Abstract
Laboratory cross-contamination by Mycobacterium tuberculosis is known to be responsible for the misdiagnosis of tuberculosis, but its impact on other contexts has not been analyzed. We present the findings of a molecular epidemiology analysis in which the recent transmission events identified by a genotyping reference center were overestimated as a result of unnoticed laboratory cross-contamination in the original diagnostic laboratories.
The phenomenon of misdiagnosing tuberculosis by laboratory cross-contamination when Mycobacterium tuberculosis is cultured has been widely reported (3, 4-8, 10, 11). The production of aerosolized particles after the processing of smear-positive specimens, cultures positive for M. tuberculosis, or positive control strains may be responsible for the inoculation of other specimens processed on the same day or of reagents used for the decontamination of specimens (5). False positivity is suspected (i) if M. tuberculosis is cultured from a sample processed together with a smear-positive specimen, (ii) if M. tuberculosis is cultured from only one of the cultures in the set (usually with a low yield of bacteria), and (iii) if the clinician is considering an alternative diagnosis, that is, a diagnosis other than tuberculosis (TB). Suspicion of false positivity is increased when two or more of these conditions are met. Finally, if molecular analysis is available, cross-contamination is confirmed when the strains cultured from both truly infected and contaminated specimens share the same genotypic pattern and no epidemiological links can be found between the cases. Several studies, some of which are based on molecular analysis, have estimated that the rate of laboratory cross-contamination for M. tuberculosis ranges from 0.1% to 3%, although massive contamination has caused up to 65% of false-positive cases (11).
False-positive results for tuberculosis have been a matter of concern because of the clinical, therapeutic, and social impacts of the misdiagnosis of tuberculosis. The economic load associated with each misdiagnosed case of tuberculosis has been estimated to be $32,618 (9). However, another area on which false positivity has an impact but which has received little attention is the misidentification of recent transmission events by molecular epidemiology studies. Molecular epidemiology is based on the analysis of the genotypes of cultured M. tuberculosis isolates to identify cases infected by the same M. tuberculosis strain. These cases are defined as clustered and are considered to be caused by recent transmission events and to belong to the same transmission chain. If an analysis to determine the existence of potential false-positive cases is not performed before molecular analysis, as a quality control of microbiological procedures, there is a risk of misassigning clustered cases. This refined preanalysis is not usually performed because molecular epidemiology studies are generally run by laboratories which are different from those which culture M. tuberculosis from clinical specimens. Therefore, the genotyping laboratory lacks the data on which the suspicion of cross-contamination is based (smear-staining features of the specimens handled on the same day, the number of positive tubes for each patient, the bacterial yield in the culture, the degree of clinical suspicion, etc).
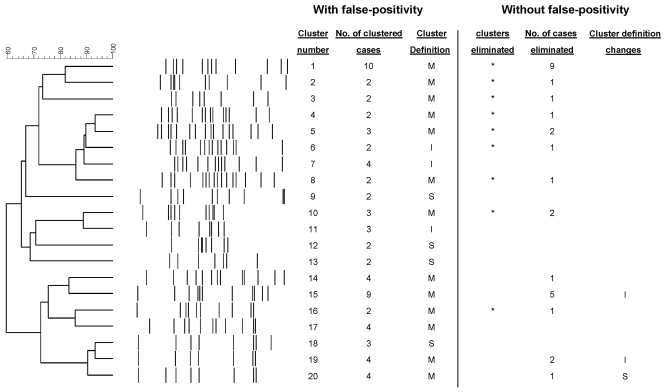
Our aim was to evaluate the possibility that the molecular analysis performed by reference centers could be overestimating recent transmission if communication with the diagnostic laboratory is poor. Our proposal was to reanalyze all fingerprinting data using laboratory observations to identify false-positive cases. Therefore, we performed a preanalysis before the initiation of a long-term molecular epidemiology study in Almería, which is in southeastern Spain. This study runs from 2005 to 2007 and aims to analyze the M. tuberculosis transmission patterns in a region of Spain where the incidence is 27.6 per 100,000 population/year and where the rate of tuberculosis in the immigrant population is very high (45% [143/316] of the cases in 2003 and 2004). The local reference mycobacteriology laboratory processed an average of 21 specimens per day (7% were acid-fast bacillus smear positive, and 54% were from other laboratories) and identified an average of 116 new cases per year. Since there are no facilities for molecular epidemiology studies in Almería, the M. tuberculosis isolates cultured from the three health care institutions in the province were sent to our institution (Hospital Gregorio Marañón, Madrid, Spain) for genotyping. During the pilot study (January 2003 to September 2004), 180 TB cases were diagnosed and 154 isolates (85.5%) were available for genotyping by IS6110-based restriction fragment length polymorphism analysis and spoligotyping. Sixty-nine cases (44.8%) were grouped in 20 clusters (each with from 2 to 10 representatives) (Fig. 1). Three clusters included only immigrant cases, 4 clusters were autochthonous, and the remaining 13 clusters (65%) included both immigrant and autochthonous cases (mixed clusters) (Fig. 1).
FIG. 1.
Dendrogram with all the clustered cases found in the study before and after the identification of the false-positive cases. The eliminated clusters and cases after documentation of false-positive cross-contaminations are indicated. I, international (clusters that included only foreign cases); M, mixed (clusters that included both Spanish and foreign cases); S; Spanish born (clusters that included only autochthonous cases).
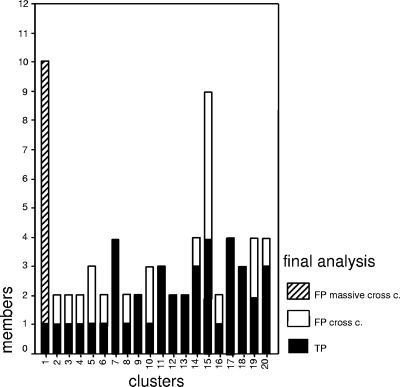
The results of the molecular analysis in the preanalytical study were sent to the diagnostic laboratories in Almería for reanalysis of all clustered cases to identify cases incorrectly clustered because of false positivity. That analysis revealed that in 13 clusters (65%), at least one case was suspected of being false positive due to laboratory cross-contamination (Fig. 1 and 2). This observation was supported by the finding of a close chronological proximity between the handling of the corresponding specimens with M. tuberculosis isolates sharing identical genotypes, and all fulfilled the criteria for consideration as a false-positive case (the specimen was coprocessed with a smear-positive specimen, M. tuberculosis grew from only one of the cultures in the set, and the clinician considered a diagnosis other than tuberculosis). An extensive false cluster (cluster 1) was caused by massive contamination affecting 10 cases. The clinicians confirmed that an alternative diagnosis was possible for all the suspected false-positive cases. Furthermore, the epidemiological study ruled out any connection between the case and its cluster companions. When the cluster analysis was purged of the false-positive cases, the number of clustered cases fell from 69 to 32 (from 44.8 to 20.7%) and the number of clusters fell from 20 to 11 (Fig. 1 and 2). Most of these eliminated clusters included foreign and autochthonous cases (mixed clusters, which are potential indicators of cross-transmission between both populations) (Table 1; Fig. 1). In summary, if the pilot study had not been performed, the recent transmission patterns obtained in Almería would not have reflected the real ongoing transmission, due to a high rate of unnoticed laboratory cross-transmission.
FIG. 2.
Distribution of clustered cases according to their true-positive (TP) or false-positive (FP) status. The cluster caused by a massive cross-contamination (cross c.) event is specified.
TABLE 1.
Nationalities of the clustered cases
| Cluster no. | Country(ies) |
|---|---|
| 1 | Morocco, Spain, Chile, United Kingdom |
| 2 | Russia, Spain |
| 3 | Romania, Spain |
| 4 | Bulgaria, Spain |
| 5 | Ecuador, Spain |
| 6 | Morocco, The Gambia |
| 7 | Romania, Morocco |
| 8 | Morocco, Spain |
| 9 | Spain |
| 10 | Ecuador, Spain, Colombia |
| 11 | Morocco |
| 12 | Spain |
| 13 | Spain |
| 14 | Romania, Spain |
| 15 | Morocco, Spain |
| 16 | Romania, Spain |
| 17 | Morocco, Ecuador, Spain |
| 18 | Spain |
| 19 | Mali, Morocco, Spain |
| 20 | Germany, Spain |
The frequency of false-positive cultures has been calculated to be higher for laboratories that do not process high numbers of specimens (4). It is also easier for them to detect these cases because of the low probability of having two positive specimens close in time. However, some reports (2) state that in high-incidence settings, several positive samples are frequently found in 1 day. This also happens in Almería, especially with cases from the Poniente region (48% of all cases in Almería), thus making it difficult to suspect false positivity. In this case, the laboratory cross-contamination alert was activated only after the molecular pilot study. On the other hand, if the molecular genotyping center (which was blinded to the laboratory features related to the handling of specimens and cultures) had not requested a retrospective reanalysis of the clustered cases, (i) the recent transmission would have been overestimated, (ii) several false clusters would have been defined, and (iii) the rate of cross-transmission between individuals of different nationalities and between foreigners and the autochthonous population would have been miscalculated. In conclusion, all the objectives of the molecular epidemiology analysis would have been misinterpreted.
Recommendations for the minimization of laboratory cross-contamination have been made. These include separation of the locations for the handling of stain-positive and -negative specimens and the use of independent cabinets when work is performed with positive cultures and specimens (1, 2). These findings led the microbiology laboratory in Almería to activate a system to minimize cross-contamination, including the activation of the previously indicated recommendations (1, 2, 12), and to precisely track the days on which the specimens were handled and the time when common reagents were used. An alert was activated when two or more positive cultures corresponded to specimens handled over a 3-day period, and urgent genotyping was requested to quickly identify potential cross-contamination and to rule out the inclusion of false-positive cases in the molecular epidemiology analysis.
This refinement before the initiation of the molecular analysis ensured that in the epidemiological research in Almería, the clusters defined at the end of the study will reflect the real situation of ongoing transmission. This kind of pilot study should be performed before molecular analysis of recent transmission in order to guarantee the rigor of the findings.
Acknowledgments
We are indebted to Thomas O’Boyle for revision of the English of the manuscript.
The study has been partially financed by Fondo de Investigaciones Sanitarias (PI030654, PI030986), Junta de Andalucía (248-03) and Fundación Progreso y Salud (14033).
REFERENCES
- 1.Carroll, N. M., M. Richardson, E. Engelke, M. de Kock, C. Lombard, and P. D. van Helden. 2002. Reduction of the rate of false-positive cultures of Mycobacterium tuberculosis in a laboratory with a high culture positivity rate. Clin. Chem. Lab. Med. 40:888-892. [DOI] [PubMed] [Google Scholar]
- 2.Carroll, N. M., M. Richardson, and P. D. van Helden. 2003. Criteria for identification of cross-contamination of cultures of Mycobacterium tuberculosis in routine microbiology laboratories. J. Clin. Microbiol. 41:2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention 2000. Misdiagnoses of tuberculosis resulting from laboratory cross-contamination of Mycobacterium tuberculosis cultures—New Jersey, 1998. Morb. Mortal. Wkly. Rep. 49:413-416. [PubMed] [Google Scholar]
- 4.de Boer, A. S., B. Blommerde, P. E. de Haas, M. M. Sebek, K. S. Lambregts-van Weezenbeek, M. Dessens, and D. van Soolingen. 2002. False-positive Mycobacterium tuberculosis cultures in 44 laboratories in The Netherlands (1993 to 2000): incidence, risk factors, and consequences. J. Clin. Microbiol. 40:4004-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de C. Ramos, M., H. Soini, G. C. Roscanni, M. Jaques, M. C. Villares, and J. M. Musser. 1999. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J. Clin. Microbiol. 37:916-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick, L., C. Braden, W. Cronin, J. English, E. Campbell, S. Valway, and I. Onorato. 2004. Investigation of laboratory cross-contamination of Mycobacterium tuberculosis cultures. Clin. Infect. Dis. 38:e52-e54. [DOI] [PubMed] [Google Scholar]
- 7.Jasmer, R. M., M. Roemer, J. Hamilton, J. Bunter, C. R. Braden, T. M. Shinnick, and E. P. Desmond. 2002. A prospective, multicenter study of laboratory cross-contamination of Mycobacterium tuberculosis cultures. Emerg. Infect. Dis. 8:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nivin, B., P. I. Fujiwara, J. Hannifin, and B. N. Kreiswirth. 1998. Cross-contamination with Mycobacterium tuberculosis: an epidemiological and laboratory investigation. Infect. Control Hosp. Epidemiol. 19:500-503. [DOI] [PubMed] [Google Scholar]
- 9.Northrup, J. M., A. C. Miller, E. Nardell, S. Sharnprapai, S. Etkind, J. Driscoll, M. McGarry, H. W. Taber, P. Elvin, N. L. Qualls, and C. R. Braden. 2002. Estimated costs of false laboratory diagnoses of tuberculosis in three patients. Emerg. Infect. Dis. 8:1264-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poynten, M., D. N. Andresen, and T. Gottlieb. 2002. Laboratory cross-contamination of Mycobacterium tuberculosis: an investigation and analysis of causes and consequences. Int. Med. J. 32:512-519. [DOI] [PubMed] [Google Scholar]
- 11.Ruddy, M., T. D. McHugh, J. W. Dale, D. Banerjee, H. Maguire, P. Wilson, F. Drobniewski, P. Butcher, and S. H. Gillespie. 2002. Estimation of the rate of unrecognized cross-contamination with Mycobacterium tuberculosis in London microbiology laboratories. J. Clin. Microbiol. 40:4100-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Small, P. M., N. B. McClenny, S. P. Singh, G. K. Schoolnik, L. S. Tompkins, and P. A. Mickelsen. 1993. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J. Clin. Microbiol. 31:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]