Abstract
In common with other diagnostic tests, detection of mycobacteria in tissue by microscopic examination is susceptible to spectrum bias. Since Crohn's disease is defined by the absence of detectable pathogenic organisms, the use of in situ techniques to search for Mycobacterium avium subsp. paratuberculosis in Crohn's disease samples requires validation of methods in a paucibacillary setting. To generate paucibacillary infection, C57BL/6 mice were artificially infected with Mycobacterium avium subsp. paratuberculosis strain K10 and M. tuberculosis H37Rv, yielding tissues harboring fewer than one bacillus per oil immersion field. Serial sections of organs were then studied by cell wall-based staining techniques (Ziehl-Neelsen and auramine rhodamine) and nucleic acid-based staining techniques (in situ hybridization [ISH] and indirect in situ PCR [IS PCR]). Microscopic examination and measurement of morphometric parameters of bacilli revealed that for all methods, Mycobacterium avium subsp. paratuberculosis bacilli were observed to be shorter, smaller, and less rod shaped than M. tuberculosis bacilli. Ziehl-Neelsen, auramine rhodamine stains, ISH targeting rRNA, and IS-PCR targeting the IS900 element afforded comparable sensitivities, but for all methods, visualization of individual bacterial forms required magnification ×1,000. Auramine rhodamine staining and IS-PCR generated positive signals in negative controls, indicating the nonspecificity of these assays. Together, our results indicate that detection of Mycobacterium avium subsp. paratuberculosis bacilli in tissue requires oil immersion microscopy, that rRNA-ISH provides sensitivity and specificity comparable to those of Ziehl-Neelsen staining, and that the microscopic detection limit for Mycobacterium avium subsp. paratuberculosis in tissue is governed more by bacterial burden than by staining method.
For nearly a century, investigators have explored a potential link between Mycobacterium avium subsp. paratuberculosis and inflammatory bowel disease of humans (8). Efforts have focused particularly on Crohn's disease, largely stimulated by the histopathologic similarity between human Crohn's disease and certain forms of paratuberculosis (13). Although a number of different laboratory methodologies have been employed to search for Mycobacterium avium subsp. paratuberculosis in Crohn's disease, the published results have been conflicting, resulting in neither confirmation nor rejection of this hypothesis to date (41).
The recent availability of genetic and genomic data for Mycobacterium avium subsp. paratuberculosis offered promise that molecular diagnostic strategies might overcome the limitations of conventional microbiologic tests for this fastidious organism (45). The insertion element IS900, found at 14 to 18 copies per genome, has been widely used as a target for PCR (4-7, 17, 27, 30, 38, 39, 42, 44) and in situ hybridization (19-21, 37, 43), but for both applications, variable results have resulted in as many questions as answers (52). An important limitation of studies looking for novel pathogens is that information about sensitivity and specificity of assays applied is generally lacking. For Mycobacterium avium subsp. paratuberculosis, the greatest experience resides in laboratories that employ conventional and molecular methods for detecting the organism in cattle and sheep (16, 53). Although these methods can be directly applied to human samples, their utility in a human disease that is defined by the absence of detectable pathogens requires consideration of spectrum bias, both for optimizing techniques and for interpreting the results.
Spectrum bias, also known as spectrum effect, refers to the observation that the operating parameters of a diagnostic assay vary as a function of the disease state (28, 36). The impact of spectrum bias has been well established for the diagnosis of a number of infectious diseases (15, 23), including the mycobacterial diseases leprosy and tuberculosis (TB). In leprosy, the sensitivity of tissue microscopy for acid-fast bacilli depends on the bacterial burden and is therefore greater in lepromatous than tuberculoid disease (31). Likewise, the sensitivity of nucleic-acid-based tests is also influenced by the bacterial burden, as exemplified by the compromised sensitivity of PCR-based assays for sputum smear-negative TB (11, 22, 32). By extension, assays that reliably detect abundant Mycobacterium avium subsp. paratuberculosis organisms in livestock with Johne's disease may not provide sufficient sensitivity to study human Crohn's tissue.
To date, in situ studies of Mycobacterium avium subsp. paratuberculosis in Crohn's disease have validated their methods using naturally infected pluribacillary livestock samples (49), tissue blocks artificially injected with high numbers of Mycobacterium avium subsp. paratuberculosis bacteria (20), or serial dilutions of bacteria in vitro (37). In contrast, an assay for Mycobacterium avium subsp. paratuberculosis in Crohn's must be able to specifically detect small numbers of organisms within the tissue, near or below the threshold of microscopic detection. To address this, we have produced artificial mycobacterial infections in mice where the bacterial burden was near the threshold of microscopic detection. The availability of tissue with certain paucibacillary Mycobacterium avium subsp. paratuberculosis infection permitted us to directly compare the sensitivity of cell wall-based (Ziehl-Neelsen [ZN] and auramine rhodamine) and nucleic acid-based staining (in situ hybridization and indirect in situ PCR) methods. Additionally, tissue from uninfected and M. tuberculosis-infected animals permitted us to determine whether these same methods were prone to false-positive results in the absence of Mycobacterium avium subsp. paratuberculosis.
MATERIALS AND METHODS
Generation of M. avium paratuberculosis- and M. tuberculosis-infected tissue.
As part of separate studies on the relative virulence of Mycobacterium avium complex strains and the efficacy of different BCG vaccines, 8-week-old C57BL/6 mice were infected with Mycobacterium avium subsp. paratuberculosis strain K10 and with M. tuberculosis H37Rv. Phosphate-buffed saline (PBS) injections (100 microliters intravenously and intraperitoneally, respectively) provided uninfected control samples from animals housed in separate cages of the same facility. Mycobacterium avium subsp. paratuberculosis-infected mice were housed in the conventional rodent facility of the McGill University Health Centre Research Institute; M. tuberculosis infections were done in our level 3 containment facility, where mice were then housed and subsequently sacrificed. All procedures were approved by the Facility Animal Care Committee as recommended by the Canadian Council on Animal Care. Two separate experiments permitted us to establish the spectrum of bacterial burden in the organs. A total of 10 mice were infected by the intravenous and intraperitoneal routes with Mycobacterium avium subsp. paratuberculosis strain K10 (1 × 106 CFU in 0.1 ml PBS). Additionally, a group of five mice infected by the intravenous route with virulent M. tuberculosis strain H37Rv (1 × 106 CFU in 0.1 ml PBS) and five mice injected with PBS provided us with specificity controls. Finally, to compare the morphology of Mycobacterium avium subsp. paratuberculosis organisms in our murine infections to that with natural Johne's disease, tissue samples were obtained from culled sheep diagnosed with pluribacillary paratuberculosis based on the presence of granulomatous ileitis, demonstration of bacteria by Ziehl-Neelsen staining in the lesions, and detection of Mycobacterium avium subsp. paratuberculosis-specific DNA by IS900-based PCR.
Determination of CFU in mice organs.
At 4 and 8 weeks postinfection, mice were sacrificed by asphyxiation with carbon dioxide gas. For four mice from each experiment group, the spleen, lung, and liver were aseptically removed and weighed prior to processing for quantitative culture. Weighing the whole organ permitted us to calculate the bacterial burden per gram of tissue and thereby to estimate the bacterial burden of the fifth mouse that served for histological examination. Culture determinations followed standard procedures. In brief, organs were homogenized with a Polytron homogenizer (Glen Mills, Inc.) in sterile 50-ml tubes containing 4 ml isotonic saline. Dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H10 agar (Difco) enriched with 10% OADC (oleic acid, bovine serum albumin [fraction V], dextrose, and catalase) (Becton, Dickinson and Company) and mycobactin J (2 μg/ml) (Allied Monitor, Inc.). Plates were incubated at 37°C until colonies appeared, usually after 3 to 6 weeks. The identity of bacterial cultures was confirmed both before injection and after recovery from mice, using strain-specific genetic markers, described elsewhere (45).
Preparation of smears of cultures for cell wall-based and rRNA staining.
To estimate the morphometric parameters of bacillus, smears were made on microscopic slides using log-phase culture of Mycobacterium avium subsp. paratuberculosis strain K10 and Mycobacterium bovis BCG Russia. Smears were air dried and heat fixed and then subjected to auramine rhodamine staining. For rRNA staining, aliquots of log-phase cultures were centrifuged at 4,000 rpm for 15 min and washed twice in PBS. Ten microliters of resuspended pellet in PBS was spotted on silane (Sigma-Aldrich)-coated slides and air dried. Smears on the slides were fixed in 10% buffered formalin (Fisher) for 2 to 3 h, washed briefly in PBS, and immersed in ice-cold ethanol for 10 min at −20°C. Air-dried slides were then stored in a tight container at −20°C until use (35). The slides were dipped in xylene for 1 h at 60°C and then immersed in fresh xylene (30 min at 37°C) and rehydrated through graded alcohol (100%, 75%, 50%, and 25% ethanol diluted in water). Cells were rendered permeable by incubation for 10 min at room temperature in 0.02 M HCl and for 90 s in 0.01% Triton X-100. Then, proteins were depleted by incubation with proteinase K (Sigma-Aldrich) (2 μg/ml, for 30 min at 37°C). The proteinase was inactivated by addition of 0.2% glycine (Sigma-Aldrich). Free DNA in the cells was fixed with 4% buffered formalin to prevent washing off. Slides were washed in PBS and dehydrated through graded alcohol (25%, 50%, 75%, and 100%) and air dried.
Preparation of tissue for histology and microbiology.
For histological study, we removed from the fifth mouse of each group the lung, spleen, and liver and immersed them in 10% buffered formalin (Fisher) for 24 h prior to embedding in paraffin. Serial 3- to 4-μm sections from each tissue specimen of mice and sheep were cut, placed on silane-coated microscope slides (Sigma-Aldrich), and incubated in an oven at 50 to 60°C for 36 to 48 h to ensure maximum tissue adhesion on the slide. The first three serial sections were subjected to hematoxylin and eosin, auramine rhodamine, and ZN staining, respectively. Auramine rhodamine staining was performed using the TB Fluorostain kit (Polyscience, Inc.).
Preparation of tissue for mycobacterial DNA and rRNA detection by in situ assays.
For nucleic acid staining, tissue sections were processed in coplin jars as described for M. tuberculosis study by Hernandez-Pando and colleagues (18). Briefly, sections were deparaffinized (18 h at 60°C in xylene) and then immersed in fresh xylene (30 min at 37°C) and rehydrated through graded alcohol (100%, 75%, 50%, and 25% ethanol diluted in water). Cells were rendered permeable by incubation for 10 min at room temperature in 0 · 02 M HCl and for 90 s in 0.01% Triton X-100. Then, proteins were depleted by incubation with proteinase K (Sigma-Aldrich) (5 to 20 μg/ml for 30 min at 37°C). The proteinase was inactivated by addition of 0.2% glycine (Sigma-Aldrich). Endogenous alkaline phosphatase was inactivated by treatment with 20% acetic acid for 15 s. Free DNA in the cells was fixed with 4% formaldehyde to prevent washing off. Slides were washed in PBS, dehydrated through graded alcohol (25%, 50%, 75%, and 100%), and air dried.
Preparation of DIG-labeled probes.
Digoxigenin (DIG)-labeled double-stranded DNA probes for the Mycobacterium avium subsp. paratuberculosis-specific insertion element IS900 were synthesized according to the manufacturer's instructions using the PCR DIG probe synthesis kit (Roche). Primer sequences and PCR conditions used for probe synthesis are provided in Table 1. The PCR was performed in a total volume of 50 μl containing 5 μl of Mycobacterium avium subsp. paratuberculosis strain K10 template DNA (10 ng/μl) and 0.5 μM of each primer. Amplified products were purified using QIAGEN PCR purification kit (QIAGEN) and visualized after electrophoresis at 80 V for 90 min in a 2% agarose gel containing ethidium bromide. To confirm that the probes were synthesized correctly, amplified products were hybridized to genomic DNA of Mycobacterium avium subsp. paratuberculosis strain K10 and to PCR amplicons of primer pair L1/L2 (Table 1) blotted onto a nitrocellulose membrane (Hybond+; Amersham Biosciences) according to the manufacturer's instruction. The DNA concentration of the purified product was estimated using spectrometer, and the probe was used at a concentration of 1 ng/μl in the hybridization procedure.
TABLE 1.
PCR primers and PCR conditions to generate probes and target amplification in tissue for IS900-based assays
| Target sequence | Primer sequence (5′-3′) | Product size (bp) | PCR conditions | Reference |
|---|---|---|---|---|
| IS900 (for probe) | AV1: ATGTGGTTGCTGTGTTGGATGG | 298 | 35 cycles of 94°C, 1 min; 58°C, 1 min; | 29 |
| AV2: CTGGAGTTGATTGCGGCGG | 72°C, 3 min of 35 cycles plus final | |||
| extension at 72°C, 3 min | ||||
| IS900 (for IS-PCR) | L1: CTTTCTTGAAGGGTGTTCGG | 400 | Same, except annealing at 60°C | 29 |
| L2: ATGGAGGCGAGGTCACGT |
DIG-labeled double-stranded IS900 probe in situ hybridization.
Hybridization was performed as described by Hulten and colleagues (21). The tissue sections processed for nucleic acid staining were hybridized with denatured double-stranded DIG-labeled probe (1 ng/μl) in hybridization buffer (50% deionized formamide, 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 0.25 μg/μl of yeast tRNA [Sigma-Aldrich], 0.5 μg/μl of denatured salmon sperm DNA [heated for 10 min at 95°C and chilled for 10 min] [Sigma-Aldrich], and 1× Denhardt's solution [Sigma-Aldrich]). The probe was boiled for 10 min and cooled on ice for 10 min before application. The sections with the probe were sealed with Frame-Seal incubation chambers (MJ Research, Inc.), heated for 10 min at 95°C, and chilled for 10 min. The hybridization was performed overnight at 37°C on a flat block of a thermocycler (MJ Research, Inc.). The washing steps included washes with 2× and 1× SSC at room temperature for 15 min in each and with 0.3× SSC for 15 min at 40°C, followed by another wash at room temperature for 15 min with 0.3× SSC. This was followed by blocking of nonspecific sites with blocking buffer (3% bovine serum albumin, 100 mM Tris [pH 7.5], 150 mM NaCl, 0.3% Triton X-100) at room temperature for 30 min. Fresh blocking buffer containing a 1:300 dilution of antidigoxigenin-alkaline phosphatase Fab fragments (Roche) was added to the slides, and the slides were incubated at room temperature for 2 to 3 h. The slides were then washed with buffer 1 (100 mM Tris [pH 7.5], 150 mM NaCl, 0.3% Triton X-100) for 15 min, followed by another wash with buffer 2 (100 mM Tris [pH 9.5], 150 mM NaCl, 50 mM MgCl2) for 15 min. The slides were then incubated with 5-bromo-4-chloro-3-indolylphosphate-2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (Roche) (75 μl per 10 ml buffer 2 and 0.2 mg of levamisole [Sigma-Aldrich] per ml) in the dark at room temperature for 4 to 12 h. The reaction was stopped by washing the slides in distilled water. The slides were counterstained with nuclear fast red (Sigma-Aldrich), mounted with Cytoseal 60 (Richard-Allan Scientific), and examined under magnification ×1,000 with a bright-field microscope.
Indirect in situ PCR detection of internal fragment of IS900.
Sections processed for nucleic acid staining were subjected to PCR in order to amplify a 400-bp sequence of the IS900 insertion element containing the probe target sequence. The PCR was carried out by incubating the 4-μm section with 165 μl of PCR mixture (each 50 μl of PCR mixture contained 1× reaction buffer [AmpliTAq Gold; Applied Biosystems], 3 mM MgCl2, 200 μM of deoxynucleoside triphosphate, and 0.5 μM of each primer for IS900 (L1 and L2) and 12 U Taq DNA polymerase [AmpliTAq Gold; Applied Biosystems]) sealed with Frame-Seal incubation chambers (MJ Research, Inc.) on a flat block of a thermocycler (MJ Research, Inc.). Primer sequences and PCR conditions used are found in Table 1. After PCR, sections were washed in PBS for 1 min at room temperature and then subjected to in situ hybridization with the internal digoxigenin-labeled double-stranded IS900 probe as described above.
rRNA-specific oligonucleotide probe in situ hybridization.
Sequences of oligonucleotide probes used in this study are given in Table 2. Probes were obtained from Integrated DNA Technologies, Inc., and were labeled at the 5′ and 3′ ends with either 6-carboxyfluorescein, Cy3, or Texas Red. For in situ hybridization with rRNA-specific oligonucleotide probes, sections were subjected to hybridization as described by St Amand et al. (49) except that tissue sections were permeabilized by incubation in xylene overnight and digestion with proteinase K (Sigma-Aldrich) (5 to 20 μg/ml, for 30 min at 37°C). Smears of cultures and tissue sections processed for rRNA staining were then hybridized with probes at a final concentration of 1 ng/μl in the hybridization buffer (900 mM NaCl, 20 mM Tris [pH 8.0], 0.01% sodium dodecyl sulfate, and 20% formamide) in an MJ Research, Inc., flat block thermocycler. Hybridization temperatures for each probe are specified in Table 2. Following hybridization, the slides were washed with 225 mM NaCl, 5 mM EDTA, 0.01% sodium dodecyl sulfate, and 20 mM Tris (pH 8.0) for 20 min at either 39°C for M. tuberculosis-specific probes or 41°C for M. avium complex-specific and eubacterial probes. The slides were then immersed briefly in cold 20 mM Tris (pH 8.0) in order to remove excess salt. Colorimetric hybridization of the 6-carboxyfluorescein-labeled oligonucleotide probes was carried out as described by St Amand et al. (49) except that antifluorescein-labeled alkaline phosphatase Fab fragments (Roche) were used in a 1:300 dilution. Colorimetric signals were visualized using a Zeiss Axioscope microscope (Carl Zeiss). Tissue sections subjected to fluorimetric hybridization were mounted with Citifluor (Electron Microscopy Sciences) antifading reagent and for examination under an epifluorescence microscope (Nikon Eclipse E600 microscope).
TABLE 2.
rRNA-based probes used for in situ hybridization
| Probe | rRNA probe sequence (5′-3′) | Hybridization temp (°C) | Reference |
|---|---|---|---|
| M. avium | MAVP187ssu: TGC GTC TTG AGG TCC TAT CC (16S) | 40 | 49 |
| MAVP515lsu: TGT CCA TGC ATG CGG TTT (23S) | 40 | ||
| M. tuberculosis | MTB226: CCA CAC CGC TAA AGC GC (16S) | 38 | 48 |
| MTB187: TGC ATC CCG TGG TCC TAT CC (16S) | 38 | ||
| MTB770: CAC TAT TCA CAC GCG CGT (23S) | 38 | ||
| Eubacteria | EUB338: CTG CTG CCT CCC GTA GGA GT (16S) | 40 | 2 |
Controls for nucleic acid staining assays.
The specificity control for IS900 in situ hybridization, indirect in situ PCR (IS PCR), and rRNA-based probes consisted of performing the whole procedure with tissue sections from uninfected and M. tuberculosis-infected mice. Additional negative controls included hybridizing test samples with unlabeled probe targeting IS900 to determine whether aggregation of colorimetric dye could interfere with signal interpretation. For the rRNA hybridization study, uninfected and M. tuberculosis-infected mice served as negative controls, a universal eubacterial probe (EUB338) was used as an internal positive control, and M. tuberculosis-specific oligonucleotide probes (MTB226, MTB187, and MTB770) applied to Mycobacterium avium subsp. paratuberculosis-infected tissue served as an additional negative control.
Microscopic and photographic details.
To look for organisms, we first studied hematoxylin-and-eosin-stained slides by using a Zeiss Axioscope microscope (Carl Zeiss), to mark areas of inflammatory infiltrates. Corresponding sites on the slides stained with ZN were examined by light microscopy (Zeiss Axioscope microscope), using 40× and 100× objectives and a 10× eyepiece, resulting in total magnifications of ×400 and ×1,000, respectively, to visualize mycobacterial forms. To further characterize bacterial forms, auramine rhodamine-stained tissue sections and smear of in vitro-grown Mycobacterium avium subsp. paratuberculosis and M. bovis BCG were also examined under magnifications ×400 and ×1,000 using an epifluorescence microscope (Nikon Eclipse E600 microscope) equipped with a Retiga digital camera (QImaging Corporation). We then used captured images from this analysis to determine the visual attributes of bacillary forms. Using Emplix Imaging Northern Eclipse software (Empix Imaging, Inc.), we determined morphometric parameters for 31 M. tuberculosis bacilli in murine tissue, 30 M. bovis BCG organisms in a smear of log-phase in vitro culture, 37 Mycobacterium avium subsp. paratuberculosis bacilli, and 25 Mycobacterium avium subsp. paratuberculosis coccobacilli in murine tissue, 52 Mycobacterium avium subsp. paratuberculosis bacilli in ovine tissue, and 30 Mycobacterium avium subsp. paratuberculosis organisms in a smear of log-phase in vitro culture. These included the following: (i) the length of the bacilli, defined as the longest line through an object parallel to its orientation, (ii) the width, defined as the longest line through an object that is perpendicular to its orientation, (iii) the area, defined as the integrated zone of signal, and (iv) the shape factor, defined as 4π × area/perimeter2. The shape factor serves as a measure of circularity and is designed to produce a result of 1 for a perfect circle, 0.78 for a square, and 0.50 for a “4 × 1” rectangle. All color images were taken using Zeiss Axioscope microscopes equipped with an Axiocam MR digital camera (Carl Zeiss).
To directly compare the capacity to detect organisms with rRNA-based staining versus the ZN stain, we enumerated bacillary forms by examining 100 fields of ×1,000 oil immersion fields of sequential sections from the same blocks of liver and spleen. Numbers were compiled independently and were compared as organisms detected per granuloma by the two methods.
RESULTS
Characterization of bacterial burden in model infections.
Counts of CFU for livers and spleens from mice infected intraperitoneally with Mycobacterium avium subsp. paratuberculosis revealed 102 to 103 organisms per spleen and undetectable bacterial infection in the liver. This translated into less than 10 CFU per mg of spleen tissue. At this tissue burden, no mycobacteria could be detected in a thorough microscopic examination of a 4-μm acid-fast-stained tissue section using either ZN or auramine rhodamine-based study. In contrast, intravenous infection with Mycobacterium avium subsp. paratuberculosis and M. tuberculosis generated CFU that were approximately 2 logs higher, such that the bacterial burden was consistently between 102 and 103 CFU/mg of tissue. For studies of detection of Mycobacterium avium subsp. paratuberculosis presented below, the mean number of bacteria per mg of splenic tissue was 396 (standard errors of the mean [SEM] = 193). At this bacterial burden, approximately 5 to 10 mycobacteria were visualized per 25 oil immersion fields examined. Samples of this tissue were thus considered to represent paucibacillary mycobacterial infection and were used in the in situ studies detailed below.
Cell wall-based staining methods for detection of mycobacteria in tissue samples.
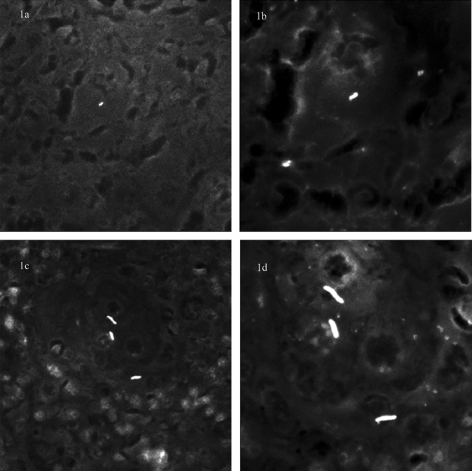
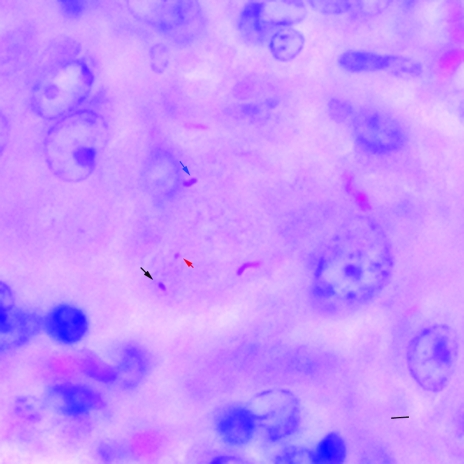
Examination of ZN-stained sections of samples from paucibacillary murine infections revealed that individual mycobacteria could only be visualized by careful examination under ×1,000 oil immersion. Occasional aggregates of several mycobacteria were noted and could be detected under magnification ×400, consistent with our experience with sections of multibacillary Johne's disease, where aggregates of mycobacteria are readily visualized under magnification ×400. By comparison, examination of tissue samples derived from M. tuberculosis-infected mice revealed that individual mycobacteria were detectable using magnification ×400. When tissue samples from both M. avium subsp. paratuberculosis- and M. tuberculosis-infected mice were subjected to auranime rhodamine staining, examination under magnification ×400 was sufficient to visualize fluorescent signals for both organisms. However, while M. tuberculosis signals could be recognized as mycobacteria based on their morphology, signals derived from M. avium subsp. paratuberculosis were half as long and less rod-like; therefore, it was difficult to distinguish bacterial signals from artifact (Fig. 1a and c). Thus, for both ZN and auramine-based visualization, isolated M. avium subsp. paratuberculosis organisms were only reliably visualized under ×1,000 oil immersion (Fig. 1b and d). To understand the need for magnification-×1,000 microscopy, we digitally captured mycobacterial signals and estimated their morphometric parameters. By measuring mycobacterial forms from these two infections, we could determine that M. avium subsp. paratuberculosis signals were shorter (1.59 μms ± 0.06 SEM versus 3.59 μm × 0.15 SEM), of a smaller area (1.03 × 0.07 μm2 versus 2.26 ± 0.09 μm2), and had a higher shape factor (0.67 ± 0.01 versus 0.41 ± 0.02) than M. tuberculosis signals (all P values were <0.01; see Table 3). Measuring M. avium subsp. paratuberculosis in ovine samples and in vitro cultures provided the same estimates as murine samples (Table 3). Additionally, for mice with few rod-shaped forms, we also observed smaller coccobacillary forms (Fig. 2); these forms were 1.12 μm (SEM = 0.07) in length by 0.78 μm (SEM = 0.06) in width, yielding an average area of just 0.63 μm2 (SEM = 0.08).
FIG. 1.
Comparison of morphometric perception of M. tuberculosis and M. avium subsp. paratuberculosis under 40× and 100× objectives. Auramine-rhodamine-stained sections of spleen from M. avium paratuberculosis- and M. tuberculosis-infected mice. (a) Under a 40× objective, providing ×400 total magnification, individual M. avium paratuberculosis forms are difficult to distinguish from artifacts. (b) Under an oil immersion objective, with total magnification ×1,000, individual M. avium paratuberculosis organisms are detectable. (c) In contrast, individual M. tuberculosis bacilli can be seen using a 40× objective and are further resolved using oil immersion (d).
TABLE 3.
Morphometric parameters of mycobacterial signals: dimensions and shape of bacillary forms in tissue
| Bacterium | State | Length (μm) (±SEM) | Width (μm) (±SEM) | Area (μm2) (±SEM) | Shape factora ± SEM |
|---|---|---|---|---|---|
| M. tuberculosis | In vitro | 3.10 ± 0.11 | 0.88 ± 0.03 | 1.92 ± 0.11 | 0.44 ± 0.01 |
| Murine infection | 3.59 ± 0.15 | 0.99 ± 0.02 | 2.26 ± 0.09 | 0.41 ± 0.02 | |
| Mycobacterium avium subsp. paratuberculosis | In vitro | 1.54 ± 0.08 | 0.74 ± 0.03 | 0.81 ± 0.07 | 0.62 ± 0.01 |
| Ovine infection | 1.66 ± 0.08 | 1.00 ± 0.05 | 1.25 ± 0.10 | 0.69 ± 0.02 | |
| Murine infection | 1.59 ± 0.06 | 0.91 ± 0.03 | 1.03 ± 0.07 | 0.67 ± 0.01 |
Shape factor = 4π × area/perimeter2.
FIG. 2.
Morphologically variable forms of M. avium paratuberculosis in tissue. Ziehl-Neelsen-stained section of a liver from an M. avium paratuberculosis-infected mouse showing bacillary (blue arrow), coccobacillary (black arrow), and coccoid forms (red arrow) of the organism. Bar, 2 μm; magnification, ×1,000.
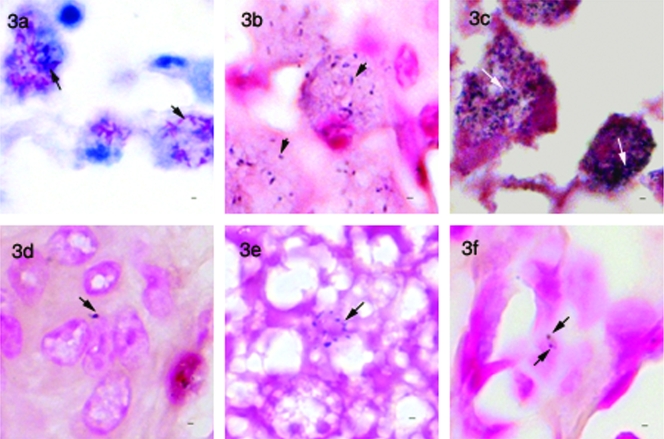
Sensitivity and specificity of IS900 in situ hybridization (ISH) and indirect IS PCR.
Microscopic analysis of tissue samples from Johne's sheep subjected to direct hybridization with the IS900 probe showed variable shapes of signals ranging from rods to undefined shapes in areas found to be positive by ZN staining (Fig. 3b). However, the sensitivity, as estimated by comparing the number of positive signals across the two modalities, was much lower by IS900 ISH than by ZN staining (Fig. 3a and b). To increase the sensitivity, we also employed indirect IS PCR, where the tissue target is first subjected to in situ amplification, before probing for the IS900 element. Indirect IS PCR provided sensitivity comparable to that of ZN staining, but signals were mostly granular in appearance (Fig. 3c), as has been described by Sanna et al. (40). Application of both of these methods to tissue samples from M. avium subsp. paratuberculosis-infected mice resulted in the appearance of signals resembling the specific signals (Fig. 3d); however, similar signals were observed in uninfected mice and in M. tuberculosis-infected tissue (Fig. 3e and f). To determine the cause of these nonspecific signals, we repeated this process using an IS900 probe without DIG; tissue samples from ovine naturally infected with M. avium subsp. paratuberculosis now failed to produce these signals, indirectly suggesting that they resulted from nonspecific binding of the IS900 probe, as has been described elsewhere (49).
FIG. 3.
IS900-based in situ staining assays. (a) Ziehl-Neelsen-stained tissue section of ovine intestinal tissue showing a large number of mycobacteria in macrophages (arrows). (b) IS900-probe-based in situ hybridization of a sequential section of the same tissue, showing positive labeling (arrow) but a reduced number of signals compared to the Ziehl-Neelsen-stained section. (c) Indirect in situ PCR for IS900 of the same sample showing granular signals (arrows) and sensitivity comparable to that with Ziehl-Neelsen staining. (d) Section of liver from an M. avium paratuberculosis-infected mouse showing positive signal (arrow) by IS900-probe-based in situ hybridization. (e) Section of liver from an uninfected mouse and (f) section of lung from a mouse infected with M. tuberculosis showing nonspecific signals with the IS900-probe-based in situ hybridization method. Bar, 1 μm; magnification, ×950.
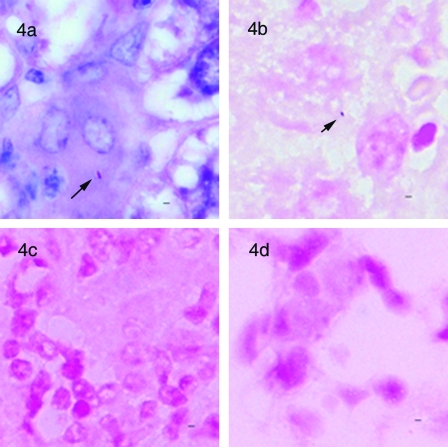
Sensitivity and specificity of rRNA ISH.
Morphology of signals derived by rRNA staining of M. avium subsp. paratuberculosis in mouse tissue was consistent with that of signals visualized in smears of M. avium subsp. paratuberculosis cultures. A preliminary comparison of colorimetric signals observed in ovine tissue with those for Johne's disease suggested that the distribution of acid-fast bacilli, as revealed by ZN staining, was similar to the number of bacillary signals observed with rRNA in situ hybridization. To quantitatively compare the sensitivities of these two methods in paucibacillary infection, ISH signals and acid-fast bacilli in the same area of serial tissue sections of liver and spleen from mice infected with M. avium subsp. paratuberculosis were independently enumerated (Fig. 4a and b), revealing that the sensitivities of the two methods were comparable (ZN = 64 bacilli/45 granulomas; ISH = 53 bacilli/45 granulomas; median of 1 bacillus per granuloma by both methods; difference not significant). To determine the specificity of the rRNA-based ISH method, probes specific for M. tuberculosis were applied to tissue sections from mice infected with M. avium subsp. paratuberculosis and, conversely, the M. avium probes were applied to tissue sections from uninfected and M. tuberculosis-infected mice. In both cases, no blue-purple precipitate was evident in any of these tissues (Fig. 4c and d). The absence of nonspecific signals in the control tissues by this assay allowed unequivocal interpretation of rod and coccoid-like signals when they were observed in the test samples. As expected, this assay also failed to detect M. avium subsp. paratuberculosis in tissue sections obtained from mice harboring less than 10 CFU/mg of tissue that were negative by acid-fast staining.
FIG. 4.
rRNA-based in situ hybridization. (a) Ziehl-Neelsen-stained section of liver from an M. avium paratuberculosis-infected mouse showing an individual bacillary form in a granuloma. (b) In situ hybridization with M. avium complex-specific rRNA probes (MAVP187ssu and MAVP515lsu) on a sequential section from the same block revealing a positive signal (arrow). (c) M. tuberculosis-specific oligonucleotide probes applied to M. avium paratuberculosis-infected mouse tissue did not generate signals. (d) M. avium complex-specific oligonucleotide probes applied to M. tuberculosis-infected mouse tissue did not generate signals. Bar, 1 μm; magnification, ×1,000.
Fluorescence in situ hybridization using fluorescence-labeled, rRNA-targeted probes provided sensitivity and specificity comparable to those of the colorimetric method. Although some background autofluorescence was useful to localize the signals within tissue sections, it generally decreased the signal-to-noise ratio and hampered detection of specific fluorescent signals.
DISCUSSION
The results presented in this study demonstrate that M. avium subsp. paratuberculosis infection of tissue can be detected by microscopic methods, subject to bacterial burden, and demonstrate the limitations of various staining and visualization methods advanced as being superior to ZN staining. Our results are consistent with that for most bacteria, where oil immersion is essential in achieving sufficient resolution to properly visualize bacterial forms. In the specific case of mycobacteria, while sputum examination of fluorochrome stained smears employs magnification ×250 to ×400 for screening followed by confirmation with oil immersion, ZN-stained smears require examination under oil immersion when looking for M. tuberculosis (9). By comparison, M. avium subsp. paratuberculosis organisms are smaller and more elliptical, translating into a greater diagnostic challenge, especially in tissue samples. Our microscopic observations, supported by considerations of size and geometry, lead us to conclude that single M. avium subsp. paratuberculosis bacilli are essentially undetectable under magnification ×400.
An important consideration when interpreting these results is whether these experimental infections adequately modeled the process under study. Since neither Johne's nor Crohn's diseases result from parenteral inoculations of live bacteria, our infections are clearly not models of pathogenesis. However, certain aspects of our model infections concurred with our understanding of mycobacterial diagnosis. First, microscopic examination of sheep with histopathologic grade 3c (paucibacillary) Johne's disease reveals either no bacteria or a few scattered organisms (33); therefore, our experimental infections resulted in a similar diagnostic challenge. Second, bacillary forms measured from murine infections had the same size and shape as organisms seen in natural ovine disease and as in vitro-grown organisms. Third, experience from M. tuberculosis indicates that sputum samples with less than 10,000 bacilli per ml are typically microscopy negative (1, 26), consistent with our CFU determinations where infections with less than 10 M. avium subsp. paratuberculosis per mg of tissue (10,000 bacteria per gram) were microscopy negative but culture positive. It is recognized that our model infections of “sterile organs” did not replicate the challenges faced when examining intestinal tissue, where high quantities of enteric bacteria populate the lumen and can occasionally translocate into adjacent tissues when the mucosal barrier is damaged (14). However, we consider that the use of tissue with an otherwise clean background provided a simpler setting for rating the relative sensitivity of methods, and more importantly, the absence of other bacterial forms provided the optimal substrate to determine the specificity of these modalities.
To overcome the limitations of conventional ZN staining for the detection of M. avium subsp. paratuberculosis in tissues, a number of molecularly based assays have been developed, including in situ hybridization using probes targeting the IS900 insertion element (20). In separate studies, we have shown that the IS900 element is genomically specific for M. avium subsp. paratuberculosis (51) and that IS900 sequences from a heterogeneous collection of M. avium subsp. paratuberculosis isolates are invariant (46). However, despite these favorable considerations, the IS900-based in situ probe was prone to nonspecific hybridization, compromising the utility of IS900-based in situ hybridization and indirect in situ PCR (49). In contrast, probes targeting rRNA provided excellent specificity, resulting in forms that were morphologically consistent with ZN-positive organisms on adjacent sections. A theoretical advantage of molecularly based assays would be the ability to detect greater numbers of organisms than are seen by ZN staining, if, as has been proposed, M. avium subsp. paratuberculosis adopts a cell wall-deficient (acid-fast-negative) form in tissue (10, 39). Unfortunately, we were not able to obtain any evidence in support of this notion with our murine infections, since enumeration of sequential sections of tissue for ZN-positive and rRNA-based ISH-positive bacilli revealed similar numbers of organisms by these two modalities.
An alternative means of increasing the sensitivity of microscopic detection involves the use of fluorescence microscopy, as has been done in the clinical mycobacteriology laboratory, where prolonged examination of slides using oil immersion objectives by light microscopy has been widely replaced by a fluorescence screening step under magnification ×400. Often described as a more-sensitive method, fluorescence microscopy is probably better considered more time efficient, since it permits the microscopist to conclude a negative result in a much shorter time frame, thereby generating fewer positive slides to confirm under oil immersion by light microscopy (47). In comparing the colorimetry-based ribosomal ISH to fluorimetry-based ribosomal ISH, the colorimetric method was preferred for confidently affirming the presence of M. avium subsp. paratuberculosis organisms and colocalizing these signals in the context of the histopathology. However, when numbers of signals were near the threshold of microscopic detection, thorough examination using oil immersion was time consuming and led to observer fatigue. The fluorimetry-based ribosomal ISH assay offered the possibility that signal detection might be achieved under magnification ×400, affording an opportunity to efficiently scan slides at this lower power. In our experience, with multibacillary ovine tissue, this fluorescence ISH-based approach appeared promising, but for paucibacillary infections, individual bacterial signals were too weak to be detectable at lower power. The intensity of signals is determined by a number of factors, specifically, the ribosomal content of cells, which is related to the metabolic state of the bacteria (24), penetration of probes inside the cells (35), and accessibility of the probes to the target sites on the ribosome (12). Since equivalent signal intensities were obtained using the eubacterial and M. avium-specific oligonucleotide probes, we could not determine factors that could be limiting the signal intensity. Recently, a number of groups have reported that substitution of DNA oligonucleotide probes with peptide nucleic acid (PNA) probes results in greater signal intensity in a number of bacterial species (24) due to their greater permeability through the bacterial cell wall (50) and target accessibility (3, 34). Since PNA probes have recently been described for the detection of M. avium complex organisms (25), the application of these newer in situ probes to tissue samples represents a promising avenue towards improved detection of M. avium subsp. paratuberculosis infection. The use of PNA probes and other newer modalities to detect M. avium subsp. paratuberculosis in tissue will gain from a thorough evaluation of their validity, with special attention to spectrum effect, prior to their application in epidemiologic studies.
Acknowledgments
This work was supported by grants from Crohn's and Colitis Foundation of Canada and the Broad Medical Research Foundation. C.T. is supported by a Lloyd-Carr-Harris McGill Major Fellowship and an F. C. Harrison scholarship from the McGill Department of Microbiology and Immunology, and M.B. is a New Investigator of the Canadian Institutes of Health Research.
None of the authors has a conflict of interest or any commercial association that may pose a conflict of interest.
We thank Danielle Charlet, Elizabeth Fidalgo, and Makeda Semret for their input and suggestions.
REFERENCES
- 1.Allen, J. L. 1992. A modified Ziehl-Neelsen stain for mycobacteria. Med. Lab. Sci. 49:99-102. [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage, B. A. 2003. The impact of nucleic acid secondary structure on PNA hybridization. Drug Discov. Today 8:222-228. [DOI] [PubMed] [Google Scholar]
- 4.Autschbach, F., S. Eisold, U. Hinz, S. Zinser, M. Linnebacher, T. Giese, T. Loffler, M. W. Buchler, and J. Schmidt. 2005. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn's disease. Gut 54:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull, T. J., J. Hermon-Taylor, I. Pavlik, F. El Zaatari, and M. Tizard. 2000. Characterization of IS900 loci in Mycobacterium avium subsp. paratuberculosis and development of multiplex PCR typing. Microbiology 146:2185-2197. [DOI] [PubMed] [Google Scholar]
- 6.Bull, T. J., E. J. McMinn, K. Sidi-Boumedine, A. Skull, D. Durkin, P. Neild, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 41:2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiodini, R. J., H. J. Van Kruiningen, W. R. Thayer, and J. A. Coutu. 1986. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J. Clin. Microbiol. 24:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalziel, T. K. 1913. Chronic intestinal enteritis. Brit. Med. J. 2:1068-1070. [Google Scholar]
- 9.Della-Latta, P. 2004. Acid-fast staining, p. 7.2.1-7.2. 4. In H. D. Isenberg (ed.), Clinical microbiology procedure handbook. American Society for Microbiology, Washington, D.C.
- 10.Dell'Isola, B., C. Poyart, O. Goulet, J. F. Mougenot, E. Sadoun-Journo, N. Brousse, J. Schmitz, C. Ricour, and P. Berche. 1994. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn's disease. J. Infect. Dis. 169:449-451. [DOI] [PubMed] [Google Scholar]
- 11.Forbes, B. A. 1997. Critical assessment of gene amplification approaches on the diagnosis of tuberculosis. Immunol. Investig. 26:105-116. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenstein, R. J. 2003. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect. Dis. 3:507-514. [DOI] [PubMed] [Google Scholar]
- 14.Guarner, F., and J. R. Malagelada. 2003. Gut flora in health and disease. Lancet 361:512-519. [DOI] [PubMed] [Google Scholar]
- 15.Hall, M. C., B. Kieke, R. Gonzales, and E. A. Belongia. 2004. Spectrum bias of a rapid antigen detection test for group A beta-hemolytic streptococcal pharyngitis in a pediatric population. Pediatrics 114:182-186. [DOI] [PubMed] [Google Scholar]
- 16.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermon-Taylor, J., M. Moss, M. Tizard, Z. Malik, and J. Sanderson. 1990. Molecular biology of Crohn's disease mycobacteria. Baillieres Clin. Gastroenterol. 4:23-42. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 19.Hulten, K., H. M. El Zimaity, T. J. Karttunen, A. Almashhrawi, M. R. Schwartz, D. Y. Graham, and F. A. El Zaatari. 2001. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn's diseased tissues by in situ hybridization. Am. J. Gastroenterol. 96:1529-1535. [DOI] [PubMed] [Google Scholar]
- 20.Hulten, K., T. J. Karttunen, H. M. El Zimaity, S. A. Naser, A. Almashhrawi, D. Y. Graham, and F. A. El Zaatari. 2000. In situ hybridization method for studies of cell wall deficient M. paratuberculosis in tissue samples. Vet. Microbiol. 77:513-518. [DOI] [PubMed] [Google Scholar]
- 21.Hulten, K., T. J. Karttunen, H. M. El Zimaity, S. A. Naser, M. T. Collins, D. Y. Graham, and F. A. El Zaatari. 2000. Identification of cell wall deficient forms of M. avium subsp. paratuberculosis in paraffin embedded tissues from animals with Johne's disease by in situ hybridization. J. Microbiol. Methods 42:185-195. [DOI] [PubMed] [Google Scholar]
- 22.Ieven, M., and H. Goossens. 1997. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clin. Microbiol. Rev. 10:242-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachs, M. S., I. Nachamkin, P. H. Edelstein, J. Goldman, A. R. Feinstein, and J. S. Schwartz. 1992. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Ann. Intern. Med. 117:135-140. [DOI] [PubMed] [Google Scholar]
- 24.Lehtola, M. J., C. J. Loades, and C. W. Keevil. 2005. Advantages of peptide nucleic acid oligonucleotides for sensitive site directed 16S rRNA fluorescence in situ hybridization (FISH) detection of Campylobacter jejuni, Campylobacter coli and Campylobacter lari. J. Microbiol. Methods 62:211-219. [DOI] [PubMed] [Google Scholar]
- 25.Lehtola, M. J., E. Torvinen, I. T. Miettinen, and C. W. Keevil. 2006. Fluorescence in situ hybridization using peptide nucleic acid probes for rapid detection of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis in potable-water biofilms. Appl. Environ. Microbiol. 72:848-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks, J. 1974. Notes on the Ziehl-Neelsen staining of sputum. Tubercle 55:241-244. [DOI] [PubMed] [Google Scholar]
- 27.Moss, M. T., E. P. Green, M. L. Tizard, Z. P. Malik, and J. Hermon-Taylor. 1991. Specific detection of Mycobacterium paratuberculosis by DNA hybridisation with a fragment of the insertion element IS900. Gut 32:395-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulherin, S. A., and W. C. Miller. 2002. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann. Intern. Med. 137:598-602. [DOI] [PubMed] [Google Scholar]
- 29.Mura, M., T. J. Bull, H. Evans, K. Sidi-Boumedine, L. McMinn, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2006. Replication and long-term persistence of bovine and human strains of Mycobacterium avium subsp. paratuberculosis within Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72:854-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naser, S. A., G. Ghobrial, C. Romero, and J. F. Valentine. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 364:1039-1044. [DOI] [PubMed] [Google Scholar]
- 31.Nayak, S. V., A. S. Shivarudrappa, and A. S. Mukkamil. 2003. Role of fluorescent microscopy in detecting Mycobacterium leprae in tissue sections. Ann. Diagn. Pathol. 7:78-81. [DOI] [PubMed] [Google Scholar]
- 32.Noordhoek, G. T., S. Mulder, P. Wallace, and A. M. van Loon. 2004. Multicentre quality control study for detection of Mycobacterium tuberculosis in clinical samples by nucleic amplification methods. Clin. Microbiol. Infect. 10:295-301. [DOI] [PubMed] [Google Scholar]
- 33.Perez, V., J. F. Garcia Marin, and J. J. Badiola. 1996. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 114:107-122. [DOI] [PubMed] [Google Scholar]
- 34.Perry-O'Keefe, H., S. Rigby, K. Oliveira, D. Sorensen, H. Stender, J. Coull, and J. J. Hyldig-Nielsen. 2001. Identification of indicator microorganisms using a standardized PNA FISH method. J. Microbiol. Methods 47:281-292. [DOI] [PubMed] [Google Scholar]
- 35.Ramage, G., S. Patrick, and S. Houston. 1998. Combined fluorescent in situ hybridisation and immunolabelling of Bacteroides fragilis. J. Immunol. Methods 212:139-147. [DOI] [PubMed] [Google Scholar]
- 36.Ransohoff, D. F., and A. R. Feinstein. 1978. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N. Engl. J. Med. 299:926-930. [DOI] [PubMed] [Google Scholar]
- 37.Romero, C., A. Hamdi, J. F. Valentine, and S. A. Naser. 2005. Evaluation of surgical tissue from patients with Crohn's disease for the presence of Mycobacterium avium subspecies paratuberculosis DNA by in situ hybridization and nested polymerase chain reaction. Inflamm. Bowel. Dis. 11:116-125. [DOI] [PubMed] [Google Scholar]
- 38.Ryan, P., S. Aarons, M. W. Bennett, G. Lee, G. C. O'Sullivan, J. O'Connell, and F. Shanahan. 2002. Mycobacterium paratuberculosis detected by nested PCR in intestinal granulomas isolated by LCM in cases of Crohn's disease. Methods Mol. Biol. 193:205-211. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson, J. D., and J. Hermon-Taylor. 1992. Mycobacterial diseases of the gut: some impact from molecular biology. Gut 33:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanna, E., C. J. Woodall, N. J. Watt, C. J. Clarke, M. Pittau, A. Leoni, and A. M. Nieddu. 2000. In situ-PCR for the detection of Mycobacterium paratuberculosis DNA in paraffin-embedded tissues. Eur. J. Histochem. 44:179-184. [PubMed] [Google Scholar]
- 41.Sartor, R. B. 2005. Does Mycobacterium avium subspecies paratuberculosis cause Crohn's disease? Gut 54:896-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sechi, L. A., M. Mura, E. Tanda, A. Lissia, G. Fadda, and S. Zanetti. 2004. Mycobacterium avium sub. paratuberculosis in tissue samples of Crohn's disease patients. New Microbiol. 27:75-77. [PubMed] [Google Scholar]
- 43.Sechi, L. A., M. Mura, F. Tanda, A. Lissia, A. Solinas, G. Fadda, and S. Zanetti. 2001. Identification of Mycobacterium avium subsp. paratuberculosis in biopsy specimens from patients with Crohn's disease identified by in situ hybridization. J. Clin. Microbiol. 39:4514-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sechi, L. A., A. M. Scanu, P. Molicotti, S. Cannas, M. Mura, G. Dettori, G. Fadda, and S. Zanetti. 2005. Detection and isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. Am. J. Gastroenterol. 100:1529-1536. [DOI] [PubMed] [Google Scholar]
- 45.Semret, M., D. C. Alexander, C. Y. Turenne, P. de Haas, P. Overduin, D. van Soolingen, D. Cousins, and M. A. Behr. 2005. Genomic polymorphisms for Mycobacterium avium subsp. paratuberculosis diagnostics. J. Clin. Microbiol. 43:3704-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semret, M., C. Y. Turenne, and M. A. Behr. 2006. Insertion sequence IS900 revisited. J. Clin. Microbiol. 44:1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somoskovi, A., J. E. Hotaling, M. Fitzgerald, D. O'Donnell, L. M. Parsons, and M. Salfinger. 2001. Lessons from a proficiency testing event for acid-fast microscopy. Chest 120:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Amand, A. L., D. N. Frank, M. A. De Groote, R. J. Basaraba, I. M. Orme, and N. R. Pace. 2005. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium tuberculosis in culture and tissue specimens. J. Clin. Microbiol. 43:5369-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St Amand, A. L., D. N. Frank, M. A. De Groote, and N. R. Pace. 2005. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium avium complex organisms in tissue. J. Clin. Microbiol. 43:1505-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stender, H., M. Fiandaca, J. J. Hyldig-Nielsen, and J. Coull. 2002. PNA for rapid microbiology. J. Microbiol. Methods 48:1-17. [DOI] [PubMed] [Google Scholar]
- 51.Turenne, C. Y., M. Semret, D. V. Cousins, D. M. Collins, and M. A. Behr. 2006. Sequencing of hsp65 distinguishes among subsets of the Mycobacterium avium complex. J. Clin. Microbiol. 44:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinh, D. C., and C. N. Bernstein. 2005. Crohn's disease and M. paratuberculosis: where's the beef? Inflamm. Bowel. Dis. 11:1025-1027. [DOI] [PubMed] [Google Scholar]
- 53.Whittington, R. J., I. B. Marsh, P. J. Taylor, D. J. Marshall, C. Taragel, and L. A. Reddacliff. 2003. Isolation of Mycobacterium avium subsp. paratuberculosis from environmental samples collected from farms before and after destocking sheep with paratuberculosis. Aust. Vet. J. 81:559-563. [DOI] [PubMed] [Google Scholar]