Abstract
Insulin-like growth factor 1 (IGF1) has been proposed as a “G1-progression factor” and as a mediator of estradiol’s (E2) mitogenic effects on the uterus. To test these hypotheses, we compared E2’s mitogenic effects on the uteri of Igf1-targeted gene deletion (null) and wild-type littermate mice. The proportion of uterine cells involved in the cell cycle and G1- and S-phase kinetics were not significantly different in wild-type and Igf1-null mice. However, the appearance of E2-induced mitotic figures and cell number increases were profoundly retarded in Igf1-null uterine tissue. There was a significant increase in nuclear DNA concentration in Igf1-null cells, consistent with a G2 arrest. Interestingly, apoptotic cells were also significantly reduced in abundance, and the normal massive apoptotic response to E2 withdrawal was absent in the Igf1-null uterus. These data show that Igf1 is an essential mediator of E2’s mitogenic effects, with a critical role not in G1 progression but in G2 progression.
Estradiol (E2) has potent stimulatory effects on uterine growth, with unopposed E2 action culminating in uterine neoplasia. Media from E2-treated uterine explants contains factors capable of stimulating tumor cell proliferation, suggesting that E2’s mitogenic effects are mediated by local growth factor production (1). Insulin-like growth factor 1 (IGF1) has been implicated as a potential mediator of E2’s effects on uterine growth [“estromedin” (2)] because E2 induces uterine IGF1 expression (3–11) in a pattern that shows a significant spatiotemporal correlation with E2-induced cell proliferation (11). Furthermore, Igf1-targeted gene deletion mice demonstrate a disproportionate reduction in uterine size, although it is not clear whether this is caused by reduced estrogen production (12) or by impaired estrogen effect on the uterus secondary to absent local IGF1. To test the hypothesis that IGF1is required for E2-induced mitogenesis and to identify the cell-cycle stages in which IGF1 acts in vivo, we compared the effects of exogenous E2 treatment on uterine cell-cycle parameters in homozygous Igf1 null and wild-type (wt) littermate mice.
METHODS AND RESULTS
The mice used in this study were from an Igf1 deletion line derived and genotyped as previously described (13). Postnatal day 40 (P40) Igf1 null and wt littermate female mice were treated with E2 or vehicle and killed 21 h later, 1 h after [3H]thymidine injection. The proportion of uterine epithelial, stromal, and myometrial cells in S phase, determined by the 3H-labeling index was equal in vehicle-treated wt and null mice (Table 1). E2 treatment stimulated an ≈7-fold increase in the percentage of labeled epithelial cells and had minor effects on stromal and myometrial cell labeling in both genotypes (Fig. 1; Table 1). Given the conventional view of IGF1 as a “G1-progression factor” (14), finding equal proportions of cells in S phase in Igf1-null and wt uteri was unexpected. We considered the possibility that Igf1-null cells might have a prolonged S phase, increasing the probability of detecting cells in S phase and potentially obscuring a smaller proliferative fraction. However, cumulative labeling experiments (15) employing [3H]thymidine and BrdUrd administered in series showed that S-phase influx, duration, and efflux were not significantly different in Igf1-null and wt mice (Fig. 2; Table 2). Because previous work has shown that E2 has maximal effects on uterine-cell proliferation in prepubertal mice (16), the experiments were repeated in P20 mice. E2-evoked DNA synthesis was equally robust in Igf1-null and wt uteri, except for a slightly reduced effect in Igf1-null myometrial cells (Fig. 3A).
Table 1.
E2 effects on DNA labeling index (LI) in wt and Igf1−/− uteri
| LI | Vehicle
|
E2
|
||||
|---|---|---|---|---|---|---|
| Epi | Stro | Myo | Epi | Stro | Myo | |
| wt | 7.27 ± 0.26 | 5.42 ± 2.06 | 7.23 ± 3.38 | 47.07 ± 4.47 | 7.07 ± 1.64 | 6.96 ± 2.19 |
| Igf1−/− | 5.08 ± 1.35 | 7.16 ± 2.50 | 2.42 ± 0.37 | 49.92 ± 3.45 | 9.20 ± 3.28 | 5.25 ± 0.95 |
| P | 0.53 | 0.56 | 0.18 | 0.64 | 0.6 | 0.46 |
There were no significant differences in LI between wt and Igf1−/− uterine cell populations either at baseline (vehicle-treated) or 21 h after E2 treatment. The LI was obtained for each uterine cell type (Epi, epithelial; Stro, stromal; and Myo, myometrial) by scoring >400 nuclei (meaning values for at least two sections per animal) for the number of positive (>5 silver grains) nuclei. Data representing 5–8 mice per group are expressed as mean ± SEM in percent labeling.
Figure 1.
Autoradiographic visualization of E2-induced DNA synthesis in wt (A, C, and E) and Igf1-null (B, D, and F) uteri. A and C and B and D are paired bright- and dark-field low-magnification photomicrographs. E and F are higher magnification dark-field views showing localization of tritium-positive red nuclei in epithelium (ep), stroma (st), and myometrium (my). Mice were given a single intraperitoneal injection of 17-β-estradiol (1 μg/g) at 0 h and injected with [3H]thymidine (2 μCi/g; 1 Ci = 37 GBq) 1 h before sacrifice at 21 h. Sections from the middle third of each uterine horn were exposed to photographic emulsion for 3 weeks, developed, and stained with hematoxylin/eosin and photographed through a red filter. Magnification ×50 (A–D) and ×200 (E and F).
Figure 2.
To compare S-phase duration and flux in wt and Igf1−/− uteri, mice were given [3H]thymidine (2 μCi/g) 3 h before and BrdUrd (Amersham Pharmacia RPN 201, 10 μl/g) 1 h before sacrifice. Uterine sections were processed for immunodetection of BrdUrd and then for autoradiographic detection of 3H-labeled nuclei. 3H-only-positive nuclei (>5 silver grains) are indicated by solid arrowheads, BrdUrd-only-positive nuclei by open arrowheads, and doubly labeled nuclei by twin arrowheads. wt (A) and Igf1-null (B) uteri demonstrate equal proportions of cells in each category (see Table 2). 3H-only-positive nuclei represent cells that exited S phase and BrdUrd-only positive nuclei represent cells that entered S phase during the course of the experiment. Doubly labeled nuclei represent cells that were in S phase for over 2 h (the time between thymidine and BrdUrd injection) during the course of the experiment. The percentage of doubly labeled cells reflects of S-phase duration. (Bar = 25 μm.)
Table 2.
S-phase kinetics in E2-treated wt and Igf1-null mice
| LI1
|
LIcum
|
Influx
|
Efflux
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epi | Stro | Myo | Epi | Stro | Myo | Epi | Stro | Myo | Epi | Stro | Myo | |
| wt | 50.6 ± 1.5 | 13.2 ± 1.8 | 9.8 ± 2.2 | 64.8 ± 2.3 | 15.0 ± 1.5 | 11.9 ± 1.3 | 14.3 ± 0.9 | 1.8 ± 0.4 | 2.1 ± 1.1 | 17.7 ± 3.4 | 7.9 ± 2.6 | 4.9 ± 1.9 |
| Igf1−/− | 58.5 ± 4.8 | 15.3 ± 3.1 | 6.0 ± 1.0 | 72.5 ± 3.5 | 17.6 ± 3.9 | 8.4 ± 0.7 | 14.0 ± 1.7 | 2.5 ± 1.0 | 2.4 ± 1.0 | 22.6 ± 4.6 | 8.4 ± 1.7 | 3.2 ± 0.9 |
The cumulative labeling index (LIcum, all labeled nuclei/total nuclei) is equally increased over the single labeling index (LI1, 3H-labeled nuclei/total nuclei) in E2-treated Igf1−/− and wt mice, showing that S-phase duration is equal in the two groups. A further estimate of S-phase duration, given by the percentage of double-labeled nuclei (simultaneously BrdUrd and 3H-positive) also was equal in both groups (data not shown). S-phase influx, represented by the percentage of BrdUrd-only positive nuclei, and efflux, represented by 3H-only positive nuclei, also are equal in the two groups. All data are given as mean ± SEM (percentage of all nuclei for each cell type). There were 3–6 mice in each group, and no statistically significant differences were found for any comparison between the two genotypes. Abbreviations are as in Table 1.
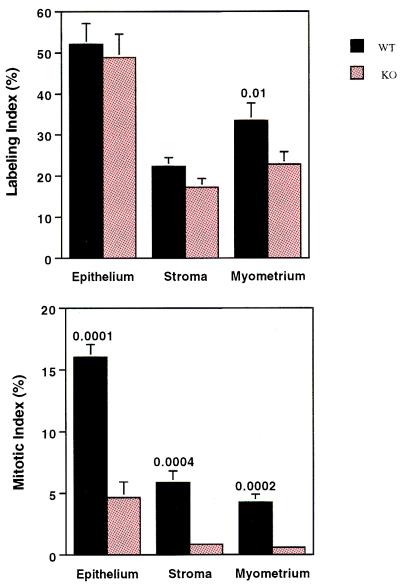
Figure 3.
Comparison of labeling index and mitotic index in the different cell types of wt and Igf1-null uteri 21 h after E2 stimulation. These data are from P20 mice, at which age the greatest proliferative response to E2 occurs. Similar data (14.7 ± 2.1 vs. 4.0 ± 1.3, respectively) were obtained from the analysis of the mitotic index in P40 wt and Igf1-null uterine epithelium. The mitotic index in other uterine cell types at P40 was so low in both wt and Igf1-null uteri that it was not counted. The values shown are means ± SEM for 8–12 mice per group.
These data show that G1 progression is normal despite IGF1 deletion. Furthermore, the double-labeling data show that Igf1-null cells transit S phase normally. There is, however, a problem between S and M phase, because mitotic figures are profoundly reduced in Igf1-null uteri (Fig. 3B). The mitotic index was 3–4-fold higher in wt epithelium and approximately 7-fold higher in wt stroma and myometrium 21 h post-E2 treatment. Normally, the mitotic response peaks 20–24 h after a dose of E2 and returns to baseline by 48 h (17), at which time extensive apoptosis occurs in response to E2 withdrawal (18). This was the pattern observed in wt mice. In Igf1-null mice, however, the MI was only modestly increased at 21 h, but remained significantly elevated 48 h post-E2 (Fig. 4; Table 3), suggesting that some cells were still progressing from G2 to M phase long after the E2 stimulus. One possible explanation for the discrepancy between the robust S-phase response and the dearth of mitotic figures is that large numbers of Igf1-null cells undergo programmed cell death after S phase. Intense scrutiny of uterine tissue by using in situ end labeling for detection of fragmented DNA as well as 4′,6-diamidino-2-phenylindole (DAPI) and hematoxylin staining for apoptotic bodies demonstrated that apoptosis was consistently more frequent in wt than in Igf1-null mice. Therefore, increased cell death is not responsible for the arrested cell cycle in the Igf1-null murine uterus. Interestingly, the massive apoptosis normally seen 48 h after E2 treatment failed to appear in the Igf1-null uterus (Fig. 4; Table 3), suggesting, as a matter of speculation, that this response to E2 withdrawal depends on completion of the E2-induced mitotic cycle.
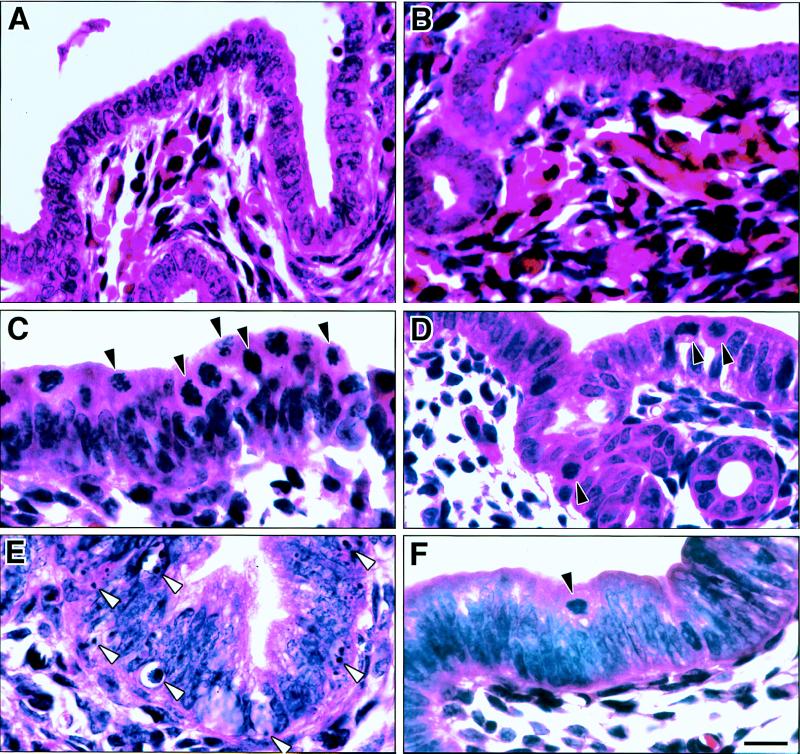
Figure 4.
Mitosis and apoptosis in wt (A, C, and E) and Igf1-null (B, D, and F) uterine epithelium. Mice were treated with vehicle (A and B) or E2 for 21 h (C and D) or 48 h (E and F). Solid arrowheads indicate some mitotic figures, and open arrowheads show examples of apoptotic bodies. The significantly greater occurrence of apoptosis in wt uteri shown here by hematoxylin/eosin staining was confirmed by analysis of DAPI-stained sections and by in situ end-labeling of fragmented DNA. (Bar = 10 μm.)
Table 3.
Time course of mitotic and apoptotic responses in Igf1−/− and wt uterine epithelium
| Treatment | MI, %
|
DI, %
|
Cell no.
|
Cell size
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt | Igf1−/− | P | wt | Igf1−/− | P | wt | Igf1−/− | P | wt | Igf1−/− | P | |
| Vehicle | 0.87 ± 0.24 | 0.22 ± 0.18 | 0.1 | 0.42 ± 0.22 | 0.13 ± 0.08 | 0.3 | 264 ± 13 | 247 ± 46 | 0.73 | 9.2 ± 1.5 μ | 6.8 ± 0.4 μ | 0.17 |
| 21 h post-E2 | 16.03 ± 1.03 | 4.64 ± 1.26 | 0.0001 | 1.21 ± 0.16 | 0.50 ± 0.08 | 0.44 | 400 ± 49 | 230 ± 19 | 0.003 | 16.5 ± 1.2 μ | 10.5 ± 1.0 μ | 0.003 |
| 48 h post-E2 | 1.19 ± 0.18 | 4.60 ± 0.72 | 0.015 | 80.33 ± 6.3 | 2.08 ± 0.75 | 0.0001 | 492 ± 78 | 357 ± 28 | 0.055 | 17.1 ± 13 μ | 14.6 ± 1.0 μ | 0.19 |
The mitotic index (MI) and death index (DI) were obtained by scoring the number of mitotic and apoptotic figures per 100 epithelial nuclei in hemotoxylinleosin-stained uterine sections. Evaluation of mitotic and apoptotic figures by DAPI (4,6-diamidino-2-phenylindole) staining confirmed the findings with hemotoxylinleosin. The cell number data represents the number of luminal epithelial cells present in anatomically matched uterine cross sections. The cell size data represents the length of luminal epithelial cells from apical to basement membranes. All data are mean ± SEM, with 3–12 mice in each group.
An alternative explanation for the discrepancy between S and M phase is that cells are arrested or retarded in the G2 phase of the cell cycle in Igf1-null mice. This would explain the prolonged mitotic response, which is apparent 2 days after the E2 stimulus in these mice. Additional support for the concept of a G2 delay because of IGF1 deletion is the finding that epithelial cell numbers do not significantly increase in the Igf1-null uterus until 2 days after E2 treatment (Table 3). More direct support for the G2-arrest hypothesis is provided by the fact that mean DNA concentration is significantly increased in E2-treated Igf1-null vs. wt epithelial nuclei, as shown by image-analysis of DAPI-stained sections (92.7 ± 2.4 vs. 79.6 ± 3.6 arbitrary density units, P < 0.03). Similar data were obtained by using Feulgen-stained tissue for the analysis of nuclear DNA concentration. Signaling molecules such as hormones or growth factors have not been previously implicated in regulation of G2. Yeast demonstrate a G2 checkpoint based on cell size (19), which may be relevant to the present observations, because we have observed a consistent reduction in cell size in the Igf1-null mice. The first day after E2 treatment, wt epithelial cell length from apical to basement membrane had increased by almost 200%, whereas Igf1-null epithelial cells had increased only ≈50% at this time point (Table 3). Of interest, wt epithelial height declined after the 21 h timepoint, whereas Igf1-null epithelium continued to grow at least through the 48-h time point (Table 3). These data show that retardation in mitoses is correlated with retardation of cellular somatic growth in the absence of IGF1, suggesting that a G2 checkpoint based on cell size, similar to that seen in yeast, might operate in this complex mammalian system.
CONCLUSIONS
Studies in growth-arrested fibroblast cell lines established the view that growth factors act primarily in the G1 phase of the cell cycle (14, 20). Peptides such as PDGF, FGF, and EGF, termed “competence factors,” stimulated quiescent cells to enter G1, whereas IGF1 spurred progression through G1 to S phase and therefore was deemed a G1-progression factor (14, 20, 21). The present study analyzed the effects of IGF1 deletion on cell cycle dynamics in vivo, and has shown that progression through G1 and S phases is normal in Igf1-null uterine tissue, indicating that this peptide is not essential as a G1-progression factor. However, Igf1-null cells are profoundly retarded in their transit through G2, suggesting that IGF1 is required for timely progression through G2/M phases of the cell cycle. It is likely that immortalized cell lines under artificial growth-arrest conditions demonstrate unique features of cell-cycle regulation, which may not be relevant to normal cells in their natural environments. In addition, many in vitro studies limited observations to G1 and the initiation of S phase, and thus major growth factor effects on later phases would have been missed. Illustrating this point, a recent study examining all phases of the cell cycle reported that IGF1 receptor-null fibroblasts demonstrate a G2/M phase duration 4-fold longer than comparable wt cells, supporting the view that IGF1 plays an important role in later phases of the cell cycle (22).
In addition to defining a major role for IGF1 in G2 as opposed to G1 progression, this study demonstrates that IGF1 is in fact an important mediator of E2’s mitogenic effects in the uterus. Together with previous evidence that E2 induces uterine IGF1 expression (3–11), the present data shows that E2 induces uterine growth in two phases, with early events through S phase independent of IGF1, whereas later events culminating in mitosis are dependent on E2-induced IGF1 synthesis and action.
Acknowledgments
We thank Ricardo Dreyfuss for expert photomicrography.
ABBREVIATIONS
- E2
estradiol, IGF1, insulin-like growth facor 1
- wt
wild type
- P
postnatal day
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Murphy L J, Ghahary A. Endocr Rev. 1990;11:443–455. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 2.Sirbasku D A. Proc Natl Acad Sci USA. 1978;75:3786–3789. doi: 10.1073/pnas.75.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy L J, Murphy L C, Freisen H G. Endocrinology. 1987;1:445–451. doi: 10.1210/mend-1-7-445. [DOI] [PubMed] [Google Scholar]
- 4.Norstedt G, Levinovitz A, Erikson H. Acta Endocrinol. 1989;120:466–473. doi: 10.1530/acta.0.1200466. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Tamada H, Dey S K, Andrews G K. Biol Reprod. 1992;46:208–211. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- 6.Simmen R C M, Simmen F A, Hofig A, Farmer S J, Bazer F W. Endocrinology. 1990;127:2166–2172. doi: 10.1210/endo-127-5-2166. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson K R, Gilmour R S, Wathes D C. Endocrinology. 1994;134:1655–1661. doi: 10.1210/endo.134.4.8137728. [DOI] [PubMed] [Google Scholar]
- 8.Giudice L C, Dsupin B A, Jin I H, Vu T H, Hoffman A R. J Clin Endocrinol Metab. 1993;76:1115–1120. doi: 10.1210/jcem.76.5.8496300. [DOI] [PubMed] [Google Scholar]
- 9.Vollenhoven B J, Herington A C, Healy D L. J Clin Endocrinol Metab. 1993;76:1106–1111. doi: 10.1210/jcem.76.5.7684390. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Dsupin B A, Giudice L C, Bondy C A. J Clin Endocrinol Metab. 1994;79:1723–1728. doi: 10.1210/jcem.79.6.7527408. [DOI] [PubMed] [Google Scholar]
- 11.Adesanya O O, Zhou J, Bondy C A. J Clin Endocrinol Metab. 1996;81:1967. doi: 10.1210/jcem.81.5.8626866. [DOI] [PubMed] [Google Scholar]
- 12.Baker J, Hardy M P, Zhou J, Bondy C A, Lupu F, Bellve A R, Efstratiadis A. Mol Endocrinol. 1996;10:903–909. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 13.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart T A. Genes Dev. 1993;7:2609–2616. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 14.Stiles C D, Capone G T, Scher C D, Antoniades H N, Van Wyk J J, Pledger W J. Proc Natl Acad Sci USA. 1979;76:1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hume W J, Thompson J. Cell Tissue Kinet. 1990;23:313. doi: 10.1111/j.1365-2184.1990.tb01127.x. [DOI] [PubMed] [Google Scholar]
- 16.Quarmby V, Korach K S. Endocrinology. 1984;114:694–701. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- 17.Kaye A M, Sheratzky D, Lindner H R. Biochim Biophys Acta. 1972;261:475–479. doi: 10.1016/0304-4165(72)90072-4. [DOI] [PubMed] [Google Scholar]
- 18.Rotello R J, Lieberman R C, Lepoff R B, Gerschenson L E. Am J Pathol. 1992;140:449–456. [PMC free article] [PubMed] [Google Scholar]
- 19.Svelcer A, Novak B, Mitchison J M. J Cell Sci. 1996;109:2947–2957. doi: 10.1242/jcs.109.12.2947. [DOI] [PubMed] [Google Scholar]
- 20.Pardee A B. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 21.Rubin R, Baserga R. Lab Invest. 1995;73:311–315. [PubMed] [Google Scholar]
- 22.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Mol Cell Biol. 1994;14:3604–3608. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]