Abstract
Functional MRI was used to test whether instructing subjects to attend to one or another location in a visual scene would affect neural activity in human primary visual cortex. Stimuli were moving gratings restricted to a pair of peripheral, circular apertures, positioned to the right and to the left of a central fixation point. Subjects were trained to perform a motion discrimination task, attending (without moving their eyes) at any moment to one of the two stimulus apertures. Functional MRI responses were recorded while subjects were cued to alternate their attention between the two apertures. Primary visual cortex responses in each hemisphere modulated with the alternation of the cue; responses were greater when the subject attended to the stimuli in the contralateral hemifield. The attentional modulation of the brain activity was about 25% of that evoked by alternating the stimulus with a uniform field.
Our ability to perform a visual discrimination task is improved when we are cued in advance toward the spatial location of the stimulus (1). Neuronal correlates of this phenomenon have been studied by recording the electrophysiological responses of individual neurons in monkeys trained to perform various visual discrimination tasks. Neurons in secondary (extrastriate) areas of the visual cortex have been found to respond more strongly to stimuli within their receptive fields when monkeys are directed to attend to those stimuli as opposed to when they are directed to attend elsewhere (2–6). This effect, when pooled across many neurons, can yield changes in brain activity that have been detected by neuroimaging (7–11) and event-related potentials (12–15).
It remains unclear, however, whether attentional instructions influence the responses of neurons in the primary visual cortex (V1). One common view holds that the visual system up to and including V1 acts as a passive and automatic image-processing machine that is unaffected by cognitive influences and task demands. We used functional MRI (fMRI) (16–19) to test this point of view. We found that instructing subjects to attend to one or another location in a visual scene caused a consistent and systematic change in V1 brain activity.
MATERIALS AND METHODS
Main Experiment.
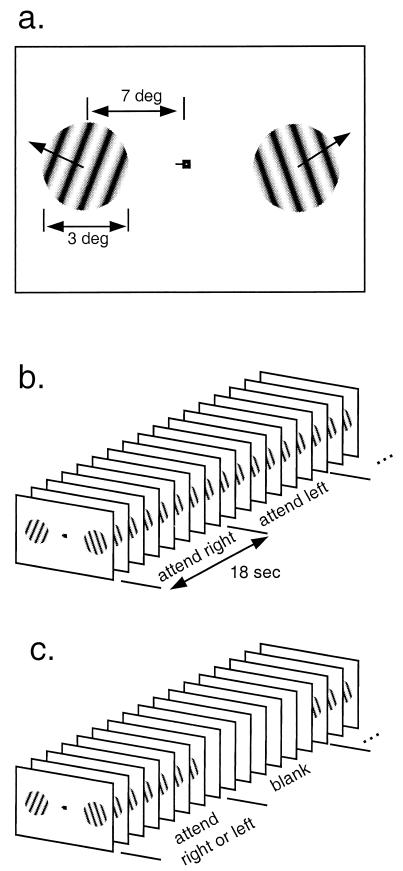
Stimuli were moving sinusoidal gratings (0.4 cycles per degree) on a uniform gray background, displayed on a screen made of rear-projection material positioned at the opening of the bore of the MRI scanner near the subjects’ knees. The gratings were presented within a pair of apertures, one positioned to the left and one positioned to the right of the center of fixation (Fig. 1).
Figure 1.
Experimental design and protocol. (a) Stimuli were moving sinusoidal gratings restricted to two peripheral, circular apertures (3° diameter, centered at 7° eccentricity). Fixation mark indicates “attend left.” (b) Main experiment: Subjects performed a speed discrimination task, attending alternately for a series of trials to the right, then for a series of trials to the left, and so on. (c) Baseline experiment: Subjects alternately performed the speed discrimination task for a series of trials (as in the main experiment) and then viewed a uniform gray field.
Subjects, lying on their backs, looked directly up into an angled mirror to see the screen. Subjects fixated a small high-contrast fixation mark. A bite bar was used to stabilize the subjects’ heads.
Subjects performed a speed discrimination task. Each trial consisted of two 750-msec stimulus intervals separated in time by 250 msec. The stimulus moved at a base speed in one interval and at a slightly faster test speed in the other interval. The base speed varied randomly (10.375°/sec ± 25%) from trial to trial. Subjects had 821 msec immediately after each trial to press a button indicating which interval contained the greater speed. Feedback was provided immediately after the button press. The shape of the fixation mark cued subjects to attend to either the left or the right aperture. The cue was presented throughout the 821-msec response period in between trials. Stimuli were presented simultaneously in both apertures on each trial. The speed of the uncued stimulus also varied; it was irrelevant for a correct response because the base speeds in the two apertures were independently randomized and the order of base/test in the two apertures was independently randomized.
Subjects were cued to attend to the aperture on the right for the first seven trials lasting a total of 18 sec, then to the left for the next 18 sec, and so on. Each fMRI scan consisted of seven 36-sec cycles of the right–left alternation (98 trials total).
Subjects practiced the task extensively (approximately 2000 trials outside of the scanner, 500 trials in the scanner) until their speed discrimination thresholds reached asymptotic levels. The stimulus speeds that were used during the fMRI scans were chosen based on these asymptotic performance levels so that the subjects would perform with an accuracy of approximately 80% correct.
Spatial Uncertainty Experiment.
To confirm that subjects were relying on the cue to optimize their performance in the speed discrimination task, we performed a separate series of measurements in which subjects were required to attend to both apertures simultaneously (20). A defining behavioral effect of spatial attention is that performance is better when the subject is cued to the correct aperture, while keeping the stimulus and response identical (21).
The stimuli in our spatial uncertainty experiments were virtually identical to those in the main experiment except that a speed change occurred in only one of the two apertures and the speed increment was adjusted to maintain the 80% correct performance level. The aperture with the speed increment was varied randomly from trial to trial. Although the stimuli were slightly different from those used in the main experiment, these differences in the speed increments do not present a confound because the speed increments were always small compared with the randomization of the base speed. As in the main experiment, subjects made a single response after each trial by pressing one of two buttons to indicate which of the two intervals contained the stimulus that moved faster. Subjects practiced the main experiment and the spatial uncertainty experiment (in alternate practice blocks) for approximately the same number of trials until their thresholds reached asymptotic levels (see above). The data reported below were then collected while subjects were in the scanner.
Data Acquisition.
MRI was performed on a standard clinical General Electric 1.5 T Signa scanner with a standard General Electric head coil. Each subject participated in several scanning sessions: one to obtain a standard, high-resolution, anatomical scan, one to functionally define the early visual areas including V1 (see below), one to define the motion-sensitive visual area (MT+; see below), and four to measure fMRI responses in the various experiments. The main experiment was performed during the first and third scanning sessions, the spatial uncertainty experiment was performed during the second session, and the baseline experiment (see below) was performed during the last session.
Functional magnetic resonance images were recorded with a T2*-sensitive gradient recalled echo pulse sequence (1500-msec repetition time, 40-msec echo time, 90° flip angle, two interleaves, in-plane resolution = 1.02 × 1.02 mm, slice thickness = 4 mm) with a spiral readout (22, 23). Eight adjacent planes of fMRI data were collected with the most ventral slice positioned along the boundary between the occipital lobe and the cerebellum.
Structural images were also acquired during each scanning session with a T1-weighted spin echo pulse sequence (500-msec repetition time, minimum echo time, 90° flip angle) in the same slices and at the same resolution as the functional images. These in-plane anatomical images were registered to the high-resolution anatomical scan of each subject’s brain so that all magnetic resonance images (across multiple scanning sessions) from a given subject were aligned to a common three-dimensional coordinate grid.
FMRI Data Analysis.
Each fMRI scan lasted 252 sec. Data from the first 36-sec cycle were discarded to minimize effects of magnetic saturation and visual adaptation. During the remaining six cycles of each scan, 72 functional images (one every 3 sec) were recorded for each slice. For a given fMRI voxel (corresponding to a 1- × 1- × 4-mm brain volume), the image intensity changed over time and made up a time series of data.
The data were analyzed separately in each of four identifiable visual areas: left hemisphere V1, right hemisphere V1, left hemisphere MT+, and right hemisphere MT+. We computed the fMRI response amplitudes and phases by (i) removing the linear trend in the time series, (ii) dividing each voxel’s time series by its mean intensity, (iii) averaging the resulting time series over the set of voxels corresponding to the stimulus representation within a visual area (V1 or MT+), and then (iv) calculating the amplitude and phase of the best fitting 36-sec period sinusoid. The first step (removing the linear trend) is important because the fMRI signal tends to drift, for unknown reasons, very slowly over time. The second step is important because dividing by the mean converts the data from arbitrary (image intensity) units to units of fractional signal change.
Localizing the V1 Representation of the Stimulus.
Following well established methods (24–28), we measured the polar angle component of the retinotopic map by recording fMRI responses as a stimulus rotated slowly (like the second hand of a clock) in the visual field. To visualize these retinotopy measurements, the gray matter from a high-resolution MRI of each subject’s brain was computationally flattened (28). Area V1 within each hemisphere was identified as a large region of cortex in/near the calcarine sulcus with a retinotopic map spanning half the visual field. To be conservative, we selected the region of V1 that represented the visual field within 60° on either side of the horizontal meridian, thereby staying away from the vertical meridian representation at the V1/V2 border.
These procedures to define V1 were performed only once per subject. Because the fMRI data recorded during successive scanning sessions in a given subject were all aligned to a common three-dimensional coordinate grid (see above), we could localize V1 across scanning sessions.
The V1 region was further restricted to the representation of the two circular apertures, based on responses to a reference stimulus. The reference stimulus was a contrast-reversing 8.3-Hz, 1-cycle/degree checkerboard restricted to the same peripheral apertures as used in the main experiment. The stimulus alternated (18 sec on, 18 sec off) with a uniform gray field. Voxels within V1 that were unresponsive to the reference stimulus were excluded from the analysis. Unresponsive voxels were defined as those that were weakly correlated (r < 0.23 and/or >9-sec time lags) with respect to a 36-sec period sinusoid, although correlation thresholds from r < 0 to r < 0.4 produced similar results. In this way, we excluded voxels corresponding to regions outside the two stimulus apertures and voxels that had too little overlap with gray matter. The reference scan was run at the beginning of each scanning session, and it was used to restrict the V1 region for all subsequent scans in that scanning session.
Localizing the Human MT+ Complex.
The fMRI data were analyzed separately in visual area MT+. Several lines of evidence suggest that this area may be homologous to monkey MT along with adjacent motion-sensitive brain areas (26, 27, 29–33).
Area MT+ was identified, according to data from previous studies (29, 30, 32), by measuring fMRI responses to stimuli that alternated in time between moving (10°/sec, radially inward and outward) and stationary dot patterns (white dots on a black background). Specifically, area MT+ was selected as a contiguous group of voxels lateral to the parietal-occipital sulcus and beyond the retinotopically organized visual areas with a time series that correlated (r > 0.35, with a 0- to 9-sec time lag) with the temporal alternation (moving vs. stationary) of the stimulus. As for V1, the MT+ region was further restricted based on responses to the contrast-reversing checkerboard reference stimulus (see above).
Baseline Experiment and Attentional Modulation Index.
The fMRI amplitudes measured in the main experiment indicate the difference in the fMRI signal to the attended vs. the ignored stimulus aperture. These amplitudes are, however, difficult to interpret on their own. A small amplitude could be caused by a small attentional effect, or it could be that the stimulus evokes only a small response to begin with. Therefore, we computed an attentional modulation index by comparing the fMRI responses from the main experiment with those recorded in a baseline experiment. This attentional modulation index is analogous to those often used to quantify attentional effects in single-cell physiology studies (5).
In the baseline experiments, the stimulus alternated between 18-sec periods when gratings were presented and 18-sec of a uniform gray field (Fig. 1c). The gratings were presented simultaneously in both apertures in a series of 750-msec intervals, as in the main experiment. Subjects performed the same speed discrimination task as in the main experiment, but they were cued throughout an entire scan to one aperture, either on the right (baseline-right) or the left (baseline-left). Two repeats of the baseline-right and the baseline-left experiments were performed for each subject.
The attentional modulation index was computed as a ratio between the response amplitude from one of the repeats of the main experiment and the response amplitude from the baseline experiments. To compute this ratio, we first had to covert the bivariate (amplitude and phase) responses from the baseline and main experiments into univariate response amplitudes. First, we computed a reference phase as the average response phase for the aforementioned contrast-reversing checkerboard reference stimulus. Second, we computed the bivariate response amplitude and phase for each baseline experiment, averaging across voxels in the contralateral hemisphere (e.g., the baseline-right measurements were analyzed in the left hemisphere) and averaging across the two repeats of each measurement. Third, we computed a component amplitude from each of the bivariate responses, as the component of the response with 0-phase lag relative to the reference phase. Fourth, we computed ratios (main/baseline) of these component amplitudes, separately for each hemisphere and for each repeat of the main experiment.
RESULTS
The purpose of the spatial uncertainty experiment was to demonstrate that subjects were relying on the cue to optimize their performance in the speed discrimination task. The speed increments in the main experiment were chosen (based on the asymptotic performance levels after practice) to be 6% and 7% for subjects S.P.G. and G.M.B., respectively. The speed increments in the spatial uncertainty experiment were considerably higher: 13% and 11.5%, respectively. By design, the (percentage correct) performance of both subjects in both experiments was about the same: 73% and 78% for S.P.G. and G.M.B., respectively, in the main experiment; 78% and 74% in the spatial uncertainty experiment.
If brain activity in V1 modulates with the spatial cue, then we would expect the fMRI response in the left hemisphere first to increase and then to decrease as the subject attended first to the right and then to the left. Conversely, in right hemisphere V1, we would expect the fMRI response first to decrease and then to increase. In other words, the temporal phase of the modulation in the left hemisphere should be near 0°, whereas the temporal phase in the right hemisphere should be near 180°.
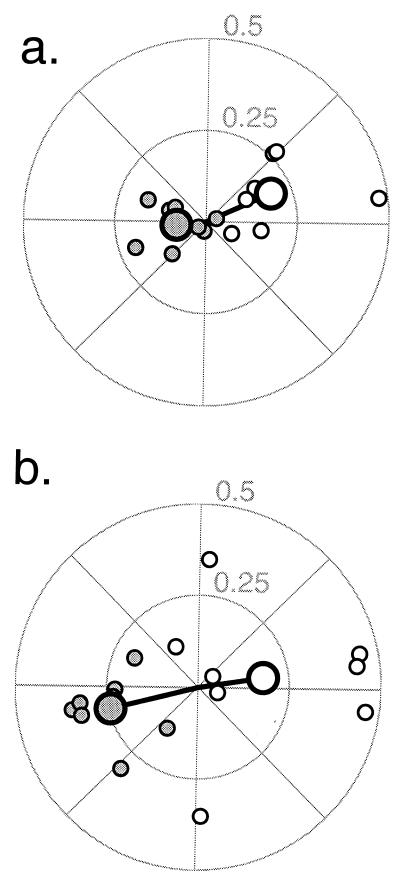
The results of the main experiment, plotted in Fig. 2, demonstrate that V1 brain activity modulated as subjects followed the attentional cue. In the polar plots, the fMRI response amplitude is represented by the radial distance from the origin, and the fMRI response phase is represented by the angle counterclockwise from the horizontal axis. Phases with a polar angle near 0° (right half of each plot) indicate greater brain activity when subjects were attending to the right aperture. The fMRI responses in the left hemisphere (open symbols) were in phase with the cue to attend right. Conversely, the fMRI responses in the right hemisphere (filled symbols) fall on the left side of the figures, in phase with the cue to attend left. The slight counterclockwise phase shift of fMRI responses relative to the horizontal axis is the result of the 3- to 4-sec temporal lag that is characteristic of the sluggishness of the hemodynamic-mediated fMRI signal (34, 35). Nearly all eight repeated measurements (small symbols) from each of the two hemispheres of each of the two subjects are on the expected side of the polar plot, and the vector means (large symbols) of the eight repeats clearly show the effect (P < 0.01 for both subjects, Hotelling t test for significant difference between the two bivariate distributions).
Figure 2.
Attentional modulation in V1. Polar plots of fMRI responses while subjects alternated attention between the right and left stimulus apertures. (a) subject S.P.G., (b) subject G.M.B. The response amplitude (percentage magnetic resonance signal modulation) indicated by radial distance from the origin and response temporal phase indicated by the angle from the horizontal axis. Responses from left hemisphere V1 (open symbols) are near 0°, in phase with the cue to attend right. Responses from right hemisphere V1 (filled symbols) are near 180°, in phase with the cue to attend left. Small symbols plot results from each of eight repeated measurements (separate scans), for each of the two subjects. Large symbols represent the vector mean of the eight repeats.
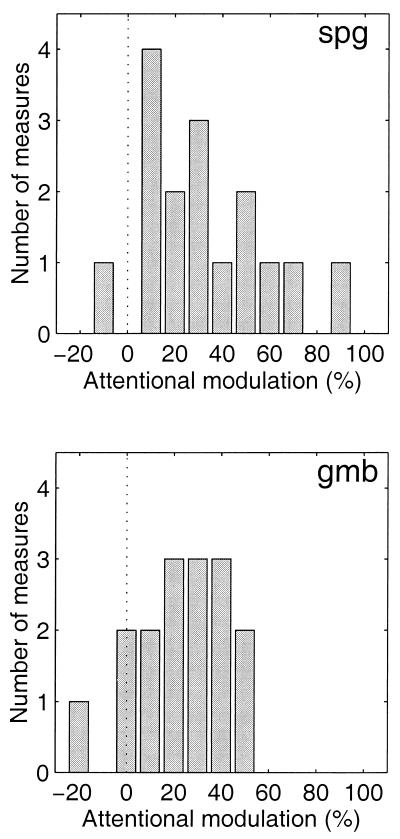
To quantify the effect, we computed an attentional modulation index (see Materials and Methods). A value of 0 for the attentional modulation index means that the attentional task has no effect on fMRI response. A value of 1 indicates that there is no response to the ignored stimulus aperture, mimicking the fMRI response during the baseline experiments when the stimuli were presented alternately with a uniform field. Negative values mean that the attentional modulation is more than 90° from the expected phase and correspond to data points on the wrong side of the polar plots in Fig. 2. The resulting distributions of the attentional modulation index (n = 16 measurements, 8 in each hemisphere) are plotted in Fig. 3. The mean attentional modulation indices in V1 were 32% (SEM = 4%) and 23% (SEM = 4%) for subjects S.P.G. and G.M.B., respectively. Behavioral performance during the baseline experiments was 75% correct for S.P.G., i.e., not significantly different from his performance in the main experiment (P > 0.3). However, subject G.M.B. did perform significantly better (85% correct, P < 0.05) during the baseline experiments. This improvement in performance might be because the subject was able to rest during the periods of the baseline experiment when the gratings were not being presented. We could not, unfortunately, control for the attentional state of the subject during these rest periods of the baseline experiments. Hence, the precise values of the attentional modulation indices should be taken only as estimates of the attentional effects.
Figure 3.
Distributions of the V1 attentional modulation index for the 16 repeated measurements (eight scans, two hemispheres) in each subject. Each panel corresponds to one of the two subjects. The dotted vertical line indicates an attentional modulation index of 0.
Previous studies have reported that attending to moving stimuli modulates brain activity both in human MT+ and in monkey MT (5, 10, 36, 37). We found that the mean attentional modulation indices in MT+ were 49% (SEM = 5%) and 24% (SEM = 6%) for subjects S.P.G. and G.M.B., respectively. Only S.P.G. had a greater modulation in MT+ than in V1.
It is conceivable that systematic eye movements could account for our results. More cortex in V1 is dedicated to representing the center of the visual field than peripheral locations (28, 38). If a subject were to make a large eye movement toward the cued aperture, the stimulus on that side would be displaced toward the fovea and would evoke activity over a larger cortical region. This would, in turn, modulate the fMRI signal in a way that could mimic our results.
To rule out this possibility, we collected eye movement data while subjects repeated the main experiment in a psychophysics laboratory. Ideally, these eye-movement measurements should have been made during the functional scans, but that was not possible with the equipment we had available at the time. With a high-resolution IR eye-tracking system (Ober2, Timra, Sweden), we measured the amplitude of modulation of the horizontal component of the eye position at the frequency corresponding to the alternation of the attentional cue (seven cycles per scan) and at the frequency corresponding to the stimulus presentations (98 trials per scan). These modulations were <0.15° in both subjects. From measurements of the cortical magnification factor in human V1 (28), one can estimate that this would cause a change of <4% in the cortical area representing one of our stimulus apertures. Hence, the eye movements alone would mimic less than a 4% attentional modulation index, compared with the 23–32% attentional modulation index that we observed.
Note, however, that the fMRI measurements were performed separately and under different circumstances (i.e., with subjects lying on their backs in the noisy scanner, while staying as still as possible). If, during the fMRI scans, subjects had moved their eyes toward the target stimulus, then they would have presumably performed better than when they were known to hold fixation, but this was not found to be the case. Performance during eye tracking was 70% and 74.5% correct (n = 49 trials) for subjects S.P.G. and G.M.B., respectively. This is not statistically different from the performance (73% and 78% correct of 392 trials) obtained during the fMRI scans (P > 0.39 for S.P.G. and P > 0.28 for G.M.B., one-tailed test of the null hypothesis that the two performance levels are drawn from the same binomial distribution). One might expect the task to be more difficult in the scanner. If so, it is possible that the performance in the scanner equaled the performance in the laboratory because the subject made small eye movements toward the target during the scans, compensating for the greater difficulty while in the scanner. We believe that this is unlikely because (i) our subjects are experienced psychophysical observers who have spent dozens of hours performing perceptual discrimination tasks in the scanner and (ii) we have repeatedly found in other experiments that performance is similar in the scanner and the laboratory.
DISCUSSION
Previous functional neuroimaging and event-related potential studies have found no effect of selective spatial attention in human V1 (7–15). There are several possible explanations. First, it may be that attentional effects in V1 increase with increasing attentional demand. If so, then it is critical to demonstrate, as we have done with our spatial uncertainty experiment, that subjects rely on the cue to optimize their performance.
Second, our fMRI measurements permit better spatial localization than some of the other human neuroimaging techniques. Our analysis was restricted to a small region of cortex corresponding to the V1 representation of the stimulus.
Third, attention may cause elevated maintained neuronal firing rates without increasing the stimulus-evoked neuronal responses, as has been demonstrated in extrastriate cortex (6). The hemodynamic/fMRI response would presumably be affected by increasing either the maintained rates or the stimulus-evoked firing rates. Event-related potentials, on the other hand, measure only stimulus-evoked activity.
Fourth, our fMRI measurements might reflect reactivation of V1 caused by feedback from extrastriate cortical areas (39). Event-related potentials cannot determine whether the source of these longer latency signals are from delayed responses in V1 or from responses in extrastriate areas.
In addition, some previous claims of attentional effects in V1 may be flawed. It is essential to control for the overall arousal state of the subject (40). Some studies have compared conditions in which subjects were alternately instructed to “attend” and to “passively view” the same stimulus (e.g., refs. 41, 42), but this design confounds effects of selective attention with the overall level of “arousal” or “vigilance.” In our study, subjects were cued to attend alternately between two spatial locations; hence, the level of arousal should have remained approximately constant.
Single-cell neurophysiology studies in awake-behaving monkeys have determined conflicting results about the effects of spatial attention in V1. Motter (3) found that the firing rates of a minority of V1 neurons are affected by attention, whereas Luck et al. (6) found no effect in V1. Small eye movements, equal in size to the V1 receptive field itself, present one of the primary difficulties in measuring attentional effects in individual V1 neurons (43). The responses of some neurons will be enhanced, and others will be suppressed, depending on whether their receptive fields were shifted toward or away from the stimulus. These biases might be confounded with the effects of spatial attention in single neurons. A recent single-cell study found an attentional effect in V1, even after carefully accounting for small eye movements (44). Small eye movements do not present a difficulty in our experiments because they should have a negligible effect on fMRI measurements of pooled neuronal activity.
Our results lead one to hypothesize that the observed modulation of brain activity directly causes the observed improvement in behavioral performance. Such a causal link would depend on a number of factors, including the issue of whether attention affects maintained firing rates or stimulus-evoked responses (see above), the nature of the noise in the neuronal activity that limits behavioral performance, and the decision rule governing how the signals corresponding to the two stimulus apertures are combined to determine the subject’s choice on each trial.
In one scenario, modulation of brain activity would cause an improved signal-to-noise ratio and, hence, improved behavioral performance if (i) the observed change in brain activity was caused by elevated stimulus-evoked neuronal responses, (ii) the neuronal noise was not affected by attention, i.e., added after the attentional modulation, and (iii) the subject adopted an ideal observer decision rule, i.e., made optimal use of the resulting noisy neuronal activity coming from the cued aperture.
In another scenario, modulation of brain activity would cause improved performance if the subject’s decisions depended on an appropriate sum of the neuronal signals corresponding to the two stimulus apertures (20). With attention, the neuronal signals corresponding to the cued aperture are greater than, and would therefore dominate, the neuronal signals corresponding to the uncued aperture. Hence, performance would improve simply because the relevant signals coming from the cued side are elevated relative to the irrelevant signals (i.e., noise) coming from the uncued side (4, 6, 45, 46).
On the other hand, it is certainly possible that the V1 modulation we observed might have nothing to do with the improved behavioral performance. For example, the memory load differs between the tasks in the main experiment and the spatial uncertainty experiment. In the spatial uncertainty experiment, subjects must remember two speeds instead of one during the 250-msec interstimulus interval. This difference in memory load might be causing the improved behavioral performance. It is difficult, however, to imagine that such a significant modulation of activity in visual cortex would fail to have consequences on perceptual thresholds.
A different form of selection, called featural attention, is known to enhance performance when subjects are instructed to attend to a specific feature (e.g., shape, motion, color) of a stimulus. There is accumulating evidence that featural attention, like spatial attention, can selectively modulate activity in several visual areas (2, 10, 36, 37, 41, 47). Corbetta et al. (37), in particular, found greater activity in MT+, but not in V1, while subjects attended to stimulus speed as opposed to color or shape.
In our study, subjects were required to attend to a specific feature (speed) at a specific location. We found modulation of brain activity both in MT+ and in V1. It remains to be seen whether attending to different stimulus features (e.g., contrast, color, shape) in our paradigm would have differential effects in these and other brain areas.
Acknowledgments
Special thanks to B. A. Wandell, J. Palmer, S. Luck, B. Press, and the anonymous reviewers for their helpful comments and to G. H. Glover (and the Richard M. Lucas Center for Magnetic Resonance Spectroscopy and Imaging, supported by a National Institutes of Health National Center for Research Resources grant) for technical support. S.P.G. was supported by a Stanford University undergraduate research opportunities grant. G.M.B. was supported by a National Institute of Mental Health postdoctoral research fellowship. D.J.H. was supported by a National Eye Institute grant (R01-EY11794) and a National Institute of Mental Health grant (R29-MH50228).
ABBREVIATIONS
- fMRI
functional MRI
- MT+
motion-sensitive visual area
- V1
primary visual cortex
Footnotes
A Commentary on this article begins on page 2585.
References
- 1.Graham N. Visual Pattern Analyzers. New York: Oxford Univ. Press; 1989. [Google Scholar]
- 2.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 3.Motter B C. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 4.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 5.Treue S, Maunsell J H R. Nature (London) 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 6.Luck S J, Chelazzi L, Hillyard S A, Desimone R. J Neurophysiol. 1997;7:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Corbetta M, Miezin F M, Shulman G L, Petersen S E. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinze H J, Mangun G R, Burchert W, Hinrichs H, Scholz M, Munte T F, Gos A, Scherg M, Johannes S, Hundeshagen H. Nature (London) 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 9.Woldorff M G, Fox P T, Matzke M, Lancaster J L, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao J H, Martin C C, et al. Hum Brain Mapp. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Beauchamp M S, Cox R W, DeYoe E A. J Neurophysiol. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- 11.Gratton G. NeuroReport. 1997;8:1749–1753. doi: 10.1097/00001756-199705060-00036. [DOI] [PubMed] [Google Scholar]
- 12.Mangun G R, Hillyard S A, Luck S J. In: Attention and Performance. Meyer D, Kornblum S, editors. XIV. Cambridge, MA: MIT Press; 1993. pp. 219–243. [Google Scholar]
- 13.Gomez Gonzalez C M, Clark V P, Fan S, Luck S J, Hillyard S A. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- 14.Clark V P, Hillyard S A. J Cognit Neurosci. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- 15.Mangun G R, Hopfinger J B, Kussmaul C L, Fletcher E M, Heinze H J. Hum Brain Mapp. 1997;2:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Lee T M, Kay A R, Tank D W. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa S, Tank D W, Menon R, Ellermann J M, Kim S G, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belliveau J W, Kennedy D N, Mckinstry R C, Buchbinder B R, Weisskoff R M, Cohen M S, Vevea J M, Brady T J, Rosen B R. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 19.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R, et al. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verghese P, Stone L S. Vis Res. 1995;35:2811–2823. doi: 10.1016/0042-6989(95)00038-2. [DOI] [PubMed] [Google Scholar]
- 21.Palmer J. Curr Dir Psychol Sci. 1995;4:118–123. [Google Scholar]
- 22.Noll D C, Cohen J D, Meyer C H, Schneider W. J Magn Reson Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- 23.Glover G H, Lai S. Magn Res Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 24.Schneider W, Noll D C, Cohen J D. Nature (London) 1993;365:150–153. doi: 10.1038/365150a0. [DOI] [PubMed] [Google Scholar]
- 25.Engel S A, Rumelhart D E, Wandell B A, Lee A T, Glover G H, Chichilnisky E J, Shadlen M N. Nature (London) 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- 26.Sereno M I, Dale A M, Reppas J B, Kwong K K, Belliveau J W, Brady T J, Rosen B R, Tootell R B H. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 27.Deyoe E A, Carman G J, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Proc Natl Acad Sci USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel S A, Glover G H, Wandell B A. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- 29.Zeki S, Watson J D G, Lueck C J, Friston K J, Kennard C, Frackowiak R S. J Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson J D G, Myers R, Frackowiak R S J, Hajnal J V, Woods R P, Mazziotta J C, Shipp S, Zeki S. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy G, Spicer M, Adrignolo A, Luby M, Gore J, Allison T. Hum Brain Mapp. 1995;2:234–243. [Google Scholar]
- 32.Tootell R B H, Reppas J B, Kwong K K, Malach R, Born R T, Brady T J, Rosen B R, Belliveau J W. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tootell R B, Taylor J B. Cereb Cortex. 1995;5:39–55. doi: 10.1093/cercor/5.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Boynton G M, Engel S A, Glover G H, Heeger D J. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malonek D, Grinvald A. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 36.O’Craven K M, Rosen B R, Kwong K K, Treisman A, Savoy R L. Neuron. 1997;18:591–598. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Miezin F, Dobmeyer S, Shulman G, Petersen S. Science. 1990;248:1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- 38.Horton J C, Hoyt W F. Arch Ophthalmol. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- 39.Aine C J, Supek S, George J S. Int J Neurosci. 1995;80:79–104. doi: 10.3109/00207459508986095. [DOI] [PubMed] [Google Scholar]
- 40.Pardo, J. V., Fox, P. T. & Raichle, M. E. Nature (London)349, 61–64. [DOI] [PubMed]
- 41.Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M R, Miyakawa S. J Neurophysiol. 1998;79:2218–2221. doi: 10.1152/jn.1998.79.4.2218. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Harner A M, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, Mukai I. Proc Natl Acad Sci USA. 1998;95:11489–11492. doi: 10.1073/pnas.95.19.11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maunsell J H R. Science. 1995;270:764–769. doi: 10.1126/science.270.5237.764. [DOI] [PubMed] [Google Scholar]
- 44.Press W A. Ph.D. thesis. Pasadena: Calif. Inst. Technol.; 1998. [Google Scholar]
- 45.Palmer J, Ames C T, Lindsey D T. J Exp Psychol. 1993;19:108–130. doi: 10.1037//0096-1523.19.1.108. [DOI] [PubMed] [Google Scholar]
- 46.Shaw M L. In: Attention and Performance. Nickerson R S, editor. VIII. Hillsdale, NJ: Erlbaum; 1980. pp. 277–296. [Google Scholar]
- 47.Haenny P E, Schiller P H. Exp Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]