly interacts with caveolin-1, requiring amino acid residues 323–434 of NOSTRIN and the N-terminal part of caveolin-1 (positions 1–61). (A) The domain structure of NOSTRIN and caveolin-1 and the constructs used in the GST pull-down and yeast two-hybrid assays are schematically shown. (B) GST pull-down of caveolin-1 from lysates of NIH-3T3 cells using GST-NOSTRIN constructs. (C) In vitro pull-down of (His)6-NOSTRIN and GST-caveolin-1 constructs. (D) Yeast two-hybrid analysis mapping the interaction sites of caveolin-1 and NOSTRIN. FCH, FES/CIP homology domain.
In a yeast two-hybrid assay, the N-terminal portion, caveolin-11-101, was sufficient for binding to NOSTRIN, whereas the C-terminal segment, caveolin-1137-178, did not interact (Figure 1D). Similar to the GST pull-down assay, amino acids 323–434 of NOSTRIN were required for caveolin binding, whereas the N-terminal (1–288) and C-terminal portions (433–506) lacked binding affinity (Figure 1D). Together, these experiments demonstrate that the direct interaction between NOSTRIN and caveolin-1 involves the central region and the N terminus, respectively, but spares their corresponding eNOS binding sites, i.e., the SH3 domain of NOSTRIN and the scaffolding domain of caveolin-1.
NOSTRIN Colocalizes and Cofractionates with Caveolin
To analyze whether NOSTRIN and caveolin-1 display any overlap in subcellular localization, we analyzed CHO cells by means of confocal laser-scanning microscopy. Moderately overexpressed NOSTRIN and endogenous caveolin-1 extensively colocalized in peripheral vesicular structures and partially in the perinuclear region (Figure 2A; colocalization yellow). To confirm that this colocalization represents distribution to the same membrane microdomains, we used a gradient centrifugation to purify lipid rafts/caveolae (Harder et al., 1998). In this assay, CHO-eNOS cells infected with SFV-NOSTRIN were lysed using 1% of Triton X-100 at 4°C and applied to an OptiPrep density gradient. After ultracentrifugation, the lipid raft fraction floats at the top of the gradient (fraction 1), which is where caveolin-1 was almost exclusively present (Figure 2B, middle). For internal control, we used the TfR known to localize to nonraft parts of membranes (Figure 2B, bottom, fractions 5 and 6). A substantial amount of NOSTRIN was associated with the caveolae-containing fraction 1 (Figure 2B, top), whereas a minor amount was present in fractions 5 and 6. Thus, NOSTRIN and caveolin-1 display substantial overlap in localization, which is reflected by their cofractionation.
Figure 2.
NOSTRIN and caveolin-1 colocalize and cofractionate in rafts. (A) CHO cells were transfected with cDNA encoding NOSTRIN and labeled specifically anti-NOSTRIN (green) and anti-caveolin-1 (red). Bar, 10 μm. (B) Lysates of CHO-eNOS cells infected with SFV-NOSTRIN were subjected to a gradient centrifugation to purify lipid rafts/caveolae which float to the top (fraction 1) of the gradients. Immunoblots of the fractions were probed with anti-NOSTRIN, anti-caveolin-1, and anti-TfR as a nonraft marker (fractions 5 and 6).
NOSTRIN, eNOS, and Caveolin-1 Form a Ternary Complex
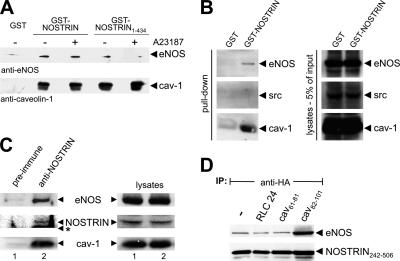
Because the direct interaction between NOSTRIN and caveolin does not involve their respective binding sites for eNOS, it was tempting to speculate that these proteins may form a ternary complex. To test this hypothesis, we did GST pull-down assays using lysates of CHO-eNOS cells and GST-NOSTRIN or GST-NOSTRIN1-434 lacking the eNOS-binding SH3 domain. Stimulation of cells with ionophore A23187 was used to displace caveolin from eNOS through competitive binding of Ca2+/calmodulin (Feron et al., 1998). Independent of stimulation with A23187, caveolin-1 interacted strongly with both GST-NOSTRIN and GST-NOSTRIN1-434 (Figure 3A). Likewise, eNOS coprecipitated with full-size NOSTRIN, pointing to the existence of a ternary complex. Pretreatment of the cells with A23187 did not promote dissociation of the complex. Using SH3-deleted NOSTRIN1-434, we observed coprecipitation of a smaller, although significant fraction of eNOS (Figure 3A). This finding can be explained by an indirect interaction between NOSTRIN and eNOS via their bridging partner caveolin-1. However, when binding of eNOS to caveolin was disrupted by stimulation of intact cells with A23187, NOSTRIN1-434 no longer precipitated eNOS beyond background level. These findings strongly suggest that a ternary complex made up of NOSTRIN, caveolin, and eNOS exists where each protein interacts with two partners. Binding of NOSTRIN to caveolin and eNOS is specific because GST-NOSTRIN did not bring down detectable amounts of other caveolae-associated proteins such as Src (Figure 3B). In caveolin-deficient endothelial cells, binding of GST-NOSTRIN to eNOS was unaffected by treatment with A23187, indicating that this interaction is Ca2+-independent also in the absence of caveolin (our unpublished data).
Figure 3.
NOSTRIN, caveolin-1, and eNOS form a ternary complex. The scaffolding domain of caveolin-1 enhances interaction of NOSTRIN and eNOS. (A) GST pull-down from lysates of CHO-eNOS cells using full-size GST-NOSTRIN and GST-NOSTRIN1-434, which lacks the eNOS binding SH3 domain. Addition of the Ca2+ ionophore A23187 abolishes the interaction of eNOS and caveolin-1. (B) Pull-down from lysates of CHO-eNOS using GST-NOSTRIN. (C) Coimmunoprecipitations from HUVECs using rabbit anti-NOSTRIN or preimmune serum for control. Western blots of immunoprecipitates and lysates were done with mouse anti-eNOS, anti-NOSTRIN, and anti-caveolin-1. The asterisk indicates the position of the immunoglobulin heavy chain. (D) Coimmunoprecipitations from CHO-eNOS cells infected with SFV-HA-NOSTRIN242-506 using anti-HA. Caveolin-1 peptides cav82-101 (scaffolding domain), cav61-81, or unrelated peptide (RLC24) were added to the lysates as indicated. Immunoblots were probed for NOSTRIN (anti-HA) and eNOS.
To show that eNOS, NOSTRIN, and caveolin-1 interact in cells endogenously expressing the three proteins, we did coimmunoprecipitations from lysates of nontransfected HUVECs. Under these conditions, anti-NOSTRIN but not preimmune serum coimmunoprecipitated eNOS and caveolin-1 demonstrating that the ternary complex exists in native HUVECs (Figure 3C).
NOSTRIN and Caveolin Cooperatively Bind to eNOS
To further characterize the ternary complex, we examined how caveolin-1 may influence the interaction between eNOS and NOSTRIN. A coimmunoprecipitation was done from cell lysates of CHO-eNOS cells transfected with HA-tagged NOSTRIN242-506. This N-terminally truncated version of NOSTRIN was used here, because we found it extremely difficult to solubilize full-size NOSTRIN from cells (Zimmermann et al., 2002). To analyze the role of caveolin-1, a molar excess of caveolin peptides cav61-81 and cav82-101 (representing the scaffolding domain) or unrelated peptide RLC24 was added to the lysates. Under these conditions, coprecipitation of eNOS with NOSTRIN242-506 was selectively enhanced in the presence of cav82-101 but not by cav61-81 or control peptide (Figure 3D). The same phenomenon was seen when the immunoprecipitation was done vice versa: Addition of cav82–101 strongly increased coprecipitation of NOSTRIN242-506 with eNOS (Supplemental Figure 1). This enhanced interaction may be due to a conformational change within eNOS upon binding of the caveolin-1 scaffolding domain, thereby inducing stronger binding of NOSTRIN. We have also studied the effect of NOSTRIN constructs on the eNOS–caveolin interaction, but we were not able to detect significant enhancement of coimmunoprecipitation (our unpublished data). This may be due to the harsh assay conditions in the presence of 60 mM N-octylglycopyranoside, because our immunofluorescence studies clearly showed enhanced colocalization of eNOS and caveolin when NOSTRIN is present (cf. Figure 6, E–H).
Figure 6.
Impact of caveolin and cholesterol on ternary complex formation. (A–H) To judge the involvement of caveolin in NOSTRIN-induced translocation of eNOS, CHO-eNOS were infected with SFV-NOSTRIN-GFP (E–H) or left uninfected (A–D). After fixation, cells were stained for caveolin-1 (A and E, red) and eNOS (C and G, blue). Fluorescence of NOSTRIN-GFP (F, green). Triple colocalization is in white. (I–P) To test the impact of the cholesterol membrane distribution on the subcellular localization of the ternary complex, CHO-eNOS cells expressing NOSTRIN-GFP were treated with filipin (I–L) or MβCD (M–P) for 30 min before fixation and specific immunolabeling for caveolin (I and M, red) and eNOS (K and O, blue). Fluorescence of NOSTRIN-GFP (J and N, green). Bars, 10 μm.
The Ternary Complex Assembles at the PM of Confluent Endothelial Cells
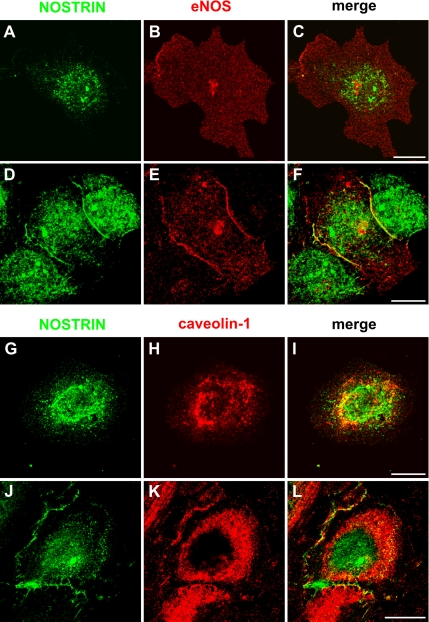
We have previously observed that the distribution of NOSTRIN within HUVECs depends on the state of confluence and that the protein colocalizes strongly with eNOS at the PM of these cells (Zimmermann et al., 2002). We now set out to analyze all three components of the newly established ternary complex in subconfluent versus confluent HUVECs and found that eNOS and NOSTRIN display little overlap under subconfluent conditions (Figure 4, A–C). The distribution patterns are clearly distinct in confluent cells where both eNOS and NOSTRIN colocalize at the PM of HUVECs (Figure 4, D–F). NOSTRIN and caveolin-1 colocalize in both confluent and subconfluent HUVECs, however, within different compartments. Although the two proteins mainly overlap in the perinuclear region of subconfluent HUVECs (Figure 4, G–I), they strongly colocalize at the PM of confluent cells (Figure 4, J–L). These findings indicate that NOSTRIN, eNOS and caveolin-1 undergo a similar change in subcellular localization when HUVECs become confluent.
Figure 4.
Differential distribution of the ternary complex of caveolin, NOSTRIN, and eNOS in endothelial cells. Subconfluent (A–C, G–I) or confluent (D–F, J–L) HUVECs were labeled for NOSTRIN and eNOS (A–F) or NOSTRIN and caveolin-1 (G–L) using specific antibodies. Transition of the subconfluent to the confluent state is associated with an extensive colocalization of the critical components at cell–cell contacts (F and L). Bars 10 μm.
Next, we asked whether translocation of the components of the ternary complex to the PM can be triggered by physiological stimuli. Indeed, application of 10 U/ml thrombin induced the redistribution and strong colocalization of NOSTRIN, eNOS (Figure 5, A–F) and caveolin (Figure 5, G–L) at the PM of subconfluent HUVECs. Coimmunoprecipitation of eNOS and caveolin-1 with NOSTRIN did not increase after thrombin stimulation of HUVECs (our unpublished data); however, this may again be due to the harsh detergent treatment required to solubilize the three proteins (see above). Together, these results indicate that the effects initially observed in overexpressing CHO-eNOS cells properly reflect the situation in native cells.
Figure 5.
Thrombin induces translocation to and assembly of the ternary complex components at the PM of endothelial cells. Subconfluent HUVECs stimulated with 10 U/ml thrombin for 30 min (D–F, J–L) or left untreated (A–C, G–I) were labeled for NOSTRIN, eNOS, and caveolin-1 using specific antibodies. Bars, 10 μm.
NOSTRIN-induced Translocation of eNOS Involves Caveolin-1
To gain insight into the mechanisms underlying eNOS translocation, and to study the relevance of the ternary complex for the subcellular trafficking of its components, we used CHO-eNOS as a well-established cell model (Dedio et al., 2001; Zimmermann et al., 2002). Colocalization of caveolin-1 and eNOS was only partial in these cells, being mainly restricted to the PM and the perinuclear region (Figure 6, A–D, colocalization pink), consistent with previous reports in different cell lines (Sowa et al., 2001; Bernatchez et al., 2005). On SFV-driven overexpression of NOSTRIN-GFP, membrane staining of both eNOS and caveolin was lost, and the triad of proteins colocalized almost exclusively at intracellular structures (Figure 6, E–H, triple colocalization white), indicating that caveolin could be involved in the NOSTRIN-induced translocation of eNOS. This is in line with our finding that intracellular structures containing NOSTRIN and eNOS are devoid of markers for conventional endosomes (our unpublished finding) and may thus well represent caveosomes.
Sequestration of Cholesterol Interferes with Ternary Complex Trafficking
The integrity of caveolae depends on their specific composition of sphingolipids and cholesterol (Simons and Ikonen, 1997). Cholesterol binding reagents frequently used to study caveolae include filipin, which binds to cholesterol leading to structural disorder of the PM, and MβCD, which extracts cholesterol from the PM, leaving caveolin levels unaltered but disrupting caveolae (Simons and Toomre, 2000; Parpal et al., 2001). In CHO-eNOS cells, filipin treatment induced a profound change in localization of NOSTRIN-GFP, eNOS, and caveolin-1: the complex preferentially resided at the PM (Figure 6, I–L), whereas it mainly localized to intracellular structures in untreated CHO-eNOS cells (Figure 6, E–H). Thus, it seems that cholesterol sequestration impairs internalization of the ternary complex without affecting its integrity.
Treatment of CHO-eNOS cells with MβCD led to a uniform distribution of caveolin-1 along the PM and to pronounced perinuclear localization (Figure 6M). Similar observations have been assigned to flattening of caveolae (Westermann et al., 2005) and redistribution of caveolin to the trans-Golgi network (TGN) in other cell types (Nuszkowski et al., 2001). NOSTRIN-GFP colocalized extensively with caveolin-1 in both compartments (Figure 6N), whereas eNOS almost completely disappeared from the PM and colocalized with NOSTRIN and caveolin at the TGN (Figure 6, O and P, inset). These findings indicate that a sizeable fraction of the complex of NOSTRIN, eNOS, and caveolin-1 remains intact when caveolae are disrupted, whereas its subcellular localization changes toward the PM (filipin) or the TGN (MβCD).
NOSTRIN Recruits Dynamin to Caveolin-positive Structures
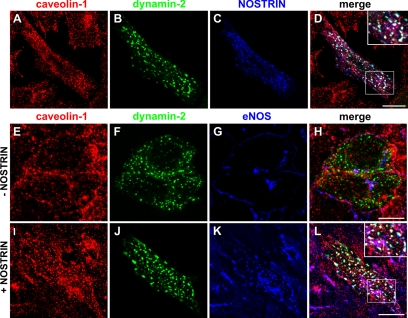
Another binding partner of the SH3 domain of NOSTRIN is dynamin (Icking et al., 2005), which led us to ask whether NOSTRIN could serve to recruit the GTPase to the ternary complex. Using CHO-eNOS cells recombinantly expressing dynamin-2-GFP and NOSTRIN, we found that the two proteins colocalized extensively with caveolin-1 (Figure 7, A–D, inset). To test the significance of this finding with respect to eNOS, we used the same setup with or without expression of NOSTRIN. In the absence of NOSTRIN, there was hardly any overlap of caveolin-1 or eNOS with dynamin-2-GFP (Figure 7, E–H). However, in the presence of NOSTRIN, eNOS localization was clearly changed in favor of vesicular and tubular structures (Figure 7, I–L) previously demonstrated to be positive for NOSTRIN (Icking et al., 2005). In these NOSTRIN-expressing cells, eNOS colocalized extensively with dynamin-2-GFP and caveolin-1 (Figure 7L, inset). We have demonstrated earlier that NOSTRIN also interacts with the K44A mutant of dynamin in a pull-down assay (Icking et al., 2005). In CHO-eNOS cells expressing dynamin-2-K44A-GFP (dyn-K44A) and NOSTRIN, eNOS and caveolin accumulated in the perinuclear region (Supplemental Figure 2), which was not surprising, because the dyn-K44A isoform has a major impact on the Golgi apparatus (Cao et al., 2000). Together, these findings suggest that NOSTRIN may serve as an adaptor recruiting dynamin to caveolin-rich membranes, many of which contain eNOS, implying a role in caveolar trafficking of eNOS.
Figure 7.
NOSTRIN functions to recruit dynamin to caveolin-positive structures, frequently containing eNOS. CHO-eNOS cells transfected with dynamin-2-GFP alone (E–H) or dynamin-2-GFP plus NOSTRIN (A–D and I–L) were labeled for caveolin-1 (A, E, and I, red), and NOSTRIN (C, blue) or eNOS (blue in G and K). (B, F, and J) Fluorescence of dynamin-2-GFP is shown in green. (E–L) Please note that NOSTRIN cannot be shown here due to a limited choice of antibodies and fluorophors. However, cells expressing NOSTRIN are easy to identify, as judged by the NOSTRIN-induced change in localization of eNOS (cf. Figure 7, G and K). Bars, 10 μm.
Depolymerization of Actin Cytoskeleton Stalls Ternary Complex Trafficking
To study whether rearrangement of actin filaments could play a role during translocation and internalization of NOSTRIN and eNOS, we treated CHO-eNOS cells expressing SFV-NOSTRIN-GFP with LatA, which disrupts microfilament organization by forming equimolar complexes with monomeric G-actin. Compared with untreated control cells (Figure 6, E–H), cells treated with LatA displayed a significantly altered distribution of the NOSTRIN/eNOS/caveolin-1 complex, which was apparently trapped at the cell periphery and in the perinuclear region (Figure 8A). This finding strongly suggests that an intact actin cytoskeleton is crucially important for trafficking of the ternary complex.
Figure 8.
Inhibition of eNOS does not require internalization by NOSTRIN. (A) CHO-eNOS cells infected with SFV-NOSTRIN-GFP were treated with LatA for 2 h before fixation. Cells were stained for caveolin-1 (red) and eNOS (blue). Fluorescence of NOSTRIN-GFP is shown in green. Bar, 10 μm. (B) Impact of actin depolymerization as in A on NOSTRIN-mediated inhibition of eNOS, measured by an arginine-citrulline conversion assay in CHO-eNOS cells. eNOS activity was stimulated by means of the Ca2+ ionophore A23187 and specifically inhibited by l-NNA. Cells were transfected with NOSTRIN or mock-transfected for control. (C) Efficiency of full-size NOSTRIN and translocation-deficient NOSTRIN242-506 in inhibiting A23187-stimulated eNOS activity, as detailed in B. Cells were infected with SFV-NOSTRIN, SFV-NOSTRIN242-506, or SFV alone (control).
Translocation Is Not Required for NOSTRIN-mediated Inhibition of eNOS
Because overexpressed NOSTRIN has a dual function on eNOS, i.e., translocation and inhibition, we asked whether inhibition is brought about by translocation. As shown above, preventing rearrangement of the actin cytoskeleton traps the ternary complex at the cell periphery and in the perinuclear region, most likely due to impaired trafficking. This led us to study how interfering with the actin cytoskeleton influences NOSTRIN-mediated inhibition of NO production. Transfection of CHO-eNOS cells with NOSTRIN yielded a 29% decrease in A23187-stimulated eNOS activity (Figure 8B). Application of LatA strongly reduced eNOS activity (Figure 8B, control versus control + LatA), which is in accordance with previous observations in porcine aortic endothelial cells (Zharikov et al., 2001). Coexpression of NOSTRIN further reduced eNOS activity by 31% (relative to control + LatA), i.e., to a similar extent of inhibition caused by NOSTRIN when the actin cytoskeleton is left intact. Because our immunofluorescence analyses showed that actin rearrangement is required for eNOS translocation (Figure 8A), this result indicates that subcellular transport is not necessarily involved in NOSTRIN-mediated attenuation of eNOS activity. To corroborate this notion, we used the deletion mutant NOSTRIN242-506, which does not induce translocation of eNOS (Zimmermann et al., 2002), although it harbors the caveolin and eNOS binding sites and colocalizes with eNOS at the PM. In spite of the mutant’s disability to induce eNOS translocation, its inhibitory potential toward eNOS was unaltered compared with that of full-length NOSTRIN (Figure 8C). Therefore, NOSTRIN can exert its inhibitory effect on eNOS before translocation of the enzyme, and the continuous association of NOSTRIN and caveolin with eNOS during translocation may help prevent uncontrolled activation of eNOS in the various subcellular compartments.
DISCUSSION
Interaction of eNOS with caveolin-1 is a well-appreciated mechanism of down-regulating NO production. We recently discovered NOSTRIN as another direct interaction partner of eNOS involved in decreasing eNOS activity. Here, we report that NOSTRIN and caveolin-1 are capable of directly binding to each other. Importantly, whereas eNOS interacts with the SH3 domain of NOSTRIN (positions 434–506), binding of caveolin to NOSTRIN depends on residues 323–434 of NOSTRIN, allowing for formation of a ternary complex of NOSTRIN, caveolin, and eNOS. At present, we do not know the precise stoichiometry of the protein complex but given that caveolin-1 oligomerizes to large complexes of up to 600 kDa (Monier et al., 1995) and that eNOS and NOSTRIN form homodimers and -trimers, respectively (Icking et al., 2005), the resultant macromolecular complex may provide a platform for dynamic recruitment of proteins involved in caveolar function.
Previous reports suggested that interaction of eNOS and caveolin may be regulated (Andries et al., 1998). NOSTRIN, being capable of interacting with both caveolin and eNOS, represents an ideal candidate to mediate this regulation. In line with this notion, the presence of the scaffolding domain of caveolin specifically increased the strength of interaction between NOSTRIN and eNOS. Because specifically the scaffolding peptides were shown to inhibit eNOS activity (Garcia-Cardena et al., 1997; Bucci et al., 2000), our finding suggests that NOSTRIN preferably interacts with eNOS in its inactivated state. Because the expression levels of NOSTRIN vary under (patho)physiological conditions (Choi et al., 2005; Xiang et al., 2005), it is well possible that the regulated expression of NOSTRIN may contribute to control ternary complex formation and availability.
We have previously observed that the localization of NOSTRIN changes in endothelial cells dependent on the status of confluence (Zimmermann et al., 2002). Other reports showed that caveolin-1 translocates to cell–cell contacts as NIH-3T3 cells become confluent (Volonte et al., 1999) and that eNOS and caveolin-1 strongly colocalize in cell–cell contacts of confluent endothelial cells (Govers et al., 2002). In line with these findings, caveolin, eNOS, and NOSTRIN meet at contact sites of confluent HUVECs. We also found that thrombin efficiently triggers the translocation to and assembly at the PM of these components in subconfluent HUVECs, whereas bradykinin or vascular endothelial growth factor failed to induce the reverse process, i.e., internalization of the preformed complex away from the PM to the cell’s interior. Clearly, a systematic approach is needed to identify more biological agonist(s) promoting the trafficking of the complex.
We have shown earlier that translocation of eNOS observed after overexpression of NOSTRIN partially reflects internalization (Icking et al., 2005). However, we cannot rule out that NOSTRIN is also involved in trafficking of eNOS to the cell surface, which was indeed indicated by accumulation of eNOS in the perinuclear region under certain experimental conditions. At any rate, our data strongly suggest that the NOSTRIN-induced translocation of eNOS involves a caveolae-based mechanism. Here, NOSTRIN seems to function in recruiting dynamin to caveolin-positive structures, which is illustrated by the fact that introduction of NOSTRIN into the CHO-eNOS cell system induced a significant colocalization of eNOS with caveolin and dynamin. NOSTRIN may therefore direct the large GTPase to its site of action, thereby facilitating membrane fission (Henley et al., 1998). This is reminiscent of observations made for intersectin, which associates with caveolae (Predescu et al., 2003), most likely acting to recruit dynamin through three of its five SH3 domains (Okamoto et al., 1999). Because we found that NOSTRIN mainly occurs as a trimer, it should be able to assemble a crucial number of dynamin molecules required for vesicle fission.
A hallmark of caveolar endocytosis is its dependence on actin rearrangement (Pelkmans et al., 2002). Consistently, we found that eNOS is unable to leave the cell periphery upon LatA treatment but still colocalizes with caveolin and NOSTRIN, suggesting that the whole complex is prevented from being internalized. The triad of proteins was also enriched in the perinuclear region under these conditions, again indicating that trafficking to the cell surface may be compromised. It is tempting to speculate that NOSTRIN may have an active role in actin polymerization during caveolar trafficking, because it is capable of recruiting N-WASP (Icking et al., 2005), i.e., a known promoter of actin polymerization. We have not further addressed this intriguing possibility.
For many signaling proteins, such as tumor necrosis factor (TNF) receptor-1, caveolar endocytosis has emerged as the main route of clearance from the PM (Parton and Richards, 2003; D’Alessio et al., 2005). In contrast, epidermal growth factor (EGF) receptors and transforming growth factor (TGF)-β receptors can each use two different pathways for internalization, i.e., via clathrin or caveolae, with opposite outcomes (Le Roy and Wrana, 2005): TGF-β signaling continues from endosomes reached by clathrin-mediated endocytosis (Di Guglielmo et al., 2003), whereas caveolar endocytosis leads to down-regulation of TGF-β receptors. Each of the two pathways involves distinct interaction partners of TGF-β receptors, and overexpression of either of them enhances one pathway in favor of the other. For eNOS, high levels of NOSTRIN seem to promote caveolar internalization with continued inhibition of the enzyme. Under these conditions, we failed to observe colocalization of eNOS with clathrin (our unpublished data), however, we cannot exclude that clathrin-mediated endocytosis may occur in the absence of NOSTRIN, possibly with a different impact on eNOS function. Interestingly, muscarinic acetylcholine receptors involved in activation of eNOS have been shown to be removed from the PM by dynamin-mediated caveolar internalization (Dessy et al., 2000). Combined with our findings, this may suggest that muscarinic receptors and eNOS jointly internalize.
Our data demonstrate that the inhibitory action of NOSTRIN toward eNOS does not necessarily require translocation of the complex. This was suggested by two experimental setups that result in partial accumulation of NOSTRIN/eNOS/caveolin at the PM but nevertheless bring about inhibition of eNOS. In many cases, endocytosis leads to degradation of signaling proteins. Because we did not observe any significant reduction in eNOS protein levels when NOSTRIN is overexpressed (Zimmermann et al., 2002), degradation of eNOS is an unlikely consequence of translocating eNOS from the PM. It is possible that eNOS is recycled back to the Golgi, similar to the mechanism proposed for Ras, which shuttles between the Golgi and the PM during a cycle of palmitoylation and depalmitoylation (Rocks et al., 2005). In this context, it is worth mentioning that two independent studies showed that stimulation with bradykinin, on the one hand, leads to translocation of eNOS to intracellular membranes (Thuringer et al., 2002), and, on the other hand, enhances depalmitoylation of eNOS (Robinson et al., 1995), i.e., two processes that may be well be linked.
In summary, we have elucidated some mechanistic details of the process of eNOS trafficking that we propose to occur through caveolar transport facilitated by NOSTRIN. Here, NOSTRIN acts as an adaptor protein for caveolin and dynamin, thereby facilitating caveolar transport. Although NOSTRIN-mediated inhibition does not require translocation of the complex, continued association of NOSTRIN with eNOS during translocation may serve to impede uncontrolled activation of eNOS in different locales of the cell.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Institute of Biochemistry II for stimulating discussions. This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Abbreviations used:
- eNOS
endothelial nitric-oxide synthase
- HUVEC
human umbilical vein endothelial cell
- Lat A
latrunculin A
- MβCD
methyl-β-cyclodextrin
- NOSTRIN
endothelial nitric-oxide synthase-trafficking inducer
- PM
plasma membrane
- TfR
transferring receptor
- TGN
trans-Golgi network.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0709) on June 28, 2006.
REFERENCES
- Andries L. J., Brutsaert D. L., Sys S. U. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circ. Res. 1998;82:195–203. doi: 10.1161/01.res.82.2.195. [DOI] [PubMed] [Google Scholar]
- Bernatchez P. N., Bauer P. M., Yu J., Prendergast J. S., He P., Sessa W. C. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc. Natl. Acad. Sci. USA. 2005;102:761–766. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M., Gratton J. P., Rudic R. D., Acevedo L., Roviezzo F., Cirino G., Sessa W. C. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Cao H., Thompson H. M., Krueger E. W., McNiven M. A. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J. Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- Choi Y. J., Cho S. Y., Kim H. W., Kim J. A., Bae S. H., Park S. S. Cloning and characterization of mouse disabled 2 interacting protein 2, a mouse orthologue of human NOSTRIN. Biochem. Biophys. Res. Commun. 2005;326:594–599. doi: 10.1016/j.bbrc.2004.11.079. [DOI] [PubMed] [Google Scholar]
- D’Alessio A., Al-Lamki R. S., Bradley J. R., Pober J. S. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedio J.K.P., Wohlfart P., Schroeder C., Kummer W., Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- Dessy C., Kelly R. A., Balligand J. L., Feron O. Dynamin mediates caveolar sequestration of muscarinic cholinergic receptors and alteration in NO signaling. EMBO J. 2000;19:4272–4280. doi: 10.1093/emboj/19.16.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Drab M., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Feron O., Belhassen L., Kobzik L., Smith T. W., Kelly R. A., Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J. Biol. Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Feron O., Michel J. B., Sase K., Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry. 1998;37:193–200. doi: 10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. USA. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Babbitt R., Zoellner S., Fontana J., Acevedo L., McCabe T. J., Iwakiri Y., Sessa W. C. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J. Biol. Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G., Martasek P., Masters B. S., Skidd P. M., Couet J., Li S., Lisanti M. P., Sessa W. C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gachhui R., Crooks C., Wu C., Lisanti M. P., Stuehr D. J. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J. Biol. Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- Govers R., Bevers L., de Bree P., Rabelink T. J. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem. J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R., Rabelink T. J. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. 2001;280:F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele P., Verkade P., Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J. R., Krueger E. W., Oswald B. J., McNiven M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icking A., Matt S., Opitz N., Wiesenthal A., Müller-Esterl W., Schilling K. NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J. Cell Sci. 2005;118:5059–5069. doi: 10.1242/jcs.02620. [DOI] [PubMed] [Google Scholar]
- Ju H., Zou R., Venema V. J., Venema R. C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Le P. U., Nabi I. R. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- Le Roy C., Wrana J. L. Clathrin- and non-clathrin-mediated regulation of endocytic cell signalling. Nat. Rev. Mol. Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Manes S., Mira E., Gomez-Mouton C., Lacalle R. A., Keller P., Labrador J. P., Martinez A. C. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S., Parton R., Vogel F., Behlke J., Henske A., Kurzchalia T. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D. I., Machleidt T., Ying Y. S., Anderson R. G., Bloom G. S. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- Nuszkowski A., Grabner R., Marsche G., Unbehaun A., Malle E., Heller R. Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J. Biol. Chem. 2001;276:14212–14221. doi: 10.1074/jbc.M007659200. [DOI] [PubMed] [Google Scholar]
- Oh P., McIntosh D. P., Schnitzer J. E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Schoch S., Sudhof T. C. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J. Biol. Chem. 1999;274:18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- Oritz P. A., Garvin J. L. Trafficking and activation of eNOS in epithelial cells. Acta Physiol. Scand. 2003;179:107–114. doi: 10.1046/j.1365-201X.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- Parpal S., Karlsson M., Thorn H., Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem. 2001;276:9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Richards A. A. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Burli T., Zerial M., Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Predescu S. A., Predescu D. N., Timblin B. K., Stan R. V., Malik A. B. Intersectin regulates fission and internalization of caveolae in endothelial cells. Mol. Biol. Cell. 2003;14:4997–5010. doi: 10.1091/mbc.E03-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. J., Busconi L., Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J. Biol. Chem. 1995;270:995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P. I. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- Schleicher M., Brundin F., Gross S., Müller-Esterl W., Oess S. Cell cycle-regulated inactivation of endothelial NO synthase through NOSIP-dependent targeting to the cytoskeleton. Mol. Cell Biol. 2005;25:8251–8258. doi: 10.1128/MCB.25.18.8251-8258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C. eNOS at a glance. J. Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- Sowa G., Pypaert M., Sessa W. C. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc. Natl. Acad. Sci. USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P., Roepstorff K., Stahlhut M., van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D., Maulon L., Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J. Biol. Chem. 2002;277:2028–2032. doi: 10.1074/jbc.M109493200. [DOI] [PubMed] [Google Scholar]
- Volonte D., Galbiati F., Lisanti M. P. Visualization of caveolin-1, a caveolar marker protein, in living cells using green fluorescent protein (GFP) chimeras. The subcellular distribution of caveolin-1 is modulated by cell-cell contact. FEBS Lett. 1999;445:431–439. doi: 10.1016/s0014-5793(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Westermann M., Steiniger F., Richter W. Belt-like localisation of caveolin in deep caveolae and its re-distribution after cholesterol depletion. Histochem. Cell Biol. 2005;123:613–620. doi: 10.1007/s00418-004-0750-5. [DOI] [PubMed] [Google Scholar]
- Xiang W., Chen H., Xu X., Zhang M., Jiang R. Expression of endothelial nitric oxide synthase traffic inducer in the placentas of women with pre-eclampsia. Int. J. Gynaecol. Obstet. 2005;89:103–107. doi: 10.1016/j.ijgo.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Zharikov S. I., Sigova A. A., Chen S., Bubb M. R., Block E. R. Cytoskeletal regulation of the L-arginine/NO pathway in pulmonary artery endothelial cells. Am. J. Physiol. 2001;280:L465–473. doi: 10.1152/ajplung.2001.280.3.L465. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Opitz N., Dedio J., Renné C., Müller-Esterl W., Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.