Abstract
Molecular typing, added to epidemiological data, can better identify transmission patterns of gonorrhea in Western countries, where the incidence has recently been rising. From September 2002 to September 2003, patients with a laboratory-confirmed diagnosis of gonorrhea at the Clinic for Sexually Transmitted Infections in Amsterdam, The Netherlands, were subjected to a questionnaire pertaining to sexual risk behavior and sexual partners in the 6 months prior to the diagnosis. The Neisseria gonorrhoeae isolates were all genotyped using PCR-restriction fragment length polymorphism of the porin and opacity genes. All patients with a completed questionnaire and genotyped isolates were included in the study. We obtained 885 N. gonorrhoeae isolates from 696 patients that revealed 88 clusters and 46 unique genotypes. Patients infected at multiple anatomical sites with one or more strains and patients infected several times during the study period were shown to pursue high-risk sexual behavior and were considered core groups. There were 11 clusters of ≥20 patients; in seven clusters, 81% to 100% of patients were men who have sex with men (MSM), three clusters contained 87 to 100% heterosexual men and women, and one cluster was formed by equal proportions of MSM and heterosexual male and female patients. However, the various clusters differed in characteristics such as types of coinfections, numbers of sexual partners, Internet use to seek sexual partners, and locations of sexual encounters. Molecular epidemiology of gonococcal isolates in Amsterdam revealed core groups and clusters of MSM and heterosexual patients that probably indicate distinct transmission networks.
A number of industrialized countries have reported considerable increases in gonorrhea cases. One explanation for the resurgence in the sexual risk behavior associated with increases in sexually transmitted infections (STIs) is the introduction of highly active antiretroviral therapy and the consequent reduction in the perceived human immunodeficiency virus (HIV)/AIDS threat (4, 14, 20-22). The identification of high-risk populations to whom prevention and intervention can be tailored is essential for managing STI outbreaks. However, studies of STI transmission that rely solely on self-reported behavioral data can yield incomplete or incorrect information due to the sensitive nature of the topic. Developments in molecular typing techniques can improve accuracy, and various new methods have already been evaluated in the search for discriminatory techniques to differentiate strains circulating in a population (12, 17, 23). The Neisseria gonorrhoeae por and opa genes, encoding the porin and opacity proteins, respectively, have proven helpful for genotyping N. gonorrhoeae (6, 15, 16), revealing links between individuals and identifying common social venues (2, 5, 25, 26).
As in other Western countries, the N. gonorrhoeae prevalence in Amsterdam, The Netherlands, has recently risen, mainly among men who have sex with men (MSM); N. gonorrhoeae diagnoses increased by 49% between 1999 (n = 706) and 2002 (n = 1049) (3, 8). We therefore started a cross-sectional study among clients of the Health Service of Amsterdam STI Clinic to identify high-risk and core groups for gonorrhea. Our goal was to improve prevention and contact tracing and to evaluate whether genotyping, added to epidemiological data, could better identify transmission patterns of N. gonorrhoeae.
MATERIALS AND METHODS
Study setting and population.
The STI Clinic in Amsterdam, The Netherlands, is a low-threshold clinic serving approximately 17,000 clients annually. All clients are routinely screened for chlamydia, gonorrhea, syphilis, and other STIs when indicated. Many clients make multiple visits a year, some are diagnosed with more than one STI at a time, and some yield positive N. gonorrhoeae cultures from more than one anatomical site. We defined a gonorrhea diagnosis as a patient who is culture positive for N. gonorrhoeae at one or more anatomical locations. In addition to N. gonorrhoeae cultures, specimens are taken for immediate microscopic evaluation for N. gonorrhoeae, and if the specimen is positive for gram-negative diplococci, the patient is treated immediately with 1,000 mg of cefotaxime by intramuscular injection. Otherwise, the patient receives treatment after laboratory confirmation.
For this study, public health nurses completed a questionnaire with gonorrhea patients after obtaining consent at the time of treatment. These questionnaires, bearing a study number, contained questions about sexual risk behavior, the last four traceable partners, and all nontraceable partners over the 6 months before the gonorrhea diagnosis. If patients had a previous gonorrhea diagnosis within those 6 months, the questionnaires pertained to the period since that diagnosis. All patients were advised to notify their sexual partners and were given notification slips (containing the patient's study number) if needed. When a sexual partner responded by visiting the STI Clinic, he/she underwent the routine STI examination and received treatment for gonorrhea pending laboratory confirmation. If gonorrhea was confirmed, a study questionnaire was then completed with that person, and the N. gonorrhoeae isolates were typed.
In 12 months from September 2002 to September 2003, 19,135 visits were made to the STI Clinic, and gonorrhea was diagnosed 872 times (824 patients, including recidivists). Of these, 734 gonorrhea diagnoses (696 patients) were included in the study. We obtained 885 isolates derived from one or more anatomical locations per diagnosis. We excluded isolates from 105 patients who lacked questionnaires (n = 100) or were treated based on positive Gram stains only (n = 3) or whose isolates could not be typed because cultures were not available for DNA sampling (n = 2). The excluded patients were more often women (30% versus 18%) and less often heterosexual men (11% versus 22%) than patients included (P < 0.001, χ2 test).
Diagnostic techniques.
For N. gonorrhoeae culture, specimens routinely taken from the cervix, rectum, pharynx, and/or urethra were directly inoculated onto GC-lect culture plates (Becton Dickinson, Germany) and incubated in candle jars at 36°C at the Public Health Laboratory of Amsterdam. After 48 h, N. gonorrhoeae colonies were confirmed morphologically and biochemically as well as by Gram staining (7). In The Netherlands, the first-line treatment for gonorrhea among general practitioners until 2004 was ciprofloxacin, and ciprofloxacin was used at the STI Clinic until 2000. Therefore, susceptibility to ciprofloxacin (Oxoid, The Netherlands) was determined using the disk diffusion method with cutoff values according to CLSI guidelines (http://www.nccls.org). Strains found to be resistant by the disk diffusion method were also tested by E-test application (M0000173-M680; AB BIODISK, Sweden) on Choc-vitox culture plates (Oxoid) inoculated with a gonococcal suspension (0.5 to 1.0 McFarland standard). MIC values of ciprofloxacin were as follows: sensitive, MIC of <0.06 μg/ml; intermediate, 0.06 μg/ml < MIC < 1.0 μg/ml; resistant, MIC of ≥1.0 μg/ml.
Molecular techniques.
After confirmation of N. gonorrhoeae on the primary plates, up to five single colonies per anatomical location were inoculated onto the secondary plate to obtain pure culture colonies. Isolates were typically harvested for DNA isolation from the pure culture plates, again taking up to five morphologically identical and single colonies depending on the size of the colonies. If no separate colonies grew on the pure culture plate, N. gonorrhoeae colonies were harvested from a tertiary plate. Colonies were lysed in 100 μl of 5 M guanidine thiocyanate buffer (BioMérieux, The Netherlands) containing 0.04 mg/ml glycogen (Roche Diagnostics, The Netherlands) and were stored at +4°C until DNA isolation was performed. Chromosomal DNA was extracted from lysates by a standard isopropanol precipitation procedure. The DNA pellet was dissolved in 50 μl T10 buffer (10 mM Tris HCl, pH 8.0) and diluted 500 times in T10 buffer.
Typing of the por gene was based on a procedure described previously by Rahman et al. (16) by using primers from Invitrogen (The Netherlands). PCR was performed with 2 μl of the undiluted DNA solution in a 25-μl mixture containing 10 mM Tris-HCl (pH 8.0), 50 mM NaCl, 2 mM MgCl2, 0.01% (wt/vol) gelatin, 20 ng of each primer, and 0.5 U Taq polymerase enzyme (Eurogentec, The Netherlands). PCR was executed in a PTC-200 machine (MJ Research, BioZym, The Netherlands) with the following cycling program: 3 min at 94°C and 30 cycles of 30 s at 93°C, 30 s at 55°C, and 50 s at 72°C, plus a final extension step for 7 min at 72°C. The size of the resulting por PCR fragment was between 900 and 1,100 bp. For restriction fragment length polymorphism (RFLP), 5 μl of the PCR product was digested with MspAlI restriction enzyme (Westburg, The Netherlands) at 37°C for 2 to 4 h. All por fragments (PCR and RFLP) were visualized with ethidium bromide on a 10% nondenaturing polyacrylamide gel.
Typing of opa genes was performed based on a method described previously by O'Rourke et al. (15). The following primers hybridized to conserved regions in each of the 11 opacity genes (opaA to opaK) flanking a hypervariable region: OPAnest-down (5′-AAG CGT CCC CAR TWG TGG TA-3′) and OPA-3 (5′-GCA GAT TAT GCC CGT TAC A-3′). The OPAnest-down antisense primer is located 30 bp downstream of the OPA-down primer used by O'Rourke et al.; similarly, the sense primer OPA-3 is located 83 bp upstream from the OPA-up primer used by O'Rourke et al. PCR was performed with 2 μl of the diluted DNA solution, 20 ng of OPA-3, and 40 ng of OPAnest-down primers, producing fragments of maximally 487 bp. RFLP digestion with TaqI restriction enzyme (Westburg) was performed by incubating 8 μl of the opa PCR product at 37°C for 2 to 4 h. opa PCR and RFLP products were visualized with ethidium bromide on a 10% polyacrylamide gel.
Identification of clusters.
PCR-RFLP fragments of all genotyped isolates were scanned, and tagged image file format (TIFF) files were digitally entered into Bionumerics software (Applied Maths, Belgium); bands were digitally identified and digitally normalized by using Bionumerics software. All RFLP patterns were visually inspected and, if necessary, manually adjusted so that only clear bands were included. Only por RFLP fragments between 100 and 1,000 bp and opa RFLP fragments between 100 and 400 bp were included for alignment. Position tolerance settings were empirically optimized to obtain sufficient discriminative power. For por RFLP, an optimization of 1.1% and a position tolerance of 2.0% were used; for opa RFLP, these settings were 1.1% and 5.0%, respectively. Phylogenetic trees (unweighted-pair group method using average linkages) were made by using Bionumerics software (Applied Maths). As opa patterns showed a higher genetic diversity, we applied a weighted experiment comparison with a 1:2 ratio of por RFLP patterns to opa RFLP patterns (thus, the opa pattern counted twice).
We compared genetic similarities between the different N. gonorrhoeae isolates in the study at three cutoff values (80%, 85%, and 88%) in order to evaluate the optimal discrimination between por and opa RFLP patterns. A cutoff value of 88% proved to be the most discriminating value; thus, N. gonorrhoeae isolates with ≥88% genetically similar por and opa RFLP patterns from any two or more patients were considered to form one cluster.
We validated our typing technique in three ways. (i) We reisolated, reamplified, and retyped DNA from the same strain of five isolates three times by using the methods described above. This resulted in reproducible por and opa RFLP patterns for all five isolates. (ii) We compared por and opa RFLP patterns of strains isolated from patients infected with N. gonorrhoeae at different anatomical locations at one clinic visit. The patterns found were also reproducible. (iii) Lastly, we compared RFLP patterns of strains isolated from known sexual partners.
Large clusters were defined as clusters containing 20 or more patients with a diagnosis of gonorrhea. N. gonorrhoeae isolates with a novel por-opa RFLP pattern were considered unique. Based on epidemiological information, patients and their traceable sexual partners of the preceding 6 months who reported to the clinic were considered components. Only those components where more than one member had a diagnosis of gonorrhea with a typed isolate and a completed questionnaire were included in the cluster analyses. Patients who had positive N. gonorrhoeae cultures from more than one anatomical site at one clinic visit were considered to be patients with multiple infections. Recidivists were patients with more than one gonorrhea diagnosis during the study period.
Statistical analysis.
Risk factor analyses for gonorrhea infection were performed for all patients at each visit to the clinic using univariate and multivariate Poisson regression analyses (19). Differences between clusters of ≥20 gonorrhea patients and clusters of 3 gonorrhea patients or less were analyzed with logistic regression analyses using SPSS software (18). Multivariate logistic models were constructed using variables with P values of ≤0.10 in univariate analyses. Variables with P values of ≤0.05 were retained in the final model. Logistic regression was performed separately for the total study population, for heterosexual patients, and for MSM, since they reflect different sexual networks. Variables with P values of ≤0.05 were considered statistically significant. Patients who belonged to a component were compared to those who did not by using χ2 tests.
RESULTS
Study population.
Among the STI clinic population, being MSM was the only significant risk factor for becoming infected with N. gonorrhoeae (infection rate ratio [IRR], 6.1 [95% confidence interval {CI}, 5.3 to 7.0]). Among heterosexual clients, men were more at risk for acquiring N. gonorrhoeae than women (IRR, 1.84 [95% CI, 1.45 to 2.33]). Younger MSM were more at risk than older MSM (per annum decline in IRR, 1.03 [95% CI, 1.02 to 1.04]).
All patients together reported a total of 1,175 traceable partners, 398 (34%) of which were steady partners; 6,758 nontraceable partners (range, 1 to 500 per patient) were also reported. Anal intercourse was reported for 2,645 (39%) nontraceable partners, with 32% reported to be unprotected. Vaginal intercourse was reported with 1,413 (21%) nontraceable partners, with 45% reported to be unprotected. The HIV serostatus of 67% of traceable and 91% of nontraceable partners was unknown to the patients (Table 1).
TABLE 1.
Characteristics of gonorrhea patients and their reported partners at the STI Clinic in Amsterdam, The Netherlands, 2002 to 2003
| Characteristic | Value |
|---|---|
| Gonorrhea patients | |
| Age (yr) | |
| Median | 32 |
| IQR | 24-48 |
| No. (%) of patients | |
| All | 696 (100) |
| Sexual orientation | |
| MSMa | 412 (59) |
| Heterosexual men | 187 (27) |
| Heterosexual women | 97 (14) |
| Ethnic background | |
| Dutch | 397 (57) |
| Surinamese/Antillean | 98 (14) |
| Otherb | 201 (29) |
| Coinfections | |
| Chlamydia trachomatis | 179 (26) |
| Treponema pallidum | 19 (3) |
| HIV serostatus | |
| Tested HIV seronegative | 170 (24) |
| Known/tested HIV seropositive | 87 (13) |
| Unknown/not tested | 439 (63) |
| No. (%) of questionnaires completed by patients | |
| All | 734 (100) |
| At least 1 traceable partner reported | 292 (40) |
| At least 1 nontraceable partner reported | 433 (59) |
| No. (%) of reported traceable partners | |
| All | 1,175 (100) |
| Steady partners | 398 (34) |
| Ethnic background | |
| Dutch | 633 (54) |
| Surinamese/Antillean | 210 (18) |
| Otherb | 331 (28) |
| HIV serostatus | |
| Known HIV seronegative | 288 (25) |
| Known HIV seropositive | 92 (8) |
| Unknown HIV serostatus | 791 (67) |
| Use of Internet | 145 (12) |
| Sex abroad | 122 (10) |
| No. (%) of reported nontraceable partners | |
| All | 6,758 (100) |
| Reported anal intercourse | 2,645 (39) |
| Unprotected | 843 (32) |
| Reported vaginal intercourse | 1,413 (21) |
| Unprotected | 638 (45) |
| HIV serostatus | |
| Known HIV seronegative | 196 (3) |
| Known HIV seropositive | 408 (6) |
| Unknown HIV serostatus | 6,130 (91) |
| Use of Internet | 737 (11) |
| Locations of encounters | |
| Darkrooms of known venues | 1,819 (27) |
| Cruising places/parks | 788 (12) |
| Sex parties | 277 (4) |
| At home | 1,561 (23) |
| Other locationsc | 1,854 (27) |
| No. of components | 58 |
| No. of patients | 116 |
| No. of isolates | 156 |
MSM, men who have sex with men, including bisexual men.
Western and Eastern European, North American, Asian, Turkish, North African, African, and South American.
Saunas, cafés, commercial sex, and abroad.
A total of 885 N. gonorrhoeae isolates were collected during the study period. These isolates were classified into 88 clusters. Clusters varied in size, containing 2 to 47 gonorrhea patients (2 to 54 isolates), and clusters with two to five isolates occurred most frequently; 46 isolates were unique. Eleven clusters were considered large (≥20 gonorrhea patients). Clusters were named according to the por RFLP pattern (A, B, C, etc.) and were then subdivided according to the opa RFLP pattern (1, 2, 3, etc., with Ui for unique isolates).
We found a total of 145 components consisting of two to four persons: 77 components consisted of a patient and a partner, both of whom had gonorrhea, and 68 components were made up of a patient and a partner without gonorrhea. We included only those components consisting of patients with gonorrhea and a typed isolate with a completed questionnaire to be able to link the por-opa RFLP patterns to the sexual risk behavior. In total, this left 58 components consisting of two members with gonorrhea that had a typed isolate and a completed questionnaire. Patients in components differed from patients that were not in components: more heterosexual women were found among patients in components than among patients not in components (25% versus 11%), and fewer MSM were part of a component (50% versus 62%; P < 0.001, χ2 test). Patients in components reported more steady traceable partners (85% versus 45%; P < 0.001, χ2 test) and were more often HIV seronegative (41% versus 20%; P < 0.001, χ2 test). Isolates of component members were classified into 54 clusters, and two isolates were unique. The isolates from the majority of components (n = 46) had at least one isolate that belonged to the same cluster (79%). The remainder were discordant: in 12 components (21%), the patients' isolates belonged to two distinct clusters.
As the largest clusters and certain risk groups were considered pivotal to N. gonorrhoeae transmission, we focused our analyses on (i) patients whose isolates belonged to the 11 largest clusters, (ii) patients diagnosed with multiple infections, in particular those whose isolates had different RFLP patterns, and (iii) recidivists.
Large N. gonorrhoeae clusters (≥20 gonorrhea patients).
The 11 largest clusters contained isolates from 321 patients. A phylogenetic tree, representing two isolates per large cluster, is shown in Fig. 1. Table 2 describes the characteristics of these patients, while Tables 3 and 4 show information on traceable and nontraceable partners, respectively, within each of the large clusters. Logistic regression analyses revealed no risk factors for belonging to a large cluster among the total gonorrhea patient population or among MSM, but being younger than 30 years of age was a risk factor among heterosexual patients (odds ratio, 2.20 [95% CI, 1.19 to 4.06]).
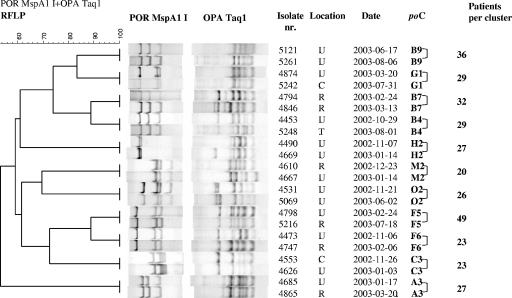
FIG. 1.
Genetic diversity among all large por-opa clusters (≥20 gonorrhea patients) as shown in a phylogenetic tree constructed for two N. gonorrhoeae isolates from each large cluster of gonorrhea patients at the STI Clinic in Amsterdam, The Netherlands. POR MspA1 I, RFLP patterns of por with restriction enzyme MspAlI; OPA Taq1, RFLP patterns of opa with restriction enzyme TaqI; Isolate nr., isolate number; Location, anatomical site of culture; C, cervix; R, rectum; T, tonsil; U, urethra; Date, date of visit to STI clinic (year-month-day); poC, por-opa cluster number. Numbers indicate the sizes of the clusters in the phylogenetic tree. The scale in the upper left-hand corner shows the percentage of similarity between the RFLP patterns of por and opa genes.
TABLE 2.
Characteristics of gonorrhea patients in large clusters at the STI Clinic in Amsterdam, The Netherlands, 2002 to 2003
| Characteristic | Value or type for cluster:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A3 | B4 | B7 | B9 | C3 | F5 | F6 | G1 | H2 | M2 | O2 | |
| No. of diagnoses | 27 | 29 | 35 | 41 | 23 | 47 | 24 | 29 | 27 | 20 | 28 |
| Median age in yrs (IQR) | 29 (26-38) | 34 (30-39) | 28 (23-36) | 36 (29-44) | 41 (28-54) | 30 (26-37) | 31 (23-37) | 22 (20-24) | 33 (29-39) | 34 (30-39) | 23 (21-41) |
| No. (%) of patients | |||||||||||
| Sexual orientation | |||||||||||
| MSMa | 22 (81) | 24 (83) | 16 (46) | 38 (93) | 3 (13) | 42 (86) | 21 (88) | 3 (10) | 28 (85) | 20 (100) | 0 |
| Heterosexual men | 5 (19) | 4 (14) | 15 (43) | 1 (2) | 17 (74) | 5 (10) | 3 (13) | 9 (31) | 4 (15) | 0 | 21 (75) |
| Heterosexual women | 0 | 1 (3) | 4 (11) | 2 (5) | 3 (13) | 2 (4) | 0 | 17 (59) | 0 | 0 | 7 (25) |
| Ethnic background | |||||||||||
| Dutch | 13 (48) | 16 (55) | 20 (57) | 29 (71) | 9 (39) | 29 (59) | 15 (63) | 15 (52) | 16 (59) | 15 (75) | 15 (54) |
| Surinamese/Antillean | 2 (7) | 1 (3) | 5 (14) | 1 (2) | 4 (17) | 1 (2) | 1 (4) | 10 (35) | 0 (0) | 0 | 8 (29) |
| Otherb | 12 (44) | 12 (41) | 10 (29) | 11 (27) | 10 (44) | 19 (39) | 8 (33) | 4 (14) | 11 (41) | 5 (25) | 5 (18) |
| Coinfections | |||||||||||
| Chlamydia trachomatis | 6 (22) | 5 (17) | 8 (23) | 8 (20) | 5 (22) | 11 (22) | 7 (29) | 11 (38) | 10 (37) | 1 (5) | 9 (31) |
| Treponema pallidum | 1 (4) | 1 (3) | 0 | 2 (5) | 1 (4) | 1 (2) | 1 (4) | 0 | 2 (7) | 1 (5) | 0 |
| HIV serostatus | |||||||||||
| Tested HIV seronegative | 3 (11) | 9 (31) | 10 (29) | 11 (27) | 3 (13) | 13 (27) | 3 (13) | 12 (41) | 7 (26) | 1 (5) | 9 (32) |
| Known/tested HIV seropositive | 7 (26) | 4 (14) | 2 (6) | 9 (22) | 2 (9) | 5 (10) | 2 (8) | 1 (3) | 6 (22) | 7 (35) | 0 |
| Unknown/not tested | 17 (63) | 16 (55) | 23 (66) | 21 (51) | 18 (78) | 31 (63) | 19 (79) | 16 (55) | 14 (52) | 12 (60) | 19 (68) |
| Total no. of patients | 27 | 29 | 32 | 36 | 23 | 49 | 23 | 29 | 27 | 20 | 26 |
| No. of isolates | 31 | 31 | 36 | 44 | 27 | 56 | 27 | 37 | 29 | 23 | 33 |
| Ciprofloxacin resistancec | S | S | S | S | S | S | R/S | S | S | S | S |
| Cluster typed | MSM | MSM | Mixed | MSM | HS | MSM | MSM | HS | MSM | MSM | HS |
MSM, men who have sex with men, including bisexual men.
Western and Eastern European, North American, Asian, Turkish, North African, African, and South American.
S, sensitive (MIC < 0.06); R, resistant (MIC > 1).
Cluster type based on sexual orientation. HS, heterosexual; Mixed, mixed heterosexual/MSM.
TABLE 3.
Characteristics of traceable partners as reported by gonorrhea patients at the time of diagnosis
| Characteristic | Value or type for cluster:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A3 | B4 | B7 | B9 | C3 | F5 | F6 | G1 | H2 | M2 | O2 | |
| Cluster type | MSM | MSM | Mixed | MSM | HS | MSM | MSM | HS | MSM | MSM | HS |
| No. (%) of partnersa | |||||||||||
| All | 41 | 44 | 48 | 67 | 29 | 76 | 42 | 51 | 49 | 34 | 56 |
| Steady partners | 9 (22) | 21 (48) | 22 (46) | 18 (27) | 7 (24) | 24 (32) | 10 (24) | 21 (41) | 14 (29) | 8 (24) | 20 (36) |
| Age category | |||||||||||
| 15-24 yr | 12 (29) | 5 (11) | 18 (37) | 9 (13) | 12 (41) | 16 (21) | 6 (14) | 32 (63) | 4 (8) | 1 (3) | 34 (61) |
| 25-34 yr | 20 (49) | 17 (39) | 19 (40) | 32 (48) | 10 (34) | 43 (57) | 23 (55) | 13 (25) | 23 (47) | 21 (62) | 11 (20) |
| >35 yr | 9 (22) | 21 (48) | 11 (23) | 26 (39) | 5 (17) | 16 (21) | 13 (31) | 6 (12) | 21 (43) | 12 (35) | 8 (14) |
| Missing valuesc | 1 (2) | 2 (7) | 1 (1) | 1 (2) | 3 (5) | ||||||
| Ethnic background | |||||||||||
| Dutch | 26 (63) | 32 (73) | 26 (54) | 43 (64) | 11 (38) | 52 (68) | 21 (50) | 18 (35) | 32 (65) | 20 (59) | 28 (50) |
| Surinamese/Antillean | 0 | 2 (4) | 8 (17) | 3 (5) | 5 (17) | 1 (1) | 2 (5) | 25 (49) | 1 (2) | 3 (9) | 21 (38) |
| Otherb | 15 (37) | 10 (23) | 14 (29) | 21 (31) | 13 (45) | 23 (30) | 19 (45) | 8 (16) | 16 (33) | 11 (32) | 7 (12) |
| HIV serostatus | |||||||||||
| Known HIV seronegative | 7 (17) | 11 (25) | 22 (46) | 15 (22) | 4 (14) | 25 (33) | 19 (45) | 6 (12) | 10 (20) | 9 (26) | 6 (11) |
| Known HIV seropositive | 6 (15) | 3 (7) | 4 (8) | 3 (5) | 2 (7) | 7 (9) | 3 (7) | 1 (2) | 6 (12) | 3 (9) | 0 (0) |
| HIV serostatus unknown | 28 (68) | 30 (68) | 22 (46) | 49 (73) | 23 (79) | 44 (58) | 20 (48) | 44 (86) | 33 (67) | 22 (65) | 50 (89) |
| Use of Internet | 13 (32) | 3 (7) | 9 (19) | 10 (15) | 2 (7) | 11 (15) | 7 (17) | 3 (6) | 16 (33) | 7 (21) | 0 (0) |
| Sex abroad | 1 (2) | 2 (5) | 0 (0) | 7 (10) | 4 (14) | 4 (5) | 10 (24) | 0 (0) | 7 (14) | 2 (6) | 0 (0) |
Number of partners reported by gonorrhea patients, per questionnaire, completed at each diagnosis; also see footnotes of Table 2 for definitions.
Western and Eastern European, North American, Asian, Turkish, North African, African, and South American.
Age data not available.
TABLE 4.
Characteristics of nontraceable partners as reported by gonorrhea patients at the time of diagnosis
| Characteristic | Value or type for cluster:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A3 | B4 | B7 | B9 | C3 | F5 | F6 | G1 | H2 | M2 | O2 | |
| Cluster type | MSM | MSM | Mixed | MSM | HS | MSM | MSM | HS | MSM | MSM | HS |
| No. (%) of partnersa | 324 | 153 | 156 | 897 | 86 | 519 | 168 | 48 | 712 | 183 | 27 |
| Anal intercourse | 140 (43) | 84 (55) | 52 (33) | 333 (37) | 14 (16) | 322 (62) | 109 (65) | 2 (4) | 111 (16) | 71 (39) | 1 (4) |
| Unprotectedb | 24 (17) | 52 (62) | 15 (29) | 96 (29) | 8 (57) | 57 (18) | 7 (6) | 2 (100) | 10 (9) | 22 (31) | 1 (100) |
| Vaginal intercourse | 39 (12) | 1 (2) | 28 (18) | 2 (0,2) | 60 (70) | 45 (9) | 1 (1) | 40 (83) | 500 (70) | 1 (1) | 27 (100) |
| Unprotectedb | 15 (39) | 0 (0) | 8 (29) | 1 (50) | 6 (10) | 26 (58) | 0 (0) | 7 (18) | 200 (40) | 0 (0) | 11 (41) |
| HIV serostatus | |||||||||||
| Known HIV seronegative | 0 (0) | 9 (6) | 11 (7) | 8 (1) | 4 (5) | 8 (1) | 21 (12) | 1 (2) | 14 (2) | 11 (6) | 1 (4) |
| Known HIV seropositive | 4 (1) | 36 (24) | 0 (0) | 14 (2) | 5 (6) | 14 (3) | 0 (0) | 0 (0) | 15 (2) | 13 (7) | 0 (0) |
| HIV serostatus unknown | 320 (99) | 103 (67) | 145 (93) | 875 (98) | 77 (89) | 496 (96) | 147 (88) | 47 (98) | 683 (96) | 159 (87) | 26 (96) |
| Missing valuesd | 5 (3) | 1 (0) | |||||||||
| Use of Interneta | 147 (45) | 18 (11) | 1 (1) | 24 (3) | 0 | 36 (7) | 39 (23) | 0 | 38 (5) | 17 (9) | 0 |
| Locations of encountersa | |||||||||||
| Dark rooms of known venues | 52 (16) | 70 (46) | 56 (36) | 483 (54) | 0 | 118 (23) | 22 (13) | 0 | 78 (11) | 71 (39) | 0 |
| Cruising places/parks | 11 (3) | 29 (19) | 28 (18) | 185 (21) | 0 | 62 (12) | 0 | 5 (10) | 4 (1) | 9 (5) | 0 |
| Sex parties | 2 (1) | 5 (3) | 0 | 6 (1) | 0 | 15 (3) | 2 (1) | 0 | 25 (4) | 3 (2) | 0 |
| At home | 104 (32) | 23 (15) | 51 (33) | 92 (10) | 42 (49) | 211 (41) | 100 (60) | 7 (15) | 104 (15) | 88 (48) | 16 (59) |
| Other locationsc | 41 (13) | 25 (16) | 18 (12) | 126 (14) | 33 (38) | 48 (9) | 9 (5) | 20 (42) | 501 (70) | 14 (8) | 8 (30) |
Number of partners reported by gonorrhea patients per questionnaire.
Missing values were not included in calculating proportions.
Saunas, cafés, commercial sex, and abroad; also see footnotes of Table 2 for definitions.
HIV serostatus data not available.
Among patients in large clusters, characteristics and sexual behavior were diverse. For instance, seven of the large clusters contained predominantly MSM (81 to 100%), three clusters consisted almost entirely of heterosexual men and women (87 to 100%), and one cluster was composed almost equally of MSM (46%) and heterosexuals (54%). Patients in mainly heterosexual clusters were younger and more often of Surinamese/Antillean ethnicity (17% to 35%) than patients in mainly MSM clusters (0% to 7%) (Table 2). Of the traceable partners who were met through the Internet, 99% were partners of MSM patients, which shows its significant role for MSM seeking their sexual partners. Patients in MSM clusters were more often known to be HIV seropositive, were more often coinfected with syphilis, and reported more sexual partners than patients in heterosexual clusters (Tables 2 and 3).
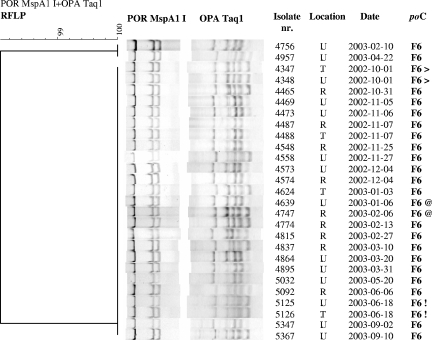
Cluster compositions varied greatly. For example, cluster F6 consisted of 27 isolates from 23 patients, mostly MSM, almost a third of whom were coinfected with chlamydia (Fig. 2). Of all large clusters, patients in cluster F6 reported the highest number of traceable partners with whom they had sex abroad, although this was in Europe and not in Southeast Asia. Some cluster F6 isolates were resistant to ciprofloxacin, and only some patients with ciprofloxacin-resistant strains reported travel abroad. Patients in cluster A3 were mostly MSM and reported much use of the Internet to seek traceable and nontraceable partners. Cluster M2, also mostly MSM, contained the highest percentage of HIV-seropositive patients but the lowest number coinfected with chlamydia. Cluster G1, a heterosexual cluster, consisted largely of young women with a median age of 20 years (interquartile range [IQR], 19 to 21 years). Notably, a third of the patients were of Surinamese/Antillean ethnicity, and this cluster had the highest percentage of patients who were coinfected with chlamydia (Tables 2, 3, and 4).
FIG. 2.
Cluster F6 of gonorrhea patients at the STI Clinic in Amsterdam, The Netherlands. The symbols following the cluster numbers (> and !) represent two isolates of multiple anatomical sites (tonsil and urethra) obtained from one individual at one visit. @ indicates two isolates from a recidivist with two isolates in the same cluster obtained at two different visits. For abbreviations, see the legend of Fig. 1.
Patients with multiple infections.
A total of 130 patients, predominantly MSM and women, were diagnosed at one visit with N. gonorrhoeae at two or more anatomical sites. Patients infected with multiple N. gonorrhoeae strains were more likely to be known to be HIV seropositive and used the Internet frequently to find sexual partners (Table 5).
TABLE 5.
Patients infected with multiple N. gonorrhoeae strains and recidivists at the STI Clinic in Amsterdam, The Netherlands, 2002 to 2003
| Characteristica | Value for:
|
|
|---|---|---|
| Patients carrying multiple strains | Recidivists | |
| No. of typed isolates | 121 | 83 |
| No. of large clusters represented among isolates | 11 | 9 |
| Gonorrhea patients | ||
| Age (yr) | ||
| Median | 30 | 33 |
| IQR | 23-35 | 26-38 |
| No. of questionnaires completed by patients | 57 | 73 |
| No. (%) of patients | ||
| Total | 52 (100) | 35 (100) |
| Sexual orientation | ||
| MSM | 38 (73) | 25 (71) |
| Heterosexual men | 0 | 10 (29) |
| Heterosexual women | 14 (27) | 0 |
| Ethnic background | ||
| Dutch | 36 (69) | 21 (60) |
| Surinamese/Antillean | 5 (10) | 2 (6) |
| Other | 11 (21) | 12 (34) |
| Coinfections | ||
| Chlamydia trachomatis | 14 (27) | 9 (26) |
| Treponema pallidum | 0 | 3 (9) |
| HIV serostatus | ||
| Tested HIV seronegative | 10 (19) | 5 (14) |
| Known/tested HIV seropositive | 9 (17) | 9 (26) |
| Unknown/not tested | 33 (64) | 21 (60) |
| Partners | ||
| No. (%) of reported traceable partners | 107 (100) | 114 (100) |
| Steady partners | 34 (32) | 37 (33) |
| Ethnic background | ||
| Dutch | 71 (66) | 74 (65) |
| Surinamese/Antillean | 13 (12) | 11 (10) |
| Other | 23 (21) | 29 (25) |
| HIV serostatus | ||
| Known HIV seronegative | 24 (22) | 31 (27) |
| Known HIV seropositive | 10 (9) | 16 (14) |
| HIV serostatus unknown | 73 (68) | 67 (59) |
| Use of Internet | 22 (21) | 16 (14) |
| Sex abroad | 11 (10) | 6 (5) |
| No. (%) of reported nontraceable partners | 1,072 (100) | 1,016 (100) |
| Anal intercourse | 464 (43) | 445 (44) |
| Unprotected | 101 (22) | 264 (59) |
| Vaginal intercourse | 312 (29)b | 32 (3) |
| Unprotected | 306 (98) | 2 (6) |
| HIV serostatus | ||
| Known HIV seronegative | 19 (2) | 9 (1) |
| Known HIV seropositive | 24 (2) | 36 (4) |
| Unknown HIV serostatus | 1,029 (96) | 971 (96) |
| Use of Internet | 269 (25) | 231 (23) |
| Locations of encounters | ||
| Dark rooms of known venues | 206 (19) | 147 (15) |
| Cruising places/parks | 27 (3) | 201 (20) |
| Sex parties | 66 (6) | 63 (6) |
| At home | 324 (30) | 236 (23) |
| Other locations | 380 (35) | 231 (23) |
Also see Table 2 footnotes for definitions.
One heterosexual patient reported unprotected vaginal sex with 300 nontraceable partners.
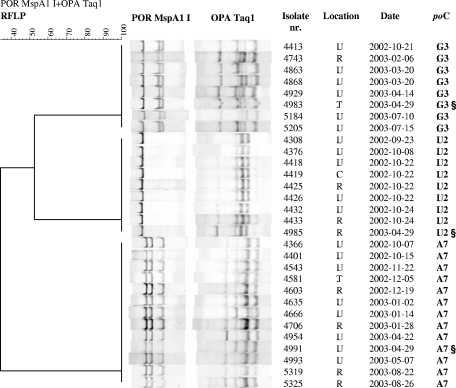
The isolates (n = 287) from these patients were distributed over 73 clusters, while nine isolates were unique. The por-opa RFLP patterns from all isolates (n = 170) of 78 patients (60%) were identical, suggesting infection with one strain (see examples in Fig. 2). The remaining 52 patients (40%) had isolates (n = 117) that belonged to more than one cluster, suggesting infection with two or more N. gonorrhoeae strains simultaneously. An infection with multiple N. gonorrhoeae strains at different anatomical locations is illustrated in Fig. 3, in which one patient has three different isolates that belong to clusters A7, G3, and U2.
FIG. 3.
Example of one gonorrhea patient (§) found to be infected with three N. gonorrhoeae strains (tonsil, rectum, and urethra) at one visit to the STI Clinic in Amsterdam, The Netherlands. The three isolates belong to clusters A7, G3, and U2; all isolates of these clusters are shown in one phylogenetic tree. See the legend of Fig. 1 for abbreviations.
Recidivists.
During the study period, 35 recidivists who were exclusively male, predominantly Dutch MSM, and relatively frequently coinfected with chlamydia and/or HIV as well as syphilis were identified. Nontraceable contacts were often recruited through the Internet, and sex took place mainly at home or at sex-cruising areas (Table 4).
All isolates (n = 83) from recidivists were classified into 36 clusters. For eight recidivists (23%), isolates obtained at two different visits fell into the same cluster, suggesting infections with the same strain (Fig. 2). The isolates of the remaining recidivists (n = 27) obtained at each new visit fell into different clusters, implying infections with different N. gonorrhoeae strains. The median time between the visits did not differ significantly between recidivists who were serially infected with the same strain and those serially infected with a different strain. For both groups, the median time between visits was 113 days (IQR, 76 to 163 days).
DISCUSSION
Genotyping of the 885 N. gonorrhoeae isolates in our study revealed 88 clusters and 46 unique isolates. Moreover, 11 clusters containing more than 20 gonorrhea patients (large clusters) were present during the total study period, suggesting that they did not represent outbreaks but rather represented continuous chains of transmission. In each of the large clusters, 80 to 100% of patients were either MSM or heterosexual men and women. Distinctions between heterosexual and MSM clusters were observed in ethnic backgrounds and types of coinfections (chlamydia versus HIV), numbers of traceable and nontraceable partners, and the awareness of partners' HIV serostatus. As for nontraceable partners, clusters differed in anal/vaginal intercourse (protected and not protected), Internet use, and locations of sexual encounters.
Our observations of distinct heterosexual and MSM clusters with shared demographic characteristics in terms of their size and persistence correspond with several N. gonorrhoeae genotyping studies done in the United Kingdom, even though opa and por RFLP typing was performed by using different molecular techniques (6, 10, 12). In our study, for example, cluster G1 contained 59% female patients, 35% of whom were from a Surinamese/Antillean ethnic background. Two persisting, homogeneous N. gonorrhoeae clusters in Sheffield (opa-1 and opa-4) also contained significantly more women (opa-1) and significantly more male heterosexual patients of black ethnic origin (opa-4) (10). Howie et al. previously found multiple clusters among MSM from the Edinburgh Genitourinary Medicine Clinic. In contrast to our study, those authors found many more small clusters and unique strains among fewer isolates when N. gonorrhoeae opa genes were typed, perhaps because only opa typing was performed and/or three restriction enzymes were used with RFLP, while we used one enzyme (6). Martin et al. also found homogeneous clusters of sequence types with shared demographic characteristics by using sequence analysis of the por and tbpB fragments of N. gonorrhoeae strains and multilocus sequence typing (MLST) for analysis (12).
Recidivists (n = 35) were also a notable group in our study population. They reported risky sexual behavior, as evidenced by the high percentage of coinfections with either syphilis or HIV, the frequency of (unprotected) anal intercourse, the unawareness of the HIV serostatus of nontraceable partners, and Internet use to contact partners. Moreover, of the 35 recidivists, 27 (77%), or 5% of the total study population, had isolates with different por-opa profiles at subsequent visits, suggesting infections with new strains. When we considered the intervals between visits, we found the turnover time of infection to be no higher than the median of 113 days. A much higher rate of reinfection with different strains was seen in South Africa although in a smaller study population: six out of seven female patients had isolates with a different opa RFLP pattern 8 to 10 days after their initial consultation (13).
We did not observe infections with multiple gonococcal strains present at one anatomical location (mixed infection). By using cultured samples, it is possible that we detected only the dominant strains. Others previously described mixed infections in specimens from 4 out of 15 male patients (11). Lynn et al. directly amplified clinical samples from 20 culture-positive women from Baltimore, Md., stored under different conditions. Those authors found that 40% of isolates from the paired cervical swab and wick samples and culture samples differed in strain type per patient, when the por gene was characterized with DNA-DNA hybridization (9). Repetition of the genotyping process with several strains in our study, however, showed that the por-opa RFLP patterns were reproducible, yielding only one strain per sampled anatomical location.
The current study did, however, reveal a high occurrence of patients simultaneously infected with multiple strains at different anatomical locations (n = 52), which is a considerable proportion (7%) of the total study population. This finding could result from high-risk sexual behavior, with several strains acquired from multiple sexual partners. The many traceable (n = 1,175) and nontraceable (n = 6,758) partners reported in the questionnaires gives an indication of this high-risk behavior. Furthermore, these patients used the Internet twice as often to seek traceable and nontraceable partners as did the total study population; they also reported more anal intercourse with nontraceable partners.
Another explanation for the frequency of infections with multiple strains at different anatomical locations can be recombination or reassortment of N. gonorrhoeae strains within one patient or between patients shortly after transmission. This bias has been described previously by several studies including those of Bhat et al., where those investigators observed that intergenic and intragenic recombination took place in the opa genes of descendants of strain MS11 in vivo and in vitro, and Martin et al., who studied N. gonorrhoeae isolates from patients attending genitourinary medicine clinics in the United Kingdom (1, 10). DNA mutation and/or recombination cannot be ruled out and will certainly occur independent of the typing technique used, as it is known that N. gonorrhoeae opacity and porin proteins are subject to host immune responses (1, 24). The persistence of the large clusters during the study period could indicate that reassortment and recombination are not extremely frequent but that, possibly, a very successful (founder) genotype circulated in the population.
O'Rourke et al. previously concluded that the differences in the repertoire of the opa genes should be reproducible, whereas genetic exchange should not (15), and our strains showed no differences in RFLP patterns after they were retyped. However, patients may be included in clusters with the same por-opa RFLP pattern without actually being (in)direct sexual contacts. This indicates that on an individual level, molecular typing is mainly a tool that helps to define linkages but cannot prove connectivity because of possible recombination. How often patients are inadvertently clustered due to recombination is unknown. By expanding the current por-opa typing with sequence-based typing methods, such as sequencing of the por IB gene, MLST, or Neisseria gonorrhoeae multiple antigenic sequence typing, the reproducibility of the observed large clusters could be verified. In the study described previously by Viscidi and Demma, such a comparison was done for 25 strains and 18 housekeeping genes (24). There appeared to be no concordance between MLST and por or opa typing, and the diversity of 18 genes, large parts of which were sequenced with MLST, was so high that all sexual partners also differed in strain types. Such a high variability is not desirable because clustering will disappear altogether. Only when just three genes were used (pilA, abcZ, and serC) were Viscidi and Demma able to molecularly link all 16 known partners (24), whereas in our study, 79% of the components clustered.
More isolates clustered with genotyping alone than when clustering was based solely on epidemiological data, as 95% of all genotyped isolates clustered, while only 58 pairs of patients, representing 17% of the included patients, actually reported being sexual contacts (constituting components). This is similar to the study described previously by Howie et al., where three linked clusters were found by conventional contact tracing, while opa RFLP typing revealed 14 clusters (6). The limited number and size of the components illustrated the relative weakness of our current partner notification system and emphasized the added value of molecular typing in our exploration of gonorrhea transmission in Amsterdam. The data collected by the structured questionnaires gave us detailed information on partner and patient behaviors within and among the detected clusters.
The probable transmission networks identified through molecular epidemiology improved possibilities for contact tracing and enhancement of STI control. For public health laboratories, using molecular epidemiology could create opportunities to investigate the spread of gonorrhea in a population, target the persons involved, and take measures at social venues where sex contacts take place. The combination of por and opa typing with epidemiological data enabled the identification of certain subgroups that otherwise would not have been disclosed: patients in large clusters, patients infected at multiple anatomical locations, and recidivists. We conclude that molecular epidemiology is an important tool that is suitable for identifying groups of patients involved in gonorrhea transmission.
Acknowledgments
We thank J. Bax, W. Bolderik, and C. de Jong, STI Clinic, Cluster of Infectious Diseases, Health Service of Amsterdam, for data management; the public health nurses at the Health Service of Amsterdam STI Clinic for collecting samples and completing the questionnaires; C. J. Aantjes, K. de Jong, and I. Peters, STI Clinic, Cluster of Infectious Diseases, Health Service of Amsterdam, for additional efforts in evaluating the contact tracing of N. gonorrhoeae patients; J. Spaargaren, J. van der Luijster, and the Public Health Laboratory personnel, Cluster of Infectious Diseases, Health Service of Amsterdam, for processing of all cultures; and L. D. Phillips for editing the manuscript.
The authors hereby state that they did not have a commercial or other association that might pose a conflict of interest regarding the study presented in this paper.
This study was supported by the Health Service of Amsterdam (GGD Amsterdam) and ZonMw (grant number 912-03-005).
REFERENCES
- 1.Bhat, K. S., C. P. Gibbs, O. Barrera, S. G. Morrison, F. Jahnig, A. Stern, E. M. Kupsch, T. F. Meyer, and J. Swanson. 1991. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 5:1889-1901. [DOI] [PubMed] [Google Scholar]
- 2.De, P., A. E. Singh, T. Wong, W. Yacoub, and A. M. Jolly. 2004. Sexual network analysis of a gonorrhoea outbreak. Sex. Transm. Infect. 80:280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fennema, J. S., I. Cairo, and R. A. Coutinho. 2000. Substantial increase in gonorrhea and syphilis among clients of Amsterdam Sexually Transmitted Diseases Clinic. Ned. Tijdschr. Geneeskd. 144:602-603. (In Dutch.) [PubMed] [Google Scholar]
- 4.Fenton, K. A., and C. M. Lowndes. 2004. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex. Transm. Infect. 80:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghani, A. C., C. A. Ison, H. Ward, G. P. Garnett, G. Bell, G. R. Kinghorn, J. Weber, and S. Day. 1996. Sexual partner networks in the transmission of sexually transmitted diseases. An analysis of gonorrhea cases in Sheffield, UK. Sex. Transm. Dis. 23:498-503. [DOI] [PubMed] [Google Scholar]
- 6.Howie, F., H. Young, and A. McMillan. 2004. The diversity of the opa gene in gonococcal isolates from men who have sex with men. Sex. Transm. Infect. 80:286-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenberg, H. D. (ed.). 1992. Clinical microbiology procedures handbook. American Society for Microbiology, Washington, D.C.
- 8.Kolader, M., P. G. Peerbooms, P. C. Vader, J. E. van Bergen, J. S. Fennema, and H. J. de Vries. 2004. The rise in fluoroquinolone-resistant Neisseria gonorrhoeae among people attending the Municipal Health Service's clinic for sexually transmitted diseases in Amsterdam, The Netherlands; cefotaxime now first-choice treatment for uncomplicated gonorrhoea. Ned. Tijdschr. Geneeskd. 148:2129-2132. (In Dutch.) [PubMed] [Google Scholar]
- 9.Lynn, F., M. M. Hobbs, J. M. Zenilman, F. M. Behets, K. Van Damme, A. Rasamindrakotroka, and M. C. Bash. 2005. Genetic typing of the porin protein of Neisseria gonorrhoeae from clinical noncultured samples for strain characterization and identification of mixed gonococcal infections. J. Clin. Microbiol. 43:368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin, I. M., A. Ghani, G. Bell, G. Kinghorn, and C. A. Ison. 2003. Persistence of two genotypes of Neisseria gonorrhoeae during transmission. J. Clin. Microbiol. 41:5609-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin, I. M. and C. A. Ison. 2003.. Detection of mixed infection of Neisseria gonorrhoeae. Sex. Transm. Infect. 79:56-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin, I. M., C. A. Ison, D. M. Aanensen, K. A. Fenton, and B. G. Spratt. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 189:1497-1505. [DOI] [PubMed] [Google Scholar]
- 13.Moodley, P., I. M. Martin, C. A. Ison, and A. W. Sturm. 2002. Typing of Neisseria gonorrhoeae reveals rapid reinfection in rural South Africa. J. Clin. Microbiol. 40:4567-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoll, A., and F. F. Hamers. 2002. Are trends in HIV, gonorrhoea, and syphilis worsening in western Europe? BMJ 324:1324-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Rourke, M., C. A. Ison, A. M. Renton, and B. G. Spratt. 1995. Opa-typing: a high-resolution tool for studying the epidemiology of gonorrhoea. Mol. Microbiol. 17:865-875. [DOI] [PubMed] [Google Scholar]
- 16.Rahman, M., A. Alam, K. Nessa, S. Nahar, D. K. Dutta, L. Yasmin, S. Monira, Z. Sultan, S. A. Khan, and M. J. Albert. 2001. Treatment failure with the use of ciprofloxacin for gonorrhea correlates with the prevalence of fluoroquinolone-resistant Neisseria gonorrhoeae strains in Bangladesh. Clin. Infect. Dis. 32:884-889. [DOI] [PubMed] [Google Scholar]
- 17.Spaargaren, J., J. Stoof, H. Fennema, R. Coutinho, and P. Savelkoul. 2001. Amplified fragment length polymorphism fingerprinting for identification of a core group of Neisseria gonorrhoeae transmitters in the population attending a clinic for treatment of sexually transmitted diseases in Amsterdam, The Netherlands. J. Clin. Microbiol. 39:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SPSS Incorporated. 2003. Statistical package for social sciences (SPSS) version 12.0 for Windows. SPSS Incorporated, Chicago, Ill.
- 19.Stata Corporation. 2005. Stata 9. [6]. Stata Press, College Station, Tex.
- 20.Stolte, I. G., N. H. Dukers, R. B. Geskus, R. A. Coutinho, and J. B. de Wit. 2004. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. AIDS 18:303-309. [DOI] [PubMed] [Google Scholar]
- 21.Van der Bij, A. K., I. G. Stolte, R. A. Coutinho, and N. H. Dukers. 2005. Increase of sexually transmitted infections, but not HIV, among young homosexual men in Amsterdam: are STIs still reliable markers for HIV transmission? Sex. Transm. Infect. 81:34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Snoek, E. M., J. B. de Wit, P. G. Mulder, and W. I. van der Meijden. 2005. Incidence of sexually transmitted diseases and HIV infection related to perceived HIV/AIDS threat since highly active antiretroviral therapy availability in men who have sex with men. Sex. Transm. Dis. 32:170-175. [DOI] [PubMed] [Google Scholar]
- 23.Van Looveren, M., C. A. Ison, M. Ieven, P. Vandamme, I. M. Martin, K. Vermeulen, A. Renton, and H. Goossens. 1999. Evaluation of the discriminatory power of typing methods for Neisseria gonorrhoeae. J. Clin. Microbiol. 37:2183-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viscidi, R. P., and J. C. Demma. 2003. Genetic diversity of Neisseria gonorrhoeae housekeeping genes. J. Clin. Microbiol. 41:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward, H., C. A. Ison, S. E. Day, I. Martin, A. C. Ghani, G. P. Garnett, G. Bell, G. Kinghorn, and J. N. Weber. 2000. A prospective social and molecular investigation of gonococcal transmission. Lancet 356:1812-1817. [DOI] [PubMed] [Google Scholar]
- 26.Wylie, J. L., and A. Jolly. 2001. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex. Transm. Dis. 28:14-24. [DOI] [PubMed] [Google Scholar]