Abstract
In India, rabies is enzootic and is a serious public health and economic problem. India has a large population of stray dogs which, together with a lack of effective control strategies, might have led to the persistence of rabies virus (RV) in the canine population. Our objective was to study the molecular epidemiology of RV isolates in India based on nucleotide sequence analysis of 29 RV isolates originating from different species of animals in four states. Here we have analyzed two sets of sequence data based upon a 132-nucleotide region of the cytoplasmic domain (CD) of the G gene (G-CD) and a 549-nucleotide region (Psi-L) that combines the noncoding G-L intergenic region (Psi) and a fragment of the polymerase gene (L). Phylogenetic analysis revealed that the RV isolates belong to genotype 1 and that they were related geographically but were not related according to host species. Five different genetic clusters distributed among three geographical regions were identified. Comparison of the deduced amino acid sequences of G-CD between RV isolates revealed three amino acid changes (amino acid 462G [aa462G], aa465H, and aa468K) that distinguished the Indian RVs from RV isolates in other parts of the world. Analysis of the data indicated that the dog rabies virus variants are the major circulating viruses in India that transmit the disease to other domestic animals and humans as well.
Rabies is enzootic and is a serious public health and economic problem in India. More than 99% of all human deaths from rabies occur in the developing world (44). A recent national survey by the Association of the Prevention and Control of Rabies in India (1) estimated that in India a total of 18,500 human deaths occur as a result of rabies each year. Although the loss of livestock due to rabies is significant, there are few publications on estimates of the incidence of rabies in livestock (17). In India, rabies occurs mainly in the urban form, although the existence of a sylvatic cycle cannot be ruled out. In the urban form, dogs play an important role as the reservoir and transmitter of the disease to humans and domestic animals.
Rabies virus (RV; genus Lyssavirus, family Rhabdoviridae) possesses a single-stranded, nonsegmented, negative-sense RNA approximately 12 kb in length (40). The viral genome encodes five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA-dependent RNA polymerase (L) (45). The G protein controls major aspects of host cell infection, such as receptor binding, antigenicity, and host adaptation (2). RNA viruses are characterized by a high mutation rate during replication due to the lack of proofreading and postreplication error correction by the RNA polymerase (11). However, the G-protein-coding region of rabies viruses and the more divergent non-rabies virus lyssaviruses consist of a well-conserved ectodomain, a variable transmembrane domain, and a cytoplasmic domain (G-CD) (41). G-CD has been shown to interact with internal proteins (10, 22, 43).
The genetic diversity seems to provide an adaptive potential for RV that can vary according to the natural history of the virus (16, 23). Nucleotide sequence analysis permits the most precise definition of virus type and helps in understanding the transmission of rabies virus from the reservoir host to other hosts, including humans and domestic animals. To date, molecular epidemiological studies of rabies viruses have been performed by sequencing different regions of RV genomes (4, 5, 8, 13, 14, 15, 24, 26, 27, 28, 29, 30, 32, 33, 36, 42).
This report describes the genetic characterization of Indian RV isolates through nucleotide sequencing of the G-CD region and of the noncoding G-L intergenic region (Psi) and the L region (Psi-L region). The phylogenetic relationships of Indian RV isolates were examined, and the regional distributions of genetic clusters thus obtained are described in detail.
MATERIALS AND METHODS
Rabies viruses.
Twenty-nine brain tissue samples were collected from four different animal species, viz., dog (13 isolates), buffalo (9 isolates), cow (3 isolates), and goat (4 isolates), from four states in India (Punjab, Andhra Pradesh, Karnataka, and Kerala). A 20% (wt/vol) homogenate of the brain tissue samples was prepared by using 2% horse serum in sterile phosphate-buffered saline and was stored in the vapor phase of liquid nitrogen until it was used for reverse transcription-PCR (RT-PCR). Those samples which tested positive for RV by both the fluorescent-antibody test (9) and the mouse inoculation test (18) were used for nucleotide sequencing (Table 1).
TABLE 1.
Epidemiological information for the rabies virus isolates
| Sample no. | Virus reference isolate | Virus identifier | Host species | Place of origin | Yr of isolation | GenBank accession no. |
|---|---|---|---|---|---|---|
| 1 | IPU-R2 | R2 | Buffalo | Ludhiana, Punjab, India | 2001 | DQ255915 |
| 2 | IPU-R3 | R3 | Buffalo | Sangrur, Punjab, India | 2001 | DQ255916 |
| 3 | IPU-R4 | R4 | Buffalo | Sangrur, Punjab, India | 2001 | DQ255917 |
| 4 | IPU-R5 | R5 | Buffalo | Ropar, Punjab, India | 2001 | DQ255918 |
| 5 | IPU-R6 | R6 | Buffalo | Bathinda, Punjab, India | 2001 | DQ255919 |
| 6 | IPU-R7 | R7 | Buffalo | Ludhiana, Punjab, India | 2002 | DQ255920 |
| 7 | IPU-R8 | R8 | Buffalo | Ludhiana, Punjab, India | 2002 | DQ255921 |
| 8 | IPU-R9 | R9 | Buffalo | Ropar, Punjab, India | 2002 | DQ255922 |
| 9 | IPU-R10 | R10 | Buffalo | Ludhiana, Punjab, India | 2002 | DQ255923 |
| 10 | IKE-R76 | R76 | Dog | Thrissur, Kerala, India | 2004 | DQ255924 |
| 11 | IKE-R81 | R81 | Dog | Thrissur, Kerala, India | 2004 | DQ255925 |
| 12 | IKE-R82 | R82 | Goat | Thrissur, Kerala, India | 2004 | DQ255926 |
| 13 | IKE-R94 | R94 | Dog | Thrissur, Kerala, India | 2004 | DQ255927 |
| 14 | IKE-R96 | R96 | Dog | Thrissur, Kerala, India | 2004 | DQ255928 |
| 15 | IKE-R97 | R97 | Dog | Thrissur, Kerala, India | 2004 | DQ255929 |
| 16 | IKE-R98 | R98 | Goat | Thrissur, Kerala, India | 2004 | DQ255930 |
| 17 | IKE-R109 | R109 | Dog | Manapoor, Kerala, India | 2004 | DQ255931 |
| 18 | IKE-R110 | R110 | Cow | Kottanallore, Kerala, India | 2004 | DQ255932 |
| 19 | IKE-R111 | R111 | Goat | Kootala, Kerala, India | 2004 | DQ255933 |
| 20 | IKE-R114 | R114 | Cow | Venginnissery, Kerala, India | 2004 | DQ255934 |
| 21 | IKE-R116 | R116 | Dog | Vadakkanchery, Kerala, India | 2004 | DQ255935 |
| 22 | IKE-R117 | R117 | Dog | Vythala, Kerala, India | 2004 | DQ255936 |
| 23 | IKE-R121 | R121 | Goat | Kuttala, Kerala, India | 2004 | DQ255937 |
| 24 | IKE-R122 | R122 | Cow | Aarattupuzha, Kerala, India | 2004 | DQ255938 |
| 25 | IKE-R123 | R123 | Dog | Chalakkudi, Kerala, India | 2004 | DQ255939 |
| 26 | IAP-R141 | R141 | Dog | Hyderabad, Andhra Pradesh, India | 2005 | DQ255940 |
| 27 | IKA-R142 | R142 | Dog | Bangalore, Karnataka, India | 2005 | DQ255941 |
| 28 | IKA-R144 | R144 | Dog | Bangalore, Karnataka, India | 2005 | DQ255942 |
| 29 | IKA-R145 | R145 | Dog | Bangalore, Karnataka, India | 2005 | DQ255943 |
| 30 | IRN1-HM | IRN1 | Human | Iran | 1988 | AF325472 |
| 31 | CHI1-BK | CHI | Goat | China | 1986 | AF325471 |
| 32 | MAL1-HM | MAL1 | Human | Malaysia | 1985 | AF325487 |
| 33 | THA1-HM | THA1 | Human | Thailand | 1983 | AF325488 |
| 34 | NEP1-DG | NEP1 | Dog | Nepal | 1989 | AF325489 |
| 35 | Mok-ZIM | MOKOLA | Cat | Zimbabwe | 1981 | S59447 |
| 36 | FRA1-FX | FRA1 | Fox | France | 1991 | AF325461 |
| 37 | HUN1-FX | HUN1 | Human | Hungary | 1992 | AF325462 |
| 38 | POL1-RD | POL1 | Raccoon dog | Poland | 1985 | AF325464 |
| 39 | SAF1-MG | SAF1 | Mongoose | South Africa | 1987 | AF325485 |
| 40 | YUG1-WF | YUG1 | Bovine | Yugoslavia | 1984 | AF325463 |
RNA extraction and RT-PCR.
The total RNA was extracted from the brain tissue homogenates by using the TRIZOL reagent (Invitrogen), according to the manufacturer's instructions. The extracted RNA was immediately used for RT-PCR with gene-specific primers (32). Briefly, about 1 μg of total RNA was used for RT-PCR (One-Step RT-PCR kit; QIAGEN, Hilden, Germany), according to the manufacturer's instructions. The following primers were used for amplification of the G-L intergenic region: forward primer G (5′-GAC TTG GGT CTC CCG AAC TGG GG-3′), which corresponds to bases 4665 to 4687 of the positive-sense G gene, and reverse primer L (5′-CAA AGG AGA GTT GAG ATT GTA GTC-3′), which corresponds to bases 5543 to 5520 of the negative-sense L gene sequences of the Pasteur virus (PV) strain (40). One cycle of reverse transcription was done at 50°C for 60 min, followed by denaturation at 94°C for 10 min. This was followed by 35 cycles of denaturation at 94°C for 30 s, primer annealing at 55°C for 40 s, and extension at 72°C for 1 min. A final 10-min extension step at 72°C was also done (42). The G-L primer pair amplified an 880-bp amplicon.
Nucleotide sequencing and phylogenetic analysis.
The amplicons were purified with a QIAquick PCR gel extraction kit (QIAGEN). The purified products were sequenced with the gene-specific primers used for RT-PCR amplification. Cycle sequencing was done with an ABI Prism BigDye Terminator cycle sequencing ready reaction kit (v3.1) and analyzed with an ABI Prism 310 genetic analyzer. For each RV isolate, the sequences included a 132-nucleotide region of G-CD corresponding to bases 4757 to 4892 of the PV genome (40) and a 519-nucleotide region of the G-L intergenic region (Psi), along with the 30 nucleotides of the adjacent L gene (herein referred to as Psi-L) of the PV genome. The G-gene region covered all 44 amino acids of G-CD. The nucleotide sequences were aligned by using the ClustalW 1.8x program (39); and a neighbor-joining tree was drawn by using the MEGA version 3.1 program (20), with confidence levels assessed by the use of 1,000 bootstrap replications. Maximum-likelihood (ML) trees obtained by use of the Tamura-Nei distance matrix (38) with the gamma distributions of the rates among the sites (with eight categories) were drawn by using TREE-PUZZLE (v5.0) software (37). The robustness of the phylogenetic tree was assessed by 10,000 puzzling steps, and the tree was drawn by using TreeView (Win 32) 1.6.6 (31). The GenBank sequences of the rabies virus isolates from various countries on the Asian and European continents and from South Africa (2) were included in the multiple-sequence alignment and for subsequent construction of the neighbor-joining tree (the tree is not shown) and the ML trees for comparison (Table 1). The ML trees are shown in Fig. 1 and Fig. 2.
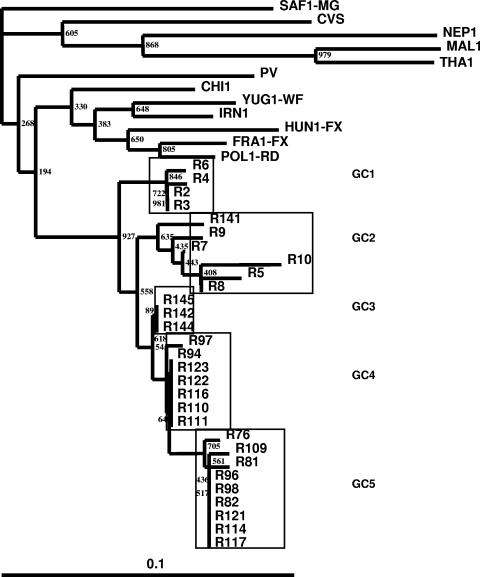
FIG. 1.
Maximum-likelihood tree showing the genetic relationship of Indian RV isolates as determined by analysis of 132 nucleotides coding for G-CD.
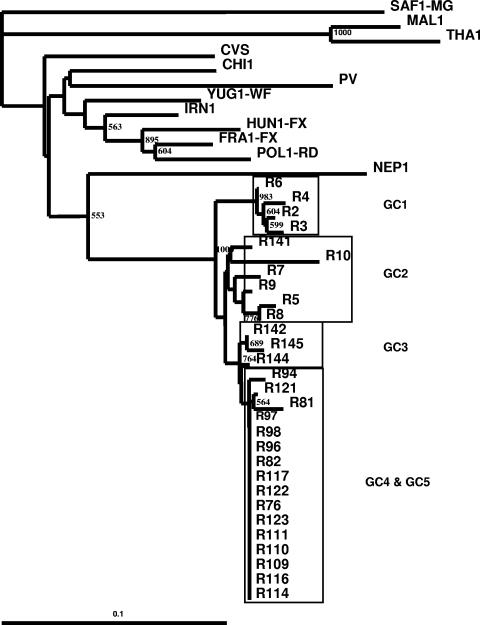
FIG. 2.
Maximum-likelihood tree showing the genetic relationship of Indian RV isolates as determined by analysis of 549 nucleotides coding for the Psi-L region.
RESULTS
Analysis of G-CD region.
The sequences of the G-CD-coding region of all the 29 RV isolates were aligned with the sequences of representatives of RV and RV-related viruses (RRVs). The ML tree showed that no Indian isolate showed homology with RRVs. All the isolates belonged to genotype 1 (<10% difference). Among the Indian RV isolates, >95% nucleotide similarity existed, even though they were from different geographical regions and different hosts (Fig. 1).
The RV isolates of Indian origin were distributed into five different genetic clusters (GCs), viz., GC1 to GC5 (Fig. 3). These clusters are very strongly supported by bootstrap values (93%, 56%, 62%, 64%, and 71%, respectively). GC1 and GC2 consisted of four and five RV isolates, respectively, from buffaloes from the state of Punjab collected in 2001 and 2002. It also included one RV isolate of dog origin from the state of Andhra Pradesh (isolate R141). GC3 consisted of three RV isolates of dog origin from the state of Karnataka collected during the year 2005. GC4 and GC5 consisted of seven and nine RV isolates, respectively; and these were isolated from three different species viz., dog, cow, and goat, from the state of Kerala during the year 2004. The geographical locations of the different genetic clusters of Indian RV isolates are provided in Fig. 4.
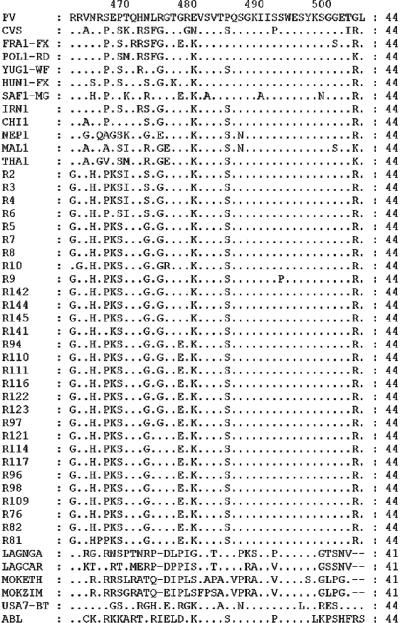
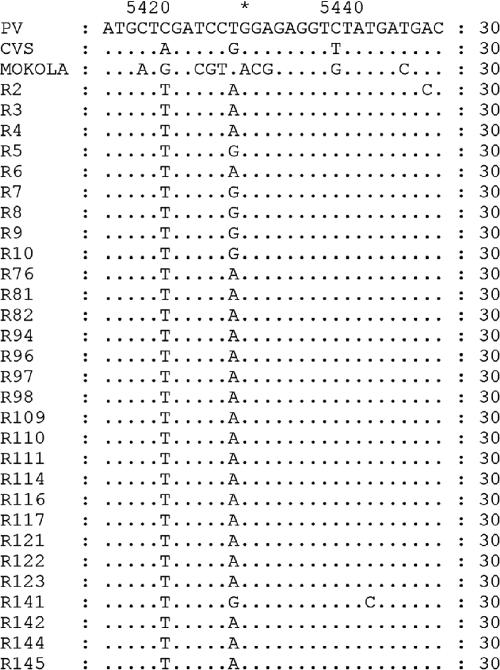
FIG. 3.
Comparison of predicted G-CD protein sequences of rabies and rabies-related viruses. Differences from the PV sequence are indicated, and dots represent identity at that position.
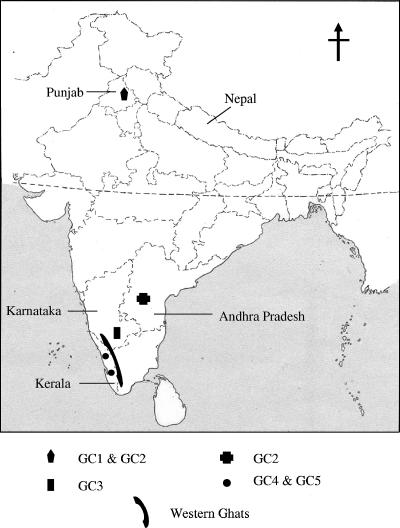
FIG. 4.
Distribution of rabies virus genetic clusters in the study area in India.
Analysis of the deduced amino acid sequences of G-CD (Fig. 3) revealed that the Indian RV isolates differed from the non-Indian RV isolates at amino acid 462 (aa462) (R/K→G), aa465 (N/S→H), and aa468 (E→K) (the E→K change was not found in isolates R2 and R6). The sequences of the GC1 RV isolates differed from those of the other clusters of isolates by two nonsynonymous changes at aa470 (T→I) and aa473 (G→S). The GC4 and GC5 RV isolates had unique nonsynonymous substitutions at aa478 (G→E). A change at aa475 (G→R) was noticed in GC5 RV isolates alone.
Analysis of Psi-L region.
The topology of the phylogenetic tree based on the nucleotide sequences of the Psi-L region was similar to that of the tree based on the G-CD region (Fig. 2). Random changes in the nucleotide sequences were noticed throughout the Psi region. However, none of the isolates examined showed any amino acid substitutions at positions 1 to 10 of the L protein, although a few synonymous mutations were detected (Fig. 5).
FIG. 5.
Alignment of partial L-protein-coding nucleotide sequence of rabies and rabies related viruses. Differences from the PV sequence are indicated, and dots represent identity at that position.
DISCUSSION
Twenty-nine brain tissue samples collected from dogs, buffaloes, cattle, and goats over a period of 5 years (2001 to 2005) were used in this study. These were collected from a northern state and three southern states of India. The nucleotide sequences of the G-CD and the Psi-L regions of these samples were studied to obtain an understanding of the epidemiological relationships of the RV isolates in the Indian subcontinent.
In India, rabies is region specific but not host specific, as is apparent from the phylogenetic trees (Fig. 1 and 2). The RV isolates formed distinct clusters which were ordered by geography, although RV isolate R141 was an exception to this, since it grouped with GC2 (Fig. 1). Similar patterns of geographical distributions based on analysis of the G-CD and Psi-L regions were reported in southern African (28) and in Israel and Middle Eastern countries (8). Our results indicate that the Indian RV isolates studied are genetically related to one another (average nucleotide similarity, >95%). None of the isolates studied were related to the RRVs, indicating the absence of a prevalence of RRVs in domestic animals, including dogs. Phylogenetic analysis of the nucleotide sequence of G-CD revealed the existence of five different genetic clusters. RV isolates of buffalo origin from the state of Punjab, in northern India, formed two distinct clusters (GC1 and GC2). The grouping of isolate R141 from southern India with GC2 shows the possibility of migration of RV reservoirs (pet dogs) within the country due to the changing socioeconomic conditions in India. The unrestricted movement of animals could be a possible reason for the transmission of the virus from one part of the country to another, which may result in the spillover of genetic clusters outside of a geographical niche.
Two nonsynonymous changes at aa470 (T→I) and aa473 (G→S) distinguished isolates in GC1 from the isolates in the other genetic clusters, including GC2 isolates, even though they belonged to the same region (Punjab). RV isolates from the state of Kerala grouped in two genetic clusters, GC4 and GC5. Unlike the RV isolates of GC1 and GC2, which were of buffalo origin, the RV isolates of GC4 and GC5 originated from three different host species, namely, dogs, cattle, and goats, clearly indicating the possible transmission of the virus from dogs to other domestic animals. The aa478 G→E mutation distinguished RV isolates of GC4 and GC5 from the rest of the isolates. RV isolates of GC5, however, had a characteristic change at aa475 (G→R). GC4 and GC5 appeared to be restricted to the state of Kerala, while the RV isolates from the bordering state of Karnataka formed a separate genetic cluster, GC3. Surprisingly, the deduced amino acid sequences revealed 100% similarity between GC3 and GC2, indicating that the groupings GC2 and GC3 were based on the synonymous changes that were present in the nucleotide sequences. However, GC3 was found to have an ancestral branch which was common to GC4 and GC5 in the phylogenetic tree drawn by use of the nucleotide sequence of G-CD (Fig. 1). With the other genetic clusters, GC1, GC2, GC4, and GC5, the amino acid substitutions paralleled the proposed phylogenetic relationships.
The G-CD amino acid sequences of Indian RV isolate were compared with those of RV isolates from Asia, Europe, the Americas, and South Africa and RRVs (complete data not shown). This comparative analysis uncovered two distinct features. First, the strong conservation of the C-terminal 28 amino acids (aa478 to aa505) of the Indian RV isolates compared with the reported conservation of 6 amino acids in the region from aa493 to aa498 of G-CD of lyssaviruses (3) was noted. This is in contrast to the observations of Nadin-Davis et al. (25), who reported greater diversity in G-CD. In addition to its role as a membrane anchor, G-CD may influence viral budding efficiency (21). Minor differences in its sequence could thus affect the rates of viral propagation within a host and transmission between hosts and, hence, could potentially influence disease incidence patterns (25). Second, the existence of three amino acids, aa462G, aa465H, and aa468K, which are unique to Indian RV isolates (with isolates R2 and R6 being exceptions) was noted; and this could possibly help in distinguishing Indian RV isolates from rabies virus isolates in other parts of the world. We propose that these three amino acid (G, H, and K) substitutions be an “epidemiological marker” which can aid in tracing the origins of RV isolates. An epidemiological marker of this kind would be more useful for the detection of human travel-related rabies acquired from countries where rabies is endemic. There has been an increase in the number of human travel-related cases of rabies contracted during travel to countries where rabies is endemic (19, 34). Earlier, Smith et al. (35) used the differential digestion of PCR-amplified products with restriction enzymes to trace the origin of rabies virus infection among immigrants to the United States. In the present study, PCR followed by DNA sequencing was used to deduce the amino acid sequences. Unique amino acid residues at certain positions were identified as possible markers for Indian isolates.
The topology of the phylogenetic tree obtained on the basis of the nucleotide sequence of the Psi-L region was exactly the same as that obtained on the basis of the nucleotide sequence of G-CD. The Psi gene is reportedly divergent among the rabies viruses reported earlier (40). As expected, random changes in the nucleotide sequence throughout the Psi region were noticed. In contrast, the L proteins of the isolates showed a high degree of conservation in the first 10 amino acids analyzed.
Certain geographic features, such as mountains and rivers, may create physical barriers to animal movement and promote localized viral evolution in specialized host niches (6). The extent of influence of physical barriers on the distribution of RV was analyzed by examining the physical features which separate the regions harboring different genetic clusters of RV. The Indian state of Kerala is bounded on the west and the south by the Arabian Sea and the Indian Ocean, respectively, and on the east and north by western Ghats (Fig. 4), and the landscape consists of wooded hilly areas with rivers and valleys. The isolation of RV isolates of GC4 and GC5 only in the state of Kerala could be probably due to these geographical barriers, which restrict the movement of infected animals. It is noteworthy that all the Indian RV isolates were distinct from a Nepalese RV isolate taken for comparison, although Nepal is linked to India by land but is separated from India by the Great Himalayan mountain range. The Indian RV isolates were distinct from other RV isolates, demonstrating the possible existence of a separate evolutionary mechanism in India.
In India, rabies is enzootic in dogs, and transmission of rabies virus to other animals and humans is primarily through the bite of a rabid dog. RV largely circulates in the dog population, and dogs account for 95% or more of all the diagnosed cases. The data presented here support the fact that dog RV variants are the single major variants circulating in India. The predominance of dog RV circulation in India could be due to the country's high population density, a constant supply of susceptible hosts and the close proximity of donor species (12), inadequate herd immunity, and unrestricted animal movement. Movements of infected animals to new uninfected areas have the potential to produce explosive, sustainable outbreaks (7) and may also result in spillover across geographical locations (as seen in the case of isolate R141).
The endemic nature of rabies in India can be attributed to the prevalence of the disease in dogs as well as other species of domestic animals. Fifty-five percent of all samples analyzed came from livestock species, and considerable numbers of livestock with a history of dog bites are treated in rabies clinics on a daily basis. Culling of livestock suspected of having rabies is not feasible in India due to socioeconomic conditions and religious beliefs. Therefore, transmission of rabies to humans by rabid livestock might happen due to various favorable ecological and societal factors. The effective control of rabies among dogs in India would be possible with the proper disposal of garbage, reductions of the stray dog and stray livestock populations, and the proper induction of “herd immunity” by mass vaccination.
In summary, dogs are the principle reservoirs of the rabies virus. RV isolates tend to form genetic clusters based on the geographical region. Analysis of more samples is essential to identify the existence of other genetic clusters and also to identify the dominant genetic cluster. The amino acids aa462G, aa465H, and aa468K may serve as epidemiological markers for RV isolates in India and could be used to trace the origins of travel-related rabies cases in humans. Studies of different genome targets (like the P gene and the N gene) must also be carried out to further characterize the RV isolates of Indian origin.
Acknowledgments
We thank S. Raju, veterinary surgeon, Kollam, Kerala, India, and C. K. Singh, assistant professor, College of Veterinary Science, Ludhiana, Punjab, India, for the gifts of the suspected RV-infected brain tissue samples for this study.
REFERENCES
- 1.Association for the Prevention and Control of Rabies in India. 2003. A national multicentric survey. Progress report. Association for the Prevention and Control of Rabies in India, Bhubaneswar, India.
- 2.Badrane, H., and N. Tordo. 2001. Host switching in lyssavirus history from the Chiroptera to the Carnivora orders. J. Virol. 75:8096-8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badrane, H., C. Bahloul, P. Perrin, and N. Tordo. 2001. Evidence of two lyssavirus phylogroups with distinct pathogenecity and immunogenecity. J. Virol. 75:3268-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi, F., S. A. Nadin-Davis, A. I. Wandeler, J. Armstrong, A. A. Gomes, F. S. Lima, F. R. Nogueira, and F. H. Ito. 2005. Antigenic and genetic characterization of rabies viruses isolated from domestic and wild animals of Brazil identifies the hoary fox as a rabies reservoir. J. Gen. Virol. 86:3153-3162. [DOI] [PubMed] [Google Scholar]
- 5.Bourhy, H., B. Kissi, and N. Tordo. 1993. Molecular diversity of the Lyssavirus genus. Virology 194:70-81. [DOI] [PubMed] [Google Scholar]
- 6.Bourhy, H., B. Kissi, L. Audry, M. Smreczak, M. S. Todys, K. Kulonen, N. Tordo, J. F. Zmudzinski, and E. C. Holmes. 1999. Ecology and evolution of rabies virus in Europe. J. Gen. Virol. 80:2545-2557. [DOI] [PubMed] [Google Scholar]
- 7.Childs, J. J., A. T. Curns, M. E. Dey, L. A. Real, L. Feinstein, O. N. Bjornstad, and J. W. Krebs. 2000. Predicting the local dynamics of epizootic rabies among raccoons in the United States. Proc. Natl. Acad. Sci. USA 97:13666-13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David, D., B. A. Yakobson, L. Gershkovich, and S. Gayer. 2004. Tracing the regional source of rabies infection in an Israeli dog by viral analysis. Vet. Rec. 155:496-497. [DOI] [PubMed] [Google Scholar]
- 9.Dean, D. J., M. K. Abelseth, and P. Atanasiu. 1996. The fluorescent antibody test, p. 88-95. In F. X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 10.Delagneau, J. F., P. Perrin, and P. Atanasiu. 1981. Structure of rabies virus: spatial relationships of the proteins G, M1, M2 and N. Ann. Virol. (Paris) 132E:473-493. [Google Scholar]
- 11.Domingo, E., and J. J. Holland. 1994. Mutation rates and rapid evolution of RNA viruses, p. 161-184. In S. S. Morse (ed.), The evolutionary biology of viruses. Raven Press, New York, N.Y.
- 12.Holmes, E. C., C. H. Woelk, R. Kassis, and H. Bourhy. 2002. Genetic constraints and the adaptive evolution of rabies virus in nature. Virology 292:247-257. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, G. J., A. Paez, J. Boshell, and C. E. Rupprecht. 2004. A phylogenetic reconstruction of the epidemiological history of canine rabies virus variants in Colombia. Infect. Genet. Evol. 4:45-51. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, N., L. M. McElhinney, Y. H. Ali, I. K. Saeed, and A. R. Fooks. 2004. Molecular epidemiology of canid rabies in Sudan: evidence for a common origin of rabies with Ethiopia. Virus Res. 104:201-205. [DOI] [PubMed] [Google Scholar]
- 15.Kissi, B., N. Tordo, and H. Bourhy. 1995. Genetic polymorphism in the rabies virus nucleoprotein gene. Virology 209:526-537. [DOI] [PubMed] [Google Scholar]
- 16.Kissi, B., H. Badrane, L. Audry, A. Lavenu, N. Tordo, M. Brahimi, and H. Bourhy. 1999. Dynamics of rabies virus quasispecies during serial passage in heterologous hosts. J. Gen. Virol. 80:2041-2050. [DOI] [PubMed] [Google Scholar]
- 17.Knobel, D. L., S. Cleaveland, P. G. Coleman, E. M. Fevre, M. I. Meltzer, M. E. G. Miranda, A. Shaw, J. Zinsstag, and F. X. Meslin. 2005. Re-evaluating the burden of rabies in Africa and Asia. Bull. W. H. O. 83:360-368. [PMC free article] [PubMed] [Google Scholar]
- 18.Koprowski, H. 1996. Mouse inoculation test, p. 80-86. In F. X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 19.Krause, R., Z. Bagó, S. R. Fernández, A. Loitsch, F. Allerberger, P. Kaufmann, K. H. Smolle, G. Brunner, and G. J. Krejs. 2005. Travel-associated rabies in Austrian man. Emerg. Infect. Dis. 11:719-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Mebatsion, T., M. Konig, and K. K. Conzelmann. 1996. Budding of rabies virus particles in the particles in the absence of the spike glycoprotein. Cell 84:941-951. [DOI] [PubMed] [Google Scholar]
- 22.Mebatsion, T., F. Weiland, and K. K. Conzelmann. 1999. Matrix protein pf rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J. Virol. 73:242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto, K., D. C. Hooper, H. Carbaugh, Z. F. Fu, H. Koprowski, and B. Dietzschold. 1998. Rabies virus quasispecies: implications for pathogenesis. Proc. Natl. Acad. Sci. USA 95:3152-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadin-Davis, S. A., G. A. Casey, and A. I. Wandeler. 1993. Identification of regional variants of the rabies virus with in the Canadian province of Ontario. J. Gen. Virol. 74:829-837. [DOI] [PubMed] [Google Scholar]
- 25.Nadin-Davis, S. A., M. I. Sampath, G. A. Casey, R. R. Tinline, and A. I. Wandeler. 1999. Phylogeographic patterns exhibited by Ontario rabies virus variants. Epidmiol. Infect. 123:325-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadin-Davis, S. A., W. Huang, J. Armstrong, G. A. Casey, C. Bahloul, N. Tordo, and A. I. Wandeler. 2001. Antigenic and genetic divergence of rabies from bat species indigenous to Canada. Virus Res. 74:139-156. [DOI] [PubMed] [Google Scholar]
- 27.Nadin-Davis, S. A., M. Abdel-Malik, J. Armstrong, and A. I. Wandeler. 2002. Lyssavirus P gene characterization provides insights into the phylogeny of the genus and identifies structural similarities and diversity within the encoded phosphoprotein. Virology 298:286-305. [DOI] [PubMed] [Google Scholar]
- 28.Nel, L. H., C. T. Sabeta, B. Von Teichman, J. B. Jaftha, C. E. Rupprecht, and J. Bingham. 2005. Mongoose rabies in Southern Africa: a re-evaluation based on molecular epidemiology. Virus Res. 109:165-173. [DOI] [PubMed] [Google Scholar]
- 29.Paez, A., C. Nunez, C. Garcia, and J. Boshell. 2003. Molecular epidemiology of rabies epizootics in Columbia: evidence for human and dog rabies associated with bats. J. Gen. Virol. 84:795-802. [DOI] [PubMed] [Google Scholar]
- 30.Paez, A., C. Saad, C. Nunez, and J. Boshell. 2005. Molecular epidemiology of rabies in northern Columbia 1994-2003. Evidence for human and fox rabies associated with dogs. Epidemiol. Infect. 133:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. http://taxonomy.zoology.gla.ac.uk/rod/treeview.html. [DOI] [PubMed] [Google Scholar]
- 32.Sacramento, D., H. Bourhy, and N. Tordo. 1991. PCR technique as an alternative method for diagnosis and molecular epidemiology. Mol. Cell. Probes 5:229-240. [DOI] [PubMed] [Google Scholar]
- 33.Sacramento, D., H. Badrane, H. Bourhy, and N. Tordo. 1992. Molecular epidemiology of rabies virus in France: comparison with vaccine strains. J. Gen. Virol. 73:1149-1158. [DOI] [PubMed] [Google Scholar]
- 34.Smith, J., L. McElhinny, G. Parsons, N. Brink, T. Doherty, D. Agranoff, M. E. Miranda, and A. R. Fooks. 2003. Case report. Rapid ante-mortem diagnosis of a human case of rabies imported into the UK from the Philippines. J. Med. Virol. 69:150-155. [DOI] [PubMed] [Google Scholar]
- 35.Smith, J. S., D. B. Fishbein, C. E. Rupprecht, and K. Clark. 1991. Unexplained rabies in three immigrants in the United States. A virologic investigation. N. Engl. J. Med. 324:204-211. [DOI] [PubMed] [Google Scholar]
- 36.Smith, J. S., L. A. Orciari, P. A. Yager, H. D. Seidel, and C. K. Warner. 1992. Epidemiologic and historical relationships among 97 rabies virus isolates as determined by limited sequence analysis. J. Infect. Dis. 166:296-307. [DOI] [PubMed] [Google Scholar]
- 37.Strimmer, K., and A. V. Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. http://tree-puzzle.de/. [Google Scholar]
- 38.Tamura, K., and M. Nei. 1993. Estimation of the number of nucleotide substitution in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10:512-526. [DOI] [PubMed] [Google Scholar]
- 39.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tordo, N., O. Poch, A. Ermine, G. Keith, and F. Rougeon. 1986. Walking along the rabies genome: is the large G-L intergenic region a remnant gene? Proc. Natl. Acad. Sci. USA 83:3914-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tordo, N., H. Badrane, H. Bourhy, and D. Sacramento. 1993. Molecular epidemiology of lyssaviruses: focus on the glycoprotein and pseudogenes. Onderstepoort J. Vet. Res. 60:315-323. [PubMed] [Google Scholar]
- 42.Tordo, N., D. Sacramento, and H. Bourhy. 1996. The polymerase chain reaction (PCR) technique for diagnosis, typing and experimental studies of rabies, p. 28-51. In F. X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 43.Whitt, M. A., L. Buonocore, C. Prehaud, and J. K. Rose. 1991. Membrane fusion activity, oligomerization, and assembly of the rabies virus glycoprotein. Virology 185:681-688. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1998. World survey of rabies no. 32 for the year 1996. WHO document EMC/ZDI/98.4. World Health Organization, Geneva, Switzerland.
- 45.Wunner, W. H., J. K. Larson, B. Dietzschold, and C. L. Smith. 1988. The molecular biology of rabies viruses. Rev. Infect. Dis. 10:S771-S784. [DOI] [PubMed] [Google Scholar]