Abstract
The microphthalmia-associated transcription factor (MITF) is required for terminal osteoclast differentiation and is a target for signaling pathways engaged by colony stimulating factor (CSF)-1 and receptor-activator of nuclear factor-κB ligand (RANKL). Work presented here demonstrates that MITF can shuttle from cytoplasm to nucleus dependent upon RANKL/CSF-1 action. 14-3-3 was identified as a binding partner of MITF in osteoclast precursors, and overexpression of 14-3-3 in a transgenic model resulted in increased cytosolic localization of MITF and decreased expression of MITF target genes. MITF/14-3-3 interaction was phosphorylation dependent, and Ser173 residue, within the minimal interaction region of amino acid residues 141–191, was required. The Cdc25C-associated kinase (C-TAK)1 interacted with an overlapping region of MITF. C-TAK1 increased MITF/14-3-3 complex formation and thus promoted cytoplasmic localization of MITF. C-TAK1 interaction was disrupted by RANKL/CSF-1 treatment. The results indicate that 14-3-3 regulates MITF activity by promoting the cytosolic localization of MITF in the absence of signals required for osteoclast differentiation. This work identifies a mechanism that regulates MITF activity in monocytic precursors that are capable of undergoing different terminal differentiation programs, and it provides a mechanism that allows committed precursors to rapidly respond to signals in the bone microenvironment to promote specifically osteoclast differentiation.
INTRODUCTION
The microphthalmia-associated transcription factor (MITF) is a basic helix-loop-helix leucine zipper (bHLHZip) protein closely related to the transcription factor E (TFE) family, made up of Tfe3, TfeB, and TfeC gene products (Carr and Sharp, 1990; Hodgkinson et al., 1993). MITF has been implicated in the survival and differentiation of developmentally unrelated cell types, including melanocytes and osteoclasts (Hershey and Fisher, 2004). Osteoclasts are myeloid-derived hematopoietic cells closely related to monocytes and macrophages that along with the bone-forming cells, osteoblasts, play a reciprocal role in bone remodeling necessary for maintaining bone integrity and calcium homeostasis in vertebrates (Hall and Chambers, 1996). MITF interactions with the ETS-family transcription factor PU.1 at least partly accounts for its ability to regulate the appropriate target genes in osteoclasts (Luchin et al., 2001). MITF and PU.1 are expressed in the mononuclear precursors of both macrophages and osteoclasts, but the mechanisms regulating MITF activity in precursors to prevent expression of osteoclast target genes are ill-defined.
Previous work has indicated that cell-specific signaling events may play a critical role in controlling MITF activity during osteoclast differentiation. Two factors produced by osteoblasts, colony stimulating factor (CSF-1 or M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) are essential for osteoclast differentiation (Boyle et al., 2003; Quinn et al., 1998). CSF-1 signaling leads to phosphorylation of MITF at Ser73 mediated by extracellular signal-regulated kinases (ERKs) and results in recruitment of coactivator p300/CBP (Hemesath et al., 1998; Price et al., 1998). Our group has demonstrated that RANKL signaling leads to p38-dependent phosphorylation at residue Ser307, resulting in increased MITF activity (Mansky et al., 2002).
14-3-3 proteins are abundant, chaperone-like adaptor molecules that act as homo- and heterodimers assembled from multiple 14-3-3 isoforms (Fu et al., 2000). The 14-3-3s bind to serine/threonine-phosphorylated proteins in a sequence-specific manner primarily through RSxpSxP and RxxxpSxP sequence motifs (Muslin et al., 1996; Tzivion and Avruch, 2002). However, some proteins that bind to 14-3-3 in a phosphorylation-dependent manner do not contain either of these motifs, and 14-3-3s can bind to unphosphorylated motifs, including the motif WLDLE (Hallberg, 2002). 14-3-3 binding generally contributes an active regulatory input to the target protein; for example, interaction with 14-3-3s can affect the catalytic activity of target enzymes; regulate subcellular localization of target proteins; or in some cases, affect both activity and localization (Tzivion and Avruch, 2002).
14-3-3 proteins can potentially engage ∼0.6% of the human proteome (Jin et al., 2004), and in this way, they regulate many cellular processes, including cell proliferation, apoptosis, differentiation, and DNA replication. 14-3-3 proteins have been demonstrated to regulate the subcellular localization of several transcription factors, including the Forkhead transcription factors family (Brunet et al., 1999; Cahill et al., 2001), nuclear factor of activated T cells (Chow et al., 2000), and bHLHZip factors such as MondoA and WBSCR14 (Eilers et al., 2002; Merla et al., 2004). Proteomic analysis demonstrated that the MITF-related factor TFE3 is a potential 14-3-3 interacting partner, but no characterized role for 14-3-3 binding with TFE3 or other transcription factors from this subfamily has been reported (Jin et al., 2004).
In this report, we identify MITF as a 14-3-3 interacting protein. In either primary bone marrow-derived osteoclast precursors or in a RAW264.7 C4 cell culture model of osteoclast differentiation, removing both CSF-1 and RANKL resulted in MITF protein localized exclusively in the cytoplasm and bound to 14-3-3, whereas during differentiation initiated by CSF-1 and RANKL, MITF was rapidly localized to the nucleus and was no longer associated with 14-3-3. Overexpression of 14-3-3 during differentiation delayed nuclear movement of MITF, resulting in decreased expression of downstream target genes. C-TAK1 interacted physically with MITF, and this interaction promoted MITF/14-3-3 complex formation. The data support a model in which 14-3-3 regulates MITF activity by promoting its cytosolic localization in the absence of signals required for osteoclast differentiation. This work identifies a mechanism that regulates MITF activity in macrophage/osteoclast precursors before initiation of the osteoclast differentiation program.
MATERIALS AND METHODS
Cell Culture, Transfections, Luciferase Assays, and Quantitative PCR
Cells (COS-7, Phoenix-gp RAW264.7 C4, and bone marrow-derived macrophages [BMMs]) were cultured as described previously (Mansky et al., 2002; Shen et al., 2003). RAW264.7 C4 are a cell clone derived from commercially available RAW264.7 cells (American Type Culture Collection, Manassas, VA) that require both CSF-1 and RANKL for efficient differentiation to osteoclast-like cells, but not for growth or survival. These cells were a gift obtained from A. Ian Cassady and David Hume (University of Queensland, St. Lucia, Australia). Differentiation of RAW264.7 C4 cells and BMMs was optimized using 20 ng/ml soluble recombinant human RANKL (PeproTech, Rocky Hill, NJ) and 50 ng/ml recombinant human CSF-1 (a gift from David Hume). Cells were treated with 100 nM calyculin A (CalA; Biomol Research Laboratories, Plymouth Meeting, PA) for 1 h at 37°C. Whole cell cleared lysates were treated with 1 U/40 μl calf intestinal phosphatase (CIAP; Invitrogen, Carlsbad, CA) for 15 min at 37°C.
For transient expression of proteins, COS-7 cells were transfected using LipofectAMINE (Invitrogen) as detailed in the figure legends, according to the manufacturer’s instructions. For transient expression of proteins, RAW264.7 C4 cells were transfected using the Nucleofector (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer’s recommendations. For stable expression FLAG-MITF, Phoenix-gp packaging cells were transfected with pBabe-puro FLAG-MITF by using calcium phosphate precipitation as described previously (Yang et al., 1996). The harvested supernatant was used for RAW264.7 C4 cell infection. 24 h after infection RAW264.7 C4 cells were passaged and selected with 4 μg/ml puromycin (Sigma-Aldrich. St. Louis, MO). For transfection of reporter plasmids, cells were transfected using the Nucleofactor system as described above. Luciferase activity was measured with luciferase assay system (Promega, Madison, WI) according to the manufacturer’s instructions.
RNA was prepared as described previously (Mansky et al., 2002). The real-time PCR was conducted using SyBr Green Super Mix (Bio-Rad, Hercules, CA) or Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) in an Icycler iQ real-time detection system (Bio-Rad) or in 7500 real-time PCR system (Applied Biosystems). RNA expression calculated as described previously (Wei et al., 2004).
14-3-3 Transgenic Mice
To generate the transgenic vector, the Myc-tagged mouse 14-3-3ζ cDNA (Shen et al., 2003) was subcloned into a transgenic vector under the control of the EF1-α promoter (a gift from Dr. Brian Seed, Massachusetts General Hospital, Boston, MA). 14-3-3 transgene expression was confirmed by PCR and by Western blotting with either anti-Myc or anti-14-3-3ζ antibody.
Immunoprecipitation, Western Blotting, and Glutathione S-Transferase (GST) Pull-Down Assays
Whole cell lysates were prepared as described previously (Shen et al., 2003). Nuclear and cytoplasmic fractions were prepared as described previously (Andrin and Hendzel, 2004). Whole cell lysates or subcellular fractions were immunoprecipitated (IP) with anti-FLAG or anti-MITF antibody precoupled to protein A/G agarose Beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), or for pull down were incubated with glutathione-Sepharose beads (GE Healthcare) (Shen et al., 2003). For in vitro binding assays, 1 μg of the recombinant GST or GST fusion bait protein expressed in Escherichia coli from pGEX2T-GST vector was used. The proteins were eluted directly in SDS sample buffer and subjected to SDS-PAGE, transferred onto nitrocellulose membranes, and probed with antibody. Antibodies used were as follows: FLAG (monoclonal, M2; Santa Cruz Biotechnology, Santa Cruz, CA), 14-3-3 K-19 and 14-3-3 ζ C-16 (polyclonal; Santa Cruz Biotechnology), GST (monoclonal; Sigma-Aldrich), MITF (rabbit polyclonal affinity purified, 14), MITF (mouse monoclonal; Abcam, Cambridge, MA), c-Myc (monoclonal; Santa Cruz Biotechnology), hemagglutinin (HA) (monoclonal; Sigma-Aldrich), and C-TAK1 (monoclonal; Upstate Biotechnology, Lake Placid, NY).
Plasmids and Mutagenesis
The M-form of MITF was cloned into p3xFLAG-Myc-CMVTM-24 expression vector by using EcoRI/XbaI restriction sites (FLAG-MITF) or pEGFP-C2 expression vector by using EcoRI/SmaI restriction sites (GFP-MITF) or pEBG-GST expression vector by using NotI restriction sites. Deletion mutations of MITF were generated by PCR by using primers that incorporated restriction sites (Figure 2A). Single or double point mutations of MITF, C-TAK-1, or 14-3-3 were generated by QuickChange method (Stratagene, La Jolla, CA). PCR primers used for making all mutations are available upon request. C-TAK1 was cloned to the pCDNA 3.1 or pEBG-GST expression vector by using primers incorporate BamHI restriction sites. The expression vector PMT2-Myc 14-3-3ζ, dimerization-deficient pEBG-GST 14-3-3 was described previously (Shen et al., 2003).
Figure 2.
Mapping of the 14-3-3 binding domain in MITF. (A and B) COS-7 cells were transiently transfected with FLAG-MITF or various FLAG-MITF truncation mutations (A) or short targeted deletion mutations (B) and GST-14-3-3 (or GST), as indicated. 14-3-3 was pulled down (GST PD), and complexes were analyzed by Western with anti-FLAG antibody. Whole cell lysates were also immunoblotted with anti-FLAG or anti-GST antibody (input controls).
Total RNA was isolated from RAW264.7 C4 cell line by using TRIzol (Invitrogen) kit according to the manufacturer’s protocol and was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The 14-3-3s (β, ε, η, γ, ζ, ς, and θ) were amplified from the same RAW264.7 C4 cell-derived cDNA by using primers that incorporated BamHI restriction sites. Purified PCR products from each of these reactions were cloned into pEBG-GST mammalian expression vector.
Indirect Immunofluorescence
For all experiments using indirect immunofluorescence, cells were grown in appropriate media on eight-well chamber slides (Lab-Tek, Naperville, IL) at a density of 3 × 104 cells/well in 200 μl of medium, fixed in 3.7% formaldehyde, and washed with 1× phosphate-buffered saline (PBS). Cells were blocked with 1× PBS, 0.1% Triton X-100, and 1% bovine serum albumin. Primary antibodies anti-MITF or anti-FLAG were used at 1:100 and 1:500, respectively. Secondary antibody anti-rabbit or anti-mouse fluorescein isothiocyanate-labeled antibodies (Jackson ImmunoResearch, Laboratories, West Grove, PA) were used at 1:200. Nuclei were stained with 2 μg/ml 4,6-diamidino-2-phenylindole. Cells images were captured at a magnification of 60 and 40×, respectively, by using an Olympus IX50 inverted microscope (Olympus, Melville, NY) with epifluorescence attachment. A minimum of 100 stained cells were scored for each experiment reported.
All experiments in each figure were performed a minimum of three times. The results of representative experiments are shown.
RESULTS
14-3-3 Is a MITF Binding Partner
MITF is highly related to the bHLH-Zip transcription factor TFE3 that was identified as 14-3-3 binding partner by mass spectrometry analysis (Jin et al., 2004). To test the possibility that MITF is also a partner of 14-3-3, cell lysates from RAW 264.7 C4 cells, that express endogenous MITF, or control COS-7 cells, were subjected to immunoprecipitation with an anti-MITF antibody. Immunoprecipitates were analyzed by Western with both anti-14-3-3 and anti-MITF antibody (Figure 1A). 14-3-3 was detectable after immunoprecipitation with anti-MITF antibody, confirming that endogenous MITF and 14-3-3 proteins are interacting partners.
Figure 1.
MITF binds to 14-3-3 in phosphodependent manner. (A) anti-MITF immunoprecipitates prepared from RAW264 C4 cells or COS-7 cells were analyzed by immunoblot. The MITF doublet is indicated by arrows. (B) GST-fusion full length MITF proteins expressed in bacteria were incubated with whole cell extract from COS-7 cells transfected with Myc-14-3-3ζ. MITF was pulled down (GST PD), and complexes were analyzed by immunoblot with anti-Myc antibody. (C) FLAG-MITF and GST-14-3-3ζ, -β, -ε, -η, and -γ were coexpressed in COS-7; 14-3-3s were pulled down; and complexes were analyzed by immunoblot with anti-FLAG antibody. (D) FLAG-MITF expression vector and either GST-14-3-3 (wt) or dimerization-deficient GST-14-3-3 (dm) were coexpressed in COS-7 cells, 14-3-3 was pulled down, and complexes were analyzed by immunoblot. In each panel, whole cell lysates were analyzed by immunoblot with the appropriate antibodies, as indicated (input controls).
The MITF/14-3-3 complex was also identified by in vitro binding experiments by using MITF-GST fusion protein purified from bacteria and COS-7 cell extracts obtained from cells transfected with Myc-14-3-3ζ. GST-MITF, but not GST alone, specifically bound to Myc-tagged 14-3-3 (Figure 1B). To determine whether MITF interacted selectively with 14-3-3 isoforms, we cloned 14-3-3 cDNAs from RAW264.7 C4 cells by using primers specific for all seven isoforms of 14-3-3 (see Materials and Methods). 14-3-3ς and -θ isoforms were not detected in this analysis, suggesting that either these isoforms were not expressed or they were expressed at very low levels in RAW264.7 C4 cells. In GST pull-down assays, FLAG-MITF and GST-14-3-3s (ζ, ε, β, η, and γ) were coexpressed in COS-7 cells and tested for their ability to coprecipitate. GST-14-3-3s specifically bound to MITF (Figure 1C). No significant differences were observed in the interaction of MITF and the five different isoforms of 14-3-3 expressed in COS-7 cells (Figure 1C). For all subsequent experiments, the 14-3-3ζ isoform was chosen as the isoform that is most commonly expressed in various tissues in both nuclear and cytosolic compartments (van Hemert et al., 2004).
14-3-3 usually binds to protein partners as either hetero- or homodimers; however, some proteins with strong binding affinity, such as c-Raf, can bind to 14-3-3 monomers (Tzivion et al., 1998; Tzivion and Avruch, 2002). To address this issue, a mutation that blocks 14-3-3 dimerization, 14-3-3 (dm), was used in pull-down assays with MITF (Figure 1D). 14-3-3 (dm) did not form a complex with MITF in these assays, indicating that the 14-3-3 dimers interact preferentially with MITF (Figure 1D).
A Region between Amino Acids 141–191 in the N-Terminal Sequence of MITF Is Required for Complex Formation with 14-3-3
To map the region of MITF that mediated 14-3-3 interaction, a series of deletion mutations were created in the FLAG-tagged MITF vector (Supplemental Figure 1). Cotransfection of FLAG-MITF deletion mutations with 14-3-3-GST identified an overlapping region in the N-terminal portion of MITF from approximately amino acids 158–218 in MITF as necessary for interaction with 14-3-3 (Figure 2A, mutations that bind to 14-3-3 are shaded gray in Supplemental Figure 1, whereas those that do not bind are white). On the basis of these results, additional mutations were created and tested in the binding assay to determine whether this region was sufficient for 14-3-3 interactions (Figure 2B). In this analysis, MITF with a deletion of residues 158–178 did not bind to 14-3-3, whereas a region in MITF corresponding to residues 141–191 was able to interact with 14-3-3, thus defining the latter as the minimal region of MITF required for interaction with 14-3-3.
MITF Ser173 Is Critical for Phosphorylation-dependent Interactions with 14-3-3
Because 14-3-3 is known to bind proteins containing phosphoserine/threonine residues, the influence of the phosphorylation status of MITF on 14-3-3 binding was analyzed (Figure 3A). Treatment of transfected cells with the phosphatase inhibitor CalA increased the interaction between MITF and 14-3-3 (Figure 3A, lane 4 vs. lane 3). Dephosphorylation of MITF with alkaline phosphatase (Figure 3A, lane 5) or protein phosphatase 2A (our unpublished data) diminished binding to 14-3-3.
Figure 3.
Identification of S173 as a 14-3-3 binding site. In each panel, FLAG- and GST-tagged proteins, as indicated, were expressed in COS-7 cells, and complexes from whole cell lysates were pulled down (GST PD), and analyzed by Western analysis with the antibody indicated. Whole cell lysate was also immunoblotted with anti-FLAG or anti-GST antibody (input controls). The experiments in all panels were all preformed a minimum of three times, and representative results are presented. (A) FLAG-MITF and GST-14-3-3 were coexpressed. Thirty-six hours after transfection, cells were left untreated or treated with 100 nM CalA for 1 h, or the whole cell lysate was treated with 40 U/μl CIAP. (B) FLAG-MITF wild type or versions with serine or threonine point mutations (S100A, S173A, T186A, and S409A) were coexpressed with GST-14-3-3ζ, or GST alone, as indicated. (C) MITF expression vector or FLAG-MITF S173A was coexpressed with GST-14-3-3 (or GST). After 36 h, cells were either not treated or treated with 100 nM CalA for 1 h. (D) Lysates from COS-7 cells transfected with GST-14-3-3 treated or not with CalA were immobilized onto glutathione-Sepharose beads and then incubated with lysate from COS-7 cells independently transfected with FLAG-MITF, also treated or not with CalA, as indicated. (E) GST-MITF and either Myc-14-3-3 or Myc 14-3-3 K49E were coexpressed. Endogenous 14-3-3 band is marked by asterisk.
The phosphorylation-dependent binding of 14-3-3s to target proteins often requires the specific motifs RSxpSxP or RxxxpSxP (Muslin et al., 1996; Tzivion and Avruch, 2002). Evaluation of the MITF sequence by using a peptide motif-based scanning algorithm (<scansite.mit.edu>) suggested the presence of several weakly related 14-3-3 binding motifs in MITF (Supplemental Figure 2A). Based on the predictions, we analyzed whether mutations that mimicked the unphosphorylated state for each of these sites, S409A, S100A, S173A, and T186A, affected interactions with 14-3-3 subtypes in the GST binding assay. All isoforms of 14-3-3 were able to interact with wild-type MITF as well as with S409A, S100A, and T186A mutations, but in contrast, not with the MITF S173A mutation (Figures 2B and Supplemental Figure 2B, lane 4 in each figure). Additionally the S173A mutation failed to interact with 14-3-3 after CalA treatment (Figure 3C). These results demonstrated that the MITF point mutation changing Ser173 to Ala173 abrogated complex formation with 14-3-3.
Several serine residues in MITF have been also been demonstrated to be phosphorylated in response to specific signaling pathways, including Ser73 by Erk kinase, Ser298 by GSK3β, Ser307 by p38 MAPK, and Ser409 by Rsk. Mutation of these four sites, either singly or in possible double combinations, had no effect on MITF/14-3-3 complex formation (Supplemental Figure 2C).
Phosphorylation of both target protein and 14-3-3 can be required for complex formation (Aitken et al., 1995; Gu et al., 2006). To determine whether the increased complex formation observed after CalA treatment was caused by increased phosphorylation of MITF, 14-3-3, or both, an in vitro mixing experiment was performed (Figure 3D). In this experiment, GST-14-3-3 and FLAG-MITF were transfected separately into COS-7 cells, in each case either with or without CalA treatment. GST-14-3-3 contained in one cell lysate was immobilized on glutathione-Sepharose beads and then incubated with the second lysate containing FLAG-MITF. The interaction of FLAG-MITF from CalA-treated cells with 14-3-3 was markedly increased, but not when the GST-14-3-3 was isolated from cell treated with CalA (Figure 3D, compare lane 3 vs. lane 2). Identical results were obtained in the reciprocal experiment preformed with GST-MITF (our unpublished data).
As an additional approach to test whether phosphorylation of MITF is required for interaction with 14-3-3, the point mutation 14-3-3 K49E, which results in an inability to bind to phosphorylated substrates (Fu et al., 2000), was tested in the pull-down assays (Figure 3E). These experiments demonstrated that GST-MITF could interact with either exogenous Myc-tagged or endogenous 14-3-3, but not with 14-3-3 K49E mutation (Figure 3E).
Dynamics of MITF Subcellular Localization and 14-3-3 Association during Primary Osteoclast-like Cell Differentiation
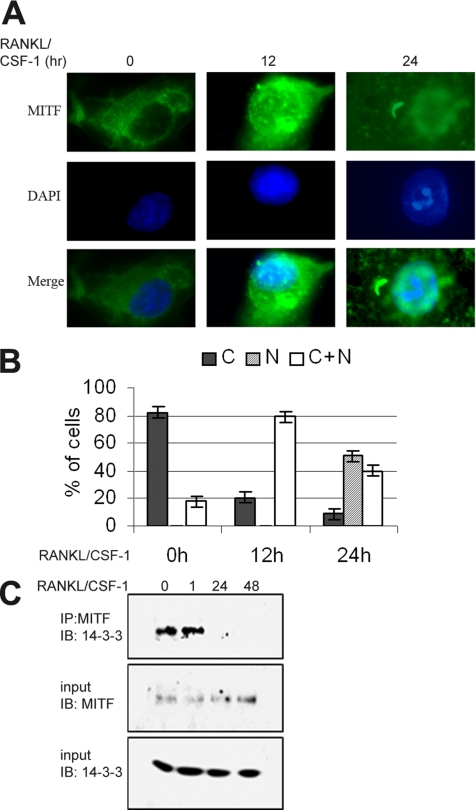
Given that 14-3-3 has been implicated in nuclear trafficking of several transcription factors (Muslin and Xing, 2000), we decided to examine the hypothesis that 14-3-3 might regulate nuclear localization of MITF in osteoclast precursor cells, primary BMMs. In previous work, both our laboratory and other groups have observed MITF localized strictly to the nucleus in both bone marrow precursors and differentiated osteoclast-like cells (Hershey and Fisher, 2004). However, these experiments were performed in the presence of CSF-1 and RANKL, cytokines that are also survival factors for these cells. When CSF-1 and RANKL were both absent from the growth media for cultured BMMs for 4–6 h (before apoptosis is irreversibly triggered), MITF was localized exclusively to the cytoplasm, as measured by indirect immunofluorescence by using anti-MITF antibody (Figure 4, A and B). The PU.1 transcription factor, a partner of MITF, remained localized only in the nucleus irrespective of withdrawal of these cytokines (our unpublished data). Adding back CSF-1 and RANKL to factor-deprived cells resulted in partial nuclear localization of MITF within 12 h, because nearly all cells examined had both nuclear and cytoplasmic staining (Figure 4, A and B). After 24 h of treatment with CSF-1/RANKL, MITF was observed only in the nucleus in ∼60% of cells (Figure 4, A and B).
Figure 4.
Dynamics of MITF localization and MITF-14-3-3 interaction during differentiation of primary osteoclast-like cells. (A and B) BMMs were grown without CSF-1 for 6 h (time 0) and then cultured with RANKL/CSF-1 for 12 or 24 h. (A) Anti-MITF was used for immunolocalization. Representative images are shown. (B) Quantitative analysis of MITF localization. Each experiment was repeated three times, and a minimum of 100 cells were counted for each condition and time point. Error bars indicate SD of the measurements. (C) BMMs were treated with RANKL/CSF-1 for times indicated (in hours). At each time point, anti-MITF IP complex was prepared and analyzed by western. Whole cell lysates were also immunoblotted with anti-MITF or anti-14-3-3 antibody.
To both confirm the results obtained by indirect immunofluorescence and examine whether MITF/14-3-3 complex formation was affected by cytokine treatment, BMMs were deprived of CSF-1 for 6 h and then grown with both RANKL and CSF-1. Cells were subsequently harvested at different times after cytokine stimulation, lysed, and subjected to immunoprecipitation with an anti-MITF antibody. Immunoprecipitates and whole cell lysates were analyzed by Western (Figure 4C). This analysis confirmed that endogenous 14-3-3 and MITF coimmunoprecipitated in CSF-1–starved cells or in cells treated for 1 h with both CSF-1 and RANKL, but not in cells treated for 1–2 d, when MITF localization was primarily nuclear.
Overexpression of 14-3-3 Regulates MITF Localization and Activity
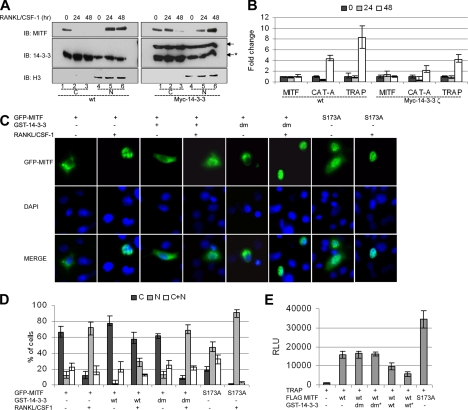
14-3-3 proteins can promote the cytoplasmic localization of many binding partners. To test the hypothesis that 14-3-3 regulated cytoplasmic localization of MITF in the absence of CSF-1/RANKL signaling, we examined whether overexpression of 14-3-3 perturbed the RANKL/CSF-1–dependent nuclear localization of MITF. BMMs were derived from wild-type mice or transgenic mice expressing Myc-14-3-3ζ under control of the EF-1α promoter (see Materials and Methods), and cytosolic and nuclear compartments were prepared and analyzed. An anti-histone H3 antibody was used to evaluate possible cross-contamination of the subcellular fractions. Both endogenous and transgenic Myc-tagged 14-3-3 were detected using the anti-14-3-3ζ antibody. This analysis indicated that Myc-14-3-3 is expressed at approximately the same level as the endogenous protein in BMMs obtained from the transgenic mice, resulting in a modest approximately twofold increase in 14-3-3ζ production (Figure 5A, compare band intensity between left and right panel). We also performed quantitative reverse transcription (qRT)-PCR analysis on 14-3-3 isoforms and found that 14-3-3ζ mRNA was indeed increased twofold in BMMs from these transgenic mice (Supplemental Figure 3A).
Figure 5.
Overexpression of 14-3-3 regulates MITF localization and activity. (A and B) BMMs were grown without CSF-1 for 6 h (time 0) and then cultured with RANKL/CSF-1 for times indicated (in hours). (A) Cytosolic (C) and nuclear (N) fractions of BMM cells were by analyzed by Western with antibodies as indicated. These experiments were repeated twice on cells derived from two different sets of mice. Endogenous 14-3-3 band is marked by asterisk. (B) Results of qRT-PCR for Mitf, TRAP or Cathepsin K (Cat A), as indicated. Results of two experiments, each performed in triplicate are presented. Results were normalized to day 0. Error bars indicate SD of the measurements. (C and D) RAW264C4 cells were transiently cotransfected with GFP-MITF and GST-14-3-3 or dimerization-deficient GST-14-3-3 (dm) or pEBG-GST vector, as indicated. Cells were left untreated or treated with RANKL/CSF-1 for 3 h. (C) Representative photomicrograph images. (D) Quantitative analysis of GFP-MITF localization. Each experiment was repeated four times, and a minimum of 100 cells were counted for each condition and time point. Error bars indicate SD of the measurements. (E) RAW C4 cells were cotransfected with 2 μg of TRAP luciferase reporter construct and 1 μg of wild-type MITF or S173A MITF, and GST-14-3-3 or dimerization-deficient GST-14-3-3 (dm) (1 or 2 μg* as indicated) or pEBG-GST empty vector (up to 5 μg total of DNA). TRAP reporter activity is presented as relative luciferase units (RLU), and the results of four experiments each performed in duplicate are presented. Error bars indicate SD of the measurements.
As in previous experiments, absence of CSF-1 in the media of the primary BMMs resulted in MITF protein being localized exclusively in the cytoplasm (Figure 5A, lane 1 in both panels). After CSF-1/RANKL treatment, MITF shifted to the nucleus in cells obtained from wild-type mice (Figure 5A, lanes 5 and 6, left). In contrast, in BMMs overexpressing Myc-14-3-3, MITF was still present in cytosol 24 h after cytokine treatment, and a fraction still was retained in the cytosol after 48 h (Figure 5A, lanes 2 and 3, right).
To determine whether the delayed movement of MITF into the nucleus in Myc-14-3-3ζ–overexpressing cells affected MITF target genes expression, the expression of two target genes, Cathepsin K and TRAP tartrate-resistant acid phosphatase (TRAP), were measured by qRT-PCR (Figure 5B). In wild-type cells, Cathepsin K and TRAP expression was increased four- and eightfold, respectively, after 48 h of cytokine treatment. In contrast, the expression Cathepsin K and TRAP was induced two- and fourfold, respectively, in cells overexpressing Myc-4–3-3ζ. Overexpression of Myc-14-3-3 did not effect Mitf mRNA expression (Figure 5B).
In a complementary approach, the effect of overexpression of 14-3-3 on MITF localization was examined in transient transfection assays. For these studies, GFP-tagged MITF was introduced into RAW264.7 C4 cells, a stable subclone of commercially available RAW264.7 cells that require both CSF-1 and RANKL for efficient differentiation in vitro, but not for cell growth or survival (see Materials and Methods). Similar to endogenous MITF in primary cells, GFP-MITF was observed in the cell cytoplasm in ∼70% of transfected cells. Furthermore, GFP-MITF relocalized to the nucleus in roughly 60% of the cells, after 3-h treatment with CSF-1/RANKL (Figure 5C, data quantification in Figure 5D). In contrast, when GST-14-3-3 was coexpressed with GFP-MITF, after CSF-1/RANKL treatment there was a threefold decrease in cells scored with strictly nuclear GFP-MITF (∼20%), and a corresponding threefold increase in number of cells with cytoplasmic GFP-MITF (Figure 5, C and D). Coexpression of the dimerization deficient 14-3-3 (dm) had no effect on GFP-MITF localization. Additionally, for GFP-MITF S173A, that does not bind to 14-3-3, only in ∼20% of cells GFP-MITF was observed in cytoplasm in the absence of cytokine treatment, and GFP-MITF was localized in the nucleus after 3 h of CSF-1/RANKL stimulation in 90% of the cells. These results were confirmed by biochemical analysis of cytosolic and nuclear extracts prepared form transfected cells (Supplemental Figure 3B).
The ability of 14-3-3 to affect MITF transcriptional activity was examined using transient transfection assays. RAW264.7 C4 cells were cotransfected with a luciferase reporter plasmid containing the TRAP promoter and FLAG-MITF, in the absence or presence of GST-14-3-3s (Figure 5E). These experiments demonstrated that 14-3-3 coexpression resulted in two- to three-fold repression of MITF-mediated activation of the TRAP promoter in a dose-dependent manner, whereas the 14-3-3 (dm) mutation had no effect on MITF transcriptional activity. As predicted, the MITF S173A mutation activated the reporter 2.5-fold more efficiently than wild-type MITF. These results were consistent with the model that 14-3-3 regulates MITF function by mediating cytoplasmic localization of the transcription factor.
Identification of C-TAK1 as Kinase Promoting MITF/14-3-3 Interaction
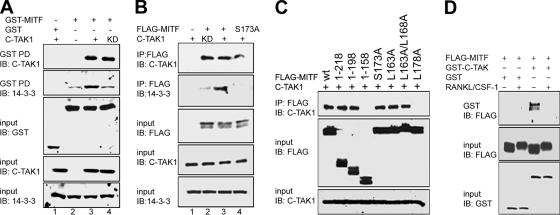
In a modified yeast-two hybrid screen designed to identify proteins that interact with the N-terminal domains of MITF, C-TAK1 was identified as a possible MITF partner (Mansky and Ostrowski, unpublished data). To verify the interaction between MITF and C-TAK1, GST pull-down assays were performed (Figure 6A). In the experiments, C-TAK1 as well as C-TAK1 KD interacted specifically with GST-MITF (Figure 6A, lane 3 and 4, top). In addition, significantly more 14-3-3 was consistently observed in the isolated complexes that contained kinase active C-TAK1 compared with the kinase-inactive form (Figure 6A, second panel). Complex formation between FLAG-MITF and C-TAK1 could also be detected in coimmunoprecipitation assays, and more endogenous 14-3-3 was again found in complex with MITF when kinase active C-TAK1 was coexpressed compared with the kinase-deficient form (Figure 6B, lane 2 vs. lane 3). The MITF-S173A mutation did not affect interaction with C-TAK1 in coimmunoprecipitation experiments, and endogenous 14-3-3 was not observed (Figure 6A, lane 4). These results are consistent with C-TAK1 as a protein kinase that can phosphorylate MITF and enhance interactions with 14-3-3, as has been reported for several other C-TAK1 substrates (Zhang et al., 1997; Peng et al., 1998; Muller et al., 2001, 2003).
Figure 6.
MITF interaction with C-TAK1 enhances formation of MITF/14-3-3 complex. (A) GST-MITF and C-TAK1 wt (+) or C-TAK1 kinase dead (KD) were coexpressed in COS-7 cells. GST-MITF was pulled down (GST PD), and complexes were analyzed by immunoblot. (B) FLAG-MITF or FLAG-MITF S173A, and C-TAK1 wt (+) or C-TAK1 kinase dead (KD) were coexpressed in COS-7. MITF was immunoprecipitated with anti FLAG antibody, and the IP complex was analyzed by immunoblot. (C) FLAG-MITF expression vector or various FLAG-MITF truncation and point mutations and C-TAK1 were coexpressed in COS-7 cells, as indicated. MITF was immunoprecipitated with anti-FLAG antibody, and the IP complex was analyzed by Western. Whole cell lysates were also immunoblotted with anti-FLAG and anti C-TAK-1 antibody (input control). (D) FLAG-MITF and GST-C-TAK were coexpressed in RAW264.7 C4 cells, either treated or not treated with CSF1/RANKL for 3 h, as indicated. GST-C-TAK1 was pulled down, and complexes were analyzed by immunoblot with anti-FLAG antibody. In the panels, whole cell lysates were also immunoblotted with anti-FLAG, anti C-TAK-1, or anti-14-3-3 antibody, as indicated (input controls).
A consensus sequence for recognition of substrates by C-TAK1 has been developed based on sequence alignment and mutagenesis studies (Supplemental Figure 4) (Muller et al., 2003). In particular, hydrophobic residues at position +5 relative to the phosphoserine residue have been implicated in substrate binding by C-TAK1 (Muller et al., 2003). MITF contains sequences with a relatively good fit to the consensus found in a region that overlaps with the 14-3-3 binding site, a common feature in these other C-TAK1 substrates. In pull-down assays with N-terminal–truncated forms of MITF, the region between residues 158–198 that contain this sequence were required for interaction with C-TAK1 (Figure 6C). The replacement of hydrophobic residue on +5-positions (MITF-L178A) abrogated C-TAK1-MITF association. Mutation of conserved leucine residues N-terminal to S173 (L163A and L158A) did not affect MITF/C-TAK1 complex formation.
As demonstrated above, CSF-1/RANKL signaling pathways regulate MITF interactions with 14-3-3 that promote cytoplasmic localization of the factor. To determine whether the MITF/C-TAK1 complex could also be regulated by CSF-1/RANKL signaling, RAW264.7 C4 cells were transiently cotransfected with GST-C-TAK1 and FLAG-MITF and treated with the two cytokines. After GST pull-down, MITF was detected using anti-FLAG antibody (Figure 6D). The association between GST C-TAK1 and wild-type MITF was detected in nontreated cells, but it was abolished in the presence of cytokines, suggesting that C-TAK1 may be negatively controlled by signals promoting differentiation of osteoclasts.
Dynamics of MITF Subcellular Localization and Interaction with 14-3-3 and C-TAK1 in a RAW264.7 C4 Cell Model
To define in detail the dynamics of MITF-14-3-3 interaction during osteoclast differentiation, we developed a model system in which RAW264.7 C4 cells stably expressed FLAG-tagged MITF (C4-MITF+ cells). We transduced FLAG-tagged MITF into these cells by using a retrovirus vector, and three clones that modestly overexpressed MITF 1.5- to 2-fold were selected for further analysis (our unpublished data). Recently, a novel role of MITF as an antiproliferative transcription factor was reported (Carreira et al., 2005). Consistent with these reports, we observed that C4-MITF+ cells had a reduced growth rate compared with control RAW264.7 C4 cells (C4-; Supplemental Figure 5A). However, the addition of RANKL/CSF-1 led to morphological changes characteristic of osteoclast differentiation (Supplemental Figure 5B), and quantification of multinuclear cells indicated that C4-MITF+ cells differentiated more rapidly than parental C4- cells, with >80% of cells multinucleated after 3 d of cytokine treatment (Supplemental Figure 5C). Increased expression of MITF regulated target genes associated with terminal osteoclast differentiation, including TRAP, OSCAR, and Cathepsin K was also observed in the C4-MITF+ cells (Supplemental Figure 5D). Results for one of the three cell clones are shown in Supplemental Figure 3, but identical results were obtained for all three cell clones with moderate 1.5- to 2-fold MITF overexpression (our unpublished data).
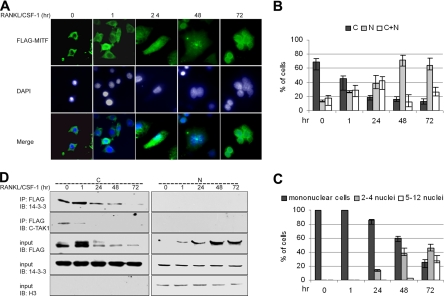
The C4-MITF+ model system was used to investigate the localization of FLAG-MITF during differentiation. Indirect immunofluorescence demonstrated that in cells grown without cytokines, FLAG-MITF localization was cytoplasmic and that treatment of cells with RANKL and CSF-1 resulted in nuclear accumulation of MITF (Figure 7, A and B), data consistent with the results obtained in primary cells (Figure 4). Three days after cytokine treatment FLAG-MITF was strictly in the nuclear compartment in >60% of cells, whereas in an additional 25% of cells FLAG-MITF was localized in both nucleus and cytoplasm (Figure 7B). Nuclear accumulation of FLAG-MITF correlates well with the extent of osteoclast differentiation (Figure 7C).
Figure 7.
14-3-3 binds to MITF in a localization- and differentiation-dependent manner. RAW C4 cells stably expressing FLAG-MITF (C4-MITF+) were left untreated or treated with RANKL/CSF-1 for the indicated times. (A) Immunolocalization of FLAG-MITF was monitored by anti-FLAG antibody. Representative photomicrograph images are presented. (B) Quantitative analysis of MITF localization. (C) Extent of differentiation, as measured by appearance of multinuclear cells. For B and C, experiments were performed three times each on three independently derived C4MITF+ cell clones, and a minimum of 100 cells were scored for each condition. Error bars indicate SD of the measurements. (D) Cytosolic and nuclear fractions of C4-MITF+ cells were prepared and anti-MITF complexes isolated and analyzed by immunoblot using anti-14-3-3 or anti C-TAK1 antibody. Nuclear and cytosolic fractions were also immunoblotted with anti-FLAG or anti-14-3-3 and anti-Histone H3 antibody. This experiment was repeated three times each on three independently derived C4MITF+ cell clones, and representative results for one cell clone are shown.
To confirm MITF localization and to analyze localization of the MITF/14-3-3 complex, cytosolic and nuclear fractions were prepared from C4-MITF+ cells treated with RANKL and CSF-1. These data confirmed the result of indirect immunofluorescence, showing that MITF shifted from predominantly cytoplasmic to nuclear location after cytokine treatment (Figure 7D). 14-3-3 was found associated with MITF primarily in the cytoplasm and was not detected in nuclear fraction. Similarly, C-TAK1 interacted with MITF in the cytoplasmic fraction of unstimulated cells and after 1 h of RANKL/CSF1 treatment, but not at times when MITF had relocalized to the nucleus. These data further support a role for C-TAK1 and 14-3-3 in maintaining MITF in the cytoplasm in the absence of differentiation signals.
DISCUSSION
MITF is involved in terminal differentiation of developmentally unrelated cell types, such as osteoclasts and melanocytes. Collaboration with lineage-specific transcription factors such as the ETS-transcription factor PU.1 defines one molecular mechanism by which MITF regulates osteoclast-specific target genes (Luchin et al., 2001). However, this mechanism raises a different problem in that mononuclear precursors of both macrophages and osteoclasts also express both MITF and PU.1. Understanding how interaction of these factors is regulated in closely related cell lineages to prevent the inappropriate expression of MITF target genes and osteoclast differentiation is a problem of general interest in organogenesis and differentiation of lineages from committed precursors. The results presented here indicate that interactions with the chaperone 14-3-3 proteins prevents accumulation of MITF in the nuclear compartment in osteoclast precursor cells, and that osteoclast-specific signaling mechanisms control 14-3-3 interactions with MITF.
Unexpectedly, MITF was observed to shuttle from the cytoplasm to the nucleus in a CSF-1/RANKL signaling-dependent manner during osteoclast differentiation. Although previous work had suggested that the molecular lesion present in the MITF-mi allele might affect nuclear localization of the encoded protein factor through disruption of a nuclear localization signal (Takebayashi et al., 1996), we and others have observed that MITF is found predominantly in the nucleus in osteoclast precursors and in other cell types (Hershey and Fisher, 2004). However, our results demonstrated that MITF nuclear accumulation depends on signaling by CSF-1 and RANKL in both primary osteoclast precursors and in the RAW264.7 C4 model. 14-3-3 was implicated in regulating MITF nuclear localization by several different lines of evidence obtained by independent experimental approaches, including biochemical fractionation studies over the course of osteoclast-like cell differentiation, the nuclear localization of the GFP-MITF S173A mutation, and 14-3-3 overexpression studies in which both nuclear localization and the transactivation potential of MITF were decreased. The data suggest a model in which 14-3-3 binding may regulate MITF nuclear localization in osteoclast precursors. Furthermore, the cytoplasmic MITF/14-3-3 complex might prevent degradation of MITF, allowing a pool of MITF to be readily available to respond to CSF-1/RANKL signals.
14-3-3 recognition of target proteins often depends on the phosphorylation status of a specific serine/threonine residue in the target, but this is not universally the case (Muslin et al., 1996; Tzivion and Avruch, 2002). Our studies are consistent with the phosphorylation of MITF as a requirement for 14-3-3 interaction, and strongly implicate pS173 as the critical residue for 14-3-3 binding. This site is in a context that does not fit precisely with the 14-3-3 binding consensus. However, the sequence ISNSCPANL that includes S173 is highly conserved in all identified MITF orthologues from human to zebrafish as well as in the relate bHLHZip factor TFE-3 (but not TFE-B or TFE-C), arguing for functional conservation of these sequences (Bronisz and Ostrowski, unpublished observation). It has recently been argued that 14-3-3 targets sites are in regions of proteins that are intrinsically disordered (Bustos and Iglesias, 2006). A disorder-to-order transition of the 14-3-3 binding partner is hypothesized to occur through hydrogen bonding interactions made with the phosphoryl group of the phosphoserine/phosphothreonine residue present in the target site, thus promoting complex formation. This may explain why 14-3-3 proteins are not able to bind to unphosphorylated targets (Bustos and Iglesias, 2006). Other residues in the consensus, for example the arginine or serine residues at the −3 or −2 position (relative to the phosphoserine residue), could have a stabilizing effect on the complex once formed, but they are likely not essential for triggering the transition. For MITF, a highly specific but low-affinity interaction with 14-3-3 may be critical for its biological role in osteoclast differentiation, providing the opportunity for precursors to respond quickly to extracellular cues in the bone microenvironment.
C-TAK1 is likely one kinase that promotes phosphodependent binding of MITF to 14-3-3 and thus promotes cytoplasmic localization of the factor. In unstimulated cells, C-TAK1–mediated phosphorylation of MITF at S173 seems to be required to maintain the cytoplasmic localization of MITF, a mechanism that is deactivated upon CSF-1/RANKL signaling. This leads to the hypothesis that C-TAK1 is inactivated by CSF-1/RANKL signaling, directly leading to destabilization of the MITF/14-3-3 complex and subsequent nuclear localization of MITF. The regulation of C-TAK1 activity by cytokines in different systems has been recently demonstrated; for example, cytokine activation of the kinase PIM-1 leads to phosphorylation and inactivation of C-TAK1 (Bachmann et al., 2004). Whether CSF-1/RANKL signaling promotes inactivation of C-TAK1 by a related mechanism involving phosphorylation of PIM-1, or whether additional mechanisms such as CSF-1/RANKL activation of protein phosphatases are involved, remains to be determined by future studies.
Mice doubly homozygous for both the Mitf hypomorphic allele vga-9 as well as a Tfe-3 knockout allele develop severe osteopetrosis, strongly indicating that these genes act redundantly during osteoclast differentiation (Hershey and Fisher, 2004). As mentioned, the region in MITF that interacts with 14-3-3 is well conserved within TFE-3, and TFE-3 has previously been identified as a 14-3-3 binding partner (Jin et al., 2004). Thus, TFE-3 may be regulated in a similar manner to MITF during osteoclast differentiation, but this hypothesis will need to be examined in future work.
Together, the data presented here demonstrate that interaction with 14-3-3 is a modification of MITF that can regulate subcellular localization of this factor, affecting the proliferation and differentiation of osteoclasts. This defines a previously unappreciated role for 14-3-3 in regulating cell lineage selection in committed myeloid precursor cells. Regulation of MITF by this type of posttranslational mechanism would allow precursor cells to respond rapidly to signals generated from osteoblasts or other stromal cells in the bone microenvironment to promote dynamic bone remodeling. Deregulation of these molecular interactions is likely to contribute to the uncoupling of osteoblast–osteoclast communication, thus contributing to common human bone disorders.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge David Hume and A. Ian Cassady for the gift of the RAW264.7 C4 cell clones and human recombinant CSF-1. This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-0447129 (to M.C.O.).
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0470) on July 5, 2006.
REFERENCES
- Aitken A., Howell S., Jones D., Madrazo J., Martin H., Patel Y., Robinson K. Post-translationally modified 14-3-3 isoforms and inhibition of PKC. Mol. Cell Biochem. 1995;149–150:41–49. doi: 10.1007/BF01076562. [DOI] [PubMed] [Google Scholar]
- Andrin C., Hendzel M. J. F-actin-dependent insolubility of chromatin-modifying components. J. Biol. Chem. 2004;279:25017–25023. doi: 10.1074/jbc.M401805200. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Hennemann H., Xing P. X., Hoffmann I., Moroy T. The oncogenic serine/threonine kinase Pim-1 phosphorylates and inhibits the activity of Cdc25C-associated kinase 1 (C-TAK1): a novel role for Pim-1 at the G2/M cell cycle checkpoint. J. Biol. Chem. 2004;279:48319–48328. doi: 10.1074/jbc.M404440200. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Simonet W. S., Lacey D. L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Bustos D. M., Iglesias A. A. Intrinsic disorder is a key characteristic in partners that bind 14-3-3 proteins. Proteins. 2006;63:35–42. doi: 10.1002/prot.20888. [DOI] [PubMed] [Google Scholar]
- Cahill C. M., Tzivion G., Nasrin N., Ogg S., Dore J., Ruvkun G., Alexander-Bridges M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J. Biol. Chem. 2001;276:13402–13410. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- Carr C. S., Sharp P. A. A helix-loop-helix protein related to the immunoglobulin E box-binding proteins. Mol. Cell Biol. 1990;10:4384–4388. doi: 10.1128/mcb.10.8.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira S., Goodall J., Aksan I., La Rocca S. A., Galibert M. D., Denat L., Larue L., Goding C. R. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005;433:764–769. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
- Chow C. W., Dong C., Flavell R. A., Davis R. J. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol. Cell Biol. 2000;20:5227–5234. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A. L., Sundwall E., Lin M., Sullivan A. A., Ayer D. E. A novel heterodimerization domain, CRM1, and 14-3-3 control subcellular localization of the MondoA-Mlx heterocomplex. Mol. Cell Biol. 2002;22:8514–8526. doi: 10.1128/MCB.22.24.8514-8526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Subramanian R. R., Masters S. C. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Gu Y. M., Jin Y. H., Choi J. K., Baek K. H., Yeo C. Y., Lee K. Y. Protein kinase A phosphorylates and regulates dimerization of 14-3-3 epsilon. FEBS Lett. 2006;580:305–310. doi: 10.1016/j.febslet.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Hall T. J., Chambers T. J. Molecular aspects of osteoclast function. Inflamm. Res. 1996;45:1–9. doi: 10.1007/BF02263497. [DOI] [PubMed] [Google Scholar]
- Hallberg B. Exoenzyme S binds its cofactor 14-3-3 through a non-phosphorylated motif. Biochem. Soc. Trans. 2002;30:401–405. doi: 10.1042/bst0300401. [DOI] [PubMed] [Google Scholar]
- Hemesath T. J., Price E. R., Takemoto C., Badalian T., Fisher D. E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- Hershey C. L., Fisher D. E. Mitf and Tfe 3, members of a b-HLH-ZIP transcription factor family essential for osteoclast development and function. Bone. 2004;34:689–696. doi: 10.1016/j.bone.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrimsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Jin J., et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr. Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Luchin A., Suchting S., Merson T., Rosol T. J., Hume D. A., Cassady A. I., Ostrowski M. C. Genetic and physical interactions between Microphthalmia transcription factor and PU. 1 are necessary for osteoclast gene expression and differentiation. J. Biol. Chem. 2001;276:36703–36710. doi: 10.1074/jbc.M106418200. [DOI] [PubMed] [Google Scholar]
- Mansky K. C., Sankar U., Han J., Ostrowski M. C. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-kappa B ligand signaling. J. Biol. Chem. 2002;277:11077–11083. doi: 10.1074/jbc.M111696200. [DOI] [PubMed] [Google Scholar]
- Merla G., Howald C., Antonarakis S. E., Reymond A. The subcellular localization of the ChoRE-binding protein, encoded by the Williams- Beuren syndrome critical region gene 14, is regulated by 14-3-3. Hum. Mol. Genet. 2004;13:1505–1514. doi: 10.1093/hmg/ddh163. [DOI] [PubMed] [Google Scholar]
- Muller J., Ory S., Copeland T., Piwnica-Worms H., Morrison D. K. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell. 2001;8:983–993. doi: 10.1016/s1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Muller J., Ritt D. A., Copeland T. D., Morrison D. K. Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. EMBO J. 2003;22:4431–4442. doi: 10.1093/emboj/cdg426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin A. J., Tanner J. W., Allen P. M., Shaw A. S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Muslin A. J., Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12:703–709. doi: 10.1016/s0898-6568(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Peng C. Y., Graves P. R., Ogg S., Thoma R. S., Byrnes M. J., 3rd, Wu Z., Stephenson M. T., Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- Price E. R., Ding H. F., Badalian T., Bhattacharya S., Takemoto C., Yao T. P., Hemesath T. J., Fisher D. E. Lineage-specific signaling in melanocytes. C-kit stimulation recruits p300/CBP to microphthalmia. J. Biol. Chem. 1998;273:17983–17986. doi: 10.1074/jbc.273.29.17983. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., Elliott J., Gillespie M. T., Martin T. J. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–4427. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- Shen Y. H., Godlewski J., Bronisz A., Zhu J., Comb M. J., Avruch J., Tzivion G. Significance of 14-3-3 self-dimerization for phosphorylation-dependent target binding. Mol. Biol. Cell. 2003;14:4721–4733. doi: 10.1091/mbc.E02-12-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi K., Chida K., Tsukamoto I., Morii E., Munakata H., Arnheiter H., Kuroki T., Kitamura Y., Nomura S. The recessive phenotype displayed by a dominant negative microphthalmia-associated transcription factor mutant is a result of impaired nucleation potential. Mol. Cell Biol. 1996;16:1203–1211. doi: 10.1128/mcb.16.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivion G., Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- Tzivion G., Luo Z., Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- van Hemert M. J., Niemantsverdriet M., Schmidt T., Backendorf C., Spaink H. P. Isoform-specific differences in rapid nucleocytoplasmic shuttling cause distinct subcellular distributions of 14-3-3 sigma and 14-3-3 zeta. J. Cell Sci. 2004;117:1411–1420. doi: 10.1242/jcs.00990. [DOI] [PubMed] [Google Scholar]
- Wei G., Guo J., Doseff A. I., Kusewitt D. F., Man A. K., Oshima R. G., Ostrowski M. C. Activated Ets2 is required for persistent inflammatory responses in the motheaten viable model. J. Immunol. 2004;173:1374–1379. doi: 10.4049/jimmunol.173.2.1374. [DOI] [PubMed] [Google Scholar]
- Yang B. S., Hauser C. A., Henkel G., Colman M. S., Van Beveren C., Stacey K. J., Hume D. A., Maki R. A., Ostrowski M. C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol. Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. H., Kobayashi R., Graves P. R., Piwnica-Worms H., Tonks N. K. Serine phosphorylation-dependent association of the band 4.1-related protein-tyrosine phosphatase PTPH1 with 14-3-3beta protein. J. Biol. Chem. 1997;272:27281–27287. doi: 10.1074/jbc.272.43.27281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.