Abstract
Candidemia studies have documented geographic differences in rates and epidemiology, underscoring the need for surveillance to monitor trends. We conducted prospective candidemia surveillance in Brazil to assess the incidence, species distribution, frequency of antifungal resistance, and risk factors for fluconazole-resistant Candida species. Prospective laboratory-based surveillance was conducted from March 2003 to December 2004 in 11 medical centers located in 9 major Brazilian cities. A case of candidemia was defined as the isolation of Candida spp. from a blood culture. Incidence rates were calculated per 1,000 admissions and 1,000 patient-days. Antifungal susceptibility tests were performed by using the broth microdilution assay, according to the Clinical and Laboratory Standards Institute guidelines. We detected 712 cases, for an overall incidence of 2.49 cases per 1,000 admissions and 0.37 cases per 1,000 patient-days. The 30-day crude mortality was 54%. C. albicans was the most common species (40.9%), followed by C. tropicalis (20.9%) and C. parapsilosis (20.5%). Overall, decreased susceptibility to fluconazole occurred in 33 (5%) of incident isolates, 6 (1%) of which were resistant. There was a linear correlation between fluconazole and voriconazole MICs (r = 0.54 and P < 0.001 [Spearman's rho]). This is the largest multicenter candidemia study conducted in Latin America and shows the substantial morbidity and mortality of candidemia in Brazil. Antifungal resistance was rare, but correlation between fluconazole and voriconazole MICs suggests cross-resistance may occur.
There is mounting evidence suggesting that Candida bloodstream infections (BSI) have become a major problem in tertiary-care hospitals worldwide (3, 6, 11, 26, 28, 37, 50). Candidemia has been observed particularly among patients hospitalized for long periods who have been exposed to antibiotics, immunosuppressive therapy, parenteral nutrition, and multiple invasive medical procedures (9, 15). Candidemia is generally difficult to diagnose and hard to treat, has a high attributable mortality rate, and is costly (18, 32, 55).
Although the incidence of candidemia among hospitalized patients increased during the 1980s (8), more-recent reports suggest that the incidence has stabilized (31). However, candidemia rates vary geographically. In The Netherlands, the incidence of Candida BSI doubled between 1987 and 1995 (53). Likewise, an increasing incidence of candidemia in Iceland has been observed during the period between 1980 and 1999 (6). On the other hand, data obtained from a national surveillance study conducted in Swiss tertiary-care hospitals suggested that the incidence of candidemia remained unchanged during the period of 1991 to 2000 (28), while a single-center study from Switzerland reported decreasing incidence rates (16). It seems therefore that differences do exist in the epidemiology of candidemia between different countries, underscoring the need for continuous surveillance to monitor trends in incidence, species distribution, and antifungal drug susceptibility profiles.
The epidemiology of candidemia has been extensively studied in the United States and Europe but not in Latin America. Data on candidemia in this region are limited to retrospective reviews of medical records or observational studies conducted in a limited number of medical centers (5, 11, 12, 46). Consequently, the incidence of candidemia in tertiary-care hospitals in Brazil is largely unknown, and no national data are available.
We conducted a prospective laboratory-based surveillance study in 11 tertiary care hospitals, representative of the public health system of 9 of the largest cities in Brazil, to assess the incidence, species distribution, frequency of antifungal resistance, and risk factors for candidemia due to fluconazole-resistant Candida species.
MATERIALS AND METHODS
Surveillance.
We performed prospective laboratory-based surveillance from March 2003 to December 2004 in 11 tertiary-care academic medical centers located in 9 cities of the south, southeast, and central regions of Brazil. All hospitals had automated blood culture systems (BACTEC or BacT-ALERT) and provide medical care to adults and children in different medical specialties. The protocol was approved by the local institutional review board of each site.
Case definitions.
A case of candidemia was defined as the incident isolation of Candida spp. from a blood culture. Candidemia occurring >30 days after the incident isolation was defined as a new case. Breakthrough candidemia was defined as the incident isolation of Candida spp. from a blood culture from a patient receiving systemic antifungal therapy for any reason. Fever was defined as a temperature of ≥37.8°C, hypotension as systolic blood pressure of <90 mm Hg, and neutropenia as an absolute neutrophil count of <500/mm3. Patients were considered adults if their age was ≥13 years.
Case accrual.
In each center, an investigator was specifically trained to have daily contact with the microbiology laboratory of the hospital and search for positive blood cultures. When a candidemia case was identified, clinical and epidemiological data were prospectively collected in a standardized case report form.
The case report form contained the following information: age, gender, date of admission, ward, date of candidemia, underlying medical conditions, exposure to invasive medical procedures, use of antibiotics or corticosteroids, management of candidemia (antifungal treatment, catheter removal), and outcome. Audits of hospital laboratories were performed periodically to ensure that no cases of candidemia were missed. In addition, audits of medical records were performed on 10% of cases to verify accuracy of data and completeness.
Yeast identification.
All Candida species recovered from blood cultures were sent to the Special Mycology Laboratory at Universidade Federal de São Paulo for confirmation of species identification and performance of antifungal agent susceptibility tests. Isolates were identified according to their microscopic morphology on cornmeal Tween 80 agar and by biochemical tests using the ID 32C system (BioMérieux AS, Marcy l'Etoile, France). A sample of 30% of all isolates was sent to the Centers for Disease Control and Prevention, Atlanta, GA, as part of a quality control program to confirm the identification.
In vitro susceptibility testing.
Antifungal susceptibility tests were performed by using the broth microdilution assay according to the methodology recommended by the CLSI (formerly known as NCCLS), document M27-A2 (33). The following antifungal drugs, supplied by the manufacturers as pure standard compounds, were tested at the indicated concentration range: amphotericin B, 0.015 to 8 μg/ml (Sigma Chemical Corporation, St. Louis, MO); flucytosine, 0.125 to 64 μg/ml (Sigma Chemical Corporation, St. Louis, MO); itraconazole, 0.03 to 16 μg/ml (Janssen Pharmaceutical, Titusville, NJ); fluconazole, 0.125 to 64 μg/ml; and voriconazole, 0.03 to 16 μg/ml (Pfizer Incorporated, New York, N.Y.). Briefly, the medium used was RPMI-1640, with l-glutamine, without bicarbonate, and buffered at pH 7.0 with 0.165 M morpholinepropanesulfonic acid. The yeast inoculum suspension was prepared by using a spectrophotometer to obtain a final yeast concentration of 0.5 × 103 to 2.5 × 103 cells/ml in each well of the microtiter plate. The assays were incubated at 35°C for 48 h. Quality-control strains (C. parapsilosis ATCC 22019 and C. krusei ATCC 6258; American Type Culture Collection, Manassas, VA) were included on each day of assay to check the accuracy of the drug dilutions and the reproducibility of the results. The MIC endpoint for amphotericin B was considered the lowest tested drug concentration able to prevent any visible growth. The MIC for azoles and flucytosine was considered the lowest tested drug concentration causing a significant reduction (approximately 50%) in growth compared to the growth of the drug-free positive control (33).
The interpretative MIC breakpoints for azoles and flucytosine were those suggested by the CLSI document M27-A2. Due to a lack of consensus about the definition of MIC breakpoints for amphotericin B, arbitrary values suggested in a previous study were used (34). Isolates with MICs of ≤8 μg/ml for fluconazole, ≤0.125 μg/ml for itraconazole, ≤1 μg/ml for voriconazole, ≤4 μg/ml for flucytosine, and ≤1 μg/ml for amphotericin B were considered susceptible. Isolates with MICs of 16 and 32 μg/ml for fluconazole, 0.25 and 0.5 μg/ml for itraconazole, and 2 μg/ml for voriconazole were considered susceptible in a dose-dependent manner (SDD). Isolates with MICs of 8 and 16 μg/ml were classified as intermediate to flucytosine. Isolates with MICs of ≥64 μg/ml for fluconazole, ≥1 μg/ml for itraconazole, ≥4 μg/ml for voriconazole, ≥32 μg/ml for flucytosine, and ≥2 μg/ml for amphotericin B were considered resistant. A sample of 30% of all isolates, as well as all strains resistant to any antifungal drug, was sent to the Centers for Disease Control and Prevention, Atlanta, GA, to confirm the accuracy of the susceptibility test results.
Statistical analysis.
To calculate incidence rates, the numbers of admissions and patient-days were collected. Incidence rates were calculated as the number of candidemias per 1,000 admissions and 1,000 patient-days. The overall incidence was determined using summed denominators of patient-days and admissions to calculate pooled mean rates. In addition, during 16 months of the surveillance, data on the number of BSI caused by different microorganisms (including bacteria and fungi) were collected from eight centers in order to estimate the relative burden of candidemia as the etiologic agent of BSIs.
Data were entered by using a web-based case report form, using the SPSS Enterprise Server 3.0 and SPSS Data Entry Builder 3.03 (SPSS, Inc. Chicago, IL). Categorical data were analyzed using Chi-square or Fisher's exact tests, as appropriate, and continuous variables were compared using the Wilcoxon test. Spearman rank-order correlation was used to measure the relationship between the MICs of fluconazole and voriconazole. We performed univariate and multivariate analysis of factors associated with candidemia caused by less-fluconazole-susceptible isolates. Variables significant at P values of <0.1 by univariate analysis were included in a multivariate model (backward and forward). Data were analyzed using the SPSS 11.0.1 software (SPSS, Inc. Chicago, IL).
RESULTS
Incidence and demographics.
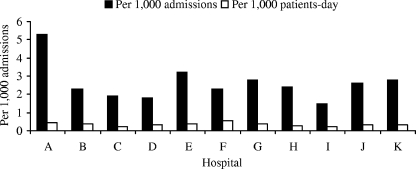
We detected a total of 712 cases of candidemia during the surveillance study, for an overall incidence of 2.49 cases per 1,000 admissions and 0.37 cases per 1,000 patient-days. As illustrated in Fig. 1, the incidence rate of candidemia in the 11 centers ranged from 1.49 to 5.30 cases per 1,000 admissions and 0.20 to 0.52 cases per 1,000 patient-days. Demographic and clinical characteristics and outcomes of 712 candidemia cases identified in the surveillance are shown in Table 1. Males comprised 56% of the case patients, and the median age was 41 years (range, 0 to 96 years). Of note, 225 case patients (32%) were children, and 147 (21%) were younger than 1 year old. The median age was 57 years among adult patients and 7 months among children.
FIG. 1.
Incidence rates of candidemia among the 11 medical centers.
TABLE 1.
Demographics, clinical characteristics, and outcomes for candidemic episodes identified during prospective sentinel surveillance conducted in Brazil in 2003 and 2004
| Variable | Value for all casesb | Value for speciesc,d
|
|||
|---|---|---|---|---|---|
| C. albicans | C. tropicalis | C. parapsilosis | C. glabrata | ||
| Median agee (range) | 41 (0-96) | 46 (0-92) | 48 (0-96) | 33 (0-89) | 52 (0-88) |
| No. of males | 400 (56) | 168 (58) | 83 (56) | 86 (59) | 18 (51) |
| Age of <1 yr | 147 (21) | 63 (22) | 18 (12)* | 30 (20) | 5 (14) |
| No. of outpatients | 16 (2) | 5 (2) | 2 (1) | 6 (4) | 1 (3) |
| Median no. of days (range) in hospital until candidemia | 20 (0-385) | 20 (0-114) | 19 (0-385) | 19 (0-47) | 19 (0-115) |
| No. of cases of underlying diseases | |||||
| Cancer | 195 (27) | 66 (23) | 47 (32)* | 39 (27) | 12 (34) |
| Hematologic malignancy | 75 (10) | 25 (9) | 19 (13) | 16 (11) | 4 (11) |
| Cardiac disease | 139 (19) | 53 (18) | 32 (22) | 34 (23) | 7 (20) |
| Pulmonary disease | 138 (19) | 63 (22) | 29 (19) | 24 (16) | 5 (14) |
| Liver disease | 56 (8) | 21 (7) | 16 (11) | 6 (4) | 5 (14) |
| Neurologic disease | 120 (17) | 54 (19) | 27 (18) | 24 (16) | 3 (9) |
| Diabetes | 93 (13) | 42 (14) | 21 (14) | 20 (14) | 6 (17) |
| Renal insufficiency | 138 (19) | 56 (19) | 25 (17) | 35 (24) | 11 (31) |
| Organ transplant | 14 (2) | 3 (1) | 1 (1) | 4 (3) | 1 (3) |
| HIVa infection | 22 (3) | 8 (3) | 6 (4) | 5 (3) | 1 (3) |
| No. of patients with characteristic | |||||
| Use of corticosteroids | 228 (32) | 100 (34) | 48 (32) | 36 (25)* | 10 (29) |
| Neutropenia | 44 (6) | 10 (3) | 12 (8)* | 10 (7) | 2 (6) |
| In the ICU at diagnosis | 317 (44) | 127 (44) | 66 (44) | 63 (43) | 15 (43) |
| Mechanical ventilation | 274 (38) | 119 (41) | 55 (37) | 45 (31)* | 18 (51) |
| Dialysis | 84 (12) | 37 (13) | 20 (13) | 20 (14) | 3 (9) |
| Previous Candida colonization | 114 (16) | 56 (19) | 26 (17) | 19 (13) | 7 (20) |
| Previous surgery | 281 (39) | 128 (44) | 52 (35) | 52 (36) | 18 (51) |
| Abdominal surgery | 180 (25) | 82 (28) | 31 (21) | 34 (23) | 14 (40) |
| Central venous catheter | 497 (70) | 216 (74) | 104 (70) | 96 (66) | 31 (89) |
| Parenteral nutrition | 150 (21) | 61 (21) | 32 (22) | 34 (23) | 8 (23) |
| Prior antibiotic therapy | 669 (94) | 277 (95) | 141 (95) | 132 (90) | 33 (94) |
| Prior fluconazole use | 80 (11) | 29 (10) | 10 (7) | 14 (10) | 9 (26)* |
| Death by day 7 of candidemia | 221 (31) | 98 (34) | 49 (33) | 32 (22)* | 15 (43) |
| Overall mortality | 383 (54) | 166 (57) | 91 (61) | 66 (45)* | 24 (69) |
HIV, human immunodeficiency virus.
A total of 712 cases were studied. Values in parentheses are percentages unless specified differently.
Values in parentheses are percentages unless specified differently. Numbers of cases are as follows: for C. albicans, 291; for C. tropicalis, 149; for C. parapsilosis, 146; for C. glabrata, 35.
*, value is significantly different (P < 0.05) from that for C. albicans.
Ages are given in years.
Candida spp. comprised the fourth-most-frequently isolated pathogen from blood cultures, preceded by coagulase-negative staphylococci (11.97 episodes per 1,000 admissions and 1.62 episodes per 1,000 patient-days), Staphylococcus aureus (7.31 per 1,000 admissions and 0.99 per 1,000 patient-days), and Klebsiella pneumoniae (2.92 per 1,000 admissions and 0.40 per 1,000 patient-days).
Coexisting exposures and underlying diseases.
At the time of the diagnosis of candidemia, 693 case patients (97%) were hospitalized, including 317 (46%) admitted in the intensive care unit (ICU) (including the neonatal intensive care unit), 161 (23%) in the medical ward, 101 (15%) in the surgical ward, 74 (11%) in the pediatrics ward, and 40 (6%) in other wards. Sixteen case-patients were outpatients, and in 3 cases no information was available. Cancer was documented for 195 case patients (27%), 75 of which (38%) were hematologic malignancies (Table 1). A total of 281 case patients (39%) had had prior surgery in the 30 days before candidemia, 180 of which (64%) were abdominal. A total of 274 case patients (38%) were on mechanical ventilation at the time of candidemia, 84 (12%) were receiving dialysis, 150 (21%) were receiving parenteral nutrition, and 497 (70%) had a central venous catheter. Neutropenia was present in only 44 case patients (6%). Candidemia was generally a late complication during hospital stay, occurring at a median of 19.5 days after admission (range, 0 to 385). A bacteremia was diagnosed on the same day of candidemia for 68 case patients (10%) and within 14 days of candidemia for 146 case patients (20%).
Excluding the 91 neonates (40% of the pediatric cases), most of the children with candidemia had cancer (31%), neurologic disease (22%), or lung disease (22%). Of the pediatric case patients, 36% had had surgery in the 30 days prior to the candidemia, 41% of the case patients were in the ICU, 25% were on mechanical ventilation, and 14% were receiving parenteral nutrition.
Clinical manifestations of candidemia.
Fever was the most frequent clinical manifestation of candidemia, occurring in 419 (62%) of the 678 case patients for which data were available. Hypotension was reported in 43 (7%) of 620 cases. Deep-seated Candida infection was documented in only 17 cases (2%). The presentations of deep-seated candidiasis were endocarditis (eight cases), endopthalmitis (five cases), disseminated candidiasis with skin lesions (two cases), and peritonitis and chronic disseminated candidiasis (one case each).
Therapy and outcome.
At the time of candidemia, 122 case patients (17%) were receiving a systemic antifungal agent and were considered breakthrough infections (fluconazole, 80 patients, 11%; amphotericin B, 36 patients, 5%; itraconazole and voriconazole, 1 patient each; and other antifungals, 4 patients).
A total of 536 case patients (75%) received antifungal therapy, started at a median of 3 days from the Candida isolation or onset of candidemia (range, 0 to 27). Excluding the 133 case patients (19%) who died within 3 days from incident candidemia, 86% of case patients (492 of 574) received treatment. Amphotericin B was the most frequently used drug as primary treatment (274 cases; 38%), followed by fluconazole (224 cases; 32%). Twenty-seven case patients received antifungal treatment as part of a double-blind randomized trial comparing an echinocandin to a lipid formulation of amphotericin B. Other drugs used as primary treatment were the following: a lipid formulation of amphotericin B (five cases), caspofungin (four cases), voriconazole (two cases), and unknown (five cases). For 22% of the 492 case patients that received treatment, the initial antifungal drug was changed after a median of 5 days of treatment. The most frequent change was the substitution of fluconazole for deoxycholate amphotericin B (47 cases) and amphotericin B for fluconazole (32 cases). The median duration of treatment was 14 days (range, 1 to 70).
The 3-day, 7-day, and 30-day mortality rates were 19%, 31%, and 54%, respectively. The 30-day mortality rate among children <1 year old was 43%, compared to 23% for children with ages between 1 and 12 years and 63% for adults (P < 0.001). Candidemia due to C. parapsilosis was associated with a lower mortality rate than that due to C. albicans (45% versus 57%; P = 0.02).
Species distribution and antifungal susceptibility testing.
Table 2 shows the species distribution. C. albicans was the most common species (291 cases; 41%), followed by C. tropicalis (149 cases; 21%) and C. parapsilosis (146 cases; 21%). C. glabrata comprised only 5% of cases. Among adult cases, the most common species was C. albicans (199 cases; 41%), followed by C. tropicalis (114; 24%) and C. parapsilosis (99; 21%). Among pediatric cases, the most common species was C. albicans (89 cases; 40% of total), followed by C. parapsilosis (47 cases; 21%), and C. tropicalis and C. pelliculosa (34 cases each; 15%).
TABLE 2.
Species distribution and incidence among 712 cases of candidemia detected during prospective sentinel surveillance in Brazil in 2003 and 2004
| Species | No. (%) of cases | Incidence per 1,000 admissions | Incidence per 1,000 patient-days |
|---|---|---|---|
| C. albicans | 291 (40.9) | 1.01 | 0.15 |
| C. tropicalis | 149 (20.9) | 0.52 | 0.07 |
| C. parapsilosis | 146 (20.5) | 0.51 | 0.07 |
| C. pelliculosa | 44 (6.2) | 0.15 | 0.02 |
| C. glabrata | 35 (4.9) | 0.12 | 0.02 |
| C. guilliermondii | 17 (2.4) | 0.06 | 0.009 |
| P. ohmeri | 9 (1.3) | 0.03 | 0.005 |
| C. krusei | 8 (1.1) | 0.03 | 0.004 |
| Othersa | 13 (1.3) |
Other Candida species: C. lusitaniae, four cases; C. lipolytica, three cases; C. zeylanoides, one case; Candida sp., five cases.
Species distribution varied substantially between centers. The proportion of cases due to C. albicans ranged between 27 and 54%, between 16 and 29% for C. tropicalis, between 7 and 40% for C. parapsilosis, between 2 and 11% for C. glabrata, and between 0 and 41% for C. pelliculosa. The incidence of candidemia due to the different species was as follows (per 1,000 admissions and per 1,000 patient-days): C. albicans, 1.01 and 0.15; C. tropicalis, 0.52 and 0.07; C. parapsilosis, 0.51 and 0.07; C. glabrata, 0.12 and 0.02; and C. pelliculosa, 0.03 and 0.004 (Table 2).
Compared with candidemia due to C. albicans, candidemia due to C. tropicalis was less likely to occur among children <1 year old (12% versus 22%; P = 0.01) and more likely to occur in patients with cancer (32% versus 23%; P = 0.04) or neutropenia (8% versus 3%; P = 0.02). Compared with those due to C. albicans, cases due to C. parapsilosis were less likely among patients on mechanical ventilation (31% versus 41%; P = 0.03) or receiving corticosteroids (25% versus 34%; P = 0.04). Case patients receiving fluconazole before developing candidemia were more likely to be infected by C. glabrata than C. albicans (26% versus 10%; P = 0.008) (Table 1).
Table 3 shows the MIC ranges, MICs at which 50% or 90% of organisms were inhibited, and percentages of Candida isolates with decreased susceptibilities to the five antifungal drugs tested. Overall, decreased susceptibility to antifungal agents was rare. Fluconazole resistance occurred in 6 (0.8%) isolates, including 1 isolate of C. albicans, 2 of C. glabrata, and 3 of C. krusei; 27 cases (4%) were classified as SDD to fluconazole (1 case of C. albicans, 15 of C. glabrata, 5 of C. krusei, 2 of C. tropicalis, 2 of C. guilliermondii, 1 of C. pelliculosa, and 1 of Pichia ohmeri). One Candida albicans isolate exhibited a MIC of ≥4 μg/ml to voriconazole; this C. albicans isolate was also resistant to fluconazole. In addition, one C. glabrata isolate that was resistant to fluconazole had a voriconazole MIC of 2 μg/ml. Overall, for each isolate, there was a linear correlation between fluconazole and voriconazole MICs (r = 0.54; P < 0.001). In addition, cases that had received fluconazole before candidemia had somewhat higher voriconazole MICs than those without previous exposure to fluconazole (MIC90s of 0.25 μg/ml and 0.06 μg/ml, respectively; P = 0.056).
TABLE 3.
Antifungal susceptibility test results for selected species of Candida isolated during prospective, sentinel surveillance in Brazil in 2003 and 2004
| Species (na) | Antifungal agent | MIC (μg/ml)
|
% Resistantb (no. of isolates) | ||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| C. albicans (291) | Amphotericin B | 0.125-1.0 | 0.5 | 1.0 | 0 |
| Fluconazole | 0.125-64 | 0.25 | 0.5 | 0.3 (1) | |
| Itraconazole | 0.03-1.0 | 0.03 | 0.03 | 0.3 (1) | |
| 5-Flucytosine | 0.125-64 | 0.25 | 2.0 | 0.3 (1) | |
| Voriconazole | 0.03-4.0 | 0.03 | 0.03 | 0.3 (1) | |
| C. tropicalis (149) | Amphotericin B | 0.125-1.0 | 0.5 | 1.0 | 0 |
| Fluconazole | 0.125-32 | 0.5 | 1.0 | 0 | |
| Itraconazole | 0.03-0.25 | 0.03 | 0.125 | 0 | |
| 5-Flucytosine | 0.05-64 | 0.25 | 1.0 | 3 (5) | |
| Voriconazole | 0.03-0.5 | 0.03 | 0.06 | 0 | |
| C. parapsilosis (146) | Amphotericin B | 0.25-1.0 | 1.0 | 1.0 | 0 |
| Fluconazole | 0.125-8.0 | 1.0 | 4.0 | 0 | |
| Itraconazole | 0.03-0.5 | 0.03 | 0.125 | 0 | |
| 5-Flucytosine | 0.125-64 | 0.25 | 0.5 | 1.4 (2) | |
| Voriconazole | 0.03-0.25 | 0.03 | 0.06 | 0 | |
| C. pelliculosa (44) | Amphotericin B | 0.125-2.0 | 0.25 | 0.75 | 0 |
| Fluconazole | 0.5-16 | 4.0 | 8.0 | 0 | |
| Itraconazole | 0.03-0.5 | 0.125 | 0.5 | 0 | |
| 5-Flucytosine | 0.125-16 | 0.125 | 0.125 | 0 | |
| Voriconazole | 0.03-0.25 | 0.06 | 0.25 | 0 | |
| C. glabrata (35) | Amphotericin B | 0.25-1.0 | 0.5 | 1.0 | 0 |
| Fluconazole | 2.0-64 | 8.0 | 32 | 6 (2) | |
| Itraconazole | 0.03-32 | 0.25 | 4 | 34 (12) | |
| 5-Flucytosine | 0.125-8.0 | 0.125 | 0.25 | 0 | |
| Voriconazole | 0.03-2.0 | 0.125 | 1.0 | 0 | |
n, no. of isolates.
Resistance breakpoints: fluconazole, MIC of ≥64 μg/ml; itraconazole, ≥1 μg/ml; flucytosine, ≥32 μg/ml; amphotericin B, ≥2 μg/ml; voriconazole, ≥4 μg/ml.
Compared to the case with susceptible isolates, candidemia caused by an isolate with decreased susceptibility to fluconazole (SDD or resistant) was associated with malignancy (9% versus 3%; P < 0.001), previous (11% versus 4%; P = 0.02) or current (16% versus 4%; P < 0.001) neutropenia, and prior fluconazole use (14% versus 3%; P < 0.001). By multivariate analysis, independent factors associated with candidemia due to an isolate with decreased susceptibility to fluconazole were malignancy (odds ratio, 2.9; 95% confidence interval, 1.4 to 5.9; P = 0.005) and prior use of fluconazole (odds ratio, 3.8; 95% confidence interval, 1.7 to 8.2; P = 0.001). Among patients who received fluconazole as treatment for the candidemia, the death rate of patients infected by a susceptible isolate or a less-susceptible isolate (SDD or resistant) was 54% or 64%, respectively (P = 0.44).
DISCUSSION
This prospective candidemia surveillance represents the largest multicenter study conducted in Latin America and reveals for the first time the large burden of candidemia in Brazilian tertiary-care hospitals. In addition, it provides the most representative and consistent data from South America on the epidemiology of candidemia to date (5, 11, 12, 39).
The first remarkable finding of our study was the high incidence of candidemia. Our rates of 2.49 cases per 1,000 admissions and 0.37 episodes per 1,000 patient-days are 2 to 15 times higher than those reported for centers in the Northern Hemisphere, including the United States (0.28 to 0.96 per 1,000 admissions) (7, 21, 41, 57), Canada (0.45 per 1,000 admissions) (27), Europe (0.20 to 0.38 per 1,000 admissions) (50), France (0.17 per 1,000 admissions) (43), Norway (0.17 per 1,000 admissions) (45), Hungary (0.20 to 0.40 per 1,000 admissions) (14), Switzerland (0.27 per 1,000 admissions) (28), Italy (0.38 per 1,000 admissions) (49), and Spain (0.76 to 0.81 per 1,000 admissions) (4, 52). A similarly high incidence rate of candidemia (2.88 cases per 1,000 discharges) was reported in a single-center study conducted in Taiwan (19). Although the reasons for our high candidemia rates are not entirely clear, it is possible that this may be related to a combination of multiple factors, including differences in resources available for medical care and training programs, difficulties in the implementation of infection control programs in hospitals of developing countries, limited number of health care workers to assist patients in critical care units, and less-aggressive practices of empirical antifungal therapy and prophylaxis for high-risk patients. Indeed, since our rates of BSI caused by other organisms are also high, it seems that these factors may have affected not just candidemia rates but the overall rates of BSI in Brazil.
The median age of our case patients was low (40 years) compared to that reported in a series from the United States (37). This is a reflection of a high proportion of children in our study (32% compared to 9% in the study from the United States), since the median age of our adults (57 years) was similar to that reported in the American study (55 years). Most of the children were premature babies (40%), but a large proportion (41%) of the older children were in an ICU and had cancer, neurologic disease, or lung disease. The adults with candidemia comprised mostly case patients admitted to an ICU (38%), with diseases such as diabetes, renal failure, cardiac diseases and lung disease. Half of the ICU case patients had surgery in the 30 days before candidemia, underscoring the importance of surgical procedures and location in the ICU in the epidemiology of candidemia (37, 50).
Over the past 10 years, some studies have reported a shift in the etiology of candidemia. While C. albicans is still considered the most common species causing candidemia, increasing rates of candidemia caused by C. tropicalis, C. parapsilosis, C. glabrata, and C. krusei have been reported worldwide (11, 44, 51). The reasons for the emergence of non-albicans species are not completely understood, but some medical conditions may consistently impact the risk of developing candidemia due to non-albicans species: C. parapsilosis fungemia has been associated with vascular catheters and parenteral nutrition (10, 17, 24), C. tropicalis candidemia is associated with cancer and neutropenia (23, 56), and C. krusei and C. glabrata fungemias are associated with previous exposure to azoles (29, 51). The findings from our surveillance are supportive of these reports.
Our series clearly consolidates the concept that candidemia due to C. glabrata is rare in Brazil, and C. tropicalis and C. parapsilosis account for the large majority (70%) of non-albicans species. Why C. glabrata is unusual in Brazil is not clear, but the wide geographic variability of species distribution suggests that factors other than the use of fluconazole may be important, including demographic characteristics (older age) (13) and the use of antibiotics (25). However, although the proportion of C. glabrata in Brazil is low, the burden of C. glabrata candidemia (0.12 cases per 1,000 admissions) is similar to rates reported in other series (4).
In contrast, we report a high proportion of C. tropicalis (21%) candidemia, even in neonates. European and North American series have consistently reported low proportions of C. tropicalis candidemia (2 to 10% in Europe and 10 to 12% in the United States and Canada) (3, 4, 6, 13, 16, 22, 26, 28, 30, 37, 42, 47, 50, 51), whereas higher proportions were observed in a study from Saudi Arabia (20.7%) (1). In our study, the proportion varied from 16 to 29% among the different centers. We were unable to determine any special distinguishing features between centers with lower and higher proportions.
Like that with C. tropicalis, C. parapsilosis candidemia was frequent in the present study, with a slightly lower proportion than in our previous study (20.5% versus 25%) (11). There is a wide variation in the proportion of C. parapsilosis as the cause of candidemia in different series, with rates varying between 7 and 21% in the United States (13, 22) and 6.9 and 30% in Europe (50). The reasons for these wide variations are not clear but may be related to the proportion of neonates in each study, as well as other local epidemiologic factors of this species, since C. parapsilosis candidemia is thought to be acquired from an external source (54).
We also observed a high proportion of candidemia caused by C. pelliculosa, the asexual form of Pichia anomala. C. pelliculosa has rarely been reported as a cause of candidemia outside of outbreaks, two of which occurred in Brazil (38, 48). Twenty-nine of our C. pelliculosa isolates were from a single institution, highlighting the possibility that this species may be associated with nosocomial outbreaks. However, further investigation is needed to understand the epidemiology of this cluster and to determine factors associated with C. pelliculosa infection.
Antifungal resistance was a rare finding in our study and was restricted to a few isolates. As with two recently published studies (3, 36), none of our Candida bloodstream isolates had MICs of ≥2 μg/ml for amphotericin B. Our proportion of fluconazole-susceptible C. glabrata isolates (51%) was somewhat lower than a report from other countries (62 to 81%) (40). However, the percentage of fluconazole-resistant isolates (5.7%) was similar to the rate observed with European isolates (5.2%) and lower than that with North American isolates (10.2%).
As reported by other authors (29), we also observed that previous exposure to fluconazole was a strong and independent factor associated with candidemia caused by fluconazole-nonsusceptible isolates. In addition, we observed that higher voriconazole MICs tended to be associated with prior exposure to fluconazole. This is of concern and illustrates the potential problem of cross-resistance between azoles. In another study with 46 fluconazole-resistant C. glabrata isolates, only 13% were susceptible to voriconazole, 4% to posaconazole, and 8.7% to ravuconazole (40). The potential for voriconazole-resistant C. glabrata to emerge as a threat in people receiving voriconazole therapy has been raised in two reports of breakthrough infections (2, 20). Nevertheless, in our study, voriconazole was the azole which exhibited the best in vitro antifungal activity, and only one of six fluconazole-resistant isolates was cross-resistant to voriconazole.
The 30-day crude mortality rate observed in our study was similar to that reported in our previous study (11), as well as by other authors (3, 11, 18, 27). Younger children and adults had higher mortality rates than children with ages between 1 and 12 years. Similar to other reports, patients with C. parapsilosis candidemia had the lowest death rates (35, 37).
These comprehensive data document important differences in the epidemiology of candidemia in Brazil compared to reports from other countries. This report shows that candidemia is a significant source of morbidity in Brazil, with a substantial burden of disease, mortality, and likely high associated costs. Although our high rates of candidemia may be related to high rates of BSI in general in Brazilian public hospitals, reasons for these high rates are not clear and warrant further study. Determining factors associated with these high rates may lead to identifying measures that can help prevent disease. In addition, our data support that fluconazole nonsusceptibility is associated with prior fluconazole exposure and suggest that such exposure may lead to voriconazole cross-resistance.
Acknowledgments
This paper was partially supported by an independent medical grant provided by Pfizer, Inc. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil (grants 300235/1993-3 and 301942/2003-0).
Contributors to the Brazilian Network Candidemia Study are as follows: Sydney H. Alves and Roselene A. Righi (Hosp. de Clínicas, Univ. Federal de Santa Maria), Flavio Queiroz-Telles and Miriam Carvalho (Hosp. de Clínicas, Univ. Federal do Paraná), Arnaldo L. Colombo and Thais Guimarães (Hosp. São Paulo, Univ. Federal de São Paulo), Thais Guimarães (Hosp. Servidor Público de São Paulo), Maria Luiza Moretti and Plínio Trabasso (Hosp. de Clínicas, Unicamp), Paulo de Tarso Oliveira e Castro and Roberto Martinez (Hosp. de Clínicas, USP-Ribeirão Preto), Antonia O. Machado and Maria Gabriela de Oliveira (Hosp. de Base, Faculdade de Medicina de São José do Rio Preto), Ana Luiza Oliveira and Simone A. Nouér (Hosp. Universitário, Univ. Federal do Rio de Janeiro), Ana Lucia Brum and Rosana Rangel (Hosp. da Lagoa, Rio de Janeiro), Mariceli Ribeiro and Reinaldo Dietze (Hosp. de Clínicas, Univ. Federal do Espírito Santo), and Julival Ribeiro and Jussara Marques (Hosp. de Base do Distrito Federal, Brasilia).
REFERENCES
- 1.Alexander, B. D., W. A. Schell, J. L. Miller, G. D. Long, and J. R. Perfect. 2005. Candida glabrata fungemia in transplant patients receiving voriconazole after fluconazole. Transplantation 80:868-871. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jasser, A. M., and N. A. Elkhizzi. 2004. Distribution of Candida species among bloodstream isolates. Saudi Med. J. 25:566-569. [PubMed] [Google Scholar]
- 3.Almirante, B., D. Rodriguez, B. J. Park, M. Cuenca-Estrella, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, P. Saballs, S. K. Fridkin, J. Morgan, J. L. Rodriguez-Tudela, D. W. Warnock, and A. Pahissa. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso-Valle, H., O. Acha, J. D. Garcia-Palomo, C. Farinas-Alvarez, C. Fernandez-Mazarrasa, and M. C. Farinas. 2003. Candidemia in a tertiary care hospital: epidemiology and factors influencing mortality. Eur. J. Clin. Microbiol. Infect. Dis. 22:254-257. [DOI] [PubMed] [Google Scholar]
- 5.Antunes, A. G., A. C. Pasqualotto, M. C. Diaz, P. A. d'Azevedo, and L. C. Severo. 2004. Candidemia in a Brazilian tertiary care hospital: species distribution and antifungal susceptibility patterns. Rev. Inst. Med. Trop. Sao Paulo 46:239-241. [DOI] [PubMed] [Google Scholar]
- 6.Asmundsdottir, L. R., H. Erlendsdottir, and M. Gottfredsson. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, W. J. Martone, et al. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. Am. J. Med. 91:86S-89S. [DOI] [PubMed] [Google Scholar]
- 8.Beck-Sague, C., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, et al. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 10.Clark, T. A., S. A. Slavinski, J. Morgan, T. Lott, B. A. Arthington-Skaggs, M. E. Brandt, R. M. Webb, M. Currier, R. H. Flowers, S. K. Fridkin, and R. A. Hajjeh. 2004. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 42:4468-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo, A. L., M. Nucci, R. Salomao, M. L. Branchini, R. Richtmann, A. Derossi, and S. B. Wey. 1999. High rate of non-albicans candidemia in Brazilian tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 34:281-286. [DOI] [PubMed] [Google Scholar]
- 12.Costa, S. F., I. Marinho, E. A. Araujo, A. E. Manrique, E. A. Medeiros, and A. S. Levin. 2000. Nosocomial fungaemia: a 2-year prospective study. J. Hosp. Infect. 45:69-72. [DOI] [PubMed] [Google Scholar]
- 13.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doczi, I., E. Dosa, E. Hajdu, and E. Nagy. 2002. Aetiology and antifungal susceptibility of yeast bloodstream infections in a Hungarian university hospital between 1996 and 2000. J. Med. Microbiol. 51:677-681. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 16.Garbino, J., L. Kolarova, P. Rohner, D. Lew, P. Pichna, and D. Pittet. 2002. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine (Baltimore) 81:425-433. [DOI] [PubMed] [Google Scholar]
- 17.Girmenia, C., P. Martino, B. F. De, G. Gentile, M. Boccanera, M. Monaco, G. Antonucci, and A. Cassone. 1996. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin. Infect. Dis. 23:506-514. [DOI] [PubMed] [Google Scholar]
- 18.Gudlaugsson, O., S. Gillespie, K. Lee, B. J. Vande, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh, P. R., L. J. Teng, P. C. Yang, S. W. Ho, and K. T. Luh. 2002. Emergence of nosocomial candidemia at a teaching hospital in Taiwan from 1981 to 2000: increased susceptibility of Candida species to fluconazole. Microb. Drug Resist. 8:311-319. [DOI] [PubMed] [Google Scholar]
- 20.Imhof, A., S. A. Balajee, D. N. Fredricks, J. A. Englund, and K. A. Marr. 2004. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin. Infect. Dis. 39:743-746. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 20:1526-1530. [DOI] [PubMed] [Google Scholar]
- 22.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 23.Komshian, S. V., A. K. Uwaydah, J. D. Sobel, and L. R. Crane. 1989. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev. Infect. Dis. 11:379-390. [DOI] [PubMed] [Google Scholar]
- 24.Levy, I., L. G. Rubin, S. Vasishtha, V. Tucci, and S. K. Sood. 1998. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin. Infect. Dis. 26:1086-1088. [DOI] [PubMed] [Google Scholar]
- 25.Lin, M. Y., Y. Carmeli, J. Zumsteg, E. L. Flores, J. Tolentino, P. Sreeramoju, and S. G. Weber. 2005. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control study. Antimicrob. Agents Chemother. 49:4555-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luzzati, R., G. Amalfitano, L. Lazzarini, F. Soldani, S. Bellino, M. Solbiati, M. C. Danzi, S. Vento, G. Todeschini, C. Vivenza, and E. Concia. 2000. Nosocomial candidemia in non-neutropenic patients at an Italian tertiary care hospital. Eur. J. Clin. Microbiol. Infect. Dis. 19:602-607. [DOI] [PubMed] [Google Scholar]
- 27.Macphail, G. L., G. D. Taylor, M. Buchanan-Chell, C. Ross, S. Wilson, and A. Kureishi. 2002. Epidemiology, treatment and outcome of candidemia: a five-year review at three Canadian hospitals. Mycoses 45:141-145. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti, O., J. Bille, U. Fluckiger, P. Eggimann, C. Ruef, J. Garbino, T. Calandra, M. P. Glauser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 29.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 30.Martin, D., F. Persat, M. A. Piens, and S. Picot. 2005. Candida species distribution in bloodstream cultures in Lyon, France, 1998-2001. Eur. J. Clin. Microbiol. Infect. Dis. 24:329-333. [DOI] [PubMed] [Google Scholar]
- 31.Morgan, J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7:429-439. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, J., M. I. Meltzer, B. D. Plikaytis, A. N. Sofair, S. Huie-White, S. Wilcox, L. H. Harrison, E. C. Seaberg, R. A. Hajjeh, and S. M. Teutsch. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 26:540-547. [DOI] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. Document M27-A2. National Committee For Clinical Laboratory Standards, Wayne, Pa.
- 34.Nguyen, M. H., C. J. Clancy, V. L. Yu, Y. C. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi. 1998. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425-430. [DOI] [PubMed] [Google Scholar]
- 35.Nucci, M., A. L. Colombo, F. Silveira, R. Richtmann, R. Salomao, M. L. Branchini, and N. Spector. 1998. Risk factors for death in patients with candidemia. Infect. Control Hosp. Epidemiol. 19:846-850. [DOI] [PubMed] [Google Scholar]
- 36.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowitz, J. E. Edwards, and W. E. Dismukes. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 38.Pasqualotto, A. C., T. C. Sukiennik, L. C. Severo, C. S. de Amorim, and A. L. Colombo. 2005. An outbreak of Pichia anomala fungemia in a Brazilian pediatric intensive care unit. Infect. Control Hosp. Epidemiol. 26:553-558. [DOI] [PubMed] [Google Scholar]
- 39.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, R. J. Hollis, and S. A. Messer for the SENTRY Participant Group. 1998. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. J. Clin. Microbiol. 36:1886-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittet, D., and R. P. Wenzel. 1995. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 155:1177-1184. [DOI] [PubMed] [Google Scholar]
- 42.Poikonen, E., O. Lyytikainen, V. J. Anttila, and P. Ruutu. 2003. Candidemia in Finland, 1995-1999. Emerg. Infect. Dis. 9:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richet, H., P. Roux, C. C. Des, Y. Esnault, and A. Andremont. 2002. Candidemia in French hospitals: incidence rates and characteristics. Clin. Microbiol. Infect. 8:405-412. [DOI] [PubMed] [Google Scholar]
- 44.Sandven, P., L. Bevanger, A. Digranes, P. Gaustad, H. H. Haukland, and M. Steinbakk. 1998. Constant low rate of fungemia in Norway, 1991 to 1996. The Norwegian Yeast Study Group. J. Clin. Microbiol. 36:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.San Miguel, L. G., J. Cobo, E. Otheo, A. Sanchez-Sousa, V. Abraira, and S. Moreno. 2005. Secular trends of candidemia in a large tertiary-care hospital from 1988 to 2000: emergence of Candida parapsilosis. Infect. Control Hosp. Epidemiol. 26:548-552. [DOI] [PubMed] [Google Scholar]
- 46.Silva, V., M. C. Diaz, and N. Febre. 2004. Invasive fungal infections in Chile: a multicenter study of fungal prevalence and susceptibility during a 1-year period. Med. Mycol. 42:333-339. [DOI] [PubMed] [Google Scholar]
- 47.Swinne, D., M. Watelle, C. Suetens, K. Mertens, P. A. Fonteyne, and N. Nolard. 2004. A one-year survey of candidemia in Belgium in 2002. Epidemiol. Infect. 132:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thuler, L. C., S. Faivichenco, E. Velasco, C. A. Martins, C. R. Nascimento, and I. A. Castilho. 1997. Fungaemia caused by Hansenula anomala—an outbreak in a cancer hospital. Mycoses 40:193-196. [DOI] [PubMed] [Google Scholar]
- 49.Tortorano, A. M., E. Biraghi, A. Astolfi, C. Ossi, M. Tejada, C. Farina, S. Perin, C. Bonaccorso, C. Cavanna, A. Raballo, and A. Grossi. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51:297-304. [DOI] [PubMed] [Google Scholar]
- 50.Tortorano, A. M., J. Peman, H. Bernhardt, L. Klingspor, C. C. Kibbler, O. Faure, E. Biraghi, E. Canton, K. Zimmermann, S. Seaton, and R. Grillot. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23:317-322. [DOI] [PubMed] [Google Scholar]
- 51.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 52.Viudes, A., J. Peman, E. Canton, P. Ubeda, J. L. Lopez-Ribot, and M. Gobernado. 2002. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 21:767-774. [DOI] [PubMed] [Google Scholar]
- 53.Voss, A., J. A. Kluytmans, J. G. Koeleman, L. Spanjaard, C. M. Vandenbroucke-Grauls, H. A. Verbrugh, M. C. Vos, A. Y. Weersink, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1996. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 54.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 55.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 56.Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115-125. [DOI] [PubMed] [Google Scholar]
- 57.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]