Abstract
Molecular methodologies, especially 16S rRNA gene sequence analysis, have allowed the recognition of many new species of Nocardia and to date have been the most precise methods for identifying isolates reliably to the species level. We describe here a novel method for identifying Nocardia isolates by using sequence analysis of a portion of the secA1 gene. A region of the secA1 gene of 30 type or reference strains of Nocardia species was amplified using secA1-specific primers. Sequence analysis of 468 bp allowed clear differentiation of all species, with a range of interspecies similarity of 85.0% to 98.7%. Corresponding 16S rRNA gene sequences of a 1,285-bp region for the same isolates showed a range of interspecies similarity of 94.4 to 99.8%. In addition to the type and reference strains, a 468-bp fragment of the secA1 gene was sequenced from 40 clinical isolates of 12 Nocardia species previously identified by 16S rRNA gene sequence analysis. The secA1 gene sequences of most isolates showed >99.0% similarity to the secA1 sequences of the type or reference strain to which their identification corresponded, with a range of 95.3 to 100%. Comparison of the deduced 156 amino acid sequences of the SecA1 proteins of the clinical isolates showed between zero and two amino acid residue differences compared to that of the corresponding type or reference strain. Sequencing of the secA1 gene, and using deduced amino acid sequences of the SecA1 protein, may provide a more discriminative and precise method for the identification of Nocardia isolates than 16S rRNA gene sequencing.
Because of the difficulty of identifying Nocardia isolates by phenotypic methods, sequence analysis of the 16S rRNA gene has become the “gold standard” for the identification of Nocardia isolates to the species level. The use of 16S rRNA gene sequencing has been instrumental in the discrimination of numerous new species of Nocardia in recent years, and many of these species have been shown to be clinically significant. However, the 16S rRNA gene sequences of some distinct Nocardia species have been shown to be quite similar; DNA-DNA hybridization studies have shown that even species with as much as 99.8% 16S rRNA gene sequence similarity (as seen with N. veterana and N. kruczakiae) can be distinct species (2, 12). The MicroSeq 500 sequencing system (Applied Biosystems, Foster City, CA) has been shown to be useful for the identification of many species of Nocardia (1); however, analysis of more than 500 bp may be necessary to clearly differentiate some species, depending on the degree of base divergence which is considered acceptable for conspecific isolates. Restriction endonuclease analysis using portions of the 16S rRNA gene and the 65-kDa heat shock protein gene has been used in the past for the identification of commonly isolated Nocardia species (4, 10). However, the usefulness of this procedure is becoming limited (8) due to the need to determine restriction fragment length polymorphisms (RFLPs) for the expanding number of described pathogenic species and the increasing number of restriction endonucleases required to make the species distinctions among these species. Therefore, sequence analysis of an alternative gene appears to be a viable adjunct to, or even a substitute for, 16S rRNA gene sequencing for the precise identification of Nocardia species.
The SecA1 protein is an essential component of the preprotein translocase ATPase that provides the driving force for the export of proteins across the bacterial cytoplasmic membrane (9). It has recently been shown that sufficient variability exists in the sequence of the secA1 gene of mycobacteria to allow discrimination of 29 species (13). We describe here a novel method of distinguishing 29 species or taxa of Nocardia, using sequence analysis of both a portion of the secA1 gene and the deduced amino acid sequence.
MATERIALS AND METHODS
Type and reference strains.
The type and reference strains evaluated are listed in Table 1.
TABLE 1.
Type and reference strains of Nocardia species used in the evaluation of the secA1 gene sequence
| Species | Type or reference strain | GenBank accession no.
|
|
|---|---|---|---|
| secA1 gene | 16S rRNA gene | ||
| Nocardia abscessus | ATCC BAA-279T | DQ360260 | DQ659895 |
| Nocardia africana | DSM 44491T | DQ360261 | AY089701 |
| Nocardia arthritidis | DSM 44731T | DQ360262 | DQ659896 |
| Nocardia asiatica | DSM 44668T | DQ360263 | DQ659897 |
| Nocardia asteroides | ATCC 19247T | DQ360267 | DQ659898 |
| Nocardia asteroides drug pattern IV | ATCC 49872 | DQ360265 | DQ659899 |
| Nocardia asteroides drug pattern VIa | ATCC 14795 | DQ360266 | DQ659900 |
| Nocardia beijingensis | JCM 10666T | DQ360268 | DQ659901 |
| Nocardia brasiliensis | ATCC 19296T | DQ360269 | DQ659902 |
| Nocardia brevicatena | ATCC 15333T | DQ360270 | DQ659903 |
| Nocardia carnea | DSM 43397T | DQ360271 | AF430035 |
| Nocardia cyriacigeorgicaa | DSM 44484T | DQ360272 | DQ659904 |
| Nocardia elegans | DSM 44890T | DQ360273 | DQ659905 |
| Nocardia farcinica | ATCC 3318T | DQ360274 | DQ659906 |
| Nocardia ignorata | DSM 44496T | DQ360275 | DQ659907 |
| Nocardia inohanensis | DSM 44667T | DQ360276 | DQ659908 |
| Nocardia kruczakiae | ATCC BAA-948T | DQ360277 | DQ659909 |
| Nocardia niigatensis | DSM 44670T | DQ360278 | DQ659910 |
| Nocardia nova | ATCC 33726T | DQ360279 | DQ659911 |
| Nocardia otitidiscaviarum | ATCC 14629T | DQ360280 | DQ659912 |
| Nocardia paucivorans | ATCC BAA-278T | DQ360281 | DQ659913 |
| Nocardia pseudobrasiliensis | ATCC 51512T | DQ360282 | DQ659914 |
| Nocardia seriolae | JCM 3360T | DQ360284 | DQ659915 |
| Nocardia sienata | DSM 44766T | DQ360285 | AB121770 |
| Nocardia testacea | DSM 44765T | DQ360286 | AB121769 |
| Nocardia transvalensis | ATCC 6865T | DQ360287 | DQ659916 |
| Nocardia vaccinii | ATCC 11092T | DQ366276 | DQ659917 |
| Nocardia veterana | DSM 44445T | DQ360288 | DQ659918 |
| Nocardia vinacea | JCM 10988T | DQ360289 | DQ659919 |
| Nocardia yamanashiensis | DSM 44669T | DQ360290 | DQ659920 |
N. asteroides drug pattern VI and N. cyriacigeorgica are thought to be conspecific.
Patient isolates.
The secA1 gene of 40 patient isolates representing 12 species or species groups was sequenced. Isolates were obtained from patients being treated at the Clinical Center of the National Institutes of Health (15 isolates), the University of Maryland Hospital, Baltimore, Maryland (1 isolate), the Children's Hospital and Regional Medical Center, Seattle, Washington (1 isolate), Hennepin County Medical Center, Minneapolis, Minnesota (1 isolate), or the Walter Reed Army Medical Center, Washington, D.C. (1 isolate), or were isolates referred for identification to the Microbiology Laboratory of the Maryland State Department of Health and Hygiene, Baltimore, Maryland (1 isolate), ARUP Laboratories, Salt Lake City, Utah (12 isolates), or the University of Texas Health Center at Tyler, Tyler, Texas (8 isolates) (Table 2).
TABLE 2.
Similarity of secA1 gene, deduced SecA1 amino acid, and 16S rRNA gene sequences for 40 clinical isolates of Nocardia species compared to type or reference strains
| Species | No. of isolates | % Similarity to type or reference strain (no. of base or amino acid differences)
|
||
|---|---|---|---|---|
|
secA1 gene
|
16S rRNA gene (DNA sequence) | |||
| DNA sequence | Amino acid sequence | |||
| N. abscessus | 2 | 96.0 (19) | 99.4 (1) | 100 (0) |
| 3 | 96.2 (18) | 99.4 (1) | 100 (0) | |
| N. asteroides drug pattern IV | 1 | 99.8 (1) | 100 (0) | 99.9 (1) |
| 2 | 100 (0) | 100 (0) | 99.9-100 (0-1) | |
| N. asteroides drug pattern VI | 2 | 97.2 (13) | 99.4 (1) | 100 (0) |
| 1 | 99.6 (2) | 100 (0) | 100 (0) | |
| 1 | 100 (0) | 100 (0) | 100 (0) | |
| N. beijingensis | 2 | 95.3 (22) | 99.4 (1) | 99.9-100 (0-1) |
| N. brasiliensis | 1 | 97.0 (14) | 98.7 (2) | 99.7 (4) |
| 2 | 99.2 (4) | 99.4 (1) | 99.9 (1) | |
| 1 | 99.6 (2) | 100 (0) | 100 (0) | |
| N. elegans | 1 | 97.7 (11) | 98.7 (2) | 100 (0) |
| N. farcinica | 2 | 99.0 (5) | 100 (0) | 100 (0) |
| 3 | 99.6 (2) | 100 (0) | 99.9-100 (0-1) | |
| N. kruczakiae | 3 | 99.8 (1) | 100 (0) | 99.9 (1) |
| N. nova | 1 | 99.4 (3) | 100 (0) | 99.5 (7) |
| 1 | 99.6 (2) | 100 (0) | 99.8 (3) | |
| 2 | 99.8 (1) | 100 (0) | 99.9-100 (0-1) | |
| N. otitidiscaviarum | 1 | 99.8 (1) | 99.4 (1) | 99.9 (1) |
| 1 | 100 (0) | 100 (0) | 99.9 (1) | |
| N. pseudobrasiliensis | 1 | 99.6 (2) | 99.4 (1) | 100 (0) |
| 2 | 99.8 (1) | 100 (0) | 99.9-100 (0-1) | |
| 1 | 100 (0) | 100 (0) | 100 (0) | |
| N. veterana | 1 | 99.4 (3) | 100 (0) | 100 (0) |
| 2 | 99.8 (1) | 100 (0) | 99.9-100 (0-1) | |
Identification of clinical isolates.
Clinical isolates were identified to the species level using sequence analysis of the 16S rRNA gene (3).
Molecular analysis of the secA1 gene.
DNA was extracted from the type or reference strains and the clinical isolates of Nocardia as previously described (4). A region of the secA1 gene (corresponding to bases 444 to 913 of the secA1 gene sequence of N. farcinica IFM 10152 [7]) was amplified using secA1-specific primers with tails containing M13 binding sites. The sequences of the primers were (sequence of the tail is indicated in bold type): 5′ GTA AAA CGA CGG CCA GGA CAG YGA GTG GAT GGG YCG SGT GCA CCG 3′ and 5′ CAG GAA ACA GCT ATG ACG CGG ACG ATG TAG TCC TTG TC 3′ (Midland Certified Reagent Company, Crawford, Texas). PCR was performed using a reaction mixture containing 2.5 mM MgCl2, 1× LightCycler-FastStart DNA Master HybProbe (Roche, Mannheim, Germany), 1 pmol of each primer, and approximately 0.2 μg of extracted DNA and ultrapure water to a final volume of 25 μl or with PuReTaq Ready-To-Go PCR beads (GE Healthcare, Fairfield, Conn.), using 1 pmol of each primer. The DNA was denatured at 95°C for 5 min and then subjected to 35 cycles of amplification (95°C for 1 min, 60°C for 1 min, and 72°C for 1 min), followed by a 10-min extension at 72°C. Aliquots (8 μl) of the resulting PCR amplification products were electrophoresed on 2% Tris acetate-EDTA gels (SeaKem GTG; Cambrex, East Rutherford, New Jersey). The resulting bands were dissected, and the DNA was purified using the GFX PCR DNA and gel band purification kit (GE Healthcare). Cycle sequencing was performed using M13-20 forward (5′ GTA AAA CGA CGG CCA G 3′) and M13 reverse (5′ CAG GAA ACA GCT ATG AC 3′) primers (Midland Certified Reagent Company). All cycle sequencing reactions were performed with the ABI Prism BigDye Terminator cycle sequencing Ready Reaction kit (PerkinElmer Applied Biosystems, Foster City, Calif). Excess dye terminators were removed by ethanol-sodium acetate precipitation according to the guidelines of the manufacturer. Fluorescence-based sequence analysis of the extension products was performed with the ABI 3100 genetic analyzer (Applied Biosystems/Hitachi, Foster City, Calif). The resulting sequences were assembled using Lasergene SeqMan II software (DNA Star, Inc., Madison, Wis.), and sequences were aligned, amino acid sequences deduced, and phylogenetic trees prepared using the CLUSTAL W algorithm with Lasergene MegAlign software (DNA Star, Inc.).
Molecular analysis of the 16S rRNA gene.
16S rRNA gene sequences were determined as previously described (3) or were obtained from GenBank (N. carnea AF430035, N. sienata AB121770, and N. testacea AB12169). For sequence comparison, all sequence lengths were adjusted to match the length of the shortest sequence (1,285 bp).
Nucleotide sequence accession numbers.
Partial secA1 and 16S rRNA gene sequences of the Nocardia type or reference strains were deposited in GenBank under accession numbers DQ360260 to DQ360263, DQ360265 to DQ360282, DQ360284 to DQ360290, DQ366276, and DQ659895 to DQ659920. The accession numbers are listed with the respective type and reference strains in Table 1.
RESULTS
secA1 gene sequences of type and reference strains.
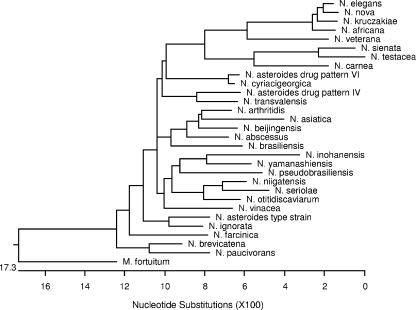
Sequence analysis and alignment of a 468-bp region of the secA1 gene for each of the type and reference strains of Nocardia species showed significant base diversity within the entire gene region among all isolates. Base divergence was significant enough to allow good separation of all strains evaluated (Fig. 1). For all type and reference strains, sequence similarity to the next most similar species ranged from 85.0 to 98.7% for the secA1 gene, compared to 94.4 to 99.8% similarity for a 1,285-bp region of the 16S rRNA gene. Species pairs previously reported to be highly similar by 16S rRNA gene sequencing (N. brevicatena/N. paucivorans, 99.5% similar; N. sienata/N. testacea, 99.7% similar; and N. kruczakiae/N. veterana, 99.8% similar) showed greater sequence diversity with the secA1 gene sequence, with 95.3, 95.7 and 91.9% similarity for the secA1 gene, respectively. N. asteroides drug pattern type VI showed 99.1% secA1 gene sequence similarity to the type strain of N. cyriacigeorgica (100% 16S rRNA gene similarity).
FIG. 1.
Phylogenetic tree of secA1 gene sequences of type and reference strains of Nocardia species.
The secA1 gene sequence of N. vaccinii, a plant pathogen shown to have considerable 16S rRNA gene sequence similarity to members of the N. nova complex (98.1 to 98.5% similarity), showed a total of 63 base insertions clustered in three areas of the gene region analyzed, resulting in an amplified region of 531 bp compared to the 468-bp region observed for other species. For N. vaccinii, the secA1 gene sequence was only between 69.3% and 76.5% similar to those of other Nocardia type or reference strains tested (data not shown).
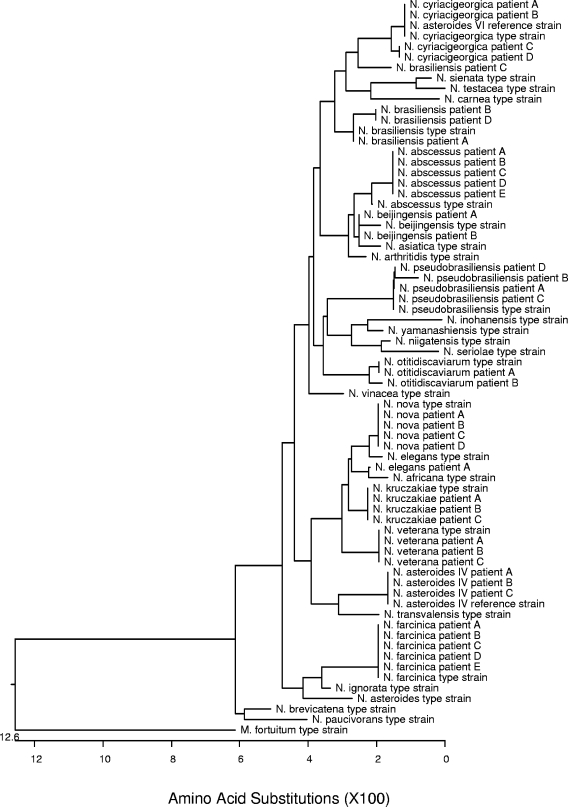
Alignment of the deduced amino acid sequence (comprised of 156 amino acid residues) of the 468-bp secA1 gene region showed good separation of all type and reference strains of Nocardia. Each type or reference strain showed a unique amino acid sequence, with similarities among the strains ranging from 91.0% (14 amino acid differences) to 99.4% (1 amino acid difference) (Fig. 2).
FIG. 2.
Phylogenetic tree of deduced SecA1 amino acid sequences of type and reference strains and clinical isolates of Nocardia species.
secA1 gene sequences of clinical isolates.
The secA1 gene sequences of 24 clinical isolates belonging to N. asteroides drug pattern IV, N. farcinica, N. kruczakiae, N. nova, N. otitidiscaviarum, N. pseudobrasiliensis, and N. veterana showed >99.0% similarity to the secA1 gene sequence of the type strain of the species to which they were determined to belong when analyzed by 16S rRNA gene sequencing. For clinical isolates belonging to the same species, the secA1 gene sequence diversity was greater than the sequence diversity seen with the 16S rRNA gene sequences (Table 2). The deduced amino acid sequences of those 24 isolates belonging to the above-mentioned species showed all isolates to be between 99.4 and 100% similar to that of the type strain (zero to one amino acid difference) (Table 2).
Seven isolates determined to belong to N. abscessus and N. beijingensis by 16S rRNA gene sequence analysis showed <99.0% secA1 gene sequence similarity to the type strains of the respective species. However, analysis of the deduced amino acid sequence for these isolates showed 99.4% similarity (one amino acid difference) to the amino acid sequences of the respective type strains.
Gene sequences of three of four isolates determined to belong to N. brasiliensis by 16S rRNA gene sequencing showed >99.0% and 99.4% similarity to the secA1 gene sequence and the deduced amino acid sequence, respectively, of the N. brasiliensis type strain. Gene sequences of one isolate determined to be N. brasiliensis (99.7% 16S rRNA gene sequence similarity to the N. brasiliensis type strain) showed only 97.0% and 98.7% similarity to the secA1 gene sequence and the deduced amino acid sequence (two amino acid differences), respectively, of the type strain of N. brasiliensis.
Two of four isolates identified as N. asteroides drug pattern VI (which is probably the same as N. cyriacigeorgica) showed >99.0% secA1 gene sequence similarity and 100% amino acid sequence similarity to the N. cyriacigeorgica type strain. Two additional isolates showed 97.2% and 99.4% secA1 gene and amino acid sequence similarities, respectively, to the type strain.
One isolate that was identified as N. elegans by 16S rRNA gene sequencing (100% sequence similarity) was more closely related to N. africana than to N. elegans by secA1 gene sequencing (98.1 and 97.7% similarity, respectively); the deduced amino acid sequence of the patient isolate showed two amino acid differences compared to the amino acid sequence of the type strain of N. elegans and one amino acid difference compared to that of the type strain of N. africana.
DISCUSSION
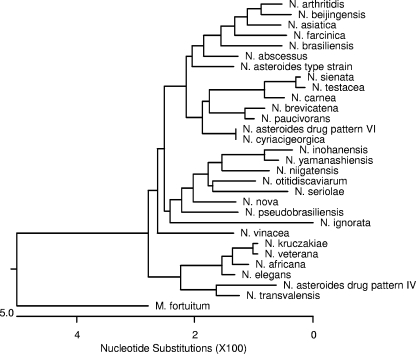
The results presented here show that secA1 gene sequence analysis gives good separation of all of the clinically relevant type and reference strains of Nocardia studied and is able to provide finer species distinctions among closely related species than 16S rRNA gene sequence analysis. With the exception of N. asteroides drug pattern VI and N. cyriacigeorgica (see below), all other type and reference strains examined showed no more than 98.7% sequence similarity to the next most closely related species (data not shown). The most distantly related species pairs showed 85.0% sequence similarity. This range of interspecies diversity among type and reference strains is considerably larger than that observed for the same species by analysis of the 1,285-bp region of the 16S rRNA gene, which showed a range of 94.4 to 99.8% similarity (data not shown).
The reference strain of N. asteroides drug pattern VI and the type strain of N. cyriacigeorgica show 100% 16S rRNA gene sequence similarity, 99.1% secA1 gene sequence similarity, and 100% SecA1 amino acid sequence similarity. Although results of DNA-DNA hybridization have not been published, the data presented here provide further evidence that these two strains most likely belong to the same species.
Members of the N. nova complex (N. africana, N. kruczakiae, N. nova, N. veterana [2], and probably N. elegans) are nearly indistinguishable by phenotypic methods and, in most cases, have been shown to share a very high level of 16S rRNA gene similarity (Table 3). Most of the secA1 gene sequences of these species also show a comparatively high level of secA1 gene sequence similarity (Table 3); N. veterana is the most divergent, with <92.0% similarity to any of the other species in the complex. The type strain of N. nova is more closely related to other members of the complex by secA1 gene sequencing than by 16S rRNA gene sequencing (Table 3).
TABLE 3.
Comparisons of the similarities of the 16S rRNA gene, the secA1 gene, and the SecA1 protein of members of the N. nova complexa
| Species | No. of base pair or amino acid differences (% similarity)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
N. elegans
|
N. kruczakiae
|
N. nova
|
N. veterana
|
|||||||||
| 16S rRNA gene | secA1 gene | SecA1 protein | 16S rRNA gene | secA1 gene | SecA1 protein | 16S rRNA gene | secA1 gene | SecA1 protein | 16S rRNA gene | secA1 gene | SecA1 protein | |
| N. africana | 6 (99.5) | 8 (98.3) | 3 (98.1) | 10 (99.2) | 12 (97.4) | 3 (98.1) | 22 (98.3) | 12 (97.4) | 3 (98.1) | 8 (99.4) | 39 (91.7) | 4 (97.5) |
| N. elegans | 14 (98.9) | 8 (98.3) | 2 (98.7) | 26 (98.0) | 6 (98.7) | 1 (99.4) | 12 (99.1) | 38 (91.9) | 3 (98.1) | |||
| N. kruczakiae | 26 (98.0) | 10 (97.9) | 2 (98.7) | 2 (99.8) | 38 (91.9) | 3 (98.1) | ||||||
| N. nova | 26 (98.0) | 38 (91.9) | 3 (98.1) | |||||||||
16S rRNA gene, 1,285 bp; secA1 gene, 468 bp; SecA1 protein, 156 amino acids.
Analysis of the secA1 gene may have usefulness in defining some phylogenetic relationships among Nocardia species. N. vaccinii, a pathogen of blueberries (6), has been shown to be most closely related to members of the N. nova complex by 16S rRNA gene sequencing. Analysis of the secA1 gene sequence resulting from amplification with the primers noted above shows insertions of a total of 63 bases in three regions of the gene, resulting in no more than 76.5% similarity to any clinically relevant Nocardia species. These additional bases presumably change the size and structure of the resulting protein, reflecting the different ecologic niche of this organism.
Analysis of the secA1 gene sequence of clinical isolates showed few isolates belonging to the same species to have identical gene sequences (data not shown). However, 27 of the 40 clinical isolates showed >99.0% similarity (zero to four base differences in a 468-bp region) to the corresponding type strain, resulting in identical deduced amino acid sequences or amino acid sequences with one residue difference (Fig. 2). Two N. farcinica clinical isolates showed five base pair differences compared to the N. farcinica type strain (99.0% similarity), with a deduced amino acid sequence identical to that of the type strain. An additional nine isolates (belonging to N. abscessus, N beijingensis, and N. cyriacigeorgica) showed significant secA1 gene sequence divergence from their corresponding type strains (13 to 22 base differences); however, the deduced amino acid sequences of these isolates differed by only one amino acid residue from the type strain. This can be explained by the fact that some amino acids are determined by more than one 3-base codon. It appears that this secA1 protein-coding gene in Nocardia has diversified along species lines but has not resulted in significant alteration of protein structure and, therefore, protein function, at least for the human pathogens evaluated.
With the limited number of clinical isolates of Nocardia studied, it appears that evaluation of the amino acid sequences of the SecA1 protein gives the most unambiguous identification. Further study with additional clinical isolates will be necessary to verify this conclusion.
Only two clinical isolates gave results with secA1 gene and amino acid analyses that suggested that the identification based on 16S rRNA gene sequencing might be incorrect. One isolate determined to belong to N. brasiliensis by 16S rRNA gene sequencing (99.7% similarity of 1,285 bases to the N. brasiliensis type strain) showed 14 secA1 gene base differences compared to the type strain of N. brasiliensis and showed a deduced amino acid sequence which differed from that of the type strain by two amino acid residues. In spite of these differences, this isolate was more similar to N. brasiliensis than to any other Nocardia species studied. One isolate identified as N. elegans (100% 16S rRNA gene similarity to the N. elegans type strain) was more closely related to the N. africana type strain than to the N. elegans type strain when the secA1 gene sequence and the amino acid sequence were evaluated. In both of these cases, DNA-DNA hybridization is needed to determine the precise species identifications.
Phylogenetic trees of the type strains of Nocardia for both the 16S rRNA gene and the secA1 gene showed similar relationships among most of the type or reference strains (Fig. 1 and 3). Some differences between the secA1 phylogenetic tree and that of the 16S rRNA gene include the placement of N. nova within a clade containing other members of the N. nova complex in the secA1 phylogenetic tree, the distinct placement of N. farcinica on a separate branch of the secA1 tree, the separation of N. abscessus and the type strain of N. asteroides on the secA1 tree, and the different relationships of N. ignorata, N. pseudobrasiliensis, and N. niigatensis to other species on the two trees. These differences may reflect the phenotypic and/or pathogenic differences that exist among these species; for example, N. nova is phenotypically similar to other members of the N. nova complex (2) and N. farcinica is known to be particularly resistant to antibiotics and the species most likely to cause disseminated disease (11).
FIG. 3.
Phylogenetic tree of 16S rRNA gene sequences of type and reference strains of Nocardia species.
Unlike the 16S rRNA gene in which there has been shown to be multiple differing copies, at least in some isolates, there is little evidence to suggest that multiple different copies of the secA1 gene exist in Nocardia species (5). Although it is unclear what the effect of multiple copies of the 16S rRNA gene has on accurate species identification, the use of the secA1 gene sequence for the identification of such isolates may provide more reliable results than can be obtained with 16S rRNA gene sequencing.
Although sequence analysis of a 500-bp region of the 16S rRNA gene is adequate for the identification of numerous Nocardia species, extended sequence analysis may be required for the identification of some isolates from closely related species, requiring multiple sequencing reactions or more-complicated cloning and sequencing. The analysis of the 468-bp region of the secA1 gene is sufficient for the identification of all pathogenic species of Nocardia analyzed, representing a more efficient and cost-effective molecular method than extended 16S rRNA gene sequencing. However, the use of this gene will require an expanded database of secA1 gene sequences from clinical isolates to provide a true evaluation of the genetic diversity of this gene.
The use of the secA1 gene may ultimately be most useful as part of a multigene approach to the identification of Nocardia isolates from human infections. Previous studies showed that the use of RFLP analysis of both the 16S rRNA and 65-kDa heat shock protein gene was useful for the identification of new and/or unusual Nocardia species (4). An identical identification obtained from sequence data from two or more gene targets may increase the confidence level of identifications obtained for closely related species and may allow detection of additional new species.
Acknowledgments
We thank the following for providing isolates for this study: Barbara Brown-Elliott, University of Texas Health Center at Tyler, Tyler, Texas; Charles Cartwright, Hennepin County Medical Center, Minneapolis, Minnesota; Joann L. Cloud, ARUP Institute for Clinical and Experimental Pathology, ARUP Laboratories, Salt Lake City, Utah; Joel T. Fishbain, Walter Reed Army Medical Center, Washington, D.C.; Nancy Hooper, Mycobacteriology Laboratory, Maryland State Health Department, Baltimore, Maryland; Patrick R. Murray, formerly of the University of Maryland Hospital, Baltimore, Maryland; and Xuan Qin, Children's Hospital and Regional Medical Center, Seattle, Washington. We thank Patrick R. Murray, Department of Laboratory Medicine, Warren G. Magnuson Clinical Center, NIH, for critically reviewing the manuscript.
The views expressed here are those of the authors and should not be construed as those of the U.S. Department of Health and Human Services.
This research was supported by the Intramural Research Program of the NIH, Warren G. Magnuson Clinical Center.
REFERENCES
- 1.Cloud, J. L., A. Croft, P. S. Conville, F. G. Witebsky, R. Yih, H. Chun, M. Martin, S. Ohlson, S. Hegewald, and K. C. Carroll. 2002. Evaluation of partial 16S ribosomal DNA sequencing for identification of Nocardia species by using the MicroSeq 500 system with an expanded database. J. Clin. Microbiol. 42:578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conville, P. S., J. M. Brown, A. G. Steigerwalt, J. W. Lee, V. L. Anderson, J. T. Fishbain, S. M. Holland, and F. G. Witebsky. 2004. Nocardia kruczakiae sp. nov., a pathogen in immunocompromised patients and a member of the “N. nova complex.” J. Clin. Microbiol. 42:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conville, P. S., J. M. Brown, A. G. Steigerwalt, J. W. Lee, D. E. Byrer, V. L. Anderson, S. E. Dorman, S. M. Holland, B. Cahill, K. C. Carroll, and F. G. Witebsky. 2003. Nocardia veterana as a pathogen in North American patients. J. Clin. Microbiol. 41:2560-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conville, P. S., S. H. Fischer, C. P. Cartwright, and F. G. Witebsky. 2000. Identification of Nocardia species by restriction endonuclease analysis of an amplified portion of the 16S rRNA gene. J. Clin. Microbiol. 38:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conville, P. S., and F. G. Witebsky. 2005. Multiple copies of the 16S rRNA gene in Nocardia nova isolates and implications for sequence-based identification procedures. J. Clin. Microbiol. 43:2881-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaree, J. B., and N. R. Smith. 1952. Nocardia vaccinii n. sp. causing galls on blueberry plants. Phytopathology 42:249-252. [Google Scholar]
- 7.Ishikawa, J., A. Yamashita, Y. Mikami, Y. Hoshino, H. Kurita, K. Hotta, T. Shiba, and M. Hattori. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 101:14925-14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Nava, V., A. Couble, G. Devulder, J.-P. Flandrois, P. Boiron, and F. Laurent. 2006. Use of PCR-restriction enzyme pattern analysis and sequencing database for hsp65 gene-based identification of Nocardia species. J. Clin. Microbiol. 44:536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt, M. G., and K. B. Kiser. 1999. SecA: the ubiquitous component of preprotein translocase in prokaryotes. Microbes Infect. 1:993-1004. [DOI] [PubMed] [Google Scholar]
- 10.Steingrube, V. A., R. W. Wilson, B. A. Brown, K. C. Jost, Jr., Z. Blacklock, J. L. Gibson, and R. J. Wallace, Jr. 1997. Rapid identification of clinically significant species and taxa of aerobic actinomycetes, including Actinomadura, Gordona, Nocardia, Rhodococcus, Streptomyces, and Tsukamurella isolates, by DNA amplification and restriction endonuclease analysis. J. Clin. Microbiol. 35:817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace, R. J., Jr., M. Tsukamura, B. A. Brown, J. Brown, V. A. Steingrube, Y. Zhang, and D. R. Nash. 1990. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J. Clin. Microbiol. 28:2726-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yassin, A. F., F. A. Rainey, J. Burghardt, H. Brzezinka, M. Mauch, and K. P. Schaal. 2000. Nocardia paucivorans sp. nov. Int. J. Syst. Evol. Microbiol. 50:803-809. [DOI] [PubMed] [Google Scholar]
- 13.Zelazny, A. M., L. B. Calhoun, L. Li, Y. R. Shea, and S. H. Fischer. 2005. Identification of Mycobacterium species by secA1 sequences. J. Clin. Microbiol. 43:1051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]