Abstract
Pneumococcal surface protein A (PspA) has been considered a potential candidate for human vaccines because of its serotype-independent protective immunity. Nasopharyngeal (NP) pneumococcal colonization is highly prevalent in infants and precedes the invasive disease. Thus, prevention of NP colonization may reduce the burden of pneumococcal disease in children. Scarce information focusing on PspA from pneumococcal carriage in humans is available. We examined the genetic diversity of PspA from NP isolates obtained during an ongoing pneumococcal surveillance study with children. PspA families and clades of 183 community-acquired Streptococcus pneumoniae NP isolates from healthy children (n = 97) and children with respiratory tract infections (n = 48), pneumonia (n = 33), or meningitis (n = 5) were investigated. Overall, 79.8% (n = 146) of the pneumococcal isolates were classified as PspA family 1 (35.5%) and family 2 (44.3%), whereas 20.2% of the isolates could not be typed. The distribution of PspA families and clades did not differ significantly according to the clinical status of the children. A dendrogram comparing the genetic relationship between the amino acid sequences of the clade-defining region of PspA from NP strains together with 24 invasive reference strains (GenBank) closely reproduced the profile of the families and clades previously reported for pneumococcal invasive strains. These findings strengthen the idea that the use of PspA as a vaccine antigen may protect children against carriage as well as invasive pneumococcal disease.
The nasopharynx of children is the ecological reservoir of Streptococcus pneumoniae and is considered the birthplace of pneumococcal drug-resistant clones (14). Nasopharyngeal (NP) colonization by pneumococci precedes invasive disease and infections such as otitis media and pneumonia (6, 13). S. pneumoniae carriage is widely present among asymptomatic young children, who spread the bacteria to susceptible individuals.
The seven-valent polysaccharide-protein conjugate vaccine has been shown to be efficacious against invasive pneumococcal diseases (5); however, protection is limited to those serotypes included in the vaccine formulation. Furthermore, replacement by nonvaccine serotypes has been described following the introduction of mass immunization (4), and the vaccine has not achieved the desired success in reducing colonization by vaccine-related serotypes (18). Recently, Dagan et al. (12) demonstrated that a nine-valent pneumococcal conjugate vaccine was able to elicit a systemic humoral immune response which reduced the carriage rate by prevention of the new acquisition of S. pneumoniae but has no effect on the duration of carriage once a serotype is acquired. The low serotype coverage and the high cost of the pneumococcal conjugate vaccines have hampered their implementation, especially in developing countries, and have prompted researchers to investigate pneumococcal protein antigens as components of vaccines which could protect against multiple serotypes at lower costs.
Pneumococcal surface protein A (PspA) is a recognized virulence factor expressed by all pneumococcal isolates. In animal models, PspA is able to elicit a protective immune response against pneumococci with different capsular serotypes (7, 21, 22). There is evidence that protective epitopes reside in the amino-terminal portion of PspA. Differences in reactivity determined by the use of several monoclonal antibodies point to the complex and serological diversity of PspA (9). In 2000, Hollingshead et al. (17) investigated the genetic basis for the serological diversity of PspA; and using the divergence of the N-terminal α-helical region of pspA nucleotide sequences, the authors identified three families of pspA (cutoff value, 40%). The main region responsible for the diversity among PspAs comprises a sequence of approximately 100 amino acids located at the end of the α-helical N-terminal region, known as the clade-defining region. Analysis of this region further subdivided the families into clades that diverged from each other by over 20% and showed that PspAs within the same clade had greater than 90% identity. Thus, according to its genetic diversity, PspA has been classified into family 1 (Fam1; clades 1 and 2), family 2 (Fam2; clades 3, 4, and 5), and family 3 (Fam3; clade 6).
Immunization of humans with PspA has induced broadly cross-reacting antibodies to heterologous PspAs, and the cross-clade reactivity roughly follows the degree of similarity among the sequences, with a tendency for a higher degree of cross-reactivity among PspAs within the same family (24). Briles et al. (10) demonstrated that sera from PspA-immunized individuals protect mice from fatal infection with S. pneumoniae expressing PspAs of different families. Studies of the local and systemic immune responses against PspA have raised evidence that antibodies to PspA could prevent NP colonization in humans and mice (20, 25). Thus, on the basis of its immunogenic properties, PspA could be an alternative antigen in a vaccine formulation aimed at attaining a strong impact on the prevention of pneumococcal NP colonization, in addition to protecting against invasive disease.
In order to establish the possible use of PspA as a vaccine candidate antigen, it is crucial to know the total array of PspAs expressed in pneumococcal strains in the community. Few studies have investigated the genetic diversity of PspA (15, 23, 26, 27), and scarce information focusing on PspA from strains derived from carriage pneumococci has been published (8). We therefore conducted this study to investigate the PspA families and clades collected from nasopharyngeal carriage isolates of community-acquired S. pneumoniae in children during an ongoing surveillance. We have also examined the clade-defining region of PspAs obtained from NP and have underscored the degree of similarity among them. These are pivotal issues in pursuing a potential pneumococcal vaccine candidate which would protect against all pneumococcal isolates, regardless of their serotype.
MATERIALS AND METHODS
Study site and selection of participants.
The investigation was carried out in the municipality of Goiânia, which is in central Brazil. Nasopharyngeal swabs were collected from children during a prospective S. pneumoniae surveillance study. Details on the pneumococcal surveillance were described elsewhere (19). Briefly, during the winter period of May 2000 to August 2001, a single NP swab was collected from children less than 5 years old admitted to hospital with acute respiratory infection or meningitis and also from healthy children who attended the immunization program in the same health center as the hospitalized children. The swabs of the hospitalized children were collected at the time of hospital admission, before antimicrobial administration.
During the surveillance period, a total of 232 NP pneumococcal isolates were obtained. For this study, in order to determine the array of PspA types colonizing the nasopharynges of children, 183 (79%) S. pneumoniae isolates were typed from children with respiratory tract infections (n = 48), pneumonia (n = 33), or meningitis (n = 5) and also from healthy children (n = 97). Written informed consent was obtained from the parents or the guardians of the children. The study protocol was approved by the regional and national ethical committees.
Collection of NP swabs and microbiological analysis.
The technical procedures used for NP swab collection, specimen transport, storage, and culture of the samples followed the standard procedures for the detection of upper respiratory tract carriage of S. pneumoniae (28). The NP specimen was collected with a pernasal, extrathin, flexible, calcium alginate swab; placed in Stuart transport medium tubes (Transwab; Medical Wire & Equipment, Whiltshire, United Kingdom); and transported to the Laboratory of Bacteriology of the Federal University of Goiás. S. pneumoniae strains were identified by their colony appearance, alpha-hemolysis, morphology in Gram stain, optochin susceptibility, and the bile solubility test. Susceptibility tests were first performed by the disk diffusion method with Oxoid (Basingstoke, United Kingdom) disks, and the penicillin MICs for the penicillin-nonsusceptible pneumococci (PNSp) were determined by broth microdilution. The results were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards) (11). Pneumococcal serotyping was performed at the Instituto Adolfo Lutz, São Paulo, Brazil, by use of the Quellung reaction with sera produced by the Statens Seruminstitut, Copenhagen, Denmark.
PspA typing by PCR and sequencing.
The bacterial cell suspension from the pneumococci isolates was prepared from 5 ml of Todd-Hewitt liquid medium containing 0.5% yeast extract (THY), in which they had been incubated overnight at 37°C. The cultures were centrifuged and resuspended in 200 μl of THY and 20% glycerol. The suspension was stored at −20°C until it was tested. DNA standards from strains Rx1 (clade 2) and BG11703 (clade 4) were used as controls for Fam1 and Fam2, respectively, in each set of PCRs. The PCR was performed with oligonucleotide primers synthesized on the basis of the published DNA sequences for PspA Fam1 (primers LSM12 and SKH63); PspA Fam2 (primers LSM12 and SKH52); and PspA Fam1, Fam2, and Fam3 (primers LSM12 and SKH2) (15, 26). The volume of the PCR mixture was 25 μl and contained PCR buffer, 3.5 mM MgCl2, 200 μM deoxyribonucleotides, 50 pmol of each primer, and 1 U of recombinant Taq polymerase (Invitrogen). A volume of 2 μl of cell suspension was used as the template. The DNA amplification (MJ Research Inc.) with primer sets LSM12/SKH63 and LSM12/SKH52 was carried out under the following conditions: an initial cycle of 94°C (10 min) and then 30 cycles of 94°C (1 min), 62°C (1 min), and 72°C (3 min), followed by a cycle of 72°C (7 min). The PCR products were resolved by electrophoresis on a 1% agarose gel for 1 h at 120 V. The gels were stained with ethidium bromide and photographed under UV light. This first set of PCRs allowed the classification of strains into PspA Fam1 or Fam2. Another PCR was performed with oligonucleotide primers LSM12 and SKH2, with the annealing temperature decreased to 58°C (15). This second PCR should amplify pspA from all strains, including Fam3. The two sets of PCRs for pneumococcal isolates and for DNA standards showed consistent results for families (Fam1 and Fam2) and clades. After three PCR attempts, pneumococcal strains which were PCR negative by LSM12/SKH2 amplification were considered nontypeable. The PCR products from primer set LSM12/SKH2 were purified from agarose gels (GFX PCR DNA and Gel Band purification kit; Amersham Biosciences) for direct sequencing by using SKH2 as the primer. Automated sequencing reactions used dye terminator chemistry, and the sequences were analyzed with a model 3100 genetic analyzer (Applied Biosytems). The DNA sequences generated were submitted to GenBank, analyzed with the Chromas program, and submitted to analysis with the ORF (open reading frame) Finder. The predicted amino acid sequence was searched against the sequence database by using BLAST software (www.ncbi.nlm.nhi.gov/BLAST) (1).
Data analysis.
Descriptive analysis was performed with EpiInfo software (version 6.04; Centers for Diseases Control and Prevention, Atlanta, GA). Differences in proportion were compared by the chi-square test or Fisher's exact test, when appropriate. P values less than 0.05 were considered statistically significant. The prevalence and the corresponding 95% confidence interval were calculated.
The diversity of pspA (clades 1 to 6) was analyzed by comparing the predicted amino acid sequences found for NP pneumococcal strains with those amino acid sequences of the clade-defining region of 24 invasive reference sequences retrieved from GenBank (accession numbers AF071802 to AF071818, AF071820, AF071821, AF071823, AF071824, AF071826, U89711, and M74122) (17). The phylogenetic analysis was also done with the DNA sequences, and the corresponding dendrogram was compared to the one generated with the nucleotide sequences. The analysis of the genetic relatedness among PspAs was done with BioNumerics software (v 4.0; Applied Maths, Sint-Martens-Latem, Belgium). Cluster analyses with Pearson's coefficient and the hierarchical unweighted pair group method (UPGMA) were performed to generate a dendrogram of the relationship between clade-defining region B of the PspAs of the NP strains and the invasive reference strains. The UPGMA dendrogram prevails on the assumption that amino acid substitution rates are the same across all branches. This methodology uses a sequential clustering algorithm, in which local topological relationships are identified in order of similarity and the tree is built in a stepwise manner. A total of 1,000 bootstrap replicates were performed and bootstrap values equal to or greater than 95% were considered significant, whereas values from 70 to 94% were considered moderately significant.
Nucleotide sequence accession numbers.
The DNA sequences generated in this study have been submitted to GenBank (accession numbers DQ459217 to DQ459257).
RESULTS
PspA and capsular typing of NP pneumococcal isolates.
Among 183 pneumococcal carriage isolates, 146 (79.8%) were identified as belonging either to PspA Fam1 (35.5%) or to Fam2 (44.3%) by PCR, whereas 37 (20.2%) of the strains could not be typed (Table 1). The amino acid sequences of the NP-derived PspA clade-defining regions were matched with those obtained from sequences of pspA invasive reference strains (17). The analysis of the predicted sequences confirmed the results of PspA family typing and showed that clade 1 prevailed in Fam1 (20.8%) and that clade 3 predominated in Fam2 (22.4%), whereas clade 5 was rarely identified. The distribution of PspA families and clades did not differ significantly according to the clinical status of the children (Table 2).
TABLE 1.
PspA families and clades of 183 S. pneumoniae isolates from nasopharynges of children
| PspA typing | No. of isolates | % of isolates | 95% CIa |
|---|---|---|---|
| Family 1 | 65 | 35.5 | 28.6-42.9 |
| Clade 1 | 38 | 20.8 | 15.1-27.4 |
| Clade 2 | 27 | 14.0 | 9.4-19.7 |
| Family 2 | 81 | 44.3 | 36.9-51.8 |
| Clade 3 | 41 | 22.4 | 16.8-29.1 |
| Clade 4 | 36 | 19.7 | 14.2-26.2 |
| Clade 5 | 4 | 2.2 | 0.6-5.5 |
| PspA NTb | 37 | 20.2 | 14.6-26.8 |
CI, confidence interval.
NT, nontypeable.
TABLE 2.
Nasopharyngeal PspA clades by clinical status of 183 children
| PspA typing | Clade | No. (%) of isolates from children with the following clinical status:
|
|||
|---|---|---|---|---|---|
| Healthy children (n = 97) | RTIa (n = 48) | Pneumonia (n = 33) | Meningitis (n = 5) | ||
| Family 1 (n = 65) | 1 | 17 (17.5) | 11 (22.9) | 8 (24.2) | 2 (40.0) |
| 2 | 12 (12.4) | 7 (14.9) | 7 (21.2) | 1 (20.0) | |
| Family 2 (n = 81) | 3 | 17 (17.5) | 17 (35.4) | 6 (18.2) | 1 (20.0) |
| 4 | 20 (20.6) | 9 (18.8) | 6 (18.2) | 1 (20.0) | |
| 5 | 3 (3.1) | 1 (3.0) | |||
| NTb (n = 37) | 28 (28.9) | 4 (8.3) | 5 (15.2) | ||
RTI, respiratory tract infection.
NT, nontypeable PspA.
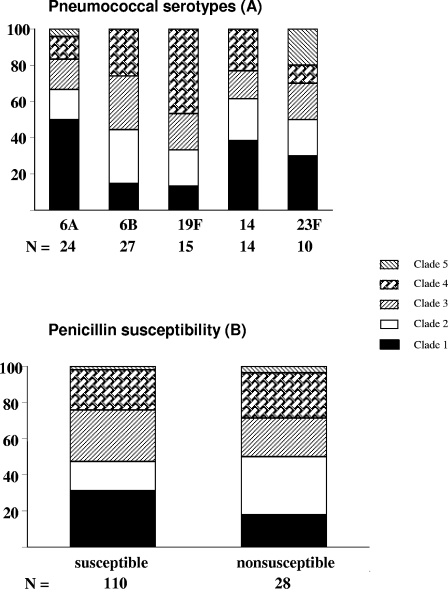
To evaluate possible differences in serotypes among the PspA families, 143 of 146 pneumococcal isolates were analyzed, yielding 24 different types. The most frequent serotypes were 6B (n = 27); 6A (n = 24); 19F (n = 15); 14 (n = 14); 23F (n = 10); 18C (n = 7); 18A (n = 6); and 9V, 9N, and 10A (n = 5 each). PspA could not be typed in 58.3% (7/12) of serotype 10A isolates and 35.7% (5/14) of serotype 14 isolates. The PspA clades among the five most frequent serotypes are shown in Fig. 1A. The diversity of PspA was higher in NP pneumococcal strains from serotypes 6A and 23F, which expressed all five clades. Clade 1 predominated in serotypes 6A and 18A; clade 3 prevailed in serotypes 9V, 9N, and 10A; and clade 4 predominated in serotypes 19F and 18C. To ascertain the penicillin susceptibility of PspA, 138 of 146 strains were assayed, yielding 28 (20.3%) PNSp isolates (serotypes 6B, 19F, 14, and 23F) with the following: clade 1 (17.9%), clade 2 (32.1%), clade 3 (21.4%), clade 4 (25.0.6%), and clade 5 (3.6%) (Fig. 1B).
FIG. 1.
Frequencies of nasopharyngeal PspA clades by capsular serotypes (A) and by penicillin susceptibility (B). Ninety nasopharyngeal pneumococcal isolates were investigated for their capsular serotypes by the Quellung reaction with sera from the Statens Seruminstitut, and PspA clades were investigated by PCR with oligonucleotide primers synthesized on the basis of the published DNA sequences for PspA Fam1 (primers LSM12 and SKH63) and PspA Fam2 (primers LSM12 and SKH52). The distribution of the PspA clades among the most frequent serotypes is shown in panel A. One hundred thirty-eight NP pneumococcal strains were investigated for their PspA clades by PCR and for their susceptibilities to penicillin by determination of the penicillin MIC by broth microdilution, with interpretation of the results by use of the criteria of CLSI. The distribution of PspA clades 1 to 5 among pneumococcal susceptible and nonsusceptible to penicillin is presented in panel B, and no significant difference (P < 0.05) was found when the susceptible and nonsusceptible NP pneumococcal strains in clades 1 to 4 were compared.
Diversity of nasopharyngeal PspA.
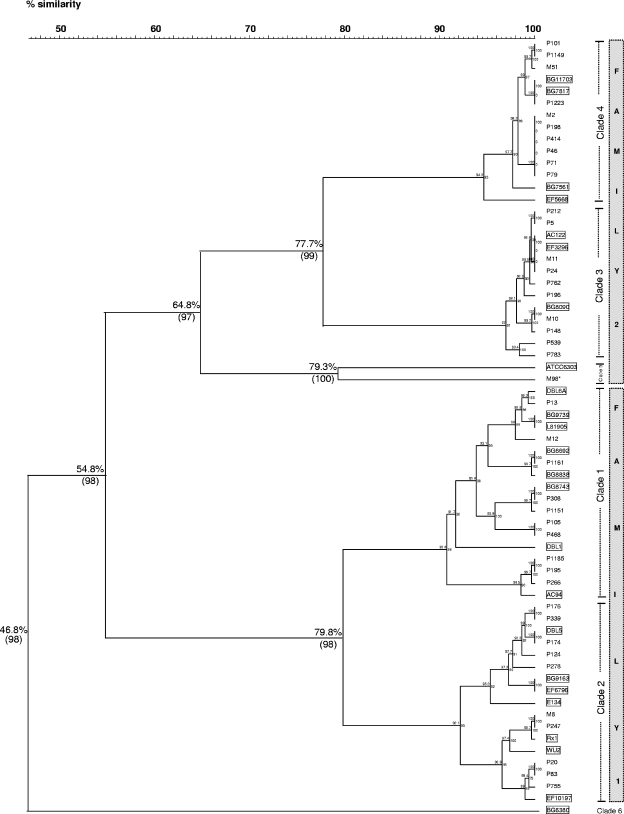
Only the complete sequences of the NP pspA clade-defining regions were used to assess their relatedness to those from invasive reference strains (GenBank). According to the dendrogram depicted in Fig. 2, the amino acid sequences of the 40 NP PspA isolates and the 24 invasive reference PspA isolates grouped in two large clusters that diverged over 45% with a 98% bootstrap value and that represented Fam1 and Fam2. In each family, two clusters were formed that diverged from each other by more than 20% (bootstrap values, 98 and 99%, respectively), represented by clades 1 and 2 (Fam1) and clades 3 and 4 (Fam2), respectively. For comparison purposes, we have also included in the dendrogram one PspA (strain M98) retrieved from the blood of one child with pneumococcal invasive disease. The sequence of strain M98 clustered with that from the ATCC 6303 invasive reference strain in clade 5, positioned in the extreme of Fam2. Within the same clade, the NP amino acid sequences and the invasive reference strains presented more than 90% identity. The clade 6 reference strain (strain BG6380; GenBank accession number AF071823) showed a high degree of divergence (53%) from all other strains and could not be matched to any PspA amino acid sequence of the NP data set. The dendrogram generated by the nucleotide sequences was similar to those obtained by grouping of the amino acid sequences (data not shown).
FIG. 2.
Dendrogram derived from comparison of 40 PspA-deduced amino acid sequences of nasopharyngeal strains and 24 sequences of PspA invasive reference strains (GenBank accession numbers AF071802 to AF071818, AF071820, AF071821, AF071823, AF071824, AF071826, U89711, and M74122). The dendrogram was constructed by using BioNumerics software (v 4.0). The names of the NP strains are indicated to the right of each branch point of the tree. Strain M98, marked with an asterisk, represents an invasive pneumococcal isolate from the blood of a child with meningitis. The invasive reference strains are boxed. Pearson's coefficient and the UPGMA clustering method were used for the analysis. Bootstrap values based on 1,000 replicates (in parentheses) and the percentages of similarity are distinguished for important nodes. The PspA families are represented by the gray column next to the corresponding clades.
DISCUSSION
To the best of our knowledge, this is the first report on the genetic diversity of PspA expressed by NP swab-derived S. pneumoniae strains obtained in a surveillance study. This is one of the strengths of this investigation, since our results are likely to represent the NP pneumococci circulating at the community level.
Concerning all NP pneumococcal isolates, the clades of PspA Fam1 and Fam2, except for clade 5, were detected at similar frequencies; clade 5 was rarely detected. These findings are in accordance with those of previous reports on invasive pneumococcal strains, in which the prevalence of PspA Fam1 ranged from 44.8 to 51.9% and that of Fam2 ranged from 43.2 to 55.2% (3, 8, 26). However, the predominance of PspA Fam1 (15, 17, 27) and the high prevalence of PspA Fam2 (85.7%) have been detected in some studies (2), mainly with adult populations. While a low proportion of nontypeable PspA has been detected among invasive pneumococcal strains, as reported in the literature (8, 16), we have found a high frequency of NP nontypeable PspA strains (20.2%), as has also been described in a previous study in Brazil, in which PspA could not be typed for 16.6% of the nasopharyngeal strains (8). Although PCR was performed three times and different strategies were tested, including pooling of cultures, amplification was not successful. In this carriage study, the nontypeable PspAs were mainly derived from serotype 10A strains, which grew poorly in cultures. Another strategy would be necessary to overcome this limitation in order to ascertain the PspA for this serotype, since pneumococcal serotype 10A is prevalent in NP carriers in our setting (19).
Our results showed no statistically significant difference between the prevalence of NP PspA families and clades in healthy and ill children, as recently reported in studies that were conducted with invasive pneumococcal isolates and that compared patients with and without sickle cell disease (26). Therefore, we could hypothesize a similar prevalence of PspA types in NP pneumococcal colonizers and invasive strains, reinforcing the usefulness of PspA as a promising candidate for the pneumococcal vaccine. The resistance to penicillin did not differ significantly among the NP pneumococcal strains expressing Fam1 and Fam2 or even clades 1, 2, 3, or 4, although in the previous study in Brazil, a significant tendency for the association of invasive PNSp strains with Fam2 was observed over the years (8). However, for unknown reasons, we found a high frequency of PspA Fam1, clade 2, in PNSp strains. Additional studies in Brazil and other countries could clarify these aspects. The NP PspA distribution was independent of the serotypes, which emphasizes the potential role of PspA as a vaccine antigen candidate that could induce protection against all S. pneumoniae serotypes.
The PspA dendrogram generated by grouping together the PspA sequences of NP and invasive reference strains closely reproduced the profiles of the families and clades reported by Hollingshead et al. (17). The families and clades were separated by divergences of over 40% and 20%, respectively. Clade 5 was more divergent from the other clades of Fam2, as reported previously (3, 17). As expected, most of the PspA sequences from NP or invasive strains presented over 90% similarity within the same clade; the exception was invasive strain M98, which diverged more than 20% from the invasive reference strain ATCC 6303 from clade 5. We are aware that only 40 of 146 PspA sequences entered into the dendrogram, and therefore, one could argue whether these strains are representative. However, since the 40 complete sequences of the PspA NP strains matched the 24 PspA sequences of the invasive strains, our assumption was that the alignment of the 146 PspA sequences would probably be similar.
Therefore, according to the diversity of the PspAs expressed by circulating NP pneumococcal strains in the community in Brazilian children, it is conceivable to anticipate that the degree of antibody cross-reactivity and/or cross-protection against different NP strains could be satisfactory and could prevent pneumococcal carriage. Furthermore, the data highlight the picture of the NP PspA array in relation to some invasive reference PspAs, indicating that these sequences share epitopes which could induce cross-immunity. The results strengthen the idea that the use of PspA, irrespective of whether it is derived from NP or invasive related strains, may protect children against carriage as well as invasive pneumococcal disease.
Acknowledgments
This investigation was sponsored by the National Council for Scientific and Technological Development/CNPq (grant MCT/CNPq/PADCT # 620023/2004-0). We are grateful to the Pan-American Health Organization (PAHO/WHO), Washington, D.C., for supporting the nasopharyngeal pneumococcal surveillance component of this investigation. A. L. S. S. de Andrade (grant 308043/2004-9), L. C. C. Leite (grant 351133/1997-6), and M. C. C. Brandileone (grant 303348/2004-6) are the recipients of fellowships from CNPq.
We thank Leonardo S. Kobashi for PspA sequence analysis.
REFERENCES
- 1.Altschul, S., F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baril, L., D. E. Briles, P. Crozier, J. King, M. Punar, S. K. Hollingshead, and J. B. McCormick. 2004. Characterization of antibodies to PspA and PsaA in adults over 50 years of age with invasive pneumococcal disease. Vaccine 23:789-793. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., G. Gherardi, R. R. Facklam, and S. K. Hollingshead. 2000. Pneumococcal PspA sequence types of the prevalent multiresistant pneumococcal strains in the United States and of internationally disseminated clones. J. Clin. Microbiol. 38:3663-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, R. Baxter, R. Austrian, L. Bracken, J. Hansen, E. Lewis, and B. Fireman. 2004. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr. Infect. Dis. J. 23:485-489. [DOI] [PubMed] [Google Scholar]
- 5.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 7.Bosarge, J. R., J. M. Watt, D. O. McDaniel, E. Swiatlo, and L. S. McDaniel. 2001. Genetic immunization with the region encoding the α-helical domain of PspA elicits protective immunity against Streptococcus pneumoniae. Infect. Immun. 69:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandileone, M. C. C., A. L. S. S. de Andrade, E. M. Teles, R. C. Zanella, T. I. Yara, J. L. DiFabio, and S. K. Hollingshead. 2004. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890-3896. [DOI] [PubMed] [Google Scholar]
- 9.Briles, D. E., R. C. Tart, E. Swiatlo, J. P. Dillard, P. Smith, K. A. Benton, B. A. Ralph, A. Brooks-Walter, M. J. Crain, S. K. Hollingshead, and L. S. McDaniel. 1998. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 11:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunizations of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing: 15th informational supplement. CLSI/NCCLS document M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 12.Dagan, R., N. Givon-Lavi, D. Fraser, M. Lipsitch, G. R. Siber, and R. Kohberger. 2005. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J. Infect. Dis. 192:367-376. [DOI] [PubMed] [Google Scholar]
- 13.de Andrade, A. L., F. C. Pimenta, M. C. Brandileone, C. A. Laval, M. L. Guerra, J. G. de Andrade, and J. L. Di Fabio. 2003. Genetic relationship between Streptococcus pneumoniae isolates from nasopharyngeal and cerebrospinal fluid of two infants with pneumococcal meningitis. J. Clin. Microbiol. 41:3970-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lencastre, H., and A. Tomasz. 2002. From ecological reservoir to disease: the nasopharynx, day-care centres and drug-resistant clones of Streptococcus pneumoniae. J. Antimicrob. Chemother. 50(Suppl. 2):75-81. [DOI] [PubMed] [Google Scholar]
- 15.Dicuonzo, G., G. Gherardi, R. E. Gertz, F. D'Ambrosio, A. Goglio, G. Lorino, S. Recchia, A. Pantosti, and B. Beall. 2002. Genotypes of invasive pneumococcal isolates recently recovered from Italian patients. J. Clin. Microbiol. 40:3660-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingshead, S. K., L. Baril, S. Ferro, J. King, P. Coan, D. E. Briles, and the Pneumococcal Proteins Epi Study Group. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215-221. [DOI] [PubMed] [Google Scholar]
- 17.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, S. S., R. Platt, S. L. Rifas-Shiman, S. I. Pelton, D. Goldmann, and J. A. Finkelstein. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408-e413. [DOI] [PubMed] [Google Scholar]
- 19.Laval, C. B., A. L. S. S. de Andrade, F. C. Pimenta, J. G. Andrade, R. M. de Oliveira, S. A. Silva, E. C. de Lima, J. L. Di Fabio, S. T. Casagrande, and M. C. C. Brandileone. 2006. Serotypes of carriage and invasive isolates of Streptococcus pneumoniae in Brazilian children in the era of pneumococcal vaccines. Clin. Microbiol. Infect. 12:50-55. [DOI] [PubMed] [Google Scholar]
- 20.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel, L. S., J. S. Sheffield, P. Delucchi, and D. E. Briles. 1991. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 59:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel, L. S., D. O. McDaniel, S. K. Hollingshead, and D. E. Briles. 1998. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect. Immun. 66:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollerach, M., M. Regueira, L. Bonofiglio, R. Callejo, J. Pace, J. L. Di Fabio, S. Hollingshead, D. Briles, and the Streptococcus pneumoniae Working Group. 2004. Invasive Streptococcus pneumoniae isolates from Argentinian children: serotypes, families of pneumococcal surface protein A (PspA) and genetic diversity. Epidemiol. Infect. 132:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of health adults with a single recombinant pneumococcal surface protein (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 25.Palaniappan, R., S. Singh, U. P. Singh, S. K. Sakthivel, E. W. Ades, D. E. Briles, S. K. Hollingshead, J. C. Paton, J. S. Sampson, and J. W. Lillard, Jr. 2005. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect. Immun. 73:1006-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne, D. B., A. Sun, J. C. Butler, S. P. Singh, S. K. Hollingshead, and D. E. Briles. 2005. PspA family typing and PCR-based DNA fingerprinting with BOX A1R primer of pneumococci from the blood of patients in the USA with and without sickle cell disease. Epidemiol. Infect. 133:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vela Coral, M. C., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, and D. E. Briles. 2001. Pneumococcal surface protein A of invasive isolates from Colombian children. Emerg. Infect. Dis. 7:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. 1994. Manual for the national surveillance of antimicrobial resistance of S. pneumoniae and H. influenzae: epidemiological and microbiological methods. Programme for the Control of Acute Respiratory Infections, Centers for Disease Control, Atlanta, Ga. [Online.] http://www.who.int/child-adolescent-health/New_Publications/CHILD_HEALTH /bact.htm.