Abstract
Here we report the development of an oligonucleotide microarray method that can identify fungal pathogens in a single reaction. Specific oligonucleotide probes targeted to internal transcribed spacer 2 were designed and synthesized. Fungal DNA was amplified by universal primers, and the PCR product was hybridized with the oligonucleotide microarray. A series of specific hybridization profiles corresponding to species were obtained. The 122 strains of fungal pathogens, including standard and clinically isolated strains, used to test the specificity, stability, and sensitivity of the microarray system belonged to 20 species representing 8 genera. We found that the microarray system can successfully discriminate among the fungal pathogens to the species level, with high specificity and stability. The sensitivity was 15 pg/ml of DNA. This oligonucleotide microarray system represents a rapid, simple, and reliable alternative to conventional methods of identifying common clinical fungal isolates.
The incidence of invasive fungal infections has increased in recent years. This increase is likely the result of several factors, including an increasing population of immunocompromised patients, intensive immunosuppressive chemotherapy, increasing awareness of fungal infections, and the widespread use of broad-spectrum antibiotics and central venous catheters (4). Invasive fungal infections have become a major cause of morbidity and mortality among immunocompromised patients (4, 13, 17, 24). Since opportunistic mycoses often have grave consequences, early, rapid, and accurate identification of the pathogenic fungus is critical for timely, appropriate clinical management. Conventional identification of pathogenic fungi in the clinical microbiology laboratory is based on phenotypic and physiological tests, which often require 3 or more days to complete and produce results that may not be accurate (9, 14, 28).
In recent years, numerous DNA-based methods have been developed to improve the identification of pathogenic fungi (16, 22). PCR methods targeting different genes for identification of fungi have been described elsewhere (15, 23, 26, 31). A number of reports have described probes, restriction fragment length polymorphisms, multiplex PCR methods (21), or other techniques to identify unique ribosomal DNA (rDNA) sequences (19, 29). Real-time PCR systems for quantitative analysis of pathogenic fungi have been developed (3). A suspension array using a novel flow cytometer with a dual-laser system to rapidly identify different varieties and genotypes of Cryptococcus neoformans has also been reported (8). Although these DNA-based methods have been useful for the identification of fungal species, they are limited in that they either identify only one species or a few kinds of fungi at a time or require a time-consuming and costly probe hybridization procedure. Because common clinical fungal pathogens belong to a wide range of genera and species, the PCR method cannot meet the requirement of quick and simultaneous identification of various infecting agents to the species level.
In this study, we describe a sensitive, specific, and high-throughput method to rapidly and simultaneously identify almost the entire assortment of common pathogenic fungi within a single oligonucleotide microarray. The method combines universal fungal primers ITS3 and ITS4, which are specific for conserved sequences in the 5.8 and 28S rRNA genes, to amplify the target DNA, and 21 species-specific probes based on the sequence variation of the internal transcribed spacer 2 (ITS2) rRNA genes.
MATERIALS AND METHODS
Organisms.
The standard fungal strains used in this study were obtained from the Chinese Medical Culture Collection Center (CMCC; Beijing, China) and are listed in Table 1. The clinical fungal strains used in this study were all isolated from specimens at the First Hospital and Research Center for Medical Mycology of Peking University (FHRCMM; Beijing, China) and were stored at FHRCMM until use (Table 2). The identification of all isolates was confirmed by conventional morphological and physiological methods (2, 30). Bacteria and nonpathogenic fungi were selected as negative controls (Table 3). All fungi were inoculated onto potato dextrose agar slants at 30°C for 72 to 120 h and were stored at 4°C until needed. Bacteria were inoculated into Luria-Bertani medium (Bacto tryptone, 10 g/liter; Bacto yeast extract, 5 g/liter; NaCl, 10 g/liter [pH 7.0]) and were shaken for 18 h at 37°C.
TABLE 1.
Standard strains used in this studya
| Strain no. | Species |
|---|---|
| C1a | Candida albicans |
| C2a | Candida tropicalis |
| C6a | Candida krusei |
| C4a | Candida parapsilosis |
| Y10 | Candida glabrata |
| D2q | Cryptococcus neoformans |
| A1 | Aspergillus fumigatus |
| A2 | Aspergillus flavus |
| A7 | Aspergillus nidulans |
| A3 | Aspergillus niger |
| D6a | Fonsecaea pedrosoi |
| D8a | Phialophora verrucosa |
| D10a | Cladosporium carrionii |
| D1 | Sporothrix schenckii |
| 3.3440 | Mucor racemosus |
| T1a | Trichophyton rubrum |
| T5a | Trichophyton mentagrophytes |
| F1d | Epidermophyton floccosum |
| M3b | Microsporum canis |
| M2b | Microsporum gypseum |
All strains listed in this table were obtained from the CMCC.
TABLE 2.
Clinically isolated strains used in this studya
| BMU no.b | Species | Source | BMU no.b | Species | Source | |
|---|---|---|---|---|---|---|
| 02991 | Candida albicans | Vagina | O2662 | Aspergillus nidulans | Ascites | |
| 00549 | Candida albicans | Vagina | 01763 | Aspergillus niger | Ears | |
| 00570 | Candida albicans | Vagina | 00614 | Aspergillus niger | Phlegm | |
| 02834 | Candida albicans | Vagina | 01633 | Aspergillus niger | Ears | |
| 00610 | Candida albicans | Vagina | 01236 | Fonsecaea pedrosoi | Skin | |
| 02850 | Candida albicans | Vagina | 01238 | Fonsecaea pedrosoi | Skin | |
| 02932 | Candida albicans | Vagina | 01237 | Fonsecaea pedrosoi | Skin | |
| 02667 | Candida albicans | Vagina | 00921 | Sporothrix schenckii | Face | |
| 00830 | Candida tropicalis | Vagina | 00497 | Sporothrix schenckii | Face | |
| 00986 | Candida tropicalis | Blood | 00644 | Sporothrix schenckii | Face | |
| 00578 | Candida tropicalis | Pus | 00061 | Sporothrix schenckii | Face | |
| 01161 | Candida tropicalis | Phlegm | 24885 | Sporothrix schenckii | Face | |
| 00572 | Candida tropicalis | Bronchial lavage | 01223 | Cladosporium carrionii | Skin | |
| 03141 | Candida parapsilosis | Belly | 01226 | Cladosporium carrionii | Skin | |
| 00608 | Candida parapsilosis | Glans | 01219 | Cladosporium carrionii | Skin | |
| 00589 | Candida parapsilosis | Glans | 01218 | Cladosporium carrionii | Skin | |
| 00827 | Candida parapsilosis | Glans | 01254 | Cladosporium carrionii | Skin | |
| 00627 | Candida krusei | Feet | 00654 | Cladosporium carrionii | Hand | |
| 00551 | Candida krusei | Feet | 01057 | Phialophora verrucosa | Skin | |
| 01268 | Candida krusei | Feet | 02669 | Phialophora verrucosa | Skin | |
| 01530 | Candida krusei | Feet | 01245 | Phialophora verrucosa | Skin | |
| 00693 | Candida glabrata | Vagina | 01246 | Phialophora verrucosa | Skin | |
| 00520 | Candida glabrata | Vagina | 00849 | Phialophora verrucosa | Skin | |
| 00526 | Candida glabrata | Urine | 01246 | Phialophora verrucosa | Skin | |
| 01121 | Cryptococcus neoformans | CSFc | 01403 | Trichophyton rubrum | Nails | |
| 01122 | Cryptococcus neoformans | CSF | 01866 | Trichophyton rubrum | Nails | |
| 01203 | Cryptococcus neoformans | CSF | 01868 | Trichophyton rubrum | Nails | |
| 02819 | Cryptococcus neoformans | CSF | 01869 | Trichophyton rubrum | Nails | |
| 03103 | Cryptococcus neoformans | CSF | 00252 | Trichophyton mentagrophytes | Feet | |
| 02055 | Cryptococcus neoformans | CSF | 01214 | Trichophyton mentagrophytes | Feet | |
| 01026 | Aspergillus fumigatus | Phlegm | 01098 | Trichophyton mentagrophytes | Feet | |
| 00967 | Aspergillus fumigatus | Phlegm | 01213 | Trichophyton mentagrophytes | Feet | |
| 00915 | Aspergillus fumigatus | Phlegm | 02036 | Epidermophyton floccosum | Feet | |
| 01021 | Aspergillus fumigatus | Phlegm | 02867 | Epidermophyton floccosum | Feet | |
| 01025 | Aspergillus fumigatus | Phlegm | 02036 | Epidermophyton floccosum | Feet | |
| 00636 | Aspergillus fumigatus | Phlegm | 02429 | Microsporum canis | Head | |
| 01762 | Aspergillus flavus | Nails | 02291 | Microsporum canis | Head | |
| 01160 | Aspergillus flavus | Secretion of eye | 02431 | Microsporum canis | Head | |
| 01606 | Aspergillus flavus | Nasal sinuses | 02463 | Microsporum canis | Head | |
| 01275 | Aspergillus flavus | Phlegm | 02036 | Microsporum gypseum | Feet | |
| 01267 | Aspergillus flavus | Phlegm | 02733 | Microsporum gypseum | Feet | |
| 00634 | Aspergillus flavus | Phlegm | 00449 | Microsporum gypseum | Feet | |
| 00597 | Aspergillus nidulans | Thoracic abscess | 00918 | Microsporum gypseum | Feet |
All strains listed in this table were obtained from the FHRCMM.
BMU, Beijing Medical University (now the Peking University Health Science Center).
CSF, cerebrospinal fluid.
TABLE 3.
Strains used as negative controls in this study
| Strain no. | Species | Origin |
|---|---|---|
| 10110 | Pseudomonas aeruginosa | CMCC |
| 49101 | Proteus sp. | CMCC |
| 52219 | Yersinia enterocolitica | CMCC |
| 32223 | Enterococcus faecalis | CMCC |
| 51081 | Shigella boydii | CMCC |
| 8099 | Escherichia coli | CMCC |
| 50304 | Salmonella sp. | CMCC |
| 3.2834 | Absidia sp. | CGMCCa |
| 3.6018 | Aspergillus giganteus | CGMCC |
| 3.2913 | Penicillium citrinum | CGMCC |
| 3.826 | Rhizopus oryzae | CGMCC |
| 2.1660 | Candida guilliermondii | CGMCC |
CGMCC, China General Microbiological Culture Collection Center.
Preparation of clinical blood samples.
The study included a total of 16 blood specimens obtained from patients at FHRCMM with suspected invasive fungal infections (seven women and nine men, aged 26 to 78 years [mean, 54 years]). However, no fungal pathogens were detected in the blood samples by culture, direct microscopy, or PCR with the universal fungal primers. In order to evaluate the practicality of the oligonucleotide microarray in the clinic, the negative blood samples were spiked with fungi and stored at −20°C before processing for DNA extraction.
Sequence analysis for the design of the primers and probes.
The sequences of ITS2 and the partial sequences of 5.8S rDNA and 28S rDNA were accessed via the GenBank database (supplemental material S1) and were aligned using Vector NTI, suite 6.0 (InforMax, Bethesda, MD). The universal fungal oligonucleotide primers were designed from 5.8S rRNA and 23S rRNA. The ITS2 regions were amplified with universal fungal primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The sequence of ITS3 is complementary to a conserved region at the end of the 5.8S rRNA gene, and ITS4 binds to a conserved region at the beginning of the 28S rRNA gene, leading to amplification of ITS2. Primers were synthesized (Takara Biotechnology Co., Ltd., Dalian, China), and primer ITS4 was labeled with 5′ Cy3 fluorescence. The specific oligonucleotide probes were designed based on the sequence data for the ITS2 region, and selection was optimized for melting temperature equivalence, lack of duplexes and hairpins, and internal stability by using Oligo (version 6.44) software. Probes were synthesized (Takara) and modified with 3′ NH2 to increase binding to the glass slide and hybridization intensity.
DNA preparation. (i) Preparation of template DNA from culture isolates.
Previously described fungal DNA isolation methods were adopted (32). Briefly, a small amount of culture was removed from the culture plates and was pelleted in a 1.5-ml Eppendorf tube. Five hundred microliters of extraction buffer (100 mM Tris-HCl [pH 9.0], 40 mM EDTA), 60 μl of 20% sodium dodecyl sulfate (SDS), and 300 μl of benzyl chloride were added to each sample. The reaction mixture was vortexed, incubated in a 50°C water bath for 40 min, and then shaken for 10 min so that the two phases were thoroughly mixed. Then 60 μl of 3 M sodium acetate (pH 5.0) was added, and the tube was kept on ice for 20 min. After centrifugation at 3,500 × g and 4°C for 15 min, the supernatant was collected and DNA was precipitated with isopropanol (1:1). The DNA pellet was resuspended in 300 μl of TE buffer (10 mM Tris-HCl [pH 7.4]-1 mM EDTA), and 1.5 μl of RNase (10 mg/ml) was added. After 5 min, the samples were extracted with phenol-chloroform (1:1 [vol/vol]) and, following chloroform extraction, were precipitated with isopropanol. The DNA pellet was resuspended in 200 μl of TE buffer.
(ii) Preparation of template DNA from blood samples.
DNA was extracted as described previously (22). Blood samples (200 μl) were lysed in 800 μl of lysis buffer (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 50 mM NaCl) at room temperature for 10 min and centrifuged at 13,000 rpm for 5 min; the supernatant was discarded; and the pellet was resuspended in 100 μl of sterile H2O. Glass beads (0.5-mm-diameter zirconium glass beads stored in 0.2% SDS) were added to the resuspended pellet, and the sample was vortexed in a minibead beater at top speed for 180 s. The suspension was removed, following bead beating, to a fresh microcentrifuge tube containing 900 μl of L7 buffer (10 M guanidium thiocyanate, 100 mM Tris-HCl [pH 6.4], 200 mM EDTA, 2.6% [wt/vol] Triton X-100, and alpha-casein [Sigma-Aldrich Ltd.]) (added to a final concentration of 1 mg/ml) and 40 μl of silica suspension for 200-μl and 1-ml samples (80 μl of silica suspension added to 5-ml samples). The sample was vortexed at maximum speed for 30 s, followed by incubation at room temperature for 10 min. The sample was vortexed again for 30 s and spun at 12,000 rpm for 1 min. The supernatant was removed, and the pellet was washed twice in 1 ml of L2 buffer (10 M guanidium thiocyanate, 100 mM Tris-HCl [pH 6.4]) while being vortexed for 30 s and spun at 12,000 rpm (12,700 × g; Heraeus Sepatech Biofuge A) for 1 min. The pellet was then washed twice in 1 ml of 70% ethanol, followed by a final wash in 1 ml of 100% acetone. The pellet was dried in a heating block at 60°C for 10 min and resuspended in 100 μl of 0.1× TE buffer for 30 min.
(iii) Preparation of DNA from bacteria.
DNA was extracted from bacteria using previously described procedures (25).
PCR amplification of the target gene.
Each 50-μl reaction mixture contained 37 μl sterile water, 5 μl 10× buffer (Takara), 200 μmol/liter deoxynucleoside triphosphate mixture (Takara), 0.02 U/μl Takara Taq (5 U/ml), 0.1 μmol/liter primer ITS3, 1 μmol/liter primer ITS4, and 2 μl supernatant, which contained fungal DNA. The PCR mixtures were subjected to 95°C for 5 min, followed by 30 cycles of 94°C for 25 s, 55°C for 30 s, and 72°C for 25 s, with a final extension step of 72°C for 5 min. The PCR product was checked by 1.5% agarose electrophoresis and visualization with ethidium bromide.
Synthesis of the oligonucleotide microarrays.
The oligonucleotides were bound to the slides as follows: 5 μl of each oligonucleotide (50 μmol/liter) was spotted onto a glass slide by an arrayer (PixSys 5500 workstation; Cartesian Technologies, Irvine, CA), with 5 mm between every two oligonucleotide spots. When all the oligonucleotides had been applied, the glass slides were left at room temperature for 24 h to permit thorough drying of the DNA onto the surfaces of the slides. After drying, the slides were washed in 0.2% SDS for 5 min, in distilled water for 5 min, in sodium borohydride solution (1.3 g Na2BH4 dissolved in 375 ml phosphate-buffered saline, followed by addition of 125 ml pure ethanol) for 5 min, in 0.2% SDS for 2 min, and in distilled water for 2 min.
Hybridization.
The fluorescently labeled amplicons were hybridized to the oligonucleotide microarrays using the following protocol. Amplicons (1 μl) were added to a tube containing 4 μl hybridization solution (UniHyb; TeleChem International, Sunnyvale, CA); then the tube was heated to 95°C for 10 min and put on ice immediately. The mixture in the tube was then transferred to the microarray and incubated for 1 h at 55°C in a hybridization cassette (TeleChem). After hybridization, unbound fluorescent amplicons were washed away by wash buffer A (1× SSC [0.15 M NaCl plus 0.015 M sodium citrate] plus 0.2% SDS) for 1 min, by wash buffer B (0.2× SSC plus 0.2% SDS) for 1 min, and by wash buffer C (0.1× SSC) for 1 min, in order.
Scanning of the microarray for fluorescent signals and scoring of hybridization results.
Slides were inserted into a ScanArray 4000B (GSI Lumonics, Billerica, MA) to scan the area of the slide containing the microarray. The scanned images were uploaded as tagged-image format files into GENEPRO software (Riverside Scientific, Bainbridge Island, WA) and examined for fluorescence intensity.
Evaluation of sensitivity of the gene chips.
We used the universal primers, ITS3 and ITS4, to amplify the target genes of A. fumigatus (A1). The concentrations of DNA from A1 were 15 ng/ml, 1.5 ng/ml, 150 pg/ml, 15 pg/ml, and 1.5 pg/ml, as determined with a UV-Vis recording spectrophotometer (UV 2100; Shimadzu Co., Kyoto, Japan). The PCR products were hybridized with the oligonucleotide probes on the gene chip.
RESULTS
Assessment of the primers.
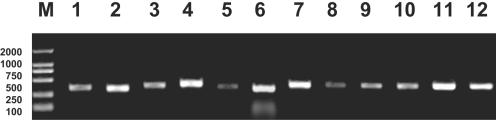
The universal primers, ITS3 and ITS4, were first assessed under the same conditions using the target genes from 122 strains of fungal pathogens belonging to 20 species and 8 genera. All the fungal pathogens tested gave PCR products with bands of 200 to 500 bp (Fig. 1).
FIG. 1.
Partial PCR amplification results for fungal pathogens. Lanes: M, DNA marker 2000; 1, C. albicans; 2, C. parapsilosis; 3, C. krusei; 4, C. glabrata; 5, C. tropicalis; 6, C. neoformans; 7, A. fumigatus; 8, A. flavus; 9, A. nidulans; 10, A. niger; 11, C. carrionii; 12, P. verrucosa.
Design of oligonucleotides and microarray.
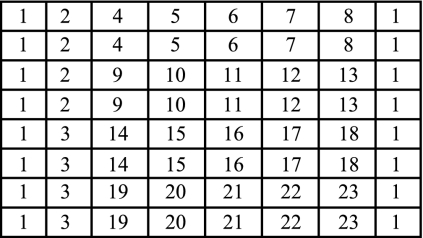
The initial choice of oligonucleotides was based on information in public databases. This information was expanded by sequencing of products from organisms in our collection of pure cultures. From these results, 22 oligonucleotides were constructed (Table 4) and the microarray model for detection of fungal pathogens was designed (Fig. 2). Five categories of designed oligonucleotide probes were identified based on their efficacies. Category 1 included oligonucleotide probes 4 to 23, a cluster of genus- or species-specific probes targeted to the sequence of ITS2 and used to identify fungi at the species level; category 2 consisted of oligonucleotide probe 2, a universal probe targeted to the portion of 28S rDNA shared by all fungi and used to detect all kinds of fungi; category 3 included oligonucleotide probes 1 and 3, a positive- and a negative-control probe used to reflect the effectiveness of this hybridization system and serving as reference coordinates for scanning.
TABLE 4.
Oligonucleotide probes used in this study
| Probe no. | Species | Sequence (5′-3′) |
|---|---|---|
| 1 | Positive control | GCATATCAATAAGCGGAGGA |
| 2 | Universal fungal probe | TTGACCTCRRATCAGGTAGGRATACCCGCTGAACTTAA |
| 3 | Negative control | |
| 4 | Candida albicans | ATTGCTTGCGGCGGTAGCGTCTACCACGTATATCTTCAAA |
| 5 | Candida tropicalis | AACGCTTATTTTGCTAGTGGCCACCACAATTTATTTCATA |
| 6 | Candida parapsilosis | TTCCACTCATTGGTACAAACTCCAAAACTTCTTCCAAA |
| 7 | Candida krusei | GACGCTTGGCGGCCGAGAGCGAGTGTTGCGAGACAACAAA |
| 8 | Candida glabrata | TAGGTTTTACCAACTCGGTGTTGATCTAGGGAGGGATAAGT |
| 9 | Cryptococcus neoformans | CCTATGGGGTAGTCTTCGGCTTGCTGATAACAACCATCTC |
| 10 | Aspergillus fumigatus | GGCCGGCGCCAGCCGACACCCAACTTTATTTTTCTAA |
| 11 | Aspergillus flavus | CCGGCGCTTGCCGAACGCAAATCAATCTTTTTCCA |
| 12 | Aspergillus niger | TGTAGGATTGGCCGGCGCCTGCCGACGTTTTCCAACCATT |
| 13 | Aspergillus nidulans | ACCCGCTCGATTAGGGCCGGCCGGGCGCCAGCCGACGTCT |
| 14 | Fonsecaea pedrosoi | GGGACTCGGTCTTCTCCCTCACGGGAACACTTTTTTCTA |
| 15 | Phialophora verrucosa | TGGACGGATTTTGGTCGTGTAACAACGGCCCCTCCTAAA |
| 16 | Cladosporium carrionii | TGGACGGTTTTGGTCGAGTGGTCTCGACCCCTCCTAAA |
| 17 | Sporothrix schenckii | CCCTGCCGTGAAAACGCGCATGACGCGCAGCTCTTTTTAC |
| 18 | Mucor racemosus | AAAGCTCTTGTAATTGACTTTGATGGGGCCTCCCAAATAA |
| 19 | Trichophyton rubrum | CCCTGGCCCCAATCTTTATATATATATATATCTTTT |
| 20 | Trichophyton mentagrophytes | CTCTGGCCTTCCCCCAAATCTCTCTGAGATATTTT |
| 21 | Epidermophyton floccosum | GCTCTCTGGCCCTAATTTCCGTCGGGAGGACGAAAGG |
| 22 | Microsporum canis | GCAGGCTGGCCTAACGCACCATGTATTATT |
| 23 | Microsporum gypseum | TAGTTTCCGTCAGAGATGTATTTCTCTGCAATTT |
FIG. 2.
Layout of the oligonucleotide probes. Numbers represent probes as follows: 1, positive control; 2, universal fungal probe; 3, negative control (3× SSC); 4, C. albicans; 5, C. tropicalis; 6, C. parapsilosis; 7, C. krusei; 8, C. glabrata; 9, Cryptococcus neoformans; 10, A. fumigatus; 11, A. flavus; 12, A. niger; 13, A. nidulans; 14, Fonsecaea pedrosoi; 15, Phialophora verrucosa; 16, Cladosporium carrionii; 17, Sporothrix schenckii; 18, Mucor racemosus; 19, T. rubrum; 20, T. mentagrophytes; 21, Epidermophyton floccosum; 22, M. canis; 23, M. gypseum.
Hybridization from enrichment broths of standard fungi.
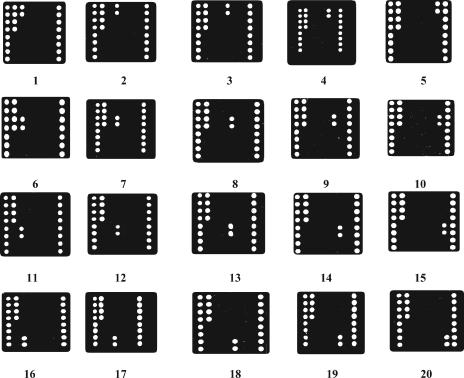
The PCR products are used to hybridize with the oligonucleotide probes on the oligonucleotide microarray under the same conditions, followed by signal acquisition using the ScanArray 4000B scanner to generate the respective hybridization maps. The results are shown in Fig. 3. A given isolate was easily identified as one of the target pathogenic fungi from the hybridization signals of the three oligonucleotide probe categories.
FIG. 3.
Typical hybridization profiles on the oligonucleotide microarray (n = 5). Panels: 1, C. albicans; 2, C. tropicalis; 3, C. parapsilosis; 4, C. krusei; 5, C. glabrata; 6, Cryptococcus neoformans; 7, A. fumigatus; 8, A. flavus; 9, A. niger; 10, A. nidulans; 11, Fonsecaea pedrosoi; 12, Phialophora verrucosa; 13, Cladosporium carrionii; 14, Sporothrix schenckii;15, Mucor racemosus; 16: T. rubrum; 17, T. mentagrophytes; 18, Epidermophyton floccosum; 19, M. canis; 20, M. gypseum.
The results were in close agreement with those predicted from the DNA sequences. For instance, in the hybridization map shown in Fig. 3, panel 1, there are strong hybridization signals at the sites corresponding to oligonucleotide probes 1, 2, and 4, so the fungal pathogen can be sequentially identified as Candida albicans. Based on the results of multiple experiments, we regarded a hybridization signal as specific if the foreground fluorescent signal at an oligonucleotide probe site was stronger than its background fluorescent signal, with a signal/noise ratio larger than 15. The fluorescent signals of specific hybridization are all 15 times stronger than those of nonspecific hybridization (supplemental material S2), making it easy to identify the specific hybridization signals directly from the hybridization maps. The strains belonged to C. albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, Candida glabrata, Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, Sporothrix schenckii, Mucor racemosus, Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, Microsporum canis, or Microsporum gypseum.
Evaluation of the reproducibility of the oligonucleotide microarray.
To evaluate the reproducibility of the assay, C. albicans, C. tropicalis, and A. fumigatus were detected by the oligonucleotide microarray. The experiment was repeated five times under the same conditions for each fungus. The coefficients of variation of the signal/noise ratio all were less than 10% (Table 5).
TABLE 5.
Evaluation of the reproducibility of the oligonucleotide microarray
| Species | Signal/noise ratio | CV (%)a |
|---|---|---|
| Candida albicans | 37.2 | 7.5 |
| 40.5 | ||
| 39.6 | ||
| 42.4 | ||
| 35.3 | ||
| Candida tropicalis | 44.3 | 8.2 |
| 50.4 | ||
| 45.6 | ||
| 43.8 | ||
| 52.1 | ||
| Aspergillus fumigatus | 55.3 | 6.4 |
| 57.4 | ||
| 60.3 | ||
| 52.1 | ||
| 54.3 |
CV, coefficient of variation.
Identification of isolated fungal pathogens from clinical samples.
Eighty-six fungal pathogens isolated from clinical samples were processed and hybridized as described above and were then identified according to their specific hybridization maps. All 86 strains were distinguished by their hybridization maps on the microarray. The comprehensive identification results by classical methods are regarded as the final standards. All the hybridization assay results were consistent with those of the conventional methods; the consistency was 100% (86/86).
Testing of spiked blood samples.
To examine the specificity of the designed probes and to assess their potential applicability in clinical testing, the performance of the array was validated by blind testing of blood samples from 16 patients at the FHRCMM. Additionally, the identification of all isolates was confirmed by conventional morphological and physiological methods. Comparison of the array results with those of conventional methods showed that the array was able to unequivocally identify the contents of all 16 samples. Ten samples were identified as C. albicans, three as Candida dubliniensis, and three as A. fumigatus. All the hybridization assay results were consistent with those of the conventional methods.
Sensitivity of the gene chips.
C. albicans (C1) and A. fumigatus (A1) were used to evaluate the sensitivity of the microarray. In some cases, the PCR product was not visualized on the ethidium bromide-stained agarose gel; however, after hybridization of the PCR products to the oligonucleotide array, hybridization signals were visualized. Thus, the PCR method, followed by hybridization of the products to the oligonucleotide array, would improve the sensitivity of detection. For the two strains tested, the detection limit was 15 pg/ml.
DISCUSSION
The oligonucleotide microarray method is based on the reversed solid hybridization of oligonucleotides (5, 6). The major advantages of gene chip technology are its miniature size, high performance, and parallelism and the ability to automate the process and evaluate the expression of hundreds of thousands of genes at a time. As a result, new gene chip applications are rapidly being advanced. The efficacy of gene chip technology depends heavily on the oligonucleotide probes residing on the gene chip. Therefore, carefully chosen target genes and carefully designed oligonucleotide probes for the target genes, including their kinds, sequences, and amounts, are cardinal factors for a good gene chip.
Various species have been implicated in fungal infections, making the ITS region a good target choice for fungal identification studies. The length of this region differs for different fungal species (29), and it contains conserved as well as variable domains, which can be exploited for family- or group-specific hybridizations. Moreover, fungi contain a high copy number of the rRNA region (10, 12, 27), which provides further amplification of the signal and improves the sensitivity of the gene chip. The transcriptional unit is composed of 18S, 5.8S, and 28S rRNA genes. Located between the 18S and 5.8S rDNA subunit genes, as well as between the 5.8S and 28S rDNA subunit genes, are ITS regions (ITS1 and ITS2) that are not translated into rRNA. Although the rRNA genes are highly conserved, the ITS regions are divergent and distinctive (1, 7, 11, 20, 29). For this study we chose ITS2 as the target gene. ITS2 is a spacer region flanked by the 5.8S and 28S rRNA genes. The ITS2 region can be amplified with the universal fungal primers ITS3 and ITS4, specific for conserved sequences in the 5.8 and 28S rRNA genes (20). The oligonucleotide probes are designed based on the sequences of ITS2 at the species level.
Interspecies variability is manifested in ITS2 regions. We utilized the variability of the ITS2 regions to design probes for the identification of pathogenic fungi. The probes on the microarrays cover most of the common clinical fungal pathogens. Sample DNAs were fluorescently labeled by PCR and then hybridized to the probes on the microarrays. Various factors were investigated to optimize the experiment and to ensure that the results were stable, specific, sensitive, and reproducible.
Recently, Leinberger et al. reported that they had developed a diagnostic microarray for the rapid and simultaneous identification of fungal pathogens involved in invasive mycoses. Oligonucleotide probes were designed against either the ITS1 or the ITS2 region, or both, or the 5.8S or 18S rRNA gene. However, only 12 pathogenic species belonging to 2 genera (Candida and Aspergillus) can be identified by that method, and only 21 samples of clinical isolates were used to examine its specificity and applicability (18).
Our findings demonstrated that a series of species-specific hybridization profiles can be obtained by an oligonucleotide microarray method. This detection system can identify the 20 common pathogenic species C. albicans, C. parapsilosis, C. krusei, C. glabrata, C. tropicalis, C. neoformans, A. fumigatus, A. flavus, A. nidulans, A. niger, C. carrionii, P. verrucosa, S. schenckii, F. pedrosoi, T. rubrum, T. mentagrophytes, M. gypseum, M. canis, E. floccosum, and M. racemosus, representing 8 genera. One hundred two fungal pathogens isolated from clinical samples were processed and identified to test the specificity of the method. Good specificity and a sensitivity of 15 pg/ml of DNA (5 to 1.2 CFU) were achieved. Further, the method was validated by using 16 clinical isolates as blind samples.
In summary, the oligonucleotide microarray hybridization protocol described here provides a sensitive, specific, and high-throughput means for the identification of fungal pathogens. The assay was demonstrated with isolates from clinical specimens and with spiked blood samples. While mixture analysis and its application to primary specimens must be performed in the future, this microarray method shows promise for application in the laborious standard fungal identification procedure. The analysis is simple to perform and can be completed in 5 h from DNA extraction, compared with 3 or more days for conventional methods. Finally, the microarray results are easy to interpret and can readily identify many fungal pathogens of clinical importance.
Supplementary Material
Acknowledgments
This study was supported by the National High Technology Research and Development Program of China (863 Program), no. 2002AA2Z2011.
We thank Shengqi Wang for technical assistance.
The authors have no conflict of interest with regard to the subject of this paper.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Accensi, F., J. Cano, L. Figuera, M. L. Abarca, and F. J. Cabañes. 1999. New PCR methods to differentiate species in the Aspergillus niger aggregate. FEMS Microbiol. Lett. 180:191-196. [DOI] [PubMed] [Google Scholar]
- 2.Ajello, L., and R. J. Hay (ed.). 1998. Medical mycology. Edward Arnold, London, United Kingdom.
- 3.Baskova, L., S. Preuner, and T. Lion. 2005. Molecular diagnosis of invasive fungal infections: species identification and quantitative analysis by broad-spectrum PCR assays. American Society of Hematology Annual Meeting Abstracts. Blood 106:A5339. [Google Scholar]
- 4.Beck-Sague, C. M., W. R. Jarvis, and the National Nosocomial Infections Surveillance System. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 5.Bowtell, D. D. 1999. Options available—from start to finish—for obtaining expression data by microarray. Nat. Genet. 21:25-32. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P. O., and D. Botstein. 1999. Exploring the new world of the genome with DNA microarrays. Nat. Genet. 21:33-37. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y. C., J. D. Eisner, M. M. Kattar, S. L. Rassoulian-Barrett, K. LaFe, S. L. Yarfitz, A. P. Limaye, and B. T. Cookson. 2000. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 38:2302-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz, M. R., and J. W. Fell. 2005. Use of a suspension array for rapid identification of the varieties and genotypes of the Cryptococcus neoformans species complex. J. Clin. Microbiol. 43:3662-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duthie, R., and D. W. Denning. 1995. Aspergillus fungemia: report of two cases and review. Clin. Infect. Dis. 20:598-605. [DOI] [PubMed] [Google Scholar]
- 10.Einsele, H., H. Hebart, G. Roller, J. Löffler, I. Rothenhöfer, C. A. Müller, R. A. Bowden, J. A. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteve-Zarzoso, B., C. Belloch, F. Uruburu, and A. Querol. 1999. Identification of yeast by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 49:329-337. [DOI] [PubMed] [Google Scholar]
- 12.Fujita, S., B. A. Lasker, T. J. Lott, E. Reiss, and C. J. Morrison. 1995. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J. Clin. Microbiol. 33:962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodrich, J. M., E. C. Reed, M. Mori, L. D. Fisher, S. Skerrett, P. S. Dandliker, B. Klis, G. W. Counts, and J. D. Meyers. 1991. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J. Infect. Dis. 164:731-740. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin, S. D., J. Fiedler-Kelly, T. H. Grasela, W. A. Schell, and J. R. Perfect. 1992. A nationwide survey of clinical laboratory methodologies for fungal infections. J. Med. Vet. Mycol. 30:153-160. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo, J. A., G. J. Alangaden, D. Eliott, R. A. Akins, J. Puklin, G. Abrams, and J. A. Vázquez. 2000. Fungal endophthalmitis diagnosis by detection of Candida albicans DNA in intraocular fluid by use of a species-specific polymerase chain reaction assay. J. Infect. Dis. 181:1198-1201. [DOI] [PubMed] [Google Scholar]
- 16.Iwen, P. C., A. G. Freifeld, T. A. Bruening, and S. H. Hinrichs. 2004. Use of a panfungal PCR assay for detection of fungal pathogens in a commercial blood culture system. J. Clin. Microbiol. 42:2292-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krcmery, V., Jr., I. Krupova, and D. W. Denning. 1999. Invasive yeast infections other than Candida spp. in acute leukaemia. J. Hosp. Infect. 41:181-194. [DOI] [PubMed] [Google Scholar]
- 18.Leinberger, D. M., U. Schumacher, I. B. Autenrieth, and T. T. Bachmann. 2005. Development of a DNA microarray for detection and identification of fungal pathogens involved in invasive mycoses. J. Clin. Microbiol. 43:4943-4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeffler, J., N. Henke, H. Hebart, D. Schmidt, L. Hagmeyer, U. Schumacher, and H. Einsele. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the LightCycler system. J. Clin. Microbiol. 38:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lott, T. J., R. J. Kuykendall, and E. Reiss. 1993. Nucleotide sequence analysis of the 5.8S rDNA and adjacent ITS2 region of Candida albicans and related species. Yeast 9:1199-1206. [DOI] [PubMed] [Google Scholar]
- 21.Lou, G., and T. G. Mitchell. 2002. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol. 40:2860-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, C., D. Roberts, M. van der Weide, R. Rossau, G. Jannes, and T. Smith. 2000. Development of a PCR-based line probe assay for identification of fungal pathogens. J. Clin. Microbiol. 38:3735-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morace, G., L. Pagano, M. Sanguinetti, B. Posteraro, L. Mele, F. Equitani, G. D'Amore, G. Leone, and G. Fadda. 1999. PCR-restriction enzyme analysis for detection of Candida DNA in blood from febrile patients with hematological malignancies. J. Clin. Microbiol. 37:1871-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nucci, M., N. Spector, A. Bueno, C. Solza, T. Perecmanis, P. C. Bacha, and W. Pulcheri. 1997. Risk factors and attributable mortality associated with superinfections in neutropenic patients with cancer. Clin. Infect. Dis. 24:575-579. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 1.25 and 9.17. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Skladny, H., D. Buchheidt, C. Baust, F. Krieg-Schneider, W. Seifarth, C. Leib-Mosch, and R. Hehlmann. 1999. Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. J. Clin. Microbiol. 37:3865-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spreadbury, C., D. Holden, A. Aufauvre-Brown, B. Bainbridge, and J. Cohen. 1993. Detection of Aspergillus fumigatus by polymerase chain reaction. J. Clin. Microbiol. 31:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaler, M., B. Pastakia, T. H. Shawker, T. O'Leary, and P. A. Pizzo. 1988. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann. Intern. Med. 108:88-100. [DOI] [PubMed] [Google Scholar]
- 29.Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 1999. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37:1846-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 31.Wildfeuer, A., R. Schlenk, and W. Friedrich. 1996. Detection of Candida albicans DNA with a yeast-specific primer system by polymerase chain reaction. Mycoses 39:341-346. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, H., F. Qu, and L. H. Zhu. 1993. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 21:5279-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.