Abstract
Potassium uptake by higher plants is the result of high- or low-affinity transport accomplished by different sets of transporters. Although K+ channels were thought to mediate low-affinity uptake only, the molecular mechanism of the high-affinity, proton-dependent K+ uptake system is still scant. Taking advantage of the high-current resolution of the patch-clamp technique when applied to the small Arabidopsis thaliana guard cells densely packed with voltage-dependent K+ channels, we could directly record channels working in the concentration range of high-affinity K+ uptake systems. Here we show that the K+ channel KAT1 expressed in Arabidopsis guard cells and yeast is capable of mediating potassium uptake from media containing as little as 10 μM of external K+. Upon reduction of the external K+ content to the micromolar level the voltage dependence of the channel remained unaffected, indicating that this channel type represents a voltage sensor rather than a K+-sensing valve. This behavior results in K+ release through K+ uptake channels whenever the Nernst potential is negative to the activation threshold of the channel. In contrast to the H+-coupled K+ symport shown to account for high-affinity K+ uptake in roots, pH-dependent K+ uptake into guard cells is a result of a shift in the voltage dependence of the K+ channel. We conclude that plant K+ channels activated by acid pH may play an essential role in K+ uptake even from dilute solutions.
Since the initial observation of Epstein that K+ uptake into plant cells can be decomposed into the activity of high- and low-affinity transport systems (1), the molecular structure of three different K+ transporters, K+ channels, and two distinct carrier types, has been identified. In 1992, AKT1 and KAT1 (2, 3) were shown to represent the first members of a large family of inward-rectifying K+ channels (4), channel subtypes that had been recognized from patch-clamp studies in almost all plant cell types studied so far (5). In the presence of millimolar K+ concentrations and upon hyperpolarization of the plasma membrane, these inward rectifiers mediate K+ uptake. Based on studies in the whole-cell configuration of the patch-clamp technique applied to Vicia faba guard cell protoplasts, Schroeder and Fang (6) calculated an affinity constant of 3.5 mM for these guard cell K+ channels. Analysis of the current–voltage relation obtained from guard cells and root cells exposed to varying external K+ concentrations revealed that the activation potential of the inward currents shifted to 20- to 30-mV more negative values with decreasing K+ concentrations (6–8), a behavior from which it was concluded that K+ channels can sense the external potassium. Thus, channel opening was supposed to occur only when the K+ gradient is inward, restricting these transporters to function as K+-sensing K+ uptake valves (9). Supporting this hypothesis, these channels were reported to not open in the absence of extracellular K+ (9). Because of this effect, K+ uptake channels were not supposed to form shunt pathways through which K+, accumulated by high-affinity carriers, can leak out of the cells.
To date, two high-affinity plant K+ carriers have been identified. In 1994, Schachtman and Schroeder reported on the discovery of HKT1, a proton-driven K+ symporter from Hordeum vulgare (10). Later, however, detailed studies revealed that HKT1 mediates Na+-driven rather than H+-driven K+ uptake (11). In search for the molecular entity generating the H+/K+ phenomenon, Escherichia coli-like K+ transporters recently were identified in barley (12) and Arabidopsis (13). Later studies showed that members of this family mediate both high- and low-affinity K+ transport (14, 15). It is, however, still unclear which members of the channel or carrier families account for acid-induced K+ uptake observed in vivo (16).
Here we report that hyperpolarization-activated K+ channels in guard cells are capable of mediating high-affinity K+ uptake, the activity of which strongly depends on the external proton concentration. Supporting this finding we show that K+ uptake-deficient yeast, when complemented with this guard cell K+ channel, regained the ability to grow in micromolar K+ media. Therefore, acid-activated K+ uptake channels might represent a molecular equivalent contributing to the H+/K+ phenomenon.
MATERIALS AND METHODS
Yeast Genetics.
Potassium-dependent growth assays were performed by using the Saccharomyces cerevisiae strain JR Y339 (17). KAT1 was subcloned into the yeast expression vector pGK (18) and transformed into JR Y339 using the lithium acetate method (19). Growth assays were performed on SDAP medium (20) containing 2% purified Agar (Sigma A7921) supplemented with potassium at concentrations indicated in the figure legends.
Patch-Clamp Experiments.
Arabidopsis thaliana seedlings (L. cv. C24; Arabidopsis Stock Center, Columbus, Ohio) were grown in a growth chamber, and guard cell protoplasts were isolated as described before (21). Current measurements were performed by using an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany) and low-pass-filtered with an eight-pole Bessel filter. Whole-cell data were low-pass-filtered with a cut-off frequency of 2 kHz. Data were sampled at 2.5 times the filter frequency (for single-channel recording this factor was 5), digitized (ITC-16; Instrutech, Mineola, NY), stored on hard disk, and analyzed with the software pulse and pulsefit from Instrutech on a Gravis TT200. Patch pipettes were prepared from Kimax-51 glass (Kimble Glass, Vineland, NJ) and coated with silicone (Sylgard 184 silicone elastomer kit; Dow-Corning). To determine membrane potentials, the command voltages were corrected off-line for series resistances and liquid junction potentials according to Neher (22). The standard pipette solution (cytoplasm) contained 300 mM potassium gluconate/2 mM MgCl2/3 mM CaCl2/5 mM EGTA/2 mM MgATP/10 mM Hepes-Tris, pH 7.5. The bathing medium contained 10 mM citrate-Tris, pH 4.5 or 6.0 or 10 mM Mes-Tris, pH 6.0, in addition to 0.26 mM MgCl2 or 2.6 mM MgCl2, respectively (0.1 mM free Mg2+). Potassium was adjusted by using potassium gluconate as indicated in the figure legends. The K+ content of all solutions was verified by atomic-absorption spectroscopy. Single-channel recordings were performed in 150 mM symmetrical potassium gluconate using standard pipette solution and a bathing medium containing 1 mM CaCl2/10 mM citrate-Tris, pH 5.0. All solutions were adjusted to 540 mosmol/kg by using d-sorbitol. Chemicals were obtained from Sigma.
Biophysical Analysis.
Relative open probabilities were deduced from a double voltage-step protocol. Time- and voltage-dependent K+ currents were elicited in response to hyperpolarization. During the second voltage step, K+ currents relaxed in a time-dependent manner. The instantaneous current–voltage relationship, obtained from extrapolating the relaxation time course of the second pulse to t = 0 with an exponential function, is proportional to the relative open probability, pO(V), at the end of the activation pulse. Relative open probabilities were fitted with a Boltzmann function:
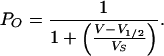 |
Here, V1/2 denotes the voltage at which 50% of the channels are active, and VS is the slope factor that is correlated to the charge of the voltage sensor.
RESULTS AND DISCUSSION
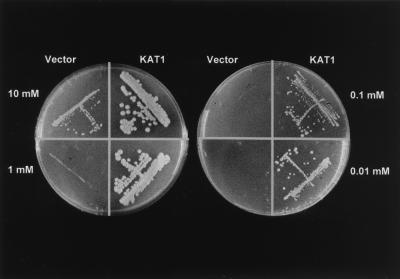
When we complemented the yeast mutant lacking the K+ transporters Δtrk1 and Δtrk2 with KAT1, a K+ channel expressed in A. thaliana guard cells (23), growth was rescued in low-K+ medium (Fig. 1), a behavior that has been used previously for cloning the first plant K+ uptake channels, KAT1 and AKT1 (2, 3). Although the mutant containing the vector alone did not grow at K+ concentrations below 10 mM, KAT1 supported yeast growth in as low as 10 μM K+ in the culture medium, indicating that K+ channels are able to catalyze high-affinity K+ uptake.
Figure 1.
Potassium concentration-dependent growth of the K+ uptake-deficient yeast mutant JR Y339 complemented with the A. thaliana guard cell K+ channel KAT1 or the empty vector.
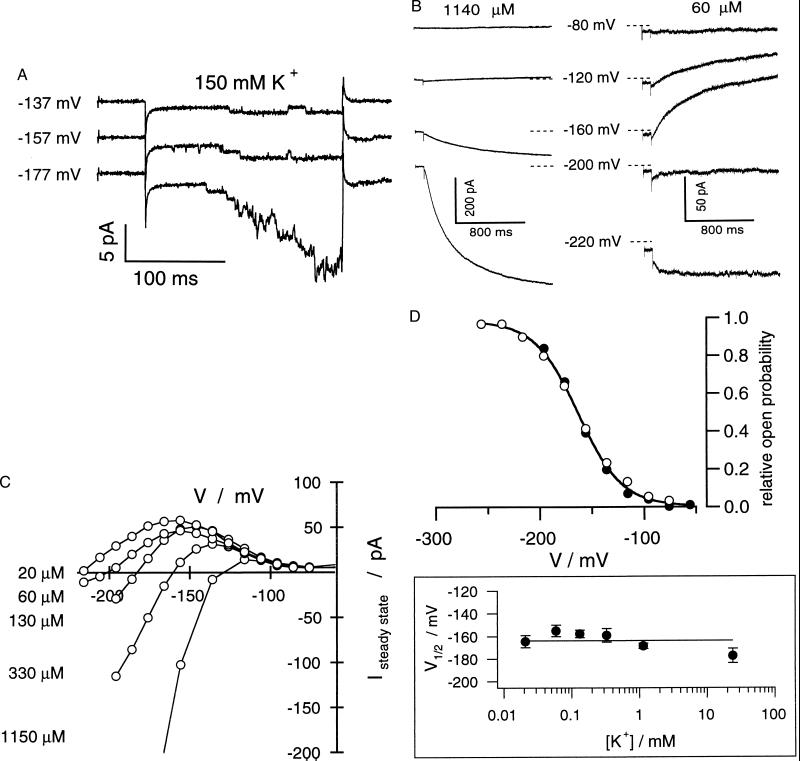
In the presence of 150 mM K+, inward-rectifying K+ channels in guard cells as well as the gene products of KAT1 and KST1 [guard cell K channel of Solanum tuberosum (24)] expressed in Xenopus oocytes are characterized by a unitary conductance in the order of 5–8 pS (Fig. 2A; ref. 21). This conductance is the result of, e.g., 1.1-pA current driven by a voltage drop across the membrane of about –180 mV (Fig. 2A, Nernst potential for K+, EK = 0). According to the substrate dependency of K+ currents (see Fig. 3A), in 100 μM K+ or even less, the same potential difference (EK – EM) would elicit single-channel K+ currents in the order of only a few femtoampere. This is far below the resolution limit of the patch-clamp technique [100 fA (25)]. To perform high-resolution current recordings at K+ concentrations representing the high-affinity K+ uptake range, we used A. thaliana guard cell protoplasts characterized by a “membrane patch-like” whole-cell surface area (capacitance = 1.4 ± 0.7 pF, n = 253) but high channel density [≈5–8 channels⋅μm−2 corresponding to around 500–1,000 channels per cell (21)].
Figure 2.
Voltage-, time-, and K+-dependent properties of the K+ uptake channel in A. thaliana guard cells. (A) Activation of single K+ uptake channels in an outside-out patch in response to hyperpolarizing voltage pulses to values indicated starting from a holding potential of –67 mV. For better resolution of current amplitudes, the K+ concentration was 150 mM K+ on both sides. The current–voltage relation of single channels from four to six individual measurements as a function of the membrane voltage corresponded to a slope conductance of 8.6 ± 0.3 pS (not shown). (B) Macroscopic outward and inward K+ currents (whole-cell configuration) through the inward rectifier elicited by 1.5-s, too pulses to voltages indicated in the presence of 1140 μM K+ (Left) and 60 μM K+ (Right). Because of the experimental conditions Kout+ channels present in the plasma membrane, too, are not active in the voltage range shown. (C) Current–voltage curves of steady-state K+ currents in the presence of different external K+ concentrations. Pulse protocols are as in B. The data shown in B and C are representative of three to six independent experiments. (D) Voltage-dependent open probabilities (Upper) and half-activation potentials (V1/2, Lower) as a function of the K+ concentration. Open probabilities were recorded in the presence of 30 mM (Upper, ○) and 20 μM K+ (Upper, ●) and 20, 60, 130, 330, and 1,140 μM K+ (Lower, n = 3–9).
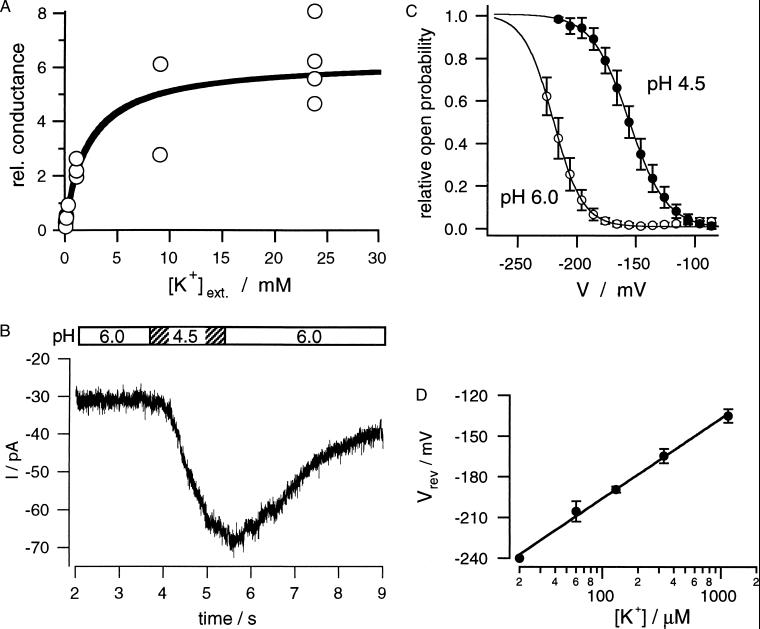
Figure 3.
High-affinity inward currents through guard cell plasma membrane K+ channels is not driven by the proton gradient. (A) Potassium-dependent saturation of the K+ channel conductance recorded in bath medium of pH 4.5 and K+ concentrations ranging from 20 μM to 30 mM. Relative conductance was determined according to ref. 6 (n = 2–6). (B) Proton-dependent stimulation of inward K+ currents during a solute pulse of pH 4.5 within 60 μM K+ and pH 6.0. The membrane potential was clamped to −220 mV. The bar illustrates the duration of the acid pulse generated by a fast perfusion system. (C) Shift in voltage-dependent open probabilities of the K+ channels in response to a pH change from 6.0 to 4.5. Relative open probabilities were normalized with respect to the maximum at pH 4.5. Lines represent Boltzmann fits to the voltage-dependent open probabilities at the two pH values. The data represent mean ± SD of seven measurements. (D) Ideal Nernstian behavior of the K+ current reversal potentials. Reversal potentials (Vrev) were deduced from steady-state current–voltage relations as shown in Fig. 2C in the presence of 20–1,140 μM external K+ at pH 4.5. The line represents a fit according to the Nernst equation, revealing a shift in Vrev of 58.9 mV per 10-fold change in the K+ concentration.
In the presence of basically 300 mM K+ in the pipette and 1.14 mM K+ in the bath, inward currents were elicited upon hyperpolarization of the plasma membrane negative to around –130 mV (Fig. 2 B and C). At potentials more positive than, e.g., –60 mV, outward-rectifying K+ channels were gated open (not shown here). Because this channel type is not involved in K+ uptake but, rather, K+ release, it will not be taken into account in this study. When we reduced the external K+ concentration to, e.g., 60 μM (Fig. 2B Right), a concentration within the working range of high-affinity K+ transporters (1), inward currents required membrane potentials negative of –200 mV because of the change in the driving force. At voltages positive to the Nernst potential for K+, channel activity resulted in outward currents, which decreased with further depolarization. Detailed analysis of the current–voltage curves revealed that channel activation is K+-independent from 30 mM down to the micromolar range (Fig. 2D; see also ref. 26). An identical behavior was seen with oocytes expressing KAT1 (down to 500 μM K+, not shown), proving high-affinity uptake to represent an intrinsic property of the K+ channel. Thus, in contrast to the current literature (see ref. 9 for review), this channel type conducts outward K+ currents positive from EK, whereas negative to the equilibrium potential it mediates K+ uptake. Additional evidence for this hyperpolarization-activated K+ channel to function as voltage sensor rather than K+ sensor was obtained from the analysis of the open probability in the presence of 20, 60, 130, 330, and 1140 μM K+ in the bath. As shown in Fig. 2D, the activation curves as well as half-activation potentials (V1/2) superimposed for K+ levels representing the high- and low-affinity range. Therefore, the voltage range at which K+ efflux occurs is a function of the difference between the voltage threshold for K+ channel activation and the Nernst potential for potassium. The whole-cell conductance of the inward current through the K+ channel plotted as a function of the K+ concentration displayed a Michaelis–Menten type of behavior characterized by an affinity constant of about 2 mM (Fig. 3A), a KM similar to that of potassium channels in V. faba guard cells (6). This channel, therefore, represents a low-affinity transport system that, because of its K+-independent gating, also operates in the high-affinity range.
Because this channel type is capable of conducting K+ currents of the same order of magnitude as described for pH-dependent K+ uptake in plants (9), we also explored the pH sensitivity of the high-affinity K+ uptake component of the K+ channel. After activating inward K+ currents through membrane hyperpolarization to −220 mV in the presence of 60 μM K+ and pH 6.0, we applied pulses of higher proton concentration to the guard cell protoplasts (Fig. 3B, pH 4.5). Upon this acidification the current amplitude increased dramatically (Fig. 3B), a feature reminiscent of H+/K+ symporters. When comparing the current–activation curves under both conditions, we could relate this increase in K+ current to an acid-induced shift in the voltage dependence by 65 mV to less-negative membrane potentials (Fig. 3C). This facilitation of K+ channel activation by protons thus is qualitatively similar to that recognized for guard cell channels from other plants as well as KAT1 and KST1 expressed in Xenopus oocytes in the presence of millimolar K+ concentrations (21, 24, 27–30). Plotting the reversal potentials of the macroscopic currents against the external K+ concentration showed that they perfectly followed the Nernst equation (Fig. 3D, 58.9 mV/decade). This indicates that acid activation of the channel is not superimposed by H+/K+ symporter activity, and, consequently, proton gradients do not energize K+ channel-dependent K+ uptake into guard cells.
From the experimental evidence presented here we conclude further that (i) K+ uptake channels are capable of mediating high-affinity K+ transport that is stimulated upon acidification, (ii) K+ uptake channels are equipped with a channel-intrinsic voltage sensor rather than a K+ sensor, (iii) the channel-intrinsic pH sensor (29, 31) is active under high- and low-affinity transport conditions, and (iv) K+ uptake channels, upon potassium starvation, could represent a K+-efflux pathway. In line with our high-resolution K+ current measurements on A. thaliana guard cells, the K+ uptake-deficient yeast mutant Δtrk1/2, when complemented with KAT1, took advantage of the voltage-dependent properties of this ion channel. Activated by hyperpolarization and acidic pH, KAT1 does not inactivate, allowing long-term K+ accumulation, which is required for yeast growth (Fig. 1).
To drive channel-mediated K+ influx with 10 μM K+ in the culture medium, yeast cells containing, e.g., 100 mM cytoplasmic K+ have to pump their membrane potential negative to −232 mV. Resting potentials even more negative have been recorded in guard cells as well as in root apex cells (32, 33). Our results also have shown that at micromolar extracellular potassium concentrations, K+ uptake channels could represent a significant K+ shunt if the membrane potential is positive to EK. K+ symporters that are driven by gradients other than or in addition to the electrical potential are able to operate in the voltage window between EK and −100 mV or even more positive (10–13, 15, 34). In A. thaliana root protoplasts, net K+ uptake currents carried by these symporters could at least balance channel-mediated K+ efflux (35). Furthermore, conditions such as “K+ depletion” or “K+ starvation” seem to induce the expression of K+ symporters probably to limit channel-mediated K+ loss (12, 15). In this context, up-regulation of nonchannel K+ transporters may explain why, in 10 μM K+, plants lacking AKT1 still reach 50% of the fresh weight compared with the wild type (32).
In summary, we conclude that acid-activated, high-affinity K+ uptake displays an intrinsic biophysical property of plant K+ uptake channels extending the dynamic range of K+ channel action. Because K+ channels operate on the basis of a K+ concentration-independent voltage sensor, K+ uptake at potentials positive to EK might represent the working range of nonchannel K+ transporters.
Acknowledgments
We are grateful to J. Vanderleyden (Leuven, Belgium) and his group for stimulating discussion as well as to D. Sanders for comments on the manuscript. For atomic absorption measurements of K+ concentrations, we thank W. Kaiser (Würzburg, Germany). We also thank J. D. Reid for providing us with the yeast mutant Δtrk1/2. This work was funded by Deutsche Forschungsgemeinschaft grants to R.H.
References
- 1.Epstein E. In: Encyclopedia of Plant Physiology. Lüttge U, Pitman N G, editors. Vol. 2. Berlin: Springer; 1976. pp. 70–94. [Google Scholar]
- 2.Anderson J A, Huprikar S S, Kochian L V, Lucas W J, Gaber R F. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J M, Gaymard F, Grignon C. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 4.Hedrich R, Hoth S, Becker D, Dreyer I, Dietrich P. In: Cellular Integration of Signalling Pathways in Plant Development, NATO ASI Series. LoSchiavo R, Last R L, Morelli G, Raiknel N V, editors. H104. Berlin: Springer; 1998. pp. 35–45. [Google Scholar]
- 5.Hedrich R, Dietrich P. Bot Acta. 1996;109:94–101. [Google Scholar]
- 6.Schroeder J I, Fang H H. Proc Natl Acad Sci USA. 1991;88:11583–11587. doi: 10.1073/pnas.88.24.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maathuis F J, Sanders D. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder J I, Ward J M, Gassman W. Annu Rev Biophys Biomol Struct. 1994;23:411–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- 9.Maathuis J M, Ichida A M, Sanders D, Schroeder J I. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schachtman D P, Schroeder J I. Nature (London) 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 11.Rubio F, Gassmann W, Schroeder J I. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 12.Santa-Maria G E, Rubio F, Dubcovsky J, Rodriguez-Navarro A. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintero F J, Blatt M R. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 14.Fu H H, Luan S. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E J, Kwak J M, Uozumi N, Schroeder J I. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker N A, Sanders D, Maathuis F J. Science. 1996;273:977–979. doi: 10.1126/science.273.5277.977. [DOI] [PubMed] [Google Scholar]
- 17.Becker D, Dreyer I, Hoth S, Reid J D, Busch H, Lehnen M, Palme K, Hedrich R. Proc Natl Acad Sci USA. 1996;93:8123–8128. doi: 10.1073/pnas.93.15.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y S, Kane J, Kurjan J, Stadel J M, Tipper D J. Mol Cell Biol. 1990;10:2582–2590. doi: 10.1128/mcb.10.6.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz R D, Schiestl R H. Yeast. 1991;7:253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Navarro A, Ramos J. J Bacteriol. 1984;159:940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brüggemann L, Dietrich P, Dreyer I, Hedrich R. Planta. 1999;207:370–376. doi: 10.1007/s004250050494. [DOI] [PubMed] [Google Scholar]
- 22.Neher E. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R L, McKendree W L J, Hirsch R E, Sedbrook J C, Gaber R F, Sussman M R. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller-Roeber B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakmann B, Neher E. Single Channel Recording. New York: Plenum; 1995. [Google Scholar]
- 26.Blatt M R. J Membr Biol. 1991;124:95–112. doi: 10.1007/BF01870455. [DOI] [PubMed] [Google Scholar]
- 27.Blatt M R. J Gen Physiol. 1992;99:615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich P, Dreyer I, Wiesner P, Hedrich R. Planta. 1998;205:287. [Google Scholar]
- 29.Hoth S, Dreyer I, Dietrich P, Becker D, Mueller-Roeber B, Hedrich R. Proc Natl Acad Sci USA. 1997;94:4806–4810. doi: 10.1073/pnas.94.9.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilan N, Schwartz A, Moran N. J Membr Biol. 1996;154:169–181. doi: 10.1007/s002329900142. [DOI] [PubMed] [Google Scholar]
- 31.Hedrich R, Moran O, Conti F, Busch H, Becker D, Gambale F, Dreyer I, Kuech A, Neuwinger K, Palme K. Eur Biophys J. 1995;24:107–115. doi: 10.1007/BF00211406. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch R E, Lewis B D, Spalding E P, Sussman M R. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 33.Lohse G, Hedrich R. Planta. 1992;188:206–214. doi: 10.1007/BF00216815. [DOI] [PubMed] [Google Scholar]
- 34.Maathuis F J, Sanders D. Proc Natl Acad Sci USA. 1994;91:9272–9276. doi: 10.1073/pnas.91.20.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maathuis F J, Sanders D, Gradmann D. Planta. 1997;203:229–236. doi: 10.1007/s004250050186. [DOI] [PubMed] [Google Scholar]