Abstract
Human blood agar (HuBA) is widely used in developing countries for the isolation of bacteria from clinical specimens. This study compared citrated sheep blood agar (CSBA) and HuBA with defibrinated horse blood agar and defibrinated sheep blood agar (DSBA) for the isolation and antibiotic susceptibility testing of reference and clinical strains of Streptococcus pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus. Reference and clinical strains of all organisms were diluted in brain heart infusion and a clinical specimen of cerebrospinal fluid and cultured on all agars. Viable counts, colony morphology, and colony size were recorded. Susceptibility testing for S. pneumoniae and S. pyogenes was performed on defibrinated sheep blood Mueller-Hinton agar, citrated sheep blood Mueller-Hinton agar (CSB MHA), and human blood Mueller-Hinton agar plates. For all organisms, the colony numbers were similar on all agars. Substantially smaller colony sizes and absent or minimal hemolysis were noted on HuBA for all organisms. Antibiotic susceptibility results for S. pneumoniae were similar for the two sheep blood agars; however, larger zone sizes were displayed on HuBA, and quality control for the reference strain failed on HuBA. For S. pyogenes, larger zone sizes were demonstrated on HuBA and CSBA than on DSBA. Poor hemolysis made interpretation of the zone sizes difficult on HuBA. CSBA is an acceptable alternative for the isolation of these organisms. The characteristic morphology is not evident, and hemolysis is poor on HuBA; and so HuBA is not recommended for use for the isolation or the susceptibility testing of any of these organisms. CSB MHA may be suitable for use for the susceptibility testing of S. pneumoniae.
The culture of organisms such as Streptococcus pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus remains the “gold standard” for the diagnosis of serious bacterial infections. The isolation of some organisms requires a source of blood as a culture medium supplement. Defibrinated sheep, horse, pig, or goat blood agar is recommended for the isolation of S. pneumoniae and S. pyogenes (1, 2, 3, 5). Agar prepared with human blood is not recommended, partly because of the safety risk to laboratory personnel, but mainly because it is said to result in poor bacterial isolation rates, although there are few published data to support this (2). Despite this, it is common practice in many developing countries to prepare bacterial culture media by using expired human blood obtained from donors for blood transfusions, because it is convenient and inexpensive. For example, human blood agar has routinely been used in bacteriology laboratories in seven developing countries that we have recently visited in the Asia-Pacific region.
Blood must be either defibrinated during collection or collected in bags containing anticoagulant to prevent clot formation. The most commonly used anticoagulant is citrate phosphate dextrose. Citric acid is often used in the food industry to inhibit the growth of bacteria (8, 9) and is therefore thought to be unsuitable for use in culture media. Commercial animal laboratories in developed countries use magnetic stirrers to defibrinate blood during collection. This specialized equipment is not easily obtained in developing countries, and the only other option for obtaining large volumes of defibrinated animal blood is to kill the animal, which is not practical or acceptable in most countries, and the blood is often contaminated. The importation of large quantities of defibrinated sheep or horse blood or the use of commercially made agar plates is also not an economically viable option for developing countries. The noncommercial collection of defibrinated animal blood requires a sterile glass bottle with glass beads or rods in situ. The bottle is manually rotated as the blood is collected so that the fibrin coats the glass beads and clot formation is prevented; this is also not a practical strategy for the collection of large volumes of blood.
In Fiji, where human blood agar is used routinely in the diagnostic bacteriology laboratory, we were unable to establish a reliable source of defibrinated sheep blood for our research laboratory. However, we could easily and reliably collect large volumes of sheep blood in commercially available human blood donor packs (which come together with a venipuncture needle and relevant tubing) containing citrate phosphate dextrose. We therefore aimed to compare blood agar prepared by using citrated human blood, citrated sheep blood, and defibrinated, noncitrated sheep blood with commercially available defibrinated, noncitrated horse blood agar. We tested each of these agars for their performance characteristics for the growth and antibiotic susceptibility testing of reference and clinical strains of S. pneumoniae, S. pyogenes, and S. aureus.
MATERIALS AND METHODS
This study was duplicated at two sites, the Colonial War Memorial Hospital (CWMH) laboratory, Suva, Fiji, and the Royal Children's Hospital (RCH), Melbourne, Australia. Photographs were taken from the RCH results.
Laboratory methods.
Each strain was cultured in serial dilutions prepared in brain heart infusion on each of four agars: defibrinated horse blood agar (HBA; Oxoid Ltd., Hampshire, United Kingdom), defibrinated sheep blood agar (DSBA), citrated sheep blood agar (CSBA), and citrated human blood agar (HuBA) plates. Each dilution of each organism on each blood agar was tested in triplicate at both laboratories. In an attempt to mimic the isolation of bacteria from a clinical sample, we repeated the same experiments using serial dilutions of the same organisms in a sample of sterile cerebrospinal fluid (CSF) obtained from a patient who had been admitted to RCH for a revision of his ventriculoperitoneal shunt. At CWMH, the CSF, obtained from a patient with suspected meningitis, had a normal appearance by microscopy and no growth on culture. Finally, we compared DSBA, CSBA, and HuBA for use in antibiotic susceptibility testing for S. pneumoniae and S. pyogenes.
Blood collection and medium preparation.
At CWMH, citrated and defibrinated sheep blood were collected by an aseptic technique from healthy sheep who were not receiving antibiotics at the time of blood collection. The defibrinated blood was collected in a sterile glass bottle containing glass rods that were manually rotated. Four hundred fifty milliliters of sheep blood was collected in a standard, sterile blood donor bag (Baxter Healthcare) containing 63 ml of citrate phosphate dextrose as an anticoagulant. Following collection, the blood was immediately chilled and transported to the CWMH laboratory, where 10 ml of blood was transferred aseptically into BacTalert (Biomerieux, Inc, Durham, NC) bottles and incubated for 7 days to ensure sterility. Expired human blood that had been collected from numerous donors in an aseptic manner and that had been stored at between 2 and 8°C was pooled and obtained from both hospitals' blood banks. Fifty-six millimeters of citrated sheep blood was added to 1 liter of agar. The blood agar plates were prepared by standard methods (Oxoid Australia Pty. Ltd). Columbia (Oxoid Ltd.) agar base was used for initial growth, and Mueller-Hinton (Oxoid Ltd.) agar was used for sensitivity testing. The agar plates were stored at 2 to 8°C until they were required.
At RCH, an expired human blood pack containing 63 ml of citrate phosphate dextrose was obtained from one donor. The citrated sheep blood and the defibrinated sheep blood were obtained from a commercial supplier (Institute of Medical and Veterinary Science, South Australia). The medium was prepared according to the methods of CWMH (Oxoid Australia Pty. Ltd.). The defibrinated HBA was made commercially (Oxoid Ltd.).
Strains and dilutions.
Three S. pneumoniae strains were used: strains ATCC 49619 (serotype 19F) and ATCC 6305 (serotype 5) and a clinical isolate (serotype 1, isolated from a blood culture). Three S. aureus strains were used: strains ATCC 25923 and ATCC 29213 and a clinical isolate (isolated from a blood culture). Two S. pyogenes strains were used: strain ATCC 19615 (emm type 80) and a clinical isolate (emm type 1, isolated from a blood culture). Each strain was inoculated in brain heart infusion (Oxoid Ltd.) broth to make a suspension equal to a 0.5 McFarland standard (∼1.2 × 108 CFU/ml). These were serially diluted before 100 μl was plated in triplicate on each agar. Similarly, each strain was serially diluted in CSF before it was plated in triplicate on each agar. The inoculated plates were incubated at 35°C in 5% CO2 for 18 to 20 h. The results were recorded in terms of viable counts, colony morphology (by description and photographs), and colony size (recorded in millimeters at the widest margin).
Antibiotic susceptibility testing.
Susceptibility testing was performed at CWMH only. Susceptibility testing for S. aureus was not performed, as blood is not required as a medium supplement for this organism. To test susceptibility, 0.5 McFarland standard concentrations were made in triplicate from S. pneumoniae strains ATCC 49619 and ATCC 6305 and a clinical isolate from a normally sterile site and from S. pyogenes strain ATCC 19615 and a clinical isolate from a normally sterile site. According to the methods of the CLSI (formerly the NCCLS) (6, 7), each sample was inoculated onto three different blood Mueller-Hinton (Oxoid Ltd.) plates. Disks containing oxacillin at 1 g, erythromycin at 15 g, chloramphenicol at 30 g, and co-trimoxazole at 1.25 g (Difco, Sparks, MD) were applied along with an optochin disk for the pneumococcal isolates. Disks containing penicillin at 1 g, erythromycin at 15 g, chloramphenicol at 30 g, and vancomycin at 30 g (Oxoid Ltd.) were applied for the S. pyogenes isolates. All plates were incubated overnight in 5% CO2 at 35°C. Zone diameters were read following 24 h if incubation. Two scientists read the plates independently.
RESULTS
The results of the isolation of all organisms were combined and the results from both CWMH and RCH were similar.
S. pneumoniae.
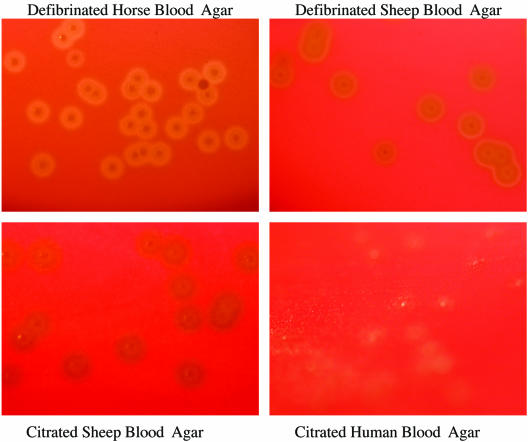
The numbers of colonies of the S. pneumoniae reference strains were similar on all blood agars at all dilutions, including the strains in CSF (Table 1). In addition, the growth of a clinical isolate of S. pneumoniae was similar on all blood agars at all dilutions and in CSF (data not shown). The morphological appearances were similar for colonies on HBA, CSBA, and DSBA. However, colonies were much smaller and alpha-hemolysis was not obvious for any strain on HuBA (Table 2; Fig. 1). Additional photographs are available on the http://www.rch.org.au/cich/pubs/ website.
TABLE 1.
Colony counts of strains tested on four different blood agar plates
| Strain and dilutiona | Colony counts on blood agar plates (range for triplicate plates)
|
|||
|---|---|---|---|---|
| HBA | CSBA | DSBA | HuBA | |
| S. pneumoniae ATCC 6305 | ||||
| 1.4 × 103 | >100 | >100 | >100 | >100 |
| 1.4 × 104 | >1,000 | >1,000 | >1,000 | >1,000 |
| 1.5 × 102 in CSF | ∼100 | ∼100 | ∼100 | ∼100 |
| 1.1 × 104 in CSF | >1,000 | >1,000 | >1,000 | >1,000 |
| S. pneumoniae ATCC 49619 | ||||
| 2.1 × 103 | >100 | >100 | >100 | >100 |
| 2.1 × 104 | >1,000 | >1,000 | >1,000 | >1,000 |
| 1.6 × 102 in CSF | ∼100 | ∼100 | ∼100 | ∼100 |
| 1.6 × 104 in CSF | >1,000 | >1,000 | >1,000 | >1,000 |
| S. aureus ATCC 25923 | ||||
| 2 × 102 | 18-21 | 22-29 | 22-32 | 13-32 |
| 2 × 104 | >1,000 | >1,000 | >1,000 | >1,000 |
| 1.1 × 102 in CSF | ∼100 | ∼100 | ∼100 | ∼100 |
| 1.1 × 104 in CSF | >1,000 | >1,000 | >1,000 | >1,000 |
| S. aureus ATCC 29213 | ||||
| 2.5 × 102 | 18-38 | 9-21 | 24-28 | 9-14 |
| 2.5 × 104 | >1,000 | >1,000 | >1,000 | >1,000 |
| 1.6 × 102 in CSF | ∼100 | ∼100 | ∼100 | ∼100 |
| 1.6 × 104 in CSF | >1,000 | >1,000 | >1,000 | >1,000 |
| S. pyogenes ATCC 19615 | ||||
| 7 × 101 | 6-8 | 6-22 | 8-24 | 1-14 |
| 7 × 103 | >100 | >100 | >100 | >100 |
| 1 × 102 in CSF | ∼100 | ∼100 | ∼100 | ∼100 |
| 1 × 104 in CSF | >100 | >100 | >100 | >100 |
| S. pyogenes strain JC20 | ||||
| 1.2 × 102 | 6-16 | 2-15 | 2-13 | 12-15 |
| 1.2 × 104 | >1,000 | >1,000 | >1,000 | >1,000 |
| 4.5 × 101 in CSF | 14-20 | 7-10 | 10-12 | 2-6 |
| 4.5 × 103 in CSF | >100 | ∼100 | ∼100 | 70 |
Results are presented for the upper and the lower dilutions only. In many cases (e.g., for all S. aureus and S. pyogenes strains) intermediate dilutions were also tested, with identical results for each agar. A clinical isolate of S. pneumoniae and a clinical isolate of S. aureus were also tested (results not shown).
TABLE 2.
Appearances of colonies and zones of hemolysis for tested strains on four different blood agar plates
| Strain and characteristica | Appearance of colonies and hemolysis on blood agar plates
|
|||
|---|---|---|---|---|
| HBA | CSBA | DSBA | HuBA | |
| S. pneumoniae ATCC 6305 | ||||
| Colony appearance | Shiny, grey | Mucoid, grey | Dull, grey | Dull, grey |
| Colony size (mm) | 1 | 1 | 1 | Pinpoint |
| Alpha-hemolysis | Small, <1 mm | Obvious, 1-5 mm | Obvious, 1 mm | Absent |
| S. pneumoniae ATCC 49619 | ||||
| Colony appearance | Shiny, mucoid grey | Dry, grey | Dry, grey | Shiny, grey |
| Colony size (mm) | 1 | 1.5 | 1 | Pinpoint |
| Alpha-hemolysis | Obvious, 1 mm | Obvious, 1-5 mm | Obvious, 1 mm | Absent |
| S. aureus ATCC 25923 | ||||
| Colony appearance | Opaque white glossy | Opaque white glossy | Opaque white glossy | Opaque white glossy |
| Colony size (mm) | 1.5 | 1.5 | 1.5 | 1.5 |
| Beta hemolysis | Faint, <1 mm | Obvious, 1 mm | Obvious, 1 mm | Absent |
| S. aureus ATCC 29213 | ||||
| Colony appearance | Opaque yellow glossy | Opaque yellow glossy | Opaque yellow glossy | Opaque yellow glossy |
| Colony size (mm) | 1.5 | 1.5 | 1.5 | 1 |
| Beta-hemolysis | Faint, <1 mm | Obvious, 1.8 mm | Obvious, 1.8 mm | Faint, <1 mm |
| S. pyogenes ATCC 19615 | ||||
| Colony appearance | Glossy white | Dry grey-white | Dry grey | Glossy white |
| Colony size (mm) | 1 | 1-2 | 1 | Pinpoint |
| Beta-hemolysis | Obvious, 5 mm | Faint, <1 mm | Obvious, 1 mm | Absent |
| S. pyogenes strain JC20 | ||||
| Colony appearance | Glossy white | Glossy white | Glossy white | Glossy white |
| Colony size (mm) | 1.5-1.8 | 1 | 1 | Pinpoint |
| Beta-hemolysis | Obvious, 1-5 mm | Obvious, 1 mm | Obvious, 1 mm | Absent |
A clinical isolate of S. pneumoniae and a clinical isolate of S. aureus were also tested (results not shown).
FIG. 1.
Growth of S. pneumoniae ATCC 6305 on the four different blood agars at a dilution of 1 × 101 CFU/ml.
S. aureus.
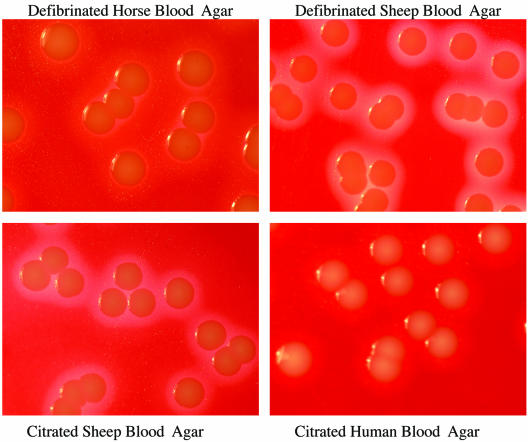
The numbers of colonies of the S. aureus reference strains were similar on all blood agars at all dilutions, including the strains in CSF (Table 1). In addition, the growth of a clinical isolate of S. aureus was similar on all blood agars at all dilutions and in CSF (data not shown). The appearances and the sizes of the colonies were similar on all agars, but hemolysis was not obvious for any strain on HuBA and was only faint on HBA (Table 2; Fig. 2). Additional photographs are available on the http://www.rch.org.au/cich/pubs/ website.
FIG. 2.
Growth of S. aureus ATCC 25923 on the four different blood agars at a dilution of 1 × 102 CFU/ml.
S. pyogenes.
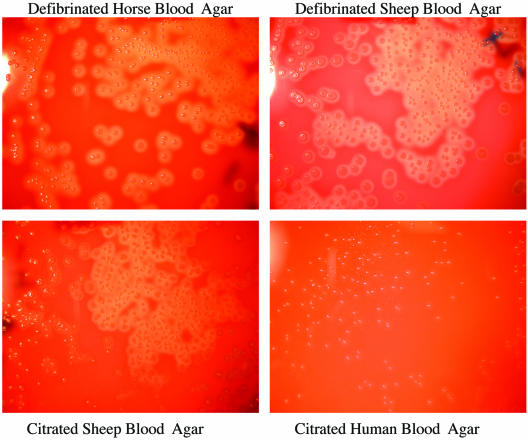
The numbers of colonies of the S. pyogenes reference and clinical strains were similar on all blood agars at all dilutions, including the strains in CSF (Table 1). The appearances and the sizes of the colonies were similar on HBA, CSBA, and DSBA. Hemolysis was most obvious on HBA and DSBA, although it was still present on CSBA. On HuBA, S. pyogenes colonies were pinpoint in size and hemolysis was absent (Table 2, Fig. 3). Additional photographs are available on the http://www.rch.org.au/cich/pubs/ website.
FIG. 3.
Growth of Streptococcus pyogenes ATCC 19615 on the four different blood agars at a dilution of 1 × 102 CFU/ml.
Antibiotic susceptibility testing results.
The antibiotic susceptibility testing results for S. pneumoniae were similar for both sheep Mueller-Hinton blood agars, but the human Mueller-Hinton blood agar (Hu MHA) demonstrated larger zone sizes for all antibiotics (Table 3). The susceptibility reference strain, ATCC 49619, failed quality control on the Hu MHA plates. The hemolysis on these plates was subtle, and it was difficult to discern where growth began.
TABLE 3.
Antibiotic disk susceptibility results for three S. pneumoniae strains
| S. pneumoniae strain and medium | Zone diam (mm)a
|
||||
|---|---|---|---|---|---|
| Oxacillin | Erythromycin | Chloramphenicol | Trimethoprim- sulfamethoxazole | Optochin | |
| ATCC 6305 | |||||
| CSB MHA | 32-35 | 31-33 | 32-33 | 26-32 | 13-20 |
| DSB MHA | 32-33 | 31-33 | 30-34 | 29-32 | 15-17 |
| Hu MHA | 32-39 | 30-35 | 30-39 | 28-40 | 14-17 |
| ATCC 49619a | |||||
| CSB MHA | 10-11 | 28-29 | 27-29 | 26-28 | 10-13 |
| DSB MHA | 10-11 | 27-29 | 26-27 | 25-27 | 10-13 |
| Hu MHA | 13-14 | 30-32 | 33-36 | 32-36 | 10-13 |
| Clinical isolate 42796 | |||||
| CSB MHA | 29-33 | 29-30 | 29-31 | 25-28 | 12-15 |
| DSB MHA | 28-31 | 29-32 | 29-32 | 28-31 | 12-14 |
| Hu MHA | 30-35 | 30-42 | 32-38 | 27-38 | 14-17 |
Values are ranges. Acceptable results for CLSI susceptibility quality control: oxacillin, ≤12 mm; erythromycin, 25 to 30 mm; chloramphenicol, 23 to 27 mm; trimethoprim-sulfamethoxazole, 20 to 28 mm.
The antibiotic susceptibility results for S. pyogenes showed that the citrated sheep Mueller-Hinton blood agar (CSB MHA) and the Hu MHA plates displayed larger zone sizes than the defibrinated Mueller-Hinton blood agar (DSB MHA) plate (Table 4). It was much easier to read the defibrinated and citrated sheep blood agars due to the hemolysis present. The Hu MHA plates were difficult to read due to the lack of beta-hemolysis.
TABLE 4.
Antibiotic disk sensitivity results for three S. pyogenes strains
| S. pyogenes strain and medium | Zone diam (mm)
|
|||
|---|---|---|---|---|
| Penicillin | Erythromycin | Chloramphenicol | Vancomycin | |
| ATCC 19615 | ||||
| CSB MHA | 38-42 | 35-36 | 28 | 22-23 |
| DSB MHA | 32-33 | 25-27 | 26-27 | 22-23 |
| Hu MHA | 35-37 | 24-25 | 27-28 | 23-24 |
| JC 20 | ||||
| CSB MHA | 33-34 | 27-29 | 22-23 | 20-21 |
| DSB MHA | 30-31 | 24-25 | 22-23 | 18-19 |
| Hu MHA | 35-36 | 24-25 | 22-24 | 20-22 |
| Clinical isolate | ||||
| CSB MHA | 38-39 | 28-29 | 27-28 | 21-22 |
| DSB MHA | 38-40 | 28 | 28-29 | 19-20 |
| Hu MHA | 31-34 | 22-25 | 27-28 | 18-21 |
DISCUSSION
The results presented here confirm that although S. pneumoniae, S. aureus, and S. pyogenes grow on HuBA, the colony sizes tend to be very small, the morphology often varies from that present on animal blood agar, and hemolysis is minimal. As colony size, colony morphology, and hemolysis are all critical to the identification of these organisms, there is a much greater chance these organisms from human specimens cultured on HuBA will be overlooked or misidentified, especially when other organisms may be present (e.g., for samples from the upper respiratory tract or skin swabs). HuBA also performed poorly compared to the performance of sheep blood agar for antibiotic susceptibility testing. These findings have profound implications for developing countries, where expired human blood is commonly used as a medium supplement. Aside from the implications for clinical care, it is likely that measurements of the disease burden due to these organisms are underestimates in countries that routinely use HuBA.
To our knowledge, this is the first time that HuBA has been shown to be suboptimal for the growth and susceptibility testing of S. pneumoniae, S. aureus, and S. pyogenes. The only published comparison of HBA, DSBA, and anticoagulated human blood agar that we could find was a study that compared the isolation of Bordetella pertussis on different blood agars (4). That study demonstrated that HuBA was inferior to defibrinated horse and sheep blood agar. It is not clear why HuBA is inferior to agar with other animal blood. It has been suggested that human blood may contain antibiotics, antibodies, or other anti-infective agents (5). The lack of hemolysis on human blood agar may be due to the age of the red cells in the expired human blood or some other factor.
By contrast, we were able for the first time to describe a practical alternative to human blood—citrated sheep blood—that most countries should be able to adapt without requiring a major investment in infrastructure. Citrate is said to have antibacterial characteristics (8, 9), but we found that CSBA performed similarly in most aspects to DSBA. Other studies have demonstrated that defibrinated pig blood and goat blood are suitable alternatives for medium supplements for S. pneumoniae (1, 3), raising the possibility that citrated blood from animals other than sheep may also be acceptable. In our experience, it is the collection of defibrinated blood rather than the availability of the animal that has posed the most difficult hurdle in providing a sustainable supply of blood.
We are confident that CSBA may be used for the isolation of all three organisms tested in this study. Our study evaluated a citrate to blood ratio of 1:10. It is not known whether a ratio lower than this may affect the growth and sensitivity pattern of the organisms; therefore, care needs to be taken at the point of collection to ensure that the correct ratio of blood is collected.
Hu MHA plates, which are used routinely in developing countries, cannot be recommended for use for the isolation or susceptibility testing of any of these organisms, which supports existing recommendations (1, 2, 3, 5). A strategy is required to phase out its use. Further work is required to assess whether CSB MHA is suitable for antibiotic susceptibility testing for S. pneumoniae. Preliminary data suggest that it is likely to perform better than Hu MHA. Our results did not confirm that CSB MHA plates could be used for susceptibility testing for S. pyogenes. Further work is required to assess whether CSBA and CSB MHA are suitable for the isolation and susceptibility testing, respectively, of a larger range of organisms. This will determine whether they could be recommended as practical alternatives for universal use.
REFERENCES
- 1.Anand, C. R., H. Gordon, H. Shaw, K. Fonseca, and M. Olsen. 2000. Pig and goat blood as substitutes for sheep blood in blood-supplemented agar media. J. Clin. Microbiol. 38:591-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1998. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. Centers for Disease Control and Prevention, Atlanta, Ga.
- 3.Gratten, M., D. Battistutta, P. Torzillo, J. Dixon, and K. Manning. 1994. Comparison of goat and horse blood as culture medium supplements for isolation and identification of Haemophilus influenzae and Streptococcus pneumoniae for upper respiratory tract secretions. J. Clin. Microbiol. 32:2871-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe, J. E., and M. Schlagenhauf. 1989. Comparison of three kinds of blood and two incubation atmospheres for cultivation of Bordetella pertussis on charcoal agar. J. Clin. Microbiol. 27:2115-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, D. R., E. L. Kaplan, J. Sramek, R. Bicova, J. Havlicek, H. Havilickova, J. Moliova, and P. Kriz. 1996. Laboratory diagnosis of group A streptococcal infections. World Health Organization, Geneva, Switzerland.
- 6.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests; approved standard, 8th ed. M2-A8, vol. 23, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing. NCCLS document M100-S13 (M2). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Phillips, C. A. 1999. The effects of citric acid, lactic acid, sodium citrate and sodium lactate, alone and in combination with nisin, on the growth of Arcobacter butzleri. Lett. Appl. Microbiol. 29:424-428. [DOI] [PubMed] [Google Scholar]
- 9.Young, K. M., and P. M. Foegeding. 1993. Acetic, lactic, and citric acids and pH inhibition of Listeria monocytogenes Scott A and the effect on intracellular pH. J. Appl. Bacteriol. 74:515-520. [PubMed] [Google Scholar]