Abstract
We typed 165 Candida albicans isolates from 44 different sources by multilocus sequence typing (MLST) and ABC typing of rRNA genes and determined their homozygosity or heterozygosity at the mating-type-like locus (MTL). The isolates represented pairs or larger sets from individual sources, which allowed the determination of strain diversity within patients. A comparison of replicate sequence data determined a reproducibility threshold for regarding isolates as MLST indistinguishable. For 36 isolate sets, MLST and ABC typing showed indistinguishable or highly related strain types among isolates from different sites or from the same site at different times from each patient. This observation included 11 sets with at least one isolate from a blood culture and a nonsterile site from the same patient. For one patient, strain replacement was evidenced in the form of two sets of isolates from different hospital admissions where the strain types within each set were nearly identical but where the two sets differed both by MLST and ABC typing. MLST therefore confirms the existing view of C. albicans strain carriage. Microvariation, evidenced as small differences between MLST types, resulted in most instances from a loss of heterozygosity at one or more of the sequenced loci. Among isolate sets that showed major strain type differences, some isolates could be excluded as likely examples of handling errors during storage. However, for a minority of isolates, intermittent differences in ABC type for tightly clustered MLST types and intermittent appearances of MTL homozygosity lead us to propose that some C. albicans isolates, or all isolates under yet-to-be-determined conditions, maintain a high level of genetic diversity by mechanisms such as recombination, gene conversion, or chromosomal ploidy change.
Candida albicans is a normal commensal of the human gut microflora that can cause invasive superficial and disseminated infections in immunologically susceptible hosts (11). A number of different technical approaches to typing C. albicans isolates have been developed since the early 1980s (78). For C. albicans, as for all microbial pathogens, a reproducible and discriminatory strain typing system is of benefit for clinical and epidemiological studies to provide information on sources, carriage, and transmission of infection and on relations between strain types and properties such as virulence and antimicrobial resistance. In many publications, multiple C. albicans isolates from longitudinal surveys of patients or from surveillance cultures of different anatomical sites have been typed. Most work of this nature has confirmed a tendency towards the persistence of a unique strain in each human host, which was suggested even in the earliest studies based on phenotypic strain typing (45, 53). Minor variations in genetically determined strain types in surveys of multiple C. albicans isolates from individual patients have been described as “microevolution” (33, 35, 37, 64, 65, 76, 78) or genotypic shuffling (37, 69). (This is not the usual sense in which the term “microevolution” is used by population geneticists.) Evidence from strain typing work confirms the view of C. albicans as a species that reproduces predominantly in a clonal manner but with a tendency for occasional genetic variation that may arise by mechanisms such as recombination, gene replacement, or cryptic mating (22-24, 29, 30, 34, 38, 39, 42, 50, 66, 79, 80, 84, 98).
Most longitudinal and/or multiple-site epidemiological work demonstrating strain maintenance in C. albicans has been done with superficial isolates, particularly with cultures from the oral cavities of human immunodeficiency virus (HIV)-infected patients (2, 3, 6, 19, 31, 48, 51, 61, 71, 72, 90, 96), with vaginal isolates (1, 37, 49, 64, 65, 73, 76, 91), with other sources of superficial isolates (4, 17, 74, 97), or with superficial surveillance cultures from hospitalized patients (60, 81, 89). Some surveys of oral isolates have shown the same strain persisting for months to years (4, 17). Occasionally, authors have suggested that multiple strain types coexist in samples from some patients (27, 69, 83), but such findings are in the minority and may reflect technical sensitivities and variabilities in the typing methodologies used in some cases. The epidemiology of disseminated C. albicans infection has been studied much less often than that of superficial sites, but the results of surveys based on karyotype electrophoretic patterns (62, 63, 93), randomly amplified PCR fragments (92), DNA fingerprinting with oligonucleotide probes (44, 67), and multilocus enzyme electrophoresis (12) consistently indicate that isolates from blood are highly similar to, or indistinguishable from, isolates from superficial sites in the same patient.
The overall picture of C. albicans colonization and infection is therefore mainly of clonal reproduction of strains, infection by the spread of endogenous, colonizing strain types, but with sporadic changes at the level of sequences of individual DNA fragments, which we shall call microvariation rather than microevolution. Microvariation refers to small but detectable changes in DNA sequences among isolates obtained longitudinally or as separate clones from individual patients (35, 65). Although the original reports of microvariation suggested that changes arose from the reorganization of repetitive DNA sequences in the genome, later work has consistently associated the loss of heterozygosity (LOH) as a common mechanism for microvariation changes (16, 21, 70, 85, 88). LOH may result from chromosome deletion or loss, recombination, and/or gene conversion events. Conjugational mating and nuclear fusion without subsequent meiosis can occur between C. albicans cells (24, 34, 42), and such mating is dependent on homozygous alleles, i.e., LOH, at the mating-type-like locus (MTL) on chromosome 5 (42, 79).
Multilocus sequence typing (MLST) is a highly discriminatory and portable approach to distinguishing strains within a microbial species (43). DNA sequences from six or seven gene fragments are compared to establish levels of similarity between isolates and results, expressed with unique numbers for individual sequences (genotypes) and for diploid sequence types (DSTs), unique combinations of the seven genotypes in each isolate (43). Several statistical procedures have been devised for the analysis of MLST data, including eBURST (20), which compiles clusters of isolates that differ from each other at only one of the sequenced loci. MLST schemes are now available for several fungi, and the approach is well developed for the typing of C. albicans strains (7-9, 68, 84, 86). The C. albicans data are stored on a central internet database (http://test1.mlst.net/) to allow free access to investigators. Results from MLST are comparable with those obtained by DNA fingerprinting with the moderately repetitive sequence Ca3, since both typing approaches assign the same sets of isolates to the same clusters of highly related strains, with the exception of DNA fingerprinting clade E, which is split between two MLST clades (84).
In the course of our ongoing work to determine DSTs by MLST for the analysis of C. albicans population structures (84), we have now typed sets of multiple isolates of the fungus from 44 different sources, including instances of superficial and blood isolates from the same patient, isolates that have been maintained and passaged through different culture collections, and isolates that have been experimentally exposed to fluconazole. We have determined the level of sequencing errors in replicate experiments to establish criteria for regarding isolates as indistinguishable. We have looked for evidence of microvariation changes and LOH in these isolates and in isolates recovered from experimental infections. In addition to MLST, we have typed the isolates for the presence or absence of an intron in the internal transcribed spacer 1 (ITS1) region of DNA sequences encoding rRNA (ABC typing) (46, 47) and for homozygosity or heterozygosity at the MTL (86). These extra data augment the MLST results by providing extra typing information for each isolate. Our findings confirm the prevailing views of strain maintenance, predominant clonality, and occasional microvariation in C. albicans and that isolates from blood cultures match those from nonsterile sites in the same patient. We hypothesize that LOH may occur among some but not all cells in the population colonizing or infecting a patient so that heterozygosity remains established in a proportion of the population. Our data also raise some caveats concerning the maintenance and distribution of isolates within and between laboratories.
MATERIALS AND METHODS
C. albicans isolates.
Details of the 165 isolates studied are shown in Table 1. In our laboratory, all the isolates were stored on beads in 50% glycerol at −80°C. For experimental use, a bead was placed onto a plate of CHROMagar Candida medium (M-Tech Diagnostics, Warrington, United Kingdom) and incubated at 37°C for 48 h. All the isolates formed green colonies on this medium, verifying their prior identification as C. albicans.
TABLE 1.
Details of C. albicans isolates studied
| Isolate set | Isolate | Yr isolated | Country of origina | Isolated fromb: | Patient detail(s)c | Storage historyd | DST | ABC type | MTL type | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IHEM16346 | 1970s | Zaire | Kidney? | May be parent of IHEM3742 | Be2 | 258 | C | a/α | |
| 1 | IHEM3742 | 1970s | Zaire | Kidney | Unknown | Be2 | 124 | C | a/α | |
| 1 | RV4688 | 1970s | Kidney | Originally IHEM3742? | Be1 | 87 | C | a/α | 58 | |
| 1 | 81/005 | Subculture of RV4688 received in 1981 | Be1, UK2, Be1 | 480 | C | a/α | ||||
| 1 | RV2LK | Recovered from experimental mouse infection with RV4688 | 269 | C | a/α | |||||
| 2 | 73/024 | 1973 | UK | Vagina | Open-heart surgery | UK1, UK2, Be1 | 145 | A | a/α | 54 |
| 2 | 73/027 | 1973 | UK | Vagina | Open-heart surgery | UK1, UK2, Be1 | 485 | A | a/α | 54 |
| 3 | 78/005 | 1978 | UK | Oropharynx | Healthy volunteer | UK2, Be1 | 96 | A | a/a | |
| 3 | 78/010 | 1978 | UK | Oropharynx | Healthy volunteer | UK2, Be1 | 517 | A | α/α | |
| 4 | 81/066 | 1980 | UK | Oropharynx | Female, STD clinic | UK2, Be1 | 396 | A | a/α | |
| 4 | 81/114 | 1980 | UK | Vagina | Female, STD clinic | UK2, Be1 | 396 | A | a/α | |
| 5 | 90/141 | 1980 | Spain | Folliculitis | Heroin addict | UK2, Be1 | 547 | A | a/α | 56 |
| 5 | 90/142 | 1980 | Spain | Abscess | Heroin addict | UK2, Be1 | 482 | A | a/α | 56 |
| 6 | L206 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L249 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L258 | 1985 | UK | Oropharynx | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L332 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L343 | 1985 | UK | Oropharynx | Patient 11 | UK2, Be1 | 80 | B | a/α | 55 |
| 6 | L475 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L485 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 256 | B | a/α | 55 |
| 6 | L527 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 257 | B | a/α | 55 |
| 6 | L531 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L548 | 1985 | UK | Oropharynx | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L586 | 1985 | UK | Feces | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 6 | L607 | 1985 | UK | Oropharynx | Patient 11 | UK2, Be1 | 255 | B | a/α | 55 |
| 7 | L344 | 1985 | UK | Oropharynx | Patient 5, March 1985 | UK2, Be1 | 273 | C | a/α | 55 |
| 7 | L345 | 1985 | UK | Sputum | Patient 5, March 1985 | UK2, Be1 | 273 | C | a/α | 55 |
| 7 | L367 | 1985 | UK | Oropharynx | Patient 5, March 1985 | UK2, Be1 | 272 | C | a/α | 55 |
| 8 | L479 | 1985 | UK | Feces | Patient 5, May 1985 | UK2, Be1 | 125 | A | a/α | 55 |
| 8 | L562 | 1985 | UK | Sputum | Patient 5, May 1985 | UK2, Be1 | 271 | A | a/α | 55 |
| 8 | L572 | 1985 | UK | Stomach | Patient 5, May 1985 | UK2, Be1 | 271 | A | a/α | 55 |
| 8 | L580 | 1985 | UK | Feces | Patient 5, May 1985 | UK2, Be1 | 270 | A | a/α | 55 |
| 9 | L217 | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 89 | B | a/α | 55 |
| 9 | L252 | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 89 | B | a/α | 55 |
| 9 | L261B | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 267 | B | a/α | 55 |
| 9 | L283 | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 268 | B | a/α | 55 |
| 9 | L289 | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 89 | B | a/a | 55 |
| 9 | L441 | 1985 | UK | Oropharynx | Patient 8 | UK2, Be1 | 89 | B | a/α | 54 |
| 9 | L463 | 1985 | UK | Feces | Patient 8 | UK2, Be1 | 89 | B | a/α | 54 |
| 10 | L121 | 1985 | UK | CSF | UK2, Be1 | 141 | B | α/α | ||
| 10 | L123 | 1985 | UK | Urine | UK2, Be1 | 515 | B | a/α | ||
| 10 | L124 | 1985 | UK | CSF | UK2, Be1 | 542 | B | α/α | ||
| 11 | L220 | 1985 | UK | Feces | Patient 6 | UK2, Be1 | 544 | A | a/α | 55 |
| 11 | L262 | 1985 | UK | Oropharynx | Patient 6 | UK2, Be1 | 545 | A | a/α | 55 |
| 11 | L353 | 1985 | UK | Oropharynx | Patient 6 | UK2, Be1 | 544 | A | a/α | 55 |
| 12 | 85/005 | 1985 | UK | Blood | UK2, Be1 | 83 | A | a/a | 10 | |
| 12 | 85/006 | 1985 | UK | Sputum | UK2, Be1 | 541 | A | a/α | 10 | |
| 13 | L513 | 1986 | UK | Sputum | UK2, Be1 | 546 | B | a/α | ||
| 13 | L516 | 1986 | UK | Blood | UK2, Be1 | 134 | B | a/α | ||
| 14 | L517 | 1986 | UK | Anus | Male, STD clinic | UK2, Be1 | 216 | A | a/α | |
| 14 | L518 | 1986 | UK | Oropharynx | Male, STD clinic | UK2, Be1 | 216 | A | a/α | |
| 15 | OD8826 | 1988 | UK | Oropharynx | UK2, Iowa, NZ | 24 | A | a/α | 72 | |
| 15 | IHEM20491 | 1989 | UK | Oropharynx | UK2, Be1, Be2 | 373 | A | a/α | 72 | |
| 15 | IHEM20493 | 1990 | UK | Oropharynx | UK2, Be1, Be2 | 372 | A | a/α | 72 | |
| 16 | B59626 | 1991 | Germany | Oropharynx | Be1 | 585 | A | a/α | ||
| 16 | B59630 | 1991 | Germany | Oropharynx | Be1 | 585 | A | a/α | ||
| 17 | C19 | 1993 | UK | Sputum | 429 | B | a/α | |||
| 17 | C25 | 1993 | UK | Catheter tip | 429 | B | a/α | |||
| 17 | C26 | 1993 | UK | Blood | 429 | B | a/α | |||
| 17 | C27 | 1993 | UK | Catheter tip | 429 | B | a/α | |||
| 18 | IHEM20486 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1, Be2 | 309 | B | a/α | |
| 18 | IHEM20487 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1, Be2 | 389 | B | a/α | |
| 18 | IHEM20488 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1, Be2 | 302 | B | a/a | |
| 18 | J932570 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1 | 489 | C | a/α | |
| 18 | J932571 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1 | 379 | B | a/α | |
| 18 | J932575 | 1993 | UK | Oropharynx | AIDS patient | UK2, Be1 | 380 | C | a/α | |
| 18 | J941383 | 1994 | UK | Oropharynx | AIDS patient | UK2, Be1 | 378 | A | a/α | |
| 19 | J942148 | 1994 | Germany | Oropharynx | AIDS patient | Be1 | 381 | A | α/α | |
| 19 | J942149 | 1994 | Germany | Oropharynx | AIDS patient | Be1 | 382 | A | α/α | |
| 20 | TW2-76 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-80 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-81 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-82 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-83 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-84 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-85 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW2-86 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW3-55 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW8-43 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW8-44 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW8-45 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW8-46 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW8-47 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 20 | TW12-79 | 1994 | USA | Oropharynx | AIDS patient | 254 | A | a/α | 95 | |
| 20 | TW12-99 | 1994 | USA | Oropharynx | AIDS patient | 79 | A | a/α | 95 | |
| 21 | S01 | 1995 | Italy | Oropharynx | Liver transplant | 88 | C | a/α | 87 | |
| 21 | S02 | 1995 | Italy | Oropharynx | Liver transplant | 264 | C | a/α | 87 | |
| 21 | S03 | 1995 | Italy | Oropharynx | Liver transplant | 88 | C | a/α | 87 | |
| 21 | S04 | 1995 | Italy | Oropharynx | Liver transplant | 264 | C | a/α | 87 | |
| 21 | S05 | 1995 | Italy | Sputum | Liver transplant | 265 | C | α/α | 87 | |
| 21 | S06 | 1995 | Italy | Sputum | Liver transplant | 88 | C | a/α | 87 | |
| 21 | S07 | 1995 | Italy | Sputum | Liver transplant | 88 | C | a/α | 87 | |
| 21 | S08 | 1995 | Italy | Sputum | Liver transplant | 266 | C | a/α | 87 | |
| 21 | S09 | 1995 | Italy | Sputum | Liver transplant | 265 | C | α/α | 87 | |
| 21 | S10 | 1995 | Italy | Bronchoalveolar lavage | Liver transplant | 88 | C | a/α | 87 | |
| 22 | T63 | 1995 | Canada | Oropharynx | AIDS patient | 222 | B | a/α | 15 | |
| 22 | T65 | 1995 | Canada | Oropharynx | AIDS patient | 222 | B | a/α | 15 | |
| 22 | T68 | 1995 | Canada | Oropharynx | AIDS patient | 223 | C | a/α | 15 | |
| 23 | T118 | 1995 | Canada | Oropharynx | AIDS patient | 151 | A | a/α | 15 | |
| 23 | D08-330 | T118 population exposed to fluconazole | 278 | A | a/α | 16 | ||||
| 23 | D09-330 | T118 population exposed to fluconazole | 278 | A | a/α | 16 | ||||
| 23 | D11-330 | T118 population exposed to fluconazole | 278 | A | a/α | 16 | ||||
| 23 | D12-165 | T118 population exposed to fluconazole | 278 | A | a/α | 16 | ||||
| 23 | D12-260 | T118 population exposed to fluconazole | 280 | A | a/α | 16 | ||||
| 23 | D12-330 | T118 population exposed to fluconazole | 279 | A | a/α | 16 | ||||
| 24 | J990102 | 1999 | Belgium | Vagina | Symptomatic vaginitis | Be1 | 45 | B | a/α | |
| 24 | J990103 | 1999 | Belgium | Cervix | Symptomatic vaginitis | Be1 | 490 | B | α/α | |
| 25 | AM2001/0019 | 2001 | UK | Sternal abscess | 527 | A | a/α | |||
| 25 | AM2001/0022 | 2001 | UK | Sternal abscess | 527 | A | a/α | |||
| 26 | WC01-202761 | 2001 | Australia | Blood | 390 | B | a/α | |||
| 26 | WC01-202762 | 2001 | Australia | Wound | 391 | B | a/α | |||
| 27 | WC02-202964 | 2002 | Australia | Blood | 420 | A | a/α | |||
| 27 | WC02-202965 | 2002 | Australia | Urine | 421 | A | a/α | |||
| 28 | WC02-202291 | 2002 | Australia | Blood | 232 | A | a/α | |||
| 28 | WC02-202292 | 2002 | Australia | Blood | 232 | A | a/α | |||
| 28 | WC02-202293 | 2002 | Australia | Blood | 430 | A | a/α | |||
| 29 | WC02-202294 | 2002 | Australia | Blood | 416 | B | a/α | |||
| 29 | WC02-202295 | 2002 | Australia | Blood | 417 | B | a/α | |||
| 29 | WC02-202296 | 2002 | Australia | Blood | 418 | B | a/α | |||
| 30 | WC02-201987 | 2002 | Australia | Urine | 414 | A | a/α | |||
| 30 | WC02-201988 | 2002 | Australia | Catheter tip | 415 | A | a/α | |||
| 30 | WC02-202225 | 2002 | Australia | Blood | 66 | A | a/α | |||
| 31 | WC02-202861 | 2002 | Australia | Ileal conduit | 419 | A | a/α | |||
| 31 | WC02-202862 | 2002 | Australia | Blood | 419 | A | a/α | |||
| 32 | WC03-200970b | 2003 | Australia | Catheter tip | 422 | B | a/α | |||
| 32 | WC03-200970d | 2003 | Australia | Blood | 423 | B | a/α | |||
| 32 | WC03-200970e | 2003 | Australia | Blood | 424 | B | a/α | |||
| 32 | WC03-200970h | 2003 | Australia | Thigh | 425 | B | a/α | |||
| 33 | WC03-202178 | 2003 | Australia | Urine | 427 | A | a/α | |||
| 33 | WC03-202179 | 2003 | Australia | Blood | 427 | A | a/α | |||
| 33 | WC03-202180 | 2003 | Australia | Blood | 427 | A | a/α | |||
| 34 | WC03-200975 | 2003 | Australia | Blood | 426 | B | a/α | |||
| 34 | WC03-201397 | 2003 | Australia | Urine | 426 | B | a/α | |||
| 35 | WC03-203422 | 2003 | Australia | Blood | 37 | A | a/α | |||
| 35 | WC03-203424 | 2003 | Australia | Urine | 428 | A | a/α | |||
| 36 | AM2003/0044 | 2003 | UK | Vagina | 172 | C | a/α | |||
| 36 | AM2003/0045 | 2003 | UK | Vagina | 172 | C | a/α | |||
| 37 | AM2003/0108 | 2003 | UK | Blood | 212 | A | a/α | |||
| 37 | AM2003/0109 | 2003 | UK | Blood | 212 | A | a/α | |||
| 38 | AM2005/0131 | 2005 | UK | Blood | 522 | A | a/α | |||
| 38 | AM2005/0134 | 2005 | UK | Blood | 522 | A | a/α | |||
| 38 | AM2005/0135 | 2005 | UK | Blood | 522 | A | a/α | |||
| 39 | AM2005/0163 | 2005 | UK | Sputum | 537 | A | a/α | |||
| 39 | AM2005/0165 | 2005 | UK | Blood | 537 | A | a/α | |||
| 40 | AM2005/0150 | 2005 | UK | Blood | 536 | A | a/α | |||
| 40 | AM2005/0151 | 2005 | UK | Catheter tip | 536 | A | a/α | |||
| 41 | AM2005/0211 | 2005 | UK | Blood | 564 | A | a/α | |||
| 41 | AM2005/0212 | 2005 | UK | Blood | 564 | A | a/α | |||
| 41 | AM2005/0213 | 2005 | UK | Blood | 565 | A | a/α | |||
| 41 | AM2005/0218 | 2005 | UK | Blood | 564 | A | a/α | |||
| 42 | AM2005/0192 | 2005 | UK | Blood | 66 | A | a/α | |||
| 42 | AM2005/0195 | 2005 | UK | Blood | 66 | A | a/α | |||
| 43 | IHEM17156 | Belgium | Guinea pig penis | Be1, Be2 | 307 | B | a/α | |||
| 43 | IHEM17157 | Belgium | Guinea pig nose | Be1, Be2 | 305 | B | a/α | |||
| 44 | 3153Abstl | Recently received as 3153, Bristol, UK | 369 | A | a/α | |||||
| 44 | 3153Alds | Recently received as 3153, Leeds, UK | 369 | A | a/α | |||||
| 44 | 3153ALndn2 | Recently received as 3153, London, UK | 286 | A | a/α | |||||
| 44 | 3153ALndn1 | 1980s culture of 3153 from London laboratory | UK2, Be1 | 286 | A | a/α | ||||
| 44 | 90/189 | Authentic 3153 | UK2, Be1 | 54 | A | a/α | 41 | |||
| 44 | 90/195 | 3153, CA05 in previous study | UK2, Be1 | 261 | A | a/α | 41 | |||
| 44 | 90/198 | 3153, CA08 in previous study | UK2, Be1 | 262 | A | a/α | 41 | |||
| 44 | 90/202 | 3153, CA12 in previous study | UK2, Be1 | 260 | B | a/α | 41 | |||
| 44 | 90/206 | 3153, CA16 in previous study | UK2, Be1 | 263 | B | a/α | 41 | |||
| 44 | IHEM20523 | 3153, CA01 in previous study | UK2, Be1, Be2 | 370 | A | a/α | 41 | |||
| 44 | IHEM20535 | 3153, CA14 in previous study | UK2, Be1, Be2 | 369 | A | a/α | 41 |
UK, United Kingdom; USA, United States.
CSF, cerebral spinal fluid.
STD, sexually transmitted disease.
UK1, Department of Microbiology, University of Leeds, United Kingdom; UK2, Department of Microbiology, University of Leicester, United Kingdom; Be1, Janssen Research Foundation, Belgium; BE2, Scientific Institute of Public Health, Brussels, Belgium.
The isolates came from 44 separate sources and are grouped in numbered sets according to source in Table 1. Most of the isolates were pairs or triplet isolates from a human subject or, in one instance, an animal. The subjects included healthy volunteers, patients with symptomatic vaginitis, patients undergoing chemotherapy for hematological malignancy, and patients with proven candidemia. Many of the isolates came from previously published studies, as indicated in Table 1. The oldest isolates in the panel were originally cultured in the 1970s. Many of the isolates have been maintained in the first author's collection of fungal strains, which was initiated in Leeds, United Kingdom, in 1973 and transported to Leicester, United Kingdom, and Beerse, Belgium, and which is now in Aberdeen, United Kingdom. With each move, all isolates in the collection were subcultured and restocked at least once (52, 57). The histories of these isolates are indicated in Table 1.
Some sets of isolates merit special comment. In set 1, isolate RV4688 was a strain used in experiments by J. Van Cutsem in Beerse, Belgium, that had become spontaneously avirulent (58). It was originally isolated in Zaire by R. Vanbreuseghem. Records at the culture collection of the Scientific Institute of Public Health (SIPH) (formerly the Institute of Hygiene and Epidemiology) in Brussels, Belgium, show that isolate IHEM3742 was received in 1988, having passed from R. Vanbreuseghem to J. Van Cutsem to the American Type Culture Collection (as strain ATCC 28516). IHEM16346 is not listed in the public SIPH catalog but came directly from the original Zaire isolate from R. Vanbreuseghem, according to SIPH collection manager N. Nolard (personal communication). 81/005 was the number given to an isolate that was received by the first author from J. Van Cutsem in 1981. From their histories, these isolates should be indistinguishable by MLST. The isolates in set 15 share a similar complex history. An oral isolate from an AIDS patient in Leicester, United Kingdom, in 1988 was sent to J. Schmid at the University of Iowa and designated OD8826. A subculture of the isolate was taken by J. Schmid to Massey University in New Zealand, and the isolate was supplied from New Zealand for the present study. Two other oral isolates from the same patient in 1988 were removed to Beerse, Belgium, and were then added to the SIPH collection and were supplied as isolates IHEM20491 and IHEM20493 for the present study. The isolates in set 18, all originally from an AIDS patient in London, United Kingdom, had also been subcultured and relocated in different culture collections before inclusion in the present study. Set 23 comprises examples of isolates derived from T118 that had been passaged for a measured number of generations in the presence of fluconazole (14, 16). For each isolate, the two digits after the letter “D” indicate the subclone number from T118, and the three final digits indicate the number of generations. Finally, set 44 comprises the isolates still available from a previous study in which examples of the widely used C. albicans strain 3153 were submitted from several laboratories for comparison by an earlier strain typing methodology (41).
Strain typing.
The isolates were tested by MLST as previously described (9, 84). The ABC type (A, B, or C) and MTL types (a/α, a/a, or α/α) were determined by PCR as previously described (86). Similarities between sequence data were analyzed in terms of p distance with MEGA version 2.1 (28), and results with 500 bootstrap replications are depicted as a dendrogram by the unweighted-pair group method with arithmetic averages (UPGMA). The analyses were based on concatenated data from all known polymorphic sites, duplicated to allow the discrimination of homozygous and heterozygous differences (84). The sequences were also analyzed to determine clonal clusters by eBURST, version 2 (20). Clusters were defined as groups of isolates with six of the seven genotype sequences being identical.
When one or more isolates within a set of isolates from a common source differed from the rest by MLST or ABC type, the sequencing chromatograms for the set were rescrutinized and/or the isolates were resequenced to ensure that the differences were reproducible.
Animal experiments.
Single colonies of isolates S09 and WC02-202294 (Table 1) were grown for 20 h at 30°C in NGY medium (0.1% neopeptone [Difco, Sparks, MD], 0.4% glucose, and 0.1% yeast extract [Difco]) (40). These isolates were chosen to represent low (S09) and high (WC02-202294) levels of heterozygosity in the MLST data. The yeast cells were washed twice in sterile saline and resuspended in saline, and concentrations were adjusted by hemocytometer count results. For each isolate, a single female BALB/c mouse weighing 20 ± 2 g was injected intravenously at a dose of 2 × 105 yeast cells/g body weight, confirmed by subsequent viable counts. Three days after challenge, the mice were humanely terminated, the kidneys and brains were removed and homogenized in saline, and 10-fold dilutions were plated onto Sabouraud agar (Oxoid, Basingstoke, United Kingdom) for incubation at 35°C. From each organ culture, six well-separated colonies were randomly selected, subcultured, and subjected to MLST.
RESULTS
Reproducibility of MLST data.
Data were obtained from 17 isolates fully sequenced in duplicate. Sequence reproducibility was perfect for 10 duplicates. For the other seven isolates, differences were at the level of a homozygous versus a heterozygous result for a single nucleotide polymorphism (SNP) in five instances, two homozygous/heterozygous discrepancies at a single locus in one instance, and a base-for-base (homozygous) SNP difference in one instance. All but one of the errors therefore resulted from a misinterpretation of double peaks, an occasional difficulty to be expected with sequencing chromatograms from a diploid organism.
The eight replicate differences occurred in a total of 2,329 bases sequenced—an accuracy of 99.66%. Since a heterozygous/homozygous difference would be scored at half the value of a homozygous difference in the calculation of distances for UPGMA analyses, the sequencing accuracy could be expressed as nine error scores among 4,658 polymorphic sites sequenced (99.8% accuracy). In the context of this study, the worst inaccuracy seen, one homozygous change or two homozygous/heterozygous changes, would erroneously suggest nonidentity between strains with a score difference of 1 across all SNPs, which corresponds with a p-distance difference of 0.005 by UPGMA nucleotide distance analysis. We have used as a worst-case value that is four times this magnitude, i.e., a p-distance difference of 0.02, as a basis for describing isolates as “indistinguishable,” although the sequence chromatograms for all isolates included in this study have been rescrutinized or the sequencing has been repeated to ensure that differences between isolates are genuine.
Similarities and differences between multiple isolates from single sources.
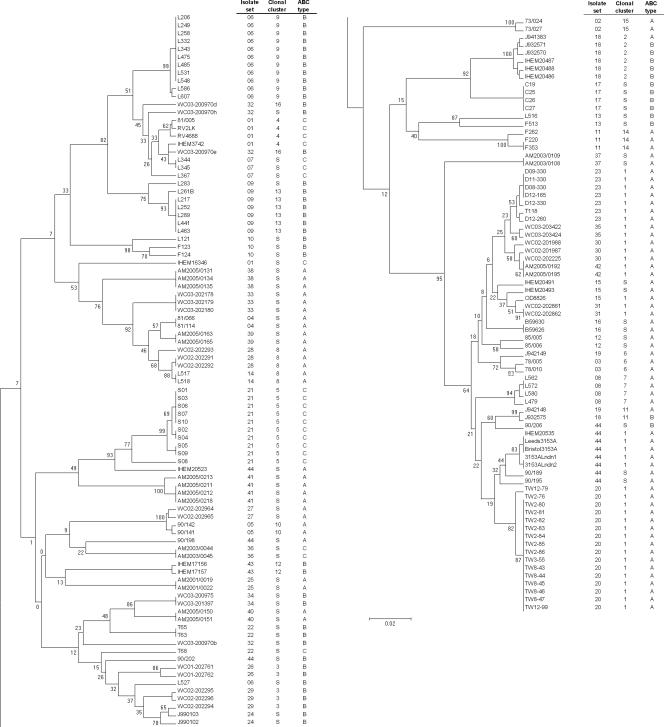
The DSTs, ABC types, and MTL types of all the isolates studied are shown in Table 1. Figure 1 shows the relationships between the isolates in the form of a UPGMA dendrogram based on MLST sequence data together with their isolate set numbers, ABC types, and eBURST clonal cluster assignments. In the majority of instances, isolates from the same source coclustered, often with the same DST or with very high levels of similarity by MLST (Fig. 1). Isolates in 36 of the 44 sets (121 of 165 isolates) were considered indistinguishable within each set because they clustered in the MLST dendrogram within our chosen limiting p distance of 0.02, they had the same ABC type, and they fell within the same eBURST clonal cluster (when a set of isolates showed sufficient sequence variation to generate a clonal cluster). This list includes 11 isolate sets (sets 12, 14, 18, 27, 28, 31, 32, 34, 35, 36, and 40) in which samples came from the bloodstream and a nonsterile site in the same patient. It also includes sets 7 and 8, which came from a patient sampled on two separate admissions to the same hospital, 2 months apart. The set from each admission comprised isolates clustering at the level of indistinguishability, but the two sets were widely separated in the UPGMA dendrogram (Fig. 1), unequivocally indicating strain replacement at all sites sampled for this patient.
FIG. 1.
UPGMA dendrogram showing clustering of 165 C. albicans isolates from 44 sources together with information on ABC type and clonal cluster. The dendrogram has been split to facilitate text legibility, with the top half at the left and the continuation at the right.
For the sets of isolates that could not be designated indistinguishable by the above-described criteria, the interisolate differences were of various types. Isolates from set 32 clustered closely, but one isolate differed from the other two in more than one genotype, so it did not conform to a clonal cluster (Fig. 1). The two isolates in sets 13 and 19 differed, with a p distance of >0.02, but were matched in ABC type (Fig. 1). In both cases, we interpret these data to be consistent with microvariation occurring in the isolate sets, which raises the number of sets of isolates that were indistinguishable or very closely related to 40/44 (125/165 isolates).
IHEM16346 did not cocluster with other isolates in set 1 (Fig. 1), but, like the rest of the set, it belonged to the least common ABC type, type C. The data therefore neither confirm nor rule out the possibility that IHEM16346 was the progenitor of the other isolates in set 1. Similarly, L527 did not cocluster with the other 11 isolates in set 6 (Fig. 1), but its ABC type, type B, matched that of the other isolates. Once again, these data do not unequivocally rule in or out an association between L527 and the other isolates in set 6; our records show that, chronologically, L527 was the last isolate received from surveillance cultures of the patient concerned, and the sample was taken 5 days after the previous isolate, so strain alteration or strain replacement is a possible interpretation.
Set 18 presented the most unusual set of findings between isolates. The seven isolates in this set originated from samples taken at different times from the same HIV-infected patient but had histories of storage in different culture collections (Table 1); no detailed information on the isolates is available. Six of the isolates clustered very tightly on the basis of MLST data and formed a single eBURST clonal cluster (Fig. 1), but J932575 was substantially different from these six isolates. Six of the isolates, including J932575, were ABC type B, while J941383 was type A. ABC typing was repeated with these isolates to confirm the different assignments. A similar finding applied to the three isolates T63, T65, and T68, all of which came from the same patient (set 22). T63 and T65, with identical DSTs (Table 1), were type B, but T68, which did not cocluster with the two other isolates, was type C.
Set 44 consisted of 11 isolates that had been supplied as “strain 3153” from various laboratories. Three isolates were recently submitted, and the remaining isolates were part of a study published in 1991 (41). Eight of the isolates (including the three recently obtained isolates) coclustered in the dendrogram (Fig. 1). Five of these isolates belonged to eBURST clonal cluster 1. Isolate 90/206, the most distant in the dendrogram cluster, was type B, while the other isolates were type A. Of the remaining three isolates in set 44, each of which appeared in isolation in the dendrogram (Fig. 1), two were type A and one was type B.
Twelve of the 165 isolates were homozygous at the MTL: nine α/α and three a/a isolates (Table 1). The random occurrence of MTL homozygosity among heterozygous isolates in sets 9, 10, 12, 18, 21, and 24 indicates that this property is not a character of use in strain typing but is a possible indicator of microadaptive change. Both oral isolates in set 3, from a healthy female volunteer in the 1970s, and in set 19, from an AIDS patient in the 1990s, were MTL homozygous; therefore, the possibility that further isolates from these individuals may also have been homozygous at the MTL cannot be excluded.
Loss of heterozygosity associated with strain microvariation.
Among the sets of multiple single-source isolates where MLST sequence diversity was most evident, differences were seen mainly as heterozygous/homozygous changes between isolates rather than as homozygous base exchanges. In set 32, isolate WC03-200970b, which differed most markedly from its three coisolates, had 15 heterozygous SNPs, compared with 4, 9, and 10 for isolates WC03-200970d, WC03-200970e, and WC03-200970h, respectively. In the pair of isolates in set 13, L516 had 20 heterozygous SNPs, compared with 25 in L513. Similarly, in set 19 J942148 had 4 heterozygous SNPs and J942149 had 10 heterozygous SNPs. For isolate set 18, IHEM16346, which may or may not have been the progenitor isolate, had 18 heterozygous SNPs, which was more than those for IHEM3742 (11 SNPs) or RV4688 (nine SNPs). Similarly, in set 22, isolate T68 had 33 heterozygous SNPs, which was many more than the 16 heterozygous SNPs in its two sister isolates, T63 and T65.
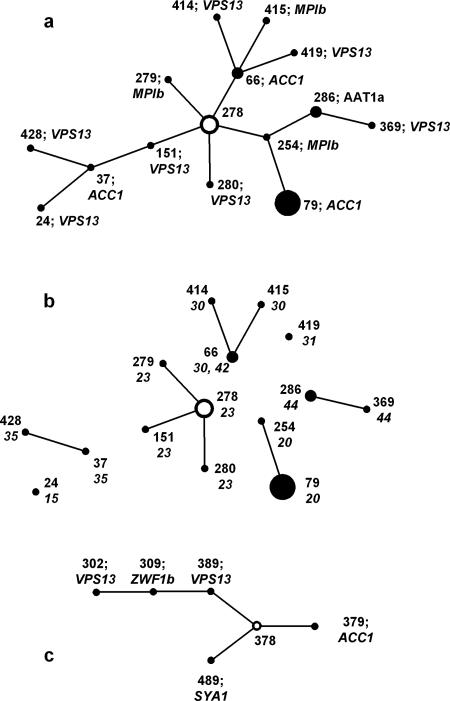
eBURST analysis of MLST data generates “clonal clusters” of isolates in which each differs from another by a single genotype among the seven sequenced. For each clonal cluster, the eBURST software determines the founder DST as the one with the greatest number of other DSTs that can be linked in steps by single locus changes (single locus variants). In the present study, most eBURST clonal clusters comprised only two isolates, but clusters 1 and 2 (Fig. 2) contained several isolates. The isolates in clonal cluster 2 (Fig. 2c) all came from a single patient. The isolates in cluster 1 (Fig. 2a) represented eight different isolate sets. The implication of clonal cluster data is of evolutionary patterns that normally show paths of isolate derivation from putative parental isolates. However, the pathways inferred from clonal cluster 1, if correct, indicate both gain and loss of heterozygosity as evolutionary mechanisms for C. albicans. For example, DST 254 in Fig. 2a differs from the putative founder DST 278 by the loss of all heterozygous SNPs at the MPIb locus. The step to DST 79 from DST 254, however, involves a gain of two heterozygous SNPs from the ACC1 locus that was fully homozygous in DST 254. Similarly, the path from DST 278 to DST 415 via DST 66 involves the gain of heterozygosity in MPIb in the final step, and the path from DST 278 to DST 37 via DST 151 requires a gain of heterozygosity in DST 37. An alternative explanation for the changes is shown in Fig. 2b, in which connecting lines between isolates from different sets have been removed so that isolates coming from a single patient or other source remain connected. The links within each set now show how the isolates could all have evolved within each patient by LOH from a different putative parent, without the clonal relationship implied by the eBURST analysis. The validity of disconnecting clonal cluster 1 in this way is emphasized by the sometimes considerable geographical distance between the isolate sources: sets 20 and 23 came from North America; sets 30, 31, and 35 came from came Australia; and sets 15 and 42 came from the United Kingdom. (Isolates in set 44 were laboratory strains.)
FIG. 2.
Clonal clusters 1 (a) and 2 (c) generated by eBURST analysis of the MLST data. For each cluster, the putative founding isolate is shown as a hollow circle. Diameters of the circles are proportionate to the numbers of isolates with the DST indicated. With each DST, the gene locus and SNP difference from the putative predecessor isolate along the path from the founder isolate are specified. The lengths of the joining lines are arbitrary. b shows clonal cluster 1 with the joining lines removed to leave different isolate sets separate; set numbers are shown next to DSTs. Only sets 30 and 42 share a common DST.
Table 2 summarizes the nature of the sequence differences between all the sets of isolates that gave nonidentical sequencing results for one or two of the test loci. The table shows that homozygous SNP changes (indicated with the letter C in the table) accounted for only four of the 38 differences found; the remaining changes were all changes from heterozygosity to homozygosity at a polymorphic base position. The loss of a single heterozygous base position, indicated with the letter A in Table 2, included those instances with only a single heterozygous base position in the entire genotype. When two or more heterozygosities were found in the sequence for one isolate and all were homozygous in the other, this was regarded as complete LOH for the locus concerned and is indicated with the letter B in Table 2. Thus, the 34 instances of putative LOH involved 21 instances in which single heterozygosities changed and 13 instances of complete LOH for a sequenced locus.
TABLE 2.
Details of differences in sequences between isolates from the same source
| Set | Clonal cluster | Nature of differencea between isolates in gene fragment
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AAT1a | ACC1 | ADP1 | MPIb | SYA1 | VPS13 | ZWF1b | Note | ||
| 1 | 4 | A | B | ||||||
| 2 | 15 | C | |||||||
| 3 | 6 | B | |||||||
| 5 | 10 | A | |||||||
| 6 | 9 | A | |||||||
| 7 | A | B | |||||||
| 8 | 7 | C | A | ||||||
| 9 | 13 | B | |||||||
| 11 | 14 | B | |||||||
| 12 | B | A | |||||||
| 18 | 2 | A | A | A | A | See Fig. 2 | |||
| 20 | 1 | B | See Fig. 2 | ||||||
| 21 | 5 | C | C | ||||||
| 23 | 1 | A | A | See Fig. 2 | |||||
| 24 | A | A | |||||||
| 26 | 3 | B | |||||||
| 27 | A | A | |||||||
| 28 | 8 | B | |||||||
| 29 | 3 | B | A | ||||||
| 30 | 1 | B | A | See Fig. 2 | |||||
| 32 | 16 | B | |||||||
| 35 | 1 | A | See Fig. 2 | ||||||
| 41 | A | B | |||||||
| 43 | 12 | A | |||||||
| 44 | See Fig. 2 | ||||||||
A, heterozygous/homozygous difference at one SNP; B, difference at all heterozygous SNPs; C, single homozygous SNP change.
MLST differences among isolates recovered from experimentally infected animals.
Because the MLST result for isolate RV2LK from set 01, recovered from the left kidney of a mouse infected with RV4688 (set 01), showed a minor difference (two more heterozygous SNPs in the recovered isolate than in the originally typed isolate), we conducted a preliminary investigation into possible MLST variations in isolates recovered from infected tissues. Isolates S09 and WC02-202294 were first spread to single colonies on Sabouraud agar, and six clones on each plate were subjected to MLST. All six clones of S09 were indistinguishable by MLST, whereas three DSTs were found among the six clones of WC02-202294. These resulted from a heterozygous/homozygous difference at one SNP in ACC1 in two instances and a similar single-site difference in ADP1 in one instance. Cloned inocula of the two strains were each injected intravenously into two mice. After 3 days, the mice were sacrificed, and colonies of C. albicans were reisolated from homogenates of kidney and brain. In repeats of MLST for five or six colonies from each organ, no differences in the DST of each strain were found.
DISCUSSION
Our study used MLST, the most dependable system for C. albicans strain differentiation, with a discriminatory power of 0.9996 (84), to investigate strain relationships in multiple isolates from single sources. None of the isolates included in this study happened to represent the most commonly encountered DST, type 69 (84), or a close relative. MLST has confirmed that sets of such isolates most often belong to a single or highly related strain type; 36 (82%) of 44 strain sets were very closely related by MLST data and had identical ABC types. MLST thus agrees with many other strain typing systems that have similarly shown the persistence of a single strain type per patient to be the most common situation. All 11 sets of isolates that included cultures from blood and nonsterile sites in the same patient fell within the 36 most closely related sets. These results clearly indicate that adult patients (none of the isolates tested came from pediatric sources) with bloodstream infections are usually infected with their own commensal isolates. This observation does not, of course, show whether the portal of endogenous infection was the patient's digestive tract or another route, such as an intravenous catheter. Results similar to ours were obtained in a recently published study from Taiwan in which both MLST and electrophoretic karyotypes of multiple isolates from nine patients in intensive care units showed high relatedness but were not always indistinguishable (13). However, multiple oral isolates taken from three HIV-positive patients over several years showed evidence of strain replacement. Our own findings are supported in the case of isolates from set 21, which were previously found by Ca3 DNA fingerprinting to be highly similar but not indistinguishable (87).
All but 2 of the 44 sets of isolates that we tested came from human sources. Among these were two sets of surveillance culture isolates (sets 7 and 8) from a patient who was admitted twice to the same hospital in March and May of 1985. While each of these individual sets of isolates clustered within this study's bounds of indistinguishability, the two sets were sufficiently different, including a change of ABC type, to provide unequivocal evidence of strain replacement between the patient's hospital admissions. No other similar, unambiguous examples of strain replacement were found. It is possible that isolate L527 (set 1) might also represent strain replacement, but the level of dissimilarity of this isolate from the rest of the set may have resulted from mishandling errors in the course of many years of maintenance. A third possibility is that the patient carried two strain types and that the less populous one was the randomly picked colony from the isolation plate only for the L527 sample.
One finding from this study concerns the storage and transmission of isolates between collections. The earliest isolates of certain date in our 44 sets were a pair of successive vaginal isolates first cultured in Leeds, United Kingdom, in 1973. Over 30 years of storage, the with removal and occasional reculture from the first author's collection in Leicester, United Kingdom, Beerse, Belgium, and now Aberdeen, United Kingdom, these isolates differed by only a single polymorphism: a change from A to G in the ADP1 sequence. Twelve other isolate sets dating from the late 1970s to the late 1980s had also been transferred between several locations and in some instances had been returned after storage in other collections. All of these isolate sets coclustered to high levels of similarity by MLST and ABC type, suggesting that their storage and maintenance had been adequate for long periods of time. By contrast, several of the isolates that had originally been received into the Leicester, United Kingdom, collection from other laboratories under the label 3153 (set 44 in the present study) were obviously subjected to handling errors at some stage in their history. In the original interlaboratory studies of these isolates (32, 41), restriction fragment length polymorphism and multilocus enzyme electrophoresis data already suggested that several of them differed from the pattern of the majority of the isolates. The MLST results now show that at least three of the isolates cannot be regarded as authentic examples of strain NCPF3153. The data for the most recently received examples of NCPF3153 define the strain as DST 369 and ABC type A. Isolate 90/189, which we received as the “authentic” strain 3153 in the 1980s, differed from this DST in two genotypes but was still type A and remained closely clustered with six other examples of “strain 3153” by MLST (Fig. 1).
The differences between the coclustered NCPF3153 isolates in Fig. 1 are of the same order of magnitude as those we interpret as microvariation in other isolate sets. The nature of these changes most often involves the loss of one or all heterozygous SNPs from one or two of the sequenced genes (Table 2). LOH as a general mechanism for microvariation changes has been previously described (16, 21, 70, 85, 88). However, the precise mechanism underlying LOH events is unknown. The list of possible mechanisms includes recombination and gene conversion. The possibility of chromosome ploidy changes must now be added to this list, since aneuploidy, including segmental aneuploidy of subchromosomal regions, has now been elegantly demonstrated in C. albicans (77). The loss of all or part of a chromosome followed by reduplication has been invoked as a mechanism underlying a range of phenotypic properties in the fungus, including sorbose utilization and fluoroorotic acid resistance (26, 94) and the generation of MTL-homozygous variants (98). If spontaneous partial ploidy changes occur as frequently in clinical isolates as was shown for the laboratory strain CAI-4 (77), the high frequency of heterozygosity changes seen among the isolates that we sequenced may not be surprising. This comment is further supported by the occasional appearance of isolates homozygous at the MTL in a temporal series of isolates that were otherwise MTL heterozygous. Wu et al. previously studied distantly located heterozygous markers on chromosome 5 on both sides of the MTL and found that whole-chromosome loss and replacement accounted for spontaneous conversion to MTL homozygosity in 15/16 isolates studied, with a recombinational event in only one isolate (98). We speculate that a complete or partial chromosome loss and reduplication, combined with occasional genetic exchange through recombination and inefficient DNA repair, account for the apparent genetic plasticity of C. albicans evidenced in our data, including apparent diploid homozygous base switches, which would be inexplicable by chance mutation. Future investigations of karyotypes and of sequences neighboring and distant from the MLST genes on the same chromosome will provide evidence for recombination, gene conversion, or aneuploidy in strain sets showing LOH and single-base homozygous changes.
Of particular note in our study are the few examples among our isolates of strains that were very closely related by MLST but that differed in ABC type (notably in sets 18 and 44). ABC type, based on the presence of one or both ITS1 sequences in rRNA genes, is usually a very stable epidemiological marker in C. albicans isolates and is sufficient information to demonstrate geographical and temporal differences among C. albicans isolates (46). It has been suggested that type C, the least common ABC type, may represent an intermediate form that is gaining or losing the type A or B ITS1 sequence (25). On this basis, and in view of our finding of two examples where types A and B were represented among types similar by MLST, we regard ABC typing as a very helpful confirmatory test in instances where isolates differ by more than one or two SNPs in MLST, but it should not be regarded as a definitive test for isolate relatedness in all instances. The rRNA genes in C. albicans are located on chromosome R; among the MLST genes, only ACC1 is located on this chromosome.
eBURST analysis of MLST data provides a useful basis for tracing putative evolutionary patterns among microbial isolates. However, in the present study, this analytical approach may have suggested invalid relationships between strains. The major clonal cluster found among our isolates (Fig. 2) suggests that there is a set of evolutionary paths between isolates that requires successive gains and losses of heterozygosity at one of the sequenced loci to be sustainable. Clonal cluster 1 is therefore unlikely to represent a true clonal relationship between these isolates of widely disparate geographical origins. One obvious mechanism for the gain of heterozygosity would be mating, but it remains uncertain to what extent mating between C. albicans isolates occurs naturally (5). If mating is the basis for the regaining of allelic heterozygosity, then the mating process would need to involve strains with dissimilar alleles; in other words, the C. albicans cells colonizing or infecting a site within a patient would need to include homozygous strains of the opposite MTL type and with different MLST DSTs. This situation appears to be quite different from the high tendency toward clonal reproduction evidenced by most isolate sets in this study as well as those described previously in other publications (66, 85). The disadvantage of the eBURST approach and other statistical methods that examine only genotype and DST assignations is that they do not distinguish whether isolates differ in sequence at a locus by a single SNP or by multiple SNPs. Hence, its value becomes limited with an organism such as C. albicans, which regularly undergoes “mutations” at multiple nucleotides within a single locus.
One hypothesis that would accommodate the otherwise conflicting evidence suggesting both clonal strain maintenance and temporal gain and loss of heterozygosities and even ABC types would be that C. albicans populations commonly exist in a state of high genetic plasticity. The phenomenon of hypermutable (“mutator”) strains is well recognized among some species of bacteria, particularly gram-negative bacilli (18, 59, 82). Hypermutability is associated with the appearance of multiple colony forms and other phenotypic variations, and it is possible that similarly variable strains exist among pathogenic Candida species. Almost all the isolates that we have studied began as single clones picked from primary isolation plates in clinical laboratories. When successive isolates from the same site in the same patient are found to be sometimes MTL homozygous and sometimes MTL heterozygous (sets 18 and 21), the most reasonable explanation is not that the clones picked randomly from a given isolation plate represent all colonies from that sample but rather that the sample contained a mixture of types. The possibility that C. albicans colonies on isolation plates often differ has been suggested by the minority of colonies that are able to undergo a white-opaque transition (36), by minor intercolony differences in DNA fingerprint patterns (the original finding characterized as “microevolution”) (35), and by colony-to-colony differences in azole antifungal susceptibility (75). In the present study, three DSTs were found among the six clones of WC02-202294 (from set 29) randomly sampled from a spread plate. If either some strains at all times or all strains in certain environments undergo recombination, gene replacement, or partial ploidy changes at elevated frequencies, the resulting mixture of strain types in the population isolated would explain the occasional superficially aberrant result in the present study. Our pilot experiment to demonstrate this phenomenon by resampling infected tissues infected with cloned C. albicans isolates failed to confirm the hypothesis; however, we plan to investigate a broader range of isolates in vivo to assess the reality, or otherwise, of strain diversity as a natural process for C. albicans. We are also investigating natural genetic diversity between colonies on primary isolation plates.
Acknowledgments
This study was supported by grants from the Wellcome Trust (69615 and 74898).
We thank individuals who have provided us with isolates, particularly James Anderson of the University of Toronto and Nicole Nolard and Francoise Symoens of the Scientific Institute of Public Health in Brussels.
REFERENCES
- 1.Asakura, K., S. I. Iwaguchi, M. Homma, T. Sukai, K. Higashide, and K. Tanaka. 1991. Electrophoretic karyotypes of clinically isolated yeasts of Candida albicans and C. glabrata. J. Gen. Microbiol. 137:2531-2538. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi, F., L. F. DiFrancesco, P. Compagnucci, D. Arzeni, O. Cirioni, and G. Scalise. 1997. Genotypic identification of sequential Candida albicans isolates from AIDS patients by polymerase chain reaction techniques. Eur. J. Clin. Microbiol. Infect. Dis. 16:601-605. [DOI] [PubMed] [Google Scholar]
- 3.Barchiesi, F., R. J. Hollis, M. Del Poeta, D. A. McGough, G. Scalise, M. G. Rinaldi, and M. A. Pfaller. 1995. Transmission of fluconazole-resistant Candida albicans between patients with AIDS and oropharyngeal candidiasis documented by pulsed-field gel electrophoresis. Clin. Infect. Dis. 21:561-564. [DOI] [PubMed] [Google Scholar]
- 4.Bartie, K. L., D. W. Williams, M. J. Wilson, A. J. C. Potts, and M. A. O. Lewis. 2001. PCR fingerprinting of Candida albicans associated with chronic hyperplastic candidosis and other oral conditions. J. Clin. Microbiol. 39:4066-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, R. J., and A. D. Johnson. 2005. Mating in Candida albicans and the search for a sexual cycle. Ann. Rev. Microbiol. 59:233-255. [DOI] [PubMed] [Google Scholar]
- 6.Boerlin, P., F. Boerlin-Petzold, J. Goudet, C. Durussel, J. L. Pagani, J. P. Chave, and J. Bille. 1996. Typing Candida albicans oral isolates from human immunodeficiency virus-infected patients by multilocus enzyme electrophoresis and DNA fingerprinting. J. Clin. Microbiol. 34:1235-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bougnoux, M.-E., D. M. Aanensen, S. Morand, M. Théraud, B. G. Spratt, and C. d'Enfert. 2004. Multilocus sequence typing of Candida albicans: strategies, data exchange and applications. Infect. Genet. Evol. 4:243-252. [DOI] [PubMed] [Google Scholar]
- 8.Bougnoux, M.-E., S. Morand, and C. d'Enfert. 2002. Usefulness of multilocus sequence typing for characterization of clinical isolates of Candida albicans. J. Clin. Microbiol. 40:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bougnoux, M.-E., A. Tavanti, C. Bouchier, N. A. R. Gow, A. Magnier, A. D. Davidson, M. C. J. Maiden, C. d'Enfert, and F. C. Odds. 2003. Collaborative consensus for optimized multilocus sequence typing of Candida albicans. J. Clin. Microbiol. 41:5265-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnie, J. P., F. C. Odds, W. Lee, C. Webster, and J. D. Williams. 1985. Outbreak of systemic Candida albicans in intensive care unit caused by cross infection. Br. Med. J. 290:746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 12.Caugant, D. A., and P. Sandven. 1993. Epidemiological analysis of Candida albicans strains by multilocus enzyme electrophoresis. J. Clin. Microbiol. 31:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, K.-W., Y.-C. Chen, H.-J. Lo, F. C. Odds, T.-H. Wang, C.-Y. Lin, and S.-Y. Li. 2006. Multilocus sequence typing for analyses of clonality of Candida albicans strains in Taiwan. J. Clin. Microbiol. 44:2172-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowen, L. E., L. M. Kohn, and J. B. Anderson. 2001. Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 183:2971-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowen, L. E., A. Nantel, M. S. Whiteway, D. Y. Thomas, D. C. Tessier, L. M. Kohn, and J. B. Anderson. 2002. Population genomics of drug resistance in Candida albicans. Proc. Natl. Acad. Sci. USA 99:9284-9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowen, L. E., D. Sanglard, D. Calabrese, C. Sirjusingh, J. B. Anderson, and L. M. Kohn. 2000. Evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 182:1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross, L. J., D. W. Williams, C. P. Sweeney, M. S. Jackson, M. A. O. Lewis, and J. Bagg. 2004. Evaluation of the recurrence of denture stomatitis and Candida colonization in a small group of patients who received itraconazole. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodont. 97:351-358. [DOI] [PubMed] [Google Scholar]
- 18.Denamur, E., S. Bonacorsi, A. Giraud, P. Duriez, F. Hilali, C. Amorin, E. Bingen, A. Andremont, B. Picard, F. Taddei, and I. Matic. 2002. High frequency of mutator strains among human uropathogenic Escherichia coli isolates. J. Bacteriol. 184:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dromer, F., L. Improvisi, B. Dupont, M. Eliaszewicz, G. Pialoux, S. Fournier, and V. Feuillie. 1997. Oral transmission of Candida albicans between partners in HIV-infected couples could contribute to dissemination of fluconazole-resistant isolates. AIDS 11:1095-1101. [DOI] [PubMed] [Google Scholar]
- 20.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forche, A., G. May, and P. T. Magee. 2005. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot. Cell 4:156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull, C. M., and J. Heitman. 2002. Fungal mating: Candida albicans flips a switch to get in the mood. Curr. Biol. 12:R782-R784. [DOI] [PubMed] [Google Scholar]
- 23.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, A. 2003. The biology of mating in Candida albicans. Nat. Rev. Microbiol. 1:106-116. [DOI] [PubMed] [Google Scholar]
- 25.Jorge, J., M. J. McCullough, and S. R. Porter. 2001. Longitudinal in vitro study of the stability of Candida albicans genotypes. Clin. Infect. Dis. 33:1195. [Google Scholar]
- 26.Kabir, M. A., A. Ahmad, J. R. Greenberg, Y. K. Wang, and E. Rustchenko. 2005. Loss and gain of chromosome 5 controls growth of Candida albicans on sorbose due to dispersed redundant negative regulators. Proc. Natl. Acad. Sci. USA 102:12147-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kam, A. P., and J. P. Xu. 2002. Diversity of commensal yeasts within and among healthy hosts. Diagn. Microbiol. Infect. Dis. 43:19-28. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 29.Lachke, S. A., S. R. Lockhart, K. J. Daniels, and D. R. Soll. 2003. Skin facilitates Candida albicans mating. Infect. Immun. 71:4970-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legrand, M., P. Lephart, A. Forche, F. M. C. Mueller, T. Walsh, P. T. Magee, and B. B. Magee. 2004. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 52:1451-1462. [DOI] [PubMed] [Google Scholar]
- 31.Leguennec, R., J. Reynes, M. Mallie, C. Pujol, F. Janbon, and J. M. Bastide. 1995. Fluconazole- and itraconazole-resistant Candida albicans strains from AIDS patients: multilocus enzyme electrophoresis analysis and antifungal susceptibilities. J. Clin. Microbiol. 33:2732-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann, P. F., L. C. Wu, and D. W. R. Mackenzie. 1991. Isoenzyme changes in Candida albicans during domestication. J. Clin. Microbiol. 29:2623-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lischewski, A., D. Harmsen, K. Wilms, G. Baier, U. Gunzer, H. Klinker, M. Wilhelm, A. Schwinn, and J. Hacker. 1999. Molecular epidemiology of Candida albicans isolates from AIDS and cancer patients using a novel standardized CARE-2 DNA fingerprinting technique. Mycoses 42:371-383. [DOI] [PubMed] [Google Scholar]
- 34.Lockhart, S. R., K. J. Daniels, R. Zhao, D. Wessels, and D. R. Soll. 2003. Cell biology of mating in Candida albicans. Eukaryot. Cell 2:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockhart, S. R., J. J. Fritch, A. S. Meier, K. Schroppel, T. Srikantha, R. Galask, and D. R. Soll. 1995. Colonizing populations of Candida albicans are clonal in origin but undergo microevolution through C1 fragment reorganization as demonstrated by DNA fingerprinting and C1 sequencing. J. Clin. Microbiol. 33:1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockhart, S. R., C. Pujol, K. J. Daniels, M. G. Miller, A. D. Johnson, M. A. Pfaller, and D. R. Soll. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockhart, S. R., B. D. Reed, C. L. Pierson, and D. R. Soll. 1996. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J. Clin. Microbiol. 34:767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lockhart, S. R., W. Wu, J. B. Radke, R. Zhao, and D. R. Soll. 2005. Increased virulence and competitive advantage of a/α over a/a or α/α offspring conserves the mating system of Candida albicans. Genetics 169:1883-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lott, T. J., R. E. Fundyga, R. J. Kuykendall, and J. Arnold. 2005. The human commensal yeast, Candida albicans, has an ancient origin. Fungal Genet. Biol. 42:444-451. [DOI] [PubMed] [Google Scholar]
- 40.MacCallum, D. M., and F. C. Odds. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48:151-161. [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie, D. W. R., and F. C. Odds. 1991. Non-identity and authentication of two major reference strains of Candida albicans. J. Med. Vet. Mycol. 29:255-261. [DOI] [PubMed] [Google Scholar]
- 42.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 43.Maiden, M. C. J. 2006. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 60:561-588. [DOI] [PubMed] [Google Scholar]
- 44.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCreight, M. C., and D. W. Warnock. 1982. Enhanced differentiation of isolates of Candida albicans using a modified resistogram method. Mykosen 25:589-598. [DOI] [PubMed] [Google Scholar]
- 46.McCullough, M., K. V. Clemons, and D. A. Stevens. 1999. Molecular epidemiology of the global and temporal diversity of Candida albicans. Clin. Infect. Dis. 29:1220-1225. [DOI] [PubMed] [Google Scholar]
- 47.McCullough, M. J., K. V. Clemons, and D. A. Stevens. 1999. Molecular and phenotypic characterization of genotypic Candida albicans subgroups and comparison with Candida dubliniensis and Candida stellatoidea. J. Clin. Microbiol. 37:417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough, M. J., B. C. Ross, B. D. Dwyer, and P. C. Reade. 1994. Genotype and phenotype of oral Candida albicans from patients infected with the human immunodeficiency virus. Microbiology 140:1195-1202. [DOI] [PubMed] [Google Scholar]
- 49.Mercure, S., S. Poirier, G. Lemay, P. Auger, S. Montplaisir, and L. Derepentigny. 1993. Application of biotyping and DNA typing of Candida albicans to the epidemiology of recurrent vulvovaginal candidiasis. J. Infect. Dis. 168:502-507. [DOI] [PubMed] [Google Scholar]
- 50.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 51.Nebavi, F., S. Arnavielhe, R. Leguennec, E. Menan, A. Kacou, P. Combe, E. Aoussi, M. Mallie, M. Kone, and J. M. Bastide. 1998. Oropharyngeal candidiasis in AIDS patients from Abidjan (Ivory Coast)—antifungal susceptibilities and multilocus enzyme electrophoresis analysis of Candida albicans isolates. Pathol. Biol. 46:307-314. [PubMed] [Google Scholar]
- 52.Odds, F. C. 1991. Long-term preservation of pathogenic yeasts under distilled water. J. Med. Vet. Mycol. 29:413-415. [DOI] [PubMed] [Google Scholar]
- 53.Odds, F. C., and A. B. Abbott. 1981. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia 18:301-317. [PubMed] [Google Scholar]
- 54.Odds, F. C., and E. G. V. Evans. 1980. Distribution of pathogenic yeasts and humoral antibodies to Candida among hospitalized patients. J. Clin. Pathol. 33:750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odds, F. C., C. C. Kibbler, E. Walker, A. Bhamra, H. G. Prentice, and P. Noone. 1989. Carriage of Candida species and C. albicans biotypes in patients undergoing chemotherapy or bone marrow transplantation for haematological disease. J. Clin. Pathol. 42:1259-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odds, F. C., A. Palacio-Hernanz, J. Cuadra, and J. Sanchez. 1987. Disseminated Candida infection syndrome in heroin addicts—dominance of a single Candida albicans biotype. J. Med. Microbiol. 23:275-277. [DOI] [PubMed] [Google Scholar]
- 57.Odds, F. C., L. Van Nuffel, and G. Dams. 1998. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J. Clin. Microbiol. 36:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odds, F. C., L. Van Nuffel, and N. A. R. Gow. 2000. Survival in experimental Candida albicans infections depends on inoculum growth conditions as well as animal host. Microbiology 146:1881-1889. [DOI] [PubMed] [Google Scholar]
- 59.Oliver, A., R. Cantón, P. Campo, F. Baquero, and J. Blázquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 60.Pfaller, M. A., I. Cabezudo, R. Hollis, B. Huston, and R. P. Wenzel. 1990. The use of biotyping and DNA fingerprinting in typing Candida albicans from hospitalized patients. Diagn. Microbiol. Infect. Dis. 13:481-48489. [DOI] [PubMed] [Google Scholar]
- 61.Pfaller, M. A., J. Rhine-Chalberg, S. W. Redding, J. Smith, G. Farinacci, A. W. Fothergill, and M. G. Rinaldi. 1994. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J. Clin. Microbiol. 32:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pittet, D., M. Monod, I. Filthuth, E. Frenk, P. M. Suter, and R. Auckenthaler. 1991. Contour-clamped homogeneous electric field gel electrophoresis as a powerful epidemiologic tool in yeast infections. Am. J. Med. 91:S256-S263. [DOI] [PubMed] [Google Scholar]
- 63.Pittet, D., M. Monod, P. M. Suter, E. Frenk, and R. Auckenthaler. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pujol, C., S. Joly, S. R. Lockhart, S. Noel, M. Tibayrenc, and D. R. Soll. 1997. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J. Clin. Microbiol. 35:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pujol, C., S. Joly, B. Nolan, T. Srikantha, and D. R. Soll. 1999. Microevolutionary changes in Candida albicans identified by the complex Ca3 fingerprinting probe involve insertions and deletions of the full-length repetitive sequence RPS at specific genomic sites. Microbiology 145:2635-2646. [DOI] [PubMed] [Google Scholar]
- 66.Pujol, C., J. Reynes, F. Renaud, M. Raymond, M. Tibayrenc, F. J. Ayala, F. Janbon, M. Mallie, and J. M. Bastide. 1993. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc. Natl. Acad. Sci. USA 90:9456-9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reagan, D. R., M. A. Pfaller, R. J. Hollis, and R. P. Wenzel. 1990. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J. Clin. Microbiol. 28:2733-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robles, J. C., L. Koreen, S. Park, and D. S. Perlin. 2004. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discrimination among strains of Candida albicans. J. Clin. Microbiol. 42:2480-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samaranayake, Y. H., L. P. Samaranayake, R. S. Dassanayake, J. Y. Y. Yau, W. K. Tsang, B. P. K. Cheung, and K. W. S. Yeung. 2003. ‘Genotypic shuffling’ of sequential clones of Candida albicans in HIV-infected individuals with and without symptomatic oral candidiasis. J. Med. Microbiol. 52:349-359. [DOI] [PubMed] [Google Scholar]
- 70.Sampaio, P., L. Gusmão, A. Correia, C. Alves, A. G. Rodrigues, C. Pina-Vaz, A. Amorim, and C. Pais. 2005. New microsatellite multiplex PCR for Candida albicans strain typing reveals microevolutionary changes. J. Clin. Microbiol. 43:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sangeorzan, J. A., S. F. Bradley, X. G. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am. J. Med. 97:339-346. [DOI] [PubMed] [Google Scholar]
- 72.Schmid, J., F. C. Odds, M. J. Wiselka, K. G. Nicholson, and D. R. Soll. 1992. Genetic similarity and maintenance of Candida albicans strains from a group of AIDS patients, demonstrated by DNA fingerprinting. J. Clin. Microbiol. 30:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid, J., M. Rotman, B. Reed, C. Pierson, and D. Soll. 1993. Genetic similarity of Candida albicans strains from vaginitis patients and their partners. J. Clin. Microbiol. 31:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schonian, G., O. Meusel, H. Tietz, W. Meyer, Y. Graser, I. Tausch, W. Presber, and T. G. Mitchell. 1993. Identification of clinical strains of Candida albicans by DNA fingerprinting with the polymerase chain reaction. Mycoses 36:171-179. [DOI] [PubMed] [Google Scholar]
- 75.Schoofs, A., F. C. Odds, R. Colebunders, M. Ieven, L. Wouters, and H. Goossens. 1997. Isolation of Candida species on media with and without added fluconazole reveals high variability in relative growth susceptibility phenotypes. Antimicrob. Agents Chemother. 41:1625-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schroppel, K., M. Rotman, R. Galask, K. Mac, and D. R. Soll. 1994. Evolution and replacement of Candida albicans strains during recurrent vaginitis demonstrated by DNA fingerprinting. J. Clin. Microbiol. 32:2646-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selmecki, A., S. Bergmann, and J. Berman. 2005. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 55:1553-1565. [DOI] [PubMed] [Google Scholar]
- 78.Soll, D. R. 2000. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 13:332-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soll, D. R. 2004. Mating-type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioessays 26:10-20. [DOI] [PubMed] [Google Scholar]
- 80.Soll, D. R., S. R. Lockhart, and R. Zhao. 2003. Relationship between switching and mating in Candida albicans. Eukaryot. Cell 2:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephan, F., M. S. Bah, C. Desterke, S. Rezaiguia-Delclaux, F. Foulet, P. Duvaldestin, and S. Bretagne. 2002. Molecular diversity and routes of colonization of Candida albicans in a surgical intensive care unit, as studied using microsatellite markers. Clin. Infect. Dis. 35:1477-1483. [DOI] [PubMed] [Google Scholar]
- 82.Taddei, F., M. Radman, J. Maynard-Smith, B. Toupance, P. H. Gouyon, and B. Godelle. 1997. Role of mutators in adaptive evolution. Nat. Biotechnol. 387:700-702. [Google Scholar]
- 83.Takasuka, T., G. G. Baily, M. Birch, M. J. Anderson, D. Law, and D. W. Denning. 1998. Variation in morphotype, karyotype and DNA type of fluconazole resistant Candida albicans from an AIDS patient. J. Infect. 36:57-62. [DOI] [PubMed] [Google Scholar]
- 84.Tavanti, A., A. D. Davidson, M. J. Fordyce, N. A. R. Gow, M. C. J. Maiden, and F. C. Odds. 2005. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J. Clin. Microbiol. 43:5601-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tavanti, A., N. A. R. Gow, M. C. J. Maiden, F. C. Odds, and D. J. Shaw. 2004. Genetic evidence for recombination in Candida albicans based on haplotype analysis. Fungal Genet. Biol. 41:553-562. [DOI] [PubMed] [Google Scholar]
- 86.Tavanti, A., N. A. R. Gow, S. Senesi, M. C. J. Maiden, and F. C. Odds. 2003. Optimization and validation of multilocus sequence typing for Candida albicans. J. Clin. Microbiol. 41:3765-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tavanti, A., A. Lupetti, E. Ghelardi, V. Corsini, P. Davini, F. Filipponi, U. Boggi, G. Biancofiore, M. Campa, and S. Senesi. 2001. Molecular monitoring of Candida albicans infections in liver transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 20:544-553. [DOI] [PubMed] [Google Scholar]
- 88.Tsang, P. W. K., B. Cao, P. Y. L. Siu, and J. Wang. 1999. Loss of heterozygosity, by mitotic gene conversion and crossing over, causes strain-specific adenine mutants in constitutive diploid Candida albicans. Microbiology 145:1623-1629. [DOI] [PubMed] [Google Scholar]
- 89.van Belkum, A., W. Melchers, B. E. Depauw, S. Scherer, W. Quint, and J. F. Meis. 1994. Genotypic characterization of sequential Candida albicans isolates from fluconazole-treated neutropenic patients. J. Infect. Dis. 169:1062-1070. [DOI] [PubMed] [Google Scholar]
- 90.Vargas, K. G., and S. Joly. 2002. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J. Clin. Microbiol. 40:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vazquez, J. A., J. D. Sobel, R. Demitriou, J. Vaishampayan, M. Lynch, and M. J. Zervos. 1994. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J. Infect. Dis. 170:1566-1569. [DOI] [PubMed] [Google Scholar]
- 92.Verma, A. K., K. N. Prasad, M. Singh, A. K. Dixit, and A. Ayyagari. 2003. Candidaemia in patients of a tertiary health care hospital from north India. Ind. J. Med. Res. 117:122-128. [PubMed] [Google Scholar]
- 93.Voss, A., R. J. Hollis, M. A. Pfaller, R. P. Wenzel, and B. N. Doebbeling. 1994. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J. Clin. Microbiol. 32:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wellington, M., and E. Rustchenko. 2005. 5-Fluoro-orotic acid induces chromosome alterations in Candida albicans. Yeast 22:57-70. [DOI] [PubMed] [Google Scholar]
- 95.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White, T. C., M. A. Pfaller, M. G. Rinaldi, J. Smith, and S. W. Redding. 1997. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 3(Suppl.):S102-S109. [DOI] [PubMed] [Google Scholar]
- 97.Wilson, M. J., D. W. Williams, M. D. L. Forbes, I. G. Finlay, and M. A. O. Lewis. 2001. A molecular epidemiological study of sequential oral isolates of Candida albicans from terminally ill patients. J. Oral Pathol. Med. 30:206-212. [DOI] [PubMed] [Google Scholar]
- 98.Wu, W., C. Pujol, S. R. Lockhart, and D. R. Soll. 2005. Chromosome loss followed by duplication is the major mechanism of spontaneous mating-type locus homozygosis in Candida albicans. Genetics 169:1311-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]