Abstract
We report on a case of gastric syphilis in a patient with chronic dyspepsia. The diagnosis was established by serology and the demonstration of spirochetes in diffusely inflammed gastric mucosa by staining with a fluorescent monoclonal antibody specific for pathogenic treponemes and by the detection of specific treponemal DNA sequences by a real-time PCR.
CASE REPORT
A 35-year-old man with dyspepsia of long duration was subjected to gastric endoscopy on two occasions in January and February 2005 at a private clinic in Portugal. He was found to have multiple, nonhealing, gastric ulcers that failed to respond to proton pump inhibitor therapy. A workup for Helicobacter pylori infection was negative, and no antibiotic therapy was prescribed. Subsequently, a diffuse gastric mucosal thickening was observed, raising suspicion for a neoplastic process. Eventually, a distal gastrectomy was performed on the patient at the University Hospital of Coimbra in Coimbra, Portugal. The antral mucosa appeared to be erythematous and edematous, with irregular nodular masses. The gastric body mucosa, however, had a normal appearance. Numerous lymph nodes were isolated from both curvatures of the stomach, as well as from the hepatic and celiac chains, for histopathologic examination. The resected gall bladder was unremarkable, and no stones were found.
No evidence of neoplasia but, rather, a diffuse destructive inflammatory gastritis was found. Notably, no parasites, viral inclusions, or H. pylori was found on initial microscopic evaluation. Paraffin-embedded material and tissue sections were sent to the Gastrointestinal Pathology Service of the Massachusetts General Hospital for evaluation. The examination confirmed the presence of a dense diffuse mucosal lymphoplasmacytic infiltrate with only scattered residual glandular elements and intraluminal abscesses (Fig. 1). An ill-defined granulomatous process was also noted. Although the inflammation was primarily mucosal, some inflammation spilled over into the superficial submucosa, where a perivascular distribution of the lymphohistiocytic and plasma cell-rich infiltrate raised the possibility of gastric syphilis. On the basis of this information, the patient was subsequently investigated and was found to be human immunodeficiency virus (HIV) negative; but serologic testing revealed a reactive polyclonal hypergammaglobulinemia, a positive Venereal Disease Research Laboratory blood test titer of 1:16, and a Treponema pallidum hemagglutination assay value of 1:1,028. Despite a noncontributory Warthin-Starry staining result, gastric syphilis was strongly considered; and the paraffin-embedded thin sections of resected tissue were forwarded to the Laboratory Reference and Research Branch, Division of STD Prevention, Centers for Disease Control and Prevention (CDC) in Atlanta, Ga., for direct fluorescent-antibody staining for pathogenic treponeme detection and PCR for treponemal DNA detection. At the CDC, formalin-fixed thin sections (thickness, 10 μm) were deparaffinized by standard procedures and were subsequently placed in 500 ml of phosphate-buffered saline (PBS; pH 7.2) and microwaved (1,400 W, 2,450 MHz) for 15 min on a “high” setting. A fluorescein isothiocyanate (FITC)-labeled mouse antitreponemal 37-kDa monoclonal antibody (5) was diluted 1:100 in PBS containing 2% Tween 80 and 1:20,000 Evans blue dye. The conjugate was placed over the sections, and the sections were incubated for 30 to 60 min at 37°C inside a moist chamber. Following the incubation, the slides were soaked in PBS for 10 min, briefly rinsed with water, and blotted. The slides were mounted in Fluoroguard (Bio-Rad) and covered with a coverslip. The slide was examined with a Nikon microscope (Eclipse E400) equipped with incident illumination with a mercury lamp (HG-100). Observations were made by using ×40/0.65, ×50/0.9 oil, or ×100/0.5 to 1.3 oil objectives and ×10 oculars. Two filter cubes were used: one (B-2A) to locate stained treponemes on or within the tissue and the other (B-2E/C) to contrast any stained treponemes against the tissue. The positive control used for immunofluorescence staining was T. pallidum harvested from rabbit testes, and tissues from healthy rabbit testes were used as a negative control. The FITC-labeled monoclonal antibody was reactive only for pathogenic treponemes, including T. pallidum subsp. pallidum, T. pallidum subsp. endemicum (which causes endemic syphilis), T. pallidum subsp. pertenue (which causes yaws), and T. pallidum subsp. carateum (which causes pinta). The last three treponemes are transmitted by nonvenereal routes, usually by direct contact during childhood; and their clinical manifestations include a spectrum of cutaneous lesions and late sequelae that are usually limited to skin, bone, and cartilage. No documented reports indicate that these nonvenereal treponemal infections cause damage to internal organs, and it is also widely accepted that they are not involved in central nervous system or congenital transmission, whereas venereal syphilis can affect practically any system or organ (1). The monoclonal antibody does not react with nonpathogenic treponemes (Treponema phagedenis, Treponema denticola, Treponema refringens) or other spirochetes (Borrelia burgdorferi and Leptospira interrogans) (5). Direct immuofluorescent-antibody test results revealed the presence of numerous spirochetes that were immunologically specific for pathogenic treponemes (Fig. 2A and B).
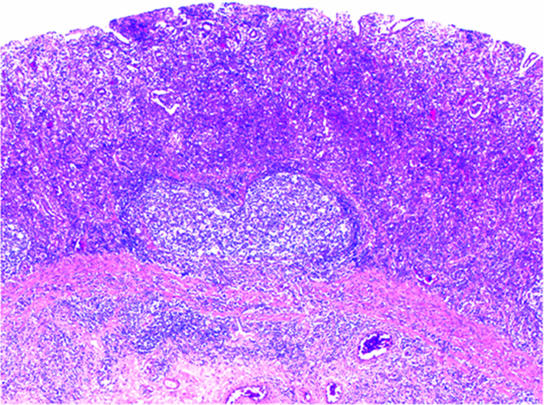
FIG. 1.
Gastric syphilis: histopathology of the stomach antrum. The mucosal glandular architecture is erased by a dense and diffuse lymphoplasmacytic infiltrate. Only residual glands with luminal neutrophilic infiltrates are seen. Inflammatory spillover is present in the submucosa. (Hematoxylin and eosin stain was used. Magnification, ×400.)
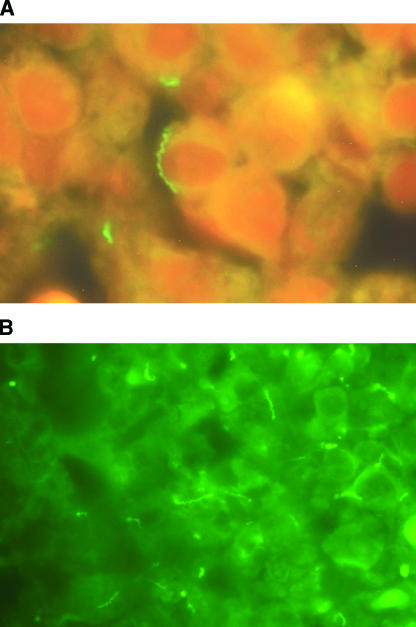
FIG. 2.
Specific immunofluorescence staining of pathogenic treponemes with monoclonal antibody. The FITC-labeled mouse antitreponemal 37-kDa monoclonal antibody stain demonstrates numerous spirochetes in thin-section specimens by the use of a ×100 oil objective and a ×10 ocular. Filter cubes B-2A and B-2C/E were used in photographs A and B, respectively.
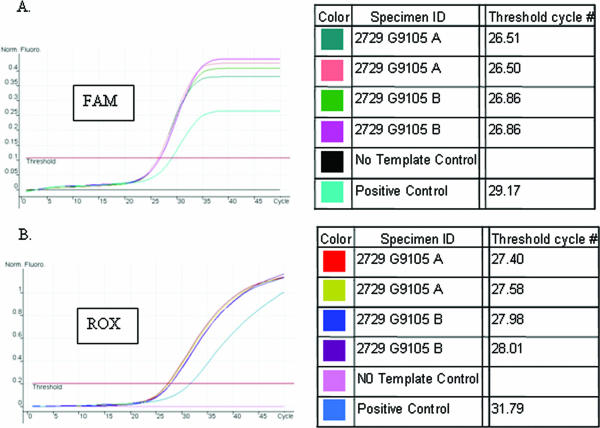
A real-time PCR specific for pathogenic treponemes detection was performed with the DNA extracted from the paraffin-embedded thin section of ulcerative tissues with a QIAamp DNA mini kit (QIAGEN) by use of the protocol for fixed tissues. Two specific DNA targets, the 47-kDa lipoprotein gene and the DNA polymerase I gene (polA) for T. pallidum, were used in the real-time simplex PCR test. A Rotor-Gene 3000 real-time PCR instrument (Corbett Research, Sydney, Australia) was used to perform the test. PCR amplifications were performed in 25-μl reaction tubes with 10 μl of DNA samples. Each reaction mixture contained the following at the indicated final concentrations: 300 nM TP-1 (CAGGATCCGGCATATGTCC), 300 nM TP-2 (AAGTGTGAGCGTCTCATCATTCC), and 200 nM TP-3 (6-carboxyfluorescein [FAM]-CTGTCATGCACCAGCTTCGACGTCTT-black hole quencher 1 [BHQ1]) for polA detection or 300 nM TP-7 (CAACACGGTCCGCTACGACTA), 300 nM TP-8 (TGCCATAACTCGCCATCAGA), and 200 nM TP-13 (6-carboxy-X-rhodamine [ROX]-ACGGTGATGACGCGAGCTACACCA-BHQ3) for 47-kDa lipoprotein gene detection; 1× PCR buffer (Applied Biosystems); 5 mM MgCl2; 200 μM each dATP, dGTP, dCTP, and dUTP; 0.5 unit of uracil-N-glycosylase (Applied Biosystems); and 1 unit of AmpliTaq Gold DNA polymerase (Applied Biosystems). Appropriate positive and no-template controls were included in each run. The following conditions were used for the PCR amplification: the first cycle was 50°C for 2 min, followed by 95°C for 10 min; and the subsequent 45 to 50 PCR cycles consisted of 95°C for 20 s and 60°C for 1 min. The analytical sensitivity of the assay for each PCR target, determined by using a serial dilution of purified T. pallidum genomic DNA, was approximately 1 to 10 genomic copies per reaction mixture. Similarly to the monoclonal antibody staining, the polA and 47-kDa lipoprotein gene PCRs are specific only for pathogenic treponemes. No PCR amplification was detected when a panel of organisms, including nonpathogenic treponemes, commensal and pathogenic microbes found in the genitourinary tract, and normal skin flora, was tested (6, 9). The real-time simplex PCR amplification curves are illustrated in Fig. 3. Both FAM- and ROX-labeled TaqMan probes detected positive fluorescent signals, indicated by arbitrary threshold cycle numbers, from duplicate DNA samples extracted from two separate paraffin-embedded thin sections as well as from the positive control DNA from the Nichols strain of T. pallidum (Fig. 3A and B). Samples without DNA template were used as negative controls.
FIG. 3.
Detection of pathogenic treponeme-specific DNA from paraffin-embedded thin sections by the real-time PCR. Amplification results of real-time simplex PCR specific for T. pallidum DNA detection are shown. (A) FAM-labeled TaqMan probed targeting polA; (B) ROX-labeled TaqMan probed targeting the 47-kDa lipoprotein gene.
Reported cases of gastric involvement by T. pallidum have been rare in the medical literature. Ikebe et al. (3) reviewed 59 cases of gastric syphilis reported between 1971 and 1990. Since 1990, only 34 cases, with 2 being HIV-infected patients, have been documented (2). Documented symptoms of gastric syphilis are usually nonspecific and include nausea, vomiting, abdominal pain, and weight loss. Complications including gastric hemorrhage, perforation, and gastric outlet obstruction have also been reported but are less common (10). Gastric syphilis may exhibit a variety of endoscopic and radiographic appearances, including mucosal erosions, shallow ulcers, rugal hypertrophy, and nodularity, that are indistinguishable from gastric lymphoma or linitis plastica. Routine staining of the gastric biopsy specimen with hematoxylin-eosin typically shows marked, diffuse, chronic inflammation composed of a dense lymphoplasmacytic cell infiltrate with or without granulomas, often in a perivascular distribution. Endarteritis obliterans may also be present. The findings are etiologically nonspecific, and a high index of clinical suspicion is needed to prompt further evaluation and establishment of a definitive diagnosis.
Warthin-Starry silver stain is capable of demonstrating spirochetes and confirming the diagnosis. However, the certainty of detecting spirochetal organisms can sometimes be compromised by an abundance of elastic and reticulum fibers in the background. In addition, this method cannot differentiate T. pallidum from contaminating oral or skin spirochetes. The treponemes have also been detected by immunofluorescence staining with FITC-labeled anti-Treponema globulin, but the stain also exhibits considerable cross-reactivity with other spirochetes, such as Borrelia burgdorferi (7). The specificity of immunofluorescent staining for pathogenic treponemes improved when Ito et al. (5) introduced the use of FITC-labeled monoclonal antibodies directed against the T. pallidum antigen with a molecular weight of 37 kDa. These monoclonal antibodies did not react with nonpathogenic treponemes and other spirochetes. Inagaki et al. (4) initially applied the use of conventional PCR as a confirmatory tool to detect T. pallidum DNA in formalin-fixed, paraffin-embedded gastric biopsy materials from two cases of gastric syphilis. A region of tmpA was used as the PCR target, as it was considered specific for members of the genus Treponema. The specificity of PCR-amplified products was verified by direct sequencing and by restriction digestion. PCR is considered more sensitive and specific than the traditional silver and immunofluorescence staining, and a properly controlled PCR would seem to be an appropriate step in making a definitive diagnosis of gastric syphilis (8). In the present study, we have used unique regions of polA and the 47-kDa protein gene as the real-time PCR targets, as they had previously been demonstrated to be specific for pathogenic treponemes but not for nonpathogenic spirochetes by PCR or multiplex PCR assays (6, 9). The limits of detection of these PCR assays were approximately 1 to 10 genomic copies per reaction. The additional perceived advantages of the real-time PCR would be rapidity, the reduced risk of contamination owing to the closed-system detection format and the use of uracil-N-glycosylase to prevent carryover contamination, high reproducibility, possible application at the point-of-care facility, and the potential of detecting multiple target organisms in one assay.
Gastric syphilis remains a rare manifestation of the secondary and tertiary forms of the disease, which may affect young adults. It is exceedingly difficult to make a definitive diagnosis of gastric syphilis on the basis of biopsy findings, since spirochetes are seen infrequently and histopathologic findings, while often suggestive, are seldom diagnostic. Unless gastric syphilis is suspected and appropriate staining or molecular testing is performed, the diagnosis cannot be made with certainty. The diagnosis has often been inferred in retrospect when examination of a gastric lesion is negative for cancer, serologic tests are positive for syphilis, and the gastric lesion resolves after therapy with penicillin. Gastric syphilis should be considered in patients at risks for sexually transmitted diseases who complain of nausea, vomiting, weight loss, and abdominal pain and in whom unusual gastric lesions or presumed peptic ulcers unresponsive to standard therapy are encountered. The clinician needs to be aware of this disease entity when he or she is evaluating patients with a prior history of sexually transmitted infections and a positive routine nontreponemal test, particularly in a population where the prevalence of syphilis is high. In this study, we have used the FITC-labeled anti-T. pallidum monoclonal antibody stain, combined with the most recent molecular diagnostic test, real-time PCR, to confirm the diagnosis of gastric syphilis in one patient. Both methods are specific for the detection of pathogenic treponemes; nevertheless, a better sensitivity and a more rapid diagnosis can be achieved by using the real-time PCR. In the case reported here, the actual stage of syphilis was not known in this patient, and information on the history of sexually transmitted infections or previous manifestations suggestive of primary or secondary syphilis in this patient was also unavailable. After a diagnosis of gastric syphilis was established, the patient was treated with antibiotic therapy and has improved. No posttherapy specimens were taken.
REFERENCES
- 1.Antal, G. M., S. A. Lukehart, and A. Z. Meheus. 2002. The endemic treponematoses. Microbes Infect. 4:83-94. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero, A. F., T. M. Straight, J. Eastone, and K. Spooner. 2005. Gastric syphilis in an HIV-infected patient. AIDS Patient Care STDs 19:281-285. [DOI] [PubMed] [Google Scholar]
- 3.Ikebe, M., T. Oiwa, M. Mori, H. Kuwan, K. Sugimachi, and T. Yao. 1994. Gastric syphilis: case report and review of the literature. Radiat. Med. 12:171-175. [PubMed] [Google Scholar]
- 4.Inagaki, H., T. kawai, M. Miyata, S. Nagaya, H. Tateyama, and T. Eimoto. 1996. Gastric syphilis: polymerase chain reaction detection of treponemal DNA in pseudolymphomatous lesions. Hum. Pathol. 27:761-765. [DOI] [PubMed] [Google Scholar]
- 5.Ito, F., E. F. Hunter, R. W. George, V. Pope, and S. A. Larsen. 1992. Specific immunofluorescent staining of pathogenic treponemes with a monoclonal antibody. J. Clin. Microbiol. 30:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, H., B. Rodes, C.-Y. Chen, and B. Steiner. 2001. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J. Clin. Microbiol. 39:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnarelli, L., J. F. Anderson, and R. C. Johnson. 1987. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J. Infect. Dis. 156:183-188. [DOI] [PubMed] [Google Scholar]
- 8.Norris, S., and S. Sell. 1996. Role of polymerase chain reaction in the diagnosis of gastric syphilis. Hum. Pathol. 27:749-750. [DOI] [PubMed] [Google Scholar]
- 9.Orle, K. A., C. A. Gates, D. H. Martin, B. A. Body, and J. B. Weiss. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus type 1 and 2 from genital ulcers. J. Clin. Microbiol. 34:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winters, H. A., V. Notar-Francesco, K. Bromberg, S. A. Rawstom, J. Vetrano, V. Prego, J. Kuan, and J.-P. Raufman. 1992. Gastric syphilis: five recent cases and a review of the literature. Ann. Intern. Med. 116:314-319. [DOI] [PubMed] [Google Scholar]