Abstract
Three hundred sixty-one quinupristin-dalfopristin (Q-D)-resistant Enterococcus faecium (QDREF) isolates were isolated from humans, turkeys, chickens, swine, dairy and beef cattle from farms, chicken carcasses, and ground pork from grocery stores in the United States from 1995 to 2003. These isolates were evaluated by pulsed-field gel electrophoresis (PFGE) to determine possible commonality between QDREF isolates from human and animal sources. PCR was performed to detect the streptogramin resistance genes vatD, vatE, and vgbA and the macrolide resistance gene ermB to determine the genetic mechanism of resistance in these isolates. QDREF from humans did not have PFGE patterns similar to those from animal sources. vatE was found in 35%, 26%, and 2% of QDREF isolates from turkeys, chickens, and humans, respectively, and was not found in QDREF isolates from other sources. ermB was commonly found in QDREF isolates from all sources. Known streptogramin resistance genes were absent in the majority of isolates, suggesting the presence of other, as-yet-undetermined, mechanisms of Q-D resistance.
Enterococci are a common cause of nosocomial infections in the United States and are a particular concern in hospital intensive care units, where they are the third-leading cause of infections (9, 25). Treatment of patients with illness caused by Enterococcus faecium isolates poses a particular challenge due to their acquired resistance to multiple antimicrobial agents. Quinupristin-dalfopristin (Q-D) is a streptogramin antibiotic used to treat hospitalized patients infected with vancomycin-resistant E. faecium (8, 10, 14, 20, 21, 22, 23). Virginiamycin, a streptogramin compound that has been used in animal feed in the United States for growth promotion, has cross-resistance with Q-D. In this study, we evaluated Q-D-resistant E. faecium (QDREF) isolates from humans, food animals, and grocery store meat for strain relatedness. We also performed PCR to determine whether the streptogramin resistance genes previously found in enterococci, vatD, vatE (both encoding resistance to streptogramin A compounds), and vgbA (encoding resistance to streptogramin B compounds), and the macrolide resistance gene ermB were present in these isolates. The horizontal transfer of these genes has previously been demonstrated in vitro (12, 13, 18, 29, 30, 37) and, for vatD, in vivo (16). Gene linkage between both vatD and ermB and vatE and ermB has also been demonstrated previously (5, 13, 18, 37). The public health concern is that QDREF isolates from a food animal reservoir could potentially cause infections in humans or transfer streptogramin resistance determinants to E. faecium in humans. This could compromise the effectiveness of Q-D and further limit treatment options for patients with multiple-antimicrobial-resistant E. faecium infections.
Three hundred sixty-one E. faecium isolates obtained from 1995 to 2003 with a Q-D MIC of ≥4 μg/ml were evaluated. Fifty-seven isolates (45 inpatients and 12 outpatients) were obtained from human stool samples from Georgia, Illinois, Massachusetts, Michigan, Minnesota, Nevada, New York, Oregon, and Pennsylvania. Two hundred eighty-three QDREF isolates were obtained from farm animals in Michigan, Indiana, and Wisconsin (15). There were 105 turkey isolates, including 31 isolates cultured from individual cloacal swabs from turkeys on a farm that used virginiamycin (34). The remaining turkey isolates were obtained from cultures of manure drag swabs collected on seven farms, four of which used virginiamycin (15). The 62 chicken isolates were obtained from cultures of manure drag swabs collected on 10 chicken farms, 7 of which used virginiamycin (15). The 62 swine isolates, 51 dairy cattle isolates, and 3 beef cattle isolates were obtained from cultures of feces from individual animals at 10 swine, 13 dairy, and 3 beef farms, none of which used virginiamycin (15). Twenty-one QDREF isolates were cultured from grocery store meat samples (18 from chicken carcasses and 3 from ground pork) purchased in Georgia, Maryland, Michigan, Minnesota, and Oregon.
Human stools, farm animal samples (feces, cloacal swabs, and manure drag swabs), and grocery store meat samples were collected and cultured using previously described methods (7, 15, 22). Enterococcosel medium (BD Diagnostics, Sparks, MD) containing 4 μg/ml Q-D was used for selecting QDREF isolates (7, 15, 22). Isolates were identified as E. faecium by using standard biochemical reactions (11). In vitro MICs of Q-D (read at 16 to 20 h) were determined for all isolates using a standardized broth microdilution method and interpretive standards described by CLSI (formerly NCCLS) (24), with an MIC of ≥4 μg/ml being resistant.
Genomic DNA was prepared using a previously described method (6). BioNumerics software (Applied Maths, Kortrijk, Belgium) was used to calculate percent similarities (Dice coefficient) of pulsed-field gel electrophoresis (PFGE) patterns. Isolates were considered to be related if their PFGE patterns were ≥80% similar. PCR was performed to determine the presence of the known enterococcal streptogramin resistance genes, vatD, vatE, and vgbA, and the macrolide resistance gene ermB by using previously described methods (3, 27, 31, 35).
Q-D MICs ranged from 4 to ≥16 μg/ml (Table 1). Ninety-six percent of isolates with MICs of ≥16 μg/ml were isolated from turkeys on farms or grocery store samples of chicken or pork. Of the 45 isolates from hospitalized patients, 39 (87%) were also vancomycin resistant. None of the QDREF isolates from outpatients, farm animals, or grocery store meats were vancomycin resistant.
TABLE 1.
Q-D susceptibility testing, comparison of PFGE strain types, and streptogramin and macrolide resistance gene content for QDREF isolates from humans, farm animals, and grocery store meat sources
| Source | Total no. of isolates | No. of isolates with Q-D MIC (μg/ml) of:
|
No. of PFGE groupsa | No. of uniqueb PFGE types (%) | No. of isolates positive for vatE (%) | No. of isolates positive for ermB (%) | ||
|---|---|---|---|---|---|---|---|---|
| 4 | 8 | ≥16 | ||||||
| Humans | 57 | 37 | 17 | 3 | 7 | 25 (44) | 1 (2) | 48 (84) |
| Hospitalized | 45 | 27 | 17 | 1 | 6 | 15 (33) | 0 | 43 (95) |
| Outpatients | 12 | 10 | 0 | 2 | 1 | 10 (83) | 1 (8) | 5 (42) |
| Farm animals | 283 | 100 | 81 | 102 | 61c | 82d | 53 (19) | 198 (70) |
| Turkeys | 105 | 13 | 31 | 61 | 24 | 27 | 37 (35) | 71 (68) |
| Chickens | 62 | 0 | 22 | 40 | 12 | 29 | 16 (26) | 23 (37) |
| Swine | 62 | 42 | 19 | 1 | 11 | 17 | 0 | 61 (98) |
| Dairy cattle | 51 | 42 | 9 | 0 | 14 | 9 | 0 | 42 (82) |
| Beef cattle | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 1 (33) |
| Grocery store meat | 21 | 3 | 4 | 14 | 6 | 10 (48) | 0 | 11 (52) |
| Chicken | 18 | 1 | 4 | 13 | 3 | 7 (39) | 0 | 8 (44) |
| Ground pork | 3 | 2 | 0 | 1 | 3 | 3 (100) | 0 | 3 (100) |
| Total | 361 | 140 | 102 | 119 | 74 | 117 (32) | 54 (15) | 257 (71) |
Each group contains more than one isolate with PFGE patterns with ≥80% similarity.
Only one isolate with this PFGE pattern.
One PFGE group of turkey isolates also contained one chicken isolate, and one strain type unique among turkeys had a related PFGE pattern compared to two chicken isolates.
One PFGE group of chicken isolates also contained one turkey isolate, and two strain types unique among chickens contained turkey isolates.
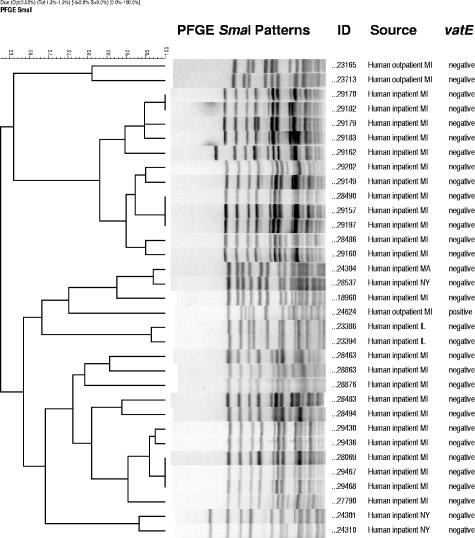
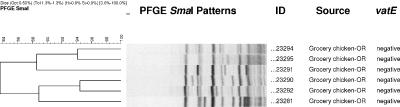
PFGE analysis produced 197 different patterns (<80% similarity) for the 361 QDREF isolates evaluated (Table 1). Seven PFGE groups of QDREF isolates from humans contained multiple isolates (Fig. 1), one with 12 isolates from four hospitals in one city in Michigan, one with eight isolates from hospitals in three cities in Michigan, one with two isolates from two outpatients in Michigan, one with three isolates from outpatients in three different states (Massachusetts, Michigan, and New York), one with two isolates from patients in one hospital in Illinois, one with two hospitalized patients in New York, and one with isolates from three hospitalized patients one city in Michigan. QDREF isolates from humans did not share PFGE patterns with QDREF isolates from farm animals or grocery store meat. QDREF isolates from farm animal sources did not share PFGE patterns with QDREF isolates from grocery store meat sources. Three PFGE groups had both turkey and chicken isolates. These three groups consisted of four QDREF isolates from three turkey farms and five QDREF isolates from three chicken farms. Otherwise, related PFGE patterns were not observed in QDREF isolates from different species of farm animals. QDREF isolates from six samples of grocery store chicken from Oregon had related PFGE patterns (Fig. 2). Related PFGE patterns were commonly seen for QDREF isolates from farm animals of the same species on the same farm and on different farms. Six grocery store chicken carcasses from Oregon yielded QDREF isolates with related PFGE patterns. All other chicken and ground pork isolates had unique PFGE patterns.
FIG. 1.
Dendrogram showing PFGE groups for quinupristin-dalfopristin-resistant E. faecium isolates from human sources.
FIG. 2.
Dendrogram showing related SmaI PFGE patterns (≥80% similarity) of QDREF isolates from grocery store chicken.
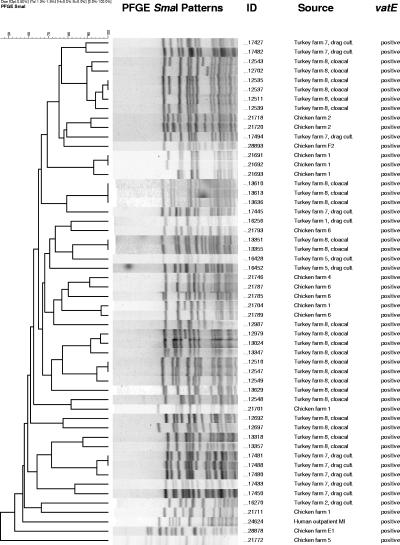
PCR results for the detection of vatE and ermB can be found in Table 1. vatE was found in one QDREF isolate from a human source (outpatient), 35% of QDREF isolates from turkeys, and 26% of QDREF isolates from chickens but not in QDREF isolates from swine, dairy or beef cattle, hospitalized patients, or grocery store meats. A comparison of SmaI PFGE patterns for vatE-positive QDREF isolates is shown in Fig. 3. ermB was commonly found (range of 33 to 100%) in QDREF isolates from all types of strain sources (Table 1). vatD and vgbA were not detected in any isolates.
FIG. 3.
Dendrogram showing SmaI PFGE patterns of quinupristin-dalfopristin-resistant E. faecium isolates positive for the streptogramin A resistance gene vatE.
Although it is clear that the use of antimicrobial agents in animal feed contributes to the emergence and dissemination of antimicrobial resistance in enterococci isolated from farm animals, the human health consequence of this resistance continues to be debated (1, 2, 4, 26, 33). Virginiamycin is still used in food animals in the United States, including chickens, turkeys, swine, and cattle, and strain relatedness and resistance gene content of QDREF isolates has not been well described. We therefore evaluated QDREF isolates from humans, farm animals, and grocery store meats from multiple locations in the United States to gain a better understanding of the epidemiology of Q-D resistance. We found a diversity of PFGE patterns among the QDREF isolates from humans outside of the hospital setting; however, 2 of 12 isolates from outpatients from Michigan had related PFGE patterns (Fig. 1). There was evidence of clonal spread within and between hospitals, and 87% of QDREF isolates from hospitalized patients were also vancomycin resistant. One PFGE group contained eight isolates from three different cities (six hospitals) in Michigan, and two other groups contained multiple isolates from one of these cities (four hospitals). Another PFGE strain type of QDREF was isolated from hospitalized patients in three states, suggesting broad dissemination between humans. Because these Q-D-resistant isolates were resistant to vancomycin in addition to Q-D, treatment of patients infected with these organisms will be a challenge, which highlights the need for precautions to control the further dissemination of Q-D- and vancomycin-resistant E. faecium.
A diversity of PFGE patterns was also found among the QDREF isolates from farm animals and grocery store meat. Although isolates from animals of the same species on the same farm often shared the same PFGE strain type and although those isolates from animals of the same species on different farms occasionally shared the same PFGE strain type, we rarely found QDREF isolates from different animal species with the same PFGE pattern. There were three PFGE groups that contained QDREF isolates from both turkeys and chickens (a total of four turkey and five chicken isolates from six farms). In our study, QDREF isolates with the same PFGE strain type were isolated from five chicken carcasses purchased from grocery stores in Oregon (Fig. 2); it is not known whether these chickens were obtained from more than one producer. Another study, which evaluated QDREF from retail poultry in the greater Washington, D.C., area, also found a diversity of PFGE patterns with some common PFGE strain types among retail chicken isolates and among retail turkey isolates (30). We found no common PFGE strain types among QDREF isolates from humans, farm animals, and grocery store meats. Similar observations have been made in other studies, demonstrating a broad diversity of PFGE patterns among enterococci with various resistance phenotypes and suggesting that it is uncommon to find shared PFGE strain types among human and animal isolates of enterococci (1, 36, 38). Evaluation of larger numbers of isolates may be needed to further elucidate clonal relationships. Nonetheless, there have been several reports from Europe and the United States of antibiotic-resistant enterococci with similar or indistinguishable PFGE patterns isolated from humans and animals or humans and retail meat sources (7, 17, 19, 28). In the United States, for example, high-level gentamicin-resistant enterococci from one human and multiple ground pork samples from Michigan grocery stores had related PFGE patterns, and high-level gentamicin-resistant enterococci from one human and one chicken carcass from an Oregon grocery store had indistinguishable PFGE patterns (7). Transient colonization of QDREF isolates of animal origin in human volunteers has been shown previously (32), which may also have implications for the transfer of resistance genes in the human intestine. In the United States, vatE has been detected only in QDREF isolates from poultry, and vatD has not been found in isolates from any source (29, 30). We found vatE in 15% of QDREF isolates (from one human and from turkey and chicken farms) and did not find vatD. This differs from findings of European studies, where most QDREF has been attributed to vatE or vatD (31, 36). We found vatE mainly among QDREF isolates from turkeys and chickens. All but one vatE-containing Q-D-resistant isolate from farm animals originated from farms using virginiamycin for growth promotion. None of the QDREF isolates from swine or dairy or beef cattle contained the vatE gene, and none of these farms used virginiamycin for growth promotion. Importantly, we found vatE in one QDREF isolate from a human outpatient. This person had no history of hospital exposure or international travel and had never been treated with Q-D. The isolate from this person was resistant to high levels of Q-D (MIC ≥ 16 μg/ml) and yielded a unique PFGE pattern. This is the first report, to our knowledge, of a vatE-positive E. faecium isolate from a human in the United States.
In this study, we found that there were multiple PFGE strain types of QDREF responsible for Q-D resistance among humans, farm animals, and grocery store meat. We found evidence of animal-to-animal transmission, clonal strains among grocery store meat, and, importantly, the spread of a Q-D-resistant and vancomycin-resistant E. faecium strain to 22 patients in six different hospitals in southeastern Michigan. The extent of this spread of an E. faecium strain with resistance to both vancomycin and Q-D has not been previously described in U.S. hospitals and is of particular concern. vatE was detected commonly in animal isolates and was also found in one human strain, suggesting that horizontal transfer of streptogramin resistance elements is a potential mechanism by which Q-D resistance is spread between humans and animals. However, because only a small proportion of isolates in this study tested positive for previously described resistance genes, it will be of great importance to identify and characterize these new mechanisms of resistance in E. faecium in order to trace the origin, epidemiology, and mechanism of QDREF isolates from humans, farm animals, and grocery store meat in the United States.
Acknowledgments
This work was supported by the Food and Drug Administration, Centers for Veterinary Medicine (FD-U-001577-01), and the Centers for Disease Control and Prevention (RS1/CCR520614-01).
REFERENCES
- 1.Aarestrup, F. M., A. M. Seyfarth, H.-D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 3.Allignet, J., V. Loncle, P. Mazodier, and N. El Solh. 1988. Nucleotide sequence of staphylococcal plasmid gene, vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid 20:271-275. [DOI] [PubMed] [Google Scholar]
- 4.Bager, F., M. Madsen, J. Christensen, and F. M. Aarestrup. 1997. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 31:95-112. [DOI] [PubMed] [Google Scholar]
- 5.Bozdogan, B., and R. Leclercq. 1999. Effects of genes encoding resistance to streptogramins A and B on the activity of quinupristin-dalfopristin against Enterococcus faecium. Antimicrob. Agents Chemother. 43:2720-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donabedian, S. M., J. W. Chow, D. M. Shlaes, M. Green, and M. J. Zervos. 1995. DNA hybridization and contour-clamped homogeneous electric field electrophoresis for identification of enterococci to the species level. J. Clin. Microbiol. 33:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donabedian, S. M., L. A. Thal, E. Hershberger, M. B. Perri, J. W. Chow, P. Bartlett, R. Jones, K. Joyce, S. Rossiter, K. Gary, J. Johnson, C. Mackinson, E. Debess, J. Madden, F. Angulo, and M. J. Zervos. 2003. Molecular characterization of gentamicin-resistant enterococci in the United States: evidence of spread from animals to humans through food. J. Clin. Microbiol. 41:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowzicky, M., G. H. Talbot, C. Feger, P. Prokocimer, J. Etienne, and R. Leclercq. 2000. Characterization of isolates associated with emerging resistance to quinupristin-dalfopristin (Synercid) during a worldwide clinical program. Diagn. Microbiol. Infect. Dis. 37:57-62. [DOI] [PubMed] [Google Scholar]
- 9.Edmond, M. B., S. E. Wallace, D. K. McKlish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., C. B. Wennersten, H. S. Gold, T. Schulin, M. Souli, M. G. Farris, S. Cerwinka, H. L. Nadler, M. Dowzicky, G. H. Talbot, and R. C. Moellering, Jr. 1998. Characterization of vancomycin-resistant Enterococcus faecium isolates from the United States and their susceptibility in vitro to quinupristin-dalfopristin. Antimicrob. Agents Chemother. 42:1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facklam, R. R., D. F. Sahm, and L. M. Teixeira. 1999. Enterococcus, p. 297-305. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 12.Hammerum, A. M., L. B. Jensen, and F. M. Aarestrup. 1998. Detection of the satA gene and transferability of virginiamycin resistance in Enterococcus faecium from food animals. FEMS Microbiol. Lett. 168:145-151. [DOI] [PubMed] [Google Scholar]
- 13.Hammerum, A. M., S. E. Flannagan, D. B. Clewell, and L. B. Jensen. 2001. Indication of transposition of a mobile DNA element containing the vat(D) and erm(B) genes in Enterococcus faecium. Antimicrob. Agents Chemother. 45:3223-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershberger, E., S. Donabedian, K. Konstantinou, and M. J. Zervos. 2004. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin. Infect. Dis. 38:92-98. [DOI] [PubMed] [Google Scholar]
- 15.Hershberger, E., S. F. Oprea, S. M. Donabedian, M. Perri, P. Bozigar, P. Bartlett, and M. J. Zervos. 2005. Epidemiology of antimicrobial resistance in enterococci of animal origin. J. Antimicrob. Chemother. 55:127-130. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen, B. L., M. Skou, A. M. Hammerum, and L. B. Jensen. 1999. Horizontal transfer of the satA gene encoding streptogramin A resistance between isogenic Enterococcus faecium strains in the gastrointestinal tract of gnotobiotic rats. Microbiol. Ecol. Health Dis. 11:241-247. [Google Scholar]
- 17.Jensen, L. B., A. M. Hammerum, F. M. Aarestrup, A. E. van den Bogaard, and E. E. Stobberingh. 1998. Occurrence of satA and vgb genes in streptogramin-resistant Enterococcus faecium isolates of animal and human origins in The Netherlands. Antimicrob. Agents Chemother. 42:3330-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen, L. B., A. M. Hammerum, and F. M. Aarestrup. 2000. Linkage of vat(E) and erm(B) in streptogramin-resistant Enterococcus faecium isolates from Europe. Antimicrob. Agents Chemother. 44:2231-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen, L. B., A. M. Hammerum, R. L. Poulsen, and H. Westh. 1999. Vancomycin-resistant Enterococcus faecium strains with highly similar pulsed-field gel electrophoresis patterns containing similar Tn1546-like elements isolated from a hospitalized patient and pigs in Denmark. Antimicrob. Agents Chemother. 43:724-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, A. P., and D. M. Livermore. 1999. Quinupristin-dalfopristin, a new addition to the antimicrobial arsenal. Lancet 354:2012-2013. [DOI] [PubMed] [Google Scholar]
- 21.Jones, R. N. 1998. Antimicrobial activity of quinupristin-dalfopristin (RP 59500, Synercid) tested against over 28,000 recent clinical isolates from 200 medical centers in the United States and Canada. Diagn. Microbiol. Infect. Dis. 31:437-451. [DOI] [PubMed] [Google Scholar]
- 22.McDonald, L. C., S. Rossiter, C. MacKinson, Y. Y. Wang, S. Johnson, M. Sullivan, R. Sokolow, E. DeBess, L. Gilbert, J. A. Benson, B. Hill, and F. J. Angulo. 2001. Quinupristin-dalfopristin-resistant Enterococcus faecium on chicken and in human stool specimens. N. Engl. J. Med. 345:1155-1160. [DOI] [PubMed] [Google Scholar]
- 23.Moellering, R. C., P. K. Linden, J. Reinhardt, E. A. Blumberg, F. Bompart, G. H. Talbot, et al. 1999. The efficacy and safety of quinupristin-dalfopristin for the treatment of infections caused by vancomycin-resistant Enterococcus faecium. J. Antimicrob. Chemother. 44:251-261. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.NNIS System. 2003. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Phillips, I., M. Casewell, T. Cox, B. De Groot, C. Friis, R. Jones, C. Nightingale, R. Preston, and J. Waddell. 2004. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 53:28-52. [DOI] [PubMed] [Google Scholar]
- 27.Rende-Fournier, R., R. Leclercq, M. Galimand, J. Duval, and P. Courvalin. 1993. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob. Agents Chemother. 37:2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robredo, B., K. V. Singh, C. Torres, and B. E. Murray. 2000. Streptogramin resistance and shared pulsed-field gel electrophoresis patterns in vanA-containing Enterococcus faecium and Enterococcus hirae isolated from humans and animals in Spain. Microb. Drug Resist. 6:305-311. [DOI] [PubMed] [Google Scholar]
- 29.Simjee, S., D. G. White, D. D. Wagner, J. Meng, S. Qaiyumi, S. Zhao, and P. F. McDermott. 2002. Identification of vat(E) in Enterococcus faecalis isolates from retail poultry and its transferability to Enterococcus faecium. Antimicrob. Agents Chemother. 46:3823-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simjee, S., D. G. White, J. Meng, D. D. Wagner, S. Qaiyumi, S. Zhao, J. R. Hayes, and P. F. McDermott. 2002. Prevalence of streptogramin resistance genes among Enterococcus isolates recovered from retail meats in the greater Washington, DC area. J. Antimicrob. Chemother. 50:877-882. [DOI] [PubMed] [Google Scholar]
- 31.Soltani, M., D. Beighton, J. Philpott-Howard, and N. Woodford. 2000. Mechanisms of resistance to quinupristin-dalfopristin among isolates of Enterococcus faecium from animals, raw meat, and hospital patients in Western Europe. Antimicrob. Agents Chemother. 44:433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen, T. L., M. Blom, D. L. Monnet, N. Frimoldt-Moller, R. L. Poulsen, and F. Espersen. 2001. Transient carriage after ingestion of antibiotic-resistant Enterococcus faecium from chicken and pork. N. Engl. J. Med. 345:1161-1166. [DOI] [PubMed] [Google Scholar]
- 33.van den Bogaard, A. E., N. Bruinsma, and E. E. Stobberingh. 2002. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:146-147. [DOI] [PubMed] [Google Scholar]
- 34.Welton, L. A., L. A. Thal, M. B. Perri, S. M. Donabedian, J. McMahon, J. W. Chow, and M. J. Zervos. 1998. Antimicrobial resistance in enterococci isolated from turkey flocks fed virginiamycin. Antimicrob. Agents Chemother. 42:705-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner, G., and W. Witte. 1999. Characterization of a new enterococcal gene, satG, encoding a putative acetyltransferase conferring resistance to streptogramin A compounds. Antimicrob. Agents Chemother. 43:1813-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner, G., I. Klare, H. Heier, K. H. Hinz, G. Bohme, M. Wendt, et al. 2000. Quinupristin-dalfopristin-resistant enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb. Drug Resist. 6:37-47. [DOI] [PubMed] [Google Scholar]
- 37.Werner, G., B. Hildebrandt, I. Klare, and W. Witte. 2000. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int. J. Med. Microbiol. 290:543-548. [DOI] [PubMed] [Google Scholar]
- 38.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van den Bogaard, and J. D. A. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]