Abstract
Angiotensin II (AngII) type 1 receptors (AT1) regulate cell growth through the extracellular signal-regulated kinase (ERK)1/2 and phosphatidylinositol 3-kinase (PI3K) pathways. ERK1/2 and Akt/protein kinase B, downstream of PI3K, are independently activated but both required for mediating AngII-induced proliferation when expressed at endogenous levels. We investigate the effect of an increase in the expression of wild-type Akt1 by using Chinese hamster ovary (CHO)-AT1 cells. Unexpectedly, Akt overexpression inhibits the AT1-mediated proliferation. This effect could be generated by a cross-talk between the PI3K and ERK1/2 pathways. A functional partner is the phosphoprotein enriched in astrocytes of 15 kDa (PEA-15), an Akt substrate known to bind ERK1/2 and to regulate their nuclear translocation. We report that Akt binds to PEA-15 and that Akt activation leads to PEA-15 stabilization, independently of PEA-15 interaction with ERK1/2. Akt cross-talk with PEA-15 does not affect ERK1/2 activation but decreases their nuclear activity as a result of the blockade of ERK1/2 nuclear accumulation. In response to AngII, PEA-15 overexpression displays the same functional consequences on ERK1/2 signaling as Akt overactivation. Thus, Akt overactivation prevents the nuclear translocation of ERK1/2 and the AngII-induced proliferation through interaction with and stabilization of endogenous PEA-15.
INTRODUCTION
Proliferation is controlled by many cellular signaling pathways involving several serine/threonine kinase cascades, including the phosphatidylinositol 3-kinases (PI3K)/Akt and the mitogen-activated protein kinase (MAPK) pathways. Akt, also known as protein kinase B, is a major downstream target of PI3K activated in response to various stimuli, growth factors, and hormones. The amino acid sequence analysis of Akt reveals an N-terminal region with homology to a modular domain termed the pleckstrin homology (PH) domain. Binding of PI3K phospholipid products to the PH domain of Akt results in transient translocation of Akt to the plasma membrane, where it is activated by phosphorylation through upstream kinases such as the phosphoinositide-dependent kinase-1. Once activated, Akt phosphorylates many cytosolic and nuclear substrates that are involved in numerous cellular responses, including promotion of cell survival, control of cell cycle progression, and regulation of cell growth.
Other key mediators of growth are the extracellular signal-regulated kinases (ERKs) 1/2. ERK1/2 belong to the MAPK family and lie downstream of the cascade of Ras/Raf/MEK kinases. The nuclear translocation of ERK1/2 is a critical step to transduce cell growth (Brunet et al., 1999). In their phosphorylated and activated forms, ERK1/2 transmit extracellular stimuli from the plasma membrane to the nucleus by phosphorylating and activating a variety of transcription factors. Among them, Elk-1 is a key element involved in the induction of immediate early genes such as cFos (Pearson et al., 2001; Peyssonnaux and Eychene, 2001).
The stimulation of both pathways by many common ligands raises the possibility that cross-talk between the PI3K/Akt and ERK1/2 pathways could play a major role in regulating cell proliferation under particular conditions. Functional interactions between these two pathways have been reported for the regulation of various cellular functions depending on cell types. Indeed, constitutively active Akt can negatively regulate the Ras/Raf/MEF/ERK1/2 cascade via phosphorylation and inactivation of the kinase Raf (Zimmermann and Moelling, 1999), leading to the phenotype modulation of differentiated myotubes (Rommel et al., 1999) or of vascular smooth muscle cells (Reusch et al., 2001). An additional level of interaction between Akt and the ERK1/2 pathway has been reported downstream of Ras, Raf, and MEK that involves the down-regulation of the transcription factor Elk-1 (Figueroa and Vojtek, 2003; Galetic et al., 2003). To date, the molecular mechanisms and the functional cellular consequences of this cross-talk remain poorly investigated.
A good candidate for mediating direct cross-talk between Akt and ERK1/2 is the phosphoprotein enriched in astrocytes of 15 kDa, PEA-15 (also called PED). PEA-15 is a small protein abundantly present in brain astrocytes (Araujo et al., 1993) but also widely expressed in other human tissues (Danziger et al., 1995; Estelles et al., 1996; Ramos et al., 1998). PEA-15 regulates multiple cellular functions (Renault et al., 2003), including Ras suppression of integrin activation, and protection against Fas- and tumor necrosis factor α-induced apoptosis (Condorelli et al., 1999; Estelles et al., 1999; Kitsberg et al., 1999). Besides, we have already demonstrated that PEA-15 binds to ERK1/2 and prevents their nuclear translocation, which results in blockade of ERK-dependent transcription and cell proliferation in response to serum (Formstecher et al., 2001). More recently, PEA-15 has been identified as a novel Akt substrate (Trencia et al., 2003). These authors reported that phosphorylation by Akt of PEA-15 on Ser116 determines resistance to apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand or serum deprivation. However, the balanced cross-talk between Akt, PEA-15 and ERK1/2 as well as its consequences on ERK1/2 subcellular localization and cell responses (proliferation/survival) has not been defined.
We have previously reported that angiotensin II (AngII) stimulates cell proliferation through the angiotensin type 1A receptor (AT1A) by activation of the endogenous PI3K and ERK1/2 pathways in transfected Chinese hamster ovary (CHO) cells (CHO-AT1A) and in rat aortic smooth muscle cells (Dugourd et al., 2003). In this process, endogenous Akt and ERK1/2 were independently stimulated but were both necessary for AngII-induced cell proliferation. Therefore, changes in the balance of activation between Akt and ERK1/2 could generate new cross-talk, possibly through PEA-15, and have drastic functional consequences.
The aim of this study was to analyze the effects of modifying the balance between the PI3K/Akt and ERK1/2 pathways on the proliferative response to AngII by possible cross-talk through recruitment of endogenous PEA-15. We found that Akt1 overexpression in CHO-AT1A decreased cell proliferation induced by AngII. Investigating the molecular mechanisms lying between Akt and PEA-15 revealed that Akt bound to PEA-15 and that their binding did not require interaction of PEA-15 with ERK1/2. Furthermore, both ERK1/2 binding to PEA-15 and Akt activation elevated PEA-15 half-life and its protein level. Stabilization of endogenous PEA-15 by overexpressed Akt resulted in similar functional effects than overexpression of PEA-15 itself, namely, inhibition of Elk-1–dependent transcription and cFos induction. This mechanism due to the blockade of ERK nuclear accumulation led to the inhibition of AngII-induced proliferation. Importantly, the use of PEA-15 antisense counteracting endogenous PEA-15 stabilization upon Akt overactivation increased cFos induction. Together, these results demonstrate the important role of Akt/PEA-15 cross-talk in controlling ERK1/2 nuclear activity.
MATERIALS AND METHODS
cDNA Constructs, Antibodies, and Reagents
Hemagglutinin (HA)-tagged human Akt1 and FLAG-tagged human PEA-15 were cloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA). Green fluorescent protein (GFP)-PEA-15, glutathione S-transferase (GST)-PEA-15, and similar constructions with a D74A single-point mutant of PEA-15, called GFP-D74A or GST-D74A, were made as described previously (Formstecher et al., 2001). PEA-15 antisense (5′-TGACGCCTCTGGAGCTGAGC-3′) and mock (5′-GACATGGCCTTGCACGCGTG-3′) oligonucleotides were a kind gift from Prof. Francesco Beguinot (University of Naples, Naples, Italy). The antibodies directed against the following molecules were used: polyclonal anti-ERK1/2, monoclonal anti-phospho-ERK1/2, and polyclonal anti-glycogen synthase kinase (GSK)3α antibodies from Upstate Biotechnology (Charlottesville, VA); polyclonal anti-phospho-GSK3α/β (Ser21/9) antibody, polyclonal anti-Akt, and polyclonal and monoclonal anti-phospho-Ser473 Akt antibodies from Cell Signaling Technology (Beverly, MA); polyclonal anti-HA, anti-ERK1, and anti-cFos antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal anti-GFP and anti-HA antibodies from Roche Molecular Biochemicals (Mannheim, Germany); monoclonal M2 anti-FLAG antibody from Sigma-Aldrich (St. Louis, MO); and monoclonal anti-Smad2 antibody from BD Biosciences (Palo Alto, CA). The polyclonal antibody directed against PEA-15 was described previously (Formstecher et al., 2001). AngII and IGF-1 were obtained from Sigma-Aldrich. LY294002 and U0216 were obtained from Calbiochem (San Diego, CA) and Promega (Madison, WI), respectively.
Cell Culture and Establishment of Stable Cell Lines
CHO-AT1A cells, stably expressing the rat AT1A receptor (Teutsch et al., 1992) were maintained at 37°C in 5% CO2, under 750 μg/ml G418 selection (Invitrogen) in Ham’s F-12 medium (Invitrogen) supplemented with 7.5% fetal calf serum, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Roche Molecular Biochemicals). CHO-AT1A cells were stably or transiently transfected using the LipofectAMINE 2000 agent according to the manufacturer’s recommendations (Invitrogen). For establishment of stable clones, HA-Akt1, GFP, GFP-PEA-15, and GFP-D74A and FLAG-PEA-15–transfected CHO-AT1A cell lines were cultivated under 500 μg/ml hygromycin selection (Invitrogen) and cloned either by limiting dilution for HA-Akt1 or by fluorescence-activated cell sorting for the GFP-containing constructs. Clones were then screened by immunoblotting and by immunofluorescence to select cells with homogenous expression. Two independent clones were analyzed for each construct. NCI H295R cells were generously provided by Dr. Alessandro Capponi (Faculty of Medicine, University of Geneva, Geneva, Switzerland). Cells were grown at 37°C in 5% CO2 in DMEM/Ham’s F-12 containing 1% ITS (BD Biosciences), 2% UltroSer SF (Ciphergen, Fremont, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone. Transfection of PEA-15 antisense or mock DNAs in H295R cells was performed using the jetPEI reagent according to the manufacturer’s recommendations (Polyplus transfection, Illkirch, France).
Immunoblotting
Before agonist stimulation, cells were maintained for 4 h of serum starvation. Cell lysates were prepared with 1% SDS containing 1 mM Na3VO4 and 10 mM β-glycerophosphate or with ice-cold 1% Nonidet P-40 in 50 mM Tris-HCl, pH 7.4, containing 120 mM NaCl, 1 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were then subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed overnight with primary antibody at 4°C. After incubation with peroxidase- or alkaline phosphatase-linked secondary antibodies, immunoreactive proteins were visualized by ECL reagent (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) or nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Promega) substrates, respectively. If necessary, quantitative analysis was performed using QuantityOne software (Bio-Rad, Hercules, CA).
Proliferation Assays
Cell growth was arrested by 48 h of serum starvation. For DNA synthesis quantification, [3H]thymidine incorporation experiments were performed as described previously (Dugourd et al., 2003). After G0 arrest, cells were stimulated with 100 nM AngII for 16 h and labeled with 1 μCi/ml [3H]thymidine for 2 h. After washing with trichloroacetic acid, radioactivity was measured by liquid scintillation counting. For cell number quantification, the Cell Titer 96 Aqueous NonRadioactive Cell Proliferation Assay (Promega) was used. After G0 arrest, cells were stimulated with 100 nM AngII for 24 h. After 1 h of incubation with a phenazine methosulfate/3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium salt (MTS) mix, the absorbance was measured at 490 nm.
Transcription Assay
Elk-1 transcription assay was performed as described previously (Inman et al., 2002). Eighty percent confluent cells were cotransfected with 150 ng of Nlex.ElkC, 750 ng of Lex-Op-luciferase reporter, and 100 ng of β-galactosidase by using the LipofectAMINE 2000 agent. Nlex.ElkC encodes a fusion protein of the LexA DNA binding domain fused to the activation domain of Elk-1. Lex-Op-Luciferase has the luciferase reporter gene under the control of a synthetic promoter containing two repeats of the LexA operator. The β-galactosidase plasmid was cotransfected to normalize the transfection efficiency. Cells were serum starved for 4 h and stimulated with 100 nM AngII for 6 h. Elk-1 transcription was measured by the expression of active luciferase using the Promega luciferase assay system according to the manufacturer’s instructions. β-Galactosidase activity was assayed by adding the substrate chlorophenol-β-d-galactopyranoside to 20 μl of cell lysate and incubating at 37°C before measuring at 595 nm.
Immunofluorescence
CHO-AT1A cells stably expressing HA-Akt1, GFP-PEA-15 or GFP-D74A were seeded on 14-mm coverslips and cultivated for 40–48 h before analysis. In some experiments, 300,000 CHO-AT1A cells stably expressing HA-Akt1 were transiently transfected with GFP-PEA-15 and seeded 24 h later on 14-mm coverslips. After cell growth arrest induced by 16-h serum starvation, cells were stimulated by 100 nM AngII for 3 h in the presence or absence of the PI3K inhibitor LY294002 (10 μM). Cells were then fixed with ice-cold methanol for 3.5 min and washed with phosphate-buffered saline (PBS). Nonspecific binding was saturated with 10% normal goat serum (NGS) in PBS. Cells were then incubated for 2 h at room temperature with the polyclonal anti-ERK1 antibody in 1% NGS in PBS. After PBS washes, cells were incubated for 1 h at room temperature with a goat anti-rabbit secondary antibody coupled to Alexa Fluor-555 (Invitrogen). In some experiments, double immunostaining was performed by incubating the cells with the monoclonal anti HA-antibody in 1% NGS in PBS for 1 h at room temperature. After PBS washes, cells were incubated with a goat anti-mouse secondary antibody coupled to Alexa Fluo-647 (Invitrogen). Coverslips were washed with PBS and mounted with Mowiol (Sigma-Aldrich) for confocal microscopy.
Cells were examined with a Leica TCS SP2 confocal microscope (Leica Microsystems, Deerfield, IL). For double or triple detection of Alexa Fluor-555, Alexa Fluor-647, and GFP signal, fluorescence was analyzed in sequential scanning mode. Images (1024 × 1024 pixels) were obtained with an 63× oil immersion objective lens. Each confocal image corresponded to the medium level of the cells.
Metabolic Labeling and Immunoprecipitation
CHO-AT1A cells (150,000) stably expressing GFP-PEA-15 or GFP-D74A were seeded in 12-well plates. Forty hours later, cells were growth arrested by 4-h serum starvation and then labeled for 20 min with 100 μCi/ml [35S]methionine and cysteine (pulse) (ProMix; GE Healthcare). Cells were stimulated with 10 nM insulin-like growth factor (IGF)-1 for various times (0, 4, 15, 25, and 40 h) in the presence or absence of 10 μM LY294002 (chase) and extracted on ice with 1% Nonidet P-40 in 50 mM Tris-HCl, pH 7.4, containing 120 mM NaCl, 1 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, and 0.5 mM PMSF. Lysates were centrifuged for 10 min at 13,000 rpm at 4°C. The supernatants were then incubated for 2 h with anti-GFP antibody, and the immune complexes were precipitated using protein G coupled to Sepharose (GE Healthcare). Immunoprecipitated proteins were resolved by SDS-PAGE. Gels were dried and exposed to a Biomax film (Eastman Kodak, Rochester, NY). In some experiments, 600,000 CHO-AT1A cells were transiently transfected with FLAG-PEA-15 and split 24 h later into 12-well plates. Cells were then processed for metabolic labeling as described above with different times for the chase (0, 4, 8, 16, and 24 h). Immunoprecipitation of FLAG-PEA-15 was performed using a polyclonal anti-PEA-15 antibody. To measure the degradation rate of PEA-15, quantitative analysis of radiolabeled material was performed using an FX Molecular Imager and QuantityOne software (Bio-Rad). Protein half-life was calculated from each degradation–chase time curve by linear regression analysis.
In Vitro Binding Assays
Using the plasmid pcDNA3.1-HA-Akt1, [35S]methionine-radiolabeled proteins were synthesized in a coupled in vitro transcription/translation reaction (TnT T7; Promega). Radiolabeled proteins (2 μl of 50 μl of translation reaction) were incubated with 1 μg of GST-PEA-15, 1 μg of GST-D74A, or 1 μg of GST alone (negative control) adsorbed on glutathione-Sepharose beads as described previously (Formstecher et al., 2001). Proteins were resolved by SDS-PAGE. Gels were dried and exposed to a Biomax film (Eastman Kodak). Radiolabeled HA-Akt was detected using an FX Molecular Imager (Bio-Rad). Alternatively, cell lysates of CHO-AT1A cells stably expressing HA-Akt1 were prepared with 1% Nonidet P-40. Lysates (500 μg) were incubated with 50 μl of Sepharose beads bound to the GST constructs described above (∼2 μg) for 2 h at 4°C. Proteins were resolved by SDS-PAGE, transferred to PVDF membranes, and processed by standard immunoblotting technique with polyclonal anti-HA antibody.
Statistical Analysis
Quantitative data were expressed as mean ± SEM and compared using a one-way analysis of variance followed by intergroup comparisons with a Student’s t test. p < 0.05 was considered statistically significant.
RESULTS
Akt Overexpression Decreases Cell Proliferation Induced by AngII
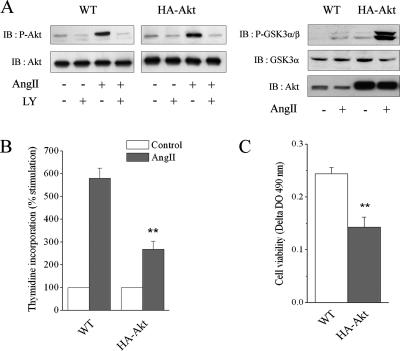
We have previously shown that endogenous PI3K/Akt and ERK1/2 pathways are both necessary for mediating proliferation in CHO cells stably expressing the rat type 1 AngII receptor (CHO-AT1A) (Dugourd et al., 2003). The modification of the precise balance between these two endogenous pathways remains poorly investigated even though it could lead to dramatic changes in the cellular response. For this purpose, we stably transfected the CHO-AT1A cell line with human recombinant HA-tagged Akt1. Twenty-four clones expressing various levels of HA-Akt1 were isolated and analyzed. As shown in Figure 1A (right), one representative cell line of this clone expressed a 10-fold increase in Akt level compared with the endogenous level detected in wild-type CHO-AT1A cells. When an equivalent amount of total Akt was loaded, CHO-AT1A cells stably expressing Akt1 showed a similar level of Akt phosphorylation to wild-type CHO-AT1A cells upon AngII stimulation (Figure 1A, left), indicating an overall increase of Akt activation in response to AngII in cells overexpressing Akt. In both cell lines, phosphorylated Akt was inhibited by the PI3K inhibitor LY294002 (Figure 1A, left) but unaffected by the MEK inhibitor U0126 (our unpublished data), as already shown for endogenous Akt (Dugourd et al., 2003). These results indicate that Akt overexpression led to an increased Akt activation upon AngII stimulation that is PI3K dependent and MEK/ERK1/2 independent. In CHO-AT1A overexpressing Akt, the increased level of AngII-induced Akt phosphorylation was correlated with an enhanced ability of Akt to phosphorylate its cytosolic substrates, GSK3α and -β (Figure 1A, right). Thus, Akt overexpression in CHO-AT1A cells correlated with an enhanced level of Akt activity upon AngII stimulation.
Figure 1.
Akt overexpression decreases cell proliferation induced by AngII. CHO-AT1A cells (WT) or stable clone overexpressing HA-Akt1 were used. (A) Cells were treated or not with 10 μM LY294002 for 30 min and stimulated with 100 nM AngII for 10 min. Cell lysates were prepared with 1% SDS. Proteins (50 and 25 μg) from WT cells were separated by SDS-PAGE and immunoblotted with anti-phospho-Ser473 Akt or anti-Akt antibody. To load equivalent level of total Akt, 10-fold less protein (5 and 2.5 μg) from HA-Akt cells was resolved using the same procedure (left). Cells were stimulated with 100 nM AngII for 10 min, and cell lysates were prepared with 1% SDS. Proteins (40, 20, and 30 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-GSK3α/β, anti-GSK3α, or anti-Akt antibody, respectively (right). (B) [3H]Thymidine incorporation was assessed in cells stimulated with 100 nM AngII for 16 h (AngII). The results are expressed as percentage of unstimulated value (control), set as 100%. (C) Cell number increase was assessed in cells stimulated with 100 nM AngII for 24 h using an MTS cell proliferation assay. The results are expressed as corrected 490-nm absorbance from which the values of unstimulated cells were subtracted. For B and C, data show mean ± SEM values of at least five independent experiments performed in triplicate. **p < 0.01 versus WT cells.
The influence of Akt overactivation on cell proliferation was investigated using two approaches: measurement of DNA synthesis by [3H]thymidine incorporation and determination of the cell number by MTS assay. Increased Akt expression did not affect basal cell proliferation, as demonstrated by the similar level of thymidine incorporation in wild-type and Akt-overexpressing CHO-AT1A cells under serum deprivation (2925 ± 509 and 2951 ± 618 cpm, respectively, n = 7, NS). Similarly, basal cell viability was unaffected by Akt overexpression (OD levels at 490 nm: 0.349 ± 0.026 and 0.383 ± 0.048 in wild-type and Akt-overexpressing CHO-AT1A, respectively, n = 5, NS). On AngII stimulation, Akt overexpression surprisingly reduced DNA synthesis (54% reduction; p < 0.01) as well as cell number (41% reduction; p < 0.01) compared with wild-type cells (Figure 1, B and C). This effect was not clone specific, because similar results were obtained with another independent clone overexpressing Akt (our unpublished data). Thus, our results demonstrate that overactivation of Akt decreased the proliferative response mediated by the AngII AT1 receptor.
PEA-15 Directly Binds Akt Independently of Its Binding to ERK1/2
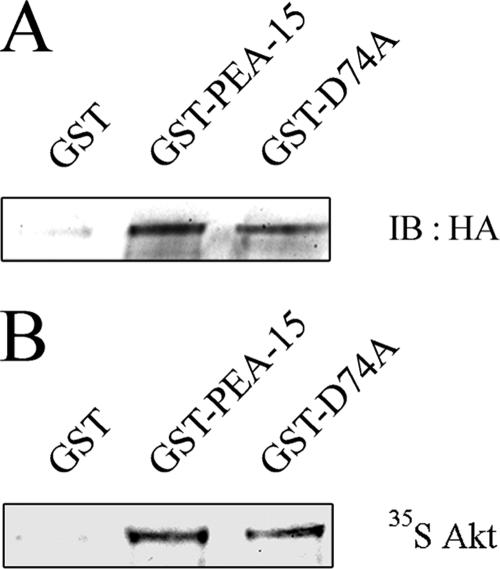
We previously reported that PEA-15 blocks serum-induced proliferation through its binding with ERK1/2 and the prevention of ERK1/2 nuclear localization (Formstecher et al., 2001). More recently, PEA-15 was identified as an Akt substrate (Trencia et al., 2003). To elucidate the molecular mechanisms leading to the inhibition of cell proliferation induced by Akt overexpression, we investigated possible cross-talk between the PI3K/Akt and the ERK1/2 signaling pathways. We first tested the interaction between Akt and PEA-15 by using pull-down assay. Lysates from CHO-AT1A cells overexpressing HA-Akt1 were incubated with GST-PEA-15 fusion protein. Bound proteins were blotted with anti-HA antibody. Recombinant HA-Akt1 specifically bound GST-PEA-15 but not GST alone (Figure 2A). Furthermore, 35S in vitro-labeled HA-Akt1 bound to immobilized GST-PEA-15 fusion protein but not to GST alone, demonstrating a direct interaction between Akt and PEA-15 (Figure 2B). To examine whether this interaction required ERK1/2 binding to PEA-15, the same experiments were performed with the D74A mutant of PEA-15. We have previously shown that this mutation of an aspartate in the death effector domain of PEA-15 abrogates its binding to ERK1/2 (Formstecher et al., 2001), although this mutant possesses the same structure as wild-type PEA-15 protein (Hill et al., 2002). Similar to wild-type PEA-15, the D74A mutant was able to interact with HA-Akt1 using either cell lysates or 35S in vitro-labeled HA-Akt1 (Figure 2, A and B). These results demonstrate the direct binding of PEA-15 to Akt. Furthermore, we show for the first time that this interaction did not require ERK1/2 binding to PEA-15.
Figure 2.
PEA-15 directly binds Akt independently of its binding to ERK1/2. (A) Cell lysates were prepared from CHO-AT1A cells overexpressing HA-Akt1 grown in complete medium and incubated with GST-PEA-15, GST-D74A or GST-coated beads. Bound Akt was visualized with anti-HA antibody. (B) In vitro-translated [35S]methionine-radiolabeled HA-Akt1 was incubated with immobilized GST-PEA-15–, GST-D74A–, or GST-coated beads. Bound proteins were separated by SDS-PAGE and detected by autoradiography.
Endogenous Active Akt and ERK1/2 Binding to PEA-15 Both Increase PEA-15 Half-Life
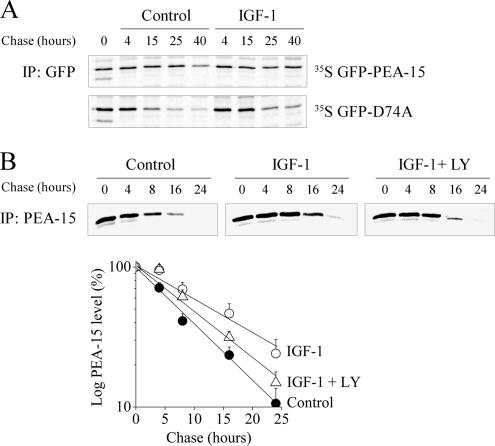
To analyze the role of Akt binding to PEA-15 on its stability, the degradation rate of PEA-15 was measured in CHO-AT1A cell line stably expressing GFP-PEA-15, in the presence or absence of IGF-1. IGF-1 is a potent and long-lasting activator of the PI3K/Akt pathway in our cell system. On IGF-1 stimulation, the half-life of GFP-PEA-15 increased: after a 40-h chase, PEA-15 was clearly detectable, whereas it was weakly present under serum starvation (Figure 3A, top). To test whether ERK1/2 binding was also required for the stabilization of PEA-15, the same experiments were performed in CHO-AT1A cell line stably expressing the GFP-D74A mutant. In serum-starved cells, GFP-D74A displayed a shorter half-life than wild-type GFP-PEA-15, because it was weakly present after a 15-h chase (Figure 3A, bottom). As for wild-type PEA-15, IGF-1 stimulation increased GFP-D74A half-life as demonstrated by the clear band remaining visible after a 15-h chase. Because GFP tag was rather bulky compared with PEA-15 and could thus affect the regulation of PEA-15 stability, we performed similar experiments in CHO-AT1A transiently expressing FLAG-PEA-15.
Figure 3.
Active Akt increases PEA-15 half-life independently of its binding to ERK1/2. (A) Stable clones of CHO-AT1A cells expressing GFP-PEA-15 or GFP-D74A were metabolically labeled for 30 min and chased for the indicated time periods in serum-free medium (control) or in the presence of 10 nM IGF-1. PEA-15 and D74A were immunoprecipitated with anti-GFP antibody. Immunoprecipitates were separated by SDS-PAGE and radiolabeled proteins were detected by autoradiography. (B) Top, CHO-AT1A cells transiently transfected with FLAG-PEA-15 were metabolically labeled as described in A, in serum-free medium, or in the presence of 10 nM IGF-1 with or without 10 μM LY294002. FLAG-PEA-15 was immunoprecipitated with anti-PEA-15 antibody and detected as described in A. Bottom, FLAG-PEA-15 half-life was quantified as described in Materials and Methods. Data are expressed as mean ± SEM of eight independent experiments.
The half-life of FLAG-PEA-15 under serum starvation was reduced compared with GFP-PEA-15 under the same conditions. Despite its shorter half-life, FLAG-PEA-15 displayed the same regulation upon IGF-1 stimulation as GFP-PEA-15, i.e., stabilization of PEA-15 (Figure 3B, top). Quantitative analysis was performed on eight independent experiments. The half-life of FLAG-PEA-15 increased from 7.5 h to 14 h upon IGF-1 stimulation (Figure 3B, bottom). In addition, IGF-1-induced increase of FLAG-PEA-15 half-life was partially abolished after LY294002 pretreatment (half-life of 10 h), demonstrating the involvement of active Akt in regulating PEA-15 stability. Together, these results show that endogenous PI3K-dependent Akt phosphorylation and ERK1/2 binding to PEA-15 exerted additive effects on PEA-15 protein stability.
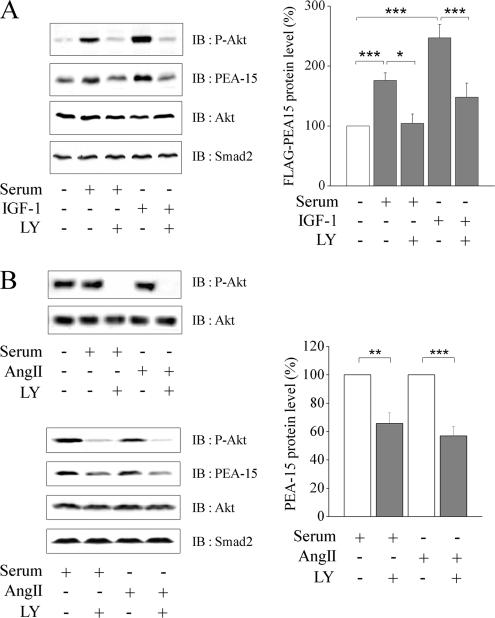
We then analyzed whether this increase of PEA-15 half-life had a detectable consequence on its expression level. In CHO-AT1A cell line stably expressing FLAG-PEA-15, PEA-15 protein levels were significantly higher under serum or IGF-1 exposure than under serum starvation (Figure 4A). These increases were associated with Akt phosphorylation as shown on Figure 4A (left). In addition, IGF-1– or serum-induced increase of PEA-15 level was reversed by LY294002 pretreatment. These results indicate that the active form of Akt was associated with the stabilization of PEA-15 and subsequently with an increase of its intracellular protein content. We then investigated the cellular relevance of the overactivation of endogenous Akt on endogenous PEA-15 protein level. For this purpose, we used a human adrenocortical carcinoma cell line H295R known to activate several signaling pathways through the AngII AT1 receptor that leads notably to the secretion of aldosterone (Bird et al., 1993). In those cells, endogenous phosphorylation of Akt was already detectable under serum deprivation and was not further increased after serum or AngII stimulation (Figure 4B, top), as reported previously (Zheng and Bollag, 2003). We further showed that, although constitutive, Akt phosphorylation was still PI3K-dependent, because it was blocked by LY294002. Interestingly, blockade of AngII- or serum-induced Akt phosphorylation by 24-h LY294002 treatment was associated with a concomitant significant reduction of the cellular level of PEA-15 (Figure 4B, bottom left and right). Inhibiting Akt activity decreased PEA-15 protein levels only by regulating the protein stability, because no modification of its mRNA expression was observed after LY294002 treatment (our unpublished data). Moreover, modulation of PEA-15 protein level by overactive Akt is specific for PEA-15. Indeed, Smad2 level is unaffected by similar treatments, although it is another protein with a short half-life subject to proteasomal degradation (Figure 4, A and B, left). Together, our results indicate that, in CHO or H295R cells, the variation of the level of phosphorylated Akt was associated with the regulation of the cellular protein level of recombinant tagged PEA-15 as well as endogenous PEA-15.
Figure 4.
Active Akt increases PEA-15 level. (A) Stable clones of CHO-AT1A cells expressing FLAG-PEA-15 were stimulated for 48 h with 10% serum or 10 nM IGF-1, in the presence or absence of 10 μM LY294002. Left, cell lysates were prepared in 1% SDS. Proteins (60 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-Ser473 Akt, anti-PEA-15, anti-Akt, and anti-Smad2 antibodies, respectively. Right, quantitative analysis of FLAG-PEA-15 protein level was performed on 10 independent experiments and corrected for Akt level. Data are expressed as mean ± SEM *p < 0.05; ***p < 0.001. (B) Serum-starved H295R cells were treated or not with 10 μM LY294002 for 30 min and then stimulated with 10% serum or 100 nM AngII for 30 min (top) or 24 h (bottom). Left, cell lysates were prepared in 1% SDS. Proteins (50 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-Ser473 Akt, anti-PEA-15, anti-Akt, and anti-Smad2 antibodies. Right, quantitative analysis of PEA-15 protein level after 24 h of stimulation was performed on eight independent experiments and corrected for Akt level. Smad2 was used as negative control in the degradation experiments. Data are expressed as mean ± SEM *p < 0.05, **p < 0.01, or ***p < 0.001 versus corresponding value.
PEA-15 Overexpression Abrogates AngII-induced Transcription and Proliferation by Blocking ERK1/2 Nuclear Translocation
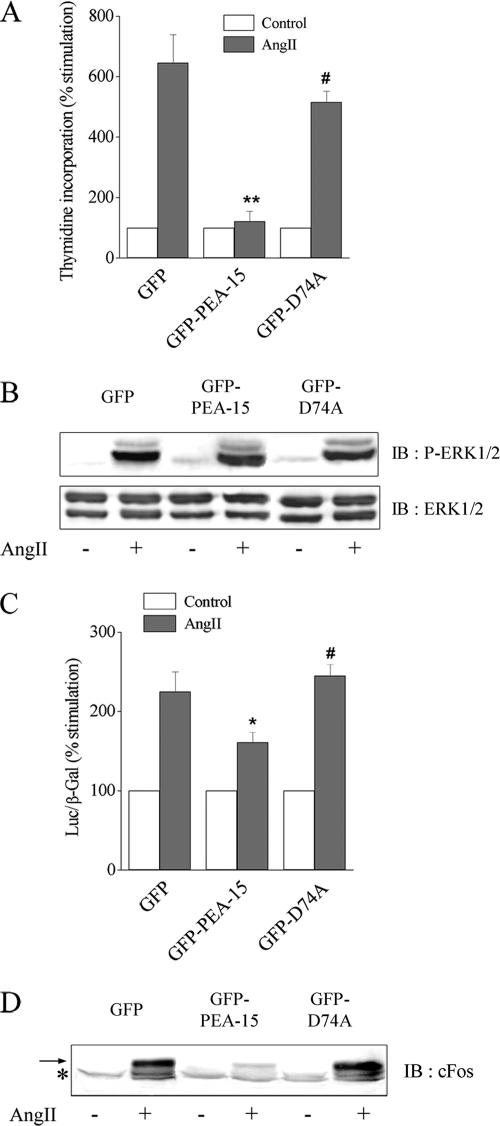
Because active Akt increased the stability and the level of PEA-15, we then tested whether PEA-15 overexpression would affect AngII-induced cell proliferation, as observed for Akt overexpression. We thus measured thymidine incorporation in CHO-AT1A cells expressing GFP-PEA-15, GFP-D74A, or GFP alone. Our results demonstrate that increased GFP-PEA-15 expression abolished the proliferative response to AngII, unlike expression of GFP alone or GFP-D74A (Figure 5A). To characterize the effects of PEA-15 overexpression on the ERK1/2 pathway, AngII-induced ERK1/2 activation was analyzed in the three cell lines. Similar levels of phosphorylation were detectable after AngII stimulation in CHO-AT1A cell line stably expressing GFP, GFP-PEA-15, or GFP-D74A (Figure 5B). In addition, AngII-induced ERK1/2 phosphorylation was inhibited by U0126 but unaffected by LY294002 in the three cell lines (our unpublished data). These results show that PEA-15 or D74A overexpression did not alter AngII-induced ERK1/2 activation that remained MEK-dependent but PI3K-independent, as observed previously in wild-type CHO-AT1A cells (Dugourd et al., 2003). We then analyzed the functional consequences of PEA-15 overexpression on the activation of the nuclear targets of ERK1/2, such as Elk-1. In response to AngII, PEA-15 overexpression decreased Elk-1–dependent transcription and suppressed, downstream of Elk-1, cFos induction (Figure 5, C and D). The induction of cFos by AngII was abolished by U0126 but unaffected by LY294002 in both wild-type and PEA-15–overexpressing cells (Supplemental Figure 1). The ability of PEA-15 to down-regulate Elk-1–dependent transcription relied on its capacity to bind to ERK1/2, because the D74A mutant, unable to bind ERK, did not modify Elk-1–dependent transcription and cFos expression in response to AngII (Figure 5, C and D).
Figure 5.
PEA-15 overexpression decreases Elk-1–dependent transcription, abolishes cFos expression, and inhibits cell proliferation induced by AngII without affecting ERK1/2 phosphorylation. Stable clones of CHO-AT1A cells expressing GFP, GFP-PEA-15, and GFP-D74A were used. (A) [3H]Thymidine incorporation was assessed in cells stimulated with 100 nM AngII for 16 h. The results are expressed as percentage of unstimulated value (control), set as 100%. (B) Cells were stimulated with 100 nM AngII for 10 min. Cell lysates were prepared with 1% SDS. Fifty and 20 μg were separated by SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 and anti-ERK1/2 antibody, respectively. (C) Elk-1–dependent transcriptional activity was measured, as described in Materials and Methods, from 100 nM AngII-stimulated and unstimulated cell lysates. (D) Cells were stimulated with 100 nM AngII for 1 h. Cell lysates were prepared with 1% SDS. Proteins (100 μg) were separated by SDS-PAGE and immunoblotted with anti-cFos antibody. For A and C, data show mean ± SEM values of at least four independent experiments performed in triplicate. *p < 0.05 or **p < 0.01 versus GFP-transfected cells and #p < 0.05 versus GFP-PEA-15–transfected cells. The arrow points out the specific band of cFos, whereas the asterisk indicates a common nonspecific signal.
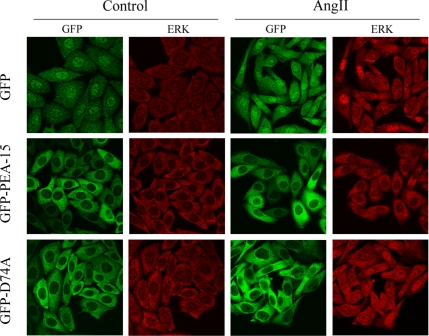
We have previously reported the ability of PEA-15 to retain ERK1/2 into the cytoplasm after serum stimulation (Formstecher et al., 2001). To test whether PEA-15 overexpression decreased ERK1/2 nuclear activity by inhibiting their nuclear ERK1/2 accumulation, we assessed the subcellular localization of ERK1/2 in CHO-AT1A cells expressing GFP-PEA-15, GFP-D74A, or GFP alone under AngII stimulation. Immunofluorescence revealed that in the presence of GFP alone, ERK1/2 localization was predominantly cytosolic in serum-starved cells and mostly nuclear in response to AngII for 3 h (Figure 6, top). In contrast, AngII failed to stimulate nuclear accumulation of ERK1/2 in cells overexpressing GFP-PEA-15 (Figure 6, middle). The cytosolic localization of GFP-PEA-15 was unaffected by the presence or absence of AngII. The cytosolic retention of ERK1/2 required its binding to PEA-15, because the D74A mutant failed to block ERK1/2 nuclear localization in response to AngII (Figure 6, bottom). As for wild-type PEA-15, the subcellular localization of GFP-D74A remained cytosolic in the presence or absence of AngII. ERK1/2 nuclear localization was unaffected by LY294002 pretreatment in the three cell lines (Supplemental Figure 2). Thus, overexpression of PEA-15–regulated ERK1/2 nuclear localization induced by AngII through the AT1 receptor. Together, our results demonstrate for the first time that increased expression of PEA-15 was able to down-regulate AngII-induced Elk1-dependent transcription, cFos induction, and cell proliferation through impaired ERK accumulation in the nucleus without any modification of their phosphorylation state.
Figure 6.
PEA-15 overexpression abrogates AngII-induced ERK1/2 nuclear accumulation. Serum-starved clones of CHO-AT1A cells expressing GFP, GFP-PEA-15, or GFP-D74A were unstimulated (control) or stimulated with 100 nM AngII for 3 h (AngII). Cells were fixed with ice-cold methanol, and ERK immunoreactivity was detected using a polyclonal anti-ERK1 antibody (red). GFP fluorescence was directly imaged (green).
Overexpressed Akt Activity Inhibits Induction of Elk-1 Transcription Factor and of cFos through the Regulation of PEA-15 Protein Level
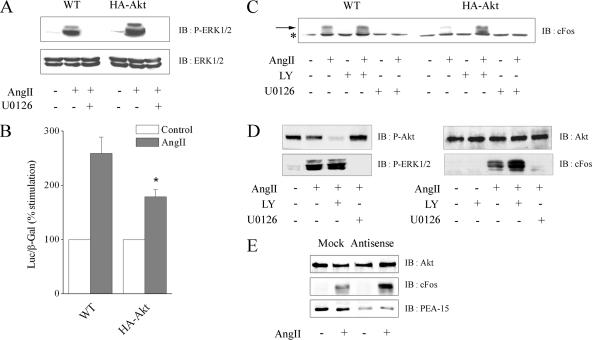
Common mechanisms may be involved in the inhibition of AngII-induced proliferation by PEA-15 or Akt overexpression. To test such hypothesis, we characterized ERK1/2 activity in wild-type or Akt-overexpressing CHO-AT1A cell lines. Parental and transfected CHO cell lines express similar level of endogenous PEA-15 protein (our unpublished data). Akt overexpression did not affect ERK1/2 protein level and their phosphorylation state after AngII stimulation (Figure 7A). In both cell lines, ERK1/2 phosphorylation was dependent of MEK but independent of PI3K, because completely inhibited by U0126 (Figure 7A) but unaffected by LY294002 (our unpublished data). Although ERK1/2 phosphorylation was not modified by the variation of the protein level of Akt, we tested the effect of Akt overexpression on the ability of ERK1/2 to activate its nuclear substrates. Overexpression of Akt significantly decreased Elk-1–dependent transcription and cFos induction after AngII stimulation (Figure 7, B and C). LY294002 pretreatment restored cFos induction in cells overexpressing Akt, corroborating the inhibitory effect of enhanced activation of Akt on cFos expression. As expected, cFos induction was dependent on ERK1/2 activation in our cell system, because it was abolished in the presence of U0126 (Figure 7C). Thus, our results show that Akt overactivation blocked the ability of ERK1/2 to stimulate transcriptional events without affecting their phosphorylation. To test ERK1/2 activity in a physiological context of high basal Akt activity, we assessed the cFos induction in H295R cells. AngII-induced ERK1/2 phosphorylation was unaffected by LY294002 (Figure 7D, left). In contrast, AngII-mediated cFos induction was potentiated after LY294002 pretreatment (Figure 7D, right), indicating that in H295R cells, constitutive active Akt down-regulates ERK1/2 nuclear activity. We then tested whether the blockade of ERK1/2-dependent transcription was correlated with the enhanced expression level of PEA-15 induced by overactive Akt. Treatment with a specific PEA-15 antisense oligonucleotide effectively blocked PEA-15 expression and restored ERK1/2-dependent cFos induction upon Akt overactivation in H295R cells (Figure 7E). Thus, overactive Akt down-regulates ERK1/2 nuclear activity through endogenous PEA-15 stabilization.
Figure 7.
Akt overexpression through PEA-15 decreases Elk-1–dependent transcription and cFos expression without affecting ERK1/2 activation. CHO-AT1A cells (WT) or stable clones overexpressing HA-Akt1 were used in experiments presented in A–C. H295R cells were used in D and E. (A) Cells were pretreated with or without 20 μM U0126 for 30 min and stimulated with 100 nM AngII for 10 min. Cell lysates were prepared with 1% SDS. Fifty and 20 μg were separated by SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 or anti-ERK1/2 antibody, respectively. (B) Elk-1–dependent transcriptional activity was measured, as described in Materials and Methods, from 100 nM AngII-stimulated and unstimulated cell lysates. Data show mean ± SEM values of at least four independent experiments performed in triplicate. *p < 0.05 versus WT cells. (C) Cells were treated or not with 10 μM LY294002 or 20 μM U0126 for 30 min and stimulated with 100 nM AngII for 1 h. Cell lysates were prepared with 1% SDS. Proteins (80 μg) were separated by SDS-PAGE and immunoblotted with anti-cFos antibody. The arrow points out the specific band of cFos, whereas the asterisk indicates a common nonspecific signal. (D) Cells were treated or not with 10 μM LY294002 or 20 μM U0126 for 30 min and stimulated with 100 nM AngII for 10 min (left) or 1 h (right). Cell lysates were prepared with 1% SDS. Proteins (50 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-Ser473 Akt, anti-Akt, anti-phospho-ERK1/2, or anti-cFos antibody. (E) Cells were transfected twice 24 and 48 h after seeding with PEA-15 antisense or mock oligonucleotides. At 72 h, cells were serum-starved for 3 h and stimulated with 100 nM AngII for 1 h. Cell lysates were prepared with 1% SDS. Proteins (80 μg) were separated by SDS-PAGE and immunoblotted with anti-Akt, anti-PEA-15, or anti-cFos antibody.
Overexpressed Akt Activity Blocks AngII-stimulated ERK1/2 Accumulation in the Nucleus
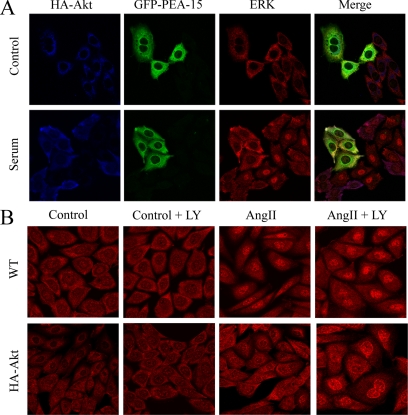
To elucidate the mechanism leading to the down-regulation of ERK1/2 nuclear activity through PEA-15 by overactive Akt, we first analyzed the similarity of effects between PEA-15 and Akt overexpressions on ERK nuclear localization upon serum stimulation in CHO-AT1A cells overexpressing Akt and transiently transfected with GFP-PEA-15. As expected, under serum depletion, ERK1/2 were exclusively detected in the cytosol of cells expressing HA-Akt1 alone as well as in those expressing both GFP-PEA-15 and HA-Akt1 (Figure 8A, top). On serum stimulation, ERK1/2 nuclear accumulation was clearly modified by Akt1 and PEA-15 overexpressions. Indeed, in cells expressing a low amount of recombinant Akt1 and no PEA-15, the staining of ERK1/2 was largely nuclear (Figure 8A, bottom). In contrast, the nuclear ERK1/2 staining was weak in cells expressing high level of Akt1 and absent in cells expressing a high level of both Akt1 and PEA-15. These results indicate that serum-induced ERK1/2 nuclear localization was all the more weak, because Akt and PEA-15 levels were high. We then assessed whether the inhibition of the AngII-induced proliferation observed in the CHO-AT1A cells stably expressing HA-Akt1 was due to the same mechanism. We thus examined by immunostaining the subcellular localization of ERK after AngII stimulation in wild-type and Akt-overexpressing CHO-AT1A cells. Under serum depletion, ERK1/2 were confined to the cytosol of CHO-AT1A cells, at any level of Akt (Figure 8B, left). In contrast, in presence of AngII, ERK1/2 subcellular distribution was affected by the level of expression of Akt. Indeed, after AngII stimulation, ERK1/2 were localized into the nucleus of wild-type CHO-AT1A cells (Figure 8B, top) but failed to translocate into the nucleus of CHO-AT1A cells overexpressing Akt (Figure 8B, bottom). The cytosolic sequestration of ERK was due to the active form of Akt, because LY294002 pretreatment blocking Akt phosphorylation restored AngII-induced nuclear ERK accumulation (Figure 8B, right).
Figure 8.
Akt overexpression abolishes serum- and AngII-induced ERK1/2 accumulation in the nucleus. (A) CHO-AT1A cells overexpressing HA-Akt1 were transiently transfected with GFP-PEA-15. After 16 h of serum starvation, cells were stimulated with 20% serum for 3 h or maintained in serum-free medium (control). Cells were then fixed with ice-cold methanol. HA-Akt1 or ERK immunoreactivity was detected using a polyclonal anti-ERK1 (red) or a monoclonal anti-HA (blue) antibody, and GFP fluorescence was directly imaged (green) by using confocal microscopy. (B) Serum-starved CHO-AT1A cells (WT) or clones overexpressing HA-Akt1 were pretreated or not with 10 μM LY294002 for 30 min. Subsequently, cells were stimulated with 100 nM AngII for 3 h (AngII and AngII + LY, respectively) or maintained in serum-free medium (control and control + LY, respectively). Cells were fixed with ice-cold methanol, and ERK immunoreactivity was detected using a polyclonal anti-ERK1 antibody (red).
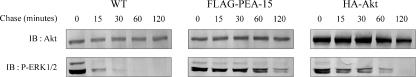
Inactivation of phosphorylated ERK1/2 occurs, at least in part, in the nucleus through exposure to phosphatases such as MAPK kinase phosphatase-1 (Keyse, 2000). We thus tested whether the cytosolic retention of ERK1/2 induced by overexpressed Akt would lead to a sustained activation of ERK1/2 in the cytosol, as a result of a decreased nuclear ERK1/2 inactivation. After 5 min of AngII exposure, CHO-AT1A cells overexpressing or not HA-Akt1 or FLAG-PEA-15 were rinsed and maintained in serum-free medium for various times to detect ERK1/2 dephosphorylation rate in the cytosol. Overexpression of PEA-15 in CHO-AT1A cells prolonged AngII-induced ERK1/2 phosphorylation in the cytosol (Figure 9, middle), in a similar way to those observed under serum stimulation (Ramos et al., 2000; Formstecher et al., 2001). Interestingly, overexpression of Akt led to the same effect than PEA-15 overexpression, i.e., a decrease in ERK1/2 dephosphorylation kinetics in the cytosol (Figure 9, right). Together, these results indicate that Akt overactivation through PEA-15 down-regulated ERK1/2 nuclear localization, leading to the suppression of AngII-induced proliferative responses.
Figure 9.
Akt overexpression decreases ERK1/2 inactivation in the cytosol. Serum-starved CHO-AT1A cells (WT) or stable clones overexpressing FLAG-PEA-15 or HA-Akt1 were stimulated with 100 nM AngII for 5 min, rinsed twice, and chased for the indicated times in serum-free medium. Cell lysates were prepared with 1% Nonidet NP-40. Proteins (60 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-ERK and anti-Akt antibody, respectively.
DISCUSSION
Precise regulation of the PI3K/Akt and MEK/ERK1/2 pathways controls cell growth, differentiation, and survival. We have previously shown that endogenous Akt and ERK1/2 are independently activated but are both required for AngII-induced cell proliferation in CHO-AT1A or rat aortic smooth muscle cells (Dugourd et al., 2003). The present study shows that overexpression of Akt sequestered ERK1/2 in the cytosol of CHO-AT1A cells via PEA-15 with a consequent abolition of the mitogenic response to AngII. This study is the first report to delineate the molecular mechanism responsible for the down-regulation of ERK1/2 transcriptional activity induced by Akt without inhibiting ERK1/2 activation.
Cross-talk between the PI3K/Akt and Ras/Raf/MEK/ERK1/2 occurs at different levels and exerts cooperative or antagonistic effects depending on external stimuli and cellular background. PI3K has been shown to stimulate integrin-mediated Raf activation in synergy with Ras (Chaudhary et al., 2000). Cooperative effects between these two signaling cascades have also been demonstrated in the regulation of the platelet-derived growth factor-induced proliferation (Choudhury et al., 1997) or in cell cycle progression and transformation (Sheng et al., 2001a, b). Alternatively, Akt has been shown to phosphorylate Raf-1, leading to the down-regulation of the ERK pathway in phorbol 12-myristate 13-acetate–stimulated MCF-7 cells (Zimmermann and Moelling, 1999) or in differentiated myotubes (Rommel et al., 1999). B-Raf activity is also inhibited by epidermal growth factor-induced Akt stimulation (Guan et al., 2000). Our study reveals an additional mechanism, because we show that overexpressed Akt down-regulated ERK/Elk-1–dependent transcriptional activity, an effect that was undetectable under endogenous Akt activation. In our system, inhibition of Ras, Raf, or MEK by overexpressed Akt cannot account for this negative regulation, because ERK1/2 phosphorylation was not affected in CHO-AT1A overexpressing Akt. In contrast, this result suggests that Akt acted downstream of ERK1/2 activation in the cytosol. In agreement with our data, a recent study reported that constitutively active Akt does not modify ERK1/2 phosphorylation (Galetic et al., 2003). In our study, the inhibition of ERK1/2 nuclear activity by overexpressed Akt resulted in a decrease in Elk-1–dependent transcription with a subsequent abolition of cFos expression. Increased Akt activation has already been shown to down-regulate the Elk-1 transcription factor by decreasing either its expression (Figueroa and Vojtek, 2003) or its activation (Galetic et al., 2003). Therefore, the critical step for the negative regulation of ERK1/2 by Akt lies between the cytosolic activation of ERK1/2 and its activity on nuclear substrates, such as Elk-1. In this context, our study describes a new cellular process of down-regulation of ERK/Elk-1–dependent transcription that implies the cytosolic retention of active ERK1/2 by active Akt (Figure 10). Indeed, only the phosphorylated form of Akt was able to retain ERK1/2 into the cytosol, because pretreatment with the PI3K inhibitor LY294002 that blocks Akt phosphorylation restored ERK1/2 nuclear translocation induced by AngII.
Figure 10.
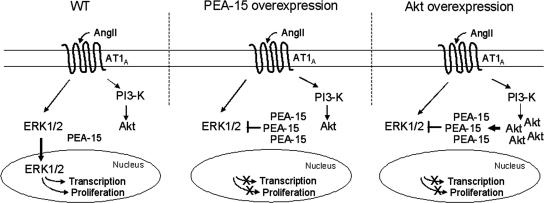
Model for the negative regulation by Akt of ERK1/2 nuclear localization and cell proliferation through PEA-15. 1) In wild-type CHO-AT1A cells, ERK1/2 are activated by AngII and translocate to the nucleus where they stimulate transcription and cell proliferation. 2) In CHO-AT1A cells overexpressing PEA-15, ERK1/2 are activated by AngII but are sequestered into the cytosol through their binding to PEA-15. This interaction abolishes the transcriptional and proliferative signals. 3) In CHO-AT1A cells overexpressing Akt, ERK1/2 activation by AngII is not affected. Overactivated Akt binds to and stabilizes PEA-15, leading to an increase in its cellular level. This interaction between PEA-15 and Akt prevents ERK1/2 nuclear accumulation and thus abolishes AngII-induced cell proliferation.
A protein partner that could account for the negative regulation of ERK1/2 by Akt is the phosphoprotein PEA-15. Indeed, Trencia et al. (2003) recently reported that PEA-15 is a new Akt substrate and that its phosphorylation and stabilization by Akt participate in Akt-mediated survival signaling. Moreover, we have previously demonstrated that PEA-15 binds ERK1/2 and abolishes their nuclear translocation in response to serum (Formstecher et al., 2001). In the present study, we show that Akt binds to PEA-15 in vitro, in agreement with Trencia et al. (2003). This interaction did not require ERK1/2 binding to PEA-15, because the D74A mutant of PEA-15, which cannot bind to ERK1/2, was still able to bind Akt. Furthermore, the interaction between recombinant GST-PEA-15 and in vitro-radiolabeled Akt allowed us to conclude that a direct interaction occurs between Akt and PEA-15. Therefore, PEA-15 is a pivotal protein functionally linking Akt and ERK1/2. Although the molecular interactions between various motifs of ERK1/2 and PEA-15 have been investigated for their direct binding (Hill et al., 2002; Chou et al., 2003), the mechanisms of molecular interactions between Akt and PEA-15 remain to be determined. An important issue will be to assess whether the phosphorylation state of Akt affects its binding to PEA-15 and/or the binding of PEA-15 to ERK1/2. A better understanding of this interaction will clarify the preferential regulation of ERK1/2 or Akt binding to PEA-15, which could be modulated by kinase expression levels and lead to differential cellular responses.
Regarding the functional consequence of this interaction, we show that Akt binds to PEA-15 and increases PEA-15 half-life. We further demonstrate that only the active form of Akt was responsible for PEA-15 stabilization, because IGF-1–induced increase in PEA-15 half-life was abrogated by LY294002 pretreatment. Interestingly, the half-life of the D74A mutant was also affected by the activation state of Akt. Together, these results demonstrate for the first time that endogenous Akt phosphorylation as well as ERK1/2 binding to PEA-15 exerted additive effects on the stability of PEA-15. Stabilization of PEA-15 by overexpressed Akt amplified the functional consequences of PEA-15 binding to ERK1/2. Overexpression of Akt resulted in the same regulation pattern of the ERK1/2 signaling as increased PEA-15 expression, i.e., exclusion of ERK1/2 from the nucleus and the consequent inhibition of Elk-1–dependent transcription and of cFos induction. When stabilization of PEA-15 by overactive Akt is blocked by specific PEA-15 antisense, ERK1/2-dependent transcription is rescued, demonstrating the important role of Akt/PEA-15 cross-talk in controlling ERK1/2 nuclear activity. Moreover, the lack of effect of LY294002 on ERK1/2 nuclear translocation and activity when PEA-15 is overexpressed rules out the possible involvement of parallel regulators of ERK1/2 signaling, other than PEA-15, downstream of Akt activation.
Finally, this study is the first to report the functional consequence of the cross-talk between Akt, PEA-15, and ERK1/2. Negative regulation of ERK1/2 by overactivated Akt was associated with the inhibition of cell proliferation induced by AngII. Hence, changes in the expression of Akt can modulate the mitogenic response by decreasing ERK1/2 nuclear activity through their cytosolic retention. This result is important considering that Akt is overexpressed and/or overactivated under many pathophysiological circumstances, ranging from tumor growth to vascular diseases (Hixon et al., 2000; Testa and Bellacosa, 2001). Whereas Akt overexpression is mainly associated with an oncogenic phenotype, this pathological context is usually associated with mutation or modification of the expression of other components of the Akt pathway, such as PI3K and/or phosphatase and tensin homologue deleted on chromosome 10 (Luo et al., 2003). Besides, the dual involvement of Akt in mediating proliferative and/or antiapoptotic responses has to be considered inasmuch as both responses are intricate (Hu et al., 2004). Zhu et al. (2004) reported that Gab2 overexpression, which enhanced the phosphorylation of Akt but not of ERK1/2, reduced cell proliferation induced by granulocyte colony-stimulated factor (Zhu et al., 2004). Our results are in agreement with this recent study. Moreover, the molecular mechanism that we propose could be involved in the lack of proliferative effect observed upon Akt up-regulation in vascular smooth muscle cells (Hixon et al., 2000). This dual effect of Akt has to be correlated to contradictory effects of PEA-15. Indeed, PEA-15 is a strong inhibitor of death receptor-dependent apoptosis (Condorelli et al., 1999; Estelles et al., 1999; Kitsberg et al., 1999) but is associated with decreased cell proliferation (Formstecher et al., 2001; Gaumont-Leclerc et al., 2004). Furthermore, a recent study showed that sustained level of PEA-15 participated in Akt-dependent chemoresistance in human breast cancer cells (Stassi et al., 2005). Hence, according to our model of Akt/PEA-15 interaction, we hypothesized that, in some physiopathological context, overexpression of Akt could slow proliferation and render the cells quiescent and resistant to certain forms of apoptosis.
Akt overexpression generated a cross-talk between the PI3K and the MAPK pathways that relies on the pivotal role of PEA-15, because this protein could directly interact with Akt and with ERK1/2 (Figure 10). Variations in the expression of one of the three proteins can result in a modified balance of these complexes and shift the cell toward a proliferative or nonproliferative phenotype. Thus, in the presence of basal endogenous PEA-15 or Akt protein levels, the cross-talk between the ERK1/2 and Akt pathways does not occur. This is due to a high amount of ERK1/2 whose nuclear translocation cannot be clearly affected by PEA-15 binding and relocalization. However, higher amount of PEA-15, either by overexpression of the protein or by increase of its half-life regulated by overactivated Akt, can lead to a blockade of the ERK1/2 nuclear translocation and a subsequent blockade of cFos induction and cell proliferation. This new cross-talk between two main kinases mediated by a small noncatalytic protein leads to a better understanding of the cellular mechanisms necessary to switch the cell from a proliferative phenotype to a quiescent phenotype.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eric Etienne for confocal microscopy, Drs. Etienne Formstecher and Alessandro Capponi for very helpful discussions, and Dr. Juliette Hadchouel for help with the cDNA constructs. We thank Dr. Joe Ramos for sharing the wild-type and mutant PEA-15 constructs. This work was supported by an Association pour la Recherche contre le Cancer fellowship (to C. D.) and by a grant (no. 3500) from the Association pour la Recherche Contre le Cancer (to H. C.).
Abbreviations used:
- AngII
angiotensin II
- CHO
Chinese hamster ovary cells
- ERK
extracellular signal-regulated kinases
- GSK
glycogen synthase kinase
- PEA-15
phosphoprotein enriched in astrocytes of 15 kDa
- PI3K
phosphatidylinositol 3-kinase.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0501) on July 5, 2006.
REFERENCES
- Araujo H., Danziger N., Cordier J., Glowinski J., Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C (PKC) in astrocytes. J. Biol. Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- Bird I. M., Hanley N. A., Word R. A., Mathis J. M., McCarthy J. L., Mason J. I., Rainey W. E. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- Brunet A., Roux D., Lenormand P., Dowd S., Keyse S., Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A., King W. G., Mattaliano M. D., Frost J. A., Diaz B., Morrison D. K., Cobb M. H., Marshall M. S., Brugge J. S. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 2000;10:551–554. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- Chou F. L., Hill J. M., Hsieh J. C., Pouyssegur J., Brunet A., Glading A., Uberall F., Ramos J. W., Werner M. H., Ginsberg M. H. PEA-15 binding to ERK1/2 MAPKs is required for its modulation of integrin activation. J. Biol. Chem. 2003;278:52587–52597. doi: 10.1074/jbc.M309322200. [DOI] [PubMed] [Google Scholar]
- Choudhury G. G., Karamitsos C., Hernandez J., Gentilini A., Bardgette J., Abboud H. E. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am. J. Physiol. 1997;273:F931–F938. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- Condorelli G., Vigliotta G., Cafieri A., Trencia A., Andalo P., Oriente F., Miele C., Caruso M., Formisano P., Beguinot F. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene. 1999;18:4409–4415. doi: 10.1038/sj.onc.1202831. [DOI] [PubMed] [Google Scholar]
- Danziger N., Yokoyama M., Jay T., Cordier J., Glowinski J., Chneiweiss H. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and PKC substrate. J. Neurochem. 1995;64:1016–1025. doi: 10.1046/j.1471-4159.1995.64031016.x. [DOI] [PubMed] [Google Scholar]
- Dugourd C., Gervais M., Corvol P., Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension. 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- Estelles A., Charlton C. A., Blau H. M. The phosphoprotein protein PEA-15 inhibits Fas- but increases TNF-R1-mediated caspase-8 activity and apoptosis. Dev. Biol. 1999;216:16–28. doi: 10.1006/dbio.1999.9510. [DOI] [PubMed] [Google Scholar]
- Estelles A., Yokoyama M., Nothias F., Vincent J. D., Glowinski J., Vernier P., Chneiweiss H. The major astrocytic phosphoprotein PEA-15 is encoded by two mRNAs conserved on their full length in mouse and human. J. Biol. Chem. 1996;271:14800–14806. doi: 10.1074/jbc.271.25.14800. [DOI] [PubMed] [Google Scholar]
- Figueroa C., Vojtek A. B. Akt negatively regulates translation of the ternary complex factor Elk-1. Oncogene. 2003;22:5554–5561. doi: 10.1038/sj.onc.1206496. [DOI] [PubMed] [Google Scholar]
- Formstecher E., et al. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- Galetic I., Maira S. M., Andjelkovic M., Hemmings B. A. Negative regulation of ERK and Elk by protein kinase B modulates c-Fos transcription. J. Biol. Chem. 2003;278:4416–4423. doi: 10.1074/jbc.M210578200. [DOI] [PubMed] [Google Scholar]
- Gaumont-Leclerc M. F., Mukhopadhyay U. K., Goumard S., Ferbeyre G. PEA-15 is inhibited by adenovirus E1A and plays a role in ERK nuclear export and Ras-induced senescence. J. Biol. Chem. 2004;279:46802–46809. doi: 10.1074/jbc.M403893200. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Figueroa C., Brtva T. R., Zhu T., Taylor J., Barber T. D., Vojtek A. B. Negative regulation of the serine/threonine kinase B-Raf by Akt. J. Biol. Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Vaidyanathan H., Ramos J. W., Ginsberg M. H., Werner M. H. Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J. 2002;21:6494–6504. doi: 10.1093/emboj/cdf641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixon M. L., Muro-Cacho C., Wagner M. W., Obejero-Paz C., Millie E., Fujio Y., Kureishi Y., Hassold T., Walsh K., Gualberto A. Akt1/PKB upregulation leads to vascular smooth muscle cell hypertrophy and polyploidization. J. Clin. Investig. 2000;106:1011–1020. doi: 10.1172/JCI8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. L., Cowan R. G., Harman R. M., Quirk S. M. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol. Endocrinol. 2004;18:326–338. doi: 10.1210/me.2003-0178. [DOI] [PubMed] [Google Scholar]
- Inman G. J., Nicolas F. J., Hill C. S. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol. Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Keyse S. M. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Kitsberg D., Formstecher E., Fauquet M., Kubes M., Cordier J., Canton B., Pan G., Rolli M., Glowinski J., Chneiweiss H. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J. Neurosci. 1999;19:8244–8251. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Manning B. D., Cantley L. C. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C., Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- Ramos J. W., Hughes P. E., Renshaw M. W., Schwartz M. A., Formstecher E., Chneiweiss H., Ginsberg M. H. Death effector domain protein PEA-15 potentiates Ras activation of extracellular signal receptor-activated kinase by an adhesion-independent mechanism. Mol. Biol. Cell. 2000;11:2863–2872. doi: 10.1091/mbc.11.9.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. W., Kojima T. K., Hughes P. E., Fenczik C. A., Ginsberg M. H. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J. Biol. Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- Renault F., Formstecher E., Callebaut I., Junier M. P., Chneiweiss H. The multifunctional protein PEA-15 is involved in the control of apoptosis and cell cycle in astrocytes. Biochem. Pharmacol. 2003;66:1581–1588. doi: 10.1016/s0006-2952(03)00514-8. [DOI] [PubMed] [Google Scholar]
- Reusch H. P., Zimmermann S., Schaefer M., Paul M., Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J. Biol. Chem. 2001;276:33630–33637. doi: 10.1074/jbc.M105322200. [DOI] [PubMed] [Google Scholar]
- Rommel C., Clarke B. A., Zimmermann S., Nunez L., Rossman R., Reid K., Moelling K., Yancopoulos G. D., Glass D. J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Sheng H., Shao J., DuBois R. N. Akt/PKB activity is required for Ha-Ras-mediated transformation of intestinal epithelial cells. J. Biol. Chem. 2001a;276:14498–14504. doi: 10.1074/jbc.M010093200. [DOI] [PubMed] [Google Scholar]
- Sheng H., Shao J., Dubois R. N. K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res. 2001b;61:2670–2675. [PubMed] [Google Scholar]
- Stassi G., Garofalo M., Zerilli M., Ricci-Vitiani L., Zanca C., Todaro M., Aragona F., Limite G., Petrella G., Condorelli G. PED mediates AKT-dependent chemoresistance in human breast cancer cells. Cancer Res. 2005;65:6668–6675. doi: 10.1158/0008-5472.CAN-04-4009. [DOI] [PubMed] [Google Scholar]
- Testa J. R., Bellacosa A. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch B., Bihoreau C., Monnot C., Bernstein K. E., Murphy T. J., Alexander R. W., Corvol P., Clauser E. A recombinant rat vascular AT1 receptor confers growth properties to angiotensin II in Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 1992;187:1381–1388. doi: 10.1016/0006-291x(92)90455-t. [DOI] [PubMed] [Google Scholar]
- Trencia A., et al. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol. Cell Biol. 2003;23:4511–4521. doi: 10.1128/MCB.23.13.4511-4521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Bollag W. B. AngII induces transient phospholipase D activity in the H295R glomerulosa cell model. Mol. Cell Endocrinol. 2003;206:113–122. doi: 10.1016/s0303-7207(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Zhu Q. S., Robinson L. J., Roginskaya V., Corey S. J. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood. 2004;103:3305–3312. doi: 10.1182/blood-2003-06-1861. [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.