Abstract
With fluorescently labeled PNA (peptide nucleic acid) probes targeting 16S rRNA, we established a 3-h fluorescence in situ hybridization (FISH) procedure for specific visualization of members of the Mycobacterium tuberculosis complex, M. leprae, M. avium, and M. kansasii. Probe specificity was tested against a panel of 25 Mycobacterium spp. and 10 gram-positive organisms. After validation, probes were used to identify 52 mycobacterial culture isolates. Results were compared to conventional genotypic identification with amplification-based methods. All isolates (M. tuberculosis complex, n = 24; M. avium, n = 7; M. kansasii, n = 1) were correctly identified by FISH. In addition, the technique was used successfully for visualization of mycobacteria in biopsies from infected humans or animals. In conclusion, PNA-FISH is a fast and accurate tool for species-specific identification of culture-grown mycobacteria and for direct visualization of these organisms in tissue sections. It may be used successfully for both research and clinical microbiology.
Mycobacterial infections are associated with chronic disease, often with a fatal outcome. Tuberculosis is a growing global public health problem, with an estimated 8 million new cases and about 2 million deaths each year (23). According to the WHO website on leprosy (http://www.who.int/lep/), 407,791 new cases of leprosy were detected during 2004. Mycobacterium avium subsp. avium and M. kansasii are important pathogens causing severe disease in immunocompromised patients (1), and M. avium subsp. paratuberculosis, the causative agent of Johne's disease in ruminants, is responsible for significant economic losses in the livestock industry worldwide (22).
Detection of mycobacteria in clinical specimens by conventional methods is difficult because of the low numbers of bacilli available, their slow growth, and their fastidious metabolism. Cultivation of M. leprae is still impossible. Recently, conventional methods such as acid-fast staining, culture, and phenotypic differentiation have been complemented by nucleic acid probes and amplification-based methods, substantially reducing the time to diagnosis (11).
Specific visualization of mycobacteria, e.g., by fluorescence in situ hybridization (FISH), would be a great help in directly identifying bacteria in clinical and environmental samples (11, 12). However, conventional oligonucleotide probes barely penetrate bacteria with cell walls containing mycolic acids. The relative hydrophobic character of PNA (peptide nucleic acid) probes compared to DNA analogues allows better diffusion through the hydrophobic cell wall of mycobacteria (19, 20). However, the FISH assays available so far are restricted to differentiation of tuberculous from nontuberculous Mycobacterium species in acid-fast bacillus-positive sputum smears or in culture (2, 6, 15, 21), as well as M. avium in potable-water biofilms (9).
There are several reports describing the detection of M. tuberculosis and M. avium subsp. paratuberculosis in tissue sections by staining with antibodies or in situ hybridization (ISH). Seiler and colleagues (18) used a polyclonal anti-M. bovis Bacille Calmette-Guerin serum for detection of cell wall-deficient M. tuberculosis in mouse tissue. Naser and colleagues (13) demonstrated M. avium subsp. paratuberculosis in tissue specimens from patients with Crohn's disease with a polyclonal antibody. Several authors have described the detection of mycobacterial DNA or RNA in tissue specimens of human or animal origin with ISH or in situ PCR techniques (3, 4, 5, 7, 8, 17). One paper reported on ISH with PNA probes, followed by signal amplification, to differentiate between M. tuberculosis complex and nontuberculous Mycobacterium spp. in archival biopsy and autopsy samples (24). All of the methods described so far either lack specificity (antibody-based staining), are laborious and time consuming, or do not distinguish single mycobacteria. Bacteria are not resolved properly but appear as a stained mass of uncertain identity.
Here we present an improved method using fluorescently labeled PNA probes for fast visualization and identification of members of the M. tuberculosis complex, M. avium, M. kansasii, and M. leprae in smears and tissue biopsies. A rapid (3-h) FISH procedure was established and evaluated by using mycobacteria cultured from clinical specimens. M. tuberculosis complex-, M. leprae-, and M. avium-specific PNA probes were used successfully for specific visualization of the respective mycobacteria in tissue sections from infected humans or animals. This technical improvement may have a great impact on the detection of mycobacteria in research or diagnostic laboratories, particularly in those which are unable or unwilling to invest into a highly sophisticated molecular genetic facility.
MATERIALS AND METHODS
Bacterial strains and liquid cultures of clinical specimens.
Mycobacterium sp. reference strains (n = 17) and mycobacterial isolates (n = 9) from our clinical microbiology laboratory (see Table 2), as well as 10 gram-positive organisms (Actinomyces israelii [clinical isolate], Actinomyces odontolyticus [ATCC 17929], Corynebacterium matruchotii [DSM20635], Corynebacterium pseudodiphtheriticum [clinical isolate], Enterococcus faecalis [ATCC 29121], Erysipelothrix rhusiopathiae [clinical isolate], Lactobacillus paracasei [clinical isolate], Nocardia brasiliensis [clinical isolate], Staphylococcus aureus [ATCC 25923], and Staphylococcus epidermidis [ATCC 12228]) were used to validate the FISH procedure. The species identities of all strains or isolates were confirmed by 16S rRNA gene PCR and sequencing. The FISH assay was validated by using 52 liquid cultures positive for acid-fast bacilli from a variety of clinical specimens. PNA-FISH was compared to standard molecular genetic identification methods based upon nucleic acid amplification (Roche COBAS Amplicor for members of the M. tuberculosis complex, M. avium, and M. intracellulare and sequencing of 16S rRNA genes for other species). All mycobacteria were grown in liquid medium (MGIT liquid medium; Becton Dickinson Europe, France) with an enrichment supplement (MGIT system oleic acid-albumin-dextrose-citric acid) and an antimicrobial supplement (MGIT system PANTA [polymyxin B, nalidixic acid, trimethoprim, and azlocillin]) at 37°C or 30°C for Mycobacterium spp. and M. marinum, respectively.
TABLE 2.
Evaluation of PNA probe specificity
| Organism | Source | MTBCCy3a
|
MAVTAMRAb
|
MKATAMRAc
|
MLEPTAMRAd
|
BacUni-1Fluo resulte | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resultf | MMg | Result | MM | Result | MM | Result | MM | |||
| M. abscessus | DSM 44196 | n | 4 | n | 6 | n | 2 | n | 7 | +-+++ |
| M. avium subsp. avium | DSM 44156 | n | 4 | +-+++ | 0 | n | 2 | n | 2 | +-+++ |
| M. avium subsp. avium | ATCC 15769 | n | 4 | +-+++ | 0 | n | 2 | n | 2 | +-+++ |
| M. bovis | Clinical isolateh | +-+++ | 0 | n | 4 | n | 2 | n | 5 | +-++ |
| M. celatum | DSM 44243 | n-(+) | 1 (pos. 8) | n | 5 | n | 2 | n | 7 | +-++ |
| M. chubuense | DSM 44219 | n | 1 (pos. 5) | n | 5 | n | 3 | n | 5 | +-+++ |
| M. flavescens | Clinical isolate | n | 4 | n | 6 | n | 3 | n | 4 | +-++ |
| M. fortuitum subsp. fortuitum | DSM 46621 | n | 4 | n | 6 | n | 2 | n | 8 | +-+++ |
| M. gordonae | DSM 44160 | n | 3 | n | 3 | n-(+) | 1 (pos. 8) | n | 5 | +-+++ |
| M. haemophilum | DSM 44634 | n | 4 | n-(+) | 1 (pos. 6) | n | 2 | n-+ | 1 (pos. 9) | +-++ |
| M. interjectum | DSM 44064 | n | 4 | n | 2 | n | 2 | n | 2 | +-+++ |
| M. intermedium | DSM 44049 | n | 4 | n | 3 | n-(+) | 1 (pos. 7) | n | 3 | +-+++ |
| M. intracellulare | DSM 43223 | n | 4 | n | 3 | n | 2 | n | 3 | +-+++ |
| M. kansasii | DSM 44162 | n | 4 | n | 4 | +-+++ | 0 | n | 4 | +-+++ |
| M. marinum | DSM 44344 | n-(+) | 1 (pos. 2) | n | 5 | n | 2 | n | 7 | +-+++ |
| M. nonchromogenicum | Clinical isolate | n | 4 | n | 4 | n | 3 | n | 5 | +-++ |
| M. phlei | Clinical isolate | n | 4 | n | 6 | n | 2 | n | 5 | +-++ |
| M. scrofulaceum | DSM 43992 | n | 4 | n | 4 | n | 2 | n | 4 | +-+++ |
| M. septicum | Clinical isolate | n | 4 | n | 6 | n | 3 | n | 4 | +-++ |
| M. simiae | Clinical isolate | n | 4 | n | 4 | n | 2 | n | 4 | (+)-++ |
| M. smegmatis | DSM 43756 | n | 4 | n | 7 | n | 3 | n | 9 | +-++ |
| M. terrae | Clinical isolate | n-(+) | 1 (pos. 8) | n | 5 | n | 2 | n | 4 | +-++ |
| M. triviale | Clinical isolate | n | 4 | n | 3 | n | 2 | n | 4 | +-++ |
| M. tuberculosis | H37Rv | +-++++ | 0 | n | 4 | n | 2 | n | 5 | +-++ |
| M. vaccae | Clinical isolate | n | 4 | n | 6 | n | 3 | n | 4 | +-++ |
| M. xenopi | DSM 43995 | n-(+) | 4 | n | 5 | n | 2 | n | 6 | +-+++ |
M. tuberculosis complex-specific PNA probe.
M. avium-specific PNA probe.
M. kansasii-specific PNA probe.
M. leprae-specific probe.
Eubacterial probe.
n = negative. Fluorescence signal intensities: (+), slightly above background fluorescence; +, clearly visible; ++, strong; +++, very strong.
MM, number of mismatches; pos., position from 5′ end of probe.
From a clinical specimen; identification by 16S rRNA gene sequencing.
Fixation of bacteria.
Bacteria from a positive MGIT vial were concentrated by centrifugation (3,300 × g, 20 min), washed once with 1 ml phosphate-buffered saline (pH 7.4, 3,300 × g, 20 min). Fixation solution (100 to 500 μl depending on pellet size) consisting of 3.7% (vol/vol) formaldehyde in 50% ethanol in phosphate-buffered saline (pH 7.4) was added. The pellet was dissolved and stored at −20°C for a minimum of 15 min. Fixation efficiency was examined by subculturing complete pellets of fixed M. tuberculosis and M. fortuitum cells. Fixed mycobacteria were resuspended in MGIT liquid medium (800 μl) with enrichment supplement and streaked onto Lowenstein-Jensen and Stonebrink slants (100 μl each). After an incubation period of 8 weeks at 37°C, no growth was recorded on either liquid or solid culture medium.
Mycobacteria in cells and in tissue sections.
Adherent human antigen-presenting cells (APC) grown on microscope slides were infected with M. bovis BCG and fixed in 4% (vol/vol) paraformaldehyde for PNA-FISH. Sections (4 to 5 μm) of paraffin-embedded tissue from skin biopsy samples from a patient with leprosy, from lungs of M. tuberculosis-infected C57BL/6 mice (18), or from animals with Johne's disease were deparaffinized by immersion in xylol 100% (vol/vol, 10 min) and ethanol 100% (vol/vol, 5 min) three times. Lung tissue from one patient with culture-proven tuberculosis was embedded in cold polymerizing resin (Technovit 8100; Kulzer, Germany) as described by Moter and Göbel (11) and sectioned (3- to 4-μm thickness).
PNA probes.
Probes MTBCCy3, MLEPTAMRA, MAVTAMRA, and MKATAMRA were designed for specific detection of members of the M. tuberculosis complex, M. leprae, M. avium, and M. kansasii 16S rRNA, respectively, with the 16S rRNA sequence database and the probe design program of the ARB software (http://www.arb-home.de/). Probes were chosen with regard to purine content and secondary structure, avoiding hairpin formations and inverse repeats. To assess their specificity, all probe sequences were compared to all of the 16S rRNA entries in the EMBL and GenBank databases currently (June 2006) accessible by using the programs BLASTN and FASTA of the HUSAR (version 4.0; Heidelberg UNIX Sequence Analysis Resources) program package (DKFZ, Heidelberg, Germany). The PNA probe BacUni-1Fluo (fluorescein), complementary to a region of the 16S rRNA gene conserved in the domain Bacteria (eubacterial probe), was used as a positive control (BacUni-1Fluo [5′-CTG CCT CCC GTA GGA-3′]) (14, 16). MTBCCy3, MLEPTAMRA, and BacUni-1Fluo were purchased from Applied Biosystems (Foster City, Calif.), and MAVTAMRA and MKATAMRA were synthesized by TIB MOLBIOL (Berlin, Germany).
ISH.
Culture-grown and fixed bacteria (2 to 5 μl) were spotted onto six-field microscope slides (Paul Marienfeld KG, Bad Mergentheim, Germany), air dried, dehydrated in 100% (vol/vol) methanol for 1 min and 100% (vol/vol) ethanol for 5 min, air dried again, and preheated to hybridization temperature. Slides with APC and tissue sections were preheated to hybridization temperature. Aliquots (10 μl for cultured bacteria and 20 to 60 μl for APC and tissue sections) of a hybridization mixture containing 10% (wt/vol) dextran sulfate (Merck, Darmstadt, Germany), 10 mM NaCl (Merck), 30 to 50% (vol/vol) formamide (Roth, Karlsruhe, Germany) (Table 1), 0.1% (wt/vol) sodium pyrophosphate (Merck), 0.2% (wt/vol) polyvinylpyrrolidone (Sigma Chemical Co., St. Louis, Mo.), 0.2% (wt/vol) Ficoll (Fluka Chemie AG, Basel, Switzerland), 5 mM disodium EDTA (Roth, Karlsruhe, Germany), 0.1% (vol/vol) Triton X-100 (Serva, Heidelberg, Germany), 50 mM Tris-HCl (pH 7.5), and a fluorescent probe(s) with a final concentration of 1 to 1.5 μmol/liter (Table 1) were applied to each sample. Slides were incubated at a temperature optimized for each PNA probe (Table 1) in a preheated moisture chamber in the dark for 30 min. After brief immersion in double-distilled water, slides were washed in preheated washing buffer (5 mM Tris, 15 mM NaCl, 0.1% [vol/vol] Triton X-100 [pH 10; Serva]) at hybridization temperature for 10 min. Following a brief immersion in double-distilled water, slides were air dried and mounted with 1 drop of Vectashield (Vector Laboratories, Inc., Burlingame, Calif.). Slides were incubated at hybridization temperature accordingly for 10 min.
TABLE 1.
PNA probe sequences and hybridization conditionsa
| Probe (concn [μM]) | Sequence (orientation) | Target species or control | Hybridization and washing temp (°C) | % Formamide (vol/vol) |
|---|---|---|---|---|
| MTBCCy3 (1.0) | TCC TGG TGC CCT ACG-Cy3 (3′-5′) | MTBCCy3 | 60 | 50 |
| AGG ACC ACG GGA TGC (5′-3′) | M. tuberculosis complex | |||
| AGG ACC ACG GGA TTC (5′-3′) | M. marinum | |||
| AGG ACC ACG GCA TGC (5′-3′) | M. chubuense | |||
| AGG ACC ATG GGA TGC (5′-3′) | M. celatum | |||
| MAVTAMRA (1.5) | CTG GAG TTC TGC GTA-TAMRA (3′-5′) | MAVTAMRA | 55 | 30 |
| GAC CTC AAG ACG CAT (5′-3′) | M. avium | |||
| GAC CTC AAG GCG CAT (5′-3′) | M. haemophilum | |||
| MKATAMRA (1.5) | GAC GTG TGG CCC TAT-TAMRA (3′-5′) | MKATAMRA | 55 | 40 |
| CTG CAC ACC GGG ATA (5′-3′) | M. kansasii | |||
| CTG CAC ACT GGG ATA (5′-3′) | M. intermedium | |||
| CTG CAC ATC GGG ATA (5′-3′) | M. gordonae | |||
| MLEPTAMRA (1.5) | ATC CTG AAG TTC CGC-TAMRA (3′-5′) | MLEPTAMRA | 55 | 30 |
| TAG GAC TTC AAG GCG (5′-3′) | M. leprae | |||
| TAG GAC CTC AAG GCG (5′-3′) | M. haemophilum |
The probe concentrations, hybridization temperatures, and formamide concentrations in the hybridization buffer shown are the optimal hybridization conditions for FISH experiments.
Microscopy.
Microscopy was performed with an epifluorescence microscope (Axioplan 2 imaging; Zeiss, Jena, Germany) equipped with a 100-W lamp (HBO 103; Osram) and 10×, 40×, and 100× objectives (Zeiss). Narrow-band HQ and F41-007 or F41-001 filter sets (AHF Analysentechnik, Tübingen, Germany) were used to analyze fluorescein or Cy3 and 6-carboxytetramethylrhodamine (TAMRA) signals, respectively. Digital images were taken with an Axiocam HRc (Zeiss) and image acquisition was performed with Axiovision 4.0 software (Zeiss).
RESULTS
Probe design and evaluation.
Probes of 15 bp each were designed for members of the M. tuberculosis complex (M. africanum, M. bovis, M. bovis BCG, “M. canetti,” M. caprae, M. microti, M. pinnipedii, and M. tuberculosis), M. avium (M. avium, M. paratuberculosis, and M. silvaticum), and M. leprae. In addition, MTBCCy3 and MAVTAMRA showed a 100% match with sequences of M. asiaticum and M. lepraemurium, respectively. However, M. asiaticum has so far only rarely been isolated from patient specimens (10; www.ridom-rdna.de). M. lepraemurium causes endemic disease in rats and other animals and has not been identified from human sources yet (www.ridom-rdna.de). As M. kansasii and M. gastri share identical 16S rRNA sequences, a probe specific for both species was designed. Searches of publicly available databases with BLASTN and FASTA of the HUSAR sequence analysis package did not reveal any other bacterial 16S rRNA gene sequences with a 100% match to MLEPTAMRA and MKATAMRA.
For specificity control, we chose cultured Mycobacterium species with 16S rRNA sequences containing single base mismatches in the target region. Table 1 compiles probe sequences, complementary 16S rRNA sequences of target species and controls, and optimized hybridization conditions for sequence-specific FISH. M. marinum shows a single base mismatch at position 2 (from the 5′ probe end) with probe MTBCCy3. To prevent unspecific hybridization of MTBCCy3 to M. marinum, high-stringency hybridization conditions (60°C, 50% formamide) were required. For M. chubuense and M. celatum, exhibiting single mismatches at positions 5 and 8 of the MTBCCy3 probe sequence, less-stringent hybridization conditions (55°C, 40% formamide) were sufficient to prevent unspecific binding (data not shown). For probes MAVTAMRA and MKATAMRA, exhibiting single base mismatches to M. haemophilum and M. intermedium or M. gordonae, respectively, unspecific binding was avoided by hybridization at 55°C and use of a formamide concentration of 30% or 40%, respectively. Since M. leprae has not yet been cultured, we used M. haemophilum (one mismatch at position 9) for evaluation of probe MLEPTAMRA. Low-stringency hybridization conditions (50°C, no formamide) resulted in strong fluorescence signals in M. haemophilum and showed the applicability of the probe for FISH. Increasing the temperature to 55°C and the formamide concentration to 30% resulted in a weak fluorescence signal with M. haemophilum, indicating M. leprae-specific FISH. Hybridization with a PNA probe for specific visualization of M. intracellulare was not successful. The probe sequence chosen had single mismatches to many different mycobacterial species at positions 1 and 2 from the 5′ probe end. We were not able to establish hybridization conditions avoiding unspecific binding (data not shown).
The specificity of all probes and hybridization conditions was evaluated with a panel of 25 different mycobacterial species (Table 2) and 10 different gram-positive organisms (see Materials and Methods). Species with single mismatches to the probe were included in every FISH experiment to control for unspecific binding. In FISH experiments lacking a PNA probe, fixed bacterial cells showed no or little autofluorescence (data not shown). Eubacterial probe BacUni-1Fluo was used to check for rRNA content, permeability of fixed bacteria, and a successful FISH procedure in positive and negative controls in every experiment. All fixed bacteria were stained by the eubacterial probe and visible as single cells or clusters. However, microscopy with the Cy3-TAMRA filter set (specific probes) revealed bright fluorescence of the target organisms only. No or weak signals were observed for mycobacterial species with single mismatches in the probe binding region. All other species showed no fluorescence with the specific probes.
Identification of mycobacteria cultured from clinical specimens.
PNA-FISH results for mycobacteria cultured from patient materials are listed in Table 3. Mycobacterial isolates (n = 52) were fixed and hybridized with probes MTBCCy3 and BacUni-1Fluo. If the MTBCCy3 FISH was negative, M. avium- and M. kansasii-specific probes combined with BacUni-1Fluo were used. All FISH experiments were done in a blinded fashion, and results were compared to conventional identification after completion. FISH with probes MTBCCy3, MAVTAMRA, and MKATAMRA resulted in the correct identification of all M. tuberculosis, M. avium, and M. kansasii isolates, respectively (Table 3).
TABLE 3.
Identification by PNA-FISH of mycobacteria from 52 positive liquid cultures
| Conventional identification | No. of isolates | PNA-FISHd result obtained with:
|
||
|---|---|---|---|---|
| MTBCCy3a | MAVTAMRAb | MKATAMRAc | ||
| M. avium | 7 | − | +++ | − |
| M. gordonae | 8 | − | − | − |
| M. intracellulare | 10 | − | − | − |
| M. kansasii | 1 | − | − | +++ |
| M. marinum | 1 | − | − | − |
| M. tuberculosis | 24 | +++ | − | − |
| M. xenopi | 1 | − | − | − |
M. tuberculosis complex-specific PNA probe.
M. avium-specific PNA probe.
M. kansasii-specific PNA probe.
− = negative. For fluorescence signal intensity definitions, see Table 2, footnote f.
Mycobacteria in cells and in tissue sections.
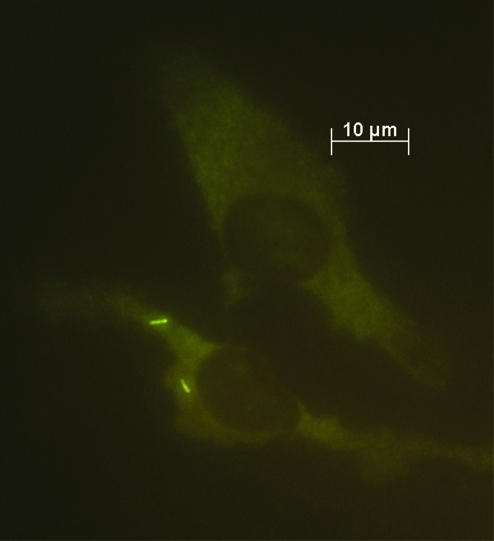
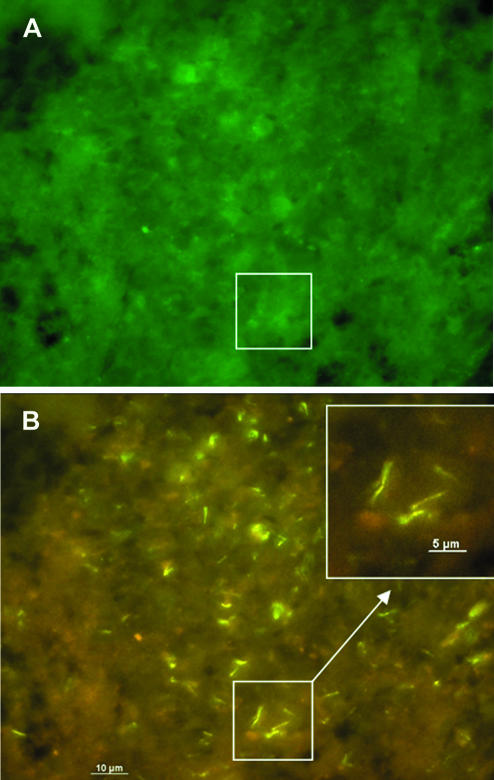
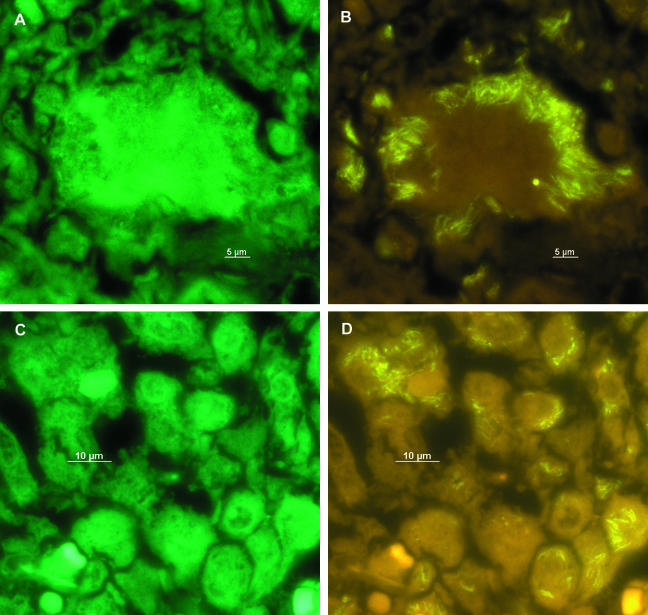
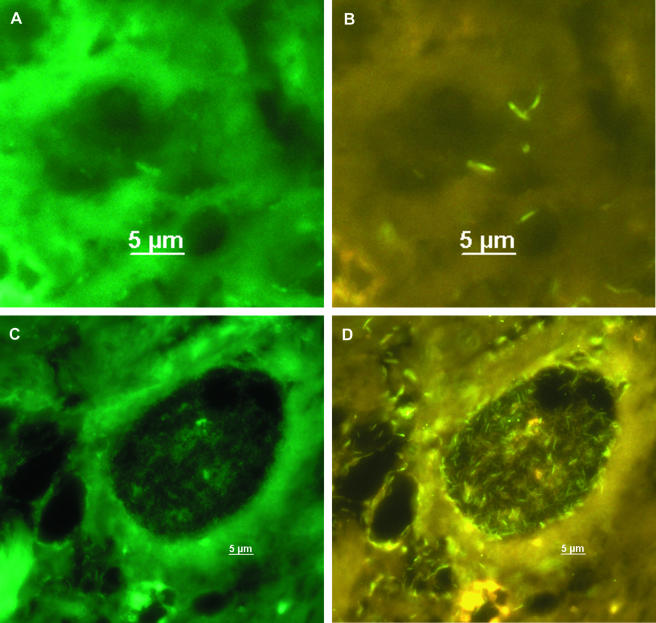
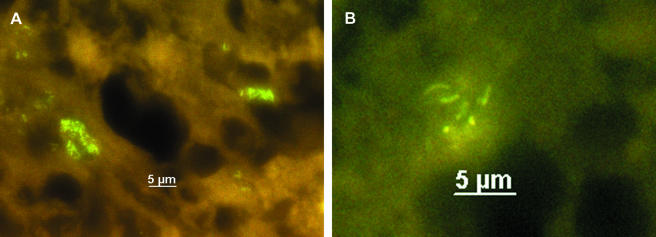
After evaluation, PNA-FISH was used to visualize mycobacteria in fixed cells and tissue sections. Slides carrying M. bovis BCG-infected APC were hybridized with probe MTBCCy3 (Fig. 1). M. bovis BCG showed a strong fluorescence on a weak background fluorescence of APC. Lung sections of mice infected with M. tuberculosis H37Rv were examined with probe MTBCCy3, showing the spatial distribution of mycobacteria (Fig. 2B). Lung sections from a culture-proven case of human tuberculosis were stained with probe MTBCCy3 as well. M. tuberculosis cells were found at the rim of the necrosis (Fig. 3A and B) and inside macrophages (Fig. 3C and D). Probe MLEPTAMRA was used for examination of a skin biopsy sample from a case of human leprosy, and single mycobacterial cells could be demonstrated (Fig. 4). Mycobacteria were demonstrated in corresponding tissue sections as single bacteria (Fig. 4A and B) or in groups (Fig. 4C and D). Furthermore, with MAVTAMRA, M. paratuberculosis was visualized successfully in a case of ruminant Johne's disease (Fig. 5).
FIG. 1.
Visualization by FISH (probe MTBCCy3) of human APC experimentally infected with M. bovis BCG.
FIG. 2.
Visualization by FISH (probe MTBCCy3) of M. tuberculosis H37Rv in a lung biopsy from an experimentally infected mouse. Same microscopic field visualized with a fluorescein filter set (A), showing background fluorescence allowing orientation within the tissue, and a Cy3-TAMRA filter set (B). The inset shows single mycobacterial cells.
FIG. 3.
Visualization by FISH (probe MTBCCy3) of M. tuberculosis in a lung biopsy from a tuberculosis patient. A and C, fluorescein filter set; B and D, Cy3-TAMRA filter set.
FIG. 4.
FISH (probe MLEPTAMRA) for detection of M. leprae in a tissue section from a case of leprosy. A and C, fluorescein filter set; B and D, Cy3-TAMRA filter set.
FIG. 5.
Detection of M. avium in a tissue section from a case of Johne's disease in a cow by FISH (probe MAVTAMRA). A and B, Cy3-TAMRA filter set.
DISCUSSION
FISH detection of mycobacteria with oligonucleotide probes is difficult since probe penetration is hampered by long-chain mycolic acids in mycobacterial cell walls. The development of PNA probes that enter mycobacteria without further pretreatment was, hence, a breakthrough. With a fluorescein-labeled PNA probe, Stender et al. (20) were able to differentiate between tuberculous and nontuberculous mycobacteria. The successful use of these probes was shown in different studies thereafter (2, 6, 15, 21).
Here we used four novel PNA probes to identify and visualize bacteria of the M. tuberculosis complex, as well as M. leprae, M. avium, and M. kansasii, in cultures and tissue sections.
To verify PNA-FISH for the detection of mycobacteria in the context of human cells, we first visualized single mycobacteria in BCG-infected APC as a well-defined experimental setting. Then we specifically detected M. tuberculosis, M. leprae, and M. avium subsp. paratuberculosis in tissue sections of human and animal origin. So far, conventional ISH procedures (3, 4, 7, 8, 17) or antibody-based methods (13, 18) have shown only colored spots. Bacterial morphology could not be assessed. FISH with fluorescein-labeled PNA probes improved the detection but needed a signal amplification step because of low fluorescence intensity (24). Labeling of our probes with TAMRA or Cy3 resulted in advanced signal intensity and succeeded in direct FISH detection of mycobacteria in tissues. Although some of these tissues were formaldehyde fixed and embedded years ago (human tuberculosis in 1999, leprosy in 2001, Johne's disease in 1999), we were able to detect mycobacteria with single-cell resolution.
In contrast to microscopic techniques like FISH, nucleic acid amplification-based methods are prone to contamination. In addition, they may be falsely negative because of the presence of amplification inhibitors such as eukaryotic and prokaryotic nontarget DNAs. In contrast, visualization of mycobacteria in a histopathological context allows unequivocal discrimination between true infection and contamination.
In addition, fluorescently labeled PNA probes represent an economical way to identify mycobacterial cultures isolated from clinical specimens. All 52 of the isolates included in this study were identified unequivocally as M. tuberculosis, M. avium, or M. kansasii, resulting in 100% sensitivity and specificity. Assuming a time to result of about 2.5 h with only 40 min of hands-on time for a FISH procedure (including fixation, hybridization, and microscopy) and considering its low cost, FISH might be an alternative to amplification-based methods for fast identification of commonly isolated mycobacterial species. An important advantage of using FISH is the fact that no biosafety level 3 laboratory is required.
The PNA-FISH probes shown here were able to distinguish between target sequences and sequences with single base mismatches. Probe composition and location of mismatches can limit the utility of PNA probes, and single base mismatches close to the 5′ end of a probe complicated or even prevented specific hybridization, as shown by our experience with probes designed for M. intracellulare. There is little information on the effect of mismatch localization in PNA probes influencing hybridization specificity. According to our limited data set, a mismatch located in the middle of the probe has a better discriminative effect than a mismatch close to the 5′ end.
Preliminary data show, however, that not all Ziehl-Neelsen-positive cells are visualized with PNA-FISH (data not shown). Further experiments will evaluate the performance of PNA-FISH in comparison with other methods (acid-fast staining, ISH with DNA probes, and amplification-based methods) for examination of tissue specimens.
In conclusion, we developed and validated four new PNA probes in an improved FISH procedure to identify and visualize mycobacteria in cultures from clinical specimens and directly within tissue sections. PNA-FISH was shown to be a fast and versatile tool for both clinical microbiology diagnostics and research purposes.
Acknowledgments
We thank Gitina Fiedler and Angela Pohlisch for excellent technical assistance. We are thankful to H. Audring (Klinik für Dermatologie, Venerologie und Allergologie, CCM, Charité, Berlin, Germany) and R. Rudolph (Veterinär-Pathologie, FB Veterinärmedizin, FU-Berlin, Berlin, Germany) for providing leprosy and Johne's disease tissue sections, respectively.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Optimierte molekulare Bibliotheken, DFG no. GO 363/8-4) to U. B. Göbel. The epifluorescence microscope was a gift from the Sonnenfeld-Stiftung.
REFERENCES
- 1.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:1-25. [DOI] [PubMed] [Google Scholar]
- 2.Drobniewski, F. A., P. G. More, and G. S. Harris. 2000. Differentiation of Mycobacterium tuberculosis complex and nontuberculous mycobacterial liquid cultures by using peptide nucleic acid-fluorescence in situ hybridization probes. J. Clinical Microbiol. 38:444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenhalls, G., L. Stevens-Muller, R. Warren, N. Carrol, J. Bezuidenhout, P. Van Helden, and P. Bardin. 2002. Localization of mycobacterial DNA and mRNA in human tuberculous granulomas. J. Microbiol. Methods 51:197-208.12133612 [Google Scholar]
- 4.Fuller, C. L., J. L. Flynn, and T. A. Reinhart. 2003. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect. Immun. 71:7023-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 6.Hongmanee, P., H. Stender, and O. F. Rasmussen. 2001. Evaluation of a fluorescence in situ hybridization assay for differentiation between tuberculous and nontuberculous Mycobacterium species in smears of Lowenstein-Jensen and Mycobacteria Growth Indicator Tube cultures using peptide nucleic acid probes. J. Clinical Microbiol. 39:1032-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulten, K., H. M. El-Zimaity, T. J. Karttunen, A. Almashhrawi, M. R. Schwartz, D. Y. Graham, and F. A. El-Zaatari. 2001. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn's diseased tissues by in situ hybridization. Am. J. Gastroenterol. 96:1529-1535. [DOI] [PubMed] [Google Scholar]
- 8.Hulten, K., T. J. Karttunen, H. M. El-Zimaity, S. A. Naser, M. T. Collins, D. Y. Graham, and F. A. El-Zaatari. 2000. Identification of cell wall deficient forms of M. avium subsp. paratuberculosis in paraffin embedded tissues from animals with Johne's disease by in situ hybridization. J. Microbiol. Methods 42:185-195. [DOI] [PubMed] [Google Scholar]
- 9.Lethola, M. J., E. Torvinen, I. T. Miettinen, and C. W. Keevil. 2006. Fluorescence in situ hybridization using peptide nucleic acid probes for rapid detection of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis in potable-water biofilms. Appl. Environ. Microbiol. 72:848-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metchock, B. G., F. S. Nolte, and R. J. Wallace. 1999. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 11.Moter, A., and U. B. Göbel. 2000. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods 41:85-112. [DOI] [PubMed] [Google Scholar]
- 12.Moter, A., C. Hoenig, B. K. Choi, B. Riep, and U. B. Göbel. 1998. Molecular epidemiology of oral treponemes associated with periodontal disease. J. Clinical Microbiol. 36:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naser, S. A., I. Shafran, D. Schwartz, F. El-Zaatari, and J. Biggerstaff. 2002. In situ identification of mycobacteria in Crohn's disease patient tissue using confocal scanning laser microscopy. Mol. Cell. Probes 16:41-48. [DOI] [PubMed] [Google Scholar]
- 14.Oda, Y., S. J. Slagman, W. G. Meijer, L. J. Forney, and J. C. Gottschal. 2000. Influence of growth rate and starvation on fluorescent in situ hybridization of Rhodopseudomonas palustris. FEMS Microbiol. Ecol. 32:205-213. [DOI] [PubMed] [Google Scholar]
- 15.Padilla, E., J. M. Manterola, O. F. Rasmussen, J. Lonca, J. Dominguez, L. Matas, A. Hernández, and V. Ausina. 2000. Evaluation of a fluorescence hybridization assay using peptide nucleic acid probes for identification and differentiation of tuberculous and non-tuberculous mycobacteria in liquid cultures. Eur. J. Clinical Microbiol. Infect. Dis. 19:140-145. [DOI] [PubMed] [Google Scholar]
- 16.Perry-O'Keefe, H., H. Stender, A. Broomer, K. Oliveira, J. Coull, and J. J. Hyldig-Nielsen. 2001. Filter-based PNA in situ hybridization for rapid detection, identification and enumeration of specific microorganisms. J. Appl. Microbiol. 90:180-189. [DOI] [PubMed] [Google Scholar]
- 17.Sechi, L. A., M. Mura, F. Tanda, A. Lissia, A. Solinas, G. Fadda, and S. Zanetti. 2001. Identification of Mycobacterium avium subsp. paratuberculosis in biopsy specimens from patients with Crohn's disease identified by in situ hybridization. J. Clinical Microbiol. 39:4514-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiler, P., T. Ulrichs, S. Bandermann, L. Pradl, S. Jorg, V. Krenn, L. Morawietz, S. H. Kaufmann, and P. Aichele. 2003. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J. Infect. Dis. 188:1326-1331. [DOI] [PubMed] [Google Scholar]
- 19.Stender, H., M. Fiandaca, J. J. Hyldig-Nielsen, and J. Coull. 2002. PNA for rapid microbiology. J. Microbiol. Methods 48:1-17. [DOI] [PubMed] [Google Scholar]
- 20.Stender, H., K. Lund, K. H. Petersen, O. F. Rasmussen, P. Hongmanee, H. Miörner, and S. E. Godtfredsen. 1999. Fluorescence in situ hybridization assay using peptide nucleic acid probes for differentiation between tuberculous and nontuberculous Mycobacterium species in smears of Mycobacterium cultures. J. Clinical Microbiol. 37:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stender, H., T. A. Mollerup, K. Lund, K. H. Petersen, P. Hongmanee, and S. E. Godtfredsen. 1999. Direct detection and identification of Mycobacterium tuberculosis in smear-positive sputum samples by fluorescence in situ hybridization (FISH) using peptide nucleic acid (PNA) probes. Int. J. Tuberc. Lung Dis. 3:830-837. [PubMed] [Google Scholar]
- 22.Sweeney, R. W. 1996. Transmission of paratuberculosis. Vet. Clinical N. Am. Food Anim. Pract. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Revised August. 2002. Tuberculosis fact sheet no. 104. World Health Organization, Geneva, Switzerland.
- 24.Zerbi, P., A. Schonau, S. Bonetto, A. Gori, G. Costanzi, P. Duca, and L. Vago. 2001. Amplified in situ hybridization with peptide nucleic acid probes for differentiation of Mycobacterium tuberculosis complex and nontuberculous Mycobacterium species on formalin-fixed, paraffin embedded archival biopsy and autopsy samples. Am. J. Clinical Pathol. 116:770-775. [DOI] [PubMed] [Google Scholar]