Abstract
GC-MS on the Viking 1976 Mars missions did not detect organic molecules on the Martian surface, even those expected from meteorite bombardment. This result suggested that the Martian regolith might hold a potent oxidant that converts all organic molecules to carbon dioxide rapidly relative to the rate at which they arrive. This conclusion is influencing the design of Mars missions. We reexamine this conclusion in light of what is known about the oxidation of organic compounds generally and the nature of organics likely to come to Mars via meteorite. We conclude that nonvolatile salts of benzenecarboxylic acids, and perhaps oxalic and acetic acid, should be metastable intermediates of meteoritic organics under oxidizing conditions. Salts of these organic acids would have been largely invisible to GC-MS. Experiments show that one of these, benzenehexacarboxylic acid (mellitic acid), is generated by oxidation of organic matter known to come to Mars, is rather stable to further oxidation, and would not have been easily detected by the Viking experiments. Approximately 2 kg of meteorite-derived mellitic acid may have been generated per m2 of Martian surface over 3 billion years. How much remains depends on decomposition rates under Martian conditions. As available data do not require that the surface of Mars be very strongly oxidizing, some organic molecules might be found near the surface of Mars, perhaps in amounts sufficient to be a resource. Missions should seek these and recognize that these complicate the search for organics from entirely hypothetical Martian life.
Keywords: Viking, organic compounds, exobiology, astrobiology
The Viking 1976 missions to Mars performed several experiments designed to assess the potential for life on the planet. The results were puzzling. Samples of soil from the top 10 cm of the Martian surface released dioxygen when exposed to humidity (1). At least one compound in a set of radiolabeled organic compounds (formate, d,l-lactate, glycolate, glycine, and d,l-alanine) released radiolabeled carbon dioxide when placed in aqueous solution on the Martian surface, evidently via oxidative processes (2). Both results were initially thought to indicate the presence of life. However, a GC-MS experiment looking for volatile products from a sample of soil heated for 30 s (sometimes repeatedly) at 200°, 350°, and 500°C did not detect any organic molecules (3). This result was (and remains) strong evidence against life on Mars, at least at the surface.
The failure to detect organic molecules by GC-MS was especially surprising, because some 2.4 × 108 g of reduced carbon comes to Mars each year via meteor (Table 1; refs. 4–6). Many meteoritic organic compounds are volatile and should have been detected by GC-MS (7). Pyrolysis should have generated volatile products from many of the nonvolatile compounds, including the polymeric organic substance known as “kerogen,” which accounts for the majority of organic material coming to Mars via meteorite and as much as 1–3% of the weight of some meteorites (8). These products too should have been detected by Viking but were not.
Table 1.
Expected Metastable Products from Organic Substances in the Murchison meteorite5,6
| Substance | Concentration (parts per million) | Metastable Products |
|---|---|---|
| Acid insoluble kerogen | 14500 | Benzenecarboxylic acids |
| Aliphatic hydrocarbons | 12–35 | Acetate |
| Aromatic hydrocarbons | 15–28 | Benzenecarboxylic acids |
| Monocarboxylic acids | ≈330 | Acetate/oxalate |
| 2-Hydroxycarboxylic acids | 14.6 | Acetate/carbonate |
| Alcohols (primary) | 11 | Acetate |
| Aldehydes | 11 | Acetate |
| Ketones | 16 | Acetate, benzenecarboxylic acids |
| Amines | 10.7 | Acetate |
| Urea | 25 | Carbonate |
| Heterocycles | 12 | Carbonate, other products |
These results have been interpreted as evidence that the Martian surface contains no organic molecules of any kind, presumably because the Martian regolith carries an oxidant powerful enough to convert all organics to carbon dioxide. Coupled with the absence of liquid water on the surface of Mars and with the irradiation of the surface by ultraviolet light, the failure to detect organic substances led many to conclude that one must dig deeply (and perhaps very deeply) below the Martian surface to have a chance of encountering any organic molecules that may have arisen from life on Mars (perhaps present several billion years ago, when the surface of Mars was more like the surface of Earth at that time and when life almost certainly had emerged on Earth) or of encountering organic molecules that may have been delivered to Mars via meteorite (9, 10).
Because this interpretation is influencing the design of missions to Mars, it is timely to reexamine it in light of what is known about the oxidation of organic compounds generally, the nature of organic substances likely to come to Mars, and the features of the Viking 1976 analysis that determined the kinds of organic molecules that it could have detected. The examination suggests that organic compounds that arrive on Mars via meteorite are most likely to be converted to carboxylic acid derivatives that would not be easily detected by GC-MS. Organic molecules generated on Mars itself by nonbiological (11–14) or (entirely hypothetical) biological synthesis (15) should suffer similar fates at or near the surface.
The Generic Oxidation Pathway.
As in any organic reaction, the specific oxidant, specific ambient conditions, and specific catalysts determine what intermediates will accumulate in the oxidative degradation of organic compounds on Mars. Only by missions to Mars can we learn these specifics to decide what has actually happened to meteoritic organics and, by inference, to other organics that might have come to the Martian surface.
Because we must today design missions to do that, we must extract as much as possible from general knowledge of organic reactivity and experimental data obtained in terrestrial laboratories under “non-Martian” conditions (very often in liquid water) to make a best guess as to how organic molecules will be transformed on the Martian surface. To this end, we infer here some generic pathways for the oxidation of organic molecules on Mars. As substrates for these pathways, we consider meteoritic organics, because this assumption avoids the need to presume the presence of organic compounds from Martian life.
We begin with the fact that the surface of Mars is exposed to ultraviolet radiation with sufficient energy to cleave water to give H⋅ and HO⋅ radicals. Some of the H⋅ radicals must recombine to give dihydrogen (H2), which escapes into space (16, 17). This process leaves behind the HO⋅ radical, which could react directly with organic substances, dimerize to give H2O2 (18), or generate peroxides or other oxidizing species through combination with elements in the Martian soil.
The HO⋅ radical reacts directly with most organic molecules. In aqueous solution under terrestrial conditions, the second order rate constants range from 107 to 1010 liter⋅mol−1⋅s−1 for reactions that include hydrogen atom abstractions and additions to double bonds (19). The concentration of HO⋅ on Mars is 1 × 105 to 2 × 105 cm−3, a number similar to the concentration of HO⋅ radical in the atmosphere at the surface of Earth (17).
Let us consider five types of organic compounds (Table 1) known to come to Mars via meteorites: alkanes, alkylbenzenes, naphthalene and higher polycyclic aromatic hydrocarbons, kerogen, and amino acids and hydroxyacids. We shall ask how HO⋅ and H2O2 might transform these in generic oxidation pathways and whether metastable intermediates in these pathways might accumulate.
Alkanes.
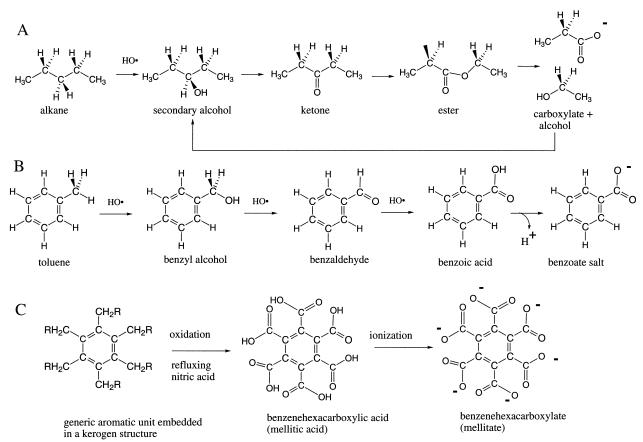
Alkanes react generically with the HO⋅ radical via abstraction of a hydrogen radical (H⋅) at a tertiary center (preferentially), then a secondary center, and last a primary center. This relative reactivity reflects the relative stability of the radical products and occurs under a wide range of conditions. Thus, a straight chain alkane would lose an internal hydrogen in the generic mechanism (Fig. 1A).
Figure 1.
Oxidative degradation of the generic alkane (represented here by pentane) to acetic acid (A), toluene to benzoic acid (B), and kerogen to benzenehexacarboxylic acid (mellitic acid) (C).
The resulting secondary radical is extremely reactive and will be trapped by almost anything available. It will react with another HO⋅ radical to yield a secondary alcohol. It can transfer an electron to (for example) Fe3+, generating a carbocation that can be trapped by water (for example, from a hydrated mineral), also generating the secondary alcohol. Other products are possible, but a secondary alcohol is the generic intermediate in the oxidative degradation of n-alkanes (Fig. 1A).
HO⋅ abstracts an H⋅ from the carbon attached to the alcohol oxygen more readily than it abstracts H⋅ from the parent alkene. Thus, the secondary alcohol (under generic conditions) is expected to react faster than the parent. It will therefore not accumulate but yield a ketone. The ketone, in turn, should undergo further oxidation to generate an ester, which will be cleaved to give a carboxylic acid and a primary alcohol, which will be oxidized directly to another carboxylic acid. Alternatively, the ketone might enolize, suffer oxidation, and then lead to a fragmentation to generate two carboxylic acids (Fig. 1A).
By these steps, the generic oxidation pathway for alkanes leads to carboxylic acids. These are, of course, subject to further oxidation. The abstraction of an H⋅ from the carbon attached to the COOH group is expected to be an important mode of oxidation involving HO⋅. This oxidation will ultimately generate the next shorter carboxylic acid. Depending on the trap, the product would be an alkane (and the process would resume) or another more easily oxidized derivative.
In this cascade of intermediates, the carboxylic acid is the first that is slower to degrade than to be formed. Under typical Fenton conditions, for example, acetic acid reacts with the HO⋅ radical 100 times more slowly than does ethanol (19). Carboxylic acids are therefore likely to accumulate. Further, acetate is more stable to further reaction under generic conditions than propanoic acid and longer alkylcarboxylic acids. Thus, acetic acid accumulates especially effectively.
Exemplifying the generic oxidation pathway are some “brand name” oxidations. In a Kuhn–Roth oxidation, for example, an alkane is refluxed in a solution of concentrated chromic acid (20). Insignificant amounts of ketone or alcohol products can be isolated as intermediates in the oxidation cascade that follows; these are too unstable with respect to further oxidation. Organic alkanecarboxylic acids (butanoic acid and propanoic acid, for example) can be isolated as metastable intermediates, however. On incubation for longer times, these are further degraded to acetic acid. Acetic acid too can be oxidized to give carbon dioxide. Nevertheless, acetate is more stable than longer chain alkanecarboxylic acids and accumulates. This accumulation makes the Kuhn–Roth oxidation useful for elucidating the structure of natural products. The amount of acetate produced from a known amount of alkane corresponds to the number of methyl groups in the alkane.
Alkylbenzenes.
The HO⋅ radical abstracts a benzylic H⋅ from the alkyl group of alkylbenzenes (such as toluene) to give a rather stable benzyl radical (Fig. 1B). This radical may trap HO⋅ or lose an electron to Fe3+ and then trap water, in each case forming benzyl alcohol. Benzyl alcohol is more reactive than toluene under generic conditions. It is not expected to accumulate but rather to be converted to benzaldehyde. Benzaldehyde is also unstable with respect to further oxidation and also should not accumulate in the generic process. Rather, it should be converted to benzoic acid.
Benzoic acid no longer has a benzylic hydrogen to lose to a radical oxidant. It is still thermodynamically unstable in the presence of oxidants to conversion to carbon dioxide. But it is metastable, resists further oxidation, and accumulates. Because benzoic acid has no hydrogen on the carbon adjacent to the COOH group, it also lacks a path available to alkanecarboxylic acids for further oxidative degradation. Further generic oxidative degradation involves a one-electron oxidation of the benzoate anion, which decarboxylates to yield the phenyl radical, which then can be converted to benzene or phenol.
This generic pathway can be illustrated by a specific oxidative process with commercial importance. Benzoic acid is synthesized on ton scales via the oxidation of toluene. The stability of benzoic acid under these oxidizing conditions is sufficient to allow benzoic acid to accumulate in the industrial process (21).
Naphthalene and higher polycyclic aromatic hydrocarbons.
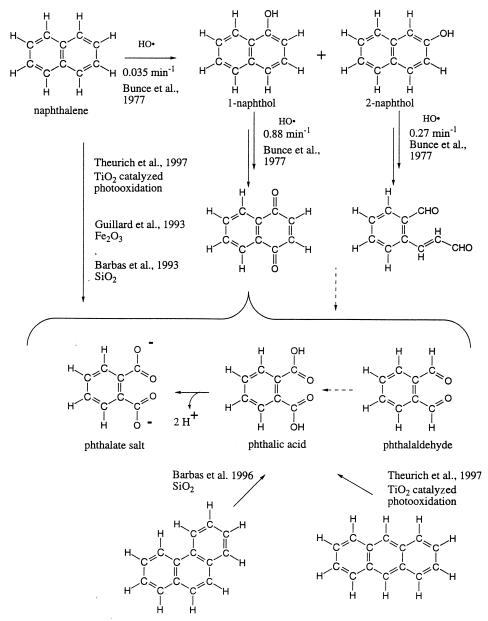
The generic oxidation of polycyclic aromatic hydrocarbons involves the addition of the HO⋅ radical to give a hydroxycyclohexadienyl radical. This radical suffers further oxidation to give eventually single core aromatic rings to which carboxylic acids are attached wherever a second ring was fused. Thus, naphthalene, phenanthrene (22), and anthracene (23) all give phthalic acid in the generic oxidation process. Higher polycyclic aromatic hydrocarbons give benzenetricarboxylic, tetracarboxylic, pentacarboxylic, and hexacarboxylic acids (Fig. 1; ref. 24).
The generic pathway can be exemplified with laboratory reactions of naphthalene, which is 1–6 ppm in some carbonaceous chondrites (25). The pseudo first-order rate constant for the first step in the reaction between naphthalene and the HO⋅ radical (Fig. 2) is 0.035 min−1 (26). The rate constants for further oxidation of 1- and 2-naphthol are higher (0.88 and 0.27 min−1). These higher rate constants imply that neither 1- nor 2-naphthol will accumulate. The metastable end products are phthaldehyde and phthalic acid.
Figure 2.
Oxidative degradation of naphthalene to phthalic acid. Solid arrows indicate reactions documented in the literature with citations. Dotted arrows indicate transformations presumed to occur but without documentation in the cited literature (from refs. 23 and 26).
Analogous outcomes are observed under a variety of other conditions [oxidation catalyzed by TiO2, by SiO2 (27), and by Fe2O3 (28)]. This uniformity in outcome argues that the oxidation of naphthalene will generically yield phthalic acid as a metastable intermediate (29). The metastability of phthalic acid to further oxidation has commercial significance. An important industrial synthesis of phthalic acid begins with the oxidation of naphthalene (30). Phthalic acid is also produced from naphthalene under simulated Martian conditions (31).
Kerogen.
Polymeric organic material (kerogen) has no defined structure. On Earth, kerogen (coal, for example) comes via metamorphosis of biological matter. Under generic oxidation conditions, the aromatic portions of kerogen generate benzenecarboxylic acids, with one carboxylic acid group for every position on the core benzene ring that was attached to a carbon in the parent structure. These are metastable, accumulate, and are isolated and quantitated when defining the structure of kerogens. For example, treating coal with alkaline permanganate oxidized its carbon to carbonic acid (H2CO3, 42% vol/vol), acetic acid (CH3COOH, 2% vol/vol), oxalic acid (HOOC-COOH, 7% vol/vol), and benzenecarboxylic acids (48% vol/vol), with a trace of succinic acid (HOOC-CH2-CH2-COOH; ref. 32). Kerogen is the most abundant organic substance in meteorites. As with terrestrial kerogen, the kerogen from the Murchison meteorite gives benzenecarboxylic acid products when oxidized (33, 34). These are stable in refluxing nitric acid for 27 h.
Amino acids and hydroxyacids.
Polyfunctional molecules are easier to oxidize than unfunctionalized carboxylic acids. Thus, hydrogen peroxide (a mild oxidant) will, in the presence of iron salts, catalyze the oxidative decarboxylation of α-hydroxyacids to give carbon dioxide and the shorter aldehyde. This reaction, well known in sugar chemistry, has a brand name (the “Ruff degradation;” ref. 35).
Polyfunctionalized compounds are more rapidly converted to carbon dioxide under generic oxidation conditions. Oxalic acid is likely to be metastable, however, where iron is abundant (36).
The Amounts and Fates of Organic Carboxylic Acids.
This discussion makes the case that aromatic and aliphatic carboxylic acids are the metastable products of generic oxidation of meteoritic organic compounds. The generic oxidation pathway is exemplified by so many specific (admittedly terrestrial) reactions and is so well supported by organic structure theory that it seems plausible that it is followed on Mars as well.
If meteorites bring 2.4 × 108 g/year of organic carbon to Mars (4) and the mass yield of benzenecarboxylic acid from this material is 10%, then ≈7.2 × 1016 g of benzenecarboxylic acids should have been generated on Mars since its surface dried 3 billion years ago. The surface area of Mars is 3.6 × 1013 m2, corresponding to 2 kg of benzenecarboxylic acids per m2 of the Martian surface.
What would be the fate of these organic molecules? It is certain that these compounds were diluted by wind and impact into the Martian regolith. The meteoritic kerogen would have been accompanied by at least 70 times more inorganic meteoritic material. The inorganic composition of meteorites is very different than from that of a range of carbonaceous chondrites. These data provide a minimum measure of dilution. If mixed in the regolith to a depth of 1 m, 2 kg of benzenecarboxylates would contribute ≈500 ppm by weight of the first meter of surface of Mars (the density of Mars is ≈4 g/cm3). If gardening mixes the material to a depth of 1 km, benzenecarboxylates will be present at a concentration of 500 ppb. Analytical tools sensitive to the ppb level should detect these.
Other processes might have removed organic carboxylic acids from the immediate surface. Carboxylic acids react with metal oxides to form salts (37, 38). These often have some solubility in water. If the Martian surface has been exposed to water in the past, organic salts may have been removed from the surface by leaching and concentrated in subsurface environments. Examples on Earth include highly soluble salts (e.g., halite) and poorly soluble salts (e.g., gypsum). This process is almost certainly less important than gardening in the recent past, because surface water on Mars has been scarce for billions of years, and iron salts of benzenecarboxylic acids are poorly soluble (39, 40).
Most important, of course, are chemical reactions that would degrade the carbon skeleton. The Fenton reaction serves as a model, even recognizing that it is best known as an aqueous process (19). The Fenton reaction is believed to involve HO⋅ radical generated from H2O2 (41). Hematite and goethite (both iron oxides believed to exist on the Martian surface) are effective catalysts (42, 43). Fenton chemistry degrades organic molecules ranging from 2-methylnaphthalene (an aromatic compound) to n-hexadecane (an aliphatic compound) entirely to carbon dioxide, if given sufficient time. Even relatively resistant molecules can be degraded. For example, trinitrotoluene (TNT) is converted by H2O2 in water to trinitrobenzoic acid, from there to trinitrobenzene, and from there to oxalic acid as the primary organic end product. The oxalic acid is removed only when the mixture is exposed to light (44). Fenton chemistry converts benzoic acid into hydroxybenzoic acid and guanosine into 8-oxoguanosine (45). A UV-accelerated Fenton reaction is also known in aqueous solution and is proposed to generate Fe2+ by photoreduction (46).
The efficiency of the Fenton reaction depends on the ligands to iron (47, 48). For example, ferric oxalate initiates the destruction of other molecules (49, 50). Benzoic acid inhibits the Fenton reaction in certain terrestrial experiments (51). Aromatics are protected against degradation by more easily oxidized species (52). Thus, it is difficult to predict the consequences of Fenton chemistry on Mars, even if we assume that the process is analogous to the aqueous process known in the laboratory.
An alternative path for the oxidative degradation of carboxylic acids involves the one-electron oxidation of the carboxylate anion to give the carboxylate radical, which would lose carbon dioxide to give the radical of the shorter-by-one-carbon alkane (53). These will lose carbon dioxide to generate an organic radical, which will then be trapped as part of the oxidative cascade. Photons can accelerate this process and are likely to be important on the UV-irradiated surface of Mars (31, 54). Further, sand storms transfer dust to high altitudes, where it is intensely irradiated. Alkanecarboxylic acids are particularly susceptible to photochemical degradation. Many benzenecarboxylic acids are quite stable to photochemical degradation, however (55). Phthalic acid derivatives, for example, yield phthalic anhydride under prolonged irradiation (56, 57) but undergo no degradation of the aromatic core.
Absence of Organic Molecules Detectable by the Viking 1976 Mission.
If laboratory reactions are taken as examples of the generic oxidation pathway, the rates for the destruction of benzenecarboxylates are at least 103- to 106-fold slower than their rates of formation. Depending on the tempo of chemistry overall on Mars (and remembering that the billion years available for the accumulation of meteoritic organics is also available for the destruction of the derived benzenecarboxylates), substantial amounts of the 2 kg of benzenecarboxylates expected to be generated per m2 should have survived. Their concentration would fall below the nominal sensitivity of the Viking 1976 MS only if more than 99% of these were destroyed and if gardening diluted these to an average depth of 1 km or greater.
To gain access to the Viking MS, however, the organic molecule must first pass through a GC. Only volatile molecules can do so. Salts of organic carboxylic acids are not volatile. Thus, the salts of benzenecarboxylic acids, oxalic acid, and acetic acid would not be directly detectable by the Viking GC-MS experiments, even if they had been present.
The ability of the Viking experiments to detect organic carboxylates therefore depends on whether these carboxylates generate volatile products in the sample preparation (pyrolysis for 30 s at 200°, 350°, and 500°C). Even considering possible thermal degradation pathways, the three principal metastable intermediates in the generic oxidation process, benzenecarboxylic acids, acetic acid, and oxalic acid, could be detected only with difficulty by the Viking GC-MS. Oxalic acid generates carbon dioxide, carbon monoxide, and water under pyrolysis. These were in fact detected, but all are also components of the Martian atmosphere.
Higher benzenecarboxylic acids also do not easily yield volatile pyrolysis products. Benzenehexacarboxylate, a nonvolatile compound, will eventually release carbon dioxide on pyrolysis and become benzenepentacarboxylate (58) and then benzenetetracarboxylate. The salts of these, however, are also not volatile.
Acetic acid and its salts may be pyrolyzed to give volatile products. At high concentrations, acetone is formed (59). However, the iron (II) acetate and iron (II) propionate salts are reported to be “amazingly stable up to 400–500°C” (60).
For these reasons, the Viking experiments do not exclude the possibility that the soil being tested contained organic carboxylic acids, especially benzenecarboxylic acids in substantial amounts. To examine this conclusion experimentally, iron (III) phthalate, mellitate, and benzene-1,2,4-tricarboxylate were all synthesized by the method of Galwey (61). Iron (II) acetate and iron (III) oxalate were purchased from Aldrich. These were subjected to thermolysis MS after heating from 25° to 400°C in 30 s. The phthalate, mellitate, and oxalate salts were separately heated to 400°C in a quartz capillary in a direct insertion probe. Under these conditions, which give the MS better access to the probe than the Viking MS had, no signal was observed from the iron (III) salts of mellitic acid and benzene-1,2,4-tricarboxylic acid. Iron (III) phthalate yielded a small amount (1–2%) of phthalic anhydride that might have been but was not detected by the Viking GC-MS. Iron (II) acetate generated acetone, acetic acid, and acetic anhydride, all of which should have been detectable by the Viking GC-MS. Iron (III) oxalate releases carbon monoxide as well as carbon dioxide and water, which suggests that the Viking experiments can rule out substantial amounts of acetate on the surface (the top 10 cm) and modest amounts of phthalate but not higher benzenecarboxylates or oxalate.
These considerations do not alter the current interpretation of the other Viking 1976 results. No model has been presented to date that can quantitatively explain the details of the gas exchange and label release experiments (see review in ref. 9). Some models combine both a thermally stable source of oxygen (e.g., KO2 or CaO2) and a thermally labile oxidizing agent to explain the label release results (62). A generic perspective, however, notes that the compounds used in the label release experiments (aqueous formate, glycolate, glycine, d-alanine, l-alanine, d-lactate acid, and l-lactate at pH 6.2) are all “easily oxidizable” (63). For example, lactate is well known to be oxidized by H2O2 in the presence of iron to give carbon dioxide, conditions where benzenecarboxylates are stable.
Significance.
The notion that organic compounds from meteorites undergo a partial oxidative diagenesis to give compounds that are not directly detectable by GC-MS and do not easily generate volatile pyrolysis products can be extended to organic molecules that might be generated endogenously on Mars, either by biological or nonbiological sources. For example, if life exists on subsurface Mars or existed several billion years ago when the planet more resembled Earth at that time, bioorganic molecules that may have come to the surface adventitiously would be subject to similar diagenesis.
Conclusions.
Benzenecarboxylates, oxalates, and perhaps acetates are likely to have been formed on the surface of Mars via oxidation of organic material arriving to Mars via meteorite. On Earth, oxidation of meteoritic kerogen gives these compounds. These would not have been easily detected by the Viking 1976 experiments. The failure of the Viking 1976 experiments to find organics should not, therefore, be taken as a strong argument against the presence of all organic substances on Mars. In particular, we expect that benzenecarboxylates will be found on Mars by an appropriately designed search. Finally, organic substances derived from Martian life (if it exists) are expected to undergo a diagenesis analogous to that described for organics of meteoritic origin. This analysis does not suggest how oxidation products of organic material from meteorites could be distinguished from biomolecules arising from putative life on Mars. It does, however, provide a model for a background from which any surviving Martian biomolecules must be differentiated.
Acknowledgments
We are indebted to conversations with many members of the Mars Architecture Definition Team. This work was funded in part by the National Aeronautics and Space Administration Exobiology and Astrobiology programs.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040539497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040539497
References
- 1.Oyama V I, Berdahl B J. J Geophys Res. 1977;82:4669–4676. [Google Scholar]
- 2.Levin G V, Straat P A. Science. 1979;194:1322–1329. doi: 10.1126/science.194.4271.1322. [DOI] [PubMed] [Google Scholar]
- 3.Biemann K, Oró J, Toulmin P, III, Orgel L E, Nier A O, Anderson D M, Simmonds P G, Flory D, Diaz A V, Rushneck D R, et al. J Geophys Res. 1977;82:4641–4658. [Google Scholar]
- 4.Flynn G J. Earth Moon Planets. 1996;72:469–474. doi: 10.1007/BF00117551. [DOI] [PubMed] [Google Scholar]
- 5.Hayatsu R, Anders E. Topics Curr Chem. 1981;99:1–37. [Google Scholar]
- 6.Mullie F, Reisse J. Topics Curr Chem. 1987;139:85–117. [Google Scholar]
- 7.Sephton M A, Pillinger C T, Gilmour I. Geochim Cosmochim Acta. 1998;62:1821–1828. [Google Scholar]
- 8.Hayes J M, Biemann K. Geochim Cosmochim Acta. 1968;32:239–267. [Google Scholar]
- 9.McKay C P, Grunthaner F J, Lane A L, Herring M, Bartman R K, Ksendzov A, Manning C M, Lamb J L, Williams R M, Ricco A J, et al. Planet Space Sci. 1998;46:769–777. doi: 10.1016/s0032-0633(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 10.Kieffer S W, Jakosky B M, Snyder C W, Matthews M S, editors. Mars. Tucson, AZ: Univ. Arizona Press; 1992. [Google Scholar]
- 11.Hubbard J S, Hardy J, Voecks G E, Golub E E. J Mol Evol. 1973;2:149–166. doi: 10.1007/BF01653995. [DOI] [PubMed] [Google Scholar]
- 12.Chyba C, Sagan C. Nature (London) 1992;355:125–132. doi: 10.1038/355125a0. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard J S, Hardy J P, Horowitz N H. Proc Natl Acad Sci USA. 1971;68:574–578. doi: 10.1073/pnas.68.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz N H, Hobby G L. J Geophys Res. 1977;82:4659–4662. [Google Scholar]
- 15.Levin G V. Proc SPIE Int Soc Opt Eng. 1997;3111:146–161. [Google Scholar]
- 16.Hunten D M. Rev Geophys Space Phys. 1974;12:529–535. [Google Scholar]
- 17.Hunten D M. J Mol Evol. 1979;14:57–64. doi: 10.1007/BF01732369. [DOI] [PubMed] [Google Scholar]
- 18.McDonald G D, de Vanssay E, Buckley J R. Icarus. 1998;132:170–175. [Google Scholar]
- 19.Walling C. Acc Chem Res. 1997;8:125–131. [Google Scholar]
- 20.Kirsten W, Stenhagen E. Acta Chem Scand. 1952;6:682–689. [Google Scholar]
- 21.Heberger K, Nemeth A, Cotarca L, Delogu P. Appl Catal A. 1994;119:L7–L12. [Google Scholar]
- 22.Barbas J T, Sigman M E, Dabestani R. Environ Sci Technol. 1996;30:1776–1780. [Google Scholar]
- 23.Theurich J, Bahnemann D W, Vogel R, Ehamed F E, Alhakimi G, Rajab I. Res Chem Intermed. 1997;23:247–274. [Google Scholar]
- 24.Juettner B. J Am Chem Soc. 1937;59:208–213. [Google Scholar]
- 25.Pering K L, Ponnamperuma C. Science. 1971;173:237–239. doi: 10.1126/science.173.3993.237. [DOI] [PubMed] [Google Scholar]
- 26.Bunce N J, Liu L, Zhu J, Lane D A. Environ Sci Technol. 1997;31:2252–2259. [Google Scholar]
- 27.Barbas J T, Sigman M E, Buchanan A C, Chevis E A. Photochem Photobiol. 1993;58:155–158. [Google Scholar]
- 28.Guillard C, Delprat H, Can H V, Pichat P. J Atmos Chem. 1993;16:47–59. [Google Scholar]
- 29.Lane D A, Fielder S S, Townsend S J, Bunce N J, Zhu J, Liu L, Wiens B, Pond P. Polycyclic Aromat Compd. 1996;9:53–59. [Google Scholar]
- 30.Lowenheim F A, Moran M K, editors. Faith, Keyes and Clark's Industrial Chemicals. 4th Ed. New York: Wiley; 1975. [Google Scholar]
- 31.Oró J, Holzer G. J Mol Evol. 1979;14:153–160. doi: 10.1007/BF01732374. [DOI] [PubMed] [Google Scholar]
- 32.Bone W A, Horton L, Ward S G. Proc R Soc London Ser A. 1930;127:480–510. [Google Scholar]
- 33.Hayatsu R, Matsuoka S, Scott R G, Studier M H, Anders E. Geochim Cosmochim Acta. 1977;41:1325–1339. [Google Scholar]
- 34.Hayatsu R, Winans R E, Scott R G, McBeth R L, Moore L P, Studier M H. Science. 1980;207:1202–1204. doi: 10.1126/science.207.4436.1202. [DOI] [PubMed] [Google Scholar]
- 35.Wieland H, Franke W. Ann Chem. 1927;457:1–70. [Google Scholar]
- 36.Walton J H, Graham D P. J Am Chem Soc. 1928;50:1641–1648. [Google Scholar]
- 37.Martell A E, Smith R M. Critical Stability Constants. Vol. 3. New York: Plenum; 1977. [Google Scholar]
- 38.Avdeef A. J Pharmacol Sci. 1993;82:183–190. doi: 10.1002/jps.2600820214. [DOI] [PubMed] [Google Scholar]
- 39.Giammar D E, Dzombak D A. J Solution Chem. 1998;27:89–105. [Google Scholar]
- 40.Wu L P, Munakata M, Kuroda-Sowa T, Maekawa M, Suenaga Y. Inorg Chim Acta. 1996;249:183–189. [Google Scholar]
- 41.Chen C T, Tafuri A N, Rahman M, Foerst M B. J Environ Sci Health Part A. 1998;33:987–1008. [Google Scholar]
- 42.Lin S S, Gurol M D. Environ Sci Technol. 1998;32:1417–1423. [Google Scholar]
- 43.Watts R J, Jones A P, Chen P H, Kenny A. Water Environ Res. 1997;69:269–275. [Google Scholar]
- 44.Li Z M, Comfort S D, Shea P J. J Environ Qual. 1997;26:480–487. [Google Scholar]
- 45.Sandstrom B E, Svoboda P, Granstrom M, Harms-Ringdahl M, Candeias L P. Free Radical Biol Med. 1997;23:744–753. doi: 10.1016/s0891-5849(97)00058-0. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y F, Pignatello J J. J Agric Food Chem. 1993;41:308–312. [Google Scholar]
- 47.Pignatello J J, Baehr K. J Environ Qual. 1994;23:365–370. [Google Scholar]
- 48.Dean R T, Nicholson P. Free Radical Res. 1994;20:83–101. doi: 10.3109/10715769409147506. [DOI] [PubMed] [Google Scholar]
- 49.Safarzadeh-Amiri A, Bolton J R, Cater S R. Water Res. 1997;31:787–798. [Google Scholar]
- 50.Safarzadeh-Amiri A, Bolton J R, Cater S R. Solar Energy. 1996;56:439–443. [Google Scholar]
- 51.Zakharov I V, Kumpan Y V. Kinet Catal. 1996;37:174–178. [Google Scholar]
- 52.Owen R W, Wimonwatwatee T, Spiegelhalder B, Bartsch H. Eur J Cancer Prev. 1996;5:233–240. doi: 10.1097/00008469-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lamrini R, Lacan P, Francina A, Guilluy R, Desage M, Michon J, Becchi M, Brazier J L. Free Radical Biol Med. 1998;24:280–289. doi: 10.1016/s0891-5849(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 54.Bullock M A, Stoker C R, McKay C P, Zent A P. Icarus. 1994;107:142–154. doi: 10.1006/icar.1994.1012. [DOI] [PubMed] [Google Scholar]
- 55.Jeevarajan A S, Fessenden R W. J Am Chem Soc. 1992;114:10461–10470. [Google Scholar]
- 56.Balabanovich A, Schnabel W. J Photochem Photobiol A. 1998;113:145–153. [Google Scholar]
- 57.Balabanovich A I, Denizligil S, Schnabel W. J Vinyl Additive Technol. 1997;3:42–52. [Google Scholar]
- 58.Manion J A, McMillen D F, Malhotra R. Energy Fuels. 1996;10:776–788. [Google Scholar]
- 59.Davis R, Schultz H P. J Org Chem. 1962;27:854–857. [Google Scholar]
- 60.Granito C, Schultz H P. J Org Chem. 1963;28:879–881. [Google Scholar]
- 61.Galwey A K. J. Chem. Soc. 1965. 4235–4239. [Google Scholar]
- 62.Klein H P. Icarus. 1978;34:666–674. [Google Scholar]
- 63.Kultyugin A A, Sokolova L N. Arch Sci Biol USSR. 1936;41:145–149. [Google Scholar]