Abstract
We present 2 cases of primary pulmonary non-O1 Vibrio cholerae infection. We believe that these are the first documented cases of primary pulmonary infection due to this organism from a freshwater source.
CASE REPORT
Case no. 1.
In August 2001, a 67-year-old man with a history of alcoholism, tobacco use, and psoriasis presented to a local hospital with generalized weakness, cough, and shortness of breath. Five days prior to presentation, he had fallen into an irrigation canal and was pulled from the canal after being face down for several seconds. He was febrile at 39°C and tachycardic. Coarse crackles were appreciated upon auscultation of the left lower lung field. Also of note on physical examination were icteric sclera and a liver edge palpated 4 cm below the right costal margin.
Initial laboratory data revealed a white blood cell count of 13,300/mm3 with 91% neutrophils, 12.1 g/dl hemoglobin, 34.5% hematocrit, a platelet count of 143,000/mm3, 132 mEq/liter sodium, 3.0 mEq/liter potassium, 91 mEq/liter chloride, 117 mg/dl glucose, 13 mg/dl blood urea nitrogen, 1.3 mg/dl creatinine, 6 gm/dl total protein, 2.5 gm/dl albumin, 8.5 mg/dl calcium, 8.7 mg/dl total bilirubin, 6.2 mg/dl direct bilirubin, 207 units/liter aspartate aminotransferase 60 units/liter alanine aminotransferase, and 191 units/liter alkaline phosphatase. The chest radiograph exposed an infiltrate in the left lower lobe. The following day, the patient's differential contained 32% band forms and a white blood cell count of 12,600/mm3.
The patient was admitted with working diagnoses of pneumonia, hypokalemia, and hepatitis. His pneumonia was suspected to be a result of aspiration of water from the irrigation canal fed by a tributary of the Pecos River in Carlsbad, New Mexico. He was started on levofloxacin. Sputum, blood, and urine were obtained for culture. Preliminary data revealed a gram-negative organism from the blood, and imipenem/cilastin was added to the antibiotic regimen. Because of gram-negative pneumonia and bacteremia, he was transferred to our critical care unit. The cultures returned a result of Vibrio cholerae non-O1, which was confirmed by the New Mexico state laboratory. The vibrio was sensitive to quinolones, and at that point, imipenem/cilastin was discontinued. The hepatitis panel and biliary workup were negative, and the liver dysfunction was attributed to alcohol abuse, with improvement of liver enzymes over the course of his hospitalization. Slow but steady progress was noted over his 6-day hospital stay.
Case no. 2.
In August 2000, a previously healthy 25-year-old white female sustained a blast injury from an exploding natural gas pipeline resulting in 50% total body surface area second and third degree burns. She had been camping next to the Pecos River at the Texas-New Mexico border. She dove into the river at the time of the blast. She was in respiratory distress when examined at the scene and was immediately intubated. She was transferred to our burn center, and treatment was initiated for the burns and lung injury. Two days after arrival, she was found to be febrile with pneumonia. Sputum Gram stain showed gram-negative rods. Empirical antibiotics, including cefazolin, aztreonam, and gentamicin, were initiated for gram-negative sepsis. Blood cultures were sterile. Cultures of the sputum grew only non-O1 V. cholerae. The Texas Department of Health Microbiology Laboratory confirmed this identification. Over the next week, her pneumonia cleared; however, the patient ultimately succumbed to her burn wounds.
The organisms recovered from the clinical specimens were initially identified by the hospital microbiology laboratory as possible vibrio using conventional microbiology techniques. They were subsequently submitted to the Texas and New Mexico state laboratories for confirmation, again using conventional microbiology techniques and V. cholerae O1 rabbit antisera provide by the CDC. After confirmation as non-O1 V. cholerae, a pulsed-field gel electrophoresis (PFGE) technique was done to compare the organisms, and the patterns were found to be different (Fig. 1). The comparison was performed by the New Mexico State Laboratory using the rapid standardized PulseNet PFGE protocol (9). Minor modifications included the following: (i) sodium dodecyl sulfate was not added to the plug agarose, (ii) the plug slices were restricted with 50 U of NotI, and (iii) running conditions were 2 s to 10 s for 14 h for block I and 21.79 s to 35 s for 5 h for block II (Kara Cooper, PulseNet, CDC, personal communication).
FIG. 1.
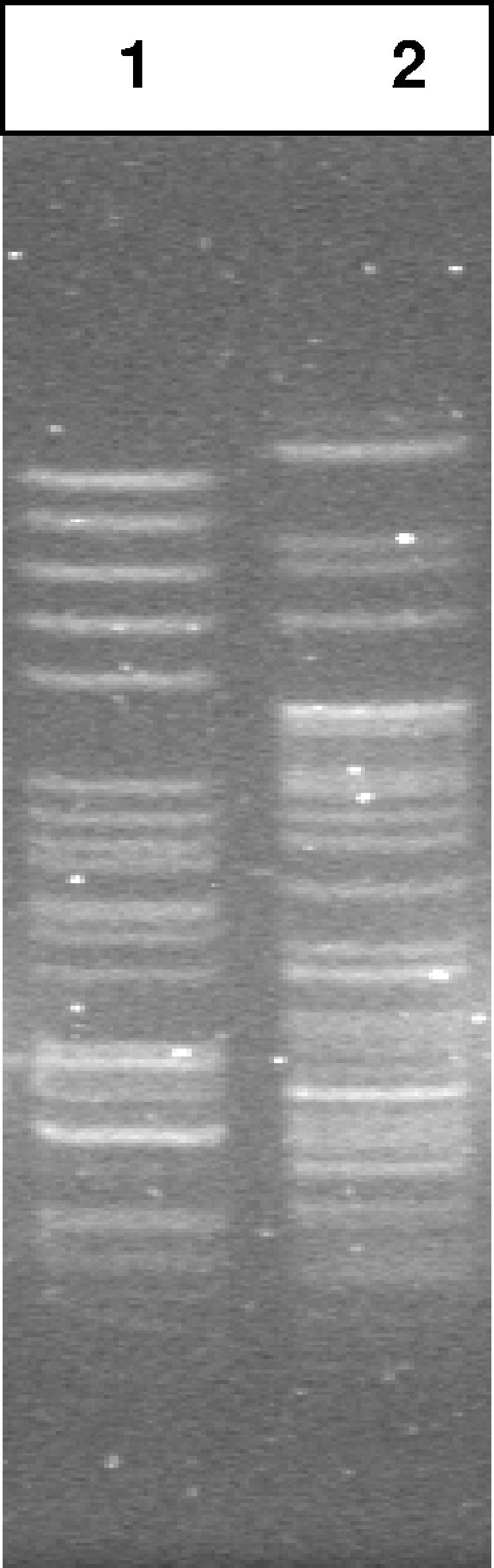
PFGE analysis showing that the non-O1 V. cholerae organisms isolated from the two patients do not share the same pattern.
V. cholerae is a gram-negative rod with a unique curved or comma shape. This organism is best known as a cause of epidemic diarrheal disease. V. cholerae O1 rarely causes extraintestinal infections, unlike its relatives, the non-O1 strains. While the non-O1 V. cholerae strains are indistinguishable both morphologically and biochemically from serogroup O1 strains, non-O1 isolates do not agglutinate in type O1 antisera. Non-O1 vibrios have been typically thought of as causing sporadic disease or as nonpathogenic.
Cases of extraintestinal non-O1 vibrio infections have multiplied over the past 20 years, and in the correct host, these vibrio are capable of causing severe disease, sepsis, and death. V. cholerae is abundant in coastal areas, and disease is commonly associated with exposure to seawater or ingestion of contaminated seafood. Most cases of wound infections are associated with estuaries and brackish waters; however, a few reports describe infections that are in association with freshwater sources with low sodium contents (1, 5, 6, 7, 8).
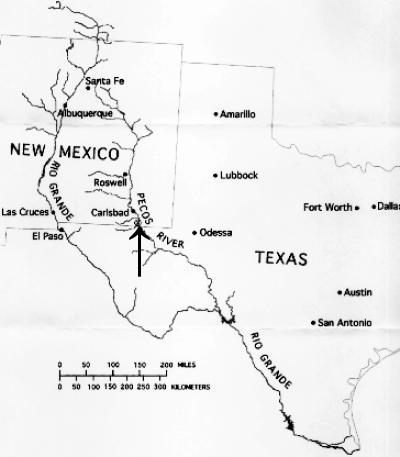
V. cholerae bacteremia is well reported (2, 3, 10); however, the occurrence of pneumonia as an entry point is rarely reported (3, 4, 10) and possibly never reported in conjunction with a fresh water source. Here, we present two cases of non-O1 V. cholerae pneumonia and subsequent bacteremia/sepsis in association with an inland water source, more specifically from the Pecos River in one case and from a tributary of the Pecos River in the other case (Fig. 2).
FIG. 2.
Map of New Mexico and West Texas showing the Pecos River and its tributaries.
We believe that our patients aspirated river water, which was contaminated with the vibrio organism. Their pneumonia developed because of this organism, and in case 2, the additional blast damage to the lungs and near drowning. With antibiotic therapy, the vibrio pneumonia cleared in both cases. These infections occurred in immunocompetent persons who aspirated fresh, flowing water from the same river source. These cases serve to alert critical care and emergency physicians to the possibility of V. cholerae as a cause of aspiration pneumonia and bacteremia in trauma victims.
Acknowledgments
We extend our gratitude to K. Hendricks and Marianne Garcia of the State Laboratory of the Texas Department of Health and to D. Horensky and Linda Nims of the Scientific Laboratory Division of the New Mexico Department of Health for their efforts in the identification, PFGE performance, and notification process. We also thank Kara Cooper of the CDC and PulseNet for information regarding the PFGE procedure.
REFERENCES
- 1.Bockemuhl, J., K. Roch, B. Wohlers, V. Aleksic, S. Aleksic, and R. Wokatsch. 1986. Seasonal distribution of facultatively enteropathogenic vibrios in the freshwater of the Elbe River at Hamburg. J. Appl. Bacteriol. 60:435-442. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Natal, I., and M. Alcoba-Leza. 1996. Non-01 Vibrio cholerae bacteraemia without diarrhoea. Lancet 948:67. [DOI] [PubMed] [Google Scholar]
- 3.Hughes, J. M., D. G. Hollis, E. J. Gangarosa, and R. E. Weaver. 1978. Non-cholera vibrio infections in the United States: clinical, epidemiologic, and laboratory features. Ann. Intern. Med. 88:602-606. [DOI] [PubMed] [Google Scholar]
- 4.Morgan, D. R., B. D. Ball, D. G. Moore, and S. Kohl. 1985. Severe Vibrio cholerae sepsis and meningitis in a young infant. Tex. Med. 81:37-38. [PubMed] [Google Scholar]
- 5.Mulder, G. D., T. M. Reis, and T. R. Beaver. 1989. Nontoxigenic Vibrio cholerae wound infection after exposure to contaminated lake water. J. Infect. Dis. 159:809-811. [DOI] [PubMed] [Google Scholar]
- 6.Patrak, D. L., and J. D. Gindorf. 1989. Bacteremic cellulites caused by non-serogroup O1 Vibrio cholerae acquired in a freshwater inland lake. J. Clin. Microbiol. 27:2874-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes, J. B., D. Schweitzer, and J. E. Ogg. 1985. Isolation of non-O1 Vibrio cholerae associated with enteric disease of herbivores in western Colorado. J. Clin. Microbiol. 22:572-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes, J. B., H. L. Smith, and J. E. Ogg. 1986. Isolation of non-O1 Vibrio cholerae serovars from surface waters in western Colorado. Appl. Environ. Microbiol. 51:1216-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribot, E. M., M. A. Fair, R. Gautom, et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59-67. [DOI] [PubMed] [Google Scholar]
- 10.Safrin, S., J. G. Morris, M. Adams, V. Pons, R. Jacobs, and J. E. Conte. 1988. Non-O1 Vibrio cholerae bacteremia: case report and review. Rev. Infect. Dis. 10:1012-1017. [DOI] [PubMed] [Google Scholar]