Abstract
The gaseous compound carbon monoxide (CO) has been identified as an important endogenous biological messenger in the brain and is a major component in regulation of cerebrovascular circulation in newborns. CO is produced endogenously by catabolism of heme to CO, free iron, and biliverdin during enzymatic degradation of heme by heme oxygenase (HO). The present study was designed to test the hypothesis that endogenously produced CO contributes to hypotension-induced vasodilation of cerebral arterioles. Experiments used anesthetized piglets with implanted, closed cranial windows. Topical application of the HO substrate, heme-L-lysinate (HLL), caused dilation of pial arterioles that was blocked by a metal porphyrin inhibitor of HO, chromium mesoporphyrin (CrMP). In normotensive piglets (arterial pressure 64±4 mm Hg), CrMP did not cause vasoconstriction of pial arterioles but rather a transient dilation. Hypotension (50% of basal blood pressure) increased cerebral CO production and dilated pial arterioles from 66±2μm to 92±7μm. In hypotensive piglets, topical CrMP or i.v. SnPP decreased cerebral CO production and produced pial arteriolar constriction to normotensive diameters. In additional experiments, since prostacyclin and nitric oxide (NO) are also key dilators that can contribute to cerebrovascular dilation, we held their levels constant. NO/prostacyclin clamp was accomplished with continuous, simultaneous application of indomethacin, Nω-nitro-L-arginine (L-NNA), and minimal dilatory concentrations of iloprost and sodium nitroprusside (SNP). With constant NO and prostacyclin, the transient dilator and prolonged constrictor responses to CrMP of normotensive and hypotensive piglets, respectively, were the same as when NO and prostaglandins were not held constant. These data suggest that endogenously produced CO contributes to cerebrovascular dilation in response to reduced perfusion pressure.
Keywords: heme oxygenase, autoregulation, pial arterioles, newborn
INTRODUCTION
Carbon monoxide (CO) is an autocrine and paracrine vasodilator in cerebral and systemic circulations and endogenously produced CO can play a role in the regulation of basal tone in resistance vessels throughout the body (24, 25). CO is produced physiologically by catabolism of heme to CO, free iron, and biliverdin during enzymatic degeneration of heme by heme oxygenase (HO) (25). The constitutive HO isoform, HO-2, is highly expressed in the newborn piglet brain (28) and piglet cerebral microvessels produce CO at rates higher than other tissues we have examined (24). HO-2 expression has been found to be regulated only by steroids (25). Endogenous CO can be produced in high enough concentration to cause vasodilation (7, 30). The vasodilator effect of CO has been attributed to both the cGMP/PKG signaling pathway (27) and activation of Ca2+ - activated K+ (Kca) channels (14). CO causes smooth muscle hyperpolarization via activation of large-conductance Kca channels (39). CO increases the Ca2+ sensitivity of Kca channels (14, 39) and may also decrease the production of vasoconstrictors such as endothelin or cytochrome P-450-derived products (8). CO contributes to cerebrovascular dilation caused by excitatory amino acids (9, 24) and thus joins other autocrine and paracrine dilators such as NO and prostaglandins, in control of cerebral vascular tone. Furthermore, there are interactions among the three (12, 21).
Prostanoids contribute to regulation of the cerebral microcirculation in a variety of ways in newborn pigs. Prostacyclin contributes to the sustained vasodilation that allows maintenance of cerebral blood flow during severe hypotension in newborn pigs (20). Endothelial prostacyclin can act as a permissive factor in cerebral microvascular smooth muscle, allowing dilation in response to another stimulus (22). The synthesis of the prostanoid or NO need not be augmented by the stimulus to allow the response to occur, since both prostacyclin and NO play permissive roles in allowing dilation to CO (21). Therefore, constrictions caused by inhibiting production of prostacyclin and / or NO could be caused, at least in part, by removing the ability of CO to produce dilation.
The goal of the present study was to address the hypothesis that HO-derived CO contributes to cerebrovascular vasodilation in response to hemorrhagic hypotension. We also investigated whether enhanced production of prostacyclin and / or NO was necessary for dilation to hypotension to occur.
MATERIALS AND METHODS
All procedures that involved animals were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center. Newborn pigs (1 to 3 days old) were anesthetized with ketamine hydrochloride (33 mg/kg IM) and acepromazine (3.3 mg/kg IM) and maintained on α-chloralose (50 mg/kg iv). A catheter was inserted into a femoral artery to monitor blood pressure and heart rate and to collect blood for measurements of Pco2, Po2, and pH. A second catheter was placed in a femoral vein for anesthetic and fluid administration. The trachea was cannulated, and the animals were mechanically ventilated and supplemented with O2, if needed, to maintain arterial pH, Pco2, and Po2 within the normal range. A heating pad was used to maintain the animals at 37.5-38.5°C, which was monitored with a rectal probe.
Cranial window placement and pial arteriolar monitoring
The scalp was surgically removed and a 2-cm-diameter craniotomy was made over the parietal cortex. The dura was incised and reflected over the bone to prevent contact between the brain surface and the cut edge of the bone. A stainless steel and glass window was implanted into the hole and cemented sequentially with bone wax and dental acrylic. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. The space under the window was filled with artificial cerebrospinal fluid (aCSF: 150 meq Na+/l, 3 meq K+/l, 2.5 meq Ca2+/l, 1.2 meq Mg2+/l 132 meq Cl−/l, 3.7 mM glucose, 6 mM urea, 25 meq HCO−3; approximately, pH 7.33; 46 mm Hg Pco2; 43 mmHg Po2, 37°C) through needles incorporated into the sides of the window. aCSF could be injected and samples collected from needle ports on the sides of the window. The volume of fluid directly beneath the window was 350μl and was contiguous with the periarachnoid space. Pial vessels were observed with a dissecting microscope. Diameters were measured with a video micrometer coupled to a television camera mounted on the microscope and a video monitor.
Chemicals
CO was purchased as compressed gas (99.5%). Water was saturated with CO to produce a 10−3 M stock solution. The stock was diluted in aCSF from 10−7 to 10−5 M. Water soluble indomethacin (indomethacin trihydrate) was a gift from Merck Sharp & Dohme Research Laboratories, Rahway, New Jersey. Iloprost was a gift from Schering AG, Berlin or was purchased from Cayman Chemical, Ann Arbor, MI. The HO substrate, heme-l-lysinate(HLL) was prepared using methods described by Tenhunen et al. (32) and was stored at −30°C. HLL stock solution (10−2M) in H2O adjusted to pH 10.0 with 0.1 NaOH was diluted with aCSF to 2× 10−6M. The HO inhibitors purchased from Frontier Scientific (Logan, UT), chromium mesoporphyrin (CrMP) and tin protoporphyrin (SnPP), were protected from light at all times and the cranial window was illuminated only during vessel diameter measurements. All other reagents were of analytical grade and purchased from Sigma Chemical Company, (St. Louis, MO, USA).
EXPERIMENTS
After implantation of the cranial window, at least 30 min were allowed for exchange and equilibrium of fluid under the window with the subarachnoid fluid before experimentation was begun. The experimental design consisted of measurements of pial arterial diameter, mean arterial pressure, arterial blood gases, and pH.
Before and after induction of hypotension, aCSF for CO measurement was collected from beneath the cranial window into an amber vial, the internal standard 13C16O (see below) was injected into the bottom of the vial, and the vial was immediately sealed with a rubberized Teflon-lined cap. The samples were heated in hot water bath (75°C) and CO production was determined by gas chromatography/mass spectrometry (GC/MS). GC/MS analysis of the headspace gas was performed on a Hewlett-Packard 5970 mass-selective ion detector interfaced to a Hewlett-Packard 5890A gas chromatograph. The separation of CO from other gases was carried out on a Varian 5A mole sieve capillary column (30 m; 0.32 mm ID) with a linear temperature gradient from 35 to 65°C at 5 degrees/min. Helium was the carrier gas at a column head pressure of 4.0 psi. Aliquots (100-μl) of the headspace gas were injected by using a gastight syringe into the splitless injector having a temperature of 120°C. Mass-to-charge ratios (m/z) 28 and 29 corresponding to 12C16O and 13C16O, respectively, were recorded via selective ion monitoring. The amount of CO in samples was calculated from the ratio of peak areas of m/z 28 and 29. The results are expressed as pmol of CO/ml CSF.
Dilatory effect of heme and involvement of HO
An initial set of experiments was performed to confirm the efficacy of CrMP in inhibiting HO (n = 6). HLL (2×10−6 M) was placed under the window without and with CrMP(2×10−5M).
Pial arteriolar responses to hypotension
Hypotension was induced by the withdrawal of blood from the venous catheter until the arterial pressure dropped to 50% of its control level. To prevent clot formation in the withdrawn blood, heparin was placed in the syringe before commencing hemorrhage. After measuring pial arteriolar diameter for the hypotensive state, topical CrMP (2×10−5M) or systemic SnPP (3mg/kg i.v.)was administered to inhibit HO activity. Control piglets were treated identically except no blood was withdrawn.
NO/PG clamp
We performed the same experiment with NO and prostacyclin held constant (NO/PG clamp). NO/PG clamp was accomplished by continuous topical application of LNA (10−3M), SNP (2×10−7M) and iloprost (10−10M) and indomethacin (5mg/kg i.v.).
To confirm prolonged inhibition of NOS and COX and adequate NO/PG to allow dilation to CO in the presence of that inhibition, CO in progressively increasing concentrations was topically applied to pial arterioles and the maximal pial arteriolar diameter reached over a 5-min period was recorded as the response to each dose. CO ascending dose-response curves were registered before and following no treatment or treatment with Nω-nitro-L-arginine (LNA) (10−3M, topically), and LNA plus sodium nitroprusside (2 ×10−7M). In other piglets the experimental approach described above for CO/LNA/SNP was used, except that LNA was replaced by indomethacin (5mg/kg) and SNP replaced by iloprost(10−10M ).
Statistical analysis
Values are presented as means ± SE. Results were subjected to a one-way ANOVA for repeated measures with Tukey's post hoc test to isolate differences between groups. A level of P< 0.05 was considered significant.
RESULTS
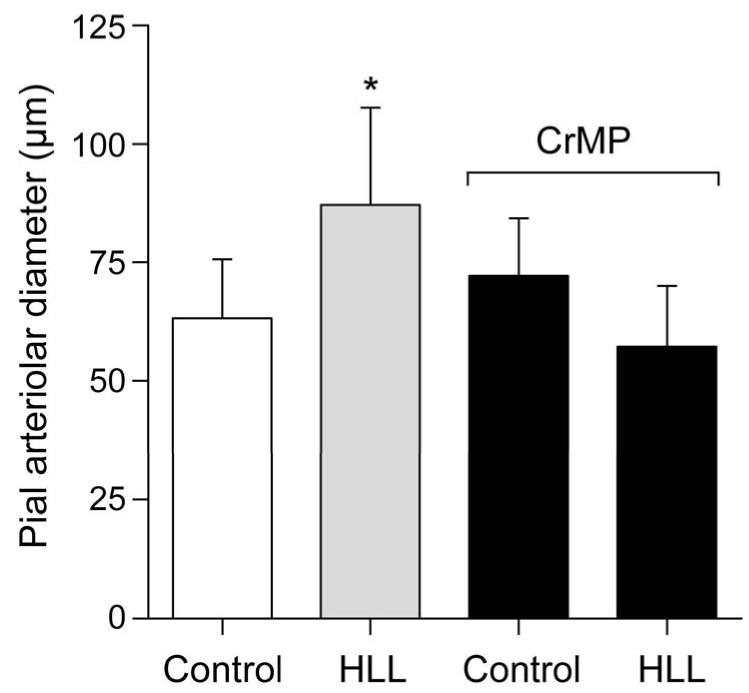
Topical application of the HO substrate, HLL, caused dilation of piglet cerebral arterioles that was blocked by the metal porphyrin inhibitor of HO, CrMP (Fig.1).
Figure 1.
Dilation to HLL (2×10−6M) of pial arterioles before and in the presence of CrMP (2×10−5 M). Mean ± SEM. N=6, *P < 0.05.
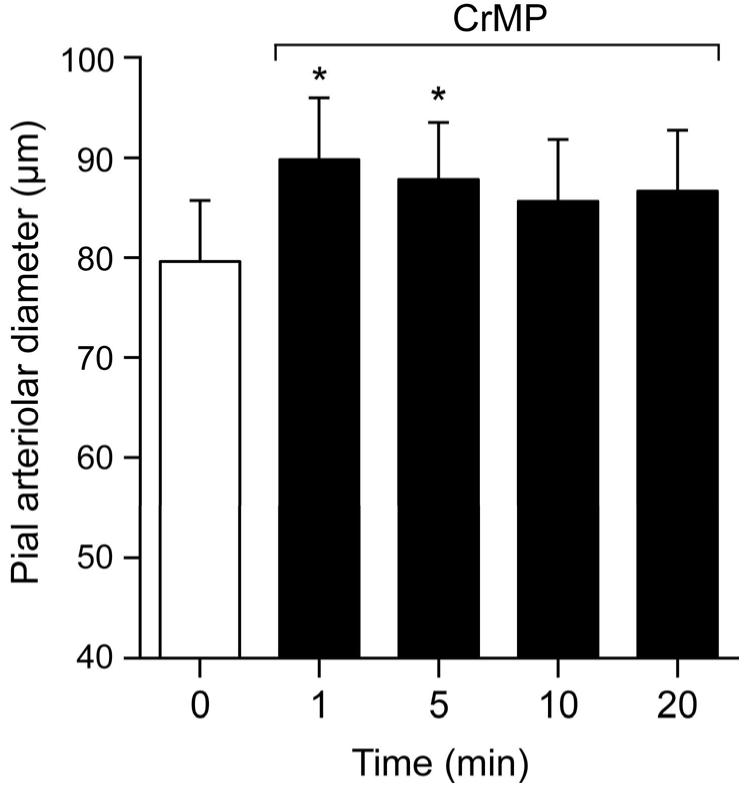
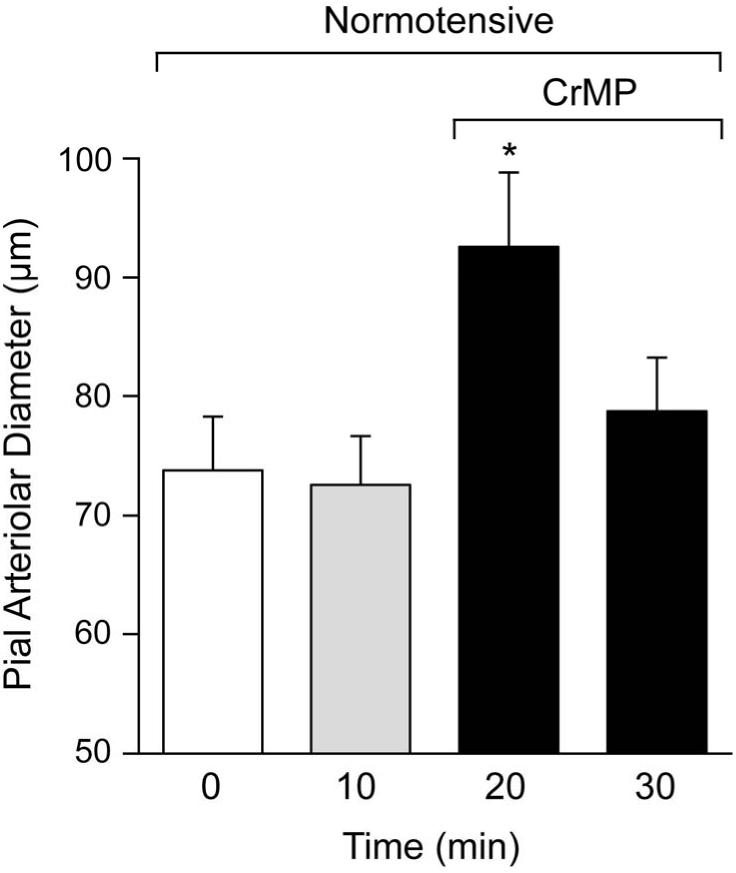
CrMP dilated pial arterioles in normotensive piglets (arterial pressure 64±4 mm Hg) at 1 and 5 min with return to diameter not significantly different from control by 10 min (Fig.2.).
Figure 2.
Effect of CrMP (2× 10−5M) on diameters of pial arterioles of normotensive piglets. Mean ± SEM. N=6 *P < 0.05 compared to zero (0) time.
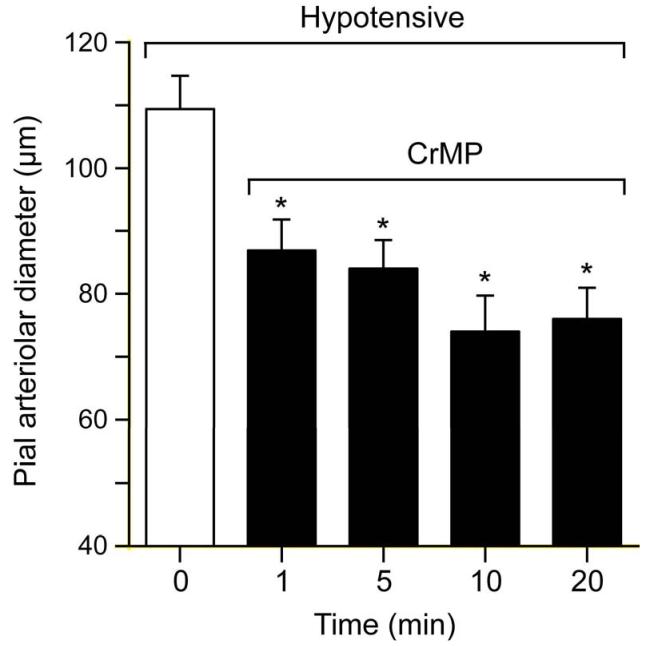
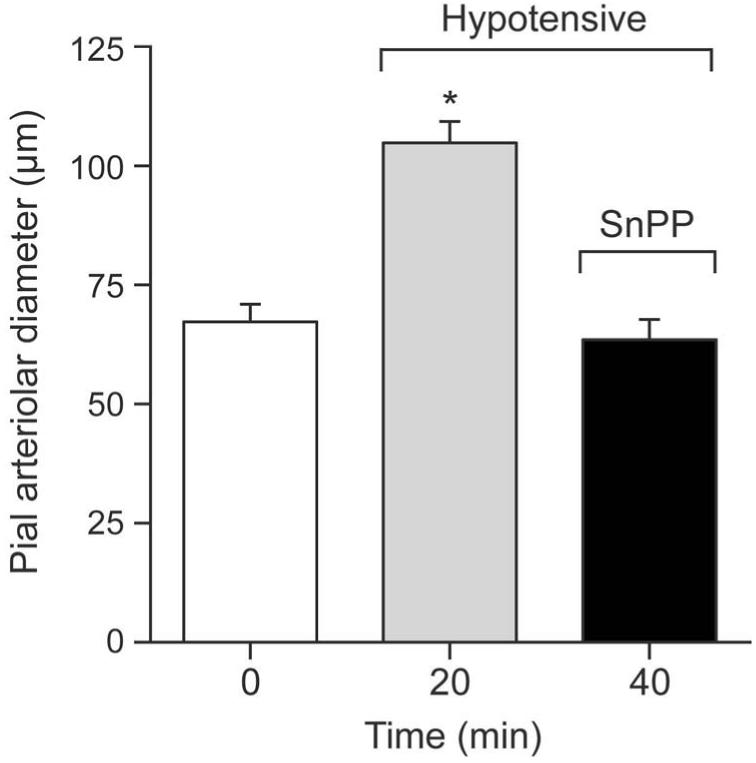
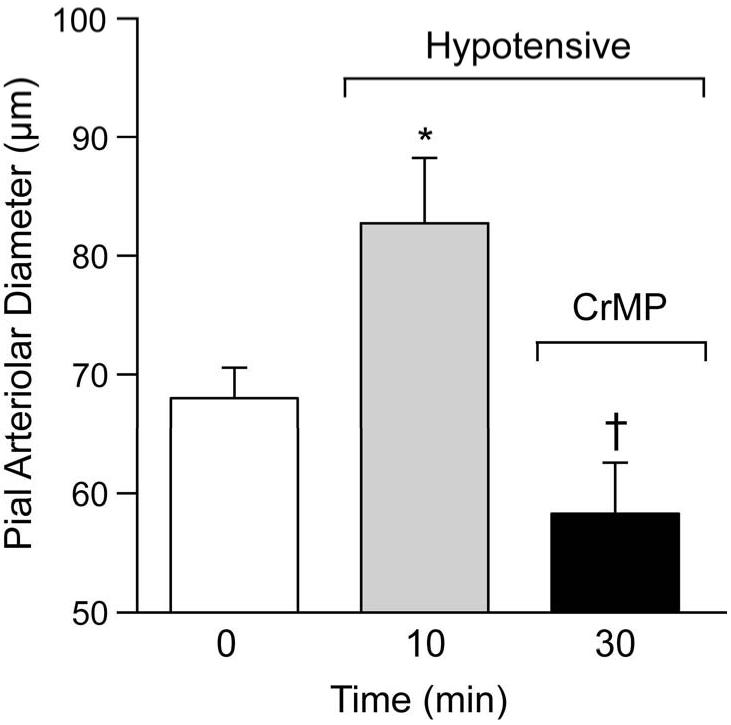
Figure 3 shows the effect of hypotension on pial arteriolar diameters and the effect of CrMP during hypotension on those diameters. Blood withdrawal produced rapid and sustained hypotension from 61±2mmHg to 33±3, 35±3, 37±3, and 38±2mmHg at 1, 5, 10, and 20 min, respectively. Concomitant with hypotension pial arterioles dilated immediately (within 1 min) from an average of 75 to about 110 μm that was sustained during 20 min of hypotension. Upon topical application of CrMP arterial pressure changed minimally (40±4, 40±5, 36±7, 38±6mmHg at 1,5,10 and 20 min, respectively) but, in contrast to normotension, pial arterioles constricted to normotensive diameters (Fig 3).
Figure 3.
Effect of hypotension and CrMP (2× 10−5M) on diameters of pial arterioles of piglets. Mean ± SEM. N=6 *P< 0.05 compared to zero (0) time.
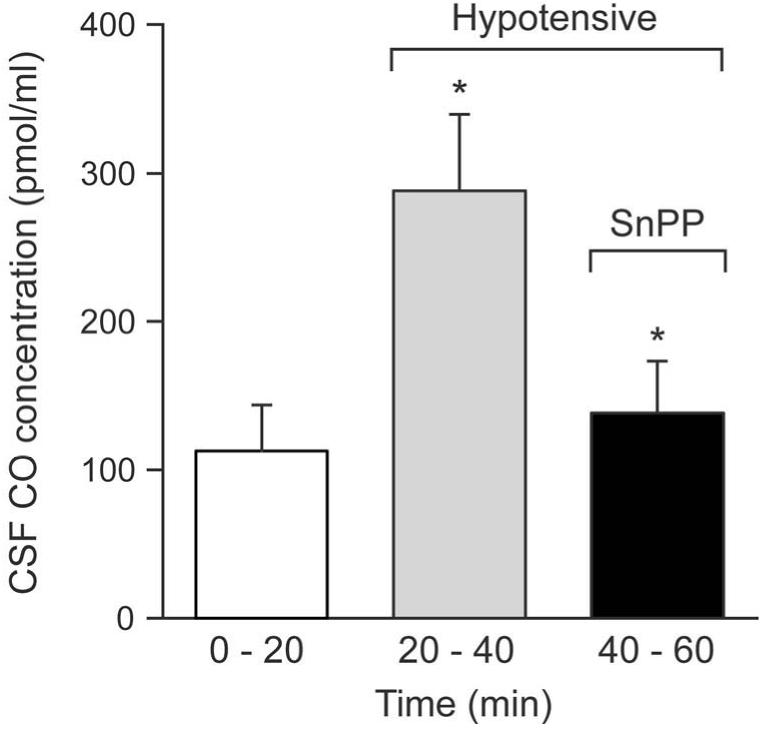
Hypotension markedly increased the CO concentration in CSF under the cranial window (Fig 4), concomitantly with the arteriolar dilation. That increase was reversed by systemic treatment with SnPP (Fig.4), that also constricted pial arterioles similarly to topical CrMP (Fig. 5).
Figure 4.
Effect of Hypotension and SnPP (3mg/kg iv) on CSF CO concentration in newborn pigs. Mean ± SEM. N=6 *P< 0.05 compared to previous column.
Figure 5.
Effect of SnPP (3mg/kg iv) on diameters of pial arterioles of hypotensive piglets. Mean ± SEM. N=6 *P< 0.05 compared to control.
Since NO and prostanoids could be involved in cerebrovascular dilation caused by CrMP or hypotension, we performed additional experiments in which both NO and prostacyclin were held constant (Methods).
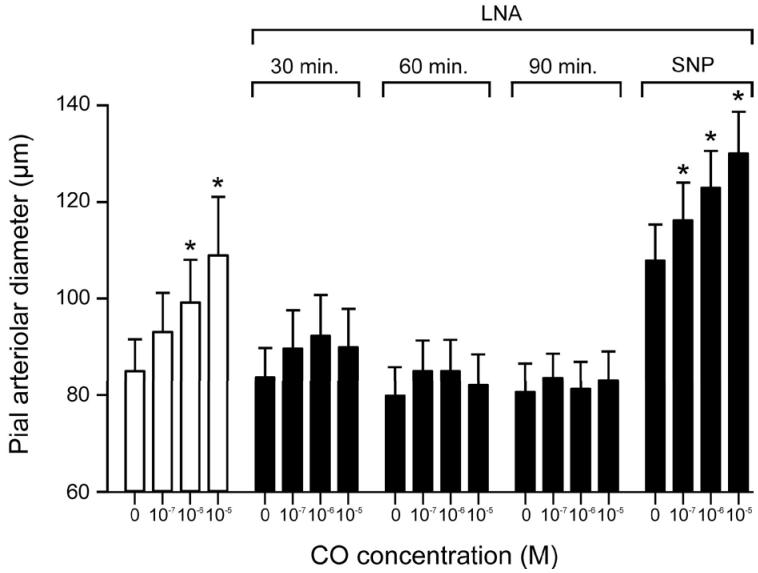
Results of the experiments conducted to confirm efficacy of procedure used to clamp NO and prostacyclin constant while not changing responses to CO are shown in Figures 6 and 7. Fig. 6 shows that dilation to CO was abolished by LNA, and this blockage was effective for at least 1.5 h. SNP (2×10−7 M) restored the dilation to CO following inhibition of NOS.
Figure 6.
Dilation to CO of pial arterioles before and in the presence of LNA(10−3M) and LNA + SNP (2×10−7M). Mean ± SEM. N=6 *P< 0.05 compared to no CO.
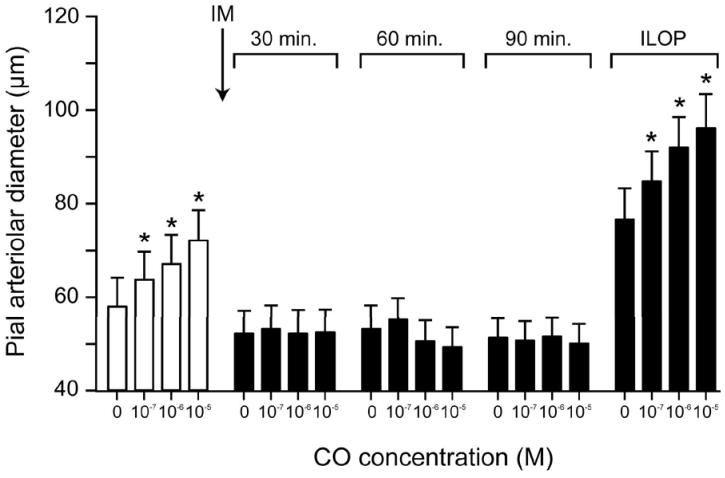
Figure 7.
Dilation to CO of pial arterioles before and after i.v. injection of indomethacin (5mg/kg i.m.) (at the arrow) and with iloprost (10−10 M). Mean ± SEM. N=6 *P< 0.05 compared to no CO.
Fig 7 shows the effect of COX inhibition with indomethacin on vasodilation of pial arterioles to CO. Dilation to CO that was blocked by indomethacin treatment was restored by 10−10M iloprost.
The effect of CrMP on normotensive piglets with NO and prostacyclin held constant is shown in Fig.8. In normotensive pigs with NO and prostacyclin held constant, CrMP produced a transient dilation (Fig.8) that was indistinguishable from intact piglets (Fig.1). Also, when prostacyclin and NO were held constant, CrMP caused vasoconstriction of pial arterioles of hypotensive pigs that was identical to that seen in piglets without NO/PG clamp (Fig. 9).
Figure 8.
Effect of CrMP (2× 10−5 M) on diameters of pial arterioles of normotensive piglets with NO/PG clamped. Mean ± SEM. N=4 *P< 0.05 compared to 10 min.
Figure 9.
Dilation to hypotension and the effect of CrMP (2× 10−5M) on diameters of pial arterioles of piglets with NO/PG clamped. Mean ± SEM. N=6 *P< 0.05 compared to control. †P< 0.05 compared to 10 min ( i.e. hypotension before CrMP treatment).
DISCUSSION
We found that 1) administration of CrMP blocked dilation to heme and caused transient dilation of pial arterioles in normotensive piglets. 2) Hypotension increased CSF CO concentration. 3) In hypotensive piglets, CrMP and SnPP, that decreased CO production, constricted pial arterioles sufficiently to completely reverse hypotension-induced dilation. 4) Clamping both NO and prostacyclin constant had no effect on the response of pial arterioles to CrMP in either normotensive or hypotensive piglets. Therefore, the present data suggest that CO contributes to hypotension-induced cerebral vasodilation in the newborn.
CO is a physiologically significant messenger molecule in the brain and contributes to the regulation of cerebrovascular circulation. CO is a potent vasodilator of newborn pig cerebral arterioles in vivo (24). CO contributes to dilation in response to excitatory amino acids, neuronal activity, and provides a modulatory vasodepressor influence that attenuates vasoconstrictor responses (6, 24, 38)
There are at least two potential endogenous sources of CO production. A small amount may be from oxidation of organic molecules (31), but the predominant source is from the degradation of heme. Results of topical application of HLL suggest endogenous HO can produce sufficient CO to dilate cerebral arterioles and CrMP as administered in the present study, blocks that production. Because no other heme metabolite has been shown to cause dilation and CrMP did not affect dilatory responses to CO, it is reasonable to propose that HO-derived CO is the mediator of the dilatory effect of HLL.
Cellular CO production results from metabolism of heme by HO. In freshly isolated cerebral microvessels from newborn pigs, as in the intact brain, in vivo, of the two known, highly catalytically active isoforms of HO, only HO-2 expression is detectable (28). HO-2 is constitutively expressed and induced by few stimuli (25), so expression of HO in the present experiments can be considered invariant.
CO production can be controlled by either regulation of substrate availability to HO, or the effective catalytic activity of HO-2 . HO-2 catalytic activity control mechanisms may be cell type and tissue specific. In neurons HO-2 activity can be stimulated by casein kinase 2 (CK2)-catalyzed phosphorylation of serine-709 (4). In neurons, glutamatergic activation of HO-2 results from metabotropic glutamate receptor-induced Ca2+ release, activation of protein kinase C (PKC), and CK2 phosphorylation (3). Conversely, in freshly isolated piglet cerebral microvessels, CO production is increased by ionotropic, but not metabotropic, glutamate receptor stimulation(18). In addition, protein tyrosine kinase inhibition decreased and tyrosine phosphatase inhibition increased basal CO production and glutamate stimulated CO production by piglet cerebral microvessels (18). Neither treatment of the cerebral microvessels with phorbol ester to activate PKC, H-7 to inhibit PKC, nor TBB to inhibit CK2 increased HO-2 catalytic activity (18).
Age related differences in mechanisms involved in cerebrovascular regulation have been observed (37, 41). CO induces cerebral arteriolar dilation in newborn pigs by activating large conductance Kca channels (14, 24). Whether the apparently increased vasodilatory sensitivity to CO observed in newborn pig cerebrovascular circulation when compared adult cerebral arteries (1, 5, 17, 24) is due to age, species, or both remains uncertain. However, developmental differences seem plausible considering low cerebrovascular sensitivity to CO is found in adult rats (1) and rabbits (5), and relative contributions of paracrine mediators in control of cerebrovascular circulation appear to be age dependent. For example, in the newborn pig contributions of prostanoids overshadow those of NO (41), while with age the importance of NO increases to the point where the relative contributions of prostacyclin and NO are similar to the adult (2, 34, 36, 37, 41). If COX is chronically inhibited in piglets, the involvement of NO is upregulated (40). COX activity stimulates HO-2 (16) and CO inhibits NOS (13) which could contribute to the reduced functional significance of NO and the increased importance of CO in the neonatal cerebrovascular circulation.
Rat gracilis muscle arteriole responses to CO are inhibited by blockers of Kca channels (35). When activated, Kca channels cause hyperpolarization and a reduction in voltage dependent Ca2+ channel activity (15). Inhibition of voltage dependent Ca2+ channels causes a reduction in intracellular Ca2+ concentration leading to dilation (15). Application of CO to freshly isolated piglet cerebral arteriolar myocytes increases the frequency and amplitude of outward K currents (STOCs), that are the results of Kca channel openings caused by Ca2+ sparks (14). CO activates Ca2+ sparks and strongly increases spark-to-STOC coupling (39).
CO may interact with other important paracrine mediators in control of cerebrovascular circulation during hypotension. Hypotensive hemorrhage stimulates the release of vascular prostanoids into cortical subarachnoid CSF concomitant with dilation of pial arterioles in anaesthesized newborn pigs (19). Prostacyclin can cause dilation directly via increases in vascular smooth muscle cAMP (29). Indomethacin, that blocks prostaglandin synthesis, reverses dilation of pial arterioles to hypotension (19). Along with prostacyclin, NO is responsible for endothelium derived relaxation of many arteries and counteracting vasoconstrictor effects of endothelin and thromboxane A2 (10, 11). In newborn pigs, neurally mediated dilation (26) involves NO. CO dose-dependently inhibits NO synthesis by rat renal arteries, although low concentrations of CO actually increase NO by causing release from a preformed pool (33).
CO-induced dilation of newborn cerebral arterioles involves interaction with the cerebral prostanoid and NO systems (23). NO and prostacyclin are obligate permissive enabling factors for CO-induced cerebrovascular dilation in newborn pigs. Inhibition of COX with indomethacin or inhibition of NOS with LNA blocks the dilatory responses of piglet cerebral arterioles to CO. Thus in the piglet cerebrovascular circulation, glutamate-induced pial arteriolar dilation can be blocked by either inhibiting (NOS) (26), which produces NO, or (HO) (30), which produces CO because the necessary permissive NO is gone following NOS inhibition. Blockage of CO-induced dilation by indomethacin and LNA can be reversed with iloprost and/or sodium nitroprusside (SNP) (present study, (21).
Therefore, to investigate the role of CO in dilation to hypotension without the complication of increasing NO and prostacyclin, we clamped NO and prostacyclin constant by blocking COX and NOS but returning the products with SNP and iloprost. Under these conditions the CO production could change but neither NO nor prostacyclin could. Nevertheless, the effects of HO inhibition were unaltered, suggesting that dilation did not involve hypotension-induced increases in NO and prostacyclin production. Further, the transient dilation to CrMP in normotension persisted, suggesting increased NO resulting from reduced CO modulation of NOS did not cause the dilation.
In summary, this study demonstrates that HO-derived CO is an important component of cerebrovascular dilation in response to reduced perfusion pressure in the newborn pig. The study impacts importantly on the regulatory influence of the heme/HO/CO system on the cerebral vasculature.
Acknowledgements
This research was supported by the National Heart, Lung, and Blood Institute/ National Institutes of Health (NHLBI/NIH). Dr. Kanu is supported by a postdoctoral training grant from NLHBI/NIH. We thank Danny Morse for preparing final figures and M. Lester for clerical assistance.
References
- 1.Andresen J, Bryan RMJ. Mouse cerebral arterioles dilate to carbon monoxide. FASEB J. 2006;20:A292. Abstract. [Google Scholar]
- 2.Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cerebral Blood Flow and Metab. 1994;14:1088–1095. doi: 10.1038/jcbfm.1994.142. [DOI] [PubMed] [Google Scholar]
- 3.Boehning D. Heme oxygenase-2 is activated by Calcium-Calmodulin. Circulation. 2004;91:2306–2309. doi: 10.1074/jbc.C400222200. [DOI] [PubMed] [Google Scholar]
- 4.Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- 5.Brian JEJ, Heistead DD, Faraci FM. Effects of carbon monoxide on rabbit cerebral arteries. Stroke. 1994;25:639–643. doi: 10.1161/01.str.25.3.639. [DOI] [PubMed] [Google Scholar]
- 6.Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol. 2003;285:H1148–1157. doi: 10.1152/ajpheart.00091.2003. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–2309. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 8.Coceani F. Control of the ductus arteriosus--a new function for cytochrome P450, endothelin and nitric oxide. Biochem Pharmacol. 1994;48:1315–1318. doi: 10.1016/0006-2952(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 9.Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 11.Ignarro LJ. Endothelium derived nitric oxide: actions and properties. FASEB J. 1989;3:31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- 12.Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuki E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilation in the adult rat cerebral circulation. Cir Res. 2005;97:e104–114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- 14.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 15.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 16.Kanu A, Gilpin D, Fedinec AL, Leffler CW. Cyclooxygenase products stimulate carbon monoxide production by piglet cerebral microvessels. Exp Biol Med. 2006;231:181–185. doi: 10.1177/153537020623100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komuro T, Borsody MK, Onon S, Marton LS, Weir BK, Zhang ZD, Paik E, MacDonald RL. The vasorelation of cerebral arteries by carbon monoxide. Exp Biol Med. 2001;226:860–865. doi: 10.1177/153537020122600909. [DOI] [PubMed] [Google Scholar]
- 18.Leffler CW, Balabanova L, Sullivan CD, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;285:H292–297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]
- 19.Leffler CW, Busija DW. Prostanoids and pial arteriolar diameter in hypotensive newborn pigs. Am J Physiol. 1987;252:H687–691. doi: 10.1152/ajpheart.1987.252.4.H687. [DOI] [PubMed] [Google Scholar]
- 20.Leffler CW, Busija DW, Beasley DG, Fletcher AM. Maintenance of cerebral circulation during hemorrhagic hypotension in newborn pigs: role of prostanoids. Circ Res. 1986;59:562–567. doi: 10.1161/01.res.59.5.562. [DOI] [PubMed] [Google Scholar]
- 21.Leffler CW, Fedinec AL, Parfenova H, Jaggar JH. Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol. 2005;289:H432–438. doi: 10.1152/ajpheart.01195.2004. [DOI] [PubMed] [Google Scholar]
- 22.Leffler CW, Mirro R, Pharris LJ, Shibata M. Permissive role of prostacyclin in cerebral vasodilation to hypercapnia in newborn pigs. Am J Physiol. 1994;267:H285–291. doi: 10.1152/ajpheart.1994.267.1.H285. [DOI] [PubMed] [Google Scholar]
- 23.Leffler CW, Nasjletti A, Johnson RA, Fedinec AL. Contributions of prostacyclin and nitric oxide to carbon monoxide-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol. 2001;280:H1490–1495. doi: 10.1152/ajpheart.2001.280.4.H1490. [DOI] [PubMed] [Google Scholar]
- 24.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol. 1999;276:H1641–1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 25.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Ann Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Tobin JR, Busija D. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through NMDA receptors. Stroke. 1995;26:857–863. doi: 10.1161/01.str.26.5.857. [DOI] [PubMed] [Google Scholar]
- 27.Morita T, Perella MA, Lee MF, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parfenova H, Neff RA, 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 29.Parfenova H, Shibata M, Zuckerman S, Mirro R, Leffler CW. Cyclic nucleotides and cerebrovascular tone in newborn pigs. Am J Physiol. 1993;265:H1972–1982. doi: 10.1152/ajpheart.1993.265.6.H1972. [DOI] [PubMed] [Google Scholar]
- 30.Robinson JS, Fedinec AL, Leffler CW. Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H2371–2376. doi: 10.1152/ajpheart.00911.2001. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers PA, Vreman HJ, Dennery PA, Stevenson DK. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994;18:2–10. [PubMed] [Google Scholar]
- 32.Tenhunen R, Tokola O, Linden IB. Heam arginate: a new stable haem compound. J Pharm Pharmacol. 1987;39:780–786. doi: 10.1111/j.2042-7158.1987.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 33.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol Renal Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Pelligrino DA, Paulson OB, Lassen NA. Comparison of the effects of NG-nitro-L-arginine and somatosensory stimuli in rats. Brain Res. 1994;641:257–264. doi: 10.1016/0006-8993(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Wang Z, Wu L. Carbon monoxide -induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol Heart Circ Physiol. 2001;281:H2366–2377. doi: 10.1152/ajpheart.2001.281.6.H2366. [DOI] [PubMed] [Google Scholar]
- 37.Willis AP, Leffler CW. NO and prostanoids: age dependence of hypercapnia and histamine-induced dilations of pig pial arterioles. Am J Physiol. 1999;277:H299–307. doi: 10.1152/ajpheart.1999.277.1.H299. [DOI] [PubMed] [Google Scholar]
- 38.Winestone JS, Bonner C, Leffler CW. Carbon monoxide as an attenuator of vasoconstriction in piglet cerebral arterioles. Exp Biol Med. 2003;228:46–50. doi: 10.1177/153537020322800106. [DOI] [PubMed] [Google Scholar]
- 39.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Leffler CW. Compensatory role of NO in cerebral circulation of piglets chronically treated with indomethacin. Am J Physiol Regul Integr Comp Physiol. 2002;282:R400–410. doi: 10.1152/ajpregu.00256.2001. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol. 1996;271:H535–540. doi: 10.1152/ajpheart.1996.271.2.H535. [DOI] [PubMed] [Google Scholar]