Abstract
Herpes simplex virus type 1 (HSV-1) is transmitted by close contact, both sexual and nonsexual, and infections are acquired during childhood and adolescence. Herpes simplex virus type 2 (HSV-2), however, is thought to be transmitted mainly by sexual contact. Most HSV-2 infections are consequently expected to occur after the onset of sexual activity. Recent reports indicate an increasing prevalence of HSV-2 on the African continent, but most studies have been performed on adult cohorts. In the present study, we collected sera from Tanzanian children and young persons from 1 to 20 years old, with at least 100 individuals in each age group. Antibodies against HSV-1 and HSV-2 were detected by an in-house Western blot method which was shown to perform well in comparison with a commercial Western blot assay. Type-specific antibodies were also analyzed by two noncommercial enzyme-linked immunosorbent assay methods based upon the antigenicities of branched synthetic oligopeptides corresponding to epitopes in glycoprotein G of HSV-1 or HSV-2. The prevalence of HSV-1 antibodies increased gradually from 73% for the age group of 1 to 4 years to 92% for the age group of 17 to 20 years. The prevalence of HSV-2 antibodies was unexpectedly high, as 15% of the children were infected by the age of 8 years, with the incidence increasing gradually to 40% in the age group of 17 to 20 years. The reason for this unexpectedly high frequency is not clear but could suggest that nonsexual transmission of HSV-2 is more common than previously thought. There was no statistically significant association between seropositivities for HSV-2 and human immunodeficiency virus.
Infections with herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are among the commonest human viral infections throughout the world (6). Classically, HSV-1 is acquired during childhood and is associated with nongenital disease, usually causing oro-labial manifestations, while HSV-2 is related to urogenital disease, although both viruses can cause either clinical syndrome (29). While most genital herpes infections are caused by HSV-2, there has been a trend toward an increasing prevalence of HSV-1 causing genital herpes (19, 23, 30, 37), suggesting changes in the age-specific epidemiology of HSV infections. Following primary infection, both types establish lifelong latent infections which periodically reactivate and may be associated with recurrent episodes of disease, with or without clinical symptoms, and as such the infection may often be transmitted without knowledge (5, 18, 26, 27). Apart from the morbidity due to symptomatic episodes, HSV infections may have severe consequences in immunosuppressed hosts or neonates (42). Recently, genital herpes has become a more prominent public health issue due to a potential facilitation effect on human immunodeficiency virus (HIV) transmission (7, 8, 28), making the development of HSV control methods a priority.
HSV-1 and HSV-2 are highly homologous genetically and antigenically (17). This results in an antigen-sharing profile for the two serotypes. Consequently, antibodies produced in response to one type exhibit extensive cross-reactivity with analogous proteins of the other type, making serological analysis difficult. The recent development of reliable HSV type-specific antibody assays using either the complete glycoprotein G (gG) (12) or specific epitopes in gG (24) has provided the means to identify past infection with a given HSV type, regardless of whether it was clinically apparent or not. To date, these assays have been used largely in epidemiological studies.
The seroprevalence of HSV-2 antibodies in adults has been studied in several places in the world and shown to vary by country and population group (35). For example, the prevalence in the general population was reported to be 22% in the United States (9), 3 to 5% in the United Kingdom (41), and between 10% and 16% in Sweden, depending upon age (14). Seven percent of pregnant women in Japan have HSV-2 antibodies, in contrast to 96% of prostitutes in Senegal (29). Infections with HSV-2 have increased during the last decades (35). In Tanzania, which is the focus of interest in the present study, HSV-2 seroprevalence was observed to increase in a 10-year period among individuals attending a sexually transmitted disease clinic, from 43 to 70% (20, 32). In contrast, very little is known about the prevalence of HSV-2 infections in childhood and adolescence in this country.
Studies on the prevalence of HSV-1 antibodies in children show variations. For children at an age of 4 to 5 years, the prevalence has been reported to be 20% in England (15), 25% in Sweden (39), 30% in Germany (43), around 35% in Estonia (40), 50% in Hong Kong (15), and 59% in Eritrea (10). In the following years, there was an increase in all studies to between 37% (Sweden) and 75% (Estonia) in European countries and 97% in Eritrea.
Geographic and socioeconomic differences affect the frequency of HSV infections, so the acquisition patterns of these infections vary greatly in different areas and populations (15, 29). Continuous changes in societies might therefore affect these acquisition patterns, and frequent studies would therefore be useful for monitoring purposes.
The patterns of acquisition of these virus infections in childhood have not previously been studied in Tanzania. The present report shows that at the age of 4 years, the majority of individuals are infected with HSV-1, and a surprisingly large number (15%) are infected with HSV-2.
MATERIALS AND METHODS
Serum samples.
A total of 565 serum specimens were collected from 1- to 20-year-old persons attending an outpatient clinic at Muhimbili National Hospital in Dar es Salaam, Tanzania. The sera were divided into five different age groups, using intervals of 4 years. There were at least 100 sera in each group. The sera were stored frozen at −20°C.
Western blot assay.
Antigens for immunoblotting were prepared by infecting baby hamster kidney cells with either HSV-1 strain 17 syn + (4) or HSV-2 strain HG52 (38). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The concentrations of acrylamide in the stacking and separation gels were 5% and 7%, respectively. The viral proteins were then blotted onto a nitrocellulose membrane (BA 85; Schleicher and Schuell, Dassel, Germany), which was subsequently cut into strips to be stored dry at 4°C. Unless stated otherwise, strips were incubated overnight with serum samples at a 1:100 dilution for detection of anti-gG-1 antibodies and a 1:50 dilution for detection of anti-gG-2 antibodies. The monoclonal antibodies LP10, directed against gG-1 (34), and AP1, directed against gG-2 (25), were used to identify the positions of gG-1 and gG-2, respectively, on the Western blot strips. Bound human antibodies were detected by their reaction with peroxidase-conjugated sheep anti-human immunoglobulin G (IgG; Amersham), and bound monoclonal antibodies were detected with peroxidase-conjugated anti-mouse IgG (Amersham). The substrate was 4-chloro-1-naphthol in both cases. All sera were analyzed by both HSV-1-specific and HSV-2-specific strips. Reactions were scored as positive when typical bands appeared. If the initial results at serum dilutions of 1:50 (HSV-2 antibodies) and 1:100 (HSV-1 antibodies) were not clear, the sera were analyzed at increasingly higher concentrations (dilutions of 1:50, 1:20, and 1:10) until conclusive results were obtained. At the end, there was one serum whose HSV-1 antibody content remained unclear. To distinguish this method from essentially similar Western blot methods used for the same purpose in other laboratories, we use the term WB-UB.
Commercial Western blot assay.
To evaluate the performance of the WB-UB method, a total of 105 sera were selected as described in Results and also analyzed with a HerpeSelect 1 and 2 Immunoblot IgG kit (ISO 9001) from MRL Diagnostics. The specific antigens were recombinant gG-1 and gG-2 proteins on nitrocellulose strips. According to the manufacturer, the sensitivity and specificity of the immunoblot strips relative to Western blotting are 99% and 92%, respectively, for HSV-1, and 98% and 94%, respectively, for HSV-2.
ELISA.
Oligopeptides corresponding to antigenic regions of gG-1 and gG-2 were used in enzyme-linked immunosorbent assays (ELISAs), as described previously, for specific detection of HSV-1 (16) or HSV-2 (24) antibodies. As described previously, peptides were made in multiple antigenic form with four copies of the antigenic peptide, with each separated from the polylysine core by a four-glycine spacer. Briefly, wells of microtiter plates (Immunlon 1B 96-well plates; Thermo Labsystems) were coated overnight at 4°C with 100 ng of peptide diluted in phosphate-buffered saline. Following incubation for 1.5 h at 37°C with phosphate-buffered saline containing 1% bovine serum albumin, 50 μl serum was added to each well, and the plate was incubated for 1.5 h at room temperature on an orbital shaker. The sera were diluted 1:10 and 1:5 for detection of HSV-1 and HSV-2 antibodies, respectively. The incubation time with biotinylated anti-human IgG and streptavidin-horseradish peroxidase (Amersham) was 90 min for each. Washing was performed six times between each of the above steps, using 150 mM NaCl containing 0.05% Tween 20. The plates were then incubated with citrate buffer containing O-phenylenediamine dihydrochloride (OPDA) and hydrogen peroxide (H2O2) at room temperature in the dark. The reaction was stopped after 15 and 10 min for detection of HSV-1 and HSV-2 antibodies, respectively, by the addition of 50 μl of 2 M H2SO4 to each well. Absorbance was measured immediately at 492 nm in a Labsystem Multiscan MS plate reader. For calculation of cutoff values, the data from the WB-UB assay were used as true results. The results from ELISA and Western blotting were then compared for each age group, using receiver operating characteristics (ROC) (44). All samples were analyzed in parallel.
Detection of HIV antibodies.
Bionor HIV type 1 (HIV-1) and HIV-2 screening and confirmatory tests were used as described by the manufacturer. These tests are enzyme-linked immunosorbent assays where magnetic beads are used as solid supports. Sensitivities and specificities of 98% or more have been reported for both tests (3, 36). All sera were analyzed in the laboratory of Bionor. In cases with limited sample amounts, the test volume was reduced from 50 μl to 30 μl and the incubation period increased from 10 to 20 min.
Statistical methods.
Pearson's chi-square (χ2) test was applied for comparing HSV-2 seropositivities in the different age groups. When comparing the seropositivities between the subgroups of children younger than 2 years and 2 to 4 years old, the strength of association was additionally estimated by calculating the odds ratio (OR) with its 95% confidence interval (95% CI). Odds ratio (95% CI) and Fisher's exact tests were used for analysis of a potential association between HIV and HSV-2 infections.
RESULTS
Performance of Western blot method.
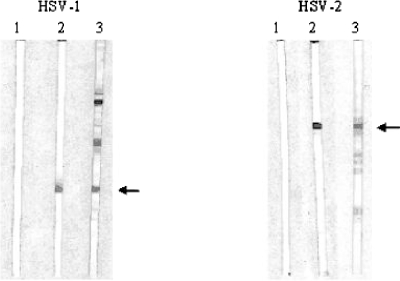
Western blotting has been reported to be an accurate and sensitive test for detecting HSV antibodies (2) and is considered the “gold standard.” Initially, we analyzed the performance of the WB-UB method. The detection of gG-1 and gG-2 proteins with this method, using type-specific monoclonal antibodies, is shown in Fig. 1. Prominent bands were observed, and in some cases, additional bands of different molecular masses were also seen. It has been described previously (34) that the monoclonal antibody LP10 also reacts with unglycosylated and fast-migrating forms of gG-1. Figure 1 also shows typical results from an analysis of human serum. Bands were detected at the positions for gG-1 and gG-2. Whenever such bands were observed, the serum was recorded as containing HSV-1 or HSV-2 antibodies, respectively. Human sera also reacted more or less strongly with proteins with molecular masses different from those of gG-1 and gG-2, which were most likely other glycoproteins of HSV-1 or HSV-2, but these reactions were not recorded or studied further.
FIG. 1.
Performance of WB-UB method. Proteins from cells infected with HSV-1 or HSV-2, as indicated, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes which were subsequently cut into strips. Strip 1 in the left panel was incubated with an HSV-1-negative serum, while strip 1 in the right panel was incubated with an HSV-2-negative serum. Strips 2 were incubated with monoclonal antibodies LP10 (left panel) and AP1 (right panel), directed against gG-1 and gG-2, respectively, while strips 3 were incubated with human sera. The positions of gG-1 and gG-2 are indicated by arrows.
The performance of the WB-UB method was compared with that of a commercial HSV-1 and HSV-2 differentiation IgG immunoblot kit from MRL Diagnostics by analyzing 105 sera by the two methods. Between 19 and 25 sera were selected from each age group. We wanted to include sera with presumably different concentrations of HSV antibodies, including high, moderate, and low levels, as well as HSV-negative sera. Selection was related to the results obtained with peptide-ELISA methods (see below). For each age group, a minimum of two sera had absorbance values that were either high, moderate, or slightly above an arbitrary value of 0.110. Negative sera had absorbance values below this cutoff. The results are shown in Table 1. For both HSV-1 and HSV-2 analysis, the two methods gave consistent results in 95% of the cases. In comparing the results from the two Western blot tests for each serum (data not included in Table 1), the WB-UB test failed to detect HSV-1 antibodies in one serum and HSV-2 antibodies in two sera which were positive in the commercial test. Furthermore, among those sera which were negative in the commercial test for both types of virus antibodies, five were HSV-1 positive and three were HSV-2 positive in the WB-UB test. We concluded that the WB-UB method was reliable for further analysis of the sera.
TABLE 1.
Comparison of commercial Western blot and WB-UB methods
| Age group (yr) | No. of samples | HSV type | No. of samples with result
|
No. of consistent results | No. of inconsistent results | ||||
|---|---|---|---|---|---|---|---|---|---|
| Commercial Western blotting
|
WB-UB
|
||||||||
| Positive | Negative | Positive | Negative | Inconclusive | |||||
| 1-4 | 25 | 1 | 10 | 15 | 11 | 14 | 0 | 24 | 1 |
| 2 | 6 | 19 | 5 | 20 | 0 | 24 | 1 | ||
| 5-8 | 20 | 1 | 10 | 10 | 10 | 9 | 1 | 18 | 1a |
| 2 | 4 | 16 | 6 | 14 | 0 | 18 | 2 | ||
| 9-12 | 20 | 1 | 10 | 10 | 11 | 9 | 0 | 19 | 1 |
| 2 | 8 | 12 | 8 | 12 | 0 | 18 | 2b | ||
| 13-16 | 21 | 1 | 10 | 11 | 11 | 10 | 0 | 20 | 1 |
| 2 | 7 | 14 | 7 | 14 | 0 | 21 | 0 | ||
| 17-20 | 19 | 1 | 10 | 9 | 11 | 8 | 0 | 18 | 1 |
| 2 | 7 | 12 | 7 | 12 | 0 | 19 | 0 | ||
One sample gave inconclusive results.
One sample was positive by commercial Western blotting and negative by WB-UB, and another gave the opposite results.
Detection of HSV-1 and HSV-2 antibodies in different age groups by using WB-UB.
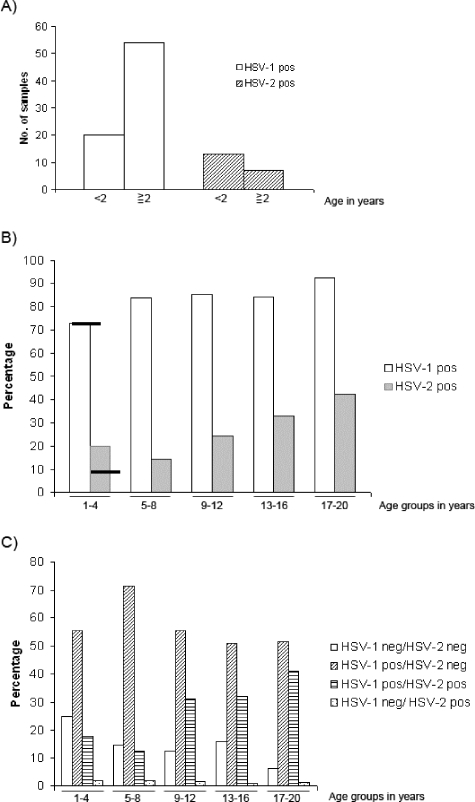
Sera from patients in the various age groups were analyzed for the presence of HSV-1 as well as HSV-2 antibodies by using the WB-UB method and serum dilutions as described in Materials and Methods. The results are shown in Fig. 2. The prevalence of HSV-1 antibodies increased gradually, from 73% for the group of 1- to 4-year-olds to 92% for the group of 17- to 20-year-olds (Fig. 2B). As expected, the prevalence of HSV-2 antibodies was markedly lower but was still close to 20% among the youngest children. There was a drop to 14% for the age group of 5- to 8-year-olds and then a nearly linear increase to 42% for children ages 17 to 20. Maternal HSV-2 antibodies were most likely present in some of the children under 2 years of age, since the number of HSV-2-positive individuals was higher for this age group than among those 2 to 4 years old (Fig. 2A). In contrast, the prevalence of HSV-1 antibodies increased markedly after 2 years of age. Still, maternal anti-HSV-1 antibodies could be present in the youngest children. Excluding individuals of <2 years of age, the remaining 73 persons in the 1- to 4-year-old age group had HSV-1 and HSV-2 prevalence rates of 74% and 8.2%, respectively, as indicated by the horizontal bars in Fig. 2B. Figure 2C shows the percentages of individuals with single or dual infections in the different age groups. Single infections with HSV-1 increased from the youngest to the second youngest age group and then decreased. In contrast, dual infections increased from 13% for the group of 5- to 8-year-olds to 41% for the oldest group. Very few individuals in each group were infected with HSV-2 only. All variations shown in Fig. 2, except that for HSV-1 in panel A, were statistically significant, as described in the figure legend. Except for the youngest group, the prevalence of HSV-2 antibodies was only slightly higher among girls than among boys, and the relative proportion of infected females did not change in the two oldest age groups (older than 13 years), where sexual activity would presumably occur (results not shown). The prevalence of HSV-2 antibodies was unexpectedly high.
FIG. 2.
Analysis of sera for antibodies against HSV-1 and/or HSV-2. An analysis of sera by WB-UB for antibodies against HSV-1 and/or HSV-2, as related to age groups, is shown in panel B. Statistical methods were used to investigate whether the differences in prevalence between the age groups were significant. For HSV-1 antibodies, the results were as follows: χ2 = 15.68, df = 4, and P = 0.003. For HSV-2 antibodies, the results were as follows: χ2 = 27.72, df = 4, and P < 0.001. The horizontal bars for the 1- to 4-year-old age group indicate the prevalence of HSV-1 and HSV-2 antibodies, respectively, in the subgroup of 2- to 4-year-olds. In panel A, the 1- to 4-year-old age group is split into two subgroups, <2 years and 2 to 4 years. Statistical methods were used to analyze the differences between the age groups. For HSV-1 antibodies, the results were as follows: P = 0.80 and OR (95% CI) = 1.14 (0.43 to 3.01). For HSV-2 antibodies, the results were as follows: P < 0.001 and OR (95% CI) = 0.12 (0.04 to 0.36). Panel C shows the percentages of uninfected and single- and double-infected individuals in each age group.
Analysis of children's sera by peptide-ELISA methods.
Alternative methods for specific detection of HSV-1 or HSV-2 antibodies include different ELISAs, both commercial methods and recently developed methods based upon the antigenicities of two different oligopeptides specific for HSV-1 and HSV-2 (16, 24). However, the performances of these methods in a pediatric population have not been evaluated. The two peptide-ELISA methods developed in our laboratories were of particular interest and were used to analyze the sera that were already characterized by the WB-UB method.
Cutoff values were calculated for each age group by using the ROC method, as described in Materials and Methods. Furthermore, all sera were also considered as a single group, for which common cutoff values were calculated for HSV-1 ELISA and HSV-2 ELISA. Positive or negative results consistent with those from the WB-UB method were categorized as truly positive or truly negative. False-positive results were positive in the ELISA but negative in the WB-UB test, and the opposite was the case for false-negative results. The results are shown in Table 2. For both ELISAs, the number of false results decreased markedly with age, from 14 and 11 for HSV-1 and HSV-2, respectively, for the group of 1- to 4-year-olds to 4 and 0, respectively, for the oldest age group. Sensitivities and specificities for the two ELISAs were calculated for each age group and also for all 565 sera as a single group. HSV-2 ELISA performed better than HSV-1 ELISA in all cases. However, the performances of both tests were markedly better for the group of 17- to 20-year-olds than for the youngest groups. With separate cutoff values for each age group, the total numbers of false-positive and false-negative HSV-1 samples were 15 and 43, respectively. A single cutoff for all 565 sera, however, led to 7 false-positive and 69 false-negative results (Table 2). This indicates that a common cutoff might be used for all age groups when analyzing HSV-2 antibodies, whereas cutoff values calculated for each age group might give more reliable results when analyzing HSV-1 antibodies.
TABLE 2.
Performances of peptide-ELISAs relative to that of the WB-UB method
| Age group (yr) | Total no. of samples | Result | Test performance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HSV-1 ELISA
|
HSV-2 ELISA
|
|||||||||
| Cutoffa,b | No. of samples | Sensitivity (%)a | Specificity (%)a | Cutoffa,b | No. of samples | Sensitivity (%)a | Specificity (%)a | |||
| 1-4 | 101 | Positive | 0.125 | 62 | 83.7 | 81.5 | 0.095 | 19 | 95.0 | 87.6 |
| Negative | 22 | 71 | ||||||||
| False positive | 5 | 10 | ||||||||
| False negative | 12 | 1 | ||||||||
| 5-8 | 111 | Positive | 0.140 | 79 | 84.9 | 88.9 | 0.095 | 16 | 100 | 94.7 |
| Negative | 16 | 90 | ||||||||
| False positive | 2 | 5 | ||||||||
| False negative | 14 | 0 | ||||||||
| 9-12 | 123 | Positive | 0.155 | 97 | 92.4 | 77.8 | 0.095 | 30 | 100 | 95.7 |
| Negative | 14 | 89 | ||||||||
| False positive | 4 | 4 | ||||||||
| False negative | 8 | 0 | ||||||||
| 13-16 | 100 | Positive | 0.110 | 79 | 94.0 | 81.3 | 0.100 | 32 | 96.7 | 100 |
| Negative | 13 | 67 | ||||||||
| False positive | 3 | 0 | ||||||||
| False negative | 5 | 1 | ||||||||
| 17-20 | 130 | Positive | 0.145 | 116 | 96.7 | 90.0 | 0.095 | 55 | 100 | 100 |
| Negative | 9 | 75 | ||||||||
| False positive | 1 | 0 | ||||||||
| False negative | 4 | 0 | ||||||||
| All samples | 565 | Positive | 0.155 | 407 | 85.5 | 92.1 | 0.095 | 153 | 99.3 | 94.6 |
| Negative | 82 | 389 | ||||||||
| False positive | 7 | 22 | ||||||||
| False negative | 69 | 1 | ||||||||
Values apply to the age group, not to a particular result within the group.
The cutoff values are measures of absorbance.
HIV antibodies.
By initial screening, 458 samples were negative for HIV and 107 samples were found to contain HIV antibodies. In 18 cases, there was an insufficient amount of serum to confirm the screening results. Of the remaining sera, 69 were confirmed as positive, 15 were indeterminate, and 5 were negative. Nine sera showed indications of seroreactivity to HIV-2 in addition to HIV-1, but the potential presence of HIV-2 was not analyzed further. The confirmed HIV seropositivity among all individuals was thus 12.2%. The relationships between antibodies against HSV-2 and those against HIV for the various age groups are shown in Table 3. The majority of the samples containing insufficient amounts of sera to confirm the HIV positivity observed in the screening test were most likely HIV positive and therefore were included as positive results in Table 3. The reason for doing this is that only 7% (5 of 69) of screening-positive samples were negative in the confirmation test. Indeterminate samples were omitted from the table. The table shows that for all age groups, HSV-2 seropositivity was higher among HIV-positive than among HIV-negative individuals. However, the differences were not significant in this situation or in the case where all samples with insufficient amounts of serum were omitted from the analysis. Furthermore, in examining all 565 samples as a single group, there was no statistically significant association between seropositivities for HSV-2 and HIV.
TABLE 3.
Presence or absence of HSV-2 antibodies in HIV-positive and HIV-negative sera
| Age group (yr) | HIV negative
|
HIV positivea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HSV-2 negativeb | HSV-2 positiveb | Totalb | % HSV-2 positive | HSV-2 negativeb | HSV-2 positiveb | Totalb | % HSV-2 positive | P value (Fisher’s exact test) | Odds ratio (95% CI) | |
| 1-4 | 67 | 15 | 82 | 18.3 | 11 | 4 | 15 | 26.7 | 0.48 | 1.62 (0.45-5.81) |
| 5-8 | 79 | 13 | 92 | 14.1 | 13 | 3 | 16 | 18.8 | 0.70 | 1.40 (0.35-5.61) |
| 9-12 | 74 | 22 | 96 | 22.9 | 15 | 7 | 22 | 31.8 | 0.42 | 1.57 (0.57-4.33) |
| 13-16 | 59 | 27 | 86 | 31.4 | 6 | 5 | 11 | 45.5 | 0.50 | 1.82 (0.51-6.49) |
| 17-20 | 61 | 45 | 106 | 42.5 | 11 | 10 | 21 | 47.6 | 0.81 | 1.23 (0.48-3.15) |
HIV-positive samples are those that were positive by screening and by the confirmation test and, additionally, those that were positive by screening but with insufficient amounts of serum for the confirmation test. Samples with indeterminate HIV status were excluded.
Values are numbers of samples.
DISCUSSION
We have studied the prevalence of antibodies against herpes simplex viruses in children and young people in Tanzania. The individuals comprising the cohort of our study presented at an outpatient clinic in Dar es Salaam, Tanzania. Mostly, they presented with symptoms of fever, and a number of individuals were suffering from malaria. Medical records were not obtained from the clinic, so we do not know if any particular diseases might have increased the frequency of HSV infections in the cohort. As such, our cohort might not be fully representative of the population in general. However, the conclusions that we drew concerning the age distribution and serotype specificity are not affected by this limitation.
The data were generated by using the WB-UB method as well as peptide-ELISA methods for specific detection of HSV antibodies. Similar Western blot methods have been used successfully in other studies (2, 21, 43). The present method performed well in comparison with a commercial Western blot method (Table 1). The HSV-2-specific ELISA method has been evaluated in several studies (31, 33) and was shown to be as sensitive and specific as commercial methods. Furthermore, the HSV-1-specific ELISA performed well in a separate study (16). Included in the commercial immunoblot kit is a standard which stains weakly and indicates the lower limit for specific detection of HSV antibodies. Since a similar low limit was not used for the WB-UB method, some sera with low levels of antibodies might score positive by this method but not by the commercial one. This could explain some of the few inconsistent results shown in Table 1. Among all seven sera that were WB-UB positive and negative in the commercial Western blot, five samples were weakly positive by the peptide-ELISA methods. The observation that the two ELISA methods perform better for older age groups than for young children (Table 2) is consistent with observations by others that analysis may be less accurate for pediatric than for adult sera (1). Many studies in which Western blotting and ELISA have been used have been performed on sera from the United States or European countries. However, Hogrefe et al. (13) analyzed 781 sera from various African countries and reported concordance between WB and ELISA in approximately 95% of the cases. These methods should therefore also be suitable for analysis of African sera.
The present results show that HSV-1 infections occur early in life in Tanzania, as 74% of the individuals aged 2 to 4 years old had specific antibodies, and >80% of children in the age group of 5 to 8 years had specific antibodies. These figures are nearly two times higher than those obtained from similar studies in Sweden (39) and Germany (43) but are rather close to the results from a study in Eritrea (10). The prevalence of HSV-2 antibodies was unexpectedly high, as 15% of the children were infected at an age of 8 years, with an increase to 40% for the group of 17- to 20-year-olds. In contrast, <5% of German and Estonian children had HSV-2 antibodies before the onset of sexual activity (40, 43), and such antibodies were detected in only 1 of 70 children's sera from Eritrea (10).
Although several reports have shown a significant association between infections with HSV-2 and HIV in adult cohorts (7, 8, 28), this was not observed in the present study of sera from children and adolescent individuals. This was not surprising, as we expected many children to have congenital HIV infections. Another reason for this difference could be that adult cohorts often contain patients attending clinics for sexually transmitted diseases.
If we excluded the infants that might have maternal antibodies, the prevalence of HSV-2 antibodies in young children was still remarkably high in our study. The reason for this is not clear. Such a high rate of sexual abuse of 1- to 5-year-old children seems very unlikely. If HSV-2 infections were related to sexual activity, one might expect a marked increase after the age when such activity starts. However, Fig. 2B shows an almost constant rate of increase of HSV-2 antibodies with age. Furthermore, the proportion of infected girls did not increase after the age of 13 years (results not shown). This might suggest that nonsexual transmission of HSV-2 could be more common than expected. Some observations support this idea. Of numerous herpesvirus isolates from fingers/hands and other extragenital regions, with the exception of the orofacial area, nearly 50% were typed as HSV-2 and the rest were typed as HSV-1 (22). It would thus be interesting to investigate possible routes of nonsexual transmission of HSV-2 in cohorts similar to that described in the present work.
Acknowledgments
We thank the Western Norway Regional Health Authority and The Norwegian State Loan Fund for financial support.
We thank Tony Minson for supplying monoclonal antibodies against gG-1 and gG-2, and we thank Maja Sommerfelt and Bionor AS for performing the analysis for HIV antibodies.
REFERENCES
- 1.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, R. L., J. Miltoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and G-specific immunodot enzyme assays for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beelaert, G., G. Vercautern, K. Fransen, M. Mangelschots, M. De Rooy, S. Garcia-Ribs, and G. van Groen. 2002. Comparative evaluation of eight commercial enzyme linked immunosorbent assays and 14 simple assays for detection of antibodies to HIV. J. Virol. Methods 105:197-206. [DOI] [PubMed] [Google Scholar]
- 4.Brown, S. M., D. A. Ritchie, and J. H. Subal-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangements into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. [DOI] [PubMed] [Google Scholar]
- 5.Brown, Z. A., J. Benedetti, R. Ashley, S. Burchett, S. Selke, S. Berry, L. A. Vontver, and L. Corey. 1991. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labour. N. Engl. J. Med. 324:1247-1252. [DOI] [PubMed] [Google Scholar]
- 6.Brugha, R., K. Keersmaekers, and A. Renton. 1997. Genital herpes infection: a review. Int. J. Epidemiol. 26:698-709. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, M. S. 1998. Sexually transmitted diseases enhance HIV transmission: no longer hypothesis. Lancet 351(Suppl. 111):5-7. [DOI] [PubMed] [Google Scholar]
- 8.Corey, L., and H. H. Handsfield. 2000. Genital herpes and public health. Addressing a global problem. JAMA 283:791-794. [DOI] [PubMed] [Google Scholar]
- 9.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1967 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 10.Ghebrekidan, H., U. Ruden, S. Cox, B. Wahren, and M. Grandien. 1999. Prevalence of herpes simplex virus types 1 and 2, cytomegalovirus, and varicella-zoster virus infections in Eritrea. J. Clin. Virol. 12:53-64. [DOI] [PubMed] [Google Scholar]
- 11.Hashido, M., F. K. Lee, A. J. Nahmias, H. Tsugami, S. Isomura, Y. Nagata, S. Sonoda, and T. Kawana. 1998. An epidemiologic study of herpes simplex virus type 1 and 2 infection in Japan based on type-specific serological assays. Epidemiol. Infect. 120:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho, D. W. T., P. R. Field, E. Sjögren-Jansson, S. Jeansson, and A. L. Cunningham. 1992. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2). J. Virol. Methods 36:249-264. [DOI] [PubMed] [Google Scholar]
- 13.Hogrefe, W., J. Su, J. Song, R. Ashley, and L. Kong. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J. Clin. Microbiol. 40:3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson, M. K., and B. Wahren. 2004. Sexually transmitted herpes simplex. Scand. J. Infect. Dis. 36:93-101. [DOI] [PubMed] [Google Scholar]
- 15.Kangro, H. O., H. K. Osman, Y. L. Lau, R. B. Heath, C. Y. Yeung, and M. H. Ng. 1994. Seroprevalence of antibodies to human herpesviruses in England and Hong Kong. J. Med. Virol. 43:91-96. [DOI] [PubMed] [Google Scholar]
- 16.Kasubi, M. J., A. Nilsen, H. S. Marsden, T. Bergström, N. Langeland, and L. Haarr. 2005. A branched, synthetic oligopeptide corresponding to a region of glycoprotein G of HSV-1 reacts sensitively and specifically with HSV-1 antibodies in an ELISA. J. Virol. Methods 125:137-143. [DOI] [PubMed] [Google Scholar]
- 17.Kieff, E., B. Hoyer, S. Bachenheimer, and B. Roizman. 1972. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J. Virol. 9:738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsky, L. A., R. L. Ashley, and K. K. Holmes. 1990. The frequency of unrecognized type 2 herpes simplex virus among women. Implications for the control of genital herpes. Sex. Transm. Dis. 17:90-94. [DOI] [PubMed] [Google Scholar]
- 19.Lafferty, W. E., L. Downey, C. Celum, and A. Wald. 2000. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J. Infect. Dis. 181:1454-1457. [DOI] [PubMed] [Google Scholar]
- 20.Langeland, N., L. Haarr, and F. Mhalu. 1998. Prevalence of HSV-2 antibodies among STD clinic patients in Tanzania. Int. J. STD AIDS 9:104-107. [DOI] [PubMed] [Google Scholar]
- 21.Liljeqvist, J.-Å., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjögren-Jansson, and T. Bergström. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 79:1215-1224. [DOI] [PubMed] [Google Scholar]
- 22.Löwhagen, G. B., P. Tunbäck, and T. Bergström. 2002. Proportion of herpes simplex virus (HSV) type 1 and 2 among genital and extragenital HSV isolates. Acta Dermato-Venereol. 82:118-120. [DOI] [PubMed] [Google Scholar]
- 23.Löwhagen, G. B., P. Tunbäck, K. Andersson, T. Bergström, and G. Johannison. 2000. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex. Transm. Infect. 27:425-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsden, H. S., K. MacAulay, J. Murray, and I. W. Smith. 1998. Identification of an immunodominant sequential epitope in glycoprotein G of herpes simplex virus type 2 that is useful for serotype-specific diagnosis. J. Med. Virol. 56:79-84. [DOI] [PubMed] [Google Scholar]
- 25.Marsden, H. S., A. Buckmaster, J. W. Palfreyman, R. G. Hope, and A. C. Minson. 1984. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J. Virol. 50:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mertz, G. J., O. Schmidt, and J. L. Jourden. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contact. Sex. Transm. Dis. 12:33-39. [DOI] [PubMed] [Google Scholar]
- 27.Mertz, G. J., R. W. Coombs, and R. Ashley. 1988. Unrecognized transmission of genital herpes in couples with one symptomatic and one asymptomatic partner: a prospective study. J. Infect. Dis. 157:1169-1177. [DOI] [PubMed] [Google Scholar]
- 28.Morse, S. A., D. L. Trees, Y. Htun, F. Radebe, K. A. Orle, Y. Dangor, C. M. Beck-Sague, S. Schmid, G. Fehler, J. B. Weiss, and R. C. Ballard. 1997. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J. Infect. Dis. 175:583-589. [DOI] [PubMed] [Google Scholar]
- 29.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiology and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. 69(Suppl.):19-36. [PubMed] [Google Scholar]
- 30.Nilsen, A., and H. Myrmel. 2000. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet. Gynecol. Scand. 79:693-696. [PubMed] [Google Scholar]
- 31.Nilsen, A., E. Ulvestad, H. Marsden, N. Langeland, H. Myrmel, R. Matre, and L. Haarr. 2003. Performance characteristics of a glycoprotein G based oligopeptide (peptide 55) and two different methods using the complete glycoprotein as assays for detection of anti-HSV-2 antibodies in human sera. J. Virol. Methods 107:21-27. [DOI] [PubMed] [Google Scholar]
- 32.Nilsen, A., D. Mwakagile, H. Marsden, N. Langeland, R. Matre, and L. Haarr. 2005. Prevalence of, and risk factors for, HSV-2 antibodies in sexually transmitted disease patients, healthy pregnant females, blood donors and medical students in Tanzania and Norway. Epidemiol. Infect. 133:915-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oladepo, D. K., P. E. Klapper, and H. Marsden. 2000. Peptide based enzyme-linked immunoassays for detection of anti-HSV-2 IgG in human sera. J. Virol. Methods 87:63-70. [DOI] [PubMed] [Google Scholar]
- 34.Richman, D. D., A. Buckmaster, S. Bell, C. Hodgman, and A. C. Minson. 1986. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J. Virol. 57:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, J. S., and J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186:S3-S28. [DOI] [PubMed] [Google Scholar]
- 36.Sommerfelt, M. A., I. Ohlsson, I. Flolid, R. Thorstensson, and B. Sorensen. 2004. A simple semi-rapid HIV-1 and 2 confirmatory immunoassay using magnetic particles. J. Virol. Methods 115:191-198. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, C. 2000. Genital herpes simplex typing in genitourinary medicine: 1995-1999. Int. J. STD AIDS 11:501-502. [DOI] [PubMed] [Google Scholar]
- 38.Timbury, M. C. 1971. Temperature-sensitive mutants of herpes simplex virus type 2. J. Gen. Virol. 13:373-376. [DOI] [PubMed] [Google Scholar]
- 39.Tunbäck, P., T. Bergström, A. S. Andersson, P. Nordin, I. Krantz, and G. B. Löwhagen. 2003. Prevalence of herpes simplex virus antibodies in childhood and adolescence: a cross-sectional study. Scand. J. Infect. Dis. 35:498-502. [DOI] [PubMed] [Google Scholar]
- 40.Uusküla, A., M. Nygard-Kibur, F. M. Cowan, P. Mayaud, R. S. French, J. N. R. Robinson, and D. W. G. Brown. 2004. The burden of infection with herpes simplex virus type 1 and type 2: seroprevalence study in Estonia. Scand. J. Infect. Dis. 36:727-732. [DOI] [PubMed] [Google Scholar]
- 41.Vyse, A. J., N. J. Gay, M. J. Slomka, R. Gropal, T. Gibbs, P. Morgan-Capner, and D. W. Brown. 2000. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex. Transm. Infect. 76:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitley, R. J., L. Corey, and A. Arvin. 1998. Changing presentation of herpes simplex virus infection in neonates. J. Infect. Dis. 158:109-116. [DOI] [PubMed] [Google Scholar]
- 43.Wutzler, P., H. W. Doerr, I. Färber, U. Eichhorn, B. Helbig, A. Sauerbrei, A. Brandstädt, and H. Rebenau. 2000. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J. Med. Virol. 61:201-207. [DOI] [PubMed] [Google Scholar]
- 44.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristics (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]